Abstract
Introduction
The second messengers cAMP and cGMP mediate fundamental aspects of brain function relevant to memory, learning and cognitive functions. Consequently, cyclic nucleotide phosphodiesterases (PDEs), the enzymes that inactivate the cyclic nucleotides, are promising targets for the development of cognition-enhancing drugs.
Areas covered
PDE4 is the largest of the eleven mammalian PDE families. This review covers the properties and functions of the PDE4 family, highlighting procognitive and memory-enhancing effects associated with their inactivation.
Expert opinion
PAN-selective PDE4 inhibitors exert a number of memory- and cognition-enhancing effects and have neuroprotective and neuroregenerative properties in preclinical models. The major hurdle for their clinical application is to target inhibitors to specific PDE4 isoforms relevant to particular cognitive disorders to realize the therapeutic potential while avoiding side effects, in particular emesis and nausea. The PDE4 family comprises four genes, PDE4A-D, each expressed as multiple variants. Progress to date stems from characterization of rodent models with selective ablation of individual PDE4 subtypes, revealing that individual subtypes exert unique and non-redundant functions in the brain. Thus, targeting specific PDE4 subtypes, as well as splicing variants or conformational states, represents a promising strategy to separate the therapeutic benefits from the side effects of PAN-PDE4 inhibitors.
Keywords: Alzheimer’s disease, schizophrenia, cognition, memory, cyclic nucleotide, cAMP, phosphodiesterase, PDE4, Rolipram
1. Introduction/Cyclic nucleotide phosphodiesterases (PDEs) and cognition
Cognition is a broad term that encompasses the ability of the brain to process and store information and then analyze this information in the context of the present to respond and plan for the future. While cognitive dysfunction is a prominent feature of all neurodegenerative and neuropsychiatric diseases [1], the nature of the dysfunction and its cause is disease specific. In autism and schizophrenia, for example, cognitive dysfunction arises from defects early or late in brain development.
As life expectancy in the industrialized world has steadily increased over the past century, so has the prevalence of age-related diseases of the brain. These are generally associated with memory loss and cognitive impairment as exemplified by Alzheimer’s disease and other forms of dementia as well as Parkinson’s disease and Huntington’s disease. Despite significant research and development efforts, there remains a large unmet need for cognition-enhancing drugs. The drugs currently in use for dementia, acetylcholinesterase inhibitors and NMDA receptor antagonists, for example, show only limited effectiveness and do not provide lasting benefits as the patient’s status inevitably declines over time, nor are there drugs that slow, arrest, or reverse the progressive neurodegeneration [2]. For autism and schizophrenia, there are no drugs to treat the cognitive deficits, let alone ameliorate the developmental defects. Cyclic nucleotide (cAMP and cGMP) signaling is fundamentally involved in brain mechanisms that actively mediate cognitive processes, as well as in brain development and homeostasis that provides the milieu for cognition. As a consequence, there has been significant interest in targeting cyclic nucleotide phosphodiesterases (PDEs), the enzymes that hydrolyze and inactivate these second messengers, as cognition-enhancing drugs [3-8].
1.1. The superfamily of mammalian PDEs
The mammalian PDEs comprise a superfamily of enzymes that are encoded by 21 genes. These are divided into eleven PDE families based on sequence homology, pharmacological properties and substrate specificity [9]. Of the eleven PDE families, three include enzymes that selectively hydrolyze cAMP (PDE4, PDE7 and PDE8), three comprise enzymes selective for cGMP (PDE5, PDE6, and PDE9) and the remaining five PDE families hydrolyze both cyclic nucleotides (PDE1, PDE2, PDE3, PDE10 and PDE11). Most PDE genes are expressed as multiple variants due to alternative splicing and use of multiple promoter/transcription start sites. As a result, up to 100 individual PDE proteins are expressed in mammals. Individual PDEs differ not only in their catalytic properties but also in their tissue and cell-specific expression pattern, their subcellular localization as determined by protein/protein or protein/lipid interactions and the regulation of their catalytic activity by post-translational modifications and allosteric regulators [10]. This complexity provides the body with an array of PDE isoforms with precise functions, and provides us with an array of unique targets for drug development.
1.2. PDEs as a target for cognition enhancement
Almost all PDE genes are expressed in the brain, which also expresses the highest level of PDE activity of any tissue. This underlines the importance of tightly controlling the second messengers cAMP and cGMP in an organ whose primary purpose is the processing of information via cell signaling. Intriguingly, almost all PDE families are being considered as targets for treating central nervous system (CNS) disorders. However, although inhibition of many PDEs produces potentially beneficial CNS effects, the mechanisms by which such effects are generated must clearly be distinct given the differences among individual PDEs, particularly their differential cellular and subcellular localization as well as substrate specificity and kinetics. In line with this idea, inhibitors of distinct PDE families have been shown to affect different stages of memory consolidation [11]. Individual brain diseases exhibit distinct forms of cognitive deficits and are associated with defective development or degeneration in different regions of the brain. Thus, distinct PDEs may be targeted for therapeutic benefits for different diseases, and matching the right PDE target with each CNS disorder is critical for drug development. This review discusses the potential of targeting members of the PDE4 family, the largest of the eleven PDE families, for cognition enhancement. See [7] for a review on other CNS PDEs as targets for drug development.
2. The PDE4 family as a target for cognition enhancement
The PDE4 family comprises four genes, PDE4A to D. PDE4s are easily distinguished from other cAMP-PDEs by their kinetic properties and, particularly, their sensitivity to inhibition by the prototypical PDE4 inhibitor, Rolipram. Soon after their discovery more than 30 years ago, several lines of evidence indicated a role for PDE4s in regulating brain function. First, PDE4s are widely expressed throughout the body, but highest concentrations are found in brain. Second, the mammalian PDE4s are orthologs of the dunce gene of Drosophila melanogaster, whose ablation produces a phenotype of impaired learning and memory in the fly [12-14]. And third, the prototypical PDE4 inhibitor Rolipram was shown to exert behavioral and antidepressant effects in rats and humans [15-17]. Since then, a plethora of other CNS effects have been reported for PAN-PDE4 inhibitors in preclinical models including memory- and cognition-enhancing effects as well as neuroprotective and neuroregenerative properties. More recently, the characterization of knock-out mice for individual PDE4 genes as well as siRNA-mediated knock-down has clearly established that individual PDE4 subtypes and splicing variants play unique and non-overlapping roles [9,18]. Thus, distinct PDE4 proteins could be specifically targeted for therapeutic benefits in different CNS diseases.
2.1. Structure and regulation of PDE4
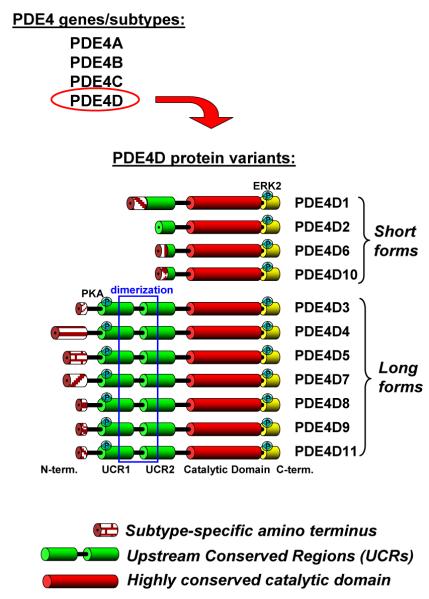
Each of the four PDE4 genes is expressed as multiple variants, together generating more than 25 individual proteins. These share a highly conserved catalytic domain and, thus, exhibit very similar kinetic properties. The catalytic domains are flanked by N- and C-terminal domains that function to regulate enzyme activity, provide mechanisms of crosstalk with other pathways, and determine the subcellular localization of these enzymes (Figure 1) [10,19]. The distinctive structural elements of the PDE4 family are two highly conserved N-terminal domains termed Upstream Conserved Regions 1 and 2 (UCR1 and UCR2). PDE4 splice variants are divided into long and short forms based on the presence or absence of the UCR domains. Long forms contain a complete set of UCR domains, whereas short forms lack UCR1 but still retain UCR2, either in its entirety or as a fragment. Highlighting the critical role of the UCR domains, the presence or absence of UCR1 entails critical structural and functional differences between long and short forms including oligomerization, regulation of enzyme activity by binding allosteric ligands or post-translational modification as well as inhibitor sensitivity. The UCR1/2 module mediates oligomerization of long PDE4 forms, whereas short PDE4s are monomers and this difference in quaternary structure is responsible for distinct sensitivities of long and short forms towards the prototypical PDE4 inhibitor Rolipram [20,21]. The UCR1 domain contains a consensus PKA-phosphorylation site that is conserved among all long forms and phosphorylation at this site induces activation of long PDE4s [22,23]. This PKA-mediated PDE4 activation constitutes a critical negative feedback loop whereby elevated levels of cAMP promote their own destruction [23-25]. The UCR1 domain also contains a docking site for phosphatidic acid that acts as an allosteric activator of long PDE4s [26,27]. Short forms not only lack the PKA- and phosphatidic acid-mediated regulation of enzyme activity, but also respond differently to post-translation modifications that they share with long forms. PDE4B, PDE4C and PDE4D variants, for example, contain a conserved ERK2 phosphorylation site at their C-termini. Phosphorylation at this ERK2 site induces inhibition of long variants, whereas phosphorylation of short forms induces enzyme activation or has no effect on enzyme activity [28,29]. The extreme N-termini of PDE4 variants are generally encoded by variant-specific exons and are, thus, unique to each individual variant. These domains are often involved in intracellular targeting of PDE4s via protein/protein or protein/lipid interactions, thus providing the cell with a diverse set of PDE4 variants that can be sequestered/localized to control distinct subcellular pools of cAMP [19].
