Abstract
Mammalian sex determination is controlled by antagonistic pathways that are initially co-expressed in the bipotential gonad and subsequently become male- or female-specific. In XY gonads, testis development is initiated by upregulation of Sox9 by SRY in pre-Sertoli cells. Disruption of either gene leads to complete male-to-female sex reversal. Ovarian development is dependent on canonical Wnt signaling through Wnt4, Rspo1 and β-catenin. However, only a partial female-to-male sex reversal results from disruption of these ovary-promoting genes. In Wnt4 and Rspo1 mutants, there is evidence of pregranulosa cell-to-Sertoli cell transdifferentiation near birth, following a severe decline in germ cells. It is currently unclear why primary sex reversal does not occur at the sex-determining stage, but instead occurs near birth in these mutants. Here we show that Wnt4-null and Rspo1-null pregranulosa cells transition through a differentiated granulosa cell state prior to transdifferentiating towards a Sertoli cell fate. This transition is preceded by a wave of germ cell death that is closely associated with the disruption of pregranulosa cell quiescence. Our results suggest that maintenance of mitotic arrest in pregranulosa cells may preclude upregulation of Sox9 in cases where female sex-determining genes are disrupted. This may explain the lack of complete sex reversal in such mutants at the sex-determining stage.
Keywords: sex determination, ovary, Wnt4, Rspo1, Sox9, meiotic germ cells
Introduction
Mammalian sex determination is controlled by opposing pathways that guide differentiation of the embryonic gonad towards a testis or ovary fate. The gonad is initially identical in XX and XY embryos and has the potential to follow either a male or female developmental pathway. Primary sex determination within the gonad dictates subsequent sexual differentiation outside of the gonad (secondary sex determination) through hormone production.
In the XY mouse gonad, male fate is determined by the expression of Sry from the Y chromosome between 10.5 and 12.5 days post coitum (dpc). Sry expression establishes Sertoli cell fate in the supporting cell lineage, shifting the bipotential gonad towards the testis fate (Hacker et al., 1995; Bullejos et al., 2001) by upregulating Sox9, a critical component of the male pathway (Sekido et al., 2004; Sekido et al., 2008). Inappropriate upregulation of Sox9/SOX9 in the XX gonad overrides female differentiation and leads to female-to-male sex reversal (Vidal et al., 2001; Qin et al., 2005), while mutation of murine (Chaboissier et al., 2004; Barrionuevo et al., 2006) or human (Foster et al., 1994; Wagner et al., 1994) Sox9/SOX9 in XY individuals leads to male-to-female sex reversal. The establishment of Sox9 expression is followed by a rapid morphological reorganization of the XY gonad: mesonephric endothelial cells migrate into the gonad to form a male-specific coelomic vessel (Martineau et al., 1997; Cool et al., 2008; Coveney et al., 2008; Combes et al., 2009), cells at the coelomic surface undergo rapid proliferation (Schmahl et al., 2000), and Sox9-expressing supporting cells cluster around germ cells forming testis cords.
In the absence of a signal to upregulate Sox9 expression, XX supporting cells upregulate several genes that establish the ovarian fate and differentiate as pregranulosa cells (reviewed in Liu et al., 2010a). Early XX gonads do not undergo obvious morphological changes. Between 14.5 dpc and birth, germ cells, surrounded by mitotically arrested pregranulosa cells, are arranged in clusters termed ovigerous cords. Throughout female reproductive life, pregranulosa cells in quiescent follicles are induced to resume proliferation as small cohorts of follicles are recruited for maturation.
Two transcriptional regulators are critical for maintaining ovarian cell fate: β-catenin and FOXL2. β-catenin activity is controlled by the canonical Wnt signaling pathway, which has been investigated from multiple perspectives including Wnt4-deficiency (Vainio et al., 1999), Rspo1-deficiency (Chassot et al., 2008b; Tomizuka et al., 2008) and conditional loss of β-catenin specifically in a subset of gonadal somatic cells (Manuylov et al., 2008; Liu et al., 2009). Loss of function of these Wnt pathway components in XX gonads leads to a partial female-to-male sex reversal characterized by ectopic coelomic vessel formation, upregulation of steroidogenic enzymes, and reduced germ cell viability. While β-catenin can directly antagonize SOX9 expression in XY gonads (Maatouk et al., 2008), a loss of canonical Wnt signaling in XX gonadal somatic cells is not sufficient to cause stable upregulation of SOX9 during primary sex determination. Near birth, ovaries lacking either Rspo1 or Wnt4 exhibit limited sex reversal marked by the appearance of cord-like structures and the expression of AMH (Vainio et al., 1999; Chassot et al., 2008b) and SOX9 (Chassot et al., 2008b). Curiously, although β-catenin is downstream of Rspo1 and Wnt4, there is no evidence of sex reversal near birth in ovaries where β-catenin is conditionally disrupted in the majority of somatic cells (Manuylov et al., 2008).
Foxl2, another regulator of ovarian differentiation, is expressed in the supporting cells of the ovary upon commitment to the female pathway. Unlike Wnt pathway mutations, loss of Foxl2 in mice does not have an embryonic phenotype in the gonad; however, one week after birth, granulosa cells begin to transdifferentiate to Sertoli-like cells and upregulate SOX9 (Schmidt et al., 2004; Ottolenghi et al., 2005; Uhlenhaut et al., 2009). This suggests that, similar to β-catenin, Foxl2 is also critical for maintaining ovarian cell fate by repressing SOX9; however, its dominant role is after birth.
Here we describe a transformation of pregranulosa cell identity that results from a Wnt/Rspo deficiency. Building off previous work demonstrating a requirement for Wnt4 in germ cell survival (Vainio et al., 1999; Yao et al., 2004), we find that the loss of germ cells is non-random and occurs in an anterior-posterior wave across the ovary, mimicking the pattern of meiotic entry observed for ovarian germ cells. This germ cell loss is accompanied by the exit of pregranulosa cells from their normal quiescent state followed by the expression of markers normally associated with proliferative granulosa cells found in growing follicles of the postnatal ovary. Near birth, these cells show evidence of transdifferentiation to a male fate with the onset of SOX9 expression in a subset of cells. Additionally, we show that Rspo1 mutants undergo a similar stepwise transformation of the pregranulosa lineage and speculate as to why these alterations are not observed in β-catenin mutants or at earlier stages of fetal ovarian development.
Materials and Methods
Mouse Strains and Genotyping
Oct4-Gfp (Yoshimizu et al., 1999), Wnt4 (Vainio et al., 1999), Wnt4GC/+ (Maatouk et al., 2012), Bax (Baxtm1Sjk/J; (Knudson et al., 1995), KitWv (Little et al., 1937), β-catflox/flox (B6.129-Ctnnb1tm2Kem/KnwJ; (Brault et al., 2001) and Sf1-Cre (Bingham et al., 2006) mice were maintained on a C57BL/6 genetic background. Rspo1 mice (Chassot et al., 2008b) were maintained on a mixed 129/C57BL6/J background (4 to 5 back-crosses to C57BL6/J background). The BRE-LacZ (BRE-Hspa1a-lacZ) transgenic line, in which LacZ expression is driven by a series of Smad1/5/8 binding sites from the Id1 promoter (Blank et al., 2008), was maintained on an outbred CD-1/ICR genetic background. Genotyping was performed on tail samples using previously published primers and standard PCR methods (see references above). For the KitWv mutation, a TaqMan SNP Genotyping Assay (Applied Biosystems) was developed and run on a StepOnePlus thermal cycler (Applied Biosystems) following the supplier's protocol. Primer and probe sequences (5′-3′) are as follows: Forward primer GCTACCTGGGCAATCACATGAATAT, Reverse primer TGAGTCTCGAGTTGCCATCTCT, FAM-conjugated probe for the KitWv allele CATGCATGGTGGGAG and VIC-conjugated probe for the Kit+ allele CATGCACGGTGGGAGG.
Matings and busulfan treatment
Wnt4 homozygous mutant embryos were generated by timed matings of Wnt4+/-or Wnt4GC/+ males and females. Rspo1-/- males are viable and fertile; therefore, Rspo1-/- embryos were generated by mating homozygous mutant males to Rspo1+/- females. Attempts to genetically ablate germ cells from Wnt4 mutant ovaries were unsuccessful (3 of 40 XY embryos were Wnt4-/-; KitWv/Wv and 0 of 58 XX embryos were Wnt4-/-; KitWv/Wv, Mendelian inheritance would predict ∼3-4 (6.3%) of the XX Wnt4-/-; KitWv/Wv embryos to be double mutants.); therefore, germ cells were depleted by treatment with busulfan. Timed pregnant Rspo1+/- and Wnt4+/ - females were intraperitoneally injected with 10 mg of busulfan (Sigma) dissolved in 50% DMSO at 11.5 dpc.
To block germ cell death in Wnt4 mutant ovaries, Wnt4+/-, Bax+/-, and Oct4-Gfp mice were bred to generate Wnt4+/-; Bax+/-; Oct4-Gfp males and females. These offspring were intercrossed, resulting in double homozygous Wnt4-/-; Bax-/-; Oct4-Gfp embryos.
Immunocytochemistry and BrdU Assay
Immunostaining and BrdU assays were performed as previously described (Mork et al., 2012). Briefly, following timed matings, gonads were dissected from embryos and fixed for several hours or overnight at 4°C in 4% paraformaldehyde. Samples were embedded in OCT and cryosectioned (18 μm) or whole-mount immunostained with antibodies listed in Table S1. For the BrdU incorporation assay, timed pregnant females were injected intraperitoneally with 1.5 mg BrdU (Sigma) at 18.5 dpc, and embryos collected 4-6 hours later.
Results
Germ cell death in Wnt4 mutant embryos occurs in an anterior-posterior wave
At 13.5 dpc, influenced by signals from the surrounding somatic cells, proliferative germ cells within the testis enter mitotic arrest while those in the ovary initiate meiosis. Progression into meiosis is non-uniform with germ cells at the anterior end of the ovary entering meiosis first, followed by a wave of meiotic entry in the posterior direction (Menke et al., 2003; Yao et al., 2003; Bullejos et al., 2004). In the ovary, meiotic germ cells undergo several waves of apoptosis that are considered a normal part of ovarian development (Rucker et al., 2000; McClellan et al., 2003). However, germ cell apoptosis is increased in ovaries homozygous for a null allele of Wnt4, where at 16.5 dpc, apoptotic germ cells are found throughout the ovary, leaving approximately 10% of the original germ cell population remaining at birth (Vainio et al., 1999; Yao et al., 2004).
