Abstract
An open question in olfactory coding is the extent of interglomerular connectivity: do olfactory glomeruli and their neurons regulate the odorant responses of neurons innervating other glomeruli? In the olfactory system of the moth Manduca sexta, the response properties of different types of antennal olfactory receptor cells are known. Likewise, a subset of antennal lobe glomeruli has been functionally characterized and the olfactory tuning of their innervating neurons identified. This provides a unique opportunity to determine functional interactions between glomeruli of known input, specifically, (1) glomeruli processing plant odors and (2) glomeruli activated by antennal stimulation with pheromone components of conspecific females. Several studies describe reciprocal inhibitory effects between different types of pheromone-responsive projection neurons suggesting lateral inhibitory interactions between pheromone component-selective glomerular neural circuits. Furthermore, antennal lobe projection neurons that respond to host plant volatiles and innervate single, ordinary glomeruli are inhibited during antennal stimulation with the female’s sex pheromone. The studies demonstrate the existence of lateral inhibitory effects in response to behaviorally significant odorant stimuli and irrespective of glomerular location in the antennal lobe. Inhibitory interactions are present within and between olfactory subsystems (pheromonal and non-pheromonal subsystems), potentially to enhance contrast and strengthen odorant discrimination.
Keywords: Electrophysiology, Glomerulus, Neural coding, Olfaction, Pheromone
Introduction
Individual olfactory glomeruli in the antennal lobes of insects and olfactory bulbs of vertebrates receive convergent input from olfactory receptor cells that express the same odorant receptor protein (reviewed in Axel 2005; Buck 2005; Mombaerts 2006; Vosshall and Stocker 2007). Thus, olfactory glomeruli serve as the address for specific features of an odorant, i.e., an individual chemical compound (Shepherd et al. 2004). Central output neurons communicate the glomerular activity patterns evoked by olfactory receptor cell stimulation to higher-order olfactory centers. Traditionally, central olfactory space has been analyzed in terms of coding qualitative aspects of odor stimuli, i.e., mixtures of individual chemicals, in olfactory glomeruli (Christensen et al. 1996; Hildebrand and Shepherd 1997; Galizia et al. 1999; Wachoviak and Cohen 2001; Christensen and Hildebrand 2002; Hansson et al.2003; Heinbockel et al. 2004). Several studies have raised questions about the existence of functional interactions between glomeruli in the antennal lobe or olfactory bulb and how these interactions shape synaptic output from the antennal lobe or olfactory bulb to downstream olfactory centers (Yokoi et al. 1995; Lei et al. 2002; Sachse and Galizia 2003; Aungst et al. 2003; Olsen et al. 2008). In the visual system, lateral inhibition between retinal ganglion cells is thought to cause the center-surround receptive field structure of retinal ganglion cells (Hartline 1949). In analogy, neural circuitry in the antennal lobe or olfactory bulb has been suggested to consist of interglomerular networks of excitatory and inhibitory neurons. These networks are thought to provide a basis for center-surround inhibition or lateral inhibition as a fundamental mechanism of sensory processing (Laurent 1997; Urban 2002; Aungst et al. 2003; Schoppa and Urban 2003; Girardin et al. 2013; Whitesell et al. 2013). Evidence has been accumulating to demonstrate refinement of glomerular output by interglomerular interactions mediated by GABAergic, inhibitory local interneurons (LNs) (Yokoi et al. 1995; Kashiwadani et al. 1999; Aungst et al. 2003; Friedrich and Laurent 2004; Nagayama et al. 2004; Wilson et al. 2004; Schoppa 2006; Vucinic et al. 2006; Silbering and Galizia 2007). While lateral inhibitory effects have been observed as a signaling mechanism in the olfactory bulb, the details of this mechanism are still elusive. It remains to be determined how olfactory bulb circuits operate to transform sensory input to olfactory bulb output.
A model olfactory system as found in the moth Manduca sexta (hereinafter referred to as Manduca) with morphologically and functionally identified glomeruli in the antennal lobes and behaviorally identified relevant odorants can directly test the hypothesis that functional interactions occur between olfactory glomeruli using natural and behaviorally relevant odorants. Here, we review the functional organization of the olfactory system of male and female Manduca to discuss glomerular interactions in the antennal lobes.
Manduca sexta as a model system for olfactory neurobiology
The neural organization of the olfactory systems of insects is surprisingly similar to those of mammals and other vertebrates (Hildebrand and Shepherd 1997). From a functional and evolutionary point of view, this striking similarity proposes the existence of specific, optimized solutions to organize olfactory systems. Based on this similarity, many investigators have decided to study the insect olfactory system as a valuable model to reveal general mechanisms of olfaction, odor-induced behavior, synaptic processing and coding of odorant information, neural plasticity, and learning and memory (Hildebrand et al. 1999; Menzel 2001).
A major advantage of insects as models for olfactory research and neuroscience in general is their relatively simpler peripheral and central nervous system compared with their vertebrate counterparts (Haupt et al. 2010). Their moderately complex nervous system gives rise to a rich behavioral repertoire, while the cost of maintaining them as experimental animals is low. Neuron numbers of insects (105–106 neurons) are higher than those of another invertebrate model system, Aplysia (2 × 104) but lower than Octopus (>108), small mammals (mouse: ca. 5 × 107), and humans (1011). Our understanding of insect brains has benefitted from individually identifiable neurons and small networks as functional units (Haupt et al. 2010).
Insects bear easily accessible sensory organs (sensilla) and individually identifiable sensory structures. The number of receptor cells mediating olfaction is relatively small (Shields and Heinbockel 2012). Likewise, the relatively small number and identifiability of olfactory glomeruli in the antennal lobes and their accessability for neurophysiological recording is of benefit. Another critical advantage lies in the known chemistry of odorant stimuli and the adaptive relevance of specific odorants for behavior. Manduca, in particular is an experimentally favorable insect species amenable to anatomical, neurophysiological, behavioral, biochemical, cytochemical, developmental, and cell-culture methods. As in many other insect species, olfaction is critical for Manduca in determining a variety of behaviors such as orientation and movement, mate finding, finding of oviposition sites, and localization of food sources (Hildebrand 1995, 1996). Female moths release sex pheromones that attract conspecific males over long distances (Kaissling 1987; Hildebrand 1996). An outstanding challenge in olfactory neurobiology is to understand how a male moth is able to locate a mate, namely, a female releasing sex pheromone. The olfactory brain of a male moth must integrate information about qualitative (chemical composition), quantitative (relative levels of components), and spatiotemporal features of an attractive blend of volatile compounds, the sex pheromone, released by a female of the same species (Hildebrand 1995, 1996; Christensen et al. 1996).
The presence of physical obstacles in the environment, movement of the animal, wind turbulence, and sometimes the pulsatile release of the sex pheromone (e.g., in the Arctiid moth Utetheisa ornatrix; Connor et al. 1980) contribute to the turbulent nature of the pheromonal signal which is virtually never a continuous stream of odorants (Murlis et al.1992; Murlis 1997). Rather, pheromone signals occur as filaments or blobs of odorant of varying concentration which, on average, diminish in concentration with increasing distance from the odor source (Murlis et al. 1992). The intermittent nature of the signal (i.e., the time during which it is absent) increases downwind from the odor source, whereas closer to the source, both the spatiotemporal frequency of odorant pulses and the concentration of pheromone within the pulse are elevated (Murlis et al. 1992). Behavioral experiments using female sex pheromones to attract male moths have demonstrated that continuous odorant plumes are inferior to intermittent pheromone stimuli in eliciting odor-modulated upwind movement of male moths toward the source (Baker et al. 1985; Kramer 1986, 1992; Kaissling 1987; Baker 1989; Kaissling and Kramer 1990). The behavioral response to changes of pheromone concentration occurs within about 100 ms (Kaissling and Kramer 1990; Mafra-Neto and Cardé 1994; Vickers and Baker 1994), and neural correlates to this rapid response have been found in Manduca and other moth species at the level of antennal olfactory receptor cells (Rumbo and Kaissling 1989; Almaas et al. 1991; Marion-Poll and Tobin 1992) and in the antennal lobes (Christensen and Hildebrand 1988, 1997; Christensen et al. 1989, 1998; Vickers et al. 1998; Heinbockel et al. 1999). Some olfactory receptor cells and central olfactory interneurons can follow stimulus pulses up to about 12 Hz (Christensen and Hildebrand 1988, 1997; Christensen et al. 1989, 1996; Rumbo and Kaissling 1989; Almaas et al. 1991), but most of these neurons follow pulse rates in the range of 3–5 Hz.
The initial central processing of information about pheromone stimuli takes place in a sexually dimorphic cluster of olfactory glomeruli, the macroglomerular complex (MGC) (Fig. 1), a male-specific olfactory subsystem in the antennal lobe (Matsumoto and Hildebrand 1981). Manduca provides an excellent model to study the early processing of olfactory information in glomerular microcircuits of the primary olfactory center, the antennal lobes, because the olfactory centers are dramatically sexually dimorphic. Input and output relationships can be precisely defined in such a neural system. The MGC receives input from antennal olfactory receptor cells (Christensen et al. 1995) that are specifically tuned to one of the two essential components of the female sex pheromone (Kaissling et al. 1989). The MGC is uniquely responsible for the primary processing of sex-pheromonal information, and it thus can serve as a model for studies of the functional architecture of glomeruli as well as the physiological relationships between glomeruli in the olfactory system (Hansson and Christensen 1999; Christensen and White 2000; Christensen and Hildebrand 2002; Heinbockel et al. 2004; Reisenman et al. 2008; Lei et al. 2010; Martin et al. 2011).
Fig. 1.
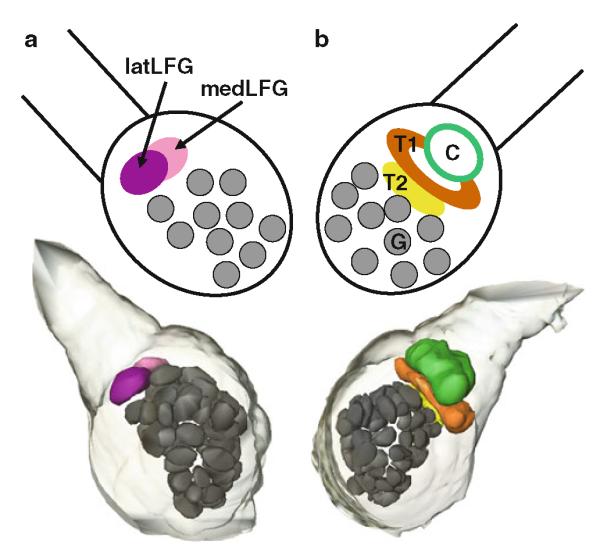
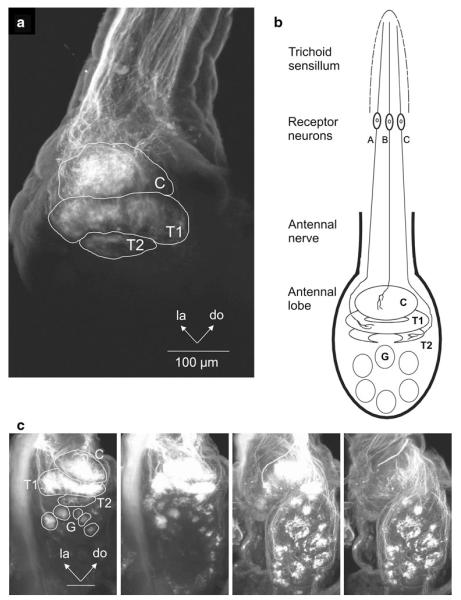
Three-dimensional reconstruction and diagrammatic representation of sexually isomorphic glomeruli (G) and sexually dimorphic glomeruli in a female and b male Manduca. latLFG lateral large female glomerulus, medLFG medial large female glomerulus, C cumulus, T1 toroid-1, T2 toroid-2, G glomerulus
Organization of the olfactory system in male M. sexta
Different species of moths can use the same chemical compounds in their sex-pheromone blend. However, the attractant signal produced by each species is unique because each blend has characteristic proportions of the components (Arn et al. 1992; Kaissling 1996). In Manduca, the sex-pheromone blend comprises eight components. Behavioral experiments revealed that two of the eight components are required to evoke odor-modulated flight in males (Tumlinson et al. 1989).