Figure 1. Members, domain organization and regulatory properties of the PDE4 family.
The PDE4 family comprises four genes, PDE4A-D, and each is expressed as multiple variants. At least six PDE4A variants (PDE4A1, PDE4A4/5, PDE4A7, PDE4A8, PDE4A10 and PDE4A11), five PDE4B variants (PDE4B1-5), three PDE4C variants (PDE4C1-3), and eleven PDE4D variants (PDE4D1-11) have been reported. Shown is the domain organization of the eleven variants generated from the PDE4D gene. Domains are depicted as ‘barrels’ connected by ‘wires’ indicating linker regions. Domains functioning as targeting sequences by mediating protein/protein interactions are indicated as red striated barrels. These are generally encoded by variant-specific first exons. Phosphorylation sites for protein kinase A (PKA) and extracellular signal-regulated kinase 2 (ERK2) are depicted as cyan circles. Long and short PDE4 variants are distinguished by the complete or partial presence of the UCR1/2 module. The regions of the UCR domains that mediate dimerization of PDE4 long forms are indicated by a blue rectangle.
2.2. Memory- and cognition-enhancing effects of PAN-selective PDE4 inhibitors
To date, the pharmacological tools to investigate the PDE4s have been limited to catalytic-site competitive inhibitors such as Rolipram with very limited selectivity for different isoforms. Nonetheless, there is now a substantial number of preclinical studies with such compounds demonstrating that PDE4 inactivation improves learning and memory in a variety of behavioral tests. These include spatial working and reference memory tests, object recognition memory tasks and passive avoidance and fear conditioning paradigms [30-34]. While most studies were performed in mice and rats, cognition-enhancing effects of PDE4 inhibitors have also been demonstrated in monkeys [35] suggesting that memory enhancement can be achieved in a range of mammalian species and this posit might also apply to humans.
Importantly, cognition- and memory-enhancing effects of PDE4 inhibition are not only observed in normal, healthy animals but have also been shown to ameliorate and/or reverse memory deficits induced by a range of manipulations that simulate age- and/or disease-related memory impairments. These include memory-deficits induced by the muscarinic acetylcholine receptor antagonist scopolamine in novel object recognition and spatial memory tasks [31,36-40] as well as memory and cognition impairments induced by the N-methyl-D-aspartate (NMDA) receptor antagonist MK-801 [39,41,42], the MEK inhibitor U0126 [43] or by acute tryptophan depletion [44]. PDE4 inhibition was also shown to ameliorate cerebral ischemia-induced neuron loss and associated memory deficits in rats suggesting a potential for PDE4 inhibitors to prevent memory-loss in stroke patients [45]. Of particular relevance for preventing age-dependent decline in memory and cognition are studies showing that PDE4 inhibition can ameliorate memory deficits due to aging [30,46], but see [47].
PDE4 inhibition also reversed memory deficits in genetic models of human disease in rodents. Rubinstein-Taybi syndrome, for example, is a genetic disease characterized by defects in the CREB-binding protein (CBP). PDE4 inhibition improves long-term memory in a mouse model of impaired CBP suggesting a potential therapeutic benefit of PDE4 inhibitors for Rubinstein-Taybi syndrome [48]. Several studies revealed a potential for PDE4 inhibitors to reverse memory deficits induced by β-amyloid, suggesting a therapeutic potential for PDE4 inhibitors in treating Alzheimers’ disease. Rolipram reverses β-amyloid-induced inhibition of the PKA/p-CREB pathway as well as long-term potentiation (LTP) in cultured hippocampal neurons [49] and PDE4 inhibitors showed similar effectiveness in a double transgenic mouse expressing amyloid precursor protein and presenilin-1 (APP(K670N/M671L)/PS1 mice) [50]. Rolipram treatment improves LTP and synaptic transmission as well as working, reference and associative memory deficits in this model, which replicates synaptic and cognitive impairments of Alzheimer’s [50]. Finally, inhibition of PDE4 reverses memory deficits induced by β-amyloid peptide infusion in the brains of rats. Rolipram treatment counteracts β-amyloid-induced deficits in CREB phosphorylation in the hippocampus as well as behavioral deficits in the Morris water maze and passive avoidance tasks [51,52]. PDE4 inhibition also reversed memory deficits and oxidative stress induced by intracerebroventricular administration of streptozotocin; a model of experimental sporadic dementia [53], suggesting a potential of PDE4 inhibition for reducing cognitive decline in a range of settings.
Importantly, memory- and cognition-enhancing effects are not only observed with Rolipram, but also with structurally unrelated PDE4 inhibitors [31-33,39,53], suggesting that these are class effects and result directly from inactivation of PDE4s. This conclusion is further confirmed by studies employing genetic downregulation or ablation of PDE4 subtypes, in particular PDE4D, as described in more detail below.
Although their cognition- and memory-enhancing effects are now well established, the mechanism of action of PDE4 inhibitors and the downstream targets that mediate their procognitive effects are less well defined. There is strong evidence that PDE4 inactivation stimulates and/or reverses deficits in PKA/p-CREB signaling induced by pharmacological agents, aging or disease [8,34,48,49,51,52,54,55] but may also act via regulation of neurotransmitter release [44,56] (Figure 2). Mechanistically, PDE4 inactivation serves to enhance LTP as well as promote synaptic plasticity [30,49,50,57,58]. Consistent with these results, pharmacologically-induced increases in PDE4, in particular PDE4D, reduce cAMP levels in the hippocampus and impair long-term memory in the water-maze test [59].
Figure 2. PDE4 inhibition, cAMP signaling and brain function.
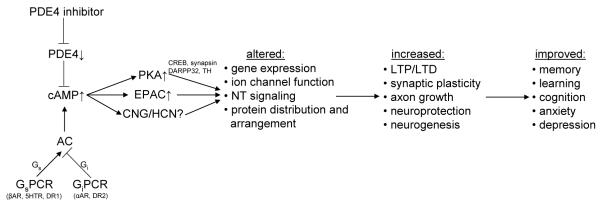
Scheme illustrating the signaling events linking PDE4 inhibition to improved brain function. Inhibition of PDE4 triggers an increase in cAMP which in turn exerts its downstream effects via activation of protein kinase A (PKA), exchange proteins activated by cAMP (EPACs) and cyclic nucleotide-gated (CNG) and hyperpolarization-activated cyclic nucleotide-modulated (HCN) channels. PKA is well known to phosphorylate a range of signaling proteins including CREB (cAMP response element binding protein), synapsin, DARPP32 (dopamine- and cAMP-regulated phosphoprotein of Mr 32 kDa) and tyrosine hydroxylase (TH) and thereby modulate gene expression, ion channel function, neurotransmitter synthesis and release as well as various other signaling events that regulate memory and cognitive processes. Recent evidence suggests that EPAC is involved in many of the same signaling paradigms previously associated with PKA, suggesting that some of the established effects of PDE4 inhibitors on memory and cognition might be mediated by EPAC rather than PKA activation [147-154]. Although a role for cyclic nucleotide-regulated channels (CNG and HCN) in brain function is well established [155,156], little is known thus far about the role of PDEs in regulating the pool of cAMP that controls the function of these channels. NT, neurotransmitter; AC, adenylyl cyclase; GsPCR, Gs protein-coupled receptor; GiPCR, Gi protein-coupled receptor; βAR, β-adrenergic receptor; αAR, α-adrenergic receptor; DR, dopamine receptor; 5HTR, serotonin type 1D receptor
2.3. Neuroprotective, neuroregenerative and anti-inflammatory properties of PAN-PDE4 inhibitors
In addition to affecting active cognitive mechanisms, PDE4 inhibition may prevent disruption of brain homeostasis that results in cognitive dysfunction. This includes promoting neurogenesis and exerting neuroprotective and regenerative as well as anti-inflammatory effects (Figure 2). In one of the earliest studies revealing a neuroprotective effect of PDE4 inhibition, Yamashita et. al. demonstrated increased survival of cultured dopaminergic neurons upon Rolipram treatment [60]. Since then, a number of studies reported neuroprotective properties of PDE4 inhibitors in preclinical models where they may act via suppression of apoptosis, promotion of neurogenesis, a reduction of oxidative stress as well as attenuation of inflammatory responses [45,52,53,61,62]. Specifically, PDE4 inhibition was shown to ameliorate cerebral ischemia-induced neuron loss in hippocampal region CA1 and associated memory deficits in rats, suggesting a potential for PDE4 inhibitors to prevent memory loss in stroke patients [45]. In addition, PDE4 inhibition reversed memory deficits and oxidative stress after intracerebroventricular administration of streptozotocin, a model of experimental sporadic dementia [53], and knockout or knock-down of PDE4D and/or inhibition of PDE4 with Rolipram has been shown to promote neurogenesis in hippocampus [34,54,55].
