Abstract
The effect of botulinum toxin type A injection on voluntary grip control was examined in a 53-year-old female, who sustained a hemorrhagic right middle cerebral artery stroke 3 years previously, which resulted in finger flexor spasticity and residual weak finger/wrist extension. The patient received 50 units of botulinum toxin type A injection each to the motor points (2 sites/muscle) of the left flexor digitorum superficialis and flexor digitorum profundus, respectively. Botulinum toxin injection led to weakness and tone reduction in the spastic finger flexors, but improved grip release time in grip initiation/release reaction time tasks. Improved release time was accompanied by shortened extensor electromyography activity, and improved release time likely correlated with blocked co-contraction of finger flexors during voluntary finger extension. This case report demonstrated that botulinum toxin injection improved voluntary motor control of the hand in a chronic stroke patient with residual finger extension.
Keywords: botulinum toxin type A, spasticity, grip, stroke, neural regeneration
Abbreviations
BT, botulinum toxin; EMG, electromyography; FDS, flexor digitorum superficialis; EDC, extensor digitorum communis
INTRODUCTION
Many stroke survivors exhibit difficulties with object manipulation when using the paretic hand for grip initiation and termination. Grip termination in the paretic hand takes approximately three-fold longer than in the non-paretic hand[1], which results in serious difficulties in releasing a grasped object in a timely fashion. Delays in initiating and terminating, i.e., relaxing, have also been observed for the paretic wrist[2] and lower limb[3] in stroke survivors. This delay in grip termination/release is attributed to sustained finger flexor muscle activity after grip[2].
Sustained finger flexor activity after a voluntary contraction, e.g., grip, has been consistently observed in stroke- survivors[1,4,5,6]. It has been ascribed to spastic hypertonia of the finger flexor muscles[4,5] and inappropriate co-activation between finger flexors and extensors during finger extension, e.g., an attempt to extend fingers paradoxically results in flexion[4].
Botulinum toxin (BT) injection is commonly used to manage focal spasticity and is recommended as the first-line treatment for post-stroke spasticity, such as finger flexor hypertonia[7]. The toxin blocks acetylcholine release pre-synaptically and inhibits muscle contraction temporarily, consequently decreasing spasticity for several months. A recent study reported that BT type A injection is useful for improving basic upper limb tasks, such as hand hygiene and facilitation of dressing. However, the study reveals that injection is unlikely to be useful for improving active upper limb functions, such as reaching and grasping[8]. The present study demonstrated improved voluntary motor control of the hand after BT injection to the spastic finger flexors in a chronic stroke patient, who exhibited severe finger flexor spasticity, but residual weak voluntary finger extension.
CASE REPORT
A 53-year-old, right-handed female (height 1.54 m; weight 48.2 kg) sustained a hemorrhagic right middle cerebral artery stroke 3 years earlier. The patient presented with left spastic hemiparesis involving, among others, the finger flexors. In addition, she exhibited weak voluntary finger and wrist extension. The patient underwent extensive physical and occupational therapy after the stroke and up to the time of presentation. A total of 50 units onabotulinum toxin A (BOTOX™, Allergan, Irvine, CA, USA) was administered to the left flexor digitorum superficialis (FDS) and flexor digitorum profundus, respectively. The injection was guided by percutaneous electrical stimulation of the motor points of these muscles.
After providing written consent, the subject attended three laboratory sessions before (Pre = Day 0), after (Post 1 = 10 days), and at the follow-up (Post 2 = 2.5 months) injection. Delays in grip initiation and release were assessed using electromyographic (EMG) signals in paretic and non-paretic sides, respectively. The Modified Ashworth Scale (MAS) was used to assess hypertonia. Bilateral grip strength was measured using a hand-held dynamometer (Lafayette Instruments, Lafayette, IN, USA), and the Functional Independence Measure (FIM) was used to evaluate clinical improvement.