The antennae of male Manduca are covered by different types of sensilla (Fig. 8) (Keil 1989; Lee and Strausfeld 1990; Shields and Hildebrand 1999a, b). The long trichoid sensilla on the male’s antennae house highly selective and sensitive olfactory receptor cells that detect one or the other of the two key pheromone components (Kaissling et al. 1989) evoking upwind flight in males (Tumlinson et al. 1989). Each of these olfactory receptor cell populations sends their axons to a different glomerulus of the MGC in the ipsilateral antennal lobe (Christensen et al. 1995). In Manduca, an array of ca. 60 sexually isomorphic glomeruli is found in each antennal lobe. In addition, sexually dimorphic structures are found in both sexes (Fig. 1). In male Manduca, the MGC exists close to the entrance of the antennal nerve into the antennal lobe and comprises three glomeruli: the cumulus, the toroid-1, and the horseshoe-shaped toroid-2 (Heinbockel et al. 1995; Homberg et al. 1995; Rössler et al. 1998). All three glomeruli of the MGC are targeted by axonal projections of olfactory receptor cells housed in the long trichoid sensilla in the antenna as revealed by specific anterograde labeling using cobalt lysine (Christensen et al. 1995) or rhodamine dextrane staining techniques (Fig. 2). In contrast, unspecific labeling of several antennal sensillum types shows stained afferents projecting to many glomeruli in the antennal lobe.
Fig. 8.
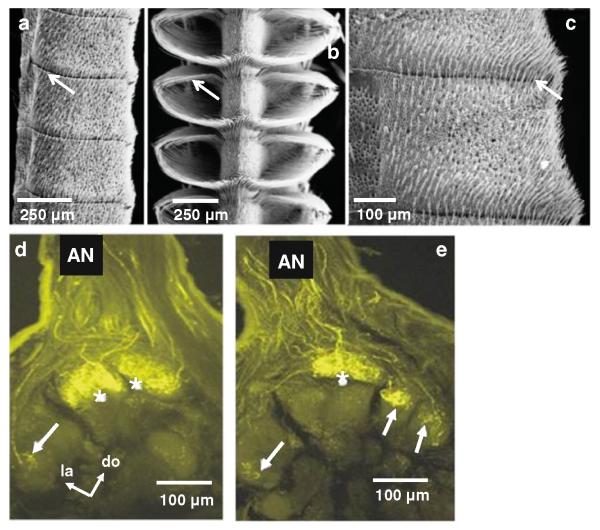
Female and male antenna of Manduca and projections of olfactory receptor cells to the female antennal lobe. a Scanning electron micrograph of a portion of an adult female antennal flagellum showing several annuli. b Portion of an adult male antennal flagellum showing several annuli. c Higher magnification view of a single annulus from a female antenna. Long, hair-like sensory organs (trichoid sensilla) are abundant on the surface of an annulus. d, e Laser scanning confocal microscope images showing serial optical sections of specimens embedded in plastic and sectioned to improve resolution, taken at different depths through the female antennal lobe. Images show central projections of olfactory receptor cell axons stained with the fluorescent dye dextran-tetramethylrhodamine. Asterisks indicate the two sexually dimorphic large female glomeruli located in the dorsolateral region of the antennal lobe, near the site of entry of the antennal nerve (AN). The arrows indicate sexually isomorphic glomeruli. do dorsal, la lateral
Fig. 2.
Anterograde labeling of antennal sensory neurons with rhodamine dextran reveals axonal projections to glomeruli in the antennal lobe of Manduca. a Labeling of receptor neurons in long antennal trichoid sensilla shows projections to the three glomeruli of the macroglomerular complex (MGC: C cumulus, T1 toroid-1, T2 toroid-2). b Schematic diagram of receptor neuron projections to the ipsilateral antennal lobe. Projections are shown to all three glomeruli of the macroglomerular complex. Receptor neurons from a given trichoid sensillum project to only one or a combination of two glomeruli in the macroglomerular complex. c Labeling of long trichoid as well as other antennal sensilla shows projections to the MGC and ordinary glomeruli in the AL. Frontal view. Optical sections at different depths from anterior to posterior through the AL from left to right. C cumulus, do dorsal, la lateral, T1 toroid-1, T2 toroid-2. Scale bar 100 μm
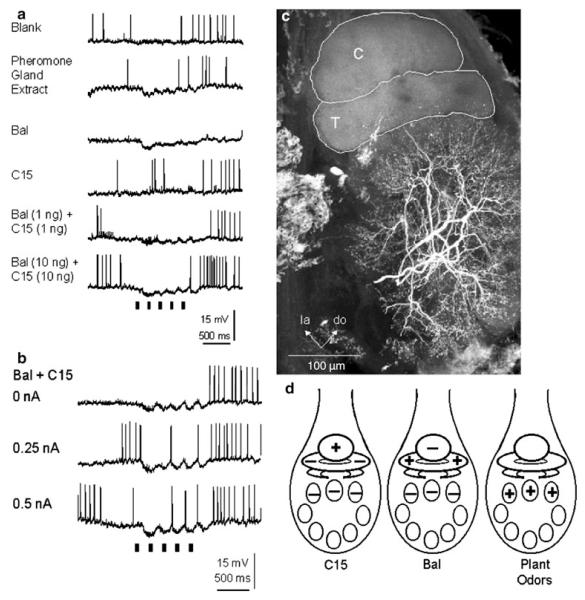
Projection neurons (PNs) that arborize in one of the glomeruli of the MGC (MGC-PNs), namely the toroid-1 (Fig. 3) (Strausfeld 1989; Homberg et al. 1995), respond to antennal stimulation with E,Z-10,12-hexadecadienal (bombykal or BAL, one of the two key components of the female’s sex pheromone) (Fig. 4). MGC-PNs that arborize in a neighboring glomerulus (the cumulus) respond selectively to E,E,Z-10,12,14-hexadecatrienal (the second key component) or its more stable mimic, E,Z-11,13-pentadecadienal (C15; Kaissling et al. 1989; Hansson et al. 1991). An MGC-PN with arborizations in both glomeruli (Fig. 3d) is activated by both bombykal and C15 (Fig. 4c) (Hansson et al. 1991; Heinbockel et al. 2004). Thus, the MGC is innervated by MGC-PNs that reach into one or more of the three glomeruli of the MGC and send axonal projections to higher brain centers. Local interneurons also send dendrites into the MGC and connect many or possibly all glomeruli in the antennal lobe (Figs. 6, 7a). Likewise, ordinary glomeruli are innervated by local interneurons (Fig. 7b), and also by uniglomerular PNs (uPNs) (Fig. 9a) (Reisenman et al. 2005, 2008) and other neuronal classes (Homberg et al. 1989).
Fig. 3.
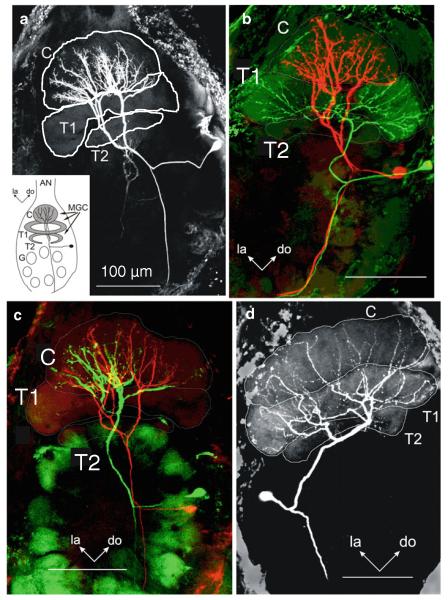
Laser-scanning confocal micrographs of antennal lobe projection neurons in the moth antennal lobe of Manduca. a Image of a C15-specialist MGC-PN with arborizations confined to the cumulus. The inset illustrates the organization of the antennal lobe with the macroglomerular complex (MGC) and other glomeruli (G). b Two specialist MGC-PNs, one neuron, stained with Lucifer Yellow (colored red here) had arborizations confined to the cumulus (C), and the other neuron, stained with biocytin (colored green here) had arborizations confined to the toroid-1 (T1). Areas of apparent overlap between the two neurons are shown in yellow and are possible sites of synaptic contact. c Morphological diversity in cumulus neurons. Image of two C15-specialist MGC-PNs with arborizations confined to the cumulus. While the branches of the two neurons apparently overlapped in certain parts of the cumulus (indicated in yellow), other parts were innervated by just one of the two neurons. The green neuron was stained with Lucifer Yellow and the red neuron with biocytin. C cumulus, T1 toroid-1, T2 toroid-2, do dorsal, la lateral. Scale bar 100 μm. d MGC-PN with arborizations in both cumulus and toroid-1. Modified from Heinbockel and Hildebrand (1998) (a); Heinbockel et al. (1999) (b, c); Heinbockel et al. (2004) (d)
Fig. 4.
Physiology of MGC-PNs in response to antennal stimulation with pheromone components bombykal and/or C15. a This MGC-PN had dendritic arborizations confined to toroid-1 and responded with excitation to bombykal (BAL). b The MGC-PN arborized in the cumulus and was activated by C15 and not bombykal. c The MGC-PN branched in both toroid-1 and cumulus and was activated by either bombykal or C15
Fig. 6.
