Abstract
Background
We previously reported higher serotonin 1A receptor (5-HT1A) binding in subjects with major depressive disorder (MDD) during a major depressive episode using positron emission tomography imaging with [11C]WAY-100635. 5-HT1A receptor binding is also associated with treatment outcome after nonstandardized antidepressant treatment. We examined whether pretreatment 5-HT1A binding is associated with treatment outcome following standardized escitalopram treatment in MDD. We also compared 5-HT1A binding between all MDD subjects in this cohort and a sample of healthy control subjects.
Methods
Twenty-four MDD subjects in a current major depressive episode and 51 previously studied healthy control subjects underwent positron emission tomography scanning with [11C]WAY-100635, acquiring a metabolite-corrected arterial input function and free-fraction measurement to estimate 5-HT1A binding potential (BPF = Bmax/KD, where Bmax = available receptors and KD = dissociation constant). Major depressive disorder subjects then received 8 weeks of treatment with escitalopram; remission was defined as a posttreatment 24-item Hamilton Depression Rating Scale <10 and ≥50% reduction in Hamilton Depression Rating Scale.
Results
Remitters to escitalopram had 33% higher baseline 5-HT1A binding in the raphe nuclei than nonremitters (p = .047). Across 12 cortical and subcortical regions, 5-HT1A binding did not differ between remitters and nonremitters (p = .86). Serotonin 1A receptor binding was higher in MDD than control subjects across all regions (p = .0003). Remitters did not differ from nonremitters in several relevant clinical measures.
Conclusions
Elevated 5-HT1A binding in raphe nuclei is associated with subsequent remission with the selective serotonin reuptake inhibitor escitalopram; this is consistent with data from a separate cohort receiving naturalistic antidepressant treatment. We confirmed our previous findings of higher 5-HT1A binding in current MDD compared with control subjects.
Keywords: Antidepressant, depression, PET imaging, prediction, serotonin 1A receptor, treatment outcome
Psychiatrists currently lack tools that predict antidepressant response to specific treatments for major depressive disorder (MDD). The National Institutes of Health has identified personalized medicine as one of its primary research goals (1), and several efforts are currently underway to characterize moderators and mediators of treatment outcome in MDD (2-4).
The serotonin 1A (5-HT1A) receptor has been implicated in the pathophysiology of MDD in both animal models and human studies (5). We have found elevated 5-HT1A binding in the brain in current MDD in two previous samples (6,7) and also in a separate remitted MDD sample (8), using positron emission tomography (PET) imaging with [11C]WAY-100635. This receptor serves an autoinhibitory role on serotonergic neurons in the raphe nuclei. Evidence also indicates a role for the 5-HT1A autoreceptor in the mechanism of action of selective serotonin reuptake inhibitors (SSRIs). In rodent models, SSRI exposure initially leads to reduced firing of serotonergic neurons via 5-HT1A autoreceptor stimulation. After approximately 14 days of SSRI exposure, the 5-HT1A receptor desensitizes and serotonergic neuronal firing rate is restored, leading to a net increase in intrasynaptic serotonin (9). This timing coincides with the clinically observed delay in SSRI antidepressant action.
We previously reported that higher baseline 5-HT1A receptor binding is associated with nonremission to naturalistic (open, nonstandardized) treatment for MDD (10). Since that publication, we have developed a method to increase precision of estimation in PET imaging, and therefore statistical power, by weighting observations according to their measurement precision, using standard errors estimated by a bootstrapping algorithm (11) (Supplement 1). The bootstrap algorithm incorporates errors associated with fitting the metabolite curve, input function, and time activity curve for each region of interest (ROI). When we reanalyzed data from this naturalistic study, weighting observations by bootstrap error, the direction of the finding was reversed in the raphe nuclei alone, with 29.5% higher raphe binding in remitters compared with nonremitters (p = .082) (12). Quantification of 5-HT1A binding in raphe nuclei may benefit particularly from incorporation of bootstrap errors, as small regions are particularly susceptible to measurement noise. This distinct finding in raphe nuclei compared with other brain regions is consistent with its distinct role as an autoreceptor in raphe nuclei (13).
In the current study, we compared baseline 5-HT1A binding between MDD remitters and nonremitters with 8 weeks of standardized pharmacotherapy with the SSRI escitalopram. Based on our naturalistic study, we hypothesized that remission would be associated with higher baseline 5-HT1A autoreceptor binding in the raphe nuclei and lower baseline binding across 12 cortical and subcortical regions in the terminal field.
The G allele of a functional promoter polymorphism in the serotonin 1A receptor gene (HTR1A, C-1019G) has been associated with increased 5-HT1A expression in raphe nucleus neurons both in vitro (14) and in vivo using PET (6,7,15). Some previous studies, including our previous naturalistic treatment study (10), have reported associations between the G allele and nonresponse to antidepressant medications (reviewed in [16]). In the current study, we examined HTR1A genotype in MDD escitalopram remitters and nonremitters, hypothesizing higher allelic frequency of the G allele among nonremitters. Finally, we compared this new cohort of MDD subjects with a sample of 51 historical control subjects (6), hypothesizing elevated 5-HT1A binding across all brain regions examined, based on our previous findings (6,7).
Methods and Materials
Sample
Participants were recruited through online or print advertisements and through referrals from neighboring outpatient clinics. Eligibility was assessed by psychiatric and medical history, chart review, physical examination, routine blood tests, pregnancy test, and urine toxicology. Axis I diagnoses were based on the Structured Clinical Interview for DSM-IV (17), conducted by doctoral- or masters’-level psychologists and reviewed in a consensus conference of research psychologists and psychiatrists. Inclusion criteria included: 1) age 18 to 65 years; 2) DSM-IV criteria for MDD in a current major depressive episode; 3) 17-item Hamilton Depression Rating Scale (HDRS) score ≥17; 4) ability to provide informed consent; and 5) ability to discontinue anticoagulant treatment, except for aspirin, for 10 days. Exclusion criteria included: 1) significant medical conditions; 2) lifetime history of alcohol abuse or dependence; 3) substance abuse or dependence (other than nicotine; Table 1) unless in complete remission for >6 months; 4) ecstasy or intravenous drug use more than two times; 5) presence of major psychiatric disorders, including schizophrenia (comorbid anxiety disorders allowed); 6) comorbid anorexia or bulimia nervosa within the past year; 7) first-degree family history of schizophrenia, if subject was <33 years old; 8) inability to remain off all psychotropic drugs that interact with serotonin transporters and/or 5-HT1A receptors for a minimum of 3 weeks; 9) fluoxetine use within 6 weeks of PET scanning; 10) pregnancy, current lactation, plans to conceive during study participation, or abortion within 2 months of enrollment; 11) medical contraindication to antidepressants; 12) dementia; 13) neurological disease or previous head injury accompanied by loss of consciousness or motor deficits; 14) exposure to 5-HT1A receptor agonist within preceding 6 months; 15) failure of more than two SSRI or other antidepressant monotherapy trials of adequate dose and duration; 16) metal implants; 17) current or past exposure to radiation; 18) active suicidality or ideation requiring inpatient admission or medication intervention; and 19) history of significant clinical decompensation in response to prior medication washout.
