Abstract
The Id family of helix-loop-helix transcription factors is upregulated in a variety of human malignancies and has been implicated in promoting tumorigenesis through effects on cell growth, differentiation, and tumor angiogenesis. While expression of Id proteins has been associated with tumorigenesis, the precise mechanistic relationship between Id expression and carcinogenesis has not been clearly delineated. We have previously shown that Id1 delays cellular senescence in primary mammalian cells through inhibition of the cell cycle regulatory protein and familial melanoma gene, p16/INK4a. We have also demonstrated that Id1 expression is upregulated in early stage primary human melanomas and may be an important marker for early malignancy. In order to further define the role of Id1 in human melanoma development, we have evaluated the function of Id1 in primary human melanocytes. Here we show that constitutive expression of Id1 in primary human melanocytes leads to delayed cellular senescence and decreased expression of the familial melanoma gene, p16/INK4a. Although melanocytes constitutively expressing Id1 are shown to possess extended lifespans, this is not associated with an appreciable change in cell growth or telomere length. We conclude that Id1 delays cellular senescence in primary human melanocytes through inhibition of p16/INK4a expression and suggest that Id1 may contribute to the malignant conversion of primary human melanocytes through extension of cellular lifespan.
Keywords: melanocyte, Id1, senescence
INTRODUCTION
The Id family of helix-loop-helix (HLH) proteins constitutes a family of transcription factors that regulate differentiation in many cell lineages (reviewed in [1]). There has been much interest in this class of transcription factors in the cancer biology community since cellular proliferation and loss of differentiation are hallmarks for cancer. The Id proteins have been shown to be highly expressed in a large number of cancers including tumors of the prostate, breast, ovary, endometrium, cervix, GI tract, pancreas, liver, upper aerodigestive tract, and thyroid; neural tumors, Ewing’s sarcoma, seminoma, leukemias, and melanoma (reviewed in [2]). In several of these cancer types, overexpression of Id1 is associated with an aggressive phenotype and poor clinical outcome [3–5]. While Id proteins, and Id1 in particular, have been found to be upregulated in a large number of tumors, little functional data exists to substantiate a specific role for Id genes in tumorigenesis. Id1 has been shown to delay senescence in primary human keratinocytes [6], human endothelial cells [7], and human diploid fibroblasts [8]. We have previously identified the cell cycle regulatory protein and familial melanoma gene, p16/INK4a, as being a downstream effector of Id1 [9], and have shown that cells null for Id1 senesce prematurely in association with increased expression of p16/INK4a. We have also shown that Id1 expression is upregulated during the early stages of melanoma in association with loss of expression of p16/INK4a [10,11]. In addition, Id1 has been associated with decreased survival in melanoma [12]. In order to define the functional significance of Id1 expression in melanocytic lesions we have evaluated the biologic consequences of Id1 expression in primary human melanocytes. Here we show that exogenously expressed Id1 extends the normal lifespan of primary human melanocytes in culture. This delay in cellular senescence is associated with repressed expression of p16/INK4a but without notable changes in cellular growth, migration, or telomere length. We conclude that aberrant expression of Id1 extends the lifespan of primary human melanocytes through downregulation of the cell cycle regulatory protein p16/INK4a which may be an important step in the malignant conversion of melanocytes to melanoma.
MATERIALS AND METHODS
Cell Culture
Monolayer cultures of primary human melanocytes were prepared from neonatal foreskins as previously outlined [13] and plated in melanocyte growth medium (Cell Applications, Inc., San Diego, CA). After G418 treatment, cells were used within 2 wk for viral infection. Lentivirus was produced in HEK293T cells using previously described protocols [13]. Infections were performed by adding virus to media along with 6 μg/mL polybrene, and incubating on cells for 8 h. Cells were given at least 5 d to recover from infection before experiments were initiated.