Intracellular recordings showing the responses of a local interneuron (LN) to antennal stimulation with pheromone. a The LN is inhibited by antennal stimulation with either bombykal (BAL), C15, or a blend of both pheromone components. Likewise, an extract of the pheromone gland of a female conspecific inhibits this LN. This neuron is able to track an intermittent odorant stimulus at a frequency of at least 5 Hz. Note the distinct periods of inactivity (arrows) evoked by consecutive odorant pulses. b In this neuron, stimulation with the pheromone blend at depolarized membrane potentials evokes a brief inhibitory response corresponding to each stimulus pulse. c Morphology of the local interneuron shown in (a, c) (frontal view). The LN has branches throughout the entire antennal lobe, except the MGC. C cumulus, do dorsal, la lateral, T1 toroid-1. Scale bar 100 μm. d Summary diagram of pheromone and odorant responses in the AL of male Manduca. Stimulation with C15 evokes a primarily excitatory response in neurons innervating the cumulus and an inhibition in neurons ramifying in the toroid-1 and other AL glomeruli. In contrast, stimulation with bombykal activates neurons in the toroid-1 and inhibits all other neurons in the AL, namely those in the cumulus and ordinary glomeruli. Projection neurons that branch in the MGC do not respond to plant odorants, whereas neurons (uPNs and LNs) that innervate ordinary glomeruli respond with excitation to plant odorants
Fig. 7.
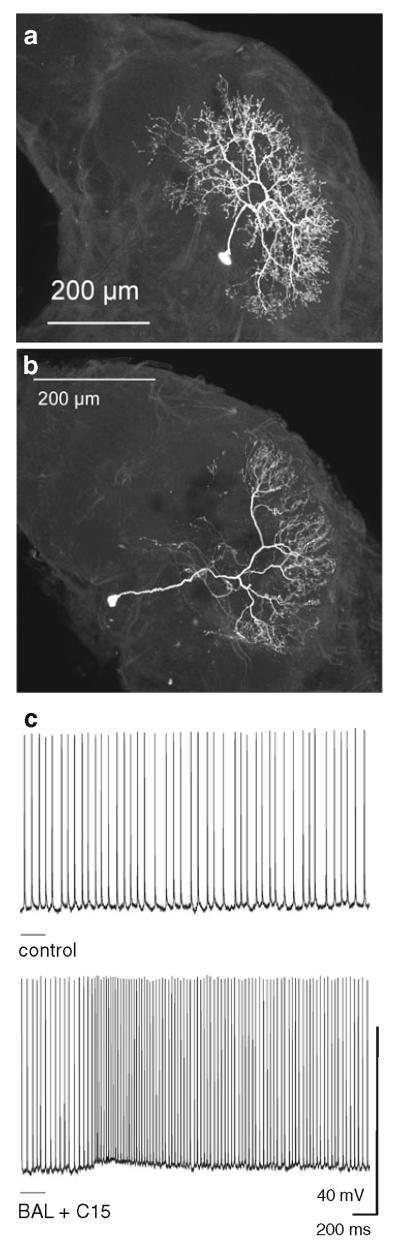
Morphology and physiology of local interneurons in the Manduca antennal lobes. a Morphology of an LN with arborizations throughout the antennal lobe including the MGC. b Morphology of an LN with arborizations restricted to isomorphic glomeruli. c Excitatory response of an LN to stimulation with the pheromone blend (bombykal, BAL plus C15)
Fig. 9.
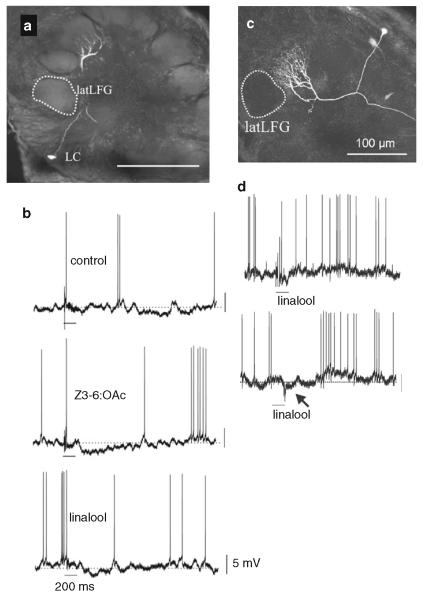
Odorant responses of projection neurons in the antennal lobe of female Manduca. a Morphology of a projection neuron with arborizations restricted to a glomerulus near the lateral Large Female Glomerulus, latLFG (dotted area) and G35 (not visible in this section; image obtained after sectioning), i.e., this PN is arborizing in a sexually isomorphic glomeruli of the female antennal lobe near latLFG and G35. The cell body is located in the lateral group of neuronal cell bodies (LC). Scale bar 200 μm. b Electrophysiological responses of this PN to stimulation with the mineral oil control, Z3-6:OAc, and [±]linalool. The odorants linalool and Z3-6:OAc strongly stimulate PNs in the latLFG and G35, respectively, and to a less extent, PNs in other glomeruli (Reisenman et al. 2004, 2005; Roche King et al. 2000). Note that both Z3-6:OAc and [±]linalool elicited hyperpolarization in this PN (deflections below the resting potential, dotted area). c Morphology of a projection neuron arborizing in the medial Large Female Glomerulus, medLFG. d Physiological responses obtained from the PN in c to a control stimulus and stimulation (duration 200 ms) with (±)-linalool, the stimulus that preferentially activates latLFG-PNs. Note that stimulation with linalool evokes an inhibitory response (membrane potential hyperpolarization) suggesting that the two large female glomeruli interact with each other similarly to the two main glomeruli of the MGC. Calibration bars 5 mV, 200 ms in all panels (b, d). Modified from Reisenman et al. (2008)
The antennal lobes of Manduca consist of two olfactory subsystems. One subsystem consists of a sexually dimorphic pathway, represented by the cumulus and toroid-1, specialized to detect and process information about the sex pheromone. The other one is a more complex pathway that processes information about plant and potentially other odorants (Hildebrand 1996). Based on the new studies discussed below, gradually a picture emerges illustrating how these two subsystems interact at the level of the antennal lobe and how their olfactory processing function is regulated by olfactory input to various antennal lobe neurons to optimize the signal-to-noise ratio and to facilitate odor discrimination.
Reciprocal inhibition of pheromone-processing glomeruli
Experimental work in Manduca has examined how activation of specific antennal lobe glomeruli affects the responses of identified antennal lobe neurons innervating neighboring glomeruli (Heinbockel et al. 1999, 2004; Heinbockel and Hildebrand 2000; Reisenman et al. 2008). Is the activity of individual olfactory neurons innervating glomeruli that process non-pheromonal stimuli (“ordinary” glomeruli in the antennal lobe) modulated by stimulation of the antenna with pheromone components of conspecific females? Using intracellular recording and staining methods (Heinbockel et al. 1999; Shields and Heinbockel 2012), this question has been addressed in several distinct classes of neurons in the antennal lobe of male Manduca.
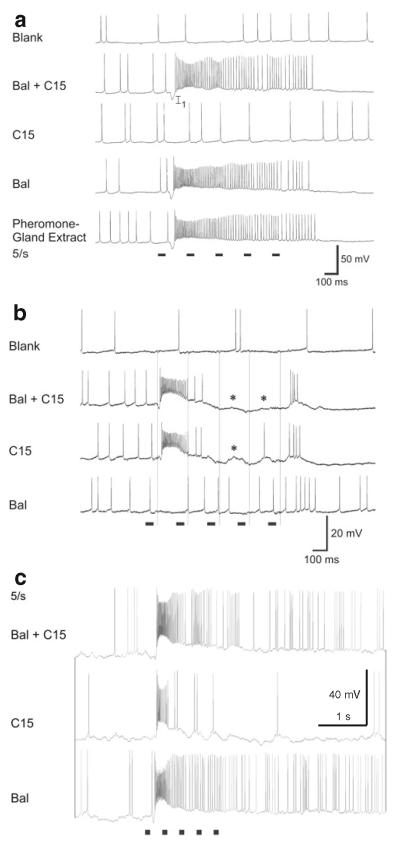
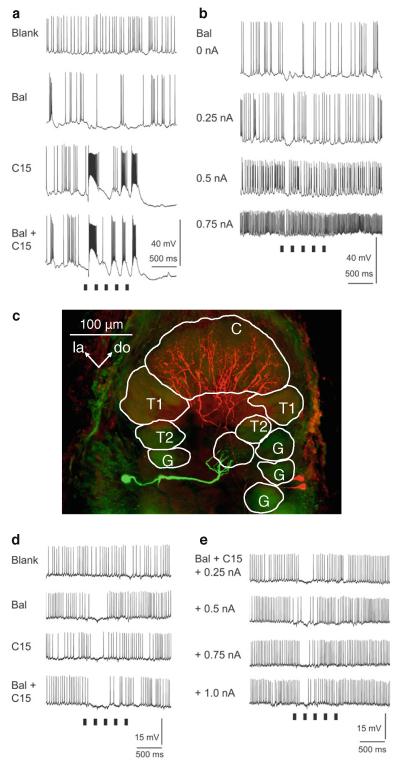
Pheromonal stimulation of the antenna with a pheromone blend comprising bombykal and C15 evokes a primarily excitatory response in MGC-PNs (Fig. 4). Stimulation with just one of the two key pheromone components, bombykal, evokes a primarily excitatory response in MGC-PNs innervating the toroid-1 (Hansson et al. 1991) and evokes no response or an inhibition in neurons innervating the neighboring cumulus (Fig. 4) (Heinbockel et al. 1999, 2004). Conversely, stimulation with C15 excites MGC-PNs in the cumulus (Hansson et al. 1991) and can inhibit MGC-PNs in the adjacent toroid-1 (Heinbockel et al. 2004) suggesting lateral inhibition within the MGC (Heinbockel et al. 1999; Lei et al. 2002). MGC-PNs with arborizations in both glomeruli, cumulus and toroid-1 (Fig. 3d) are excited by both bombykal and C15 (Fig. 4c) (Hansson et al. 1991; Heinbockel et al.2004). The lateral inhibitory effect is illustrated in Figs. 4 and 5 which show intracellular recordings of MGC-PNs during antennal stimulation with pheromone. The neuron in Fig. 5 is responsive to both C15 and bombykal. The neuron displays a purely inhibitory response to bombykal and primarily an excitation to C15. Stimulation with a blend of the two key pheromone components (C15 plus bombykal) gives a new type of response illustrating a blend effect and results in better pulse tracking of an intermittent pheromone stimulus. The inhibitory effect of bombykal persists even at high activity levels of this neuron (Fig. 5b).
Fig. 5.
