Abstract
We examined whether the combined indices of respiratory sinus arrhythmia at rest (resting RSA) and in response to a sad film (RSA reactivity) predict effective and ineffective responses to reduce sadness (adaptive vs. maladaptive mood repair) in women with histories of juvenile-onset depression (n = 74) and no history of major mental disorders (n = 75). Structural equation models were used to estimate latent resting RSA, depression, and adaptive and maladaptive mood repair and to test the study hypotheses. Results indicated that combinations of resting RSA+RSA reactivity (RSA patterns) predicted maladaptive mood repair, which in turn, mediated the effects of RSA pattern on depression. Further, RSA patterns moderated the depressogenic effects of maladaptive mood repair. RSA patterns were unrelated to adaptive mood repair. Our findings suggest that mood repair is one mechanism through which physiological vulnerabilities adversely affect mental health.
1. Introduction
A growing literature has implicated abnormalities in the autonomic nervous system, particularly its parasympathetic branch, in dysphoria (Kreibig, 2010) and clinical depression (Kemp et al., 2010; Rottenberg, 2007). One index of parasympathetic functioning, respiratory sinus arrhythmia (RSA), is a naturally occurring variation in heart rate. RSA occurs during the breathing cycle and is primarily driven by the influence of the vagus nerve and respiration. In the absence of environmental challenges, the vagus modulates the cardiac cycle by serving as a “brake” (resting RSA) on heart rate and other sympathetic nervous system activity (Porges, Doussard-Roosevelt, Portales, & Greenspan, 1996). However, the “brake” is adjusted in response to environmental contexts (RSA reactivity), in that it is withdrawn in response to most environmental challenges (Porges et al., 1996) and is strengthened or augmented following positive events (Kreibig, 2010). Thus, while resting RSA represents a relative maximum of parasympathetic nervous system gated energy reserves; RSA reactivity reflects the efficiency with which these reserves can be directed toward cognitive, emotional, and behavioral responses to environmental demands.
Resting RSA and RSA reactivity have been investigated intensively as indicators of self-regulation and vulnerability to depressive disorders), typically as separate predictors (Beauchaine, 2001; Rottenberg, 2007). Importantly, however, it has been suggested that the relationship between resting RSA and RSA reactivity may reveal more about self-regulation and its relationship to mood disorders than either RSA metric independently (El-Sheikh & Erath, 2011; Yaroslavsky, Rottenberg, & Kovacs, 2013. Theoretical models, such as that proposed by Thayer and Lane, highlight the function of resting RSA and RSA reactivity as a part of a larger autonomic space that sets constraints on the intensity of emotional responding and regulation efforts (e.g., Thayer & Lane, 2000; 2009). Ultimately, it is the bi-directional communication between neural circuits responsible for attention and goal-directed behaviors and the autonomic nervous system that sets the bounds for flexible responding to environmental challenges (Thayer & Lane, 2000; 2009).
Consistent with the neurovisceral integration model (Thayer & Lane, 2000; 2009), resting RSA and RSA reactivity have recently been examined conjointly in order to fully understand the role of the parasympathetic nervous system (PNS) in adaptive functioning (Del Giudice, Ellis, & Shirtcliff, 2011; Hinnant & El-Sheikh, 2009; Yaroslavsky et al., 2013). Based on theory and the promising initial data from these investigations (reviewed below), we propose that RSA withdrawal to negative mood triggers, combined with high resting RSA, represents an optimal RSA pattern that supports successful self-regulation and buffers against depression. Conversely, combinations of high resting RSA and RSA augmentation or low resting RSA and RSA withdrawal to negative affect represent sub-optimal patterns for self-regulation that heighten risk for depression.
1.1 RSA patterns and depression
Most studies of depression examine individual relationships between RSA and depression. High resting RSA levels correlate with effective self-regulation, while low levels of resting RSA relate to increased emotional lability and depression (Beauchaine, 2001; Kemp et al., 2010; Rottenberg, 2007; Thayer & Lane, 2000. Resting RSA is associated with activity in neural circuits that underlie emotional processing and hedonic experience (Thayer, Åhs, Fredrikson, Sollers, & Wager, 2012), and low resting RSA correlates with self-report deficits in hedonic emotions (Ingjaldsson, Laberg, & Thayer, 2003; Oveis et al., 2009). However, some studies have failed to find relationships between RSA levels and depression (e.g., Lehofer et al., 1997; Moser et al., 1998), which have led some to suggest that it may also be important to consider RSA reactivity (see Rottenberg, Clift, Bolden & Salomon, 2007). Indeed, irregular RSA reactivity is also linked to current depression (Rottenberg, et al., 2007) and is a predictor of poor depression course (Rottenberg, Salomon, Gross, & Gotlib, 2005). For example, currently depressed individuals are more likely to evidence RSA augmentation (rather than appropriate RSA withdrawal) in response to stress (Rottenberg et al., 2007), and depressed subjects who fail to show RSA withdrawal to sad film clips are more likely to remain depressed six months later (Rottenberg et al., 2005).
Only recently have studies examined the value of combining resting RSA and RSA reactivity indices to understand psychopathology. For example, subjects with optimal RSA patterns (high resting RSA+RSA withdrawal) evidenced reduced internalizing symptoms over a two-year period (Hinnant & El-Sheikh, 2009) and maintained positive affect while recalling stressful events (Cribbet, Williams, Gunn, & Rau, 2011). Conversely, subjects with sub-optimal RSA patterns (low resting RSA+RSA withdrawal; high resting RSA+RSA augmentation) evidenced the highest levels of internalizing symptoms (Hinnant & El-Sheikh, 2009). We have also shown that RSA reactivity moderates the relationship between resting RSA and depression: RSA withdrawal to a sad film in the context of high resting RSA predicted the lowest levels of depressive symptoms and the least likelihood of histories of juvenile-onset depression (Yaroslavsky et al., 2013). Importantly, results from Yaroslavsky and colleagues (2013) showed that the combination of high resting RSA and RSA reactivity was predictive of depression outcomes but neither metric was predictive on its own.
1.2 Mood repair and depression
RSA abnormalities have repeatedly been shown to relate to impairments in self-regulation (Thayer & Lane, 2009). Depression is a mood disorder (APA, 2000); thus, the connection between RSA and depression may operate through the regulation of dysphoric mood, a construct known as mood repair. Mood repair refers to the suite of self-regulatory cognitive processes, instrumental behaviors, and interpersonal interactions that can serve to reduce sadness and dysphoria (see Parkinson & Totterdell, 1999, for review). Existing research supports a differentiation between adaptive mood repair responses (adaptive repair) that generally attenuate dysphoria appropriately in the short- and long-term, and maladaptive mood repair responses (maladaptive repair) that typically have the unintended effect of prolonging or exacerbating dysphoria (Kovacs, Rottenberg, & George, 2009). Adaptive repair responses include distraction (Blagden & Craske, 1996; Joorman, Siemer, & Gotlib, 2007), cognitive reappraisal (see Gross, 2002), and seeking interpersonal support (see Marroquín, 2011). Conversely, maladaptive repair responses include suppressing distressing emotions (Ehring, Tuschen-Caffier, Schnüle, Fischer, & Gross, 2010) and rumination (Blagden & Craske, 1996; Lyubomirsky, Caldwell, & Nolen-Hoeksema, 1998).
Tendencies to engage in frequent maladaptive repair (e.g., rumination) and infrequent adaptive repair (e.g., cognitive reappraisal) are associated with depression symptoms (Aldao, Nolen-Hoeksema, & Schweizer, 2010) and predict the onset of first episodes and recurrence of depression (Mueller, Lavori, Keller, & Swartz, 1994; Nolen-Hoeksema, Schepis & McCabe, 2012; Spasojevic & Alloy, 2001; Wisco, & Lyubomirsky, 2008). Further, never-depressed youth at high familial risk for depression have extensive maladaptive repair repertoires and a limited range of adaptive responses compared to low-risk peers (Gentzler, Santucci, Kovacs, & Fox, 2009). They also more frequently engage in maladaptive strategies such as ruminative thinking (Thompson et al., 2010) and behavioral passivity (Silk, Shaw, Skuban, Oland, & Kovacs, 2006; but see Joormann, Cooney, Henry, & Gotlib, 2012). Importantly, our own work demonstrates problematic mood repair tendencies are detectable in persons remitted from depression, suggesting that these tendencies may reflect a trait-like vulnerability (Kovacs et al., 2009). Thus, it is likely that frequent use of maladaptive repair and insufficient use of adaptive mood repair contribute to depression symptoms and clinically significant episodes of depression.