Table 1.
Clinical, Demographic, and Genetic Characteristics
| Control Subjects (n = 51) |
Remitters (n = 11) |
Nonremitters (n = 13) |
Remitters Versus Nonremitters p Value |
|
|---|---|---|---|---|
| Age | 37.3 ± 14.4 | 34.7 ± 14.0 | 35.2 ± 13.3 | .92 |
| Hamilton Depression Rating Scale (24-Item) | .7 ± 1.0 | 24.6 ± 6.2 | 24.6 ± 4.7 | .99 |
| Years of Education | 16.6 ± 2.9 | 15.1 ± 2.3 | 15.5 ± 2.9 | .74 |
| Beck Depression Inventory | 1.6 ± 2.5 | 23.3 ± 10.5 | 27.1 ± 10.2 | .38 |
| Global Assessment Scale | 90.2 ± 4.8 | 60.4 ± 6.4 | 58.7 ± 5.3 | .49 |
| Beck Hopelessness Scale | 1.6 ± 2.3 | 7.6 ± 8.1 | 9.5 ± 4.8 | .53 |
| Age of Onset | – | 21.5 ± 9.2 | 26.8 ± 13.1 | .28 |
| Median Number of Major Depressive Episodes | – | 2 | 2 | .67a |
| % Female | 29 (56.9%) | 7 (63.6%) | 10 (76.9%) | .66b |
| Number of Subjects with a Family History of Major Depressive Disorder | – | 1 | 3 | .60b |
| Number Not Recently Medicated Subjects (%) | – | 10 (90.9%) | 8 (61.5%) | .24b |
| Current Anxiety Disorder Comorbidity | – | 5 (45.5%) | 3 (23.1%) | .39b |
| Final Escitalopram Dose (mg) | – | 15 ± 5.9 | 18.5 ± 3.8 | .10 |
| Current Nonsmoker (%) | 46 (90.2%) | 9 (81.8%) | 10 (76.9%) | 1b |
| C(-1019)G HTR1A Promoter Polymorphism | ||||
| CC | 17 (34.0%) | 4 (36.3%) | 2 (16.7%) | |
| CG | 29 (58.0%) | 5 (45.5%) | 7 (58.3%) | |
| GG | 4 (8.0%) | 2 (18.2%) | 3 (25.0%) | .644b |
HTR1A, serotonin 1A.
Mann-Whitney U Test p-value.
Fisher’s exact test p value.
Inclusion criteria for control subjects consisted of items 1, 4, and 5 listed above. Additionally, control subjects had no current or past psychiatric diagnosis, with the exception of specific phobia, and were medication and drug free. Exclusion criteria for control subjects were: 1) lifetime alcohol or substance use disorder other than nicotine; 2) first-degree relatives with history of major depression, schizophrenia, or suicide attempt or more than two relatives with substance dependence; and 3) items 4, 10, 13, and 17 listed above.
Based on prior medication history elicited during a semi-structured interview, MDD subjects were characterized as antidepressant-exposed (AE) if they had been exposed to an antidepressant medication for ≥2 months at a therapeutic dosage within 4 years of the scan date and as not recently medicated (NRM) if they were antidepressant-naïve or had antidepressant exposure ≥4 years before the date of PET scanning. We used this definition of NRM, as we previously found no difference in 5-HT1A binding potential BPF between antidepressant-naïve MDD subjects and MDD subjects off of antidepressants for ≥4 years (6).
Clinical Procedures
No MDD subjects were taking antidepressant medication at study enrollment. One MDD subject had stopped ineffective antidepressant medication (duloxetine) before study enrollment and remained off of medication for 23 days before PET imaging, with weekly clinical monitoring. Short-acting benzodiazepines were allowed for treatment of anxiety or insomnia up until 72 hours before scanning. Only one subject used benzodiazepines for this purpose; this subject discontinued benzodiazepines 4 days before PET scanning. Following baseline PET and magnetic resonance imaging (MRI), treatment was initiated with escitalopram at a dose of 10 mg daily for the first 4 weeks. After 4 weeks, escitalopram dose was increased to 20 mg for nonresponders (<50% decrease in HDRS) and was maintained at 10 mg for responders. At 6 weeks, subjects still taking escitalopram 10 mg who were nonremitters (HDRS ≥10 or <50% decrease in HDRS) had their escitalopram dose increased to 20 mg. The primary clinical outcome measure was remission status at 8 weeks.
Twenty-eight subjects underwent baseline PET imaging and began treatment. Two subjects were not analyzed due to 1) further history revealing a prior diagnosis of anorexia nervosa, and 2) lack of input function. Two subjects dropped out of the study before completing 4 weeks of treatment. Two subjects discontinued escitalopram after completing 6 weeks of treatment (due to intolerable side effects), and 22 subjects completed 8 weeks of treatment. We analyzed data from 24 subjects completing at least 6 weeks of SSRI treatment, using last observation carried forward to determine remission status for subjects who did not attend their week 8 visit (both were nonremitters). Three MDD subjects from the current sample were excluded from comparisons of binding between MDD and control subjects, as they overlapped with an MDD sample presented previously (6).
PET/MRI Imaging
[11C]WAY-100635 was synthesized as previously described (18). A metabolite-corrected arterial input function was acquired for use in kinetic modeling (19,20). Plasma free fraction (fP) was measured to allow estimation of the outcome measure BPF (see modeling). Injected dose (ID), injected mass (IM), and fP differed between MDD subjects and healthy control subjects but not between remitters and nonremitters (Table 2). Results from a human dosimetry study (21) necessitated a reduction in ID (and consequently IM), causing the differences between control subjects (scanned earlier) and MDD subjects (scanned later). However, no correlation was found between BPF and either ID or IM (ID: F = .135, df = 1,75, p = .71; IM: F = .33, df = 1,75, p = .95). A head holder (Soule Medical, Tampa, Florida) was molded around the subject’s head to minimize motion. Positron emission tomography images were acquired on an ECAT EXACT HR+ camera (Siemens/CTI, Knoxville, Tennessee) in three-dimensional mode. Following a 10-minute transmission scan, [11C]WAY-100635 was injected as an intravenous bolus and emission data were collected for 110 minutes. T1-weighted MRI images were acquired for co-registration with PET images, identification of ROIs, and tissue segmentation on a 1.5T Signa Advantage or a 3T Signa HDx scanner (General Electric Medical Systems, Milwaukee, Wisconsin).
Table 2.
[11C]WAY-100635 PET Scan Parameters
| Control Subjects (n = 51) |
Remitters (n = 11) |
Nonremitters (n = 13) |
Remitters Versus Nonremitters p Value |
Control Subjects Versus MDD p Value |
|
|---|---|---|---|---|---|
| Injected Dose (mCi) | 7.99 ± 3.43 | 5.68 ± 1.34 | 5.56 ± 1.62 | .85 | .002 |
| Injected Mass (μg) | 2.98 ± 1.94 | 1.30 ± 1.28 | 1.29 ± .95 | .98 | <.001 |
| Plasma Free Fraction (fP) | 8.09% ± 2.40% | 6.49% ± 1.95% | 6.34% ± 2.05% | .85 | .004 |
| Reference Region Binding | .25 ± .011 | .24 ± .023 | .28 ± .024 | .27 | .60 |
| (volume of distribution, VT(REF), Cerebellar White Matter) |
MDD, major depressive disorder; PET, positron emission tomography.
