Introduction
Gastrointestinal stromal tumors (GIST) appear to originate from interstitial cells of Cajal (ICC) or their stem cell-like precursors.1 ICC are pacemaker-like intermediates between the GI autonomic nervous system and smooth muscle cells regulating GI motility and autonomic nerve function. Approximately 95% of GIST are positive for the CD117 antigen, an epitope of KIT receptor tyrosine kinase expressed by ICC.2 KIT-positive and KIT-dependent, ICC are located around the myenteric plexus and the muscularis propria throughout the GI tract. CD 117 is not a specific marker of GIST but may be expressed by other mesenchymal tumors too. DOG1 (Discovered on GIST) is a protein of unknown function that is expressed strongly on GIST and is rarely expressed on other soft tissue tumors.3 Around 85% of GIST possess oncogenic mutation in any two of the tyrosine kinase receptors the C KIT and the platelet derived growth factor receptor alpha (PDGFRA). Activation of either of these receptors plays a central role in the pathogenesis of GIST.1 The proper identification of GIST with genotyping is very important because of the availability of specific, molecular-targeted therapy with KIT/PDGFRA tyrosine kinase inhibitors (TKI), such as imatinib mesylate or, in the case of imatinib-resistant GIST, sunitinib malate.4
Case report
A 58 years old male patient presented with a protuberant abdomen along with distension, and dragging pain in hypogastrium. No history of GI bleed or any symptoms suggestive of intestinal obstruction was found. The hemogram and biochemical investigations were within normal limits. MRI abdomen revealed a large intra-abdominal mass arising from the sigmoid colon (Fig. 1). Per-operatively there was a large mass arising from the sigmoid colon. The patient underwent sigmoid colectomy. The gross specimen weighed 4.6 kg with a size of 25 × 20 × 20 cm. The mass was found arising from the sigmoid colon (Fig. 2). Histomorphology of the tumor revealed a stromal tumor arising from the wall of the sigmoid with cells arranged in fascicles. The cells had eosinophilic cytoplasm and oval to spindle shaped nucleus with blunt ends (Fig. 3). There were scattered giant cells with 3–4 nuclei and areas with predominantly these cells. The tumor had a mitotic score of 10/50 hpf. There were areas of necrosis and myxoid change. The tumor was evaluated with Immunohistochemistry. It was weakly CD34 positive but negative for CD117 (Name of Ab CD 117 rabbit monoclonal; clone Y 145; dilution 1: 100; manufacturer-Biocare; place of manufacture – Concord U.S.A.) and DOG 1 (Name of Ab Dog1 mouse monoclonal; clone Dog 1.1; dilution 1:100; manufacturer-Biocare; place of manufacture – Concord U.S.A.). SMA and S-100 were also performed to rule out other tumors of mesenchymal origin and were found to be negative. The tumor was subjected to C KIT mutation evaluation as the imaging and histomorphology of the tumor was suggestive of GIST. The tumor was found to have mutated Exon 11 of deletion type with normal or wild type Exon 9 among the C KIT genes. Mutational analysis was outsourced. The protocol was performed on de-paraffinised section post DNA extraction. Polymerase chain reaction amplification was used followed by denaturing high-performance liquid chromatography screening, and automated sequencing.
Fig. 1.
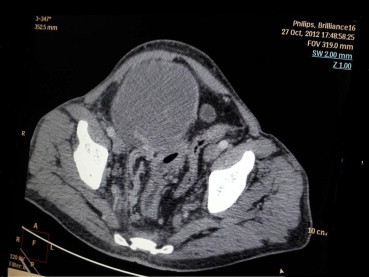
CT scan image of the tumor.
Fig. 2.
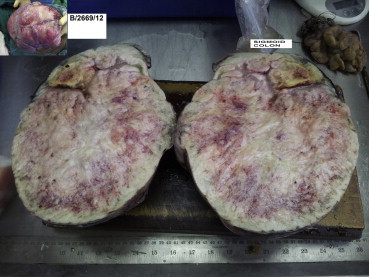
Cut surface of the tumor with sigmoid colon segment. Inset showing external surface per-operatively.