Intracellular recordings of antennal lobe projection neurons during antennal stimulation with pheromone. a This neuron is initially inhibited by a stimulus that contains C15, followed by strong depolarization and again inhibition (third and fourth trace). The neuron shows an inhibitory response to bombykal (BAL) and a blend effect to the pheromone blend of C15 + BAL. In each trace, the ipsilateral antenna receives five 50-ms stimulus pulses at 5 Hz (stimulus markers are shown beneath the records). b When the neuron is depolarized by injecting current through the recording electrode, the overall firing frequency increases and the first stimulus pulse of BAL evokes a distinct reduction in firing (inhibition). c Laser scanning confocal micrograph showing intracellular labeling of two PNs in the AL. Anatomy of the PN in (colored red here, stained with biocytin) described in a, b and a uniglomerular projection neuron described in d, e (colored green here, stained with Lucifer Yellow) (frontal view). The red neuron branches in the cumulus and not in the toroid-1 or any other glomerulus. The green neuron innvervates one ordinary glomerulus. C cumulus, do dorsal, G ordinary glomerulus, la lateral, me medial, T1 toroid-1, T2 toroid-2. Scale bar 100 μm. d Intracellular recordings from a uniglomerular projection neuron. Stimulation with bomybkal, C15, or a blend of both pheromone components reduces firing. In each trace, five identical stimulus pulses are delivered to the ipsilateral antenna at a frequency of 5 Hz (stimulus markers for the 50-ms pulses are shown beneath the records). e In the same neuron, the inhibition is also observed in response to stimulation with the pheromone blend when injecting depolarizing current into the neuron to increase the overall firing of the neuron. Only the first pheromone stimulus evokes an inhibition
Antennal stimulation with one pheromone component evokes excitation in one glomerulus of the MGC and inhibition in the other (Heinbockel et al. 1999; Lei et al. 2002). Moreover, stimulation with an individual pheromone component can evoke a mixed inhibitory–excitatory response in an MGC-PN (Heinbockel et al. 2004). The presence of both inhibitory and excitatory response phases indicates that one pheromone component triggers activity in two different populations of olfactory receptor cells, one that mediates excitatory input and another that mediates inhibitory input to an MGC-PN (Figs. 4, 5) (Heinbockel et al. 1999).
Pheromone responses of uniglomerular projection neurons
Characterization of the chemosensory tuning of projection neurons (uniglomerular PNs, uPNs) innervating an identifiable, sexually isomorphic glomerulus, G35, in the antennal lobe revealed that G35-PNs respond to low concentrations of the plant-derived volatile compound Z-3-hexenyl acetate (Z3-6:OAc) (Reisenman et al. 2005). The propionate and butyrate homologs of this compound evoke excitatory responses but only at moderate-to-high concentrations. Other plant volatiles do not evoke responses from G35-PNs. The glomerulus G35 is located adjacent to the MGC. G35-PNs are inhibited by input to Toroid-1 PNs, i.e., antennal stimulation with bombykal, whereas Toroid-1 PNs are not inhibited by input to G35 (Reisenman et al. 2008). In contrast to MGC-PNs, in projection neurons that innervate ordinary glomeruli stimulation with pheromone evokes an inhibition and no excitation, i.e., the MGC and ordinary glomeruli exhibit non-reciprocal inhibition. GABAergic local interneurons are the likely neural substrate that mediate these inhibitory interactions such that they are activated by MGC-PNs and, subsequently, inhibit their synaptic targets such as uniglomerular projection neurons.
Are inhibitory interactions (reduction of action potential firing, hyperpolarization) among the macroglomerular complex and sexually isomorphic glomeruli or between sexually isomorphic glomeruli a general phenomenon in the Manduca antennal lobe? Pheromonal stimulation inhibits uniglomerular projection neurons in sexually isomorphic glomeruli regardless of their distance to the macroglomerular complex (Reisenman et al. 2008). Likewise, stimulation with Z3-6:OAc leads to suppression of spikes and hyperpolarization in many uniglomerular projection neurons with arborizations in glomeruli distant from the macroglomerular complex and the glomerulus G35. Therefore, inhibitory interglomerular interactions are not determined by proximity of the glomeruli involved in these interactions (Reisenman et al. 2008), as has been observed in other systems (Linster et al. 2005; Girardin et al. 2013).
An example of inhibition of a uniglomerular projection neurons by pheromone stimulation is shown in Fig. 5d, e. Stimulation with bombykal, C15 or a blend of both pheromone components evokes a pronounced reduction in firing of the uniglomerular projection neurons. Not only do the two projection neurons in Fig. 5 have different morphologies, i.e., each innervates a different glomerulus in the antennal lobe (MGC-PN in cumulus, uPN in ordinary glomerulus), but also they have distinct functional differences, one being primarily excited by pheromone stimulation and one being inhibited by any pheromonal stimulus.
Pheromone responses of local interneurons
Local interneurons project to many, in some cases all glomeruli in the antennal lobe (Figs. 6, 7) and, therefore, serve an important role in interglomerular connectivity (Hoskins et al. 1986; Christensen et al. 1993). In Manduca, many local interneurons are GABA immunoreactive (Fig. 7a). However, a large subset of local interneurons is not GABA immunoreactive and perhaps excitatory (Fig. 7b) (Reisenman et al. 2011). In Manduca, local interneurons respond either with excitation (Fig. 7c) or inhibition to pheromone stimulation (Fig. 6a) (Matsumoto and Hildebrand 1981). Most local interneurons show inhibitory responses (Reisenman et al. 2011). The sparse innervation of the MGC by some local interneurons is in sharp contrast to their strong inhibitory responses to pheromone stimulation of the antenna (Fig. 6a, c). Local interneurons may not receive direct input from olfactory receptor cells in Manduca, such that MGC-PNs leaving the MGC are likely to form inhibitory synaptic contacts onto local interneurons.
In Manduca, many local interneurons have primary neurites that arborize in only a few glomeruli (Matsumoto and Hildebrand 1981; Reisenman et al. 2011). These local interneurons can serve as neural connections among the glomeruli that process the individual components of an innately significant odor blend (Reisenman et al. 2008, 2011). Since each glomerulus is innervated by different but overlapping sets of local interneurons, these neurons can facilitate a combinatorial coding scheme for odor blends. Other local interneurons in Manduca and other insect species arborize in all of the glomeruli (Matsumoto and Hildebrand 1981; Abel et al. 2001; Wilson and Laurent 2005; Chou et al. 2010; Reisenman et al. 2011). Functionally, these wide-field local interneurons and the more restricted local interneurons could serve in two separate inhibitory networks, a global one and a local, glomerulus-specific one as proposed for Drosophila melanogaster (Silbering and Galizia 2007). In addition to inhibitory local interneurons, in Drosophila a population of excitatory local interneurons has been identified that mediates excitatory interactions between olfactory processing channels through electrical coupling and is thought to enhance projection neuron output through lateral excitation (Olsen et al. 2008; Shang et al. 2007; Yaksi and Wilson 2010). While both inhibitory and excitatory local interneurons are present in Drosophila, interglomerular inhibition dominates over interglomerular excitation (Root et al. 2007; Olsen and Wilson 2008). Excitatory local interneuron input to projection neurons can increase the gain when the input is weak. In contrast, inhibitory local interneurons can suppress responses when stimulus intensity is high.
Organization of the olfactory system in female M. sexta
The main sensillum type present in female antennae, the trichoid type-A sensilla, are shorter than the long trichoid sensilla in males and are found in greatest abundance in a narrow band along the distal and proximal margins of each annulus (Fig. 8). These bands merge toward the trailing surface and form a U-shaped cul-de-sac (Shields and Hildebrand 1999b). This pattern is similar, but less conspicuous, than the pattern found in the male-specific trichoid sensilla (Lee and Strausfeld 1990; Sanes and Hildebrand 1976). In females, the type-A trichoid sensilla are also found on the leading, dorsal, and ventral surfaces of an annulus (Shields and Hildebrand 1999b). Approximately 3.0–3.4 × 105 olfactory receptor cells (Oland and Tolbert 1988) are associated with about 105 sensilla (Sanes and Hildebrand 1976; Keil 1989; Lee and Strausfeld 1990; Shields and Hildebrand 1999a, b). A single antennal annulus bears about 1,100 of trichoid type-A sensilla. Each sensillum is innervated by two olfactory receptor cells.
Based on the responses to stimulation of olfactory receptor cells with a panel of 102 individual odorants and three plant-derived odor mixtures, three different functional types of type-A trichoid sensilla are discerned (Shields and Hildebrand 2001a, b). One subset of olfactory receptor cells exhibits an apparently narrow molecular receptive range to any of the odorants tested, responding strongly to only one to two terpenoid odorants, specifically certain oxygenated mono- and sesquiterpenoids such as linalool, geraniol, and trans-nerolidol. The second subset of olfactory receptor cells is activated exclusively by aromatics and responded strongly to two to seven odorants, such as cis-3-hexenylbenzoate, 2-methylpropylbenzoate, ethyl-2-aminobenzoate, ethylsalicylate, and isoamylsalicylate. The third subset of olfactory receptor cells has a broad molecular receptive range and responds strongly to odorants belonging to several chemical classes, including terpenoids, aromatics, aliphatics, green-leaf volatiles, a furan, and N-bearing volatiles. Other receptor cells do not respond to any odorants but are spontaneously active. Some olfactory receptor cells are strongly excited by certain odorants and inhibited by others.
Olfactory receptor cells in trichoid type-A sensilla project their axons mainly to two large, distinct, and sexually dimorphic glomeruli, the medial and lateral large female glomerulus (medLFG, latLFG) (Rössler et al. 1998, 1999; Rospars and Hildebrand 2000). Other olfactory receptor cell afferents from these sensilla project to some of the 60 sexually isomorphic glomeruli in the antennal lobe (Shields and Hildebrand 2001a). The large female glomeruli reside outside the array of spheroidal ordinary glomeruli, near the entrance of the antennal nerve in a position corresponding to that of the MGC in males (Fig. 1). The large female glomeruli may play a female-specific role, such as processing of information about one or more odorants important for orientation of a female to host plants for oviposition (Kalberer et al. 2010, Reisenman et al. 2009, 2010). Recordings from projection neurons with dendritic arborizations in the lateral large female glomerulus reveal excitation in response to low concentrations of the monoterpenoid linalool (Roche King et al. 2000). Neither other monoterpenoids nor representatives of other classes of host plant volatiles of Manduca are similarly stimulatory to projection neurons in the lateral large female glomerulus, suggesting that this glomerulus has a characteristic, limited molecular receptive range. Furthermore, projection neurons in the lateral large female glomerulus show selective responses to the (+) enantiomer of linalool, and the alcohol group of this odorant molecule is the molecular feature that evokes responses from these projection neurons (Reisenman et al. 2004). Although the evidence is limited, it appears that inhibitory interactions, analogous to those described between the male pheromone-sensitive glomeruli, also operate between the medial and lateral large female glomeruli. Figure 9d shows an example of a projection neuron in the medial large female glomerulus that is hyperpolarized by antennal stimulation with low concentrations of (±)-linalool, the odorant that specifically activates projection neurons in the neighboring lateral large female glomerulus.
Responses of projection neurons in sexually isomorphic glomeruli of female antennal lobes
Lateral inhibitory interactions are also observed in recordings of female projection neurons with arborizations in glomeruli near the sexually dimorphic lateral large female glomeruli and the sexually isomorphic glomerulus G35 when the antenna is stimulated with the odorants that preferentially activate those glomeruli (Fig. 9). Such a projection neuron can be hyperpolarized by both linalool, which stimulates projection neurons in the lateral large female glomerulus (Roche King et al. 2000) and Z3-6:Oac, which stimulates projection neurons in glomerulus G35 (Reisenman et al. 2004) (Fig. 9b). The findings suggest that at least a subset of projection neurons in sexually isomorphic glomeruli receives inhibitory input from the lateral large female glomerulus and glomerulus G35 or from glomeruli activated by linalool and Z3-6:OAc, respectively (Reisenman et al. 2008). The inhibitory effect of linalool is stronger than that of Z3-6:OAc both in terms of numbers of inhibited projection neurons and amplitude of membrane hyperpolarization. As in male Manduca, the sexually dimorphic glomeruli (MGC, large female glomeruli) appear to have a more prominent inhibitory effect on sexually isomorphic antennal lobe glomeruli than the activation of ordinary glomeruli has on its neighboring glomeruli.