Image Processing
Image analysis was performed within MATLAB 2006b (The MathWorks, Natick, Massachusetts) using extensions to FSL version 3.3 (including Functional Magnetic Resonance Imaging of the Brain’s Linear Image Registration Tool [FLIRT] [22], Brain Extraction Tool [23]), as well as Statistical Parametric Mapping 5 normalization (24) and segmentation routines (25). Motion correction of PET data was achieved using de-noising filter applied to all PET images starting at frame five, as well as rigid-body FLIRT. Positron emission tomography/MRI co-registration was performed using FLIRT between a mean image of motion-corrected PET frames and the T1-weighted MRI as previously described (26).
Regions of interest were manually drawn onto individual subjects’ T1-weighted MRI images by experienced technicians trained to reliably approximate these regions using brain atlases (27,28) and published reports (29,30). A fixed volume elliptical ROI (2 cm3) was placed on raphe nuclei in the dorsal midbrain identified on a mean PET image for each subject. A cylindrical ROI was drawn in the cerebellar white matter, which was used as the reference region, because, compared with cerebellar gray matter, it has lower volume of distribution (VT), comparable nonspecific binding, and less specific binding (6,20).
Quantitative Analysis
VT values of [11C]WAY-100635 were estimated for each ROI using kinetic analysis with an arterial input function and a two-tissue compartment constrained model (for more details, see [19]). Time activity curves were fit with a two-tissue compartment constrained model in which K1/k2 ratio was constrained to that of the reference region (REF, cerebellar white matter), which was fit with a one-tissue compartment model. The primary outcome measure for this study was binding potential (BPF = Bavail/KD) where Bavail is the total number of available receptors and 1/KD is the affinity of the tracer for the receptor. BPF was calculated as (VT(ROI) – VT(REF))/fP. While we have previously provided evidence supporting the use of BPF as the outcome measure of choice with [11C]WAY-100635 (6), we repeated primary analyses with the alternative binding potential outcome measures BPP, which does not correct for fP, calculated as VT(ROI) – VT(REF), and BPND, which assumes equivalent nondisplaceable uptake across groups, calculated as [VT(ROI) – VT(REF)]/VT(REF), for comparison with other findings.
Genotyping
Genotyping of the C-1019G polymorphism of the HTR1A receptor gene was performed as previously described with allele-specific polymerase chain reaction amplification (7).
Statistics
Group comparisons of BPF (remitters vs. nonremitters, MDD vs. control subjects) were performed using mixed-effects modeling methods, with region and diagnostic group as fixed effects and subject as the random effect. Standard errors were computed for each estimated BPF value, using a bootstrap algorithm taking into account errors in plasma, metabolite, and brain data (11). To improve precision in group estimates, observations were weighted by their associated standard errors in the linear mixed effects. To determine the extent to which pretreatment raphe BPF predicted clinical outcome, linear regression was performed, using posttreatment HDRS as the dependent variable and both pretreatment HDRS and raphe BPF as independent variables. To allow for testing of proportional differences in binding across regions and to stabilize variance across regions, all analyses involving multiple regions were performed on log-transformed data. Analyses on a single region (raphe nuclei) were performed on data in the original (nontransformed) scale. Log transformation is used commonly to address skewness and unequal variance of data, both of which are generally issues with PET data. We and others have used log transformation in multiple prior studies (7,10,31-37). Other groups have used related statistical approaches, including linearizing transformation (38) and non-parametric testing (39), to address these issues in analyzing PET data. As the natural log is a monotone transformation, demonstrating a difference in log(BPF) is equivalent to demonstrating a difference (in the same direction) in BPF.
Data are presented graphically using actual (not log-transformed) BPF values. Reported p values were not adjusted for multiple comparisons. Threshold of statistical significance for all analyses was set at p < .05. Linear mixed-effects models of binding and Fisher’s exact tests were performed in R 2.1.0 (http://cran.r-project.org); t tests were performed in Excel (Microsoft, Redmond, Washington) and chi-square tests were done in SPSS Statistics (IBM Corp., Armonk, New York).
Results
Sample Characteristics
Clinical and demographic variables are presented in Table 1. There were no differences between MDD remitters and nonremitters in baseline measures of depression severity, chronicity, prior antidepressant exposure, or family history of depression. Remission rate in the sample was 46%. One MDD subject (a remitter) had past cannabis dependence in sustained remission. Three nonremitters suffered from current comorbid anxiety disorders (two with generalized anxiety disorder and one with social phobia). Five remitters suffered from current comorbid anxiety disorders (all with social phobia, one with comorbid panic disorder, and one with comorbid generalized anxiety disorder).
5-HT1A Binding and Remission Status
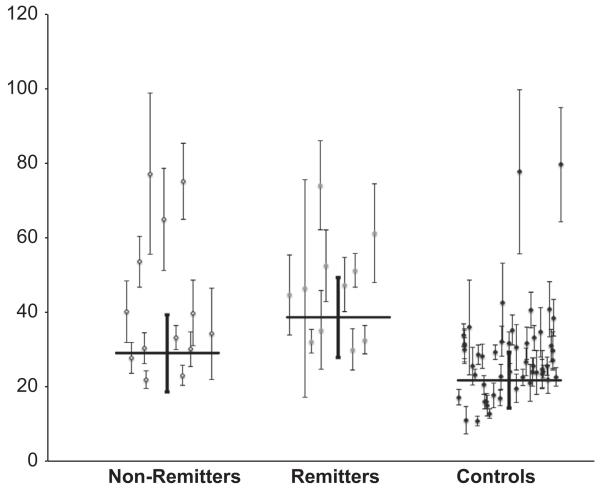
Remitters had higher 5-HT1A binding in the raphe nuclei compared with nonremitters (F = 4.43, df = 1, p = .047) (Figure 1, Table 3). In contrast, 5-HT1A binding did not differ between remitters and nonremitters across all other regions tested simultaneously (F = .033, df = 1,22, p = .86). We have previously shown that 5-HT1A binding is dependent on sex, 5-HT1A genotype, and prior medication status (7,40). Remitter/nonremitter contrasts were unchanged after including sex, genotype, and prior medication status as covariates (raphe nuclei: F = 5.36, df = 1, p = .033; other ROIs: F = .002, df = 1,17, p = .97). Reference region binding did not differ between remitters and nonremitters (Table 2).
Figure 1.
Remitters to escitalopram have higher serotonin 1A binding potential (BPF) in raphe nuclei than nonremitters (p = .047). Error bars represent standard errors computed using a bootstrap algorithm that takes into account errors in metabolite, plasma, and brain data. Horizontal bars represent weighted means.
Table 3.