1.3 RSA patterns and mood repair
Despite the logical connection between RSA patterns and mood repair, there has been no research to our knowledge that has directly examined this association. There is considerable indirect evidence from work that tests RSA reactivity and resting RSA as independent predictors of related constructs. For instance, high resting RSA and RSA withdrawal independently predict improved emotion regulation abilities in adolescence (Vasilev, Crowell, Beauchaine, Mead, Gatzke-Kopp, 2009). High resting RSA is also inversely correlated with the use of maladaptive cognitive strategies (Ingjaldsson et al., 2003; Ode, Hilmert, Zielke, & Robinson, 2010) and inappropriate interpersonal responses during distress (Gyurak & Ayduk, 2008). Further, high resting RSA is related to the use of adaptive cognitive strategies (Volokhov & Demaree, 2010) social engagement (Hopp, Shallcross, Ford, Troy, Wilhelm, & Mauss, 2013) and adaptive coping responses by reducing the intensity of negative arousal during stress (Fabes & Eisenberg, 1997). At the neural level, resting RSA levels show a strong correspondence with activity in cortical areas known to subsume emotion regulation processes that are altered in depressed subjects (e.g., ventromedial prefrontal cortex; Thayer et al., 2012; Phillips, Drevets, Rauch, & Lane, 2003).
Indirect evidence suggests that high resting RSA may moderate the effectiveness of mood repair. For example, journaling reduced depressive symptoms only for those subjects with high resting RSA (O’Connor, Allen, & Kaszniak, 2005; Sloan & Epstein 2005). These findings may indicate that high resting RSA potentiates effective emotional processing believed to underlie the beneficial effects of journaling (Esterling, L’Abate, Murray, & Pennebaker, 1999). By extension, these findings suggest the possibility that RSA patterns may moderate the relationship between mood repair and depression.
1.4 The present study
In the present study, we propose and test a framework in which the combination of RSA indicators (RSA rest and RSA reactivity) predicts risk for depression and the use of mood repair responses, and they may moderate the effects of mood repair better than each RSA indicator on its own. Within this framework, a sub-optimal RSA pattern serves a vulnerability factor for depression. This vulnerability is in-part brought about by an increased use of maladaptive mood repair strategies (mood regulation responses that prolong or exacerbate distress), and reduced adaptive mood repair strategies (responses that reduce distress). By contrast, an optimal RSA pattern would protect against depression risk and promote adaptive mood repair strategies. In our framework, the use of adaptive and maladaptive mood repair responses will partially mediate the association between RSA patterns and depression. Finally, we posit that RSA patterns may modulate the affective consequences of mood repair efforts. That is, an optimal RSA pattern attenuates distress secondary to the use of maladaptive strategies, and potentiates salubrious effects of adaptive responses.
While our previous work demonstrated the incremental utility of RSA patterns for predicting depressive symptoms and histories of depressive disorders over individual RSA indices (Yaroslavsky et al., 2013), it did not test possible mechanisms. In this study, we aimed to clarify our prior findings in two important ways: 1) mood repair was tested as one mechanism by which RSA patterns predict depression, and 2) RSA patterns were tested as moderators for mood repair outcomes. We tested our hypotheses in the context of RSA reactivity to sadness induction, as sadness is highly relevant to mood repair and depression (Kovacs et al., 2009). We defined the optimal RSA pattern to a sad mood induction as a combination of high resting RSA and RSA withdrawal. We hypothesized that this optimal RSA pattern would be inversely related to depression symptom severity, and that this relationship would a) be partially mediated through reduced maladaptive repair and increased adaptive repair, and b) attenuate the adverse effects of maladaptive repair but potentiate salubrious effects of adaptive mood repair on depressive symptoms. Conversely, we expected suboptimal RSA patterns (defined as combinations of high resting RSA+RSA augmentation or low resting RSA+RSA withdrawal) would be positively related to depression symptom severity, which in turn, would be mediated through increased maladaptive mood repair and decreased adaptive mood repair. We further hypothesized that suboptimal RSA patterns would exacerbate the adverse effects of maladaptive mood repair on depression symptoms.
Study hypotheses were tested utilizing structural equation models (SEM) in a sample of female subjects, who had been included in previous analyses of RSA combinations on depression (Yaroslavsky et al., 2013). We focused on women in order to reduce heterogeneity associated with sex differences in RSA reactivity (El-Sheikh, Hinnant & Erath, 2011; Yaroslavsky et al., 2013) and mood repair (Kovacs et al., 2009; Nolen-Hoeksema & Aldao, 2011). Further, to increase measurement precision (see Bollen, 1989), we used latent variables to represent depression, adaptive mood repair, and maladaptive mood repair. As in our prior paper (Yaroslavsky et al., 2013), a latent resting RSA factor was also estimated from multiple measurements during the study protocol.
2. Method
2.1 Participants
Participants were 149 adult females from a longitudinal Program Project (PP) on risk factors for juvenile-onset depression (JOD). In terms of demographics, 76% were Caucasian, 21% African American, and 3% represented the “other” racial category. Women with histories of JOD (Probands, n= 74) were younger (Mcontrols = 29.59, SD = 5.85; MJOD = 26.29, SD = 3.73; t[126] = 4.11, p < .001), less likely to be of African American or “other” racial descent (χ2 [2] = 7.19, p < .05), and were more likely to use psychotropic medication (ncontrol = 0 vs. nJOD = 29) than controls.
For probands, mean age at onset of the first depressive episode was 10.52 years (SD = 2.99 years), and on average, they had 3 prior depressive episodes (M=3.16, SD = 2.14). At the time of assessment for the current report, 24 (32%) were in a depressive episode while the rest were in full or partial remission, and 22 (30%) had a co-morbid anxiety disorder. The controls had no history of major psychiatric disorders.
Recruitment, clinical assessment, and diagnostic procedures have been described in detail previously (Miller et al., 2002). All diagnoses were derived by experienced, masters’ level clinicians via semi-structured psychiatric interviews (e.g., Structured Clinical Interview of DSM-IV Disorders or SCID-I, [First, Spitzer, Gibbon, & Williams, 1995]). DSM-IV criteria were used to determine diagnoses (APA, 1994). Medical records were used, when available and needed, to verify age at depression onset, and diagnoses were finalized via best-estimate consensus procedures by pairs of psychiatrists (Maziade et al., 1992). Exclusion criteria included a history of psychosis, mental retardation (IQ<70), or major systemic medical disorders.
2.2 Measures
2.2.1 Structured Clinical Interview of DSM-IV Disorders (SCID-I)
The SCID-I (First et al., 1995) is a commonly used semi-structured interview for diagnosing lifetime history of Axis-I psychiatric disorders based on DSM-IV criteria that has shown good psychometric properties (Williams, Gibbon, First, & Spitzer, 1992), and good inter-rater reliability in our study (κ = .92 for major depressive disorder and κ = .63 for dysthymic disorder).
2.2.2 Follow-Up Anxiety Scale (FAS)
The FAS is a structured interview comprised of 11 items that reflect signs and symptoms of generalized anxiety, social phobia, and panic. Items were adopted from the adult version of the Interview Schedule for Children and Adolescents and were scored along a 4-point severity scale ranging from “0=None: rare or fleeting, once in a while” to “3=Severe: pervasive/constant, impairing/disrupts functioning” (Sherrill & Kovacs, 2000). The FAS had excellent internal consistency (α = .87) and inter-rater reliability (intraclass correlation coefficient = .87) in this study.
2.2.3 Emotion Ratings
Subjects rated the subjective intensity of five discrete emotions (happy, sad, angry, fearful, and disgust) on a computer via a 0–8 point Likert-type scale. Changes in these intensity ratings were used as a manipulation check.
2.2.4 Latent Depression Factor Indicators
2.2.4.1 Beck Depression Inventory (BDI)
The BDI (Beck, Ward, Mendelson, Mock, & Erbaugh, 1961) is a widely used 21-item self-report measure of depression severity focusing on the past week, which has demonstrated adequate reliability and validity (Bumberry, Oliver, & McClure, 1978), and had excellent internal consistency in our study (α = .94).
2.2.4.2 Follow-Up Depression Scale for Adults (FDS)
The FDS is a structured interview comprised of 24 items that reflect signs and symptoms of depression and dysthymia. Items were adopted from the adult version of the Interview Schedule for Children and Adolescents and were scored along a 4-point severity scale ranging from “0=None: rare or fleeting, once in a while” to “3=Severe: pervasive/constant, impairing/disrupts functioning” (Sherrill & Kovacs, 2000). The measure displayed excellent internal consistency (α=.93) and inter-rater reliability (intraclass correlation coefficient = .97) in our study.