Fig. 3.
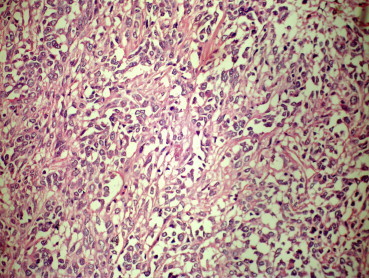
H&E stained section of the tumor 40× with oval to spindle cell morphology.
Discussion
Our case had a very large lesion which was found arising from the wall of the sigmoid colon. Colonic GISTs are extremely rare and constitute only 1–2% of these neoplasms. The commonest location being the stomach.5 On histomorphology beside the spindle cells, few scattered tumor giant cells with 2–3 nuclei were also seen. This tumor is of high risk stratification based on mitotic score, size of lesion and origin of tumor.2 The phenomenon of presence of giant cells has been reported in the past in GIST.6 The usual immuno-histochemical panel used for diagnosis of GIST and exclusion of other mesenchymal tumors are CD34, S-100, SMA, CD 117 and DOG 1. CD117 (C KIT) is the Kit protein an epitope of tyrosine kinase receptor. It is positive in 95% GIST.2 It is also found to be positive in certain normal tissues like breast ductal cells & melanocytes. Certain non-GIST tumors too stain with CD 117 antibodies like follicular & papillary carcinoma of thyroid, melanomas and small cell carcinomas. But none can usually confuse with GIST. It shows membrane and cytoplasmic positivity. DOG 1 is a protein of unknown significance but has been found up regulated in GIST. It is quite sensitive and specific for GIST.3 It shows membrane and cytoplasmic staining. In our case both CD117 and DOG 1 was negative. The tumor tissue was subjected to cytogenetic evaluation for CKIT mutations. Exon 11 showed presence of deletion mutation. Exon 9 had wild or normal sequence. Due to availability of targeted tyrosine kinase inhibitor therapy with imatinib and sunitinib, cytogenetic evaluation for C KIT mutation must be undertaken for cases where imaging and histomorphology is suggestive of GIST and the tumor is CD117 and DOG 1 negative. Review of literature for CD117 negative GISTs revealed seven out of 108 (6%) cases GIST to be negative for CD117 in a study.7 All these had KIT mutations located in the exon 11 only. In another detailed study of 81 cases diagnosed as GIST, correlation has been presented for IHC staining for CD117 and DOG 1 with C KIT and PDGFR gene mutation.8 Out of 81 cases of GISTs studied, 28 (34.5%) were negative for CD117 staining. Seventeen of these cases were also negative for DOG 1. Thus 17 out of 81 (21%) cases were negative for both CD117 and DOG 1. Out of these 17 cases, C KIT and PDGFR mutation analysis was conducted in 12 cases. These 12 cases revealed presence of C KIT, exon 11 mutation in two cases and PDGFR mutation in seven cases. The remaining three cases which were negative for CD117/DOG 1 staining and also C KIT/PDGFR mutation showed at least focal CD34 expression in association with SMA and/or Caldesmon. Review of Pub Med for Indian citations revealed two large studies of 92 and 121 cases of GIST revealed a CD117 positivity of 95% and 94% respectively.9,10 However no Indian study depicting DOG 1 and C KIT mutation pattern was found. Compared to patients with KIT exon 11 mutations, patients with exon 9 mutations have a better relapse free survival and overall survival; however, their tumors show intermediate sensitivity to imatinib Median duration of benefit from imatinib is approximately 7–12 months compared to 23 months for patients with exon 11 mutations.11 Kit exons 9,11,13 and 17 and PDGFRA exons 10,12, 14 and 18 have been sequenced to identify GIST. Our tumor qualified for high risk stratification and GISTs with Exon 11 mutations are preferentially in malignant GIST than benign ones.12 C KIT mutation studies are more sensitive than DOG 1.1 for intestinal GISTs13 and hence the cytogenetic evaluation is felt necessary in cases like ours.