These inhibitory interactions could result in non-linear processing of antennal lobe input such that synaptic output carried by projection neurons cannot be predicted by the sum of antennal lobe input from olfactory receptor cells. This hypothesis has been tested experimentally in Manduca by applying optical calcium imaging techniques to directly compare the olfactory responses in individual female moths to an artificial host odor mixture and its single components simultaneously at two different processing levels, namely olfactory receptor cell input and projection neuron output (Kuebler et al. 2012). Network modulation in the antennal lobe generates a unique “mixture feature” separate from the simple sum of individual compound identities and can have implications for olfactory behavior in Manduca as assessed by flight tunnel analyses. Such network modulation can be explained by the observation that odor mixtures are represented by each antennal lobe neuron as a unique combinatorial representation with no apparent general rule by which the network computes the mixture in comparison to single components (Kuebler et al. 2011). Single neurons differ in their responses in a variety of factors such as spatial location, frequency, latency, and temporal pattern of the response kinetics. All of these contribute to a spatiotemporal representation of host odor mixtures among single neurons in the antennal lobe as a highly combinatorial, non-linear process characterized by suppression, hypoadditivity, and synergism (Kuebler et al. 2011).
Functional implications of reciprocal and non-reciprocal inhibition
What are the functional consequences of reciprocal and non-reciprocal inhibitory glomerular interactions (Fig. 6d)? MGC-PNs receive excitatory input driven by bombykal and inhibitory input driven by C15 (or vice versa; Christensen and Hildebrand 1987; Hansson et al. 1991; Heinbockel et al. 1999; Lei et al. 2002). This convergent input from both excitatory and inhibitory pathways enhances the ability of such MGC-PNs to resolve multiple pulses of pheromone and improves the ability to correctly reflect the intermittent character of odorant stimuli in a natural environment (Christensen and Hildebrand 1997; Christensen et al. 1998; Heinbockel et al. 1999). Inhibitory interactions improve the temporal resolution of odorant pulses by contrast enhancement at the cellular and behavioral level (Lei et al. 2009). Intermittent pheromone stimulation of the antenna activates MGC-PNs which generate discrete bursts of action potentials separated by periods of inhibition. The binary burst/non-burst neural patterns are thought to better resolve the intermittency of the stimulus encountered in the odor plume. Blocking the GABA-mediated inhibitory periods disrupts the temporal pattern of MGC-PN responses, namely the spiking bursts entrained to odor pulses but not the magnitude of the responses, i.e., frequency of spiking. Furthermore, blockade of GABA receptors prevents animals to efficiently locate the odorant source, even though they can detect the odorant signal. These findings link the inhibitory interactions at the cellular level to the pulse following ability of MGC-PNs and to pheromone-modulated orientation behavior of male moths (Lei et al. 2009).
Another function of inhibitory interactions is to synchronize the outputs of the MGC. When the cumulus and toroid-1 receive their inputs simultaneously, the temporal tuning of output from each glomerulus is enhanced by reciprocal and inhibitory interglomerular interactions (Lei et al. 2002). Such synchronous firing across glomeruli may help to temporally bind multiple and spatially distributed input streams activated by a given odorant.
Even though interglomerular inhibition extends beyond a cluster of functionally related glomeruli (MGC), the inhibitory interactions between glomeruli of the MGC are larger than those seen between the MGC and sexually isomorphic glomeruli (Reisenman et al. 2008). This raises the possibility that sex-pheromonal inhibition has dual roles: to promote synchronous firing of projection neurons within the MGC (Lei et al. 2002) and to promote overall inhibition in the antennal lobe akin to contrast enhancement (Reisenman et al. 2008).
How can we interpret the inhibitory effects seen in uniglomerular projection neurons in response to pheromone stimulation in the Manduca antennal lobe? The data imply that the pheromone subsystem overrides the nonpheromone subsystem (Heinbockel and Hildebrand 2000; Reisenman et al. 2008). Processing of olfactory input to antennal lobe glomeruli appears to follow a hierarchy such that pheromonal input dominates, i.e., processing of odorants involved in reproductive behaviors (pheromones) takes priority over processing of food-related odorants (plant volatiles). Activation of the sexually dimorphic glomeruli (MGC) “shuts down” responses in glomeruli involved in detection and discrimination of plant odors (Reisenman et al. 2008). In the presence of a conspecific female, plant odors may become behaviorally less significant to a male moth. This intriguing idea still awaits testing both physiologically and behaviorally, e.g., by concurrent stimulation with sex pheromone and plant odorants. Such a dominance principle has not been observed in the olfactory subsystems of all insect species studied in this regard. Possibly, the processing hierarchy in Manduca is related to the presence of segregate parallel olfactory subsystems which contrasts the situation in hymenopteran species, e.g., the honeybee, Apis mellifera, where dual parallel olfactory subsystems are in place (Galizia and Rössler 2010). A comparison between these two olfactory guided species, Apis and Manduca, reveals distinct differences in the processing function of the two olfactory pathways (Brill et al. 2013). Even though, in the honeybee, each olfactory subsystem has a unique output tract, projection neurons from both tracts respond to all tested odorants and both tracts respond with widely overlapping response profiles to floral, pheromonal, and biologically relevant odorant mixtures. This suggests that each organization has a highly adaptive value such that specific sensory organs or processing streams analyze different chemical stimuli (segregate parallel subsystems) or similar odorant stimuli are processed but analyzed with respect to different features (dual parallel systems) (Galizia and Rössler 2010).
In other insect systems such as the olfactory pathway of the male noctuid moth Agrotis ipsilon, most MGC-PNs respond to a plant odorant such as heptanal, a behaviorally attractive floral odorant (Chaffiol et al. 2012; Deisig et al. 2012). Heptanal reduces pheromone sensitivity of central neurons independently of the mating status. The response to the mixture of pheromone plus plant odorant is generally weaker than to the pheromone alone, showing a suppressive effect of heptanal on the pheromone responses. At the same time, these MGC-PNs respond with better temporal resolution of pulsed stimuli, suggesting that better resolution of pulsed stimuli is more important than high sensitivity to the localization of a calling female.
In the antennal lobes of the sphinx moth Bombyx mori, the response of MGC-PNs innervating the toroid glomerulus to the primary pheromone component bombykol is enhanced when cis-3-hexen-1-ol, a mulberry leaf, host plant volatile, is presented as a mixture along with the pheromone component (Namiki et al. 2008). Furthermore, plant odor information can be processed under conditions of simultaneous exposure to sex pheromone since the response of projection neurons innervating ordinary glomeruli to cis-3-hexen-1-ol is unaffected when bombykol is applied along with the plant odorant. These results provide a mechanism at the level of the antennal lobe through which insect orientation to a pheromone source can be modified by coexisting plant volatiles. Host plant volatiles can also enhance responses to pheromone at the level of olfactory receptor cells and, thereby, increase the sensitivity of orientation behavior as shown for many insect species including moths (Dickens et al. 1990, 1993; Ochieng et al. 2002; Deng et al. 2004; Yang et al. 2004). Plant volatiles do not always enhance pheromone responses as shown for Heliothis virescens (Pregitzer et al. 2012). In this case, plant odorants interfere with the signaling process of the major sex-pheromone component at the receptor level indicating that plant odorants in the environment can reduce the activity of pheromone-specific olfactory receptor cells and might also impair mate finding. Similarly, in Spodoptera littoralis, linalool as a background (i.e., nonspecific) odorant reduces the maximum firing rate of pheromone-sensitive olfactory receptor cells in response to a pheromone pulse (Party et al. 2009; Rouyar et al. 2011). However, the odorant background ensures preservation of temporal character of a pulsed olfactory signal, irrespective of effects on response intensity which can enhance mate location at high pheromone density close to a pheromone source (Rouyar et al. 2011). This confirms findings that pheromone detection by moth olfactory receptor cells is influenced by odorant compounds in the environment and these odorants can affect the ability of pheromone-sensitive olfactory receptor cells to code temporal parameters of the pheromone signal. The most frequent type of interaction between general odorants and pheromone appears to be mixture suppression (Party et al. 2009; Rouyar et al. 2011). Even in situations where a cognate pheromone component and a non-cognate odorant (other conspecific/heterospecific pheromone component or host plant volatile) are used as stimuli, mixture suppression can occur as in H. virescens (Hillier and Vickers 2011).
Recent evidence suggests the existence of lateral inhibition in the peripheral olfactory system of Drosophila and Anopheles (Su et al. 2012). Here, the inhibitory effects between functionally distinct olfactory receptor cells are thought to be mediated non-synaptically through ephaptic coupling. This type of lateral inhibition in the periphery can modulate olfactory behavior and shows that integration of olfactory information can occur via lateral interactions between olfactory receptor cells. In Drosophila, olfactory receptor cells have been shown to express GABAB receptors which allow for presynaptic gain control of antennal lobe projection neurons (Root et al. 2008), i.e., feedforward input from olfactory receptor cells onto glomerular projection neurons is controlled in each glomerulus. GABAB receptor expression widens the dynamic range of olfactory receptor cell synaptic transmission that is preserved in projection neuron responses. GABAB receptor expression is particularly high in pheromone-responsive olfactory receptor cells and allows for strong presynaptic inhibition.
Overall, new studies have revealed that a variety of processing mechanisms are in place in the antennal lobe leading to synchronized activity and unique representation of odorant mixtures (Lei and Vickers 2008; Riffell 2012; see also separate papers by Lei, Vickers, and Riffell in this volume). Examples include lateral excitation, disinhibition (Namiki et al. 2008) and excitatory local interneurons that can work through electrical synapses and might spread activity of neighboring glomeruli (Olsen et al. 2008; Huang et al. 2010; Yaksi and Wilson 2010), possibly to increase gain for weak input signals. In contrast, inhibitory local interneurons can downregulate olfactory responses when synaptic or afferent input is strong. The most prominent mechanism appears to be inhibition in its various forms such as global and local inhibition through pre- and postsynaptic effects (Root et al. 2007, 2008; Silbering and Galizia 2007; Olsen and Wilson 2008; Girardin et al. 2013) and modulation of projection neurons and local interneurons by a diverse population of local interneurons (Reisenman et al. 2008; Chou et al. 2010). Inhibitory mechanisms include inhibitory intra- and interglomerular interactions that have distinct functions in signal processing for the interacting neurons and glomeruli. The rules that govern these interactions (e.g., response properties, chemical relatedness, or other functional relationships) are not yet known. The existing inhibitory and excitatory networks regulate antennal lobe output in ways that cannot be predicted based on projection neuron responses to single odorants or peripheral input. It is reasonable to speculate that patterns of glomerular interaction reflect a functional and biologically relevant organization of glomeruli since integration of signals among glomeruli is an important mechanism for processing of olfactory information about behaviorally significant, naturally occurring odorant blends.