Comparison of Binding Potential Values (Weighted Means ± SD) Across MDD Remitters, MDD Nonremitters, and Controls
| RN | AMY | HIP | PHG | TEM | ACN | CIN | DOR | MED | VPFC | INS | OCC | PAR | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BPF | |||||||||||||
| Control subjects (51) | 21.8 ± 7.5 | 36.3 ± 12.5 | 53.0 ± 16.7 | 45.6 ± 15.3 | 39.2 ± 12.1 | 34.0 ± 10.4 | 27.9 ± 8.6 | 27.6 ± 8.7 | 29.7 ± 8.9 | 28.9 ± 8.7 | 46.6 ± 13.3 | 21.6 ± 7.2 | 25.9 ± 8.4 |
| Nonremitters (13) | 29.0 ± 10.3 | 47.8 ± 14.8 | 59.9 ± 17.8 | 61.6 ± 18.4 | 49.8 ± 16.2 | 42.4 ± 13.8 | 36.6 ± 11.8 | 34.5 ± 11.4 | 35.8 ± 12.3 | 35.7 ± 12.0 | 54.2 ± 16.9 | 28.1 ± 9.8 | 33.8 ± 10.9 |
| Remitters (11) | 38.7 ± 10.7 | 48.8 ± 14.8 | 74.4 ± 19.3 | 66.8 ± 16.5 | 55.0 ± 16.4 | 50.2 ± 13.0 | 40.1 ± 12.0 | 40.3 ± 11.2 | 44.0 ± 11.5 | 41.5 ± 11.2 | 65.7 ± 18.7 | 29.8 ± 9.3 | 37.5 ± 11.6 |
| MDD (21)a | 34.1 ± 10.6 | 49.7 ± 11.8 | 68.6 ± 16.5 | 66.2 ± 14.7 | 54.5 ± 13.6 | 47.4 ± 11.8 | 40 ± 9.6 | 38.6 ± 10.3 | 39.9 ± 11.5 | 40 ± 10.5 | 61.4 ± 14.7 | 31.1 ± 7.9 | 37.2 ± 9.8 |
| BPP | |||||||||||||
| Control subjects (51) | 1.73 ± .51 | 2.73 ± .81 | 3.92 ± 1.23 | 3.46 ± 1.05 | 2.80 ± .93 | 2.46 ± .80 | 2.00 ± .67 | 1.97 ± .67 | 2.16 ± .69 | 2.06 ± .69 | 3.40 ± 1.00 | 1.56 ± .55 | 1.85 ± .62 |
| Nonremitters (13) | 1.74 ± .68 | 2.86 ± .85 | 3.68 ± .94 | 3.66 ± 1.00 | 3.02 ± .85 | 2.63 ± .73 | 2.20 ± .61 | 2.18 ± .57 | 2.27 ± .64 | 2.21 ± .59 | 3.34 ± .89 | 1.76 ± .50 | 2.10 ± .55 |
| Remitters (11) | 2.62 ± .66 | 3.20 ± .93 | 4.58 ± 1.22 | 4.30 ± 1.08 | 3.64 ± .99 | 3.17 ± .91 | 2.67 ± .69 | 2.60 ± .71 | 2.83 ± .78 | 2.63 ± .72 | 4.20 ± 1.16 | 1.96 ± .58 | 2.50 ± .69 |
| MDD (21)a | 2.0 ± .79 | 3.04 ± .84 | 4.08 ± 1.06 | 3.95 ± 1.02 | 3.34 ± .88 | 2.87 ± .79 | 2.43 ± .62 | 2.4 ± .63 | 2.5 ± .72 | 2.42 ± .64 | 3.71 ± .98 | 1.94 ± .49 | 2.33 ± .6 |
| BPND | |||||||||||||
| Control subjects (51) | 6.28 ± 1.93 | 10.68 ± 2.18 | 15.41 ± 3.27 | 14.24 ± 2.50 | 11.82 ± 2.31 | 10.19 ± 2.08 | 8.24 ± 1.86 | 8.33 ± 1.84 | 8.94 ± 1.84 | 8.58 ± 1.94 | 13.78 ± 2.61 | 6.64 ± 1.43 | 7.93 ± 1.68 |
| Nonremitters (13) | 6.69 ± 2.11 | 11.23 ± 1.75 | 13.32 ± 3.23 | 13.81 ± 2.79 | 11.51 ± 2.75 | 9.64 ± 2.69 | 8.46 ± 2.01 | 7.95 ± 2.22 | 8.40 ± 2.65 | 8.06 ± 2.33 | 12.31 ± 3.11 | 6.62 ± 1.49 | 7.93 ± 1.91 |
| Remitters (11) | 8.56 ± 2.65 | 12.12 ± 2.26 | 17.81 ± 3.60 | 15.31 ± 3.62 | 13.31 ± 3.11 | 11.88 ± 2.71 | 9.22 ± 2.46 | 9.57 ± 2.48 | 10.69 ± 2.27 | 9.56 ± 2.58 | 14.45 ± 3.81 | 7.03 ± 2.05 | 8.88 ± 2.47 |
| MDD (21)a | 7.18 ± 2.43 | 11.54 ± 2.05 | 14.44 ± 3.8 | 14.23 ± 3.16 | 12.13 ± 3.03 | 10.29 ± 2.88 | 8.69 ± 2.23 | 8.41 ± 2.44 | 9.08 ± 2.75 | 8.49 ± 2.52 | 12.97 ± 3.55 | 6.77 ± 1.73 | 8.22 ± 2.17 |
ACN, anterior cingulate; AMY, amygdala; CIN, cingulate cortex (posterior to ACN); DOR, dorsolateral prefrontal cortex; HIP, hippocampus; INS, insular cortex; MDD, major depressive disorder; MED, medial prefrontal cortex; OCC, occipital cortex; PAR, parietal cortex; PHG, parahippocampal gyrus; RN, raphe nuclei; TEM, temporal cortex; VPFC, ventral prefrontal cortex.
Three MDD subjects from the current sample were excluded from comparisons of binding between MDD and control subjects, as they overlapped with the MDD sample presented in a previous publication (6).