Intriguingly, we found that the loss of germ cells that occurs in response to disruption of Wnt4 progressed from the anterior to the posterior end of the ovary. At 15.5 dpc, the number of germ cells at the anterior end of Wnt4-/- ovaries was reduced compared to the anterior end of control gonads (Fig. 1A, D). Within one day, the area occupied by germ cells encompassed barely half of the ovary (Fig. 1B, E) and near birth only a small population of germ cells survived, clustered at the posterior end of the ovary (Fig. 1C, F). This orderly wave of germ cell loss was consistently observed using three independent markers of germ cells; the cell surface markers PECAM1 and CDH1, expressed by both XX and XY germ cells, and a marker of the meiotic synaptonemal complex, SCP3 (Fig. 1 and Fig. S1).
Figure 1. Germ cell loss proceeds in an anterior-posterior wave in XX Wnt4-null ovaries.
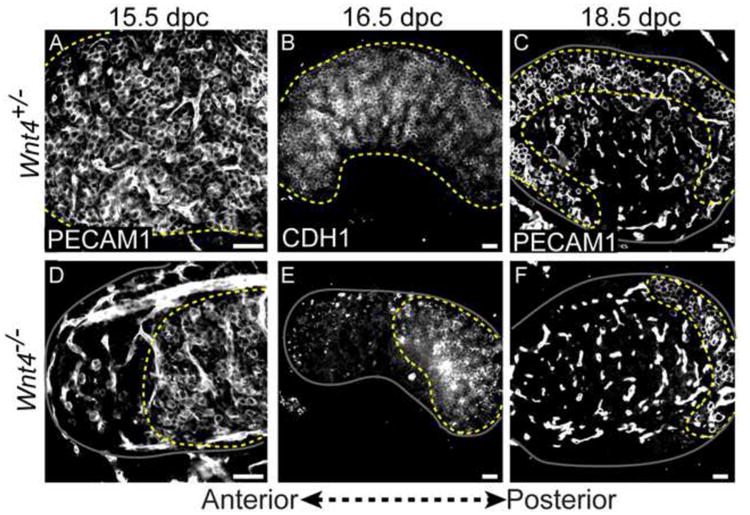
(A-C) Germ cells in Wnt4+/- ovaries were distributed throughout the ovary at 15.5-16.5 (A,B) and restricted to the ovarian cortex at 18.5 dpc (C). (D-F) In Wnt4-/- ovaries, germ cell loss was apparent at the anterior end of gonad at 15.5 dpc (D) and progressed posteriorly across the gonad at 16.5 dpc (E). Only a small population remained clustered at the posterior pole of the ovary at 18.5 dpc (F). Germ cells were labeled with two different markers: PECAM1 (A,C,D,F) or CDH1 (B,E) (PECAM1 labels both germ cells and endothelial cells). The boundary of the ovary is denoted by a solid gray line, and a dotted yellow line marks the region occupied by germ cells. Panels (A) and (D) show the anterior end of the gonad, and panels (E) and (F) are oriented with the anterior end on the left. Whole mount immunostaining was performed on samples in A, B, D and E. Cryosectioning followed by immunostaining was performed for samples in C and F. Scale bars represent 50 μm in all panels.
Because the decision to enter meiosis versus mitotic arrest depends on the somatic environment, we investigated whether a subset of germ cells in XX Wnt4 mutants (exhibiting partial sex reversal) differentiated as male germ cells and entered mitotic arrest. However, we found no evidence of germ cell sex reversal. Co-labeling of germ cells with PECAM1 and SCP3 did not reveal a population of SCP3-negative germ cells, and DNMT3L, a marker of mitotically arrested male germ cells, was not expressed in Wnt4-null ovaries (Fig. S1). These findings uncovered an interesting pattern of germ cell loss that leaves a small cohort of surviving meiotic germ cells at the posterior end of the ovary at birth.
Pregranulosa cell quiescence is disrupted specifically in agametic regions of Wnt4-null ovaries
Contrary to the Sertoli cell lineage, which proliferates throughout fetal development, the pregranulosa cell lineage is mitotically arrested and remains so until after birth when primordial follicles are selected for maturation and granulosa cells differentiate and resume proliferation (Hirshfield, 1992; Mork et al., 2012). We previously showed that mitotically arrested pregranulosa cells express an inhibitor of the cell cycle, p27 (Cdkn1b), at all fetal stages (Hirshfield, 1992; Mork et al., 2012). Loss of Wnt4 had no effect on the initial entry of pregranulosa cells into mitotic arrest; however, coincident with the loss of germ cells beginning at 15.5 dpc, we observed downregulation of p27 in a small population of FOXL2-expressing pregranulosa cells at the anterior end of the ovary (Fig. 2A, B). Similar to the wave of germ cell loss, loss of p27 expression progressed along the anterior-posterior axis (Fig. 2C, D). Near birth, only pregranulosa cells residing adjacent to surviving meiotic germ cells retained expression of the mitotic arrest marker.
Figure 2. Pregranulosa cells lose p27 expression and precociously upregulate AMH in Wnt4-null ovaries near birth.
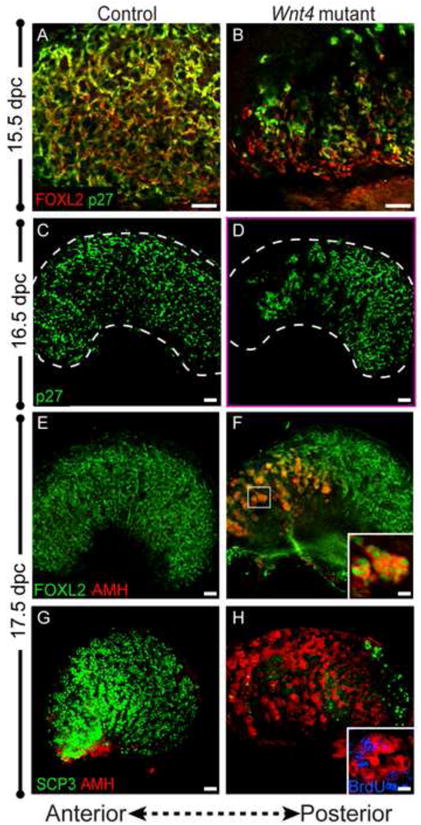
(A,B) At 15.5 dpc, FOXL2 (red) and p27 (green) are co-expressed in pregranulosa cells of control ovaries (A). In Wnt4 mutants, a small group of pregranulosa cells at the anterior of the ovary downregulate p27 (B) (Panels A and B show the anterior end of the ovary). (C,D) By 16.5, the population of pregranulosa cells that downregulated p27 (green) has expanded in an anterior-posterior direction, and continues to do so until birth (data not shown). The border of the ovary is marked by a white dotted line. (E,F) By 17.5 dpc, FOXL2-expressing pregranulosa cells (green) ectopically express AMH (red) in the anterior end of the ovary (see high magnification of boxed region, F, inset). (G,H) AMH-expressing cells (red) are found throughout the ovary near birth, but are excluded from the posterior end of the ovary occupied by meiotic germ cells (SCP3, green). These AMH-positive cells are actively proliferating and are labeled by BrdU (inset in H; blue). Whole mount immunostaining was performed for all samples. Scale bars represent 50 μm in main panels, and 5.5 μm and 2.75 μm in insets in F and H, respectively.
It was previously reported that near birth, Wnt4-null ovaries begin showing evidence of sex reversal, expressing markers of Sertoli cells such as AMH and showing morphological structures reminiscent of testis cords (Vainio et al., 1999). To localize cells that might be sex-reversing with respect to the anterior-posterior disruption of the ovarian program, we co-labeled 17.5 dpc ovaries with FOXL2 and AMH. As previously observed, AMH was ectopically expressed in Wnt4 mutant ovaries, but surprisingly, was restricted to the anterior half of the ovary which had undergone a concomitant loss of both germ cells and somatic expression of p27 (Fig. 2E, F). AMH-expressing cells were not observed at the posterior end of the ovary, where surviving meiotic germ cells resided (Fig. 2G, H).
In vitro and in vivo experiments showed that exposure of fetal ovaries to AMH leads to a loss of germ cells (Vigier et al., 1987; Behringer et al., 1990) followed by transdifferentiation of granulosa cells into Sertoli-like cells at postnatal stages (Behringer et al., 1990). To determine whether the wave of germ cell death was caused by the ectopic expression of AMH, we examined the onset of AMH expression. In 15.5 dpc Wnt4-/- ovaries, when germ cells are first lost from the anterior end of the gonad, we detected no AMH expression (Fig. S1C-D) excluding AMH as the cause of germ cell death.
The appearance of AMH/FOXL2 double-positive cells overlapped the domain where the mitotic arrest marker, p27, was lost. This suggested that the AMH/FOXL2 double-positive cells may resume proliferation. To test this, pregnant females were injected with the synthetic thymidine analog BrdU several hours before embryo collection to identify cells undergoing active DNA replication. Co-labeling for BrdU and AMH revealed BrdU/AMH double-positive cells, confirming that proliferation had resumed in a subset of pregranulosa cells (Fig. 2H, inset). Curiously, the AMH-expressing cells continued expressing the pregranulosa cell marker FOXL2 (Fig. 2F, inset), challenging the prior assertion that AMH expression in the perinatal Wnt4-null ovary is an indicator of sex reversal (Vainio et al., 1999).
Wnt4-null pregranulosa cells resemble differentiating granulosa cells rather than Sertoli cells
AMH is expressed throughout the embryonic stages of testis development but is not expressed in Sertoli cells of adult testes (reviewed in Behringer et al., 1994). In females, AMH is not expressed during embryonic stages of ovarian development; however, after birth, AMH is expressed in differentiating granulosa cells of growing follicles from the primary to early antral stages (Ueno et al., 1989a; Ueno et al., 1989b). In Wnt4-null ovaries, pregranulosa cells continue to express FOXL2, suggesting that their female fate is maintained (Fig. 2F). The aberrant proliferation that occurs concomitantly with the expression of AMH could indicate that in the absence of Wnt4, pregranulosa cells are precociously differentiating as granulosa cells of growing follicles, rather than transdifferentiating to Sertoli-like cells. To test this possibility, we investigated additional markers of granulosa and Sertoli cells.
The transcription factor DMRT1 is transiently expressed in male and female gonads around the sex-determining stage, but is downregulated in females shortly thereafter and remains on in Sertoli cells (Raymond et al., 1999; Raymond et al., 2000). In the 18.5 dpc testis, DMRT1 and AMH were co-expressed in Sertoli cells; however, in Wnt4-null ovaries, AMH-expressing cells were negative for DMRT1 (Fig. 3A, B).
Figure 3. Cells co-expressing FOXL2 and AMH in XX Wnt4 mutants resemble activated granulosa cells, not Sertoli cells.