Intriguingly, PDE4 inhibition potently reversed cognitive impairments and neuroinflammatory responses induced by treatment with β-amyloid peptides including microglia activation, activation of NFκB and release of pro-inflammatory cytokines such as TNFα. These findings strongly imply a potential for PDE4 inhibitors to treat Alzheimer’s disease [52,62].
Finally, PDE4 inhibition has been shown to promote axonal regeneration and functional recovery as well as a reduction in inflammatory responses and astrogliosis after spinal cord injury [61,63].
2.4. Not all cAMP signaling is procognitive
A complication in the development of PDE4 inhibitors as memory- and cognition-enhancing drugs is the fact that cAMP/PKA activation (in general, and after PDE4 inhibition in particular) does not exclusively exert procognitive effects. A case in point is the Drosophila dunce gene. The phosphodiesterase encoded by the dunce gene is the evolutionary ancestor of the mammalian PDE4s and its role in olfactory learning provided the first indication that PDE4s regulate CNS functions. However, while inhibition of the mammalian PDE4s is pursued as a way to improve memory and cognition, inactivating mutations in the dunce gene result in memory impairment, specifically impairment of early memory formation [12,14]. Although a large majority of studies attest to positive memory- and cognition enhancing effects of PDE4 inactivation, pharmacologic and/or genetic ablation of the mammalian PDE4s have also been shown to impair learning and memory in some paradigms [47,64-66]. Given the multitude of downstream targets and cellular functions regulated by cAMP/PKA signaling, it is not surprising that cAMP signaling does not exclusively exert beneficial effects on memory and cognition, but may also induce some untoward effects (see [67]).
Even in cAMP pathways that are therapeutically relevant, the level of PDE4 inhibition is critical to realize pro-cognitive effects. Physiological cAMP signals are generally short lived and the shape of cAMP transients is critical to induce the appropriate cellular responses [68]. Amplifying the cAMP transient may serve to amplify the cellular responses and thereby mediate therapeutic benefits. However, increasing cAMP levels above a certain threshold to supraphysiologic levels may essentially disrupt signaling, as downstream effectors are chronically switched on. Such conditions likely trigger compensatory mechanisms at other steps to desensitize the cAMP signaling cascade and these compensatory effects, rather than increased cAMP signaling per se, might induce memory and cognitive deficits (see for example [59]). This hypothesis fits well with some of the scenarios where PDE4 inactivation produces deficits. Memory deficits in R6 mice, a model of Huntington’s disease, for example, are associated with a reduced expression of hippocampal PDE4s and hyperactivation of PKA [64]. Importantly, while acute PDE4 inhibition is generally memory-enhancing, chronic treatment of wild type mice with high doses of Rolipram (22 days; 5 mg/kg/day i.p.) produced a similar upregulation of PKA activity and associated learning and memory deficits, but had no further effect in R6 mice [64]. Conversely, lower doses of Rolipram have shown neuroprotective effects in models of Huntington’s disease, including the R6 mice [69,70]. High doses of Rolipram also impaired prefrontal cortical function in aged, but not young monkeys, and the age-specific effects may be due to the fact that the cAMP/PKA pathway is already disinhibited in the aged prefrontal cortex [47,67]. Thus, PDE4 inhibition may exert deleterious effects on memory and cognition under conditions in which PDE4s are already downregulated and cAMP levels and PKA activity are elevated. On the other hand, targeting PDE4s can be expected to mediate beneficial effects in diseases characterized by cognitive deficits resulting from reduced/impaired cAMP/PKA signaling. Under these circumstances, PDE inactivation can serve to reverse memory and cognition deficits by restoring “normal” cAMP signaling. The untoward effects induced by cAMP/PKA hyperstimulation may also suggest that submaximal doses of PDE4 inhibitors that induce partial, rather than full inhibition of the enzymes might be more effective for memory- and cognition-enhancement.
2.5. Challenges in PDE4 inhibitor development
The PDE4 family is the most complex PDE family and arguably the most widely expressed PDE throughout the human body. As its members contribute a significant portion of total cAMP hydrolyzing activity in many mammalian cells and tissues, it is not surprising that PDE4 inactivation produces a range of physiological effects that can be exploited for drug development for multiple indications including, but by no means limited to memory- and cognition-enhancement [18]. At the same time however, the ubiquitous expression of PDE4s in human cells and tissues constitutes the major obstacle to the clinical application of PDE4 inhibitors for any indication, as PAN-PDE4 inactivation is bound to trigger a range of effects, including dose-limiting side effects. Of these, by far the most serious are nausea and emesis.
Adverse gastrointestinal effects including emesis, nausea and diarrhea are the main side effects associated with PAN-PDE4 inhibitors. They were first reported for the prototypical PDE4 inhibitor Rolipram [15,71,72] and are shared to varying degrees by all PAN-PDE4 inhibitors. Thus, emesis and nausea are clearly class effects of PDE4 inhibitors. They are likely due to inhibition of PDE4 in brain regions responsible for the emetic reflex, such as the area postrema and nucleus of the solitary tract in which PDE4 is highly expressed [73-75] as well as PDE4 inhibition in the gut, and remain the main obstacles for PDE4 inhibitor development today.
The finding that PDE4 inactivation can induce development of heart failure in mice [76] and promote arrhythmias in mice as well as human atrial strips ex vivo [76-78] raises the question of whether PDE4 inactivation might also induce cardiac toxicities in humans. In addition, SNPs in PDE4D that are associated with reduced PDE4D expression have been identified as a risk factor for stroke [79] and high doses of at least one PDE4 inhibitor, SCH 351591, have been shown to cause vasculitis in monkeys [80]. However, long-term clinical trials with the PDE4 inhibitor Roflumilast did not uncover significantly increased incidences of adverse cardiovascular events suggesting that, at least at the doses given, PAN-PDE4 inhibition with Roflumilast does not cause cardiovascular toxicities in humans [81,82].
In summary, there is an impressive breadth of data supporting the idea that PDE4 inhibition may improve cognitive function in a range of conditions and by multiple mechanisms. On the other hand, because of the widespread distribution of PDE4 isoforms in the brain and elsewhere in the body, PAN-PDE4 inhibitors have significant liabilities that have precluded their clinical use for the treatment of neuropsychiatric and cognitive dysfunction. Thus, interest has turned to identifying the particular PDE4 isoforms that are most relevant as targets for cognition enhancement while avoiding the side effects that have so far precluded drug development. To date, the focus has been on investigating the role of individual PDE4 subtypes, PDE4A-D. This has been enabled by the availability of knock-out mice and other genetic means of manipulating the expression of these isoforms.
3. Role of individual PDE4 subtypes
Messenger RNA and the corresponding proteins for PDE4 subtypes PDE4A, PDE4B and PDE4D are abundantly expressed and widely distributed in the mammalian brain. Detailed analyses reveal that the expression patterns of individual PDE4 subtypes are clearly distinct at the regional and cellular level suggesting that each PDE4 subtype may serve unique functions [73,83-85]. This conclusion is further reinforced when expression patterns of individual PDE4 variants, rather than all variants generated from the same gene, are determined [73,83,86-91]. Unfortunately, the subcellular distribution of PDE4 variants has remained largely unexplored, a gap that needs to be filled to facilitate rational target identification. Despite some differences, the expression patterns of PDE4A, PDE4B and PDE4D show substantial similarity in the brain of rodents, monkeys and humans. This indicates a conservation of PDE functions and suggests that findings in animal models may also apply in humans.
In line with the idea of specialized functions for individual PDE4s, characterization of rodent models with genetic inactivation of individual PDE4 subtypes confirm the idea that individual PDE4 subtypes exert unique and non-redundant functions. The effect of PDE4 subtype ablation on allergen-induced airway hyperreactivity is a good example to illustrate this point. Ablation of PDE4A, PDE4B or PDE4D in mice mimics the effect of PAN-selective PDE4 inhibition in ablating allergen-induced airway hyperreactivity [18,92-94]. However, the mechanisms of action appear to be distinct, with PDE4D inactivation ablating airway smooth muscle contractility [94], PDE4B ablation impairing inflammatory responses by reducing infiltration of immune cells and the release of inflammatory cytokines [18,93,95], and PDE4A ablation acting via a yet unknown mechanism. By analogy, it may be anticipated that ablation of the different PDE4s may overlap in mimicking the procognitive effects of PAN-PDE4 inhibitors at the behavioral level, but do so by different cellular mechanisms. The current literature on the expression and cognitive functions of individual PDE4 subtypes, which is beginning to address this question, is discussed below.