2.2.5 Latent Adaptive and Maladaptive Mood Repair Factor Indicators
2.2.5.1 Response to Depression Questionnaire (RDQ)
The RDQ (Nolen-Hoeksema & Morrow, 1991) is a 39-item self-report questionnaire of cognitive and behavioral responses to depressed mood. Individuals respond via a 4-point scale to the degree that each describes their response when they feel down, sad, or depressed item (i.e., “Think about a recent situation, wishing it had gone better”). The 19-item Rumination sub-scale used in this study has been shown to have good internal consistency and concurrent and predictive validity (Nolen-Hoeksema & Morrow, 1991; Nolen-Hoeksema, Morrow, & Fredrickson, 1993). This subscale also showed good test-retest reliability over a 1 year period in this sample (r = .83).
2.2.5.2 Feelings and Me-Adult Version (FAM)
The FAM (Kovacs et al., 2009) is a 54-item questionnaire that measures the use of adaptive and maladaptive cognitive, behavioral, and social regulation strategies that individuals typically use in response to dysphoria. Individuals rate along a 3-point scale ranging from “0 = not true of me” to “2 = many times true of me” the degree that items describe them. Items that represent adaptive responses (e.g., “when I am sad, I look for a friend or other adult to talk to”) and maladaptive responses (e.g., “when I am sad, I take pills, or drugs, or drink alcohol”) are summed into two subscales. These adaptive and maladaptive subscales display good internal consistency (α=.80 – .91) across clinical and non-clinical populations, and have been shown to be related to current depression status and episode recurrence (Kovacs et al., 2009). Further, these subscales showed good test-retest reliability over a 1 year period in this sample (Maladaptive, r = .84; Adaptive, r = .79).
2.3 Procedures
2.3.1 Psychiatric Diagnoses
Participants completed comprehensive psychiatric evaluations that included SCID-I within 1 year of the physiology protocol (M = 2.78 months, SD = 2.67). Measures of mood repair (FAM-A & RDQ) were collected as part of the psychiatric evaluation and demonstrated trait-like stability (1 year test-retest reliability rs = .74 – .84). The interval between that assessment and the physiology protocol was unrelated to all study variables.
2.3.2 Physiological Measures
The data reported in this paper were collected as part of a larger electrophysiological protocol targeting emotional reactivity and regulation. After subjects completed the clinical evaluation, they completed a questionnaire regarding recent caffeine consumption, smoking, and current medications. They were then connected to equipment, which continuously monitored multiple physiological parameters during a protocol that involved rest periods and experimental tasks. Subjects were seated upright in a comfortable chair facing a computer monitor. Resting ECG data were collected during a pre-task period at which time participants sat quietly and focused on an a fixation cross on a computer screen while being asked to sit for alternating 60s periods of eyes open or eyes closed (as this is the standard procedure for EEG baseline acquisition, which was also being acquired simultaneously). Three 60s epochs of eyes closed and three 60s epochs of eyes open were collected (order randomized), for a total of at least 6 minutes of resting baseline for each participant. Subjects participated in several tasks, including watching a sad and neutral film clip. The present study focuses on subjects’ RSA during the initial resting baseline period, two later inter-task intervals, and while watching a sad film clip. The 172s clip from “the Champ” was selected to elicit sadness based on Gross and Levenson (1995). The neutral film clip depicted activity in a train yard. We focused on subjects’ responses to the sad film clip because this challenge is relevant to depression (Rottenberg et al., 2005) and affects RSA (Kreibig, 2010). All tasks were separated by a 120s inter-task interval, during which participants sat quietly. For a manipulation check, emotion ratings after the sad film were compared to those of a preceding neutral film clip.
2.3.3 Physiological data acquisition and reduction
Standard guidelines were followed in the ECG data acquisition and reduction (Berntson et al., 1997; Task Force, 1996) using software and equipment from the James Long Company (Caroga Lake, NY). Ag/AgCl ECG electrodes were placed axially on the left and right rib cage, approximately 10–15 cm below the armpits. The bioamplifier was set for bandpass filtering with frequencies of 0.01 and 1000 Hz. The ECG signal was amplified with a gain of 500 and data were digitized with a sampling rate of 512 Hz and resampled off-line at 1000 Hz. R-waves in the ECG signal were automatically identified using a multi-pass algorithm. Automated R-wave identification was manually checked using an interactive program for missed or mislabeled R-waves, and ectopic beats were deleted and interpolated. The interbeat interval (IBI) series was resampled in equal 125 ms intervals, linearly detrended, and tapered using a Hanning window. Heart rate variability (HRV) was calculated using Fast Fourier transformation analysis of the IBI series, with spectral power values determined in in ms2/Hz (Berntson et al., 1997). Our index of cardiac parasympathetic activity, RSA, was defined as the log transformed high frequency (HF) power band of HRV (.15–.04 Hz range; see Berntson et al., 1997). Hereafter we refer to HF-HRV as RSA, since HF-HRV is the power band of HRV that occurs in the typical range of respiration. Data were processed in 60s epochs for the baseline (six 60s epochs were processed and averaged together before analyses, for a total of 6 minutes of baseline) and in a single 172s epoch for the entire sad film, Mean heart rate for the initial and the two inter-task resting periods ranged from 72.33–73.98 (SD = 9.17–9.61), and was 71.44 (SD = 9.76) during the sad film.
2.4 Statistical Analyses
Descriptive analyses were conducted in SAS version 9.3 software (SAS Institute Inc., 2012), and latent variable models were fit in Mplus version 7 software (Muthén & Muthén, 1998 – 2012). A two-step approach was taken to model associations between latent variables (Anderson & Gerbing, 1988). In the first step, measurement models were fit to relate measured variables with their latent counterparts. In the second step, structural equation models were estimated that fit regression parameters between predictors and latent outcome variables. Robust Full Information Maximum Likelihood was used to adjust parameter estimates for missing values that ranged from 0–15% of the sample and were missing completely at random (Little’s χ2 [154] = 174.73, p =.12).
Following Hu and Bentler (1999), CFI values of .95 or greater and RMSEA of .06 or lower indicated excellent model fit, and CFI greater than .90 and RMSEA below .07 was indicative of acceptable fit (Browne & Cudeck, 1993; Steiger, 2000). Latent variable interactions were modeled using Latent Moderated Structural (LMS) equations (Klein & Moosbrugger, 2000), and the incremental value of each interaction term was evaluated through Wald χ2 tests. Sample size was reduced to n=145 in latent interaction models due to missing values on a constituent of the interaction term.
2.4.1 Measurement Models
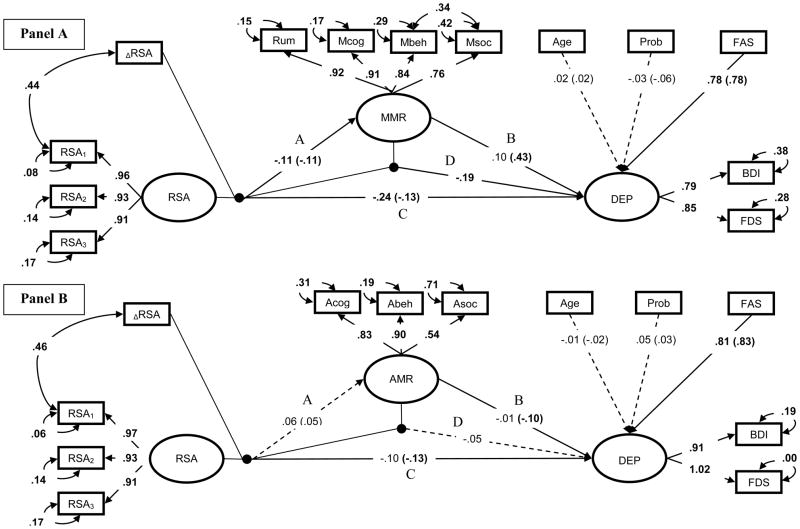
Separate measurement models were estimated for adaptive and maladaptive mood repair. Each measurement model related depression, resting RSA, and mood repair measures to their respective latent counterparts. Given tendencies for adaptive and maladaptive mood responses to cluster across cognitive, interpersonal, and behavioral domains (see Kovacs et al., 2009), the RDQ Rumination scale (MMR1), and FAM-A Cognitive, Social, and Behavioral Maladaptive Repair scales (MMR2-4) served as indicators of a latent Maladaptive Mood Repair (MMR) factor in the first measurement model (Maladaptive Model). FAM-A Cognitive, Social, and Behavioral Adaptive Repair scales (AMR1-3) comprised the latent Adaptive Mood Repair factor (AMR) in the second measurement model (Adaptive Model). In both models: BDI and FDS were used to estimate the latent depression factor; latent resting RSA was estimated using the 360s pre-task rest epoch and the two 120s inter-task intervals (RSA1, RSA2, & RSA3). RSA reactivity (ΔRSA) was defined as the difference between RSA1 and RSA during the sad film. In all models, negative ΔRSA values indicate vagal augmentation and positive values indicate vagal withdrawal.