Growth Assays
Cell proliferation was quantified using a colorimetric-based XTT proliferation kit according to manufacturers protocol (Roche Applied Science, Indianapolis, IN, catalog #11465015001) and cell counts at serial passage. For the serial passage senescence experiments, melanocytes were maintained in Melanocyte Growth Media supplemented with 1 nM cholera toxin (Sigma, St. Louis, MO, catalog # C-8052). Cells were plated at 50 000 cells per well in a 12-well dish in triplicate. One-week later, each well was trypsinized and counted individually, then each group was pooled, counted, and replated in three wells at the original number. This procedure was continued until no increase in cell number was seen for 4 wk. Population doublings were calculated from population increase/passage. For BrdU assays, chamber slides were coated for 20 min at 37°C with a 0.05% gelatin solution before cells were plated and allowed to settle overnight. Cells were stained using the 5-Bromo-2′-deoxy-uridine Labeling and Detection Kit I (Roche catalog # 11296736001) according to the manufacturer’s instructions. To ensure reproducibility, growth curves were repeated 15 times. BrdU assays were performed in triplicate.
Immunoblotting
Lysates were prepared at various timepoints in the growth of the primary melanocytes. Cells were trypsinized and spun down before adding lysis buffer (0.1% NP-40, 250 mM NaCl, 50 mM Tris–HCl, 5 mM EDTA supplemented with 5 μL/mL protease inhibitor, Sigma # P8340 and 3 μL/mL 200 μM PMSF, Sigma # P7626) and chilling on ice for 20 min. Supernatant was collected by centrifugation at 8000 RPM for 5 min and protein was quantified using BioRad Laboratories (Hercules, CA) reagent (catalog # 5000006). All gels were 15% acrylamide and run with a Hoeffer gel apparatus (model SE600). Protein was loaded at 75 μg/well. Gels were run overnight and then transferred at 300 mA for 1.5 h. Western blotting was performed using standard techniques and reactive proteins were visualized using ECL reagents (GE Healthcare, Piscataway, NJ, # RPN2106). Antibodies used include: Id-1 (Santa Cruz Biotechnology, Santa Cruz, CA, sc-488), p16 (Lab Vision Corporation, Fremont, CA, Ab-4), Actin (Amersham Life Science, Piscataway, NJ, N350), PCNA (Santa Cruz, sc-56). Westerns were developed using ECL Reagent (Amersham). Western blotting and associated experimental protocols were repeated in triplicate.
Migration Assays
Primary melanocytes were plated in a 12-well plate at approximately 80% confluency and allowed to settle overnight. The next day, a scratch was made down the middle of each well using a 200 μL pipet and the media changed twice to remove any loose cells. Photographs were taken on serial days to assess growth into the scratched area using a Nikon Eclipse TS100. Migration assays were performed in triplicate.
Promoter Methylation Experiments
Genomic DNA was obtained from late passage melanocytes using the DNA Wizard kit (Promega Corporation, Madison, WI, #A1120) according to manufacturer’s instructions. The DNA methylation pattern in the p16 promoter region was analyzed using nested PCR and then methylation-specific PCR (MSP), as reported by Herman et al. [14]. Nested PCR conditions were as follows: 57°C annealing, 35 cycles (1 min 95°C denature, 30 s 57°C anneal, 1 min 72°C extension) and primers used were 5′-AGAAAGAGGAGGGGTTGGTTGG-3′ (forward) and 5′-ACRCCCRCACCTCCTCTACC-3′ (reverse). Primers used for the methylation reaction were 5′-TTATTAGAGGGTGGGGC-GGATCGC-3′ (forward) and 5′-GACCCCGAACCGCGACCGTAA-3′ (reverse) and for the unmethylated reaction were 5′-TTATTAGAGGGTGGGGTGGATTGT-3′ (forward) and 5′-CAACCCCAAACCACAACCATAA-3′ (reverse) with PCR conditions as follows: 64°C annealing temperature with 30 cycles (1 min 95°C, 30 s 64°C annealing, 1 min 72°C). Promoter methylation of genomic DNA was performed in triplicate for a single DNA sample.