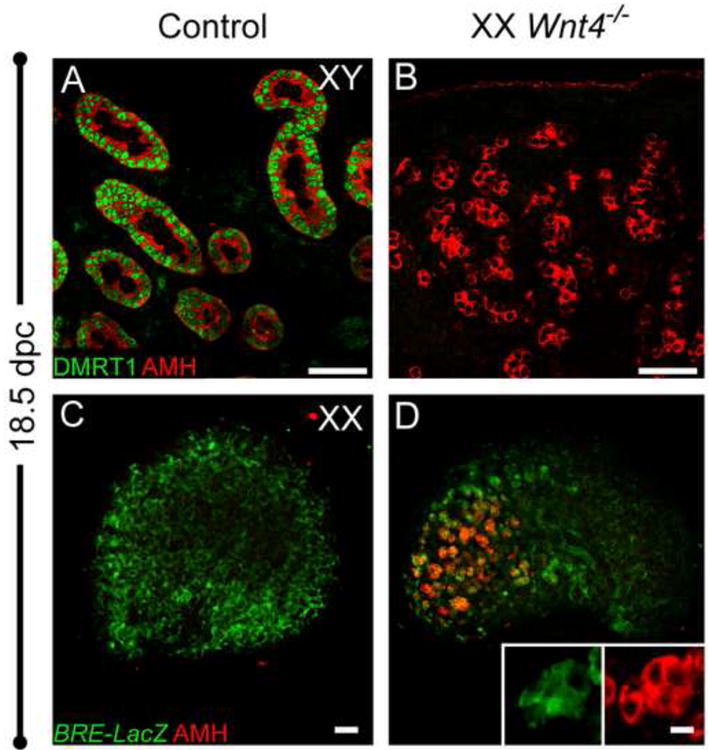
(A) At 18 5 dpc, Sertoli cells express DMRT1 (green) and AMH (red). DMRT1 expression was not detected in the AMH-expressing cells of Wnt4-/- ovaries (B). (C-D) cytoplasmic BRE-LacZ expression was detected by immunostaining for β-galactosidase (green). At 18.5 dpc, BRE-LacZ localized to somatic cells, including pregranulosa cells, of control ovaries (C) and is expressed in AMH-positive cells in Wnt4 mutants (D and Fig. S3). Whole mount immunostaining was performed for all samples. Scale bars represent 50 μm in main panels and 14 μm in insets.
Bmp signaling plays an important role during multiple stages of ovarian development (reviewed in Knight et al., 2006). In the postnatal ovary, Bmp15 in germ cells and Bmp7 in theca cells regulate follicle progression. Earlier in development, around the stage of sex determination, Bmp2 is specifically expressed immediately under the coelomic surface of the ovary, and its expression is dependent upon Wnt4 (Yao et al., 2004). Consistent with the expression of Bmp2 in the early gonad, we found that a LacZ reporter for SMAD1/5/8-dependent BMP signaling (BRE-LacZ; (Blank et al., 2008) was expressed in somatic cells of the ovary, including FOXL2-positive pregranulosa cells, and was restricted to granulosa cells of all follicle stages after birth (Fig. S2 and S3). In the absence of Wnt4, at 18.5 dpc we detected BRE-LacZ expression in germ cell-depleted regions at the anterior end of the ovary, specifically in AMH-expressing cells (Fig. 3D).
Based on these additional markers, Wnt4-null pregranulosa cells undergo a transformation that resembles the transition from quiescent to differentiating granulosa cells in postnatal growing follicles. This precocious differentiation involves escape from mitotic arrest, resumption of proliferation, and ectopic expression of AMH. Considering the continued expression of granulosa cell markers, FOXL2 and BRE-LacZ, the lack of expression of the Sertoli marker DMRT1, and the rare occurrence of SOX9-expressing cells (discussed below), our results suggest that the loss of Wnt4 leads to the precocious differentiation of pregranulosa cells.
Germ cell death is not a prerequisite for precocious differentiation of pregranulosa cells in Wnt4 mutants
In Wnt4-null mutants, Wnt4 is never expressed, yet female somatic cells initially enter mitotic arrest and activate Foxl2, similar to wild type XX gonads. This suggests that Wnt4 is not the primary or sole regulator of pregranulosa cell mitotic arrest. Near birth, we found that pregranulosa cells at the anterior end of the Wnt4-null ovary, which lacks germ cells, resemble differentiating granulosa cells, while those in the posterior end, adjacent to surviving meiotic germ cells, remain undifferentiated. The spatial correlation between regions of germ cell loss and disruption of pregranulosa cells quiescence led us to investigate whether the presence or absence of germ cells influenced the pregranulosa cell cycle state. To test this hypothesis, we produced Wnt4 mutants in which germ cell death was rescued or accelerated.
Germ cell survival is regulated by a balance between Bax (pro-apoptotic) and Bcl-x (anti-apoptotic) activity (Rucker et al., 2000). Homozygous deletion of Bax results in an increased number of germ cells in wild type gonads and rescues germ cell death when germ cells migrate to ectopic locations (Stallock et al., 2003) or in mutants where germ cell survival is compromised (Suzuki et al., 2008; Cook et al., 2009). To rescue germ cells in the Wnt4 mutant, we generated Wnt4-/-; Bax-/- double mutant embryos. In Wnt4-/-; Bax-/- double mutant gonads, germ cells were found throughout the ovary and were no longer limited to the posterior end, as in single Wnt4-/- mutants (Fig. 4). However, pregranulosa cells at the anterior end of the double mutant ovary still ectopically expressed AMH, similar to Wnt4-/- single mutants (Fig. 4C-D). Therefore, the presence of germ cells did not prevent aberrant differentiation of pregranulosa cells, suggesting that the loss of germ cells is not responsible for the precocious differentiation phenotype in Wnt4 mutants.
Figure 4. Meiotic germ cells are required for precocious pregranulosa cell differentiation.
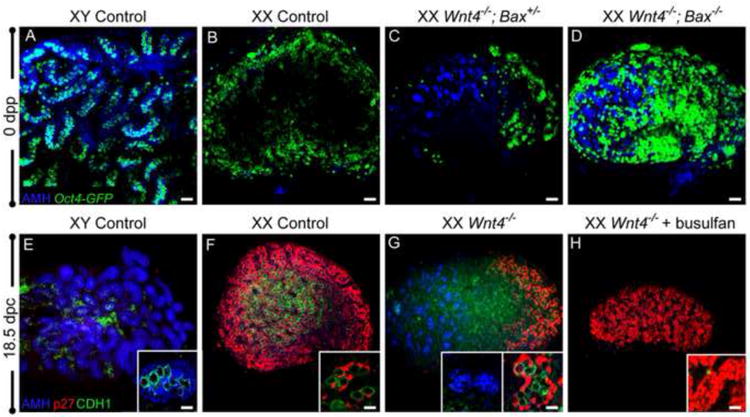
(A-D) At birth (0 days post-partum, dpp), gonads were immunostained for AMH (blue), and germ cells were visualized with Oct4-Gfp (green) to determine whether rescue of germ cells in agametic ovarian regions blocked AMH activation. AMH is expressed in Sertoli cells (A) and not in control ovaries (B). XX Wnt4-/-; Bax+/- mutants phenocopied XX Wnt4-/- mutants, with ectopic activation of AMH in agametic regions (C). In double mutant Wnt4-/-; Bax-/- ovaries, rescued germ cells were present throughout the ovary, but did not prevent ectopic activation of AMH at the anterior end of the ovary (D). (E-H) To determine whether the absence of germ cells blocked or exacerbated ectopic AMH expression, germ cells were depleted by injection of busulfan at 11.5 dpc. As expected, male and female control gonads expressed AMH (blue) and p27 (red), respectively (E,F). Wnt4-/- ovaries had ectopic AMH at the anterior end and p27 expression at the posterior end, surrounding CDH1-positive germ cells (green) (G). Following busulfan treatment, XX Wnt4-/- ovaries did not ectopically activate AMH, and all pregranulosa cells, at both the anterior and posterior ends, maintained expression of p27 (H). Whole mount immunostaining was performed for all samples. For the samples in (A) and (E), a small piece of testis was used as a control for whole mount antibody immunostaining. Scale bars represent 50 μm in main panels and 10 μm in all insets.
To examine the consequence of ablating all germ cells from Wnt4-null ovaries, we exposed developing embryos to the chemotherapeutic drug busulfan, previously used to deplete germ cells from mid-gestation embryos (Merchant, 1975; Maatouk et al., 2012). Pregnant dams were treated with busulfan at 11.5 dpc to deplete germ cells shortly after they enter the gonads. Although germ cells were absent in busulfan-treated gonads, based on staining with the germ cell marker CDH1 at 18.5 dpc (Fig. 4E-H), mitotic arrest of granulosa cells was not disrupted in control or Wnt4-mutant ovaries (data not shown and Fig. 6G). Furthermore, pregranulosa cells in Wnt4-null ovaries lacking germ cells did not activate AMH (Fig. 4E-H). The data suggests that the aberrant differentiation of pregranulosa cells in the Wnt4 mutant depends upon prior contact with germ cells; in the absence of pre-meiotic germ cells, pregranulosa cells remain in their quiescent state.
Figure 6. Pregranulosa cell activation precedes transdifferentiation in 17.5 dpc Rspo1-null ovaries near birth.
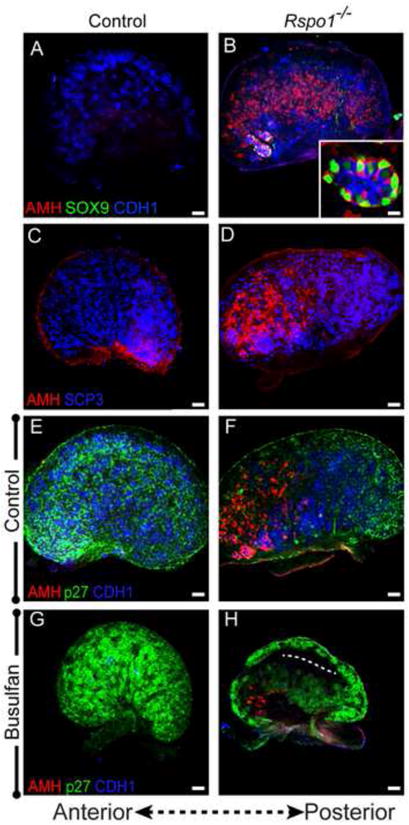
(A,B) AMH-expressing cells (red) are found in excess of SOX9-expressing cells (green) in 17.5 dpc Rspo1-/- mutant ovaries. (C,D) Immunolabeling of germ cells with SCP3 (blue) shows that XX Rspo1-/- mutants have many meiotic germ cells at 17.5 dpc. Contrary to wild type ovaries, Rspo1-/- ovaries show anteriorly restricted AMH expression (red). (E,F) p27 expression (green) is downregulated in the anterior end of Rspo1-/- ovaries, coinciding with regions of AMH upregulation (red). (G,H) Following busulfan treatment at 11.5 dpc, 17.5 dpc Rspo1-/-ovaries have fewer AMH-expressing cells. The dotted line in (H) marks the location of the ectopic vasculature. Samples were whole mount immunostained and oriented with the anterior end to the left. Scale bars represent 50 μm in main panels and 11.5 μm in the inset (D).