Conclusion
Due to availability of molecular-targeted therapy it becomes mandatory to perform cytogenetic studies in cases with imaging and histomorphology suggestive of GIST though CD117 and DOG 1 immuno-histochemical markers are negative.
Conflicts of interest
All authors have none to declare.
References
- 1.Hirota S., Isozaki K., Moriyama Y. Gain-of-function mutations of C-KIT in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 2.Corless C.L., Heinrich M.C. Molecular pathobiology of gastrointestinal stromal sarcomas. Annu Rev Pathol. 2008:557–586. doi: 10.1146/annurev.pathmechdis.3.121806.151538. [DOI] [PubMed] [Google Scholar]
- 3.West R.B., Corless C.L., Chen X. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am J Pathol. 2004;165:107–113. doi: 10.1016/S0002-9440(10)63279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demetri G.D., von Mehren M., Blanke C.D. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–480. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 5.Edge S.B., Byrd D.R., Compton C.C. American Joint Committee on Cancer Staging Manual. 7th ed. Springer; New York: 2010. Gastrointestinal stromal tumor; pp. 175–180. [Google Scholar]
- 6.Leung Kai Man, Wong Shun, Chow Tat Chong, Lee Kam Cheong. A malignant gastrointestinal stromal tumor with osteoclast-like giant cells. Arch Pathol Lab Med. 2002;126:972–974. doi: 10.5858/2002-126-0972-AMGSTW. [DOI] [PubMed] [Google Scholar]
- 7.Tzen Chin-Yuan, Mau Bey-Liing. Analysis of CD117-negative gastrointestinal stromal tumors. World J Gastroenterol. 2005;11:1052–1055. doi: 10.3748/wjg.v11.i7.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liegl Bernadette, Hornick Jason L., Corless Christopher L., Fletcher Christopher D.M. Monoclonal antibody DOG1.1 shows higher sensitivity than KIT in the diagnosis of gastrointestinal stromal tumors, including unusual subtypes. Am J Surg Pathol. 2009;33:437–446. doi: 10.1097/PAS.0b013e318186b158. [DOI] [PubMed] [Google Scholar]
- 9.Lakshmi V.A., Chacko R.T., Kurian S. Gastrointestinal stromal tumors: a 7-year experience from a tertiary care hospital. Indian J Pathol Microbiol. 2010;53:628–633. doi: 10.4103/0377-4929.72005. [DOI] [PubMed] [Google Scholar]
- 10.Vij M., Agrawal V., Kumar A., Pandey R. Gastrointestinal stromal tumors: a clinicopathological and immunohistochemical study of 121 cases. Indian J Gastroenterol. 2010;29:231–236. doi: 10.1007/s12664-010-0079-z. [DOI] [PubMed] [Google Scholar]
- 11.Heinrich M.C., Maki R.G., Corless C.L. Primary and secondary kinase genotypesrrelate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J Clin Oncol. 2008;26:5352–5359. doi: 10.1200/JCO.2007.15.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lasota Jerzy, Jasinski Marek, Sarlomo-Rikala Maarit, Miettinen Markku. Mutations in exon 11 of C-KIT occur preferentially in malignant versus benign gastrointestinal stromal tumors and do not occur in leiomyomas or leiomyosarcomas. Am J Pathol. 1999;154:53–60. doi: 10.1016/S0002-9440(10)65250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miettinen M., Wang Z.F., Lasota J. DOG1 antibody in the differential diagnosis of gastrointestinal stromal tumors: a study of 1840 cases. Am J Surg Pathol. 2009;33:1401–1408. doi: 10.1097/PAS.0b013e3181a90e1a. [DOI] [PubMed] [Google Scholar]