Telomere-Immunostaining Fluorescence In Situ Hybridization (TELI-FISH)
Cells were plated on poly-d-lysine-coated chamber well slides (Nalgene) at approximately 50% confluency and grown for 72 h. Cells were stained for telomeric DNA and quantified as described previously [15] and were performed in triplicate.
RESULTS
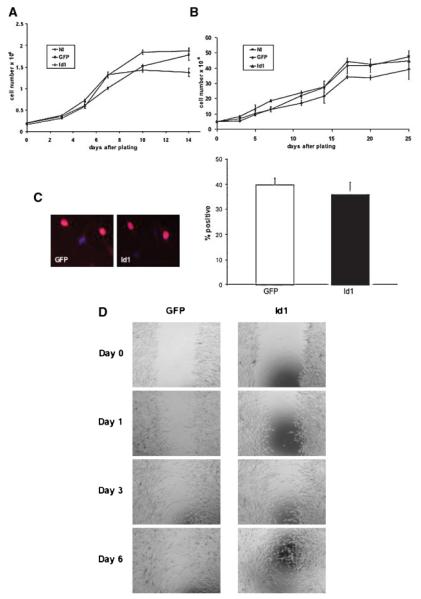
Id1 Does Not Significantly Alter Growth or Migration of Primary Human Melanocytes
In order to define the function of Id1 in primary human melanocytes, we evaluated the biologic consequences of ectopic expression of Id1 in these cells. Id1 was introduced into primary human melanocytes using a bicistronic lentiviral expression system also encoding GFP [13,16]. Cells expressing GFP alone (GFP), GFP and Id1 (Id1), and noninfected (NI) cells were serially passaged and evaluated for growth. Although Id proteins have been shown to promote cellular proliferation (reviewed in [17]), we did not observe any changes in cellular growth in Id1-transduced melanocytes at either early (Figure 1A) or later (Figure 1B) passages. Evaluation of cellular proliferation through BrdU incorporation also failed to demonstrate altered cell growth in Id1 transduced cells versus controls (Figure 1C). Since Id1 expression has been associated with aggressive tumor phenotype and poorer prognosis in a variety of malignancies we sought to determine whether expression of Id1 in primary human melanocytes altered cellular migration. Id1-transduced melanocytes and control infected cells were evaluated for migration using a nondirected migration scratch assay. All cells evaluated demonstrated equivalent migration into the cleared space over time suggesting no significant alteration in primary human melanocyte migration by Id1 (Figure 1D).
Figure 1.
Id1 does not alter cellular growth or migration of primary human melanocytes. Growth profiles of early (A) and later (B) passage melanocytes expressing Id1 versus controls. Growth profiles of primary human melanocytes infected with Id1-expressing lentivirus (Id1), GFP-expressing control lentivirus (GFP) or noninfected cells (NI) were obtained at passage-1 (A) and passage-12 (B). Representative curves are shown for experiments performed in triplicate. Note that no significant alterations in cellular growth were identified in Id1-expressing melanocytes at either passage. (C) BrdU assay and quantification for Id1-expressing melanocytes versus controls. Note Id1 expression does not significantly alter BrdU incorporation in primary human melanocytes. (D) Evaluation of cellular migration by Id1-expressing primary human melanocytes over time. Note, scratch assay for Id1-expressing melanocytes versus controls fails to distinguish alterations in cellular migration due to Id1. Error bars indicate standard deviation. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
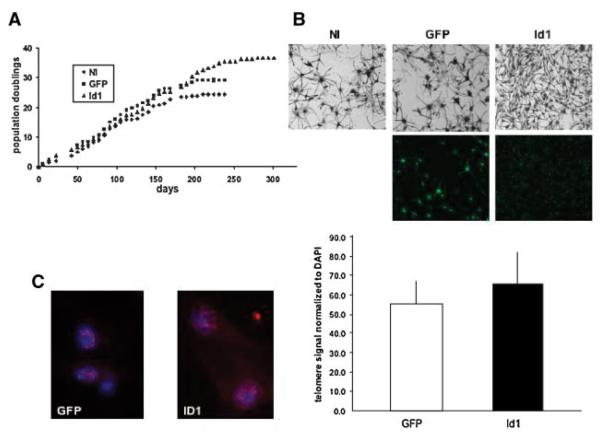
Id1 Delays Senescence of Primary Human Melanocytes
Alani and coworkers have previously identified a role for Id1 in regulating the normal lifespan of primary human cells [6–9,18]. In order to determine whether Id1 expression in primary human melanocytes altered replicative senescence in these cells, primary human melanocytes were infected with a lentiviral vector expressing Id1 and GFP (Id1), or GFP alone (GFP), subjected to serial passage, and evaluated for population doublings until they had achieved growth arrest. Noninfected and GFP-infected cells were found to arrest following 25 and 28 population doublings, respectively, while Id1-infected cells exhibited delayed growth arrest following 37 doublings (Figure 2A). Although GFP expression alone in