Acknowledgments
We are grateful for support, critical discussions, and review of an earlier version of the manuscript provided by Dr. John G. Hildebrand, Tucson, Arizona. This work was supported in part by the Whitehall Foundation and United States Public Health Service grants GM-08016, MD-007597 (T.H.), DC-007609 (V.D.C.S.), AI-23253 and DC-02751 (John G. Hildebrand).
Abbreviations
- BAL
Bombykal
- C15 (E,Z)
11,13-Pentadecadienal
- LFG
Large female glomerulus
- MGC
Macroglomerular complex
- PN
Projection neuron
Footnotes
Contribution to the special issue on “Insect olfaction in tribute to John Hildebrand”.
Contributor Information
Thomas Heinbockel, Department of Anatomy, Howard University College of Medicine, 520 W St., N.W., Washington, DC 20059, USA.
Vonnie D. C. Shields, Department of Biological Sciences, Towson University, Towson, MD 21252, USA
Carolina E. Reisenman, Department of Neuroscience, University of Arizona, Tucson, AZ 85721-0077, USA
References
- Abel R, Rybak J, Menzel R. Structure and response patterns of olfactory interneurons in the honeybee, Apis mellifera. J Comp Neurol. 2001;437:363–383. doi: 10.1002/cne.1289. [DOI] [PubMed] [Google Scholar]
- Almaas TJ, Christensen TA, Mustaparta H. Chemical communication in heliothine moths. I. Antennal receptor neurons encode several features of intra- and interspecific odorants in the male corn earworm moth Helicoverpa zea. J Comp Physiol A. 1991;169:249–258. [Google Scholar]
- Arn H, Tóth M, Priesner E. List of sex pheromones of Lepidoptera and related attractants. 2nd edn International Organization for Biological Control, West Palearctic Regional Section; Montfavet: 1992. [Google Scholar]
- Aungst JL, Heyward PM, Puche AC, Karnup SV, Hayar A, Szabo G, Shipley MT. Centre-surround inhibition among olfactory bulb glomeruli. Nature. 2003;426:623–629. doi: 10.1038/nature02185. [DOI] [PubMed] [Google Scholar]
- Axel R. Scents and sensibility: a molecular logic of olfactory perception (Nobel lecture) Angew Chem Int Ed Engl. 2005;44:6110–6127. doi: 10.1002/anie.200501726. [DOI] [PubMed] [Google Scholar]
- Baker TC. Sex pheromone communication in the Lepidoptera: new research progress. Experientia. 1989;45:248–262. [Google Scholar]
- Baker TC, Willis MA, Haynes KF, Phelan PL. A pulsed cloud of sex pheromone elicits upwind flight in male moths. Physiol Entomol. 1985;10:257–265. [Google Scholar]
- Brill MF, Rosenbaum T, Reus I, Kleineidam CJ, Nawrot MP, Rössler W. Parallel processing via a dual olfactory pathway in the honeybee. J Neurosci. 2013;33:2443–2456. doi: 10.1523/JNEUROSCI.4268-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck LB. Unraveling the sense of smell (Nobel lecture) Angew Chem Int Ed Engl. 2005;44:6128–6140. doi: 10.1002/anie.200501120. [DOI] [PubMed] [Google Scholar]
- Chaffiol A, Kropf J, Barrozo RB, Gadenne C, Rospars J-P, Anton S. Plant odour stimuli reshape pheromonal representation in neurons of the antennal lobe macroglomerular complex of a male moth. J Exp Biol. 2012;215:1670–1680. doi: 10.1242/jeb.066662. [DOI] [PubMed] [Google Scholar]
- Chou Y-H, Spletter ML, Yaksi E, Leong JCS, Wilson RI, Luo L. Diversity and wiring variability of olfactory local interneurons in the Drosophila antennal lobe. Nat Neurosci. 2010;13:439–449. doi: 10.1038/nn.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen TA, Hildebrand JG. Male-specific, sex-pheromone-selective projection neurons in the antennal lobes of the moth Manduca sexta. J Comp Physiol A. 1987;160:553–569. doi: 10.1007/BF00611929. [DOI] [PubMed] [Google Scholar]
- Christensen TA, Hildebrand JG. Frequency coding by central olfactory neurons in the sphinx moth Manduca sexta. Chem Senses. 1988;13:123–130. [Google Scholar]
- Christensen TA, Hildebrand JG. Coincident stimulation with pheromone components improves temporal pattern resolution in central olfactory neurons. J Neurophysiol. 1997;77:775–781. doi: 10.1152/jn.1997.77.2.775. [DOI] [PubMed] [Google Scholar]
- Christensen TA, Hildebrand JG. Pheromonal and host-odor processing in the insect antennal lobe: how different? Curr Opin Neurobiol. 2002;12:393–399. doi: 10.1016/s0959-4388(02)00336-7. [DOI] [PubMed] [Google Scholar]
- Christensen TA, White J. Representation of olfactory information in the brain. In: Finger TE, Silver WL, Restrepo D, editors. The neurobiology of taste and smell. Vol. 2. Wiley-Liss; New York: 2000. pp. 201–232. [Google Scholar]
- Christensen TA, Mustaparta H, Hildebrand JG. Discrimination of sex pheromone blends in the olfactory system of the moth. Chem Senses. 1989;14:463–477. [Google Scholar]
- Christensen TA, Waldrop B, Harrow ID, Hildebrand JG. Local interneurons and information processing in the olfactory glomeruli in the moth Manduca sexta. J Comp Physiol A. 1993;173:385–399. doi: 10.1007/BF00193512. [DOI] [PubMed] [Google Scholar]
- Christensen TA, Harrow ID, Cuzzocrea C, Randolph PW, Hildebrand JG. Distinct projections of two populations of olfactory receptor neurons in the antennal lobe of the sphinx moth Manduca sexta. Chem Senses. 1995;20:313–323. doi: 10.1093/chemse/20.3.313. [DOI] [PubMed] [Google Scholar]
- Christensen TA, Heinbockel T, Hildebrand JG. Olfactory information processing in the brain: encoding chemical and temporal features of odors. J Neurobiol. 1996;30:82–91. doi: 10.1002/(SICI)1097-4695(199605)30:1<82::AID-NEU8>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Christensen TA, Waldrop BR, Hildebrand JG. Multitasking in the olfactory system: context-dependent responses to odors reveal dual GABA-regulated coding mechanisms in single olfactory projection neurons. J Neurosci. 1998;18:5999–6008. doi: 10.1523/JNEUROSCI.18-15-05999.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor WE, Eisner T, Vander Meer RK, Guerrero A, Ghiringelli D, Meinwald J. Sex attractant of an Arctiid moth (Utetheisa ornatrix): a pulsed chemical signal. Behav Ecol Sociobiol. 1980;7:55–63. [Google Scholar]
- Deisig N, Kropf J, Vitecek S, Pevergne D, Rouyar A, Sandoz J-C, Lucas P, Gadenne C, Anton S, Barrozo R. Differential interactions of sex pheromone and plant odour in the olfactory pathway of a male moth. PLoS ONE. 2012;7(3):e33159. doi: 10.1371/journal.pone.0033159. doi:10.1371/journal.pone.0033159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng JY, Wei HY, Huang YP, Du JW. Enhancement of attraction to sex pheromones of Spodoptera exigua by volatile compounds produced by host plants. J Chem Ecol. 2004;30:2037–2045. doi: 10.1023/b:joec.0000045593.62422.73. [DOI] [PubMed] [Google Scholar]
- Dickens JC, Jang EB, Light DM, Alford AR. Enhancement of insect pheromone responses by green leaf volatiles. Naturwissenschaften. 1990;77:29–31. [Google Scholar]
- Dickens JC, Smith JW, Light DM. Green leaf volatiles enhance sex attractant pheromone of the tobacco budworm, Heliothis virescens (Lep.: Noctuidae) Chemoecology. 1993;4:175–177. [Google Scholar]
- Friedrich RW, Laurent G. Dynamics of olfactory bulb input and output activity during odor stimulation in zebrafish. J Neurophysiol. 2004;91:2658–2669. doi: 10.1152/jn.01143.2003. [DOI] [PubMed] [Google Scholar]
- Galizia CG, Rössler W. Parallel olfactory systems in insects: anatomy and function. Annu Rev Entomol. 2010;55:399–420. doi: 10.1146/annurev-ento-112408-085442. [DOI] [PubMed] [Google Scholar]
- Galizia CG, Sachse S, Rappert A, Menzel R. The glomerular code for odor representation is species specific in the honeybee Apis mellifera. Nat Neurosci. 1999;2:473–478. doi: 10.1038/8144. [DOI] [PubMed] [Google Scholar]
- Girardin CC, Kreissl S, Galizia CG. Inhibitory connections in the honeybee antennal lobe are spatially patchy. J Neurophysiol. 2013;109:332–343. doi: 10.1152/jn.01085.2011. [DOI] [PubMed] [Google Scholar]
- Hansson BS, Christensen TA. Functional characteristics of the antennal lobe. In: Hansson BS, editor. Insect olfaction. Springer; Berlin: 1999. pp. 126–161. [Google Scholar]
- Hansson BS, Christensen TA, Hildebrand JG. Functionally distinct subdivisions of the macroglomerular complex in the antennal lobe of the male sphinx moth Manduca sexta. J Comp Neurol. 1991;312:264–278. doi: 10.1002/cne.903120209. [DOI] [PubMed] [Google Scholar]
- Hansson BA, Carlsson MA, Kalinova B. Olfactory activation patterns in the antennal lobe of the sphinx moth Manduca sexta. J Comp Physiol A. 2003;189:301–308. doi: 10.1007/s00359-003-0403-5. [DOI] [PubMed] [Google Scholar]
- Hartline HK. Inhibition of activity of visual receptors by illuminating nearby retinal areas in the Limulus eye. Fed Proc. 1949;8:69. [Google Scholar]
- Haupt SS, Sakurai T, Namiki S, Kazawa T, Kanzaki R. Olfactory information processing in moths. In: Menini A, editor. The neurobiology of olfaction. CRC Press; Boca Raton: 2010. pp. 126–161. ch 3. [PubMed] [Google Scholar]
- Heinbockel T, Hildebrand JG. Antennal receptive fields of pheromone-responsive neurons in the antennal lobes of the sphinx moth Manduca sexta. J Comp Physiol A. 1998;183:121–133. doi: 10.1007/s003590050240. [DOI] [PubMed] [Google Scholar]
- Heinbockel T, Hildebrand JG. Cellular mechanisms of odor processing in the antennal lobes of the sphinx moth Manduca sexta. Mitt Deutsche Ges Allg Angew Entomol. 2000;12:549–553. [Google Scholar]
- Heinbockel T, Christensen TA, Hildebrand JG. Subunit architecture of the macroglomerular complex and the integrative function of output neurons in the moth, Manduca sexta. Chem Senses. 1995;20:707. [Google Scholar]
- Heinbockel T, Christensen TA, Hildebrand JG. Temporal tuning of odor responses in pheromone-responsive projection neurons in the brain of the sphinx moth Manduca sexta. J Comp Neurol. 1999;409:1–12. [PubMed] [Google Scholar]