During grip initiation and release tasks, the subject sat comfortably in a chair with a backrest to provide proper trunk support. The limb was supported and secured firmly on a customized apparatus on a desk and was placed with 45° shoulder abduction and flexion and a 90° elbow flexion. The wrist was placed and secured in a neutral position (with thumb pointing upward), and the hand was placed around a customized hand-held apparatus. The apparatus was fixed to the table and was adjusted to accommodate hand size, adjusting for comfort and a firm grip. First, the non-paretic hand was tested, followed by the impaired hand. In each 20-second trial, the subject was asked to relax the fingers for the first 10 seconds, maximally grip the apparatus and hold for 5 seconds, and then relax the hand for 10 seconds. Computer-generated audible tones cued the subject when to start and stop grip efforts. The subject was instructed to grip the apparatus as quickly as possible in response to the first tone (start), and then relax the hand as quickly as possible when the second tone (stop) was played. The patient practiced five trials prior to the grip initiation and release task, which allowed for familiarizing with the setting and task. After the practice sets, five trials for each side were performed.
Bipolar active surface electrodes (Delsys, Boston, MA, USA) were placed over the muscle bellies of FDS and extensor digitorum communis (EDC). The FDS muscle is the primary mover in power grip, and EDC muscle activity was recorded to monitor antagonist muscle activity. For consistency, electrode locations were recorded, and electrodes were placed at identical locations for all sessions. EMGs were recorded at 1 000 Hz using a Delsys amplifier and data acquisition system (National Instruments, Austin, TX, USA) controlled by custom LabView program (National Instruments). EMG signals were processed and analyzed offline using a custom Matlab program (The MathWorks, Natick, MA, USA). EMG signals were initially band-pass filtered (10-400 Hz), rectified, and then root-mean-square smoothed with a 40-ms time constant to create envelopes that clearly displayed aggregated muscle activity.
Delays in grip initiation and release were determined from the FDS EMG. The Teager-Kaiser energy operator was used to analyze surface EMG to improve the signal-to-noise ratio, as well as to optimize EMG onset/offset detection[9]. The baseline FDS EMG was calculated as values for the EMG averaged across a 0.5-second window during the initial resting period of each trial. The FDS EMG onset time was identified when the EMG value increased more than the mean value plus 3 standard deviations of the baseline values. Grip initiation delay was calculated as the time difference between EMG onset and the “start” audible tone (Figure 1).
Figure 1.
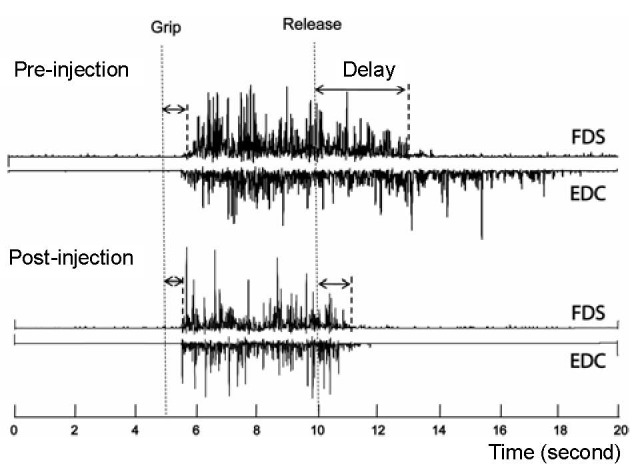
A representative electromyography of flexor digitorum superficialis (FDS) and extensor digitorum communis (EDC) before and 10 days after botulinum toxin injection.
The subject was asked to grip as soon and as hard as possible after the “grip” signal and relax after the “release” signal (dash lines). The release delay time decreases after injection, along with shortened EDC activities.
The FDS EMG offset time was identified as the time when EMG values fell below the mean plus 3 standard deviations of the baseline values. The release delay was then calculated as the time difference between EMG offset and the “stop” audible tone (Figure 1).