primary human melanocytes resulted in a slightly increased cellular lifespan (25 vs. 28 population doublings), this was significantly extended in the GFP-Id1 expressing melanocytes. At passage 32, control cells (NI and GFP) exhibited a large, flattened cellular morphology characteristic of cellular senescence, while Id1 expressing cells maintained a smaller, bipolar spindle-shaped appearance characteristic of normal primary human melanocytes in culture (Figure 2B). Since telomere length is closely associated with senescence and carcinogenesis (reviewed in [19]), we performed a telomeric quantitative fluorescence in situ hybridization (TELI-FISH) assay on GFP and Id1-infected melanocytes by staining for telomere repeats (Figure 2C). Telomere signal was quantified microscopically and normalized to DAPI signal in order to determine whether Id1 expression was associated with shortened telomeres in late passage primary human melanocytes. We found no significant difference in telomeric qFISH in GFP versus Id1-infected cells.
Figure 2.
Id1 delays replicative senescence of primary human melanocytes. (A) Growth curves for Id1 expressing melanocytes and control cells over time. Cells were counted on a weekly basis. Cell counting was continued until no increase in cell number was seen for 4 wk. Population doublings were calculated from population increase/passage. (B) Senescence-associated morphology changes in Id1-expressing versus control primary human melanocytes. Photo-micrographs were taken at passage 32. (C) Evaluation of telomere length in late passage (P-32) Id1 expressing versus control primary human melanocytes using quantitative fluorescence in-situ hybridization. TELI-FISH quantification shows no difference in telomere signal between control and Id1-expressing primary human melanocytes. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Id1 Promotes Loss of p16/INK4a Expression Without Associated Promoter Methylation in Primary Human Melanocytes
Replicative senescence has been shown to be associated with increased expression of the cell cycle regulatory proteins, p16/INK4a and p21 (reviewed in [19]). In order to evaluate cell cycle changes in primary human melanocytes exogenously expressing Id1, we evaluated the expression of Id1, p16/INK4a, p21, and proliferating cell nuclear antigen (PCNA) by Western blotting in early and late passage Id1, GFP, and NI melanocytes (Figure 3A,B). Only Id1 melanocytes were shown to express Id1 in both early and later passages. Early passage, rapidly growing melanocytes failed to express detectable levels of p16/INK4a, whereas later passage noninfected and GFP-infected cells that had undergone replicative senescence, expressed significant levels p16/INK4a (passage 32). Later passage Id1-infected melanocytes had not undergone replicative senescence and were found to express minimal levels of p16/INK4a. While early passage melanocytes failed to express the cdk inhibitor p21, later passage cells expressed appreciable levels of p21 in all cells evaluated without significant differences in p21 expression noted in the Id1 expressing cells (Figure 3B). Since epigenetic silencing of p16/INK4a has been associated with melanoma and other malignancies (reviewed in [20]), we evaluated late passage Id1 melanocytes for p16/INK4a promoter methylation. MSP was performed with primers specific for unmethylated or methylated promoter states. We failed to detect significant methylation of the p16 promoter in any of the cell populations evaluated (Figure 3C) suggesting that transcriptional repression is the primary means of Id1 downregulation of p16/INK4a expression in primary melanocytes.