- Heinbockel T, Christensen TA, Hildebrand JG. Representation of binary pheromone blends by glomerulus-specific olfactory projection neurons. J Comp Physiol A. 2004;190:1023–1037. doi: 10.1007/s00359-004-0559-7. [DOI] [PubMed] [Google Scholar]
- Hildebrand JG. Analysis of chemical signals by nervous systems. Proc Natl Acad Sci USA. 1995;92:67–74. doi: 10.1073/pnas.92.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand JG. Olfactory control of behavior in moths: central processing of odor information and the functional significance of olfactory glomeruli. J Comp Physiol A. 1996;178:5–19. doi: 10.1007/BF00189586. [DOI] [PubMed] [Google Scholar]
- Hildebrand JG, Shepherd GM. Mechanisms of olfactory discrimination: converging evidence for common principles across phyla. Annu Rev Neurosci. 1997;20:595–631. doi: 10.1146/annurev.neuro.20.1.595. [DOI] [PubMed] [Google Scholar]
- Hildebrand JG, Christensen TA, Heinbockel T, Roche King J, Mechaber W, Rössler W, Shields VDC. The olfactory neurobiology of host- and mate-attraction in moths. In: Elsner N, Eysel U, editors. From molecular neurobiology to clinical neuro-science; Proceedings of the 1st Göttingen conference of the German Neuroscience Society 1999 & 27th Göttingen Neuro-biology Conference; New York: Georg Thieme Verlag Stuttgart; 1999. pp. 56–67. [Google Scholar]
- Hillier NK, Vickers NJ. Mixture interactions in moth olfactory physiology: examining the effects of odorant mixture, concentration, distal stimulation, and antennal nerve transection on sensillar responses. Chem Senses. 2011;36:93–108. doi: 10.1093/chemse/bjq102. [DOI] [PubMed] [Google Scholar]
- Homberg U, Christensen TA, Hildebrand TA. Structure and function of the deutocerebrum in insects. Annu Rev Entomol. 1989;34:477–501. doi: 10.1146/annurev.en.34.010189.002401. [DOI] [PubMed] [Google Scholar]
- Homberg U, Hoskins SG, Hildebrand JG. Distribution of acetylcholinesterase activity in the deutocerebrum of the sphinx moth Manduca sexta. Cell Tissue Res. 1995;279:249–259. doi: 10.1007/BF00318481. [DOI] [PubMed] [Google Scholar]
- Hoskins SG, Homberg U, Kingan TG, Christensen TA, Hildebrand JG. Immunocytochemistry of GABA in the antennal lobes of the sphinx moth Manduca sexta. Cell Tissue Res. 1986;244:243–252. doi: 10.1007/BF00219199. [DOI] [PubMed] [Google Scholar]
- Huang J, Zhang W, Qiao WH, Hu AQ, Wang ZR. Functional connectivity and selective odor responses of excitatory local interneurons in Drosophila antennal lobe. Neuron. 2010;67:1021–1033. doi: 10.1016/j.neuron.2010.08.025. [DOI] [PubMed] [Google Scholar]
- Kaissling KE. In: R. H. Wright lectures on insect olfaction. Colbow, editor. Simon Fraser University; Burnaby: 1987. [Google Scholar]
- Kaissling KE. Peripheral mechanisms of pheromone reception in moths. Chem Senses. 1996;21:257–268. doi: 10.1093/chemse/21.2.257. [DOI] [PubMed] [Google Scholar]
- Kaissling KE, Kramer E. Sensory basis of pheromone-mediated orientation in moths. Verh Dtsch Zool Ges. 1990;83:109–131. [Google Scholar]
- Kaissling KE, Hildebrand JG, Tumlinson JH. Pheromone receptor cells in the male moth Manduca sexta. Arch Insect Biochem Physiol. 1989;10:273–279. [Google Scholar]
- Kalberer N, Reisenman CE, Hildebrand JG. Male moths bearing transplanted female antennae express characteristically female behaviour and central neural activity. J Exp Biol. 2010;213:1272–1280. doi: 10.1242/jeb.033167. [DOI] [PubMed] [Google Scholar]
- Kashiwadani H, Sasaki YF, Uchida N, Mori K. Synchronized oscillatory discharges of mitral/tufted cells with different molecular receptive ranges in the rabbit olfactory bulb. J Neurophysiol. 1999;82:1786–1792. doi: 10.1152/jn.1999.82.4.1786. [DOI] [PubMed] [Google Scholar]
- Keil TA. Fine structure of the pheromone-sensitive sensilla on the antenna of the hawkmoth, Manduca sexta. Tissue Cell. 1989;21:139–151. doi: 10.1016/0040-8166(89)90028-1. [DOI] [PubMed] [Google Scholar]
- Kramer E. Turbulent diffusion and pheromone triggered anemotaxis. In: Payne TL, Birch MC, Kennedy CEJ, editors. Mechanisms in insect olfaction. Oxford University Press; Oxford: 1986. pp. 59–67. [Google Scholar]
- Kramer E. Attractivity of pheromone surpassed by time-patterned application of two nonpheromone compounds. J Insect Behav. 1992;5:83–97. [Google Scholar]
- Kuebler LS, Olsson SB, Weniger R, Hansson BS. Neuronal processing of complex mixtures establishes a unique odor representation in the moth antennal lobe. Front Neural Circuits. 2011;5:7. doi: 10.3389/fncir.2011.00007. doi:10.3389/fncir.2011.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuebler LS, Schubert M, Kárpáti Z, Hansson BS, Olsson SB. Antennal lobe processing correlates to moth olfactory behavior. J Neurosci. 2012;32:5772–5782. doi: 10.1523/JNEUROSCI.6225-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent G. Olfactory processing: maps, time and codes. Curr Opin Neurobiol. 1997;7:547–553. doi: 10.1016/s0959-4388(97)80035-9. [DOI] [PubMed] [Google Scholar]
- Lee JK, Strausfeld NJ. Structure, distribution and number of surface sensilla and their receptor cells on the olfactory appendage of the male moth, Manduca sexta. J Neurocytol. 1990;19:519–538. doi: 10.1007/BF01257241. [DOI] [PubMed] [Google Scholar]
- Lei H, Vickers N. Central processing of natural odor mixtures in insects. J Chem Ecol. 2008;34:915–927. doi: 10.1007/s10886-008-9487-2. [DOI] [PubMed] [Google Scholar]
- Lei H, Christensen TA, Hildebrand JG. Local inhibition modulates odor-evoked synchronization of glomerulus-specific output neurons. Nat Neurosci. 2002;5:557–565. doi: 10.1038/nn0602-859. [DOI] [PubMed] [Google Scholar]
- Lei H, Riffell JA, Gage SL, Hildebrand JG. Contrast enhancement of stimulus intermittency in a primary olfactory network and its behavioral significance. J Biol. 2009;8:21. doi: 10.1186/jbiol120. doi:10.1186/jbiol120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei H, Oland LA, Riffell JA, Beyerlein A, Hildebrand JG. Microcircuits for olfactory information processing in the antennal lobe of Manduca sexta. In: Shepherd GM, Grillner S, editors. Handbook of brain microcircuits. Oxford University Press; New York: 2010. pp. 417–426. [Google Scholar]
- Linster C, Sachse S, Galizia CG. Computational modeling suggests that response properties rather than spatial position determine connectivity between olfactory glomeruli. J Neurophysiol. 2005;93:3410–3417. doi: 10.1152/jn.01285.2004. [DOI] [PubMed] [Google Scholar]
- Mafra-Neto A, Cardé RT. Fine-scale structure of pheromone plumes modulates upwind orientation of flying moths. Nature. 1994;369:142–144. [Google Scholar]
- Marion-Poll F, Tobin TR. Temporal coding of pheromone pulses and trains in Manduca sexta. J Comp Physiol A. 1992;171:505–512. doi: 10.1007/BF00194583. [DOI] [PubMed] [Google Scholar]
- Martin JP, Beyerlein A, Dacks AM, Reisenman CE, Riffell JA, Lei H, Hildebrand JG. The neurobiology of insect olfaction: sensory processing in a comparative context. Prog Neurobiol. 2011;95:427–447. doi: 10.1016/j.pneurobio.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Matsumoto SG, Hildebrand JG. Olfactory mechanisms in the moth Manduca sexta: response characteristics and morphology of central neurons in the antennal lobes. Proc R Soc Lond B. 1981;213:249–277. [Google Scholar]
- Menzel R. Searching for the memory trace in a mini-brain, the honeybee. Learn Mem. 2001;8:53–62. doi: 10.1101/lm.38801. [DOI] [PubMed] [Google Scholar]
- Mombaerts P. Axonal wiring in the mouse olfactory system. Annu Rev Cell Dev Biol. 2006;22:713–737. doi: 10.1146/annurev.cellbio.21.012804.093915. [DOI] [PubMed] [Google Scholar]
- Murlis J. Odor plumes and the signal they provide. In: Cardé RT, Minks AK, editors. Insect pheromone research—new directions. Chapman & Hall; New York: 1997. pp. 221–231. [Google Scholar]
- Murlis J, Elkinton JS, Cardé RT. Odor plumes and how insects use them. Annu Rev Entomol. 1992;37:505–532. [Google Scholar]
- Nagayama S, Takahashi YK, Yoshihara Y, Mori K. Mitral and tufted cells differ in the decoding manner of odor maps in the rat olfactory bulb. J Neurophysiol. 2004;91:2532–2540. doi: 10.1152/jn.01266.2003. [DOI] [PubMed] [Google Scholar]
- Namiki S, Iwabuchi S, Kanzaki R. Representation of a mixture of pheromone and host plant odor by antennal lobe projection neurons of the silkmoth Bombyx mori. J Comp Physiol A. 2008;194:501–515. doi: 10.1007/s00359-008-0325-3. [DOI] [PubMed] [Google Scholar]
- Ochieng SA, Park KC, Baker TC. Host plant volatiles synergize responses of sex pheromone specific olfactory receptor neurons in male Helicoverpa zea. J Comp Physiol A. 2002;188:325–333. doi: 10.1007/s00359-002-0308-8. [DOI] [PubMed] [Google Scholar]
- Oland LA, Tolbert LP. Effects of hydroxyurea parallel the effects of radiation in developing olfactory glomeruli. J Comp Neurol. 1988;278(3):377–387. doi: 10.1002/cne.902780307. [DOI] [PubMed] [Google Scholar]
- Olsen SR, Wilson RI. Lateral presynaptic inhibition mediates gain control in an olfactory circuit. Nature. 2008;452:956–960. doi: 10.1038/nature06864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen SR, Bhandawat V, Wilson RI. Excitatory interactions between olfactory processing channels in the Drosophila antennal lobe. Neuron. 2008;54:89–103. doi: 10.1016/j.neuron.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Party V, Hanot C, Said I, Rochat D, Renou M. Plant terpenes affect intensity and temporal parameters of pheromone detection in a moth. Chem Senses. 2009;34:763–774. doi: 10.1093/chemse/bjp060. [DOI] [PubMed] [Google Scholar]