Pregranulosa cells lacking Wnt4 transdifferentiate toward a Sertoli cell fate after passing through a transient differentiated granulosa cell state
There has been no case of complete female-to-male sex reversal upon mutation of any female gene at the sex-determining stage and the only mutations that disrupt initial female sex determination include members of the canonical Wnt signaling pathway. These mutations do not initially lead to de-repression of SOX9 in female supporting cells; however, near birth in Rspo1-/- single mutants as well as Foxl2-/-; Wnt4-/- double mutant ovaries, SOX9 expression is detected (Ottolenghi et al., 2007; Chassot et al., 2008b).
In contrast to wild type ovaries, SOX9-expressing cells were detected at perinatal stages in Wnt4 mutants (Fig. 5). SOX9-expressing cells were variable in number and individual SOX9-expressing cells were often identified in close proximity to FOXL2-expressing cells (Fig. 5B-C). While most ovaries had only a few SOX9-expressing cells, some exhibited larger areas of SOX9 expression. Occasional FOXL2/SOX9 double-positive cells were detected (Fig. 5C), likely representing a transitional state and suggesting that SOX9-positive cells were derived from the FOXL2-expressing pregranulosa cells, consistent with other reports of transdifferentiation between the granulosa and Sertoli fates (Uhlenhaut et al., 2009; Matson et al., 2011). Although SOX9-positive cells were present, AMH-expressing cells were always found in excess of cells expressing SOX9 (Fig. 5D-F). These results suggest that transdifferentiation of the pregranulosa cell lineage occurs only after the cells transition through a differentiated granulosa cell state;.
Figure 5. Transdifferentiation from a pregranulosa to Sertoli cell fate occurs sporadically in Wnt4-null ovaries.
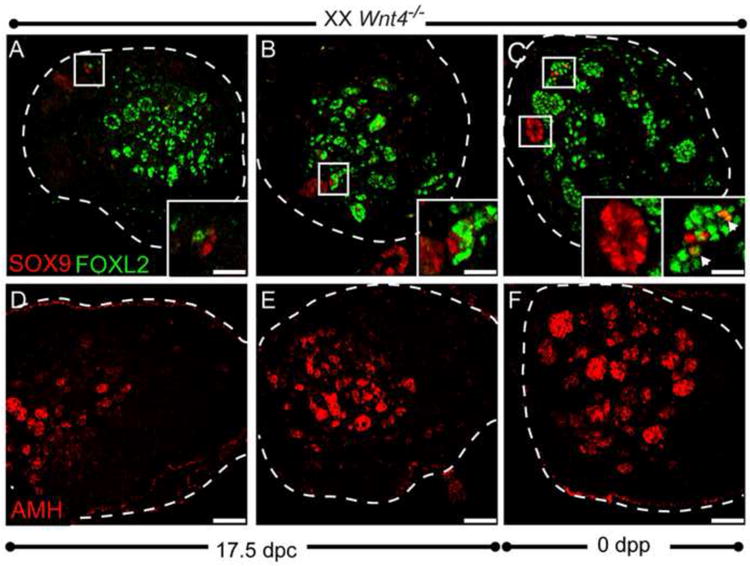
(A-C) Representative perinatal ovaries (17.5 dpc or 0 dpp) were co-immunolabeled for SOX9 (red) and FOXL2 (green). SOX9 was detected in a variable number of cells near birth in Wnt4 mutants (A-C). Insets in (A) and (B) show individual SOX9-expressing cells adjacent to FOXL2-expressing cells. The left inset in (C) shows a cluster of SOX-expressing cells that no longer express FOXL2, while the right inset shows a cluster of FOXL2-expressing cells with a few cells co-expressing SOX9 and FOXL2 (arrowheads). (D-E) Immunolabeling of representative samples with AMH shows that Wnt4 mutants have more AMH-expressing cells than SOX9. Samples in B and C carried the KitWv/+ mutation, which when heterozygous, reduces germ cell numbers, but does not alter the phenotype of Wnt4 mutant ovaries (compare A to B). All samples were cryosectioned (18 μm), followed by immunostaining. The edge of the ovary is marked by a white dotted line. Scale bars represent 50 μm in main panels and 16.5 μm in all insets
Gonads lacking R-spondin1 resemble Wnt4 mutants near birth
The phenotype of Rspo1 mutants is similar to Wnt4 mutants (Chassot et al., 2008b), therefore, we investigated whether the phenotype of Rspo1 mutants follows a similar pattern of pregranulosa cell activation prior to transdifferentiation that is also dependent upon the presence of germ cells. Co-immunolabeling with AMH and SOX9 revealed some AMH/SOX9 double-positive somatic cells arranged into cord structures in Rspo1-/-ovaries, as previously described (Fig. 6A-B and (Chassot et al., 2008b). However, we observed a far greater number of AMH-expressing cells throughout the Rspo1-/- ovaries, similar to the Wnt4 mutant. In postnatal Rspo1 mutants (14 dpp), the phenotype progressed such that few primordial follicles or FOXL2-positive clusters were present and SOX9/AMH-expressing cells occupied the bulk of the ovary (Fig. S4).
Consistent with previous observations, 17.5 dpc Rspo1-/- ovaries had much higher numbers of germ cells compared to Wnt4-/- ovaries, and germ cells were distributed throughout the ovary (i.e., not posteriorly restricted as in Wnt4-null ovaries) (Fig. 6C-D). Nonetheless, AMH was expressed in the ovary with an anterior bias, similar to Wnt4-/-ovaries (Fig. 6B, D, F). The increased number of germ cells in Rspo1-/- ovaries mimics the situation created by Bax-mediated germ cell rescue of the Wnt4 mutant (Fig. 4), further supporting our conclusion that the loss of germ cells is not a prerequisite for aberrant differentiation of pregranulosa cells in either the Wnt4 or Rspo1 mutants.
Depletion of germ cells from Wnt4-null ovaries blocked aberrant differentiation of pregranulosa cells (Fig. 4). To determine whether pregranulosa cells lacking Rspo1 behaved similarly, we treated Rspo1 mice with busulfan and examined pregranulosa cells for AMH expression. Immunostaining for CDH1 revealed a clear reduction in germ cells between control and busulfan-treated samples (Fig. 6E-H). Following busulfan treatment, fewer AMH-positive cells were observed in Rspo1-/- ovaries compared to busulfan-treated controls (Fig. 6G, H), consistent with Wnt4 mutants. These results suggest that, similar to the Wnt4 mutant, Rspo1 deficient pregranulosa cells resemble differentiating granulosa cells of the postnatal ovary prior to exhibiting signs of transdifferentiation and this transition is dependent on the presence of germ cells.
Discussion
Beginning with the generation of the Wnt4 mutation over 10 years ago (Vainio et al., 1999), canonical Wnt signaling has emerged as the predominant pathway controlling ovarian development (Chassot et al., 2008a; Tevosian et al., 2008; Liu et al., 2010a). While loss of several sex-determining genes in the XY gonad, including Sry, Sox9, or Fgf9, leads to male-to-female sex reversal, no single gene mutation leads to primary female-to-male sex reversal in the XX gonad (reviewed in Eggers et al., 2012). Wnt4-null embryos undergo partial sex reversal near the sex-determining stage (Vainio et al., 1999), but evidence of Sertoli differentiation (e.g., SOX9 expression) only occurs in sporadic cells near birth (Fig. 5). It is possible that expression of SOX9 requires an additional activating signal (such as SRY) not present in XX gonads at early stages. However, the fact that SOX9 is activated near birth in Wnt4 and Rspo1-null ovaries argues against this explanation. Alternatively, additional mechanisms may function independently of Wnt signaling to repress expression of SOX9 in XX supporting cells. One possibility is that cells are only competent to activate SOX9 when proliferative; cell fate determination and cell cycle regulation may be interdependent in bipotential supporting cells.
Loss of Wnt4 disrupts ovarian development asymmetrically along the anterior-posterior axis
The expression of Wnt4, by in situ hybridization or expression of a Wnt4-GFP reporter, shows no anterior-posterior bias (Vainio et al., 1999; Kim et al., 2006b; Maatouk et al., 2012). Therefore, the progressive disruption of pregranulosa cell quiescence and germ cell survival along the anterior-posterior axis in XX Wnt4 mutants was a surprising finding.
It is well established that entry of germ cells into meiosis occurs in an anterior-posterior wave (Menke et al., 2003; Yao et al., 2003; Bullejos et al., 2004), possibly due to the delivery of the meiosis-inducing factor through the mesonephric tubules that attach to the anterior ovary (Bowles et al., 2006; Kumar et al., 2011). However, this factor is unaffected by loss of Wnt signaling, as evidenced by the widespread expression of SCP3 observed in Wnt4 mutants (Fig. S1), Rspo1 mutants (Fig. 6B) and in p-catenin conditional mutants (Liu et al., 2010b).
A few cases of somatic cell-specific genes expressed in a wave across the ovary also have been reported. Sprr2d is transiently expressed in both sexes and is downregulated from anterior-to-posterior in females (Hyunjoo et al., 2009). Additionally, adamts19 expression is initiated in an anterior-to-posterior direction (Menke et al., 2002) and was found to be downregulated in XX Wnt4 mutants (Naillat et al., 2010). These observations suggest that regional differences exist in a gradient between the anterior and posterior ends of the ovary. Although Wnt4 may disrupt pathways that promote ovarian differentiation specifically at the anterior end of the ovary, it is likely that over time the posterior end of the ovary would be progressively affected. Because of neonatal lethality, we could not analyze Wnt4 mutants at later stages to make this determination; however, this appears to be the case in Rspo1 mutants (Fig. S4).
Wnt4 is not required to initiate pregranulosa cell quiescence
Wnt4 transcripts are detected at 10.5 dpc (Nef et al., 2005), and are upregulated in pregranulosa cells by 12.5 dpc (Bouma et al., 2010; Jameson et al., 2012b). In the absence of Wnt4, the appearance of aberrantly differentiating pregranulosa cells is delayed with respect to the timing of Wnt4 upregulation in XX gonads. If Wnt4 was a direct regulator of pregranulosa cell mitotic arrest, an early disruption of mitotic arrest would be expected. The finding that pregranulosa cells prior to 15.5 dpc maintained expression of p27, as did a small population of cells at the posterior end of the ovary near birth, suggests that Wnt4 is not directly responsible for initiating or maintaining pregranulosa cell quiescence. Failure to maintain mitotic arrest is likely a secondary consequence of other changes occurring in response to loss of Wnt4 and, based on our data, may be related to disrupted interactions between germ cells and pregranulosa cells around the time of meiotic entry.