Figure 3.
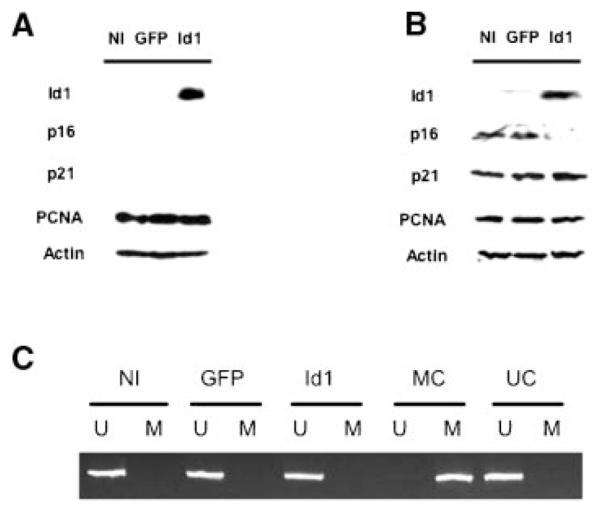
Id1 expression in primary human melanocytes leads to downregulation of p16/INK4a expression without associated promoter methylation. (A,B) Western analysis of Id1 and cell cycle regulatory protein expression of Id1-expressing and control primary human melanocytes at early passage (A), and late passage (B). Note significant upregulation of p16/INK4a expression in late passage (P-32)/senescent NI and GFP melanocytes without an associated increase in Id1-expressing cells. Actin expression is included as a control for sample loading. (C) Evaluation of p16/INK4a promoter methylation in late passage (P-32) Id1-expressing versus control primary human melanocytes. Methylation-specific PCR showed no detectable methylation of the p16/INK4a promoter in late passage cells expressing Id1 despite loss of p16/INK4a protein expression. U, unmethylated PCR; M, methylated PCR; UC, unmethylated PCR control; MC, methylated PCR control.
DISCUSSION
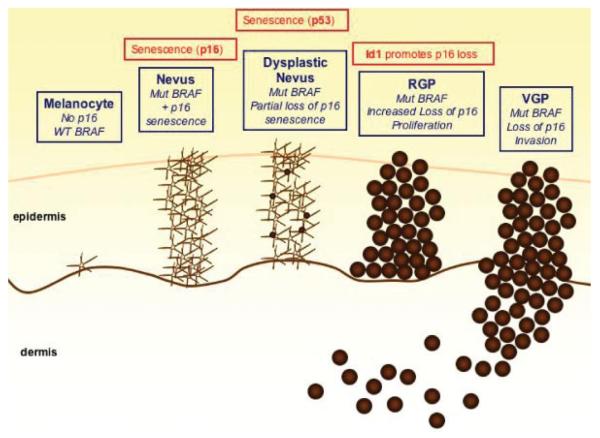
We have evaluated the functional significance of Id1 expression in primary human melanocytes in order to help define the role of Id1 in melanoma development. Our data demonstrate a dominant “Id1 phenotype” of delayed replicative senescence associated with downregulation of the cell cycle inhibitory protein p16/INK4a. Interestingly, this repression of p16/INK4a is not associated with a significant alteration in cellular growth at either early or late passage, and cells expressing Id1 at late passage do not possess significantly altered levels of the p53 effector protein and cyclin-dependent kinase inhibitor, p21. Recently, much attention has been focused on the role of cellular senescence in the growth of benign and malignant melanocytic lesions since activating mutations of BRAF kinase, a gene mutated in up to 70% of melanomas, were shown to induce a premature senescence-associated cell cycle arrest of human melanocytes in-vitro [21,22]. Benign nevi, which possess up to an 80% mutation rate of BRAF kinase [23] were also shown to display evidence of cellular senescence associated with heterogeneous elevated expression of p16/INK4a [21,22]. Although cellular senescence can occur as either a p53 or p16/INK4a-mediated event, studies of melanocytes derived from individuals with germline bi-allelic loss of functional p16/INK4a demonstrated significant extension of cellular lifespan with eventual cellular senescence being associated with elevated expression of p53 suggesting that normal senescence of human melanocytes is mediated by p16/INK4a which can be compensated