Effect of Diagnosis on 5-HT1A Binding
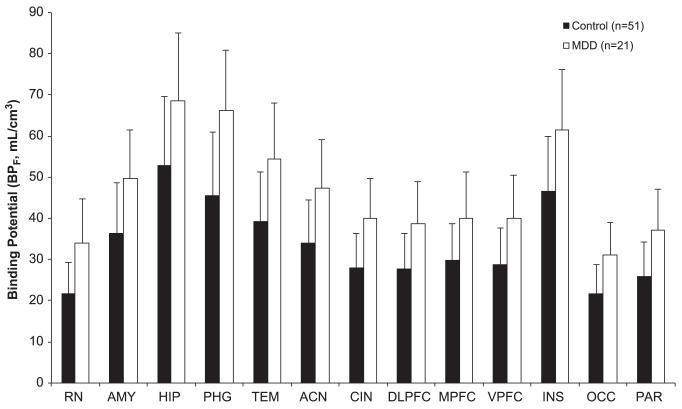
Consistent with our findings in two previous cohorts, the MDD group had higher 5-HT1A BPF than control subjects across all ROIs examined (F = 14.59, df = 1,69, p = .0003) (Figure 2). This finding was unchanged after including sex and genotype as covariates (F = 12.08, df = 1,65, p = .0009). To examine the effects of prior medication status on binding, we compared AE MDD, NRM MDD, and control subjects in a model simultaneously and found a difference in BPF across these groups (F = 7.51, df = 2,68, p = .0011). Pair-wise post hoc testing demonstrated higher binding in NRM MDD subjects compared with healthy control subjects (F = 14.39, df = 1,68, p = .0003) but not compared with AE MDD subjects (F = .896, df = 1,68, p = .35). These results were unchanged with the inclusion of sex and genotype as covariates (three-group comparison: F = 7.46, df = 2,64, p = .0012; NRM MDD vs. control subjects: F = 14.55, df = 1,64, p = .0003; NRM MDD vs. AE MDD: F = .85, df = 1,64, p = .36). Reference region binding did not differ between MDD subjects and control subjects (Table 2).
Figure 2.
Current major depressive disorder (MDD) subjects have higher serotonin 1A binding potential (BPF) than healthy control subjects across all regions of interest examined (p = .0003). Bar heights represent the weighted means for each region of interest; error bars indicate the corresponding equivalent of the standard deviations of the weighted means. ACN, anterior cingulate; AMY, amygdala; CIN, cingulate cortex (posterior to ACN); DLPFC, dorsolateral prefrontal cortex; HIP, hippocampus; INS, insular cortex; MPFC, medial prefrontal cortex; OCC, occipital cortex; PAR, parietal cortex; PHG, parahippocampal gyrus; RN, raphe nuclei; TEM, temporal cortex; VPFC, ventral prefrontal cortex.
Relationship between HTR1A Genotype and 5-HT1A Binding
Genotype at the C-1019G locus did not differ between remitters and nonremitters (Fisher’s exact, p = .64; Table 1). Consistent with our previous studies, we examined the effects of genotype on 5-HT1A binding in raphe nuclei in NRM MDD subjects and control subjects, including diagnosis as a covariate in this analysis. 5-HT1A binding was associated with genotype, with highest binding in raphe nuclei among GG homozygotes (F = 7.36, df = 1, p = .0086). This finding was unchanged including sex as an additional covariate (F = 9.09, df = 1, p = .0038).
Alternative Outcome Measures
Comparisons of binding between remitters and nonremitters yielded similar results using the alternative outcome measure BPP, but not BPND (Table 3, all with same covariates as BPF analyses; BPP: remitters vs. nonremitters in raphe: F = 5.24, df = 1, p = .035; remitters vs. nonremitters in other ROIs: F = .023, df = 1,17, p = .88; BPND: remitters vs. nonremitters in raphe: F = 3.51, df = 1, p = .40; remitters vs. nonremitters in other ROIs: F = .49, df = 1,17, p = .49). Comparisons of binding between MDD and control subjects yielded similar results using the alternative outcome measure BPND, but not BPP (Table 3; BPP: F = 2.31, df = 1,66, p = .13; BPND: F = 6.42, df = 1,66, p = .014).
Discussion
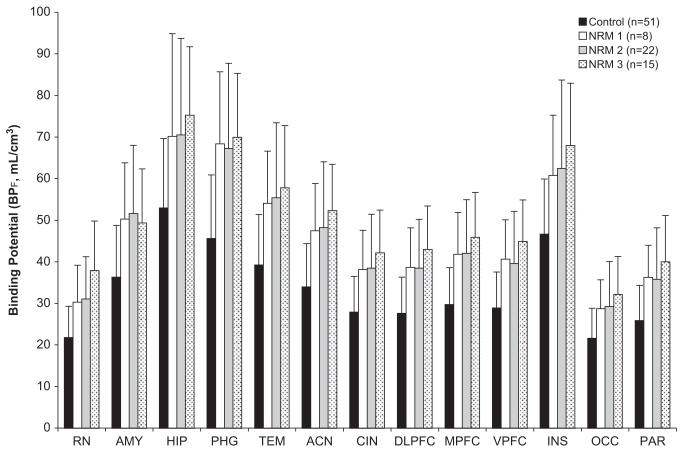
In this study, we found higher pretreatment 5-HT1A binding in raphe nuclei among MDD subjects who remit after 8 weeks of standardized SSRI treatment compared with nonremitters. We did not find differences in 5-HT1A binding between remitters and nonremitters in the other brain regions examined, where 5-HT1A is localized mostly on target neurons within the terminal field of serotonergic neurons. Finally, we found elevated 5-HT1A binding across all regions examined in this MDD cohort compared with a historical healthy volunteer comparison group, consistent with findings in two previous MDD cohorts (6,7). This is presented in Figure 3, showing binding in three independent cohorts of not recently medicated MDD subjects compared with healthy control subjects.
Figure 3.
Comparison of three independent cohorts of not recently medicated (NRM) current major depressive disorder subjects with healthy control subjects demonstrating consistent finding of elevated serotonin 1A binding potential (BPF) in major depressive disorder across samples. ACN, anterior cingulate; AMY, amygdala; CIN, cingulate cortex (posterior to ACN); DLPFC, dorsolateral prefrontal cortex; HIP, hippocampus; INS, insular cortex; MPFC, medial prefrontal cortex; OCC, occipital cortex; PAR, parietal cortex; PHG, parahippocampal gyrus; RN, raphe nuclei; TEM, temporal cortex; VPFC, ventral prefrontal cortex.
Higher pretreatment 5-HT1A binding in the raphe nuclei in MDD remitters is consistent with the trend we found in the same direction in a previous cohort that received naturalistic antidepressant treatment when analyzed using an equivalent analytic approach (10). In that study, we also found higher 5-HT1A binding across the terminal field of serotonergic neurons among nonremitters compared with remitters, a finding not replicated in the current prospective study. There are several differences between these two studies that may partially explain these discrepant findings: in the previous study, subjects received nonstandardized treatment, including a range of different pharmacologic and psychotherapeutic interventions, and remission status was assessed at 1 year (in contrast to the 8-week trial of standardized SSRI treatment in the current study). Moreover, the samples differed: the current study had a higher proportion of antidepressant-naïve individuals, which is relevant, as prior antidepressant exposure is associated with lower 5-HT1A binding (6,7).
We hypothesize the following model to explain the association between high 5-HT1A binding at baseline in raphe nuclei and subsequent remission following SSRI treatment. High 5-HT1A autoreceptor levels in raphe nuclei (seen in eventual SSRI remitters) causes lower basal firing rate of serotonergic neurons. With acute SSRI administration, serotonin reuptake inhibition activates autoreceptors in raphe nuclei, further lowering serotonergic neuron firing rate and serotonin (5-HT) release. When these raphe autoreceptors desensitize over weeks of SSRI treatment (9), there will be a progressive increase in serotonergic neuron firing rate and in 5-HT release, which combined with SSRI reuptake inhibition enhances serotonergic neurotransmission.