Meiotic germ cells may influence cell cycle regulation in pregranulosa cells
The observation that germ cell loss in Wnt4 mutants progresses in an anterior-posterior direction, in a manner resembling the progression of germ cell entry into meiosis, and the finding that mitotically-arrested pregranulosa cells were found in close proximity to surviving meiotic germ cells at the posterior end of the ovary, led us to question whether meiotic germ cells maintain mitotic arrest of pregranulosa cells.
In the XX Wnt4 mutant, pockets of precociously differentiating pregranulosa cells were found in agametic regions at the anterior end of the ovary and near birth a subset of these cells began to transdifferentiate towards a Sertoli fate. Several previous studies have reported granulosa cell transdifferentiation associated with a prior loss of germ cells. Germ cell loss precedes transdifferentiation in the ERαβKO and ArKO mouse models (Couse et al., 1999; Dupont et al., 2000; Britt et al., 2002), as well as in vivo and in vitro models of AMH overexpression (Vigier et al., 1987; Behringer et al., 1990), all of which undergo postnatal transdifferentiation. In γ-irradiated postnatal rat ovaries, primordial follicles lost germ cells and pregranulosa cells continued to differentiate; however, once granulosa cells began dividing and expressed AMH, transdifferentiation occurred and morphological characteristics of testis cords were observed (Guigon et al., 2005). The chronology of these transitions resembles the phenotype in the Wnt4 mutant, but not all cases of transdifferentiation depend on a loss of germ cells. For example, in Foxl2 mutants, granulosa cells begin expressing SOX9 one week after birth in the presence of germ cells (Ottolenghi et al., 2005; Uhlenhaut et al., 2009).
At 13.5 dpc, germ cells initiate meiosis and progress through prophase I before arresting at diplotene around birth (McLaren, 1984). Shortly after meiotic entry, germ cell numbers are reduced in Wnt4-null ovaries and, to a lesser extent, Rspo1-null ovaries. Using the Bax mutation to block germ cell death in Wnt4 mutants, we found that pregranulosa cells became activated even in the presence of near-normal numbers of germ cells, suggesting that the absence of meiotic germ cells was not the cause of the phenotype. However, when pre-meiotic germ cells were depleted by busulfan, aberrant differentiation was blocked. While we cannot exclude the possibility that busulfan treatment disrupted pregranulosa cells in a manner that blocked their ability to resume proliferation, we favor the interpretation that the differentiation of XX germ cells around the stage of meiotic entry triggers a maturation step in pregranulosa cells, rendering them competent for activation and transdifferentiation. This concept has been proposed previously based on the effect of germ cell loss on ovarian development in various models that exhibit germ cell depletion (Guigon et al., 2006). It is also possible that progression through meiosis is disrupted in Wnt4 mutants even though SCP3 expression and localization appear unaffected (Fig. S1). Other components of the meiotic machinery may be affected, or the timing of meiotic progression may be altered. Alternatively, it is possible that prenatal mutant germ cells begin to express markers of genes normally restricted to postnatal oogonia, such as Gdf9 or Bmp15 (Knight et al., 2006) that could trigger pregranulosa cells to undergo activation. After birth, communication between germ cells and granulosa cells is critical for folliculogenesis (reviewed in Matzuk et al., 2002). Such an interaction has not been defined during fetal stages, but disrupted adhesion between germ cells and somatic cells in Wnt4 mutants has been observed (Naillat et al., 2010). Our results suggest that germ cell-somatic cell interactions at prenatal meiotic stages are critical to maintain both pregranulosa cell quiescence and germ cell survival.
Precociously differentiating pregranulosa cells are found specifically in Wnt4-and Rspo1-null mutants and not in conditional β-catenin mutants
While Wnt4, Rspo1 and β-catenin conditional mutants all undergo a similar partial sex reversal near the sex-determining stage and a similar loss of germ cells, discrepancies exist among these mutants near birth. Contrary to Wnt4-null and Rspo1-null ovaries, loss of β-catenin in gonadal somatic cells causes a loss of FOXL2 expression by 13.5 dpc (Manuylov et al., 2008). Yet, even in the absence of FOXL2, pregranulosa cells maintain expression of p27 (Fig. S5). Furthermore, conditional loss of β-catenin in somatic cells did not lead to precocious differentiation of pregranulosa cells or perinatal sex reversal; β-catenin mutants lack both SOX9 and AMH expression near birth (Fig. S5 and Manuylov et al., 2008).
The lack of a similar perinatal phenotype in β-catenin conditional mutants is curious. In addition to somatic cells, β-catenin signaling is active in germ cells of wild type ovaries as evidenced by the expression of its downstream target, Axin2, by localization of an Axin2-LacZ reporter (Chassot et al., 2011) and by microarray (Jameson et al., 2012b). While the Wnt4- and Rspo1-null alleles disrupt canonical Wnt signaling in all cells of the gonad, the Sf1-Cre-driven conditional β-catenin mutation is limited to somatic cells and does not disrupt canonical Wnt signaling in germ cells. It is possible that active β-catenin signaling in germ cells of the β-catenin conditional mutant may maintain normal germ cell-somatic cell interactions, preventing precocious activation of pregranulosa cells.
Transdifferentiation of Wnt4-null pregranulosa cells towards a Sertoli fate occurs after disruption of mitotic arrest
In the bipotential gonad, components of the male and female pathways antagonize one another; Sox9 and Fgf9 in males versus Wnt4, Rspo1 and β-catenin in females (reviewed in Kim et al., 2006a). In the testis, it is clear that Wnt-signaling antagonizes SOX9 expression. Stabilization of β-catenin in XY gonadal somatic cells leads to downregulation of SOX9 (Maatouk et al., 2008). Additionally, while XY Fgf9 mutants are completely sex reversed (Colvin et al., 2001; Kim et al., 2006b), XY gonads that are null for both Fgf9 and Wnt4 develop as males (Jameson et al., 2012a). This complete rescue of sex reversal suggests that the primary function of Fgf9 is to repress Wnt4 in XY gonads, and in the absence of Fgf9, derepression of Wnt4 leads to the downregulation of SOX9. However, loss of canonical Wnt signaling is not sufficient for SOX9 upregulation in early XX gonads.
The disparate effects of Wnt4 on Sox9 expression in the testis versus the ovary may relate to different cofactors or downstream transcriptional regulators in XX versus XY gonads. Another possibility is that supporting cells are not competent to activate SOX9 unless they are actively dividing. In support of this hypothesis, transdifferentiation near birth occurred in regions of the Wnt4- and Rspo1-null ovaries where pregranulosa cells exited mitotic arrest and resumed cycling. Additionally, most other cases of granulosa cell transdifferentiation occur postnatally when granulosa cells normally resume proliferation (Vigier et al., 1987; Behringer et al., 1990; Couse et al., 1999; Dupont et al., 2000; Britt et al., 2002; Ottolenghi et al., 2005; Uhlenhaut et al., 2009). Indeed, a detailed analysis of transdifferentiation in γ-irradiated rat ovaries showed that transdifferentiation was restricted to granulosa cells in growing follicles, and did not occur in mitotically arrested primordial follicles (Guigon et al., 2005).
We suggest that XX supporting cells may be incapable of upregulating SOX9 while mitotically arrested, even in the absence of its repressor, Wnt4. Mitotic arrest may contribute to the specification and maintenance of female supporting cell fate during primary sex determination. Although disruption of p27 expression leads to premature activation of pregranulosa cells in primordial follicles and increased granulosa cell proliferation after birth (Rajareddy et al., 2007), there is no evidence of sex reversal at prenatal or postnatal stages. Therefore, disruption of mitotic arrest alone is not likely to cause sex reversal. However, we predict that disruption of the mitotic arrest program in conjunction with mutations that disrupt Wnt4, or other genes responsible for maintenance of the pregranulosa cell fate, may result in a primary female-to-male sex reversal at the sex-determining stage.
Our results suggest that female sex determination is not solely dependent on Wnt signaling. Instead, multiple independent pathways function in the early XX gonad to maintain pregranulosa cell fate. Preservation of mitotic arrest and later signals from meiotic germ cells may cooperate to protect pregranulosa cells from precocious differentiation and sex reversal. This may explain why cases of female-to-male sex reversal have not been observed at the primary sex-determining stage.
Supplementary Material
Figure S1. Germ cells in Wnt4-null ovaries express the meiotic marker SCP3. (A-D) At 15.5 dpc, germ cells in control (A) and Wnt4-/- (B) ovaries are co-labeled for PECAM1 (green) and SCP3 (blue). SCP3-positive germ cells are distributed throughout the gonad in both controls (C) and mutants (D). The absence of germ cells along the coelomic surface is due to the presence of the ectopic vasculature in Wnt4 mutants (position of coelomic vasculature is indicated by a white line, inset in D). Ectopic AMH (red), which is expressed after 16.5 dpc in Wnt4 mutants (Figure 2), is not detected at 15.5 dpc and therefore is not the cause of germ cell loss that begins at the anterior end of Wnt4 mutants at this stage. (E,F) At 18.5 dpc, surviving germ cells in Wnt4 mutants maintain expression of the germ cell markers PECAM1 (red) and SCP3 (blue), similar to controls (Syto13, shown in green, marks nuclei). The pattern of SCP3 on chromosomes of single cells appears to be unaffected by the loss of Wnt4 (insets in E and F). (G, H) At 19.5 dpc, mitotically arrested germ cells express DNMT3L (blue) in XY control males. XX Wnt4 mutants do not express DNMT3L (PECAM1, green, faintly marks germ cells, brightly labels vasculature and autofluorescent cells in the testis). Panels in C and D are oriented with the anterior end of the gonad to the left. The scale bars represent 50 μm (A-D, G-H), 25 μm (E,F), 12 μm (insets in C,D) or 8 μm (insets in E,F).
Figure S2. Time course of BRE-lacZ expression in male and female fetal gonads and prepubertal ovaries as determined by X-gal staining. Strong reporter activity was observed in the mesonephric ducts of both sexes throughout fetal development (arrow in top left panel). (A,B) At 11.75 dpc, X-gal activity was detected in the gonads of both sexes. (C, D) By 12.5 dpc, Xgal staining was stronger in the ovary than the testis. This pattern continued until birth (E-J). Xgal staining in testes became restricted to the interior vasculature and coelomic vessel (arrowhead in J). The strong X-gal staining observed in P7 ovaries (K) became restricted to follicles by P21 (L). Testes were not examined for BRE reporter activity by X-gal activity at postnatal stages. Bright-field images were all taken at the same magnification.