for by p53 [24]. Interestingly, oxidative stress has been shown to result in hypermethylation and epigenetic silencing of p16/INK4a with associated activation of the MAP kinase pathway [25] which may be relevant to early evolution of atypical melanocytic lesions. Our current studies also support a critical role for p16 in regulating the normal cellular senescence of primary human melanocytes which may be inhibited by the HLH transcription factor, Id1. Given our previous identification of Id1 expression in radial growth phase melanomas [10], we therefore propose that Id1-associated extension of cellular lifespan in melanocytes and transcriptional repression of p16/INK4a promotes the malignant conversion of primary human melanocytes in vivo through acquired increased cellular division cycles during which cells may acquire additional mutational events (Figure 4). Thus the evolution of melanomas from benign acquired nevi would include the following steps: BRAF (or N-RAS) kinase activating mutations occur as the initiating events leading to melanocyte proliferation and subsequent activation of the tumor-suppressor response of cellular senescence mediated by p16/INK4a and the clinical appearance of a benign acquired nevus. In individuals with inherited mutations of p16/INK4a, delayed cellular senescence allows for additional cell division cycles and the potential for acquiring addition mutational events associated with larger, more disordered melanocytic lesions or “dysplastic nevi.” Activation of Id1 within a dysplastic nevus allows for further loss of p16/INK4a and additional cellular division cycles with the potential for acquiring further mutations resulting in an in situ melanoma. Since Id1 has not been shown to be genetically activated in primary melanocytic tumors, Id1-mediated transcriptional repression of p16/INK4a is likely to be a transient, reversible step in melanoma development. Our model for Id1 function in melanoma suggests that this transient activation of Id1 is superceded by either genetic loss or epigenetic silencing of p16/INK4a in the progression to vertical growth phase melanoma since vertical growth phase and metastatic melanomas fail to express significant levels of Id1 yet have minimal expression of p16/INK4a [10]. Since replicative senescence of somatic cells is only one of many processes that need to be overcome during tumorigenesis, we expect that further evaluation of Id1 functions in combination with other oncogenic events in primary human melanocytes will elucidate the precise role of this and other genetic pathways in melanoma development.
Figure 4.