In contrast, relatively normal 5-HT1A autoreceptor levels in raphe nuclei (seen in eventual SSRI nonremitters) may lead to more normal firing of serotonergic neurons at baseline and therefore less serotonin deficiency. Chronic SSRI exposure in this case will cause a smaller pool of 5-HT1A autoreceptors to desensitize, causing less of an increase in serotonergic neuron firing rate and in net 5-HT release.
The long-term goal of this research is to move from identification of group differences (remitters vs. nonremitters) to prediction of outcome in individual patients. In an exploratory manner, we examined the capacity of raphe BPF to predict posttreatment HDRS, while co-varying for pretreatment HDRS, using linear regression. Raphe BPF predicted 13% of variance in posttreatment HDRS, although the regression coefficient was not significant (p = .13). While a finding of this magnitude is not yet translatable to the clinic, it is consistent with findings from a separate sample, has face validity, and was achieved without requiring the use of statistical methodology such as support vector machine for prediction.
This is the third independent cohort of current MDD subjects in which we report higher 5-HT1A BPF compared with healthy control subjects; we have also shown that the abnormality is present in unmedicated MDD in sustained remission (8). There is disagreement in the literature regarding the direction of 5-HT1A receptor abnormalities in major depression assessed by PET using [11C]WAY-100635. As described in a recent review (41), the largest differences across these studies are not in differential sampling of clinical populations, but rather in differences in the PET outcome measures employed. For the reasons described below, we believe BPF to be the optimal outcome measure for quantification of 5-HT1A receptors using [11C]WAY-100635. This series of studies provides strong support for elevated 5-HT1A receptor levels in MDD, consistent with animal models of depression (42,43), genetic findings (44), and the effectiveness of 5-HT1A-modulating medications in treating MDD (45,46).
Other groups have also examined the relationship between 5-HT1A binding and treatment response in MDD. One study reported higher [11C]WAY-100635 binding in orbitofrontal cortex in 7 treatment nonresponders compared with 15 responders following treatment with an SSRI or a serotonin-norepinephrine reuptake inhibitor using the PET outcome measure BPND (47). Another study in elderly MDD subjects found that higher pretreatment [11C]WAY-100635 BPND in the dorsal raphe nucleus was associated with longer time to achieve remission with paroxetine at a trend level (39). One difference between those studies and the present finding is the outcome measure used (BPF vs. BPND); the outcome measure BPND is most dependent on the assumption of equivalent nondisplaceable uptake between groups (48). BPND normalizes specific binding to the binding in the reference region, whereas BPF, employed in the current study, normalizes specific binding to the plasma free fraction of radiotracer. In the case of [11C]WAY-100635, reference-region binding is very low, making it particularly susceptible to noise from sources including radiometabolites and problems with scatter correction (6,41). Small differences in reference-region binding (distribution volume of nondisplaceable compartment, VND) can greatly influence the outcome measure BPND, which is defined as (VT – VND)/VND. Consistent with this, we found differences between remitters and nonremitters in raphe using both BPF and BPP, but not BPND. For more on outcome measure selection for [11C]WAY-100635, see Parsey et al. (6). An additional methodological difference with previous studies is that the current study incorporated bootstrap errors to better account for measurement error, thereby reducing noise in estimates (11), of particular importance in small regions such as raphe nuclei.
Plasma free fraction differed between MDD and control groups in this study, although it did not differ between MDD remitters and nonremitters. The difference observed in raphe BPF between remitters and nonremitters was not driven by fP, as these differences persisted using BPP, which does not correct for fP. Plasma free fraction did differ between MDD subjects and control subjects, with lower fP among MDD subjects. When using the alternative outcome measure BPP, which does not account for fP, we did not find differences between MDD subjects and control subjects in the current sample. In a previous study, however, with a larger MDD sample, we found that higher [11C] WAY BPF in not recently medicated MDD subjects was not fully explained by the observed group differences in fP, as BPP also differed between MDD and control subjects (6). There is evidence of inflammatory processes being activated in depression, with increased levels of C-reactive protein expression and certain cytokines, including interleukin-6, among individuals with MDD (49,50). One possible (and speculative) mechanism explaining low fP and possibly fND (free fraction in the nondisplaceable compartment) in MDD would be through greater nonspecific binding of radiotracer to cytokines or C-reactive protein in peripheral plasma and in the central nervous system in this group.
We did not find a significant relationship between the C1019G polymorphism in the HRT1A gene and remission status. This is a small sample for pharmacogenetic research, but at least in this study, the relationship between binding and treatment outcome is independent of this promoter polymorphism. Other factors may have led to higher raphe binding in remitters independent of C1019G genotype, including genetic variation at other regulatory sites and epigenetic factors. Larger samples would be required to more definitively test this conclusion.
Future studies will benefit from the use of a 5-HT1A agonist radioligand, such as [11C]CUMI-101, which specifically identifies high-affinity 5-HT1A receptors (51), thereby capable of measuring desensitization and not just downregulation. This may therefore be a better measure of autoreceptor effects on firing rates and may better predict treatment outcome. It also remains to be determined whether baseline 5-HT1A autoreceptor binding can predict antidepressant outcome with nonserotonergic treatments.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Mental Health R01MH074813 (Ramin V. Parsey, Principal Investigator). Some medication samples were provided by Forest Pharmaceuticals.
Dr. Miller has received financial compensation for psychiatric evaluations of subjects enrolled in medication studies sponsored by Pfizer and Orexigen Therapeutics unrelated to this article. His family owns stock in Johnson & Johnson. Ms. Hesselgrave, Dr. Ogden, and Dr. Zanderigo report no biomedical financial interests or potential conflicts of interest. Dr. Oquendo received financial compensation from Pfizer for the safety evaluation of a clinical facility, unrelated to the current manuscript, and was the recipient of a Grant from Eli Lilly to support a year of the salary for the Lilly Suicide Scholar, Enrique Baca-Garcia, M.D., Ph.D. She has received unrestricted educational grants and/or lecture fees from Astra-Zeneca, Bristol Myers Squibb, Eli Lilly, Janssen, Otsuko, Pfizer, Sanofi-Aventis, and Shire. Her family owns stock in Bristol Myers Squibb. Dr. Mann received past unrelated Grants from GlaxoSmithKline and Novartis. Dr. Parsey received past unrelated Grants from GlaxoSmithKline, Novartis, Pfizer, and Lundbeck.
Footnotes
ClinicalTrials.gov: Pre-Treatment Positron Emission Topography Scanning for Increasing Success in Antidepressant Treatment; http://www.clinicaltrials.gov/ct2/show/NCT00456014?term=NCT00456014&rank=1; NCT00456014.
Supplementary material cited in this article is available online at http://dx.doi.org/10.1016/j.biopsych.2013.03.021.