Figure S3. The BRE-LacZ reporter, for active Bmp signaling, is expressed in ovarian somatic cells at prenatal and postnatal stages. (A-E) XX gonads were immunostained for β-galactosidase to visualize the BRE-LacZ reporter (BRE; green). Somatic cells were labeled with GATA4, which marks all gonadal somatic cells, or FOXL2, which labels the female supporting cell lineage (blue). Germ cells were labeled with PECAM1, which marks germ cells and endothelial cells, or CDH1, which is specific to germ cells (purple). At all stages examined, 11.75 dpc through 21 dpp, BRELacZ co-labeled with ovarian somatic markers, and was expressed in the supporting cell lineage (FOXL2-positive), as well as in other ovarian somatic cells (GATA4-positive, FOXL2-negative). Immunostaining was performed on whole mount samples in A-C, and on cryosectioned samples in D-E. Panels on the right (ex. A′) are high magnification images from the same samples on the left. (FH) XY gonads were immunostained for β-calactosidase (BRE;green) and AMH (blue). At E13.5 dpc (F) and E15.5 dpc (G) BRE was localized to interstitial cells and not expressed in Sertoli cells. In adult testes (H) BRE localized to germ cells and was not expressed in Sertoli cells. The inset shows a high magnification image; the arrow indicates a Sertoli cell. (I,J) XX control (I) and XX Wnt4-/- (J) gonads were immunostained for β-galactosidase (BRE; green) and FOXL2 (blue). A decrease in BRE-positive cells in XX Wnt4-/- gonads 13.5 dpc is consistent with the previous observation that Bmp2 expression is lost in the absence of Wnt4 (Yao, et al, 2004). The arrow in (J) points to autofluoresence from blood in the ectopic coelomic vasculature. Scale bars represent 50 μm in main panels and 25 μm in the inset (H).
Figure S4. Transdifferentiation progresses after birth in postnatal Rspo1-null ovaries. (A-C) Gonads were collected from 14 dpp pups and immunostained for AMH (red) and CDH1 (blue). At 14 dpp, AMH is not expressed in Sertoli cells (A) but is expressed growing follicles (B). In Rspo1-null ovaries, AMH is expressed in large tubule structures (C) and only a few surviving germ cells are present. (D-F) Immunostaining of 14 dpp control and Rspo1-null gonads with SOX9 (red), FOXL2 (green) and CDH1 (blue). As expected, XY control testes had SOX9-positive Sertoli cells (D) and XX control ovaries exhibited FOXL2 expression (E). (F) XX Rspo1-null ovaries had a few small clusters of FOXL2-positive cells, whereas the bulk of the ovary was occupied by large SOX9-positive tubule structures. Scale bars represent 50 μm.
Figure S5. Loss of β-catenin in gonadal supporting cells causes downregulation of FOXL2, but does not disrupt mitotic arrest. (A-C) Gonads were collected at 13.5 dpc and immunostained for FOXL2 (green) and p27 (red). (A) p27 is expressed in all FOXL2-expressing cells and in additional somatic cells just beneath the coelomic epithelium (the coelomic surface marked by green background staining). (B-C) Ovaries lacking β-catenin in Sf1-Cre expressing somatic cells (βcatfl/fl; Sf1-Cre) had a severe reduction in FOXL2-expressing cells, but p27 was unaffected. (D-F) 18.5 dpc ovaries were dissected from control ovaries (D, βcatfl/fl) and ovaries lacking β-catenin in Sf1-Cre expressing somatic cells (E-F, βcatfl/fl; Sf1-Cre). Samples were immunostained for FOXL2 (D,E; green), or AMH (F; red). A positive control (XY βcatfl/fl; Sf1-Cre) for AMH immunostaining is shown in the inset in (F). All samples were whole mount immunostained and carried the Rosa-tdTomato reporter (RTM; kindly provided by Fan Wang, Duke University) which indicates active Cre recombination (blue). A white dotted line outlines the ovary in D-F. Scale bars represent 50 μm in all main panels, and 60 μm in inset in (F).
Highlights.
Ovary development is progressively disrupted across the A-P axis in Wnt4 mutants.
Pregranulosa cells transdifferentiate to Sertoli cells in perinatal Wnt4 mutants.
Pregranulosa cells become proliferative prior to transdifferentiating.
Interactions with meiotic germ cells may trigger pregranulosa cell differentiation.
Mitotic arrest may prevent transdifferentiation at earlier developmental stages.
Acknowledgments
We thank members of the Capel lab for their helpful comments and suggestions and David Zarkower and Dr. Shoji Tajima for kindly sharing the DMRT1 antibody and DNMT3L antibodies, respectively. Funding was provided by the National Institutes of Health (5R01-HD039963-13 to B.C. and F32-HD055791 to D.M.M.) and by Agence Nationale pour la Recherche (ANR-09-GENM-009-03 GENIDOV to M.-C.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barrionuevo F, Bagheri-Fam S, Klattig J, Kist R, Taketo MM, Englert C, Scherer G. Homozygous inactivation of Sox9 causes complete XY sex reversal in mice. Biol Reprod. 2006;74:195–201. doi: 10.1095/biolreprod.105.045930. [DOI] [PubMed] [Google Scholar]
- Behringer RR, Cate RL, Froelick GJ, Palmiter RD, Brinster RL. Abnormal sexual development in transgenic mice chronically expressing mullerian inhibiting substance. Nature. 1990;345:167–170. doi: 10.1038/345167a0. [DOI] [PubMed] [Google Scholar]
- Behringer RR, Finegold MJ, Cate RL. Mullerian-inhibiting substance function during mammalian sexual development. Cell. 1994;79:415–425. doi: 10.1016/0092-8674(94)90251-8. [DOI] [PubMed] [Google Scholar]
- Bingham NC, Verma-Kurvari S, Parada LF, Parker KL. Development of a steroidogenic factor 1/Cre transgenic mouse line. Genesis. 2006;44:419–424. doi: 10.1002/dvg.20231. [DOI] [PubMed] [Google Scholar]
- Blank U, Seto ML, Adams DC, Wojchowski DM, Karolak MJ, Oxburgh L. An in vivo reporter of BMP signaling in organogenesis reveals targets in the developing kidney. BMC Dev Biol. 2008;8:86. doi: 10.1186/1471-213X-8-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouma GJ, Hudson QJ, Washburn LL, Eicher EM. New candidate genes identified for controlling mouse gonadal sex determination and the early stages of granulosa and Sertoli cell differentiation. Biol Reprod. 2010;82:380–389. doi: 10.1095/biolreprod.109.079822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, Yashiro K, Chawengsaksophak K, Wilson MJ, Rossant J, Hamada H, Koopman P. Retinoid signaling determines germ cell fate in mice. Science. 2006;312:596–600. doi: 10.1126/science.1125691. [DOI] [PubMed] [Google Scholar]
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- Britt KL, Kerr J, O'Donnell L, Jones ME, Drummond AE, Davis SR, Simpson ER, Findlay JK. Estrogen regulates development of the somatic cell phenotype in the eutherian ovary. Faseb J. 2002;16:1389–1397. doi: 10.1096/fj.01-0992com. [DOI] [PubMed] [Google Scholar]
- Bullejos M, Koopman P. Spatially dynamic expression of Sry in mouse genital ridges. Dev Dyn. 2001;221:201–205. doi: 10.1002/dvdy.1134. [DOI] [PubMed] [Google Scholar]
- Bullejos M, Koopman P. Germ cells enter meiosis in a rostro-caudal wave during development of the mouse ovary. Mol Reprod Dev. 2004;68:422–428. doi: 10.1002/mrd.20105. [DOI] [PubMed] [Google Scholar]
- Chaboissier MC, Kobayashi A, Vidal VI, Lutzkendorf S, van de Kant HJ, Wegner M, de Rooij DG, Behringer RR, Schedl A. Functional analysis of Sox8 and Sox9 during sex determination in the mouse. Development. 2004;131:1891–1901. doi: 10.1242/dev.01087. [DOI] [PubMed] [Google Scholar]
- Chassot AA, Gregoire EP, Lavery R, Taketo MM, de Rooij DG, Adams IR, Chaboissier MC. RSPO1/beta-catenin signaling pathway regulates oogonia differentiation and entry into meiosis in the mouse fetal ovary. PLoS One. 2011;6:e25641. doi: 10.1371/journal.pone.0025641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassot AA, Gregoire EP, Magliano M, Lavery R, Chaboissier MC. Genetics of ovarian differentiation: Rspo1, a major player. Sex Dev. 2008a;2:219–227. doi: 10.1159/000152038. [DOI] [PubMed] [Google Scholar]
- Chassot AA, Ranc F, Gregoire EP, Roepers-Gajadien HL, Taketo MM, Camerino G, de Rooij DG, Schedl A, Chaboissier MC. Activation of beta-catenin signaling by Rspo1 controls differentiation of the mammalian ovary. Hum Mol Genet. 2008b;17:1264–1277. doi: 10.1093/hmg/ddn016. [DOI] [PubMed] [Google Scholar]
- Colvin JS, Green RP, Schmahl J, Capel B, Ornitz DM. Male-to-female sex reversal in mice lacking fibroblast growth factor 9. Cell. 2001;104:875–889. doi: 10.1016/s0092-8674(01)00284-7. [DOI] [PubMed] [Google Scholar]
- Combes AN, Wilhelm D, Davidson T, Dejana E, Harley V, Sinclair A, Koopman P. Endothelial cell migration directs testis cord formation. Dev Biol. 2009;326:112–120. doi: 10.1016/j.ydbio.2008.10.040. [DOI] [PubMed] [Google Scholar]
- Cook MS, Coveney D, Batchvarov I, Nadeau JH, Capel B. BAX-mediated cell death affects early germ cell loss and incidence of testicular teratomas in Dnd1(Ter/Ter) mice. Dev Biol. 2009;328:377–383. doi: 10.1016/j.ydbio.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cool J, Carmona FD, Szucsik JC, Capel B. Peritubular myoid cells are not the migrating population required for testis cord formation in the XY gonad. Sex Dev. 2008;2:128–133. doi: 10.1159/000143430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couse JF, Hewitt SC, Bunch DO, Sar M, Walker VR, Davis BJ, Korach KS. Postnatal sex reversal of the ovaries in mice lacking estrogen receptors alpha and beta. Science. 1999;286:2328–2331. doi: 10.1126/science.286.5448.2328. [DOI] [PubMed] [Google Scholar]
- Coveney D, Cool J, Oliver T, Capel B. Four-dimensional analysis of vascularization during primary development of an organ, the gonad. Proc Natl Acad Sci U S A. 2008;105:7212–7217. doi: 10.1073/pnas.0707674105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- Eggers S, Sinclair A. Mammalian sex determination-insights from humans and mice. Chromosome Res. 2012;20:215–238. doi: 10.1007/s10577-012-9274-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JW, Dominguez-Steglich MA, Guioli S, Kowk G, Weller PA, Stevanovic M, Weissenbach J, Mansour S, Young ID, Goodfellow PN, Brook JD, Schafer AJ. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature. 1994;372:525–530. doi: 10.1038/372525a0. [DOI] [PubMed] [Google Scholar]