Model for Id1 regulation of melanocyte growth and melanoma development. Normal epidermal melanocytes reside at the basement membrane and are slowly proliferating. They possess a wild-type p16/INK4a gene without significant p16/INK4a expression, and wild-type BRAF kinase. Melanocytes within benign nevi frequently possess mutant BRAF kinase which promotes cellular growth; however, cellular growth arrest (senescence) is achieved through an intact p16/INK4a gene and upregulated expression in nevomelanocytes with associated cell cycle inhibition. Dysplastic nevi possess partial loss of p16/INK4a expression/function (genetic or epigenetic loss through mutational changes in the gene encoding p16/INK4a or methylation of the p16 promoter) in conjunction with BRAF mutations leading to a proliferative lesion with eventual growth arrest induced by either residual functional p16/INK4a or p53 and associated effectors. Radial growth phase (RGP) melanomas possess mutant BRAF and associated proliferative changes in conjunction more extensive loss of p16/INK4a expression via genetic/epigenetic changes and additional reversible loss through promoter repression by Id1. Additional cell cycling achieved through loss of p16/INK4a function promotes further genetic changes and results in radial growth lesions (p53 pathway is likely to be involved here despite wildtype p53 expression in these tumors). Since Id1 regulation of p16/INK4a is transcriptional and not associated with distinctive genetic events, this stage may be functionally reversible through targeted disruption of Id1 function. Vertical growth phase tumors possess even more extensive loss of functional p16/INK4a through genetic/epigenetic silencing. Id1 expression is lost since p16/INK4a silencing is now propogated via genetic/epigenetic changes and Id1 functions would therefore be redundant. BRAF activity remains high in these tumors and proliferation induced by active BRAF kinase promotes additional genetic changes that promote tumor invasion into the dermis. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
ACKNOWLEDGMENTS
We thank members of the Alani Lab for critical review of this manuscript. Work in the Alani Lab is supported by National Institutes of Health Grants CA107017 (RA) and CA113779 (BR), the American Skin Association (RA), the Flight Attendant Medical Research Institute (RA), The Joanna M. Nicolay Melanoma Foundation, The Henry and Elaine Kaufman Foundation, and The Murren Family Foundation.
REFERENCES
- 1.Norton JD. ID helix-loop-helix proteins in cell growth, differentiation and tumorigenesis. J Cell Sci. 2000;113:3897–3905. doi: 10.1242/jcs.113.22.3897. [DOI] [PubMed] [Google Scholar]
- 2.Sikder HA, Devlin MK, Dunlap S, Ryu B, Alani RM. Id proteins in cell growth and tumorigenesis. Cancer Cell. 2003;3:525–530. doi: 10.1016/s1535-6108(03)00141-7. [DOI] [PubMed] [Google Scholar]
- 3.Schoppmann SF, Schindl M, Bayer G, et al. Overexpression of Id-1 is associated with poor clinical outcome in node negative breast cancer. Int J Cancer. 2003;104:677–682. doi: 10.1002/ijc.11009. [DOI] [PubMed] [Google Scholar]
- 4.Schindl M, Schoppmann SF, Strobel T, et al. Level of id-1 protein expression correlates with poor differentiation, enhanced malignant potential, and more aggressive clinical behavior of epithelial ovarian tumors. Clin Cancer Res. 2003;9:779–785. [PubMed] [Google Scholar]
- 5.Takai N, Miyazaki T, Fujisawa K, Nasu K, Miyakawa I. Id1 expression is associated with histological grade and invasive behavior in endometrial carcinoma. Cancer Lett. 2001;165:185–193. doi: 10.1016/s0304-3835(01)00433-5. [DOI] [PubMed] [Google Scholar]
- 6.Alani RM, Hasskarl J, Grace M, Hernandez M-C, Israel MA, Munger K. Immortalization of primary human keratinocytes by the helix-loop-helix protein, Id-1. Proc Natl Acad Sci USA. 1999;96:9637–9641. doi: 10.1073/pnas.96.17.9637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang J, Gordon GM, Nickoloff BJ, Foreman KE. The helix-loop-helix protein id-1 delays onset of replicative senescence in human endothelial cells. Lab Invest. 2002;82:1073–1079. doi: 10.1097/01.lab.0000022223.65962.3a. [DOI] [PubMed] [Google Scholar]