3.1. PDE4A
PDE4A is expressed in multiple regions of the mammalian brain with high levels found in cerebral cortex, hippocampus and cerebellum [73,84,85,88]. Multiple PDE4A variants, including PDE4A1, PDE4A4/5, PDE4A8 and PDE4A10 are present, and exhibit clearly distinct localization patterns [87,88,96]. PDE4A expression levels are altered in certain patient populations, including patients with bipolar disorder and autism [97,98], providing a first association between PDE4A function and CNS disorders.
Although its brain expression pattern suggests a role for PDE4A in cognition and memory, the effect of PDE4A ablation on cognitive functions has not been probed directly as yet because subtype-selective PDE4A inhibitors are not available and PDE4A-KO mice were generated only recently [93]. Nonetheless, there is some circumstantial evidence for a role for PDE4A in memory processes. Brief sleep deprivation, for example, produces deficits of synaptic plasticity and hippocampus-dependent memory in mice via increased expression of specific PDE4A variants and impaired cAMP/PKA signaling in the hippocampus [99]. Treatment with the PAN-PDE4 inhibitor Rolipram reverses the deficits of both synaptic plasticity and memory suggesting a role for PDE4A in this paradigm of sleep-deprivation-induced memory deficit. Inhibition of PDE4A may also be the mechanism by which PAN-PDE4 inhibitors attenuate memory deficits induced by the NMDA receptor antagonist MK-801 [39,41] given that NMDA receptor activity controls PDE4A expression in cultured neurons [100].
3.2. PDE4B
PDE4B is widely distributed throughout the brain in rodents, monkeys and humans, with high levels of expression found in the striatum, amygdala, thalamus and hypothalamus [73,84,85,90]. Long term potentiation (LTP) in hippocampal neurons was found associated with changes in expression and subcellular localization of PDE4B providing a first association between PDE4B and learning and memory [101-103]. The PAN-PDE4 inhibitor Rolipram affects synaptic plasticity by promoting both LTP as well as long-term depression (LTD) [30,57,104]. Interestingly, mice deficient in PDE4B show enhanced LTD, whereas LTP is not affected [66]. Conversely, mice deficient in PDE4D show enhanced LTP without an effect on LTD [65]. These findings may suggest that the effects of PDE4 inhibition on LTP and LTD are mediated via inactivation of distinct PDE4 subtypes, which would support the idea that individual PDE4 subtypes play unique and non-overlapping roles. However, mice deficient in PDE4B behaved similarly to wild type controls in a series of behavioral tests including passive avoidance tests, the Morris water-maze task, the hot plate and the elevated plus maze test as well as in fear conditioning models, suggesting that inactivation of PDE4B is not the primary mechanism by which PAN-PDE4 inhibitors enhance memory in these and similar models [66,105,106].
PDE4B may be attractive as a therapeutic target for memory and cognition impairment associated with age-dependent or neurodegenerative diseases through different mechanisms, namely through neuroprotective, neuroregenerative and anti-inflammatory activities. In line with this idea, an increase in the proliferation of neuronal cells in the hippocampal dentate gyrus has been reported for PDE4B-deficient mice [106] and the well-established anti-inflammatory properties of PDE4B inactivation [18,95] may limit neuroinflammation and, thus, disease progression in Alzheimer’s [107]. Indeed, recent reports have shown that β-amyloid-induced microglia activation is associated with increased expression of PDE4B and that treatment with PDE4 inhibitors may limit microglia activation by reducing TNFα release [62].
Several lines of evidence have implicated PDE4B in schizophrenia. Disruption of PDE4B by a balanced translocation resulted in schizophrenia in a Scottish patient [108] and PDE4B has been shown to interact with DISC1 (disrupted in schizophrenia 1), a well-established risk factor for schizophrenia [108,109]. In addition, several studies have identified single nucleotide polymorphisms in the PDE4B gene that were associated with schizophrenia [108,110-113], but see [114,115]. Finally, a decrease in the levels of PDE4B expression in post-mortem brains of schizophrenia patients has been reported [110]. In behavioral models relevant to schizophrenia, mice deficient in PDE4B evidenced a complex phenotype of reduced prepulse inhibition, reduced spontaneous locomotor activity but exaggerated locomotor activity in response to high doses of amphetamine [105]. Further study is warranted to understand the role of PDE4B dysfunction and the ways in which this subtype may be targeted in treatment of different aspects of schizophrenia.
Cognitive dysfunction is one of the major problems associated with chronic alcohol consumption [116]. Since Rolipram reduces alcohol consumption and alcohol preference in animals drinking excessive alcohol [117,118], PDE4 inhibitors may produce beneficial effects in alcoholics. PDE4B may be important in this paradigm given its enrichment in the striatum and nucleus accumbens, which regulate alcohol intake and cognition related to drug abuse [119].
Finally, PDE4B-KO mice display a range of anxiogenic-like behaviors including reduced exploratory activity in hole board, light-dark transition and open-field tests [106]. Clearly, PDE4B plays multiple, complex roles in brain physiology and the regulation of behavior that may be exploited for therapeutic benefit.
3.3 PDE4C
Expression of PDE4C in the brain is limited, with some mRNA detected in the olfactory bulb in rats, and in several cortical areas and the cerebellum in humans and monkeys [84,85]. Low cellular and tissue expression levels of a PDE do not exclude important functions for this enzyme per se, as PDEs may exert critical functions by acting in subcellular microdomains of signaling. The role of PDE4C is largely unexplored, however, because research tools, such as knock-out animals or subtype-selective inhibitors have not been available to study the subtype-selective inactivation of PDE4C.
3.4. PDE4D
Multiple lines of evidence indicate that PDE4D is a primary target through which PAN-selective PDE4 inhibitors exert memory- and cognition-enhancing effects. PDE4D is highly expressed in hippocampal formations [73,85,89] and mice deficient in PDE4D display enhanced LTP in hippocampal CA1 [65]. Notably, RNAi-mediated downregulation (PDE4D-KD) or genetic ablation of PDE4D (PDE4D-KO) in mice replicates a series of behavioral phenotypes that define the memory- and cognition-enhancing properties of PDE4 inhibitors, which include improvement in spatial memory and object recognition tests [34,120]. Treatment with PDE4 inhibitors improved cognitive function in wild type mice, but had no further effect in PDE4D-KO or PDE4D-KD mice, suggesting that ablation of PDE4D is sufficient to promote cognition and memory enhancements. Analogous to the effects of PDE4 inhibitors, genetic ablation of PDE4D increased cAMP/PKA/p-CREB signaling and promoted hippocampal neurogenesis [34]. Finally, PDE4 inhibitors with some selectivity for PDE4D showed similar efficacy in enhancing memory and cognition as the non-selective inhibitor Rolipram [31,36,39]. Somewhat surprisingly, knock-down of PDE4D in rats [121] did not reproduce the improvements in spatial memory observed with PDE4 inhibitors or genetic ablation of PDE4D in mice. PDE4D-KO mice also showed impaired learning in fear-conditioning paradigms [65] suggesting that PDE4D ablation may produce distinct effects in different behavioral tests depending on the type of memory probed and the brain region involved.
In addition to memory enhancement, genetic ablation of PDE4D in mice or down-regulation of PDE4D expression in mice and rats produced behavioral phenotypes indicative of antidepressant activity such as decreased immobility in tail-suspension and forced-swim-tests [120-122]. These phenotypes recapitulate the established antidepressant effects of PAN-PDE4 inhibitors in the same paradigms. PDE4 inhibitors produced no further effects in PDE4DKO mice [122], identifying PDE4D as a critical target for the antidepressant properties of PANPDE4 inhibitors.
4. Approaches for developing the next generation of PDE4 inhibitors
Current approaches for limiting the side effects associated with PAN-PDE4 inhibitors are based on the same general idea: develop compounds that selectively inhibit only a fraction of the total PDE4 present in the body. The underlying idea is to separate the therapeutically beneficial from the unwanted side effects by increasing the specificity of inhibitors for the fraction of PDE4 mediating the former, such as memory- and cognition-enhancing effects.
4.1. Targeting conformational states of PDE4 - HARBS/LARBS
PDE4s exist in at least two distinct conformational states, termed high-affinity Rolipram binding state (HARBS) and low-affinity Rolipram binding state (LARBS), which are revealed by their distinct affinities for the prototypical PDE4 inhibitor Rolipram. The potency of PDE4 inhibitors to induce emesis appears more closely correlated with their affinity for HARBS. Conversely, LARBS is prevalent in peripheral cells and tissues and mediates many of the well-established anti-inflammatory properties of PDE4 inhibitors [123,124]. This prompted the idea that compounds with bias towards LARBS over HARBS would mediate therapeutic benefits (specifically anti-inflammatory effects) without inducing emesis. Drug development efforts to this end resulted in several compounds with some selectivity towards LARBS or at least similar affinities for both conformational states. Roflumilast, which is used for the treatment of chronic obstructive pulmonary disease (COPD) and is the only PDE4 inhibitor approved for any indication to date, is an example of this class of compounds [81,82]. Although emesis and nausea remain its predominant side effects, Roflumilast has a wider therapeutic window compared to Rolipram and the fact that Roflumilast binds with similar affinity to both HARBS and LARBS is likely one of the reasons for the success of this compound.