- Guigon CJ, Coudouel N, Mazaud-Guittot S, Forest MG, Magre S. Follicular cells acquire sertoli cell characteristics after oocyte loss. Endocrinology. 2005;146:2992–3004. doi: 10.1210/en.2005-0045. [DOI] [PubMed] [Google Scholar]
- Guigon CJ, Magre S. Contribution of germ cells to the differentiation and maturation of the ovary: insights from models of germ cell depletion. Biol Reprod. 2006;74:450–458. doi: 10.1095/biolreprod.105.047134. [DOI] [PubMed] [Google Scholar]
- Hacker A, Capel B, Goodfellow P, Lovell-Badge R. Expression of Sry, the mouse sex determining gene. Development. 1995;121:1603–1614. doi: 10.1242/dev.121.6.1603. [DOI] [PubMed] [Google Scholar]
- Hirshfield AN. Heterogeneity of cell populations that contribute to the formation of primordial follicles in rats. Biol-Reprod. 1992;47:466–472. doi: 10.1095/biolreprod47.3.466. issn: 0006-3363. [DOI] [PubMed] [Google Scholar]
- Hyunjoo JL, Dorothy EP, Ravi SK, Stephanie MC, Kenneth HA. Novel markers of early ovarian pre-granulosa cells are expressed in an Sry-like pattern. Developmental Dynamics. 2009;238:812–825. doi: 10.1002/dvdy.21902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson SA, Lin YT, Capel B. Testis development requires the repression of Wnt4 by Fgf signaling. Dev Biol. 2012a;370:24–32. doi: 10.1016/j.ydbio.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson SA, Natarajan A, Cool J, DeFalco T, Maatouk DM, Mork L, Munger SC, Capel B. Temporal transcriptional profiling of somatic and germ cells reveals biased lineage priming of sexual fate in the fetal mouse gonad. PLoS Genet. 2012b;8:e1002575. doi: 10.1371/journal.pgen.1002575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Capel B. Balancing the bipotential gonad between alternative organ fates: A new perspective on an old problem. Dev Dyn. 2006a;235:2292–2300. doi: 10.1002/dvdy.20894. [DOI] [PubMed] [Google Scholar]
- Kim Y, Kobayashi A, Sekido R, DiNapoli L, Brennan J, Chaboissier MC, Poulat F, Behringer RR, Lovell-Badge R, Capel B. Fgf9 and Wnt4 act as antagonistic signals to regulate mammalian sex determination. PLoS Biol. 2006b;4:e187. doi: 10.1371/journal.pbio.0040187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight PG, Glister C. TGF-beta superfamily members and ovarian follicle development. Reproduction. 2006;132:191–206. doi: 10.1530/rep.1.01074. [DOI] [PubMed] [Google Scholar]
- Knudson CM, Tung KS, Tourtellotte WG, Brown GA, Korsmeyer SJ. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science. 1995;270:96–99. doi: 10.1126/science.270.5233.96. [DOI] [PubMed] [Google Scholar]
- Kumar S, Chatzi C, Brade T, Cunningham TJ, Zhao X, Duester G. Sex-specific timing of meiotic initiation is regulated by Cyp26b1 independent of retinoic acid signalling. Nat Commun. 2011;2:151. doi: 10.1038/ncomms1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little CC, Cloudman AM. The Occurrence of a Dominant Spotting Mutation in the House Mouse. Proc Natl Acad Sci U S A. 1937;23:535–537. doi: 10.1073/pnas.23.10.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CF, Bingham N, Parker K, Yao HH. Sex-specific roles of beta-catenin in mouse gonadal development. Hum Mol Genet. 2009;18:405–417. doi: 10.1093/hmg/ddn362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CF, Liu C, Yao HH. Building pathways for ovary organogenesis in the mouse embryo. Curr Top Dev Biol. 2010a;90:263–290. doi: 10.1016/S0070-2153(10)90007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CF, Parker K, Yao HH. WNT4/beta-catenin pathway maintains female germ cell survival by inhibiting activin betaB in the mouse fetal ovary. PLoS One. 2010b;5:e10382. doi: 10.1371/journal.pone.0010382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maatouk DM, DiNapoli L, Alvers A, Parker KL, Taketo MM, Capel B. Stabilization of beta-catenin in XY gonads causes male-to-female sex-reversal. Hum Mol Genet. 2008;17:2949–2955. doi: 10.1093/hmg/ddn193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maatouk DM, Mork L, Hinson A, Kobayashi A, McMahon AP, Capel B. Germ cells are not required to establish the female pathway in mouse fetal gonads. PLoS One. 2012;7:e47238. doi: 10.1371/journal.pone.0047238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuylov NL, Smagulova FO, Leach L, Tevosian SG. Ovarian development in mice requires the GATA4-FOG2 transcription complex. Development. 2008;135:3731–3743. doi: 10.1242/dev.024653. [DOI] [PubMed] [Google Scholar]
- Martineau J, Nordqvist K, Tilmann C, Lovell-Badge R, Capel B. Male-specific cell migration into the developing gonad. Curr Biol. 1997;7:958–968. doi: 10.1016/s0960-9822(06)00415-5. [DOI] [PubMed] [Google Scholar]
- Matson CK, Murphy MW, Sarver AL, Griswold MD, Bardwell VJ, Zarkower D. DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature. 2011;476:101–104. doi: 10.1038/nature10239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzuk MM, Burns KH, Viveiros MM, Eppig JJ. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science. 2002;296:2178–2180. doi: 10.1126/science.1071965. [DOI] [PubMed] [Google Scholar]
- McClellan KA, Gosden R, Taketo T. Continuous loss of oocytes throughout meiotic prophase in the normal mouse ovary. Dev Biol. 2003;258:334–348. doi: 10.1016/s0012-1606(03)00132-5. [DOI] [PubMed] [Google Scholar]
- McLaren A. Meiosis and differentiation of mouse germ cells. Symp Soc Exp Biol. 1984;38:7–23. [PubMed] [Google Scholar]
- Menke DB, Koubova J, Page DC. Sexual differentiation of germ cells in XX mouse gonads occurs in an anterior-to-posterior wave. Dev Biol. 2003;262:303–312. doi: 10.1016/s0012-1606(03)00391-9. [DOI] [PubMed] [Google Scholar]
- Menke DB, Page DC. Sexually dimorphic gene expression in the developing mouse gonad. Gene Expr Patterns. 2002;2:359–367. doi: 10.1016/s1567-133x(02)00022-4. [DOI] [PubMed] [Google Scholar]
- Merchant H. Rat gonadal and ovarioan organogenesis with and without germ cells. An ultrastructural study. Dev Biol. 1975;44:1–21. doi: 10.1016/0012-1606(75)90372-3. [DOI] [PubMed] [Google Scholar]
- Mork L, Maatouk DM, McMahon JA, Guo JJ, Zhang P, McMahon AP, Capel B. Temporal differences in granulosa cell specification in the ovary reflect distinct follicle fates in mice. Biol Reprod. 2012;86:37. doi: 10.1095/biolreprod.111.095208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naillat F, Prunskaite-Hyyrylainen R, Pietila I, Sormunen R, Jokela T, Shan J, Vainio SJ. Wnt4/5a signalling coordinates cell adhesion and entry into meiosis during presumptive ovarian follicle development. Hum Mol Genet. 2010;19:1539–1550. doi: 10.1093/hmg/ddq027. [DOI] [PubMed] [Google Scholar]
- Nef S, Schaad O, Stallings NR, Cederroth CR, Pitetti JL, Schaer G, Malki S, Dubois-Dauphin M, Boizet-Bonhoure B, Descombes P, Parker KL, Vassalli JD. Gene expression during sex determination reveals a robust female genetic program at the onset of ovarian development. Dev Biol. 2005;287:361–377. doi: 10.1016/j.ydbio.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Ottolenghi C, Omari S, Garcia-Ortiz JE, Uda M, Crisponi L, Forabosco A, Pilia G, Schlessinger D. Foxl2 is required for commitment to ovary differentiation. Hum Mol Genet. 2005;14:2053–2062. doi: 10.1093/hmg/ddi210. [DOI] [PubMed] [Google Scholar]
- Ottolenghi C, Pelosi E, Tran J, Colombino M, Douglass E, Nedorezov T, Cao A, Forabosco A, Schlessinger D. Loss of Wnt4 and Foxl2 leads to female-to-male sex reversal extending to germ cells. Hum Mol Genet. 2007;16:2795–2804. doi: 10.1093/hmg/ddm235. [DOI] [PubMed] [Google Scholar]
- Qin Y, Bishop CE. Sox9 is sufficient for functional testis development producing fertile male mice in the absence of Sry. Hum Mol Genet. 2005;14:1221–1229. doi: 10.1093/hmg/ddi133. [DOI] [PubMed] [Google Scholar]
- Rajareddy S, Reddy P, Du C, Liu L, Jagarlamudi K, Tang W, Shen Y, Berthet C, Peng SL, Kaldis P, Liu K. p27kip1 (cyclin-dependent kinase inhibitor 1B) controls ovarian development by suppressing follicle endowment and activation and promoting follicle atresia in mice. Mol Endocrinol. 2007;21:2189–2202. doi: 10.1210/me.2007-0172. [DOI] [PubMed] [Google Scholar]
- Raymond CS, Kettlewell JR, Hirsch B, Bardwell VJ, Zarkower D. Expression of Dmrt1 in the genital ridge of mouse and chicken embryos suggests a role in vertebrate sexual development. Dev Biol. 1999;215:208–220. doi: 10.1006/dbio.1999.9461. [DOI] [PubMed] [Google Scholar]
- Raymond CS, Murphy MW, O'Sullivan MG, Bardwell VJ, Zarkower D. Dmrt1, a gene related to worm and fly sexual regulators, is required for mammalian testis differentiation. Genes & Development. 2000;14:2587. doi: 10.1101/gad.834100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker EB, Dierisseau P, Wagner KU, Garrett L, Wynshaw-Boris A, Flaws JA, Hennighausen L. Bcl-x and Bax regulate mouse primordial germ cell survival and apoptosis during embryogenesis. Mol Endocrinol. 2000;14:1038–1052. doi: 10.1210/mend.14.7.0465. [DOI] [PubMed] [Google Scholar]
- Schmahl J, Eicher EM, Washburn LL, Capel B. Sry induces cell proliferation in the mouse gonad. Development. 2000;127:65–73. doi: 10.1242/dev.127.1.65. [DOI] [PubMed] [Google Scholar]
- Schmidt D, Ovitt CE, Anlag K, Fehsenfeld S, Gredsted L, Treier AC, Treier M. The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development. 2004;131:933–942. doi: 10.1242/dev.00969. [DOI] [PubMed] [Google Scholar]
- Sekido R, Bar I, Narvaez V, Penny G, Lovell-Badge R. SOX9 is up-regulated by the transient expression of SRY specifically in Sertoli cell precursors. Dev Biol. 2004;274:271–279. doi: 10.1016/j.ydbio.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Sekido R, Lovell-Badge R. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature. 2008;453:930–934. doi: 10.1038/nature06944. [DOI] [PubMed] [Google Scholar]