References
- 1.Hamburg MA, Collins FS. The path to personalized medicine. N Engl J Med. 2010;363:301–304. doi: 10.1056/NEJMp1006304. [DOI] [PubMed] [Google Scholar]
- 2.Williams LM, Rush AJ, Koslow SH, Wisniewski SR, Cooper NJ, Nemeroff CB, et al. International Study to Predict Optimized Treatment for Depression (iSPOT-D), a randomized clinical trial: Rationale and protocol. Trials. 2011;12:4. doi: 10.1186/1745-6215-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Establishing Moderators and Biosignatures of Antidepressant Response for Clinical Care. doi: 10.1016/j.jpsychires.2016.03.001. Available at: http://clinicaltrials.gov/ct2/show/NCT01407094. [DOI] [PMC free article] [PubMed]
- 4.Franco AR, Holtzheimer PE, Kelley ME, Dunlop BW, Craighead WE, Mayberg HS. Pretreatment resting state fMRI predicts differential response to CBT or medication. Biol Psychiatry. 2011;69:165S. [Google Scholar]
- 5.Richardson-Jones JW, Craige CP, Guiard BP, Stephen A, Metzger KL, Kung HF, et al. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron. 2010;65:40–52. doi: 10.1016/j.neuron.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parsey RV, Ogden RT, Miller JM, Tin A, Hesselgrave N, Goldstein E, et al. Higher serotonin 1A binding in a second major depression cohort: Modeling and reference region considerations. Biol Psychiatry. 2010;68:170–178. doi: 10.1016/j.biopsych.2010.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parsey RV, Oquendo MA, Ogden RT, Olvet DM, Simpson N, Huang YY, et al. Altered serotonin 1A binding in major depression: A [carbonyl-C-11]WAY100635 positron emission tomography study. Biol Psychiatry. 2006;59:106–113. doi: 10.1016/j.biopsych.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 8.Miller JM, Brennan KG, Ogden TR, Oquendo MA, Sullivan GM, Mann JJ, Parsey RV. Elevated serotonin 1A binding in remitted major depressive disorder: Evidence for a trait biological abnormality. Neuropsychopharmacology. 2009;34:2275–2284. doi: 10.1038/npp.2009.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blier P, De Montigny C. Electrophysiological investigations on the effect of repeated zimelidine administration on serotonergic neurotransmission in the rat. J Neurosci. 1983;3:1270–1278. doi: 10.1523/JNEUROSCI.03-06-01270.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parsey RV, Olvet DM, Oquendo MA, Huang YY, Ogden RT, Mann JJ. Higher 5-HT1A receptor binding potential during a major depressive episode predicts poor treatment response: Preliminary data from a naturalistic study. Neuropsychopharmacology. 2006;31:1745–1749. doi: 10.1038/sj.npp.1300992. [DOI] [PubMed] [Google Scholar]
- 11.Ogden RT, Tarpey T. Estimation in regression models with externally estimated parameters. Biostatistics. 2006;7:115–129. doi: 10.1093/biostatistics/kxi044. [DOI] [PubMed] [Google Scholar]
- 12.Miller JM, Hesselgrave N, Ogden RT, Oquendo MA, Mann JJ, Parsey RV. Elevated serotonin 1A receptor binding in raphe nuclei is associated with remission to the antidepressant escitalopram. Biol Psychiatry. 2012;71:196S. [Google Scholar]
- 13.Aghajanian GK, Sanders-Bush E. Serotonin. In: Davis KL, Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology-The Fifth Generation of Progress. Lipincott Williams & Wilkins; Philadelphia: 2002. pp. 15–34. [Google Scholar]
- 14.Lemonde S, Turecki G, Bakish D, Du L, Hrdina PD, Bown CD, et al. Impaired repression at a 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. J Neurosci. 2003;23:8788–8799. doi: 10.1523/JNEUROSCI.23-25-08788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sullivan GM, Ogden RT, Oquendo MA, Kumar JS, Simpson N, Huang YY, et al. Positron emission tomography quantification of serotonin-1A receptor binding in medication-free bipolar depression. Biol Psychiatry. 2009;66:223–230. doi: 10.1016/j.biopsych.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le François B, Czesak M, Steubl D, Albert PR. Transcriptional regulation at a HTR1A polymorphism associated with mental illness. Neuropharmacology. 2008;55:977–985. doi: 10.1016/j.neuropharm.2008.06.046. [DOI] [PubMed] [Google Scholar]
- 17.First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I/P, Version 2.0) Biometrics Research Department, New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- 18.Hwang DR, Simpson NR, Montoya J, Man JJ, Laruelle M. An improved one-pot procedure for the preparation of [11C-carbonyl]-WAY100635. Nucl Med Biol. 1999;26:815–819. doi: 10.1016/s0969-8051(99)00056-6. [DOI] [PubMed] [Google Scholar]
- 19.Parsey RV, Slifstein M, Hwang DR, Abi-Dargham A, Simpson N, Mawlawi O, et al. Validation and reproducibility of measurement of 5-HT1A receptor parameters with [carbonyl-11C]WAY-100635 in humans: Comparison of arterial and reference tisssue input functions. J Cereb Blood Flow Metab. 2000;20:1111–1133. doi: 10.1097/00004647-200007000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Parsey RV, Arango V, Olvet DM, Oquendo MA, Van Heertum RL, John Mann J. Regional heterogeneity of 5-HT1A receptors in human cerebellum as assessed by positron emission tomography. J Cereb Blood Flow Metab. 2005;25:785–793. doi: 10.1038/sj.jcbfm.9600072. [DOI] [PubMed] [Google Scholar]
- 21.Parsey RV, Belanger MJ, Sullivan GM, Simpson NR, Stabin MG, Van Heertum R, Mann JJ. Biodistribution and radiation dosimetry of 11C-WAY100,635 in humans. J Nucl Med. 2005;46:614–619. [PubMed] [Google Scholar]
- 22.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 23.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 1999;7:254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 26.DeLorenzo C, Klein A, Mikhno A, Gray N, Zanderigo F, Mann JJ, et al. A new method for assessing PET-MRI coregistration. Proc SPIE. 2009:7529. [Google Scholar]
- 27.Duvernoy H. Surface, Three-Dimensional Sectional Anatomy and MRI. Sringer-Verlag Wien; New York: 1991. The Human Brain. [Google Scholar]
- 28.Talairach J, Tournoux P. Three-dimensional Proportional System: An Approach of Cerebral Imaging. Theime Medical Publisher; New York: 1988. Co-planar Stereotactic Atlas of the Human Brain. [Google Scholar]
- 29.Kates WR, Abrams MT, Kaufmann WE, Breiter SN, Reiss AL. Reliability and validity of MRI measurement of the amygdala and hippocampus in children with fragile X syndrome. Psychiatry Res. 1997;75:31–48. doi: 10.1016/s0925-4927(97)00019-x. [DOI] [PubMed] [Google Scholar]