Latent factors and ΔRSA were centered at a zero value in all models, and no restrictions were made on latent covariance terms. Residual correlations between RSA1 and ΔRSA, and the behavioral and social MMR subscales were tested given that ΔRSA is in part predicated on RSA1 values (Wilder, 1967), and the tendency for maladaptive social and behavioral strategies to co-occur (e.g., social strategy “go off to be alone” with behavioral strategy “lay down and just feel bad”).
2.4.2 Structural Models
A series of nested latent variable models then tested mood repair as a mediator of resting RSA and RSA reactivity combinations, and the moderating effect of these combined indices on mood repair effects on the latent depression factor. Mediation effects of latent mood repair on latent depression were tested following standard procedures (Baron & Kenney, 1986; MacKinnon, Fairchild, & Fritz, 2007). First, latent depression was regressed on first- and second-order effects of resting RSA and RSA reactivity (Figure 1, path C). Significant moderation of resting RSA by RSA reactivity would provide support for testing mediating effects of mood repair. Meditational hypotheses were then tested in the second and third analytic steps. In the second step, mood repair was regressed on resting RSA/RSA reactivity combinations (Figure 1, path A), and the indirect effect of RSA combinations through mood repair was tested in the third step (Figure 1, paths A & B). Asymmetric confidence intervals of indirect effects were estimated using the PRODCLIN program (MacKinnon, Fritz, Williams, & Lockwood, 2007) as opposed to Mplus, which relies on algorithms that have been shown to bias interaction terms’ standard error (Preacher & Selig, 2012). The fourth step tested the hypothesized RSA moderation of mood repair effect on depression (Figure 1, path D). Due to sample size constraints, this series of models was tested separately for adaptive and maladaptive mood repair. Further, the major findings of this paper were replicated using only manifest variables, thus supporting the validity of the latent variable models reported below (results available upon request).
Figure 1.
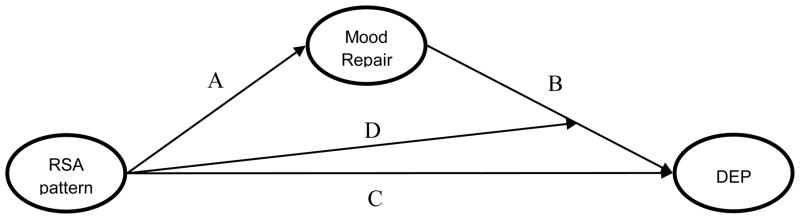
Conceptual model of: 1) Mood Repair mediating effects of RSA patterns on depression, and 2) RSA pattern moderation of Mood Repair effects on depression. RSA pattern = interaction of resting RSA and RSA reactivity, DEP = depression. Following Barron and Kenney (1986): C = effects of the resting RSA/RSA reactivity interaction on depression; A = effect of resting RSA/RSA reactivity interaction on mood repair; B = effect of Mood Repair on depression; D = resting RSA/RSA reactivity moderation of Mood Repair effects on depression.
3. Results
3.1 Preliminary Analyses
Consistent with the intended manipulation, subjects reported more intense sadness after watching the sad film clip (Mneutral film = .29, SD = 1.01, Msad film = 5.17, SD = 2.17, t[142] = 25.15, p <.001). The sad clip also elicited vagal withdrawal as expected (MΔRSA = .20, SD =.61, t[144] = 3.98, p < .001). Multivariate analyses (MANCOVAs) of demographic variables revealed significant effects of age on RSA indicators (RSA1-RSA3), maladaptive repair indicators (MMR1-MMR4), and depression factor indicators (BDI & FDS). Depressive episode status and psychotropic medication use were not significantly related to RSA variables, and were not included in subsequent analyses. Racial background was unrelated to depression measures, and caffeine consumption and smoking were unrelated to the RSA indices (not shown in Table 1). Repeated measures ANOVAs revealed that three resting RSA indicators did not differ after the effects of age were statistically covaried (F[2, 254] = 1.04, p=.35). Effects of age, anxiety symptoms, and proband status were statistically controlled as warranted in the subsequent analyses.
Table 1.
Descriptive statistics and correlations among demographic, symptom, and mood repair measures (N=126–149).
| Measures | M (SD) | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | 12. | 13. | 14. | 15. | 16. | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | 27.95 (5.17) | −.32** | −.22** | −.29** | −.30** | −.27** | −.29** | −.26** | .04 | .31** | −.25** | −.36** | −.30** | −.10 | −.17 | .00 | ||
| 2. Prob | --- | .41** | .46** | .50** | .03 | .11 | .02 | .07 | .45** | .40** | .39** | .38** | −.05 | −.16 | −.10 | |||
| 3. BDI | 8.33 (9.88) | .88** | .78** | .15 | .16 | .08 | .06 | .70** | .69** | .70** | .60** | −.20* | −.21* | −.32** | ||||
| 4. FDS | 10.98 (12.22) | .86** | .08 | .12 | .10 | .10 | .71** | .70** | .69** | .61** | −.19* | −.21* | −.26** | |||||
| 5. FAS | 5.74 (5.44) | .18* | .21* | .17 | .06 | .68** | .61** | .58** | .58** | −.12 | −.12 | −.25** | ||||||
| 6. RSA1 | 6.68 (1.24) | .89** | .88** | .12 | .12 | .13 | .09 | .24** | −.14 | .02 | .00 | |||||||
| 7. RSA2 | 6.67 (1.30) | .83** | −.03 | .19 | .16 | .13 | .23* | −.13 | .04 | .00 | ||||||||
| 8. RSA3 | 6.46 (1.25) | −.03 | .15 | .16 | .13 | .19* | .04 | .15 | .18 | |||||||||
| 9. ΔRSA | .20 (.61) | .05 | .07 | .07 | .20* | −.08 | −.11 | −.13 | ||||||||||
| 10. Rum | 36.42 (13.37) | .85** | .78** | .72** | −.03 | −.11 | −.20 | |||||||||||
| 11. Mcog | 5.44 (4.59) | .77** | .70** | −.12 | −.19* | −.23** | ||||||||||||
| 12. Mbeh | 4.38 (3.64) | .77** | −.15 | −.18* | −.25** | |||||||||||||
| 13. Msoc | 3.06 (2.41) | −.22* | −.25** | −.39** | ||||||||||||||
| 14. Acog | 7.27 (3.77) | .74** | .43** | |||||||||||||||
| 15. Abeh | 10.05 (5.26) | .48** | ||||||||||||||||
| 16. Asoc | 6.38 (3.06) | --- | ||||||||||||||||
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | 12. | 13. | 14. | 15. | 16. | |||
|
| ||||||||||||||||||
| Skew. | 2.81 | --- | 7.76 | 6.23 | 4.55 | −.55 | 1.12 | −1.25 | −3.97 | 3.55 | 3.84 | 5.09 | 2.29 | .74 | 2.08 | −.31 | ||
|
| ||||||||||||||||||
| Kurt. | −1.30 | --- | 4.51 | 2.19 | −.39 | 1.62 | .37 | −.50 | 14.30 | −1.02 | −.69 | 2.31 | −.96 | −1.34 | .17 | −1.61 | ||
Note. Prob = positive proband status, BDI = Beck Depression Inventory, FDS = Follow-up Depression Scale for Adults, FAS = Follow-up Anxiety Scale for Adults, RSA1-RSA3= pre-film, post film, and post money game resting RSA, ΔRSA = change score from pre-film RSA to RSA during the sad film, Rum = RDQ Rumination subscale, Mcog-Msoc = Feelings and Me Maladaptive Cognitive, Behavioral and Social mood repair subscales, Acog-Asoc = Feelings and Me Adaptive Cognitive, Behavioral, and Social mood repair subscales. Skew. = Skewness Z-score, Kurt. = Kurtosis Z-score.
p ≤ .01,
p ≤ .051
3.2 Measurement Models
Both measurement models demonstrated good-to-excellent fit (Maladaptive Model: χ2 [40] = 65.75, p <.01, CFI = .98, RMSEA = .07; Adaptive Model: χ2 [31] = 44.80, p = n.s., CFI = .99, RMSEA = .06). Removing residual covariance between RSA and ΔRSA significantly reduced fit (residual rs = .44–.46, Wald χ2 [1] = 4.72–11.19, ps < .01). Similar loss in fit occurred when residual covariance between maladaptive social and behavioral scales was removed (residual r = .34, Wald χ2 [1] = 4.85, p < .05).
The latent depression factor negatively correlated with age (r = −.27) and was positively associated with anxiety (r = .87–.90). Importantly, depression showed the expected inverse relationship with adaptive mood repair (r = −.26), and a strong direct relationship with maladaptive mood repair (r = .80) (ps <.001). Resting RSA was positively correlated with maladaptive mood repair (r = .18, p < .05) and anxiety (r = .23) (ps <.05), but was unrelated to adaptive mood repair. Follow-up analyses revealed that RSA correlations with maladaptive mood repair and anxiety were a function of age (ps = ns after controlling for age differences). RSA reactivity was not significantly correlated with the latent resting RSA, depression, anxiety, or mood repair factors.