- Pregitzer P, Schubert M, Breer H, Hansson BS, Sachse S, Krieger J. Plant odorants interfere with detection of sex pheromone signals by male Heliothis virescens. Front Cell Neurosci. 2012;6:42. doi: 10.3389/fncel.2012.00042. doi:10.3389/fncel.2012.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisenman CE, Christensen TA, Francke W, Hildebrand JG. Enantioselectivity of projection neurons innervating identified olfactory glomeruli. J Neurosci. 2004;24:2602–2611. doi: 10.1523/JNEUROSCI.5192-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisenman CE, Christensen TA, Hildbrand JG. Chemosensory selectivity of output neurons innervating an identified, sexually isomorphic olfactory glomerulus. J Neurosci. 2005;25:8017–8026. doi: 10.1523/JNEUROSCI.1314-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisenman CE, Heinbockel T, Hildebrand JG. Inhibitory interactions among olfactory glomeruli do not necessarily reflect spatial proximity. J Neurophysiol. 2008;100:554–564. doi: 10.1152/jn.90231.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisenman CE, Riffell JA, Hildebrand JG. Neuroethology of oviposition behavior in the moth Manduca sexta. Ann N Y Acad Sci. 2009;1170:462–467. doi: 10.1111/j.1749-6632.2009.03875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisenman CE, Riffell JA, Bernays EA, Hildebrand JG. Antagonistic effects of floral scent in an insect–plant interaction. Proc R Soc B. 2010;277:2371–2379. doi: 10.1098/rspb.2010.0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisenman CE, Dacks A, Hildebrand JG. Local interneuron diversity in the primary olfactory center of the moth Manduca sexta. J Comp Physiol A. 2011;197:653–665. doi: 10.1007/s00359-011-0625-x. [DOI] [PubMed] [Google Scholar]
- Riffell JA. Olfactory ecology and the processing of complex mixtures. Curr Opin Neurobiol. 2012;22:236–242. doi: 10.1016/j.conb.2012.02.013. [DOI] [PubMed] [Google Scholar]
- Roche King J, Christensen TA, Hildebrand JG. Response characteristics of an identified, sexually dimorphic olfactory glomerulus. J Neurosci. 2000;20:2391–2399. doi: 10.1523/JNEUROSCI.20-06-02391.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root CM, Semmelhack JL, Wong AM, Flores J, Wang JW. Propagation of olfactory information in Drosophila. Proc Natl Acad Sci USA. 2007;104:11826–11831. doi: 10.1073/pnas.0704523104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root CM, Masuyama K, Green DS, Enell LE, Nässel DR, Lee CH, Wang JW. A presynaptic gain control mechanism fine tunes olfactory behavior. Neuron. 2008;59:311–321. doi: 10.1016/j.neuron.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rospars JP, Hildebrand JG. Sexually dimorphic and isomorphic glomeruli in the antennal lobes of the sphinx moth Manduca sexta. Chem Senses. 2000;25:119–129. doi: 10.1093/chemse/25.2.119. [DOI] [PubMed] [Google Scholar]
- Rössler W, Randolph PW, Tolbert LP, Hildebrand JG. Axons of olfactory receptor cells of transsexually grafted antennae induce development of sexually dimorphic glomeruli in Manduca sexta. J Neurobiol. 1999;38(4):521–541. doi: 10.1002/(sici)1097-4695(199903)38:4<521::aid-neu8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Rössler W, Tolbert LP, Hildebrand JG. Early formation of sexually dimorphic glomeruli in the developing olfactory lobe of the brain of the moth Manduca sexta. J Comp Neurol. 1998;396(4):415–428. [PubMed] [Google Scholar]
- Rouyar A, Party V, Presern J, Blejec A, Renou M. A general odorant background affects the coding of pheromone stimulus intermittency in specialist olfactory receptor neurones. PLoS ONE. 2011;6:e26443. doi: 10.1371/journal.pone.0026443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumbo ER, Kaissling KE. Temporal resolution of odour pulses by three types of pheromone receptor cells in Antheraea polyphemus. J Comp Physiol A. 1989;165:281–291. [Google Scholar]
- Sachse S, Galizia CG. Role of inhibition for temporal and spatial odor representation in olfactory output neurons: a calcium imaging study. J Neurophysiol. 2003;87:1106–1117. doi: 10.1152/jn.00325.2001. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Hildebrand JG. Structure and development of antennae in a moth, Manduca sexta. Dev Biol. 1976;51(2):282–299. [PubMed] [Google Scholar]
- Schoppa NE. Synchronization of olfactory bulb mitral cells by precisely timed inhibitory inputs. Neuron. 2006;49:271–283. doi: 10.1016/j.neuron.2005.11.038. [DOI] [PubMed] [Google Scholar]
- Schoppa NE, Urban NN. Dendritic processing within olfactory bulb circuits. Trends Neurosci. 2003;26:501–506. doi: 10.1016/S0166-2236(03)00228-5. [DOI] [PubMed] [Google Scholar]
- Shang Y, Claridge-Chang A, Sjulson L, Pypaert M, Miesenbock G. Excitatory local circuits and their implications for olfactory processing in the fly antennal lobe. Cell. 2007;128:601–612. doi: 10.1016/j.cell.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GW, Chen WR, Greer CA. Olfactory bulb. In: Shepherd GM, editor. The synaptic organization of the brain. Oxford, New York: 2004. pp. 165–216. [Google Scholar]
- Shields VDC, Heinbockel T. Neurophysiological recording techniques applied to insect chemosensory systems. In: Maria-Dolores Garcia., editor. Zoology. Intech Open Access Publisher; Rijeka: 2012. pp. 123–162. ch. 7. [Google Scholar]
- Shields VDC, Hildebrand JG. Fine structure of antennal sensilla of the female sphinx moth, Manduca sexta (Lepidoptera: Sphingidae). I. Trichoid and basiconic sensilla. Can J Zool. 1999a;77:290–301. [Google Scholar]
- Shields VDC, Hildebrand JG. Fine structure of antennal sensilla of the female sphinx moth, Manduca sexta (Lepidoptera: Sphingidae). II. Auriculate, coeloconic, and styliform complex sensilla. Can J Zool. 1999b;77:302–313. [Google Scholar]
- Shields VDC, Hildebrand JG. Responses of a population of antennal olfactory receptor cells in the female moth Manduca sexta to plant-associated volatile organic compounds. J Comp Physiol A. 2001a;186:1135–1151. doi: 10.1007/s003590000165. [DOI] [PubMed] [Google Scholar]
- Shields VDC, Hildebrand JG. Recent advances in insect olfaction, specifically regarding the morphology and sensory physiology of antennal sensilla of the female sphinx moth Manduca sexta. Microsc Res Tech. 2001b;55:307–329. doi: 10.1002/jemt.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbering AF, Galizia CG. Processing of odor mixtures in the Drosophila antennal lobe reveals both global inhibition and glomerulus-specific interactions. J Neurosci. 2007;7:11966–11977. doi: 10.1523/JNEUROSCI.3099-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strausfeld NJ. Cellular organization in male-specific olfactory neuropil in the moth Manduca sexta. In: Elsner N, Singer W, editors. Dynamics and plasticity in neuronal systems. Thieme; Stuttgart: 1989. p. 79. [Google Scholar]
- Su CY, Menuz K, Reisert J, Carlson JR. Non-synaptic inhibition between grouped neurons in an olfactory circuit. Nature. 2012;492:66–71. doi: 10.1038/nature11712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumlinson JH, Brennan MM, Doolittle RE, Mitchell ER, Brabham A, Mazomenos BE, Baumhover AH, Jackson DM. Identification of a pheromone blend attractive to Manduca sexta (L.) males in a wind tunnel. Arch Insect Biochem Physiol. 1989;10:255–271. [Google Scholar]
- Urban NN. Lateral inhibition in the olfactory bulb and in olfaction. Physiol Beh. 2002;77:607–612. doi: 10.1016/s0031-9384(02)00895-8. [DOI] [PubMed] [Google Scholar]
- Vickers NJ, Baker TC. Reiterative responses to single strands of odor promote sustained upwind flight and odor source location by moths. Proc Natl Acad Sci USA. 1994;91:5756–5760. doi: 10.1073/pnas.91.13.5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers NJ, Christensen TA, Hildebrand TA. Combinatorial odor discrimination in the brain: attractive and antagonist odor blends are represented in distinct combinations of uniquely identifiable glomeruli. J Comp Neurol. 1998;400:35–56. [PubMed] [Google Scholar]
- Vosshall LB, Stocker RF. Molecular architecture of smell and taste in Drosophila. Annu Rev Neurosci. 2007;30:505–533. doi: 10.1146/annurev.neuro.30.051606.094306. [DOI] [PubMed] [Google Scholar]
- Vucinic D, Cohen LB, Kosmidis EK. Interglomerular center-surround inhibition shapes odorant-evoked input to the mouse olfactory bulb in vivo. J Neurophysiol. 2006;95:1881–1887. doi: 10.1152/jn.00918.2005. [DOI] [PubMed] [Google Scholar]
- Wachoviak M, Cohen LB. Representation of odorants by receptor neuron input to the mouse olfactory bulb. Neuron. 2001;32:723–735. doi: 10.1016/s0896-6273(01)00506-2. [DOI] [PubMed] [Google Scholar]
- Whitesell JD, Sorensen KA, Jarvie BC, Hentges ST, Schoppa NE. Interglomerular lateral inhibition targeted on external tufted cells in the olfactory bulb. J Neurosci. 2013;33:1552–1563. doi: 10.1523/JNEUROSCI.3410-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Laurent G. Role of GABAergic inhibition in shaping odor-evoked spatiotemporal patterns in the Drosophila antennal lobe. J Neurosci. 2005;25:9069–9079. doi: 10.1523/JNEUROSCI.2070-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Turner GC, Laurent G. Transformation of olfactory representations in the Drosophila antennal lobe. Science. 2004;303:366–370. doi: 10.1126/science.1090782. [DOI] [PubMed] [Google Scholar]
- Yaksi E, Wilson RI. Electrical coupling between olfactory glomeruli. Neuron. 2010;67:1034–1047. doi: 10.1016/j.neuron.2010.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Bengtsson M, Witzgall P. Host plant volatiles synergize response to sex pheromone in codling moth, Cydia pomonella. J Chem Ecol. 2004;30:619–629. doi: 10.1023/b:joec.0000018633.94002.af. [DOI] [PubMed] [Google Scholar]
- Yokoi M, Mori K, Nakanishi S. Refinement of odor molecule tuning by dendrodendritic synaptic inhibition in the olfactory bulb. Proc Natl Acad Sci USA. 1995;92:3371–3375. doi: 10.1073/pnas.92.8.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]