- Stallock J, Molyneaux K, Schaible K, Knudson CM, Wylie C. The pro-apoptotic gene Bax is required for the death of ectopic primordial germ cells during their migration in the mouse embryo. Development. 2003;130:6589–6597. doi: 10.1242/dev.00898. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Tsuda M, Kiso M, Saga Y. Nanos3 maintains the germ cell lineage in the mouse by suppressing both Bax-dependent and -independent apoptotic pathways. Dev Biol. 2008;318:133–142. doi: 10.1016/j.ydbio.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Tevosian SG, Manuylov NL. To beta or not to beta: canonical beta-catenin signaling pathway and ovarian development. Dev Dyn. 2008;237:3672–3680. doi: 10.1002/dvdy.21784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizuka K, Horikoshi K, Kitada R, Sugawara Y, Iba Y, Kojima A, Yoshitome A, Yamawaki K, Amagai M, Inoue A, Oshima T, Kakitani M. R-spondin1 plays an essential role in ovarian development through positively regulating Wnt-4 signaling. Hum Mol Genet. 2008;17:1278–1291. doi: 10.1093/hmg/ddn036. [DOI] [PubMed] [Google Scholar]
- Ueno S, Kuroda T, Maclaughlin DT, Ragin RC, Manganaro TF, Donahoe PK. Mullerian Inhibiting Substance in the Adult Rat Ovary During Various Stages of the Estrous Cycle. Endocrinology. 1989a;125:1060–1066. doi: 10.1210/endo-125-2-1060. [DOI] [PubMed] [Google Scholar]
- Ueno S, Takahashi M, Manganaro TF, Ragin RC, Donahoe PK. Cellular Localization of Mullerian Inhibiting Substance in the Developing Rat Ovary. Endocrinology. 1989b;124:1000–1006. doi: 10.1210/endo-124-2-1000. [DOI] [PubMed] [Google Scholar]
- Uhlenhaut NH, Jakob S, Anlag K, Eisenberger T, Sekido R, Kress J, Treier AC, Klugmann C, Klasen C, Holter NI, Riethmacher D, Schutz G, Cooney AJ, Lovell-Badge R, Treier M. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell. 2009;139:1130–1142. doi: 10.1016/j.cell.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Vainio S, Heikkila M, Kispert A, Chin N, McMahon AP. Female development in mammals is regulated by Wnt-4 signalling. Nature. 1999;397:405–409. doi: 10.1038/17068. [DOI] [PubMed] [Google Scholar]
- Vidal VP, Chaboissier MC, de Rooij DG, Schedl A. Sox9 induces testis development in XX transgenic mice. Nat Genet. 2001;28:216–217. doi: 10.1038/90046. [DOI] [PubMed] [Google Scholar]
- Vigier B, Watrin F, Magre S, Tran D, Josso N. Purified bovine AMH induces a characteristic freemartin effect in fetal rat prospective ovaries exposed to it in vitro. Development. 1987;100:43–55. doi: 10.1242/dev.100.1.43. [DOI] [PubMed] [Google Scholar]
- Wagner T, Wirth J, Meyer J, Zabel B, Held M, Zimmer J, Pasantes J, Bricarelli FD, Keutel J, Hustert E, Wolf U, Tommerup N, Schempp W, Scherer G. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell. 1994;79:1111–1120. doi: 10.1016/0092-8674(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Yao HH, DiNapoli L, Capel B. Meiotic germ cells antagonize mesonephric cell migration and testis cord formation in mouse gonads. Development. 2003;130:5895–5902. doi: 10.1242/dev.00836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao HH, Matzuk MM, Jorgez CJ, Menke DB, Page DC, Swain A, Capel B. Follistatin operates downstream of Wnt4 in mammalian ovary organogenesis. Dev Dyn. 2004;230:210–215. doi: 10.1002/dvdy.20042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimizu T, Sugiyama N, De Felice M, Il Yeom Y, Ohbo K, Masuko K, Obinata M, Abe K, Scholer HR, Matsui Y. Germline-specific expression of the Oct-4/green fluorescent protein (GFP) transgene in mice. Development Growth & Differentiation. 1999;41:675. doi: 10.1046/j.1440-169x.1999.00474.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Germ cells in Wnt4-null ovaries express the meiotic marker SCP3. (A-D) At 15.5 dpc, germ cells in control (A) and Wnt4-/- (B) ovaries are co-labeled for PECAM1 (green) and SCP3 (blue). SCP3-positive germ cells are distributed throughout the gonad in both controls (C) and mutants (D). The absence of germ cells along the coelomic surface is due to the presence of the ectopic vasculature in Wnt4 mutants (position of coelomic vasculature is indicated by a white line, inset in D). Ectopic AMH (red), which is expressed after 16.5 dpc in Wnt4 mutants (Figure 2), is not detected at 15.5 dpc and therefore is not the cause of germ cell loss that begins at the anterior end of Wnt4 mutants at this stage. (E,F) At 18.5 dpc, surviving germ cells in Wnt4 mutants maintain expression of the germ cell markers PECAM1 (red) and SCP3 (blue), similar to controls (Syto13, shown in green, marks nuclei). The pattern of SCP3 on chromosomes of single cells appears to be unaffected by the loss of Wnt4 (insets in E and F). (G, H) At 19.5 dpc, mitotically arrested germ cells express DNMT3L (blue) in XY control males. XX Wnt4 mutants do not express DNMT3L (PECAM1, green, faintly marks germ cells, brightly labels vasculature and autofluorescent cells in the testis). Panels in C and D are oriented with the anterior end of the gonad to the left. The scale bars represent 50 μm (A-D, G-H), 25 μm (E,F), 12 μm (insets in C,D) or 8 μm (insets in E,F).
Figure S2. Time course of BRE-lacZ expression in male and female fetal gonads and prepubertal ovaries as determined by X-gal staining. Strong reporter activity was observed in the mesonephric ducts of both sexes throughout fetal development (arrow in top left panel). (A,B) At 11.75 dpc, X-gal activity was detected in the gonads of both sexes. (C, D) By 12.5 dpc, Xgal staining was stronger in the ovary than the testis. This pattern continued until birth (E-J). Xgal staining in testes became restricted to the interior vasculature and coelomic vessel (arrowhead in J). The strong X-gal staining observed in P7 ovaries (K) became restricted to follicles by P21 (L). Testes were not examined for BRE reporter activity by X-gal activity at postnatal stages. Bright-field images were all taken at the same magnification.
Figure S3. The BRE-LacZ reporter, for active Bmp signaling, is expressed in ovarian somatic cells at prenatal and postnatal stages. (A-E) XX gonads were immunostained for β-galactosidase to visualize the BRE-LacZ reporter (BRE; green). Somatic cells were labeled with GATA4, which marks all gonadal somatic cells, or FOXL2, which labels the female supporting cell lineage (blue). Germ cells were labeled with PECAM1, which marks germ cells and endothelial cells, or CDH1, which is specific to germ cells (purple). At all stages examined, 11.75 dpc through 21 dpp, BRELacZ co-labeled with ovarian somatic markers, and was expressed in the supporting cell lineage (FOXL2-positive), as well as in other ovarian somatic cells (GATA4-positive, FOXL2-negative). Immunostaining was performed on whole mount samples in A-C, and on cryosectioned samples in D-E. Panels on the right (ex. A′) are high magnification images from the same samples on the left. (FH) XY gonads were immunostained for β-calactosidase (BRE;green) and AMH (blue). At E13.5 dpc (F) and E15.5 dpc (G) BRE was localized to interstitial cells and not expressed in Sertoli cells. In adult testes (H) BRE localized to germ cells and was not expressed in Sertoli cells. The inset shows a high magnification image; the arrow indicates a Sertoli cell. (I,J) XX control (I) and XX Wnt4-/- (J) gonads were immunostained for β-galactosidase (BRE; green) and FOXL2 (blue). A decrease in BRE-positive cells in XX Wnt4-/- gonads 13.5 dpc is consistent with the previous observation that Bmp2 expression is lost in the absence of Wnt4 (Yao, et al, 2004). The arrow in (J) points to autofluoresence from blood in the ectopic coelomic vasculature. Scale bars represent 50 μm in main panels and 25 μm in the inset (H).
Figure S4. Transdifferentiation progresses after birth in postnatal Rspo1-null ovaries. (A-C) Gonads were collected from 14 dpp pups and immunostained for AMH (red) and CDH1 (blue). At 14 dpp, AMH is not expressed in Sertoli cells (A) but is expressed growing follicles (B). In Rspo1-null ovaries, AMH is expressed in large tubule structures (C) and only a few surviving germ cells are present. (D-F) Immunostaining of 14 dpp control and Rspo1-null gonads with SOX9 (red), FOXL2 (green) and CDH1 (blue). As expected, XY control testes had SOX9-positive Sertoli cells (D) and XX control ovaries exhibited FOXL2 expression (E). (F) XX Rspo1-null ovaries had a few small clusters of FOXL2-positive cells, whereas the bulk of the ovary was occupied by large SOX9-positive tubule structures. Scale bars represent 50 μm.
Figure S5. Loss of β-catenin in gonadal supporting cells causes downregulation of FOXL2, but does not disrupt mitotic arrest. (A-C) Gonads were collected at 13.5 dpc and immunostained for FOXL2 (green) and p27 (red). (A) p27 is expressed in all FOXL2-expressing cells and in additional somatic cells just beneath the coelomic epithelium (the coelomic surface marked by green background staining). (B-C) Ovaries lacking β-catenin in Sf1-Cre expressing somatic cells (βcatfl/fl; Sf1-Cre) had a severe reduction in FOXL2-expressing cells, but p27 was unaffected. (D-F) 18.5 dpc ovaries were dissected from control ovaries (D, βcatfl/fl) and ovaries lacking β-catenin in Sf1-Cre expressing somatic cells (E-F, βcatfl/fl; Sf1-Cre). Samples were immunostained for FOXL2 (D,E; green), or AMH (F; red). A positive control (XY βcatfl/fl; Sf1-Cre) for AMH immunostaining is shown in the inset in (F). All samples were whole mount immunostained and carried the Rosa-tdTomato reporter (RTM; kindly provided by Fan Wang, Duke University) which indicates active Cre recombination (blue). A white dotted line outlines the ovary in D-F. Scale bars represent 50 μm in all main panels, and 60 μm in inset in (F).