Although a preferential interaction with LARBS is a worthwhile approach for the development of PDE4 inhibitors as anti-inflammatory drugs, this strategy might not be suitable for therapeutics targeting the CNS because HARBS is highly enriched in the brain and some of the therapeutically beneficial effects of PDE4 inhibitors, including their memory- and cognition-enhancing properties, may well be due to inhibition of HARBS. Indeed, the antidepressant effects of Rolipram have been associated with Rolipram-binding to HARBS [125].
4.2. Subtype-selective PDE4 inhibitors
With the characterization of knock-out mice deficient in individual PDE4 genes, it was clearly established that individual PDE4 subtypes play unique and often non-overlapping physiological and pathophysiological roles [18,95,126]. Development of subtype-selective PDE4 inhibitors may thus open the opportunity to dissect therapeutically beneficial from the side effects of PDE4 inhibitors in so far as they are mediated by distinct PDE4 subtypes. Although the catalytic domains of PDE4 subtypes are composed largely of conserved residues, several compounds with selectivity towards either PDE4B [127-130] or PDE4D [31,36,131] have now been reported suggesting that compounds with high selectivity for individual PDE4 subtypes can eventually be developed.
Importantly, the duration of α2-adrenoceptor-mediated xylazine/ketamine-induced anesthesia, a correlate of emesis in rodents, was found to be shortened in mice deficient in PDE4D, but unaffected in mice deficient in PDE4B [132]. This has led to the hypothesis that emesis is mediated by inhibition of PDE4D, but not PDE4B, and that, thus, PDE4B-selective inhibitors would be nonemetic. As ablation of PDE4B mediates many of the anti-inflammatory properties of PAN-PDE4 inhibitors, development of subtype-selective PDE4B inhibitors has become a major focus for the development of these PDE4 inhibitors as anti-inflammatory drugs. PDE4B-selective inhibitors may also be attractive therapeutics for CNS disorders, including for schizophrenia [110] as well as diseases associated with neuroinflammation including Alzheimer’s [62,107] (see Section 3.2.).
As a word of caution, PDE4A, PDE4B and PDE4D mRNAs are expressed in the area postrema and the nucleus of the solitary tract, areas associated with emetic responses [75,85,89,90,132]. Thus, the hypothesis that PDE4B inhibitors are non-emetic needs to be confirmed in humans. In addition, the role of PDE4A in emesis remains to be determined.
4.3. Allosteric modulators
Burgin and co-workers recently reported development of so-called “allosteric PDE4D modulators” that exert potent pro-cognitive and anti-inflammatory effects in animal models [31]. Allosteric modulators do not behave as pure competitive inhibitors at the active site and do not completely inhibit enzyme activity. The authors propose that the limited magnitude of PDE4 inhibition may serve to maintain basal aspects of cAMP signaling and thereby improve tolerability compared to active-site-directed “full” inhibitors. Whether this hypothesis is correct remains to be confirmed, given that the procognitive effects as well as the emetic effects of conventional PDE4 inhibitors also occur at well below maximum inhibition. It will be of interest to determine whether allosteric modulators of other PDE4 subtypes can also be developed.
4.4. Displacement of PDE4s from macro-molecular signaling complexes
There is now a large body of evidence suggesting that PDEs/PDE4s function in microdomains of signaling that are generated by macromolecular signaling complexes and to which PDEs are tethered via protein/protein or protein/lipid interactions (for a recent review see: [19]). Thus, it may be sufficient to displace PDE4s from critical signaling complexes rather than inhibit their catalytic activity to affect physiologically relevant cAMP signaling events. In line with this idea, several studies have shown that overexpression of dominant-negative PDE4s, constructs which encode catalytically inactive enzymes that act by displacing endogenous PDEs from signaling complexes, elevate cAMP signals and PKA activity in subcellular compartments controlled by anchored PDE4 [133-136]. Developing this idea a step further, several groups mapped the interaction domains between PDE4s and their scaffold proteins and developed disrupting peptides based on this information. Treatment of cells with these peptides was shown to augment discrete pools of cAMP and thereby exert specific biological functions via PDE4 displacement from signaling complexes [109,137,138]. Although no therapeutic has been generated thus far, these studies demonstrate, in proof of concept, that development of small molecule disruptors of PDE4 signaling complexes is a viable option for future drug development. The major limitation for this approach is our current lack of understanding of the relevant molecular targets that mediate the procognitive effects of PDE4 inhibitors.
4.5. Targeting drug delivery to the brain
To limit the side effects associated with systemic exposure to PDE4 inhibitors, the idea of a local application of these drugs has been previously entertained for various inflammatory diseases. These include inhalation for inflammatory lung diseases such as asthma and COPD and topical application for skin diseases such as dermatitis or psoriasis. A similarly targeted, local delivery of PDE4 inhibitors to the CNS might appear unrealistic at present, given that delivery of drugs beyond the blood-brain-barrier per se can be challenging. However, targeted drug delivery to the brain is a field of active investigation; major advances being driven in particular by the need to deliver antineoplastic drugs to treat brain tumors yet limit the systemic exposure to these cytotoxic compounds [139,140]. Advances in the delivery options for these drugs, which generally aim to overcome or circumvent the blood-brain-barrier, may eventually also benefit delivery of drugs for neurodegenerative and psychiatric diseases. These may include direct delivery to the brain via intranasal delivery or intracerebral implants that release drugs over extended periods of time [140-144]. Alternatively, nano-sized carrier and delivery systems may facilitate transport of drugs past the blood-brain-barrier and to their target cells while keeping the systemic exposure to active/free compounds low [139-144]. In this context, an advantageous delivery system could also be key for the successful development of a PDE4 inhibitor for cognition enhancement.
5. Expert opinion
5.1. What are the key findings so far?
There is now a large array of preclinical studies attesting to the potent memory- and cognition-enhancing as well as neuroprotective and neuroregenerative properties of PAN-PDE4 inhibitors (see Sections 2.2. and 2.3.). In fact, it can be argued that PDE4 inhibition is the best-replicated cognitive enhancement in preclinical studies. These compounds are effective in ameliorating or reversing pharmacologically or genetically induced impairments in a broad range of animal models of human diseases, suggesting a therapeutic potential of targeting PDE4s for a spectrum of CNS defects including Alzheimer’s disease and other forms of dementia, as well as neuropsychiatric conditions such as schizophrenia and depression. Indeed, the PAN-PDE4 inhibitor Rolipram often serves as a ‘standard’ in preclinical studies against which other cognitive enhancers are compared.
5.2. What are the key weaknesses in this field?
Despite this significant body of preclinical research, there is no PDE4 inhibitor in clinical use for the treatment of any cognitive disorder. To date, all of the PDE4 inhibitors examined in clinical trials of cognitive and neuropsychiatric disorders have been PAN-selective. These compounds suffer from significant side effects, in particular emesis and nausea, which also largely prevented their clinical application for non-CNS diseases.
5.3. What is the ultimate goal of PDE4 inhibitor development?
Given that PDE4s are widely expressed throughout the human body, systemic exposure to PAN-selective PDE4 inhibitors is bound to affect cAMP signaling in many cells and tissues and, therefore, produce a range of biological effects including those that are therapeutically beneficial but also untoward side effects. Thus, the conceptually simple strategy to overcome this hurdle is the development of inhibitors that selectively block only that portion of total PDE4 activity that is therapeutically relevant to the particular disease being targeted (see Sections 4.1.-4.5.). The practical steps to accomplish this goal involve identifying PDE4-regulated signaling cascades underlying specific, disease-related cognitive dysfunctions and then developing means of selectively targeting PDE4 inhibitors to these disease-related pathways. Progress is being made in regards to both of these steps, despite the considerable complexity.
5.4. What research or knowledge is needed to achieve this goal?
To date, progress towards identifying disease-relevant PDE4 signaling pathways has come primarily from the study of mice deficient in individual PDE4 subtypes and the knock-down of individual PDE4 subtypes and splicing variants [34,65,66,105,106,121,122,132]. The four genes that comprise the PDE4 family are expressed as more than 25 individual proteins [10]. Through the characterization of genetically modified mouse models, it is now well established that individual PDE4s serve unique and non-redundant physiological and pathological roles in the body. In fact, the literature reviewed here has begun to demonstrate that targeting individual PDE4 subtypes and variants opens the opportunity to identify PDE4 targets that are therapeutically beneficial (see Sections 3.1. to 3.4.). It remains to be determined whether identification of these targets will also yield compounds that lack the side effects of non-selective PDE4 inhibitors. To date, only PDE4D subtype-selective inhibitors have been available for clinical testing in airway inflammatory disorders such as COPD [145]. Unfortunately, these compounds proved to be as emetic as PAN-PDE4 inhibitors and were not viable for therapeutic use for airway disorders [7,146]. It has not been determined whether such compounds have procognitive effects in humans, as predicted from the preclinical studies in PDE4D-KO mice reviewed here (see Section 3.4.). If such were the case, it would still be critical to determine whether there may be a therapeutic index for such effects relative to emesis and nausea.