3.3 Do mood repair processes mediate effects of RSA patterns on depression?1
To establish a relation between RSA patterns and depression (Figure 1, path C), first- and second-order effects of resting RSA and RSA reactivity were evaluated in series of SEMs that controlled for effects of age, anxiety symptoms, mood repair, and proband status. First, structural models were fit to test the first-order effects of resting RSA and RSA reactivity on the latent depression factor. These models fit the data well and served as the base for comparison with the second-order models (Maladaptive Model: χ2[54] = 78.13, p = .02, CFI = .98, RMSEA = .06; Adaptive Model: χ2[56] = 66.12, p = .17, CFI = .99, RMSEA = .04). The RSA metrics were nonsignificant as first-order predictors of depression. Of the predictors, only anxiety symptoms, adaptive mood repair, and maladaptive mood repair significantly predicted latent depression (Maladaptive Model: βANX = .64, βMMR = .38 ps < .01; Adaptive Model: βANX = .85, βAMR = −.11, ps < .01).
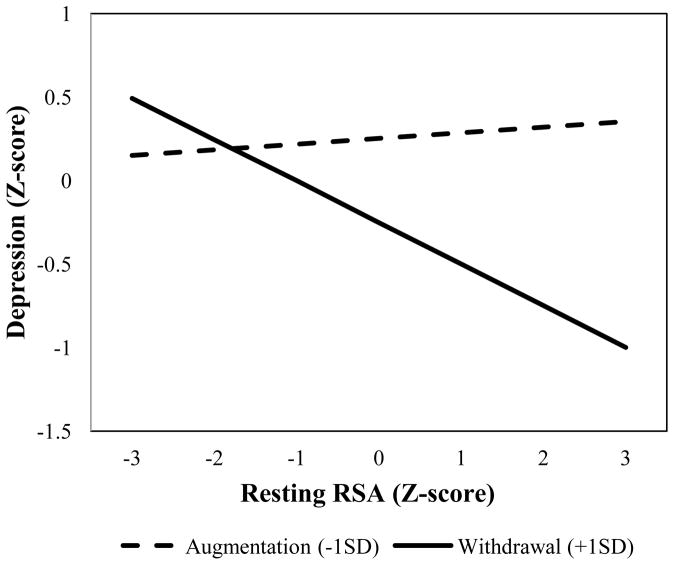
Second-order effects of resting RSA and RSA reactivity were then added to the first-order models (Figure 2, path C). RSA reactivity significantly moderated effects of resting RSA on depression in both models and significantly improved model fit (Maladaptive & Adaptive Models: βRSA × ΔRSA = - .13, ps < .05, Wald χ2 [1] = 5.02 – 6.09, ps < .05). Post hoc probes revealed that high resting RSA was associated with reduced depression symptoms as a function of RSA withdrawal (see Figure 3). Thus, as predicted, our theorized optimal RSA pattern was associated with lower depressive symptoms than sub-optimal RSA patterns.
Figure 2.
Standardized SEMs of second- and third-order effects of RSA predicting latent mood repair and depressive symptoms (Panel A: Maladaptive Model; Panel B: Adaptive Model). Black circles represent latent variable interactions. Regression paths between covariates and latent predictors, select first-order effects, and residual covariances omitted to improve interpretability. Effects of categorical predictors standardized with respect to the outcome variable. Parameters within parentheses are from the second-order mediation models. Parameters outside parentheses are from the third-order moderation models. Dashed lines represent non-significant paths. Bold parameters, significant at p ≤ .01; and parameters in plain text, p >.05. Prob = proband group membership, Rum = RDQ Rumination subscale, Mcog-Msoc = Feelings and Me Maladaptive Cognitive, Behavioral and Social mood repair subscales, Acog-Asoc = Feelings and Me Adaptive Cognitive, Behavioral, and Social mood repair subscales, RSA1 - RSA3 = pre-film and inter-task interval resting RSA, ΔRSA = change score from pre-film RSA to RSA during the sad film, BDI = Beck Depression Inventory, FDS = Follow-up Depression Scale for Adults, FAS = Follow-up Anxiety Scale for Adults, Resting RSA = latent resting RSA factor, MMR= latent maladaptive repair factor, AMR= latent adaptive repair factor, DEP = latent depression factor.
Figure 3.
RSA reactivity moderation of resting RSA on depression.
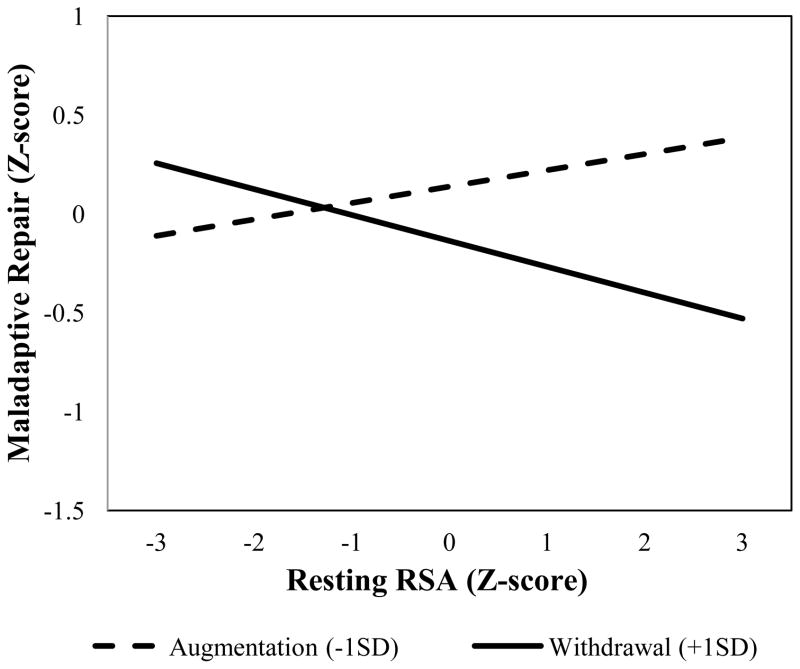
To establish a relation between RSA patterns and mood repair (Figure 1, path A), first- and second-order effects of resting RSA and RSA reactivity on adaptive and maladaptive repair were added to the second-order models described above. Results showed that RSA withdrawal significantly moderated effects of resting RSA on maladaptive repair, but not on adaptive repair (Maladaptive Model: βRSA × ΔRSA = −.11, p = .05, Wald χ2 [1] =3.82, p = .05; Adaptive Model: Wald χ2 [1] =.19, p = .66) (Figure 2, path A). Post hoc probes revealed that consistent with prediction, high resting RSA was associated with reduced use of maladaptive repair as a function of RSA withdrawal and increased use of maladaptive repair as a function of RSA augmentation (see Figure 4). These findings show that the theorized optimal RSA pattern is associated with a reduced use of maladaptive mood repair, while other RSA patterns are associated with increased use of maladaptive strategies.
Figure 4.
RSA reactivity moderation of resting RSA on maladaptive repair.
To determine whether maladaptive mood repair mediates the effects of RSA patterns on depression severity (Figure 2, paths A & B), regression weights and their respective standard errors were submitted to the PRODCLIN program. Consistent with expectation, maladaptive repair significantly mediated physiological pattern effects on depression (βMMR, RSA × ΔRSA = −.05, 95% CI −.01 – −.12). Indirect effects analysis of the simple slopes revealed that resting RSA reduced depression by decreasing maladaptive mood repair in the context of RSA withdrawal (β = −.06); resting RSA predicted increased maladaptive mood repair and depression in the context of RSA augmentation (β = .04). Thus, results are consistent with the idea that the salubrious effects of the optimal RSA pattern on depression reflect a reduced use of maladaptive mood repair strategies. On the other hand, sub-optimal RSA patterns were associated with increased maladaptive repair, and the accompanying worsening of depressive symptoms.