After BT injection to the finger flexor muscles (FDS and flexor digitorum profundus) of the paretic left hand, grip strength decreased at 10 days post-injection and returned to pre-injection levels at 2.5 months post-injection (Pre vs. Post 1 vs. Post 2: 2.3 vs. 1.0 vs. 2.2 kg), while grip strength of the non-paretic hand remained stable (Pre vs. Post 1 vs. Post 2: 22.7 vs. 20.4 vs. 22.2 kg). In parallel, the MAS scores of the affected finger flexors were as follows: Pre vs. Post 1 vs. Post 2: 2 vs. 0 vs. 1 for FDS; 2 vs. 0 vs. 1 for flexor digitorum profundus. FIM scores remained unchanged (FIM = 6 for Pre, Post 1, and Post 2), but the subject reported an improved overall ability for handling objects, as well as bimanual activities at home while performing activities of daily living in Post 1 and Post 2 measures.
Release delay time in the paretic hand resulted in dynamic changes (Pre: 2 223 ms; Post 1:1 024 ms; Post 2: 1 333 ms), without dramatic changes in grip initiation delay times in the paretic hand (Pre: 417 ms; Post 1: 439 ms; Post 2: 378 ms). It is noteworthy to mention that a decreased FDS release delay time was paralleled by shortened EDC and EDC EMGs (Figure 1). In contrast, both grip initiation and release delay times in the non-impaired hand remained relatively stable in all measurements (Pre vs. Post 1 vs. Post 2: 468 ms vs. 404 ms vs. 371 ms for grip initiation delay; 725 ms vs. 740 ms vs. 691 ms for release delay).
DISCUSSION
Results from the present case report demonstrated that BT injection to spastic finger flexors in a chronic stroke patient improved grip initiation and termination. The magnitude of grip initiation and release delay time in the paretic hand was within previously published ranges[1]. The pattern of changes in initiation and release delay time after the BT injection, however, could shed light on the mechanisms underlying grip release delay in chronic stroke survivors. Given the unchanged grip initiation delay time in the paretic hand after BT injection, as well as stable grip initiation and release delay time in the non-paretic hand in the follow-up measurements, improvement in grip release time was not likely related to practice/learning effect. Previous results have shown that BT injections can paralyze afferent fibers[10], in addition to blocking acetylcholine release pre-synaptically at neuromuscular junctions. This would release the presynaptic and/or reciprocal inhibitory effect exerted by the finger flexor afferents on the motoneurons of finger extensors during attempted voluntary finger extension, i.e., reduced inhibition from paralyzed finger flexors after BT injection. This interpretation is further supported by two findings in this case study: (1) duration of extensor activity was dramatically shortened. This apparently resulted from a reduced need of the finger extensors to counteract reciprocal inhibition from the finger flexors; (2) grip release delay time increased in conjunction with the finger flexor MAS score at 2.5 months post-injection. This parallel change suggested that increased reciprocal inhibition from hypertonic finger flexors resulted in prolonged grip release delay. Functional improvement of the hand after BT injection is likely to be case-specific. Selected patients with spastic finger flexors, who exhibit residual voluntary finger extension, are likely to have this potential as a result of reduced reciprocal inhibition from paralyzed finger flexors after the injection.
Following the BT injection to spastic finger flexors in a chronic stroke patient, grip strength of the paretic hand decreased at 10 days post-injection and returned to the pre-injection level at 2.5 months post-injection. Dynamic changes in paretic hand-grip strength correlated with dynamic changes in finger flexor hypertonia. These were consistent with previously described changes and common clinical observations after BT[11]. The unchanging FIM scores after BT injection could be explained by the ceiling effects of this measurement. However, patient subjective improvement in overall motor control correlated with laboratory measurements of improved release delay time in the paretic hand. Given that the patient suffered from residual weak voluntary finger extension, it is possible that reduced reciprocal inhibition from the finger flexors after BT injection could result in increased ability to handle objects and bimanual activities.