5.5. Where do we see the field going in the coming years?
An increasingly diverse array of PDE4 inhibitors with unique pharmacology is becoming available. These include compounds selective for individual PDE4 subtypes, in particular for PDE4B or PDE4D, as well as compounds that may modulate activity by mechanisms that go beyond simple competitive inhibition at the substrate binding site [31,36,127-131]. The study of these compounds in preclinical models will undoubtedly shed new light on the roles of different subtypes in mediating cognitive processes. Clinical evaluation of such compounds, for use in cognitive disorders or other diseases, will also help clarify the role of PDE4 isotypes in producing nausea and emesis in humans.
5.6. What areas of research are of particular interest?
PDE drug development efforts are increasingly benefiting from knowledge derived from the atomic structures of these enzymes. This includes crystal structures encompassing areas of the enzymes beyond the catalytic domains. Indeed, such data has already proven particularly useful in elucidating the mechanism of subtype-selective inhibition for a novel class of PDE4D-selective inhibitors [31]. More complete PDE structures are also beginning to reveal mechanisms of allosteric regulation of catalytic activity by the N-terminal regulatory domains [31]. Such knowledge may soon make it feasible to target the N-terminal domains independently of the catalytic sites for the discovery of novel PDE allosteric modulators.
Another area of high interest for drug discovery is the identification of the mechanisms and protein partners involved in the subcellular localization of PDE isoforms and the significance of macromolecular protein complexes containing PDEs in regulating the spatial dynamics of cyclic nucleotide signaling [19]. This knowledge offers the hope of identifying new classes of compounds that can modulate PDE activity (and thereby cAMP levels) within very discrete signaling domains such as via disruption of specific signaling complexes. However, as disruption of protein-protein interactions has been diffciualt to achieve through traditional small molecule intervention, this avenue of research may require new thinking in delivering relevant molecules to such drug targets.
5.7. What potential does this research hold?
Given the extensive body of preclinical literature indicating that PDE4 inhibition exerts procognitive efficacy in a number of neurodegenerative and neuropsychiatric disorders, PDE4 remains a target of high interest for CNS drug development. Research that reveals means to better target specific PDE4 isoforms associated with disease-relevant signaling pathways holds the promise of delivering a new generation of PDE4 inhibitors for the treatment of various CNS diseases.
Article highlights box.
The PDE4 family comprises four genes, PDE4A to D, which together are expressed as more than 25 splicing variants that each exert unique and non-redundant physiological functions.
PAN-PDE4 inhibitors exert memory- and cognition-enhancing as well as neuroprotective, neuroregenerative and anti-inflammatory effects in preclinical models.
Inactivation of PDE4D replicates a significant number of the behavioral phenotypes that define the memory- and cognition-enhancing properties of PDE4 inhibitors, identifying PDE4D as a promising target for memory- and cognition-enhancing drugs.
Emesis and nausea are dose-limiting side effects of PAN-PDE4 inhibitors.
Targeting subpopulations of PDE4 activity, such as individual PDE4 subtypes, splice variants or conformational states, promises drugs/inhibitors with reduced side effects and a wider therapeutic window.
Footnotes
Declaration of Interest Work in the authors’ laboratories is supported by NIH grants HL107960 (WR), AG031687 and AA020042 (HTZ), and HL0927088 (MC). Frank Menniti is an employee of Mnemosyne Pharmaceuticals, Inc., Providence, RI. Han-Ting Zhang is a consultant for Asubio Pharamaceuticals, Paramus, NJ and TAKEDA Pharmaceuticals, Tokyo, Japan, and received financial support from Lundbeck, Copenhagen, Denmark. Marco Conti is a consultant for Grünenthal GmbH, Aachen, Germany.
Bibliography
- 1.Buccafusco JJ. Emerging cognitive enhancing drugs. Expert Opin Emerg Drugs. 2009;14:577–89. doi: 10.1517/14728210903257796. [DOI] [PubMed] [Google Scholar]
- 2.Plath N, Lerdrup L, Larsen PH, et al. Can small molecules provide truly effective enhancement of cognition? Current achievements and future directions. Expert Opin Investig Drugs. 2011;20:795–811. doi: 10.1517/13543784.2011.574612. [DOI] [PubMed] [Google Scholar]
- 3.Blokland A, Menniti FS, Prickaerts J. PDE inhibition and cognition enhancement. Expert Opin Ther Pat. 2012;22:349–54. doi: 10.1517/13543776.2012.674514. [DOI] [PubMed] [Google Scholar]
- 4.Blokland A, Schreiber R, Prickaerts J. Improving memory: a role for phosphodiesterases. Curr Pharm Des. 2006;12:2511–23. doi: 10.2174/138161206777698855. [DOI] [PubMed] [Google Scholar]
- 5.Ghavami A, Hirst WD, Novak TJ. Selective phosphodiesterase (PDE)-4 inhibitors: a novel approach to treating memory deficit? Drugs R D. 2006;7:63–71. doi: 10.2165/00126839-200607020-00001. [DOI] [PubMed] [Google Scholar]
- 6.Kleppisch T. Phosphodiesterases in the central nervous system. Handb Exp Pharmacol. 2009:71–92. doi: 10.1007/978-3-540-68964-5_5. [DOI] [PubMed] [Google Scholar]
- 7.Menniti FS, Faraci WS, Schmidt CJ. Phosphodiesterases in the CNS: targets for drug development. Nat Rev Drug Discov. 2006;5:660–70. doi: 10.1038/nrd2058. ** A comprehensive review on PDEs as drug targets for CNS diseases.
- 8.Xu Y, Zhang HT, O’Donnell JM. Phosphodiesterases in the central nervous system: implications in mood and cognitive disorders. Handb Exp Pharmacol. 2011:447–85. doi: 10.1007/978-3-642-17969-3_19. [DOI] [PubMed] [Google Scholar]
- 9.Conti M, Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem. 2007;76:481–511. doi: 10.1146/annurev.biochem.76.060305.150444. ** A comprehensive review of the structure and function of the mammalian PDEs.
- 10.Conti M, Richter W, Mehats C, et al. Cyclic AMP-specific PDE4 phosphodiesterases as critical components of cyclic AMP signaling. J Biol Chem. 2003;278:5493–6. doi: 10.1074/jbc.R200029200. [DOI] [PubMed] [Google Scholar]
- 11.Rutten K, Prickaerts J, Hendrix M, et al. Time-dependent involvement of cAMP and cGMP in consolidation of object memory: studies using selective phosphodiesterase type 2, 4 and 5 inhibitors. Eur J Pharmacol. 2007;558:107–12. doi: 10.1016/j.ejphar.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 12.Davis RL, Cherry J, Dauwalder B, et al. The cyclic AMP system and Drosophila learning. Mol Cell Biochem. 1995;149-150:271–8. doi: 10.1007/978-1-4615-2015-3_31. [DOI] [PubMed] [Google Scholar]
- 13.Dudai Y, Jan YN, Byers D, et al. dunce, a mutant of Drosophila deficient in learning. Proc Natl Acad Sci U S A. 1976;73:1684–8. doi: 10.1073/pnas.73.5.1684. ** An early report associating the dunce gene, the fly ortholog of the mammalian PDE4s, with memory processes.
- 14.Kauvar LM. Defective cyclic adenosine 3′:5′-monophosphate phosphodiesterase in the Drosophila memory mutant dunce. J Neurosci. 1982;2:1347–58. doi: 10.1523/JNEUROSCI.02-10-01347.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wachtel H. Characteristic behavioural alterations in rats induced by rolipram and other selective adenosine cyclic 3′, 5′-monophosphate phosphodiesterase inhibitors. Psychopharmacology (Berl) 1982;77:309–16. doi: 10.1007/BF00432761. ** First report of CNS effects of the prototypal PDE4 inhibitor Rolipram.