3.4 Do RSA patterns moderate mood repair effects on depression?1
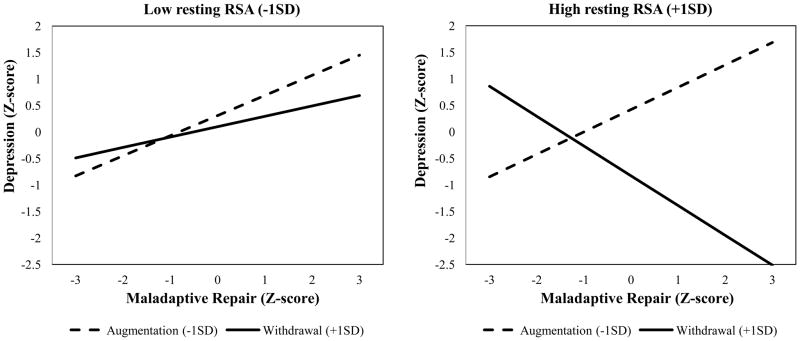
To evaluate whether RSA patterns moderate mood repair effects on depression (Figure 1, path D), third-order effects between resting RSA, RSA reactivity, and the two mood repair factors were added to the mediation models described above. Results showed that resting RSA patterns significantly moderated adverse effects of maladaptive mood repair, but not adaptive repair (Maladaptive Model: βMMR × RSA × ΔRSA = −.19, p = .02, Wald χ2 [1] =5.78, p < .05; Adaptive Model: Wald χ2 [1] =1.39, p = .24) (Figure 2, path D).
Post hoc probes revealed that in the context of RSA withdrawal, high resting RSA buffered against adverse maladaptive repair effects on depressive symptoms (see Figure 5). Thus, an optimal RSA pattern reduced adverse effects of maladaptive repair. These buffering effects were not noted for sub-optimal RSA patterns, which predicted increased depression as a function of maladaptive repair.
Figure 5.
Resting RSA+RSA reactivity moderation of maladaptive repair on depression.
4. Discussion
Using a bio-behavioral framework for understanding depression, this study addressed two questions: 1) does mood repair mediate the relationship between RSA patterns and depression, and 2) do RSA patterns moderate the effects of mood repair on depressive symptoms? Results were largely as hypothesized. The proposed optimal RSA pattern (high resting RSA in the context of RSA withdrawal to a negative emotion trigger) was associated with a reduced use of maladaptive repair responses, and robustly buffered the depressogenic effects of maladaptive mood repair. In turn, RSA patterns considered sub-optimal (high resting RSA+RSA augmentation or low resting RSA+RSA withdrawal combinations) predicted greater use of maladaptive repair, and more depressive symptoms. In other words, while individuals with optimal RSA patterns were less likely to report maladaptive repair responses and depressive symptoms, those with sup-optimal RSA patterns were more likely to engage in maladaptive repair and have higher levels of depression. Contrary to expectation, RSA patterns were unrelated to indices of adaptive mood repair. Together, these findings support the utility of using multiple RSA indices in studying depression and extend our understanding of the parasympathetic nervous system’s role in mood regulation.
4.1 Maladaptive mood repair mediates RSA pattern effects on depression
We see this finding as consistent with several possible mechanisms within our bio-behavioral framework for understanding depression. Thayer and Lane (2000; 2009) proposed that the autonomic nervous system influences the selection of responses to environmental demands, which is supported by the associations between RSA and emotion regulation in our study and in the findings by others (e.g., Vasilev et al., 2009). Given the positive association between RSA and executive functioning (Hansen, Johnsen, & Thayer, 2003), and emotion processing (Dywan, Mathewson, Choma, Rosenfeld, & Segalowitz, 2008; Thayer et al., 2012), an optimal RSA pattern may enable access to a wide array of regulatory responses during distress. This access may be constrained for subjects with sub-optimal patterns. Indeed, Fabes and Eisenberg (1997) showed that high resting RSA reduced stress-related negative emotional arousal, which, in turn, reduced the use of emotional venting and substances to reduce distress. Thus, the beneficial effects of high resting RSA appear to be gated by RSA reactivity.
The association of maladaptive repair and sub-optimal RSA patterns (high resting RSA+ RSA augmentation and low resting RSA+RSA withdrawal combinations) is consistent with extant knowledge. First, RSA augmentation is an atypical response to sadness (Kreibig, 2010), particularly in the context of high resting RSA (Wilder, 1967), and RSA withdrawal in the context of low resting RSA may indicate an overtaxing of the self-regulation system (e.g., Beauchaine, Gatzke-Kopp, & Mead, 2007). Because high resting RSA is associated with reduced baseline emotional arousal (Fabes & Eisenberg, 1997), and vagal augmentation is associated with the use of avoidant maladaptive strategies (Verkuil, Brosschot, de Beurs, & Thayer, 2009), it is plausible that the high resting RSA+RSA augmentation pattern promotes dissociation or avoidance of emotion-relevant stimuli. On the other hand, as low resting RSA levels are associated with increased negative emotional arousal (Fabes & Eisenberg, 1997) and excess RSA withdrawal is associated with panic disorder (Yeragani et al.,1993), a low resting RSA+RSA withdrawal pattern may lead to exaggerated reactivity to certain environmental triggers. This RSA combination may thus potentiate the use of maladaptive regulatory responses (e.g., venting and alcohol use, Fabes & Eisenberg, 1997) and inhibit access to adaptive mood repair responses that require greater use of executive resources (e.g., reappraisal). These possibilities will be important to address in future investigations.
Our results did not support the hypothesized mediating role of adaptive repair in the relationship between RSA patterns and depression. This null finding, however, is commensurate with the mixed literature on the association between adaptive mood repair strategies and depression (e.g., Aldao et al., 2010; Kovacs et al., 2009). As one explanation, it has been proposed that while the association between maladaptive repair and psychopathology is robust across situations, the salubrious effects of adaptive responses vary as a function of context (Aldao & Nolen-Hoeksema, 2012). Because our study measured general tendencies to engage in adaptive repair, the role of context in the relationship between RSA patterns and adaptive mood repair warrants further scrutiny.
4.2 RSA patterns moderate mood repair effects on depression
The moderation of maladaptive repair effects by RSA patterns is consistent with the role of autonomic processes in emotional experience (Thayer & Lane, 2000; 2009). Maladaptive mood repair responses prolong and exacerbate dysphoria (e.g., Lyubomirsky et al., 1998), which can intensify to clinical levels. Others have reported that resting RSA mediates the relationship between cortical areas responsible for the intensity of dysphoric emotions (Dywan et al., 2008; Thayer et al., 2012), as well as aversive emotional arousal in the natural environment (Fabes & Eisenberg, 1997). Together, these findings suggest that RSA modulates the intensity of dysphoric emotions that result from deploying maladaptive mood repair responses. Our findings suggest that individuals with high resting RSA may feel less distressed relative to their low resting RSA counterparts after engaging in a maladaptive response, such as ruminative thinking. The buffering effect of an optimal RSA pattern (high resting RSA+RSA withdrawal) is consistent with findings showing buffering effects of resting RSA on negative emotions (Dywan et al., 2008; Fabes & Eisenberg, 1997). Given that RSA reactivity is an indicator of emotional responsiveness (Weinberg & Tronick, 1996), RSA withdrawal in response to a trigger may potentiate effects of resting RSA to attenuate dysphoria that is generated by frequent maladaptive mood repair. This possibility is consistent with our findings: advantageous effects of resting RSA on depression increased as a function of RSA withdrawal. Conversely, sub-optimal RSA patterns, particularly involving RSA augmentation, did not buffer the adverse effects of maladaptive repair across resting RSA levels. This pattern may suggest that RSA augmentation inhibits the beneficial effects of high resting RSA on emotional intensity, as appropriate to context.
Our findings should be interpreted in the context of several limitations. Because we did not measure respiration, we cannot rule out the possibility that it influenced our findings. We also did not consider effects of psychiatric comorbidity, or sympathetic nervous system activity, on the association between RSA, mood repair, and depression. Further, due to our use of female juvenile-onset depression subjects, our findings have unknown generalizability to adult-onset depression or to males. Finally, methodological limitations in the form of lags between the collection of mood repair and physiology data prevent us from making causal statements about the relationship between RSA, mood repair, and depression. Future studies that address the above noted limitations could help to further clarify the role of RSA in mood repair and depression.
Despite these limitations, this study has a number of notable strengths. First, we used a sample of probands that controlled for age at onset, a key contributor to heterogeneity in depression phenotypes. Second, our multi-method analytic approach and structural equation modeling attenuated measurement error and increased the precision of our findings. Further, our analyses controlled for key variables known to confound RSA. Finally, a sadness-inducing film challenge as a means to probe RSA reactivity has both ecological and empirical validity. To our knowledge, this study is the first to investigate potential mechanisms by which RSA level and RSA reactivity jointly predict depression and impact the use of maladaptive repair strategies. This study extends past work on the role of RSA in depression by proposing mood repair as one mechanism through which physiological vulnerabilities adversely affect mental health.
Highlights.
Resting RSA and RSA reactivity jointly predict depression and mood regulation.
Neither RSA metric predicts either outcome on its own.
Mood regulation partially mediates joint effects of RSA indices on depression.
RSA indices jointly buffer depressogenic effects of ineffective mood regulation.
Acknowledgments
The first author wishes to thank Dr. Robert Rosenbaum at the University of Pittsburgh for providing consultation on the data analyses.