- 30.Killiany RJ, Moss MB, Nicholson T, Jolesz F, Sandor T. An interactive procedure for extracting features of the brain from magnetic resonance images: The lobes. Hum Brain Mapp. 1997;5:355–363. doi: 10.1002/(SICI)1097-0193(1997)5:5<355::AID-HBM4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 31.Parsey RV, Hastings RS, Oquendo MA, Hu X, Goldman D, Huang YY, et al. Effect of a triallelic functional polymorphism of the serotonin-transporter-linked promoter region on expression of serotonin transporter in the human brain. Am J Psychiatry. 2006;163:48–51. doi: 10.1176/appi.ajp.163.1.48. [DOI] [PubMed] [Google Scholar]
- 32.Hirvonen J, Karlsson H, Kajander J, Lepola A, Markkula J, Rasi-Hakala H, et al. Decreased brain serotonin 5-HT1A receptor availability in medication-naive patients with major depressive disorder: An in-vivo imaging study using PET and [carbonyl-11C]WAY-100635. Int J Neuropsychopharmacol. 2008;11:465–476. doi: 10.1017/S1461145707008140. [DOI] [PubMed] [Google Scholar]
- 33.Oquendo MA, Hastings RS, Huang YY, Simpson N, Ogden RT, Hu XZ, et al. Brain serotonin transporter binding in depressed patients with bipolar disorder using positron emission tomography. Arch Gen Psychiatry. 2007;64:201–208. doi: 10.1001/archpsyc.64.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parsey RV, Hastings RS, Oquendo MA, Huang YY, Simpson N, Arcement J, et al. Lower serotonin transporter binding potential in the human brain during major depressive episodes. Am J Psychiatry. 2006;163:52–58. doi: 10.1176/appi.ajp.163.1.52. [DOI] [PubMed] [Google Scholar]
- 35.Miller JM, Oquendo MA, Ogden RT, Mann JJ, Parsey RV. Serotonin transporter binding as a possible predictor of one-year remission in major depressive disorder. J Psychiatr Res. 2008;42:1137–1144. doi: 10.1016/j.jpsychires.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sullivan GM, Oquendo MA, Simpson N, Van Heertum RL, Mann JJ, Parsey RV. Brain serotonin1A receptor binding in major depression is related to psychic and somatic anxiety. Biol Psychiatry. 2005;58:947–954. doi: 10.1016/j.biopsych.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 37.Milak MS, Severance AJ, Prabhakaran J, Kumar JS, Majo VJ, Ogden RT, et al. In vivo serotonin-sensitive binding of [11C]CUMI-101: A serotonin 1A receptor agonist positron emission tomography radiotracer. J Cereb Blood Flow Metab. 2011;31:243–249. doi: 10.1038/jcbfm.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rabiner EA, Wilkins MR, Turkheimer F, Gunn RN, Udo de Haes J, de Vries M, Grasby PM. 5-Hydroxytryptamine1A receptor occupancy by novel full antagonist 2-[4-[4-(7-chloro-2,3-dihydro-1,4-benzdioxyn-5-yl)-1-piperazinyl]butyl]-1,2-benzi sothiazol-3-(2H)-one-1,1-dioxide: a[11C] [O-methyl-3H]-N-(2-(4-(2-methoxyphenyl)-1-piperazinyl)ethyl)-N-(2-pyridinyl) cyclohexanecarboxamide trihydrochloride (WAY-100635) positron emission tomography study in humans. J Pharmacol Exp Ther. 2002;301:1144–1150. doi: 10.1124/jpet.301.3.1144. [DOI] [PubMed] [Google Scholar]
- 39.Meltzer CC, Price JC, Mathis CA, Butters MA, Ziolko SK, Moses-Kolko E, et al. Serotonin 1A receptor binding and treatment response in late-life depression. Neuropsychopharmacology. 2004;29:2258–2265. doi: 10.1038/sj.npp.1300556. [DOI] [PubMed] [Google Scholar]
- 40.Parsey RV, Oquendo MA, Simpson NR, Ogden RT, Van Heertum R, Arango V, Mann JJ. Effects of sex, age, and aggressive traits in man on brain serotonin 5-HT(1A) receptor binding potential measured by PET using [C-11]WAY-100635. Brain Res. 2002;954:173–182. doi: 10.1016/s0006-8993(02)03243-2. [DOI] [PubMed] [Google Scholar]
- 41.Shrestha S, Hirvonen J, Hines CS, Henter ID, Svenningsson P, Pike VW, Innis RB. Serotonin-1A receptors in major depression quantified using PET: Controversies, confounds, and recommendations. Neuroimage. 2012;59:3243–3251. doi: 10.1016/j.neuroimage.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naudon L, El Yacoubi M, Vaugeois JM, Leroux-Nicollet I, Costentin J. A chronic treatment with fluoxetine decreases 5-HT(1A) receptors labeling in mice selected as a genetic model of helplessness. Brain Res. 2002;936:68. doi: 10.1016/s0006-8993(02)02548-9. [DOI] [PubMed] [Google Scholar]
- 43.Shishkina GT, Kalinina TS, Dygalo NN. Serotonergic changes produced by repeated exposure to forced swimming: Correlation with behavior. Ann N Y Acad Sci. 2008;1148:148–153. doi: 10.1196/annals.1410.074. [DOI] [PubMed] [Google Scholar]
- 44.Albert PR, Lemonde S. 5-HT1A receptors, gene repression, and depression: Guilt by association. Neuroscientist. 2004;10:575–593. doi: 10.1177/1073858404267382. [DOI] [PubMed] [Google Scholar]
- 45.Khan A, Cutler AJ, Kajdasz DK, Gallipoli S, Athanasiou M, Robinson DS, et al. A randomized, double-blind, placebo-controlled, 8-week study of vilazodone, a serotonergic agent for the treatment of major depressive disorder. J Clin Psychiatry. 2011;72:441–447. doi: 10.4088/JCP.10m06596. [DOI] [PubMed] [Google Scholar]
- 46.Trivedi MH, Fava M, Wisniewski SR, Thase ME, Quitkin F, Warden D, et al. Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006;354:1243–1252. doi: 10.1056/NEJMoa052964. [DOI] [PubMed] [Google Scholar]
- 47.Moses-Kolko EL, Price JC, Thase ME, Meltzer CC, Kupfer DJ, Mathis CA, et al. Measurement of 5-HT1A receptor binding in depressed adults before and after antidepressant drug treatment using positron emission tomography and [11C]WAY-100635. Synapse. 2007;61:523–530. doi: 10.1002/syn.20398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- 49.Berk M, Wadee AA, Kuschke RH, O’Neill-Kerr A. Acute phase proteins in major depression. J Psychosom Res. 1997;43:529–534. doi: 10.1016/s0022-3999(97)00139-6. [DOI] [PubMed] [Google Scholar]
- 50.Karlovic D, Serretti A, Vrkic N, Martinac M, Marcinko D. Serum concentrations of CRP, IL-6, TNF-alpha and cortisol in major depressive disorder with melancholic or atypical features. Psychiatry Res. 2012;198:74–80. doi: 10.1016/j.psychres.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 51.Milak MS, DeLorenzo C, Zanderigo F, Prabhakaran J, Kumar JS, Majo VJ, et al. In vivo quantification of human serotonin 1A receptor using 11C-CUMI-101, an agonist PET radiotracer. J Nucl Med. 2010;51:1892–1900. doi: 10.2967/jnumed.110.076257. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.