CONCLUSION
The present case report demonstrated that BT injection improved voluntary motor control of the hand in a chronic stroke patient with residual finger extension.
Footnotes
Funding: This study was supported in part by NIH grants (NIH/NINDS R01NS060774; NIH/NICHD/NCMRR R24 HD050821-08) under subcontract with the Rehabilitation Institute of Chicago.
Conflicts of interest: None declared.
(Edited by Acosta GB, Chen JC, Zhan SQ, Bai H/Song LP)
REFERENCES
- [1].Seo NJ, Rymer WZ, Kamper DG. Delays in grip initiation and termination in persons with stroke: effects of arm support and active muscle stretch exercise. J Neurophysiol. 2009;101(6):3108–3115. doi: 10.1152/jn.91108.2008. [DOI] [PubMed] [Google Scholar]
- [2].Chae J, Yang G, Park BK, et al. Delay in initiation and termination of muscle contraction, motor impairment, and physical disability in upper limb hemiparesis. Muscle Nerve. 2002;25(4):568–575. doi: 10.1002/mus.10061. [DOI] [PubMed] [Google Scholar]
- [3].Chae J, Quinn A, El-Hayek K, et al. Delay in initiation and termination of tibialis anterior contraction in lower-limb hemiparesis: relationship to lower-limb motor impairment and mobility. Arch Phys Med Rehabil. 2006;87(9):1230–1234. doi: 10.1016/j.apmr.2006.05.007. [DOI] [PubMed] [Google Scholar]
- [4].Kamper DG, Rymer WZ. Impairment of voluntary control of finger motion following stroke: role of inappropriate muscle coactivation. Muscle Nerve. 2001;24(5):673–681. doi: 10.1002/mus.1054. [DOI] [PubMed] [Google Scholar]
- [5].Kamper DG, Harvey RL, Suresh S, et al. Relative contributions of neural mechanisms versus muscle mechanics in promoting finger extension deficits following stroke. Muscle Nerve. 2003;28(3):309–318. doi: 10.1002/mus.10443. [DOI] [PubMed] [Google Scholar]
- [6].Kline TL, Schmit BD, Kamper DG. Exaggerated interlimb neural coupling following stroke. Brain. 2007;130(Pt 1):159–169. doi: 10.1093/brain/awl278. [DOI] [PubMed] [Google Scholar]
- [7].Sheean G. Botulinum toxin should be first-line treatment for poststroke spasticity. J Neurol Neurosurg Psychiatry. 2009;80(4):359. doi: 10.1136/jnnp.2008.164889. [DOI] [PubMed] [Google Scholar]
- [8].Shaw LC, Price CI, van Wijck FM, et al. Botulinum Toxin for the Upper Limb after Stroke (BoTULS) Trial: effect on impairment, activity limitation, and pain. Stroke. 2011;42(5):1371–1379. doi: 10.1161/STROKEAHA.110.582197. [DOI] [PubMed] [Google Scholar]
- [9].Li X, Zhou P, Aruin AS. Teager-Kaiser energy operation of surface EMG improves muscle activity onset detection. Ann Biomed Eng. 2007;35(9):1532–1538. doi: 10.1007/s10439-007-9320-z. [DOI] [PubMed] [Google Scholar]
- [10].Filippi GM, Errico P, Santarelli R, et al. Botulinum A toxin effects on rat jaw muscle spindles. Acta Otolaryngol. 1993;113(3):400–404. doi: 10.3109/00016489309135834. [DOI] [PubMed] [Google Scholar]
- [11].Varghese-Kroll E, Elovic EP. Contralateral weakness and fatigue after high-dose botulinum toxin injection for management of poststroke spasticity. Am J Phys Med Rehabil. 2009;88(6):495–499. doi: 10.1097/PHM.0b013e3181a5b056. [DOI] [PubMed] [Google Scholar]