- 16.Wachtel H. Potential antidepressant activity of rolipram and other selective cyclic adenosine 3′,5′-monophosphate phosphodiesterase inhibitors. Neuropharmacology. 1983;22:267–72. doi: 10.1016/0028-3908(83)90239-3. [DOI] [PubMed] [Google Scholar]
- 17.Zeller E, Stief HJ, Pflug B, et al. Results of a phase II study of the antidepressant effect of rolipram. Pharmacopsychiatry. 1984;17:188–90. doi: 10.1055/s-2007-1017435. [DOI] [PubMed] [Google Scholar]
- 18.Jin SLC, Richter W, Conti M. Insights into the physiological functions of PDE4 from knockout mice. In: Beavo J, Francis S, Houslay M, editors. Cyclic Nucleotide Phosphodiesterases in Health and Disease. CRC Press; 2006. pp. 323–346. [Google Scholar]
- 19.Houslay MD. Underpinning compartmentalised cAMP signalling through targeted cAMP breakdown. Trends Biochem Sci. 2010;35:91–100. doi: 10.1016/j.tibs.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Richter W, Conti M. Dimerization of the type 4 cAMP-specific phosphodiesterases is mediated by the upstream conserved regions (UCRs) J Biol Chem. 2002;277:40212–21. doi: 10.1074/jbc.M203585200. [DOI] [PubMed] [Google Scholar]
- 21.Richter W, Conti M. The oligomerization state determines regulatory properties and inhibitor sensitivity of type 4 cAMP-specific phosphodiesterases. J Biol Chem. 2004;279:30338–48. doi: 10.1074/jbc.M312687200. [DOI] [PubMed] [Google Scholar]
- 22.MacKenzie SJ, Baillie GS, McPhee I, et al. Long PDE4 cAMP specific phosphodiesterases are activated by protein kinase A-mediated phosphorylation of a single serine residue in Upstream Conserved Region 1 (UCR1) Br J Pharmacol. 2002;136:421–33. doi: 10.1038/sj.bjp.0704743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sette C, Conti M. Phosphorylation and activation of a cAMP-specific phosphodiesterase by the cAMP-dependent protein kinase. Involvement of serine 54 in the enzyme activation. J.Biol.Chem. 1996;271:16526–16534. doi: 10.1074/jbc.271.28.16526. [DOI] [PubMed] [Google Scholar]
- 24.Bruss MD, Richter W, Horner K, et al. Critical role of PDE4D in beta2-adrenoceptor-dependent cAMP signaling in mouse embryonic fibroblasts. J Biol Chem. 2008;283:22430–42. doi: 10.1074/jbc.M803306200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sette C, Iona S, Conti M. The short-term activation of a rolipram-sensitive, cAMP-specific phosphodiesterase by thyroid-stimulating hormone in thyroid FRTL- 5 cells is mediated by a cAMP-dependent phosphorylation. J Biol.Chem. 1994;269:9245–9252. [PubMed] [Google Scholar]
- 26.Grange M, Sette C, Cuomo M, et al. The cAMP-specific phosphodiesterase PDE4D3 is regulated by phosphatidic acid binding. Consequences for cAMP signaling pathway and characterization of a phosphatidic acid binding site. J Biol Chem. 2000;275:33379–87. doi: 10.1074/jbc.M006329200. [DOI] [PubMed] [Google Scholar]
- 27.Grange M, Sette C, Prigent AF, et al. Regulation of cAMP-phosphodiesterases by phosphatidic acid binding. Lipids. 1999;34(Suppl):S83. doi: 10.1007/BF02562239. [DOI] [PubMed] [Google Scholar]
- 28.Baillie GS, MacKenzie SJ, McPhee I, et al. Sub-family selective actions in the ability of Erk2 MAP kinase to phosphorylate and regulate the activity of PDE4 cyclic AMP-specific phosphodiesterases. Br J Pharmacol. 2000;131:811–9. doi: 10.1038/sj.bjp.0703636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffmann R, Baillie GS, MacKenzie SJ, et al. The MAP kinase ERK2 inhibits the cyclic AMP-specific phosphodiesterase HSPDE4D3 by phosphorylating it at Ser579. Embo J. 1999;18:893–903. doi: 10.1093/emboj/18.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barad M, Bourtchouladze R, Winder DG, et al. Rolipram, a type IV-specific phosphodiesterase inhibitor, facilitates the establishment of long-lasting long-term potentiation and improves memory. Proc Natl Acad Sci U S A. 1998;95:15020–5. doi: 10.1073/pnas.95.25.15020. * Demonstration that PDE4 inhibition facilitates LTP and improves memory.
- 31.Burgin AB, Magnusson OT, Singh J, et al. Design of phosphodiesterase 4D (PDE4D) allosteric modulators for enhancing cognition with improved safety. Nat Biotechnol. 2010;28:63–70. doi: 10.1038/nbt.1598. ** This paper suggests that therapeutic and side-effects of PDE4D inhibition can be dissociated.
- 32.Gallant M, Aspiotis R, Day S, et al. Discovery of MK-0952, a selective PDE4 inhibitor for the treatment of long-term memory loss and mild cognitive impairment. Bioorg Med Chem Lett. 2010;20:6387–93. doi: 10.1016/j.bmcl.2010.09.087. [DOI] [PubMed] [Google Scholar]
- 33.Huang Z, Dias R, Jones T, et al. L-454,560, a potent and selective PDE4 inhibitor with in vivo efficacy in animal models of asthma and cognition. Biochem Pharmacol. 2007;73:1971–81. doi: 10.1016/j.bcp.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 34.Li YF, Cheng YF, Huang Y, et al. Phosphodiesterase-4D knock-out and RNA interference-mediated knock-down enhance memory and increase hippocampal neurogenesis via increased cAMP signaling. J Neurosci. 2011;31:172–83. doi: 10.1523/JNEUROSCI.5236-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rutten K, Basile JL, Prickaerts J, et al. Selective PDE inhibitors rolipram and sildenafil improve object retrieval performance in adult cynomolgus macaques. Psychopharmacology (Berl) 2008;196:643–8. doi: 10.1007/s00213-007-0999-1. * Confirmation that PDE4 inhibition improves memory also in monkeys.
- 36.Bruno O, Fedele E, Prickaerts J, et al. GEBR-7b, a novel PDE4D selective inhibitor that improves memory in rodents at non-emetic doses. Br J Pharmacol. 2011;164:2054–63. doi: 10.1111/j.1476-5381.2011.01524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Egawa T, Mishima K, Matsumoto Y, et al. Rolipram and its optical isomers, phosphodiesterase 4 inhibitors, attenuated the scopolamine-induced impairments of learning and memory in rats. Jpn J Pharmacol. 1997;75:275–81. doi: 10.1254/jjp.75.275. [DOI] [PubMed] [Google Scholar]
- 38.Imanishi T, Sawa A, Ichimaru Y, et al. Ameliorating effects of rolipram on experimentally induced impairments of learning and memory in rodents. Eur J Pharmacol. 1997;321:273–8. doi: 10.1016/s0014-2999(96)00969-7. [DOI] [PubMed] [Google Scholar]
- 39.Zhang HT, Huang Y, Suvarna NU, et al. Effects of the novel PDE4 inhibitors MEM1018 and MEM1091 on memory in the radial-arm maze and inhibitory avoidance tests in rats. Psychopharmacology (Berl) 2005;179:613–9. doi: 10.1007/s00213-004-2085-2. [DOI] [PubMed] [Google Scholar]
- 40.Zhang HT, O’Donnell JM. Effects of rolipram on scopolamine-induced impairment of working and reference memory in the radial-arm maze tests in rats. Psychopharmacology (Berl) 2000;150:311–6. doi: 10.1007/s002130000414. [DOI] [PubMed] [Google Scholar]
- 41.Zhang HT, Crissman AM, Dorairaj NR, et al. Inhibition of cyclic AMP phosphodiesterase (PDE4) reverses memory deficits associated with NMDA receptor antagonism. Neuropsychopharmacology. 2000;23:198–204. doi: 10.1016/S0893-133X(00)00108-1. [DOI] [PubMed] [Google Scholar]
- 42.Davis JA, Gould TJ. Rolipram attenuates MK-801-induced deficits in latent inhibition. Behav Neurosci. 2005;119:595–602. doi: 10.1037/0735-7044.119.2.595. [DOI] [PubMed] [Google Scholar]
- 43.Zhang HT, Zhao Y, Huang Y, et al. Inhibition of the phosphodiesterase 4 (PDE4) enzyme reverses memory deficits produced by infusion of the MEK inhibitor U0126 into the CA1 subregion of the rat hippocampus. Neuropsychopharmacology. 2004;29:1432–9. doi: 10.1038/sj.npp.1300440. [DOI] [PubMed] [Google Scholar]
- 44.Rutten K, Lieben C, Smits L, et al. The PDE4 inhibitor rolipram reverses object memory impairment induced by acute tryptophan depletion in the rat. Psychopharmacology (Berl) 2007;192:275–82. doi: 10.1007/s00213-006-0697-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li LX, Cheng YF, Lin HB, et al. Prevention of cerebral ischemia-induced memory deficits by inhibition of phosphodiesterase-4 in rats. Metab Brain Dis. 2011;26:37–47. doi: 10.1007/s11011-011-9235-0. [DOI] [PubMed] [Google Scholar]
- 46.de Lima MN, Presti-Torres J, Garcia VA, et al. Amelioration of recognition memory impairment associated with iron loading or aging by the type 4-specific phosphodiesterase inhibitor rolipram in rats. Neuropharmacology. 2008;55:788–92. doi: 10.1016/j.neuropharm.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 47.Ramos BP, Birnbaum SG, Lindenmayer I, et al. Dysregulation of protein kinase a signaling in the aged prefrontal cortex: new strategy for treating age-related cognitive decline. Neuron. 2003;40:835–45. doi: 10.1016/s0896-6273(03)00694-9. [DOI] [PubMed] [Google Scholar]
- 48.Bourtchouladze R, Lidge R, Catapano R, et al. A mouse model of Rubinstein-Taybi syndrome: defective long-term memory is ameliorated by inhibitors of phosphodiesterase 4. Proc Natl Acad Sci U S A. 2003;100:10518–22. doi: 10.1073/pnas.1834280100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vitolo OV, Sant’Angelo A, Costanzo V, et al. Amyloid beta -peptide inhibition of the PKA/CREB pathway and long-term potentiation: reversibility by drugs that enhance cAMP signaling. Proc Natl Acad Sci U S A. 2002;99:13217–21. doi: 10.1073/pnas.172504199. * PDE4 inhibition reverses β-amyloid-induced inhibition of PKA/CREB signaling and LTP.