This research was supported by NIMH Program Project MH056193 (Dr. Nathan Fox was the PI of the Physiological Sub-Study); grantsMH085722 and MH077669.
Footnotes
In response to reviewers’ comments, we examined the specificity of RSA patterns’ effects when these patterns were comprised of RSA reactivity to the sad film clip versus a fear-evocative film clip (“Cliff Hanger”). Consistent with the intended manipulation, the fear film clip evoked more intense fear-ratings relative to the neutral film (Mneutral film = .13, SD = .79, Mfear film = 5.08, SD = 2.65, t[142] = 21.58, p <.001, d = 2.09), and elicited vagal withdrawal (MΔRSA = .48, SD =.58, t[144] = 9.97, p < .001). RSA patterns comprised of resting RSA and RSA reactivity to the fear film clip did not predict depression symptoms (p = .68), nor did they moderate mood repair effects on depression (p = .17). This pattern of results provides preliminary evidence for the specificity of RSA reactivity to sadness as a predictor of depression, rather than reactivity to any negative emotion.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aldao A, Nolen-Hoeksema S. The influence of context on the implementation of adaptive emotion regulation strategies. Behaviour Research and Therapy. 2012;50:276–281. doi: 10.1016/j.brat.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Aldao A, Nolen-Hoeksema S, Schweizer S. Emotion-regulation strategies across psychopathology: A meta-analytic review. Clinical Psychology Review. 2010;30:217–237. doi: 10.1016/j.cpr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 2000. Text Revision. [Google Scholar]
- Anderson JC, Gerbing DW. Structural equation modeling in practice: A review and recommended two-step approach. Psychological Bulletin. 1988;103:411–423. [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP. Vagal tone, development, and gray’s motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology. 2001;13:183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Gatzke-Kopp LM, Mead HK. Polyvagal theory and developmental psychopathology: Emotion dysregulation and conduct problems from preschool to adolescence. Biological Psychology. 2007;74:174–184. doi: 10.1016/j.biopsycho.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Eckberg DL, Grossman P, Kaufmann PG, Malik M, van der Molen MW. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Blagden C, Craske MG. Active and passive distraction and rumination: A replication in anxious mood. Journal of Anxiety Disorders. 1996;10:243–252. [Google Scholar]
- Bollen KA. Structural Equations with Latent Variables. New York: John Wiley & Sons, Inc; 1989. [Google Scholar]
- Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing Structural Equation Models. Newbury Park, CA: Sage; 1993. pp. 136–162. [Google Scholar]
- Bumberry W, Oliver JM, McClure JN. Validation of the Beck Depression Inventory in a university population using psychiatric estimate as the criterion. Journal of Consulting and Clinical Psychology. 1978;46:150–155. [Google Scholar]
- Cribbet MR, Williams PG, Gunn HE, Rau HK. Effects of tonic and phasic respiratory sinus arrhythmia on affective stress responses. Emotion. 2011;11:188–193. doi: 10.1037/a0021789. [DOI] [PubMed] [Google Scholar]
- Del Giudice M, Ellis BJ, Shirtcliff EA. The Adaptive Calibration Model of stress responsivity. Neuroscience & Biobehavioral Reviews. 2011;35:1562–1592. doi: 10.1016/j.neubiorev.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dywan J, Mathewson K, Choma BL, Rosenfeld B, Segalowitz S. Autonomic and electrophysiological correlates of emotional intensity in older and younger adults. Psychophysiology. 2008;45:389–397. doi: 10.1111/j.1469-8986.2007.00637.x. [DOI] [PubMed] [Google Scholar]
- Ehring T, Tuschen-Caffier B, Schnulle J, Fischer S, Gross JJ. Emotion regulation and vulnerability to depression: Spontaneous versus instructed use of emotion suppression and reappraisal. Emotion. 2010;10:563–572. doi: 10.1037/a0019010. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Erath SA. Family conflict, autonomic nervous system functioning, and child adaptation: State of the science and future directions. Development and Psychopathology. 2011;23:703–721. doi: 10.1017/S0954579411000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M, Hinnant JB, Erath S. Developmental trajectories of delinquency symptoms in childhood: The role of marital conflict and autonomic nervous system activity. Journal of Abnormal Psychology. 2011;120:16–32. doi: 10.1037/a0020626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterling BA, L’Abate L, Murray EJ, Pennebaker JW. Empirical foundations for writing in prevention and psychotherapy: Mental and physical health outcomes. Clinical Psychology Review. 1999;19:79–96. doi: 10.1016/s0272-7358(98)00015-4. [DOI] [PubMed] [Google Scholar]
- Fabes RA, Eisenberg N. Regulatory control and adults’ stress-related responses to daily life events. Journal of Personality and Social Psychology. 1997;73:1107–1117. doi: 10.1037//0022-3514.73.5.1107. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis I disorders—Patient edition (SCID-I/P, Version 2.0) Biometrics Research Department, New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- Gentzler AL, Santucci AK, Kovacs M, Fox N. Respiratory sinus arrhythmia reactivity predicts emotion regulation and depressive symptoms in at-risk and control children. Biological Psychology. 2009;82:156–163. doi: 10.1016/j.biopsycho.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ. Emotion regulation: Affective, cognitive, and social consequences. Psychophysiology. 2002;39:281–291. doi: 10.1017/s0048577201393198. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Levenson RW. Emotion elicitation using films. Cognition and Emotion. 1995;9:87–108. [Google Scholar]
- Gyurak A, Ayduk Ö. Resting respiratory sinus arrhythmia buffers against rejection sensitivity via emotion control. Emotion. 2008;8:458–467. doi: 10.1037/1528-3542.8.4.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen AL, Johnsen BH, Thayer JF. Vagal influence on working memory and attention. International Journal of Psychophysiology. 2003;48:263–274. doi: 10.1016/s0167-8760(03)00073-4. [DOI] [PubMed] [Google Scholar]
- Hinnant JB, El-Sheikh M. Children’s externalizing and internalizing symptoms over time: The role of individual differences in patterns of RSA responding. Journal of Abnormal Child Psychology. 2009;37:1049–1061. doi: 10.1007/s10802-009-9341-1. [DOI] [PubMed] [Google Scholar]
- Hopp H, Shallcross AJ, Ford BQ, Troy AS, Wilhelm FH, Mauss IB. High cardiac vagal control protects against future depressive symptoms under conditions of high social support. Biological Psychology. 2013 doi: 10.1016/j.biopsycho.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- Ingjaldsson JT, Laberg JC, Thayer JF. Reduced heart rate variability in chronic alcohol abuse: Relationship with negative mood, chronic thought suppression, and compulsive drinking. Biological Psychiatry. 2003;54:1427–1436. doi: 10.1016/s0006-3223(02)01926-1. [DOI] [PubMed] [Google Scholar]
- Joormann J, Cooney RE, Henry ML, Gotlib IH. Neural correlates of automatic mood regulation in girls at high risk for depression. Journal of Abnormal Psychology. 2012;121:61–72. doi: 10.1037/a0025294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Siemer M, Gotlib IH. Mood regulation in depression: Differential effects of distraction and recall of happy memories on sad mood. Journal of Abnormal Psychology. 2007;116:484–490. doi: 10.1037/0021-843X.116.3.484. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. Impact of depression and antidepressant treatment on heart rate variability: A review and meta-analysis. Biological Psychiatry. 2010;67:1067–1074. doi: 10.1016/j.biopsych.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Klein A, Moosbrugger H. Maximum likelihood estimation of latent interaction effects with the LMS method. Psychometrika. 2000;65:457–474. [Google Scholar]
- Kovacs M, Rottenberg J, George C. Maladaptive mood repair responses distinguish young adults with early onset depressive disorders and predict future depressive outcomes. Psychological Medicine. 2009;39:1841–1854. doi: 10.1017/S0033291709005789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreibig SD. Autonomic nervous system activity in emotion: A review. Biological Psychology Special Issue: The Biopsychology of Emotion: Current Theoretical and Empirical Perspectives. 2010;84:394–421. doi: 10.1016/j.biopsycho.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Lehofer M, Moser M, Hoehn-Saric R, McLeod D, Liebmann P, Drnorvsek B, Egner S, Hildebrandt G, Zapotoczky HG. Major depression and cardiac autonomic control. Biological Psychology. 1997;42:914–919. doi: 10.1016/S0006-3223(96)00494-5. [DOI] [PubMed] [Google Scholar]