- 8.Zheng W, Wang H, Xue L, Zhang Z, Tong T. Regulation of cellular senescence and p16(INK4a) expression by Id1 and E47 proteins in human diploid fibroblast. J Biol Chem. 2004;279:31524–31532. doi: 10.1074/jbc.M400365200. [DOI] [PubMed] [Google Scholar]
- 9.Alani RM, Young AZ, Shifflett CB. Id1 regulation of cellular senescence through transcriptional repression of p16/Ink4a. Proc Natl Acad Sci USA. 2001;98:7812–7816. doi: 10.1073/pnas.141235398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polsky D, Young AZ, Busam KJ, Alani RM. The transcriptional repressor of p16/Ink4a, Id1, is upregulated in early melanomas. Cancer Res. 2001;61:6008–6011. [PubMed] [Google Scholar]
- 11.Ryu B, Kim DS, Deluca AM, et al. Id1 expression is transcriptionally regulated in radial growth phase melanomas. Int J Cancer. 2007;121:1705–1709. doi: 10.1002/ijc.22875. [DOI] [PubMed] [Google Scholar]
- 12.Straume O, Akslen LA. Strong expression of ID1 protein is associated with decreased survival, increased expression of ephrin-A1/EPHA2, and reduced thrombospondin-1 in malignant melanoma. Br J Cancer. 2005;93:933–938. doi: 10.1038/sj.bjc.6602792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunlap S, Yu X-B, Cheng L-Z, Civin CI, Alani RM. High-efficiency stable gene transduction in primary human melanocytes using a lentiviral expression system. J Invest Dermatol. 2004;122:549–551. doi: 10.1046/j.0022-202X.2004.22214.x. [DOI] [PubMed] [Google Scholar]
- 14.Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB. Methylation-specific PCR: A novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA. 1996;93:9821–9826. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meeker AK, Hicks JL, Platz EA, et al. Telomere shortening is an early somatic DNA alteration in human prostate tumorigenesis. Cancer Res. 2002;62:6405–6409. [PubMed] [Google Scholar]
- 16.Yu X, Zhan X, D’Costa J, et al. Lentiviral vectors with two independent internal promoters transfer high-level expression of multiple transgenes to human hematopoietic stem-progenitor cells. Mol Ther. 2003;7:827–838. doi: 10.1016/s1525-0016(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 17.Yokota Y, Mori S. Role of Id family proteins in growth control. J Cell Physiol. 2002;190:21–28. doi: 10.1002/jcp.10042. [DOI] [PubMed] [Google Scholar]
- 18.Ohtani N, Zebedee Z, Huot TJ, et al. Opposing effects of Ets and Id proteins on p16INK4a expression during cellular senescence. Nature. 2001;409:1067–1070. doi: 10.1038/35059131. [DOI] [PubMed] [Google Scholar]
- 19.Campisi J. Senescent cells, tumor suppression, and organismal aging: Good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Rocco JW, Sidransky D. p16(MTS-1/CDKN2/INK4a) in cancer progression. Exp Cell Res. 2001;264:42–55. doi: 10.1006/excr.2000.5149. [DOI] [PubMed] [Google Scholar]
- 21.Michaloglou C, Vredeveld LC, Soengas MS, et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436:720–724. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- 22.Gray-Schopfer VC, Cheong SC, Chong H, et al. Cellular senescence in naevi and immortalisation in melanoma: A role for p16? Br J Cancer. 2006;95:496–505. doi: 10.1038/sj.bjc.6603283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pollock PM, Harper UL, Hansen KS, et al. High frequency of BRAF mutations in nevi. Nat Genet. 2003;33:19–20. doi: 10.1038/ng1054. [DOI] [PubMed] [Google Scholar]
- 24.Sviderskaya EV, Hill SP, Evans-Whipp TJ, et al. p16(Ink4a) in melanocyte senescence and differentiation. J Natl Cancer Inst. 2002;94:446–454. doi: 10.1093/jnci/94.6.446. [DOI] [PubMed] [Google Scholar]
- 25.Govindarajan B, Klafter R, Miller MS, et al. Reactive oxygen-induced carcinogenesis causes hypermethylation of p16(Ink4a) and activation of MAP kinase. Mol Med. 2002;8:1–8. [PMC free article] [PubMed] [Google Scholar]