- Lyubomirsky S, Caldwell ND, Nolen-Hoeksema S. Effects of ruminative and distracting responses to depressed mood on retrieval of autobiographical memories. Journal of Personality and Social Psychology. 1998;75:166–177. doi: 10.1037//0022-3514.75.1.166. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annual Review of Psychology. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Fritz MS, Williams J, Lockwood CM. Distribution of the product confidence limits for the indirect effect: Program PRODCLIN. Behavior Research Methods. 2007;39:384–389. doi: 10.3758/bf03193007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marroquín B. Interpersonal emotion regulation as a mechanism of social support in depression. Clinical Psychology Review. 2011;31:1276–1290. doi: 10.1016/j.cpr.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Maziade M, Roy MA, Fournier JP, Cliche D, Merette C, Caron C, Raymond V. Reliability of best-estimate diagnosis in genetic linkage studies of major psychoses: Results from the Quebec Pedigree Studies. American Journal of Psychiatry. 1992;149:1674–1686. doi: 10.1176/ajp.149.12.1674. [DOI] [PubMed] [Google Scholar]
- Miller A, Fox NA, Cohn JF, Forbes EE, Sherrill JT, Kovacs M. Regional patterns of brain activity in adults with a history of childhood-onset depression: Gender differences and clinical variability. The American Journal of Psychiatry. 2002;159:934–940. doi: 10.1176/appi.ajp.159.6.934. [DOI] [PubMed] [Google Scholar]
- Moser M, Lehofer M, Hoehn-Saric R, McLeod DR, Hildebrandt G, Steinbrenner B, Zapotoczky HG. Increased heart rate in depressed subjects in spite of unchanged autonomic balance? Journal of Affective Disorders. 1998;48:115–124. doi: 10.1016/s0165-0327(97)00164-x. [DOI] [PubMed] [Google Scholar]
- Mueller TI, Lavori PW, Keller MB, Swartz A. Prognostic effect of the variable course of alcoholism on the 10-year course of depression. The American Journal of Psychiatry. 1994;151:701–706. doi: 10.1176/ajp.151.5.701. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. 7. Los Angeles, CA: Muthén & Muthén; 1998–2012. [Google Scholar]
- Nolen-Hoeksema S, Aldao A. Gender and age differences in emotion regulation strategies and their relationship to depressive symptoms. Personality and Individual Differences. 2011;51:704–708. [Google Scholar]
- Nolen-Hoeksema S, Morrow J. A prospective study of depression and posttraumatic stress symptoms after a natural disaster: The 1989 loma prieta earthquake. Journal of Personality and Social Psychology. 1991;61:115–121. doi: 10.1037//0022-3514.61.1.115. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Morrow J, Fredrickson BL. Response styles and the duration of episodes of depressed mood. Journal of Abnormal Psychology. 1993;102:20–20. doi: 10.1037//0021-843x.102.1.20. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking rumination. Perspectives on Psychological Science. 2008;3:400–424. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- O’Connor M, Allen JJB, Kaszniak AW. Emotional disclosure for whom? A study of vagal tone in bereavement. Biological Psychology. 2005;68:135–146. doi: 10.1016/j.biopsycho.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Ode S, Hilmert CJ, Zielke DJ, Robinson MD. Neuroticism’s importance in understanding the daily life correlates of heart rate variability. Emotion. 2010;10:536–543. doi: 10.1037/a0018698. [DOI] [PubMed] [Google Scholar]
- Oveis C, Cohen AB, Gruber J, Shiota MN, Haidt J, Keltner D. Resting respiratory sinus arrhythmia is associated with tonic positive emotionality. Emotion. 2009;9:265–270. doi: 10.1037/a0015383. [DOI] [PubMed] [Google Scholar]
- Parkinson B, Totterdell P. Classifying affect-regulation strategies. Cognition and Emotion. 1999;13:277–303. [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biological Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Selig JP. Advantages of Monte Carlo confidence intervals for indirect effects. Communication Methods and Measures. 2012;6:77–98. [Google Scholar]
- Porges SW, Doussard-Roosevelt J, Portales AL, Greenspan SI. Infant regulation of the vagal “brake” predicts child behavior problems: A psychobiological model of social behavior. Developmental Psychobiology. 1996;29:697–712. doi: 10.1002/(SICI)1098-2302(199612)29:8<697::AID-DEV5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Rottenberg J. Cardiac vagal control in depression: A critical analysis. Biological Psychology. 2007;74:200–211. doi: 10.1016/j.biopsycho.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Clift A, Bolden S, Salomon K. RSA fluctuation in major depressive disorder. Psychophysiology. 2007;44:450–458. doi: 10.1111/j.1469-8986.2007.00509.x. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Salomon K, Gross JJ, Gotlib IH. Vagal withdrawal to a sad film predicts subsequent recovery from depression. Psychophysiology. 2005;42:277–281. doi: 10.1111/j.1469-8986.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- SAS® 9.3. System Options: Reference. 2. Cary, NC: SAS Institute Inc; 2013. [Google Scholar]
- Schepis TS, McCabe SE. Exploring age of onset as a causal link between major depression and nonmedical use of prescription medications. Drug and Alcohol Dependence. 2012;120:99–104. doi: 10.1016/j.drugalcdep.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrill JT, Kovacs M. Interview Schedule for Children and Adolescents (ISCA) Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:67–75. doi: 10.1097/00004583-200001000-00018. [DOI] [PubMed] [Google Scholar]
- Silk JS, Shaw DS, Skuban EM, Oland AA, Kovacs M. Emotion regulation strategies in offspring of childhood-onset depressed mothers. Journal of Child Psychology and Psychiatry. 2006;47:69–78. doi: 10.1111/j.1469-7610.2005.01440.x. [DOI] [PubMed] [Google Scholar]
- Sloan DM, Epstein EM. Respiratory sinus arrhythmia predicts written disclosure outcome. Psychophysiology. 2005;42:611–615. doi: 10.1111/j.1469-8986.2005.00347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spasojević J, Alloy LB. Rumination as a common mechanism relating depressive risk factors to depression. Emotion. 2001;1:25–37. doi: 10.1037/1528-3542.1.1.25. [DOI] [PubMed] [Google Scholar]
- Steiger JH. Point estimation, hypothesis testing, and interval estimation using the RMSEA: Some comments and a reply to Hayduck and Glaser. Structural Equation Modeling. 2000;7:149–162. [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- Thayer JF, Åhs F, Fredrikson M, Sollers JJ, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neuroscience and Biobehavioral Reviews. 2012;36:747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders Special Issue: Arousal in Anxiety. 2000;61:201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. Claude Bernard and the heart–brain connection: further elaboration of a model of neurovisceral integration. Neuroscience Biobehavioral Review. 2009;33:81–88. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Thompson RJ, Mata J, Jaeggi SM, Buschkuehl M, Jonides J, Gotlib IH. Maladaptive coping, adaptive coping, and depressive symptoms: Variations across age and depressive state. Behavior Research and Therapy. 2010;48:459–466. doi: 10.1016/j.brat.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilev CA, Crowell SE, Beauchaine TP, Mead HK, Gatzke-Kopp L. Correspondence between physiological and self-report measures of emotion dysregulation: A longitudinal investigation of youth with and without psychopathology. Journal of Child Psychology and Psychiatry. 2009;50:1357–1364. doi: 10.1111/j.1469-7610.2009.02172.x. [DOI] [PubMed] [Google Scholar]
- Verkuil B, Brosschot JF, de Beurs DP, Thayer JF. Effects of explicit and implicit perseverative cognition on cardiac recovery after cognitive stress. International Journal Psychophysiology. 2009;74:220–228. doi: 10.1016/j.ijpsycho.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Volokhov RN, Demaree HA. Spontaneous emotion regulation to positive and negative stimuli. Brain & Cognition. 2010;73:1–6. doi: 10.1016/j.bandc.2009.10.015. [DOI] [PubMed] [Google Scholar]
- Weinberg KM, Tronick EZ. Infant affective reactions to the resumption of maternal interaction after the still-face. Child Development. 1996;67:905–914. [PubMed] [Google Scholar]
- Wilder J. Stimulus and response: The law of initial value. Bristol: U.K: Wright; 1967. [Google Scholar]
- Williams JB, Gibbon M, First MB, Spitzer RL. The structured clinical interview for DSM-III—R (SCID): II. multisite test-retest reliability. Archives of General Psychiatry. 1992;49:630–636. doi: 10.1001/archpsyc.1992.01820080038006. [DOI] [PubMed] [Google Scholar]
- Yaroslavsky I, Rottenberg J, Kovacs M. The utility of combining RSA indices in depression prediction. Journal of Abnormal Psychology. 2013;122:314–321. doi: 10.1037/a0032385. [DOI] [PubMed] [Google Scholar]
- Yeragani VK, Pohl R, Berger RD, Balon R, Ramesh C, Glitz D, Weinberg P. Decreased heart rate variability in panic disorder patients: A study of power-spectral analysis of heart rate. Psychiatry Research. 1993;46:89–103. doi: 10.1016/0165-1781(93)90011-5. [DOI] [PubMed] [Google Scholar]