- 50.Gong B, Vitolo OV, Trinchese F, et al. Persistent improvement in synaptic and cognitive functions in an Alzheimer mouse model after rolipram treatment. J Clin Invest. 2004;114:1624–34. doi: 10.1172/JCI22831. ** PDE4 inhibitors are effective in a mouse model of Alzheimer’s.
- 51.Cheng YF, Wang C, Lin HB, et al. Inhibition of phosphodiesterase-4 reverses memory deficits produced by Abeta25-35 or Abeta1-40 peptide in rats. Psychopharmacology (Berl) 2010;212:181–91. doi: 10.1007/s00213-010-1943-3. [DOI] [PubMed] [Google Scholar]
- 52.Wang C, Yang XM, Zhuo YY, et al. The phosphodiesterase-4 inhibitor rolipram reverses Abeta-induced cognitive impairment and neuroinflammatory and apoptotic responses in rats. Int J Neuropsychopharmacol. 2012;15:749–66. doi: 10.1017/S1461145711000836. [DOI] [PubMed] [Google Scholar]
- 53.Sharma V, Bala A, Deshmukh R, et al. Neuroprotective effect of RO-20-1724-a phosphodiesterase4 inhibitor against intracerebroventricular streptozotocin induced cognitive deficit and oxidative stress in rats. Pharmacol Biochem Behav. 2012;101:239–45. doi: 10.1016/j.pbb.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 54.Li YF, Huang Y, Amsdell SL, et al. Antidepressant- and anxiolytic-like effects of the phosphodiesterase-4 inhibitor rolipram on behavior depend on cyclic AMP response element binding protein-mediated neurogenesis in the hippocampus. Neuropsychopharmacology. 2009;34:2404–19. doi: 10.1038/npp.2009.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakagawa S, Kim JE, Lee R, et al. Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. J Neurosci. 2002;22:3673–82. doi: 10.1523/JNEUROSCI.22-09-03673.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rutten K, Prickaerts J, Blokland A. Rolipram reverses scopolamine-induced and time-dependent memory deficits in object recognition by different mechanisms of action. Neurobiol Learn Mem. 2006;85:132–8. doi: 10.1016/j.nlm.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 57.Navakkode S, Sajikumar S, Frey JU. The type IV-specific phosphodiesterase inhibitor rolipram and its effect on hippocampal long-term potentiation and synaptic tagging. J Neurosci. 2004;24:7740–4. doi: 10.1523/JNEUROSCI.1796-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wiescholleck V, Manahan-Vaughan D. PDE4 inhibition enhances hippocampal synaptic plasticity in vivo and rescues MK801-induced impairment of long-term potentiation and object recognition memory in an animal model of psychosis. Transl Psychiatry. 2012;2:e89. doi: 10.1038/tp.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Giorgi M, Modica A, Pompili A, et al. The induction of cyclic nucleotide phosphodiesterase 4 gene (PDE4D) impairs memory in a water maze task. Behav Brain Res. 2004;154:99–106. doi: 10.1016/j.bbr.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 60.Yamashita N, Hayashi A, Baba J, et al. Rolipram, a phosphodiesterase-4-selective inhibitor, promotes the survival of cultured rat dopaminergic neurons. Jpn J Pharmacol. 1997;75:155–9. doi: 10.1254/jjp.75.155. * An early report suggesting neuroprotective effects of PDE4 inhibitors.
- 61.Schaal SM, Garg MS, Ghosh M, et al. The therapeutic profile of rolipram, PDE target and mechanism of action as a neuroprotectant following spinal cord injury. PLoS One. 2012;7:e43634. doi: 10.1371/journal.pone.0043634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sebastiani G, Morissette C, Lagace C, et al. The cAMP-specific phosphodiesterase 4B mediates Abeta-induced microglial activation. Neurobiol Aging. 2006;27:691–701. doi: 10.1016/j.neurobiolaging.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 63.Nikulina E, Tidwell JL, Dai HN, et al. The phosphodiesterase inhibitor rolipram delivered after a spinal cord lesion promotes axonal regeneration and functional recovery. Proc Natl Acad Sci U S A. 2004;101:8786–90. doi: 10.1073/pnas.0402595101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Giralt A, Saavedra A, Carreton O, et al. Increased PKA signaling disrupts recognition memory and spatial memory: role in Huntington’s disease. Hum Mol Genet. 2011;20:4232–47. doi: 10.1093/hmg/ddr351. [DOI] [PubMed] [Google Scholar]
- 65.Rutten K, Misner DL, Works M, et al. Enhanced long-term potentiation and impaired learning in phosphodiesterase 4D-knockout (PDE4D) mice. Eur J Neurosci. 2008;28:625–32. doi: 10.1111/j.1460-9568.2008.06349.x. [DOI] [PubMed] [Google Scholar]
- 66.Rutten K, Wallace TL, Works M, et al. Enhanced long-term depression and impaired reversal learning in phosphodiesterase 4B-knockout (PDE4B-/-) mice. Neuropharmacology. 2011;61:138–47. doi: 10.1016/j.neuropharm.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 67.Arnsten AF, Ramos BP, Birnbaum SG, et al. Protein kinase A as a therapeutic target for memory disorders: rationale and challenges. Trends Mol Med. 2005;11:121–8. doi: 10.1016/j.molmed.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 68.Hotte M, Dauphin F, Freret T, et al. A biphasic and brain-region selective down-regulation of cyclic adenosine monophosphate concentrations supports object recognition in the rat. PLoS One. 2012;7:e32244. doi: 10.1371/journal.pone.0032244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.DeMarch Z, Giampa C, Patassini S, et al. Beneficial effects of rolipram in the R6/2 mouse model of Huntington’s disease. Neurobiol Dis. 2008;30:375–87. doi: 10.1016/j.nbd.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 70.DeMarch Z, Giampa C, Patassini S, et al. Beneficial effects of rolipram in a quinolinic acid model of striatal excitotoxicity. Neurobiol Dis. 2007;25:266–73. doi: 10.1016/j.nbd.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 71.Heaslip RJ, Evans DY. Emetic, central nervous system, and pulmonary activities of rolipram in the dog. Eur J Pharmacol. 1995;286:281–90. doi: 10.1016/0014-2999(95)00457-2. [DOI] [PubMed] [Google Scholar]
- 72.Hebenstreit GF, Fellerer K, Fichte K, et al. Rolipram in major depressive disorder: results of a double-blind comparative study with imipramine. Pharmacopsychiatry. 1989;22:156–60. doi: 10.1055/s-2007-1014599. [DOI] [PubMed] [Google Scholar]
- 73.Cherry JA, Davis RL. Cyclic AMP phosphodiesterases are localized in regions of the mouse brain associated with reinforcement, movement, and affect. J Comp Neurol. 1999;407:287–301. [PubMed] [Google Scholar]
- 74.Lamontagne S, Meadows E, Luk P, et al. Localization of phosphodiesterase-4 isoforms in the medulla and nodose ganglion of the squirrel monkey. Brain Res. 2001;920:84–96. doi: 10.1016/s0006-8993(01)03023-2. [DOI] [PubMed] [Google Scholar]
- 75.Mori F, Perez-Torres S, De Caro R, et al. The human area postrema and other nuclei related to the emetic reflex express cAMP phosphodiesterases 4B and 4D. J Chem Neuroanat. 2010;40:36–42. doi: 10.1016/j.jchemneu.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 76.Lehnart SE, Wehrens XH, Reiken S, et al. Phosphodiesterase 4D deficiency in the ryanodine-receptor complex promotes heart failure and arrhythmias. Cell. 2005;123:25–35. doi: 10.1016/j.cell.2005.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leroy J, Richter W, Mika D, et al. Phosphodiesterase 4B in the cardiac L-type Ca(2)(+) channel complex regulates Ca(2)(+) current and protects against ventricular arrhythmias in mice. J Clin Invest. 2011;121:2651–61. doi: 10.1172/JCI44747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Molina CE, Leroy J, Richter W, et al. Cyclic adenosine monophosphate phosphodiesterase type 4 protects against atrial arrhythmias. J Am Coll Cardiol. 2012;59:2182–90. doi: 10.1016/j.jacc.2012.01.060. [DOI] [PubMed] [Google Scholar]
- 79.Gretarsdottir S, Thorleifsson G, Reynisdottir ST, et al. The gene encoding phosphodiesterase 4D confers risk of ischemic stroke. Nat Genet. 2003;35:131–8. doi: 10.1038/ng1245. [DOI] [PubMed] [Google Scholar]
- 80.Losco PE, Evans EW, Barat SA, et al. The toxicity of SCH 351591, a novel phosphodiesterase-4 inhibitor, in Cynomolgus monkeys. Toxicol Pathol. 2004;32:295–308. doi: 10.1080/01926230490431493. [DOI] [PubMed] [Google Scholar]
- 81.Calverley PM, Rabe KF, Goehring UM, et al. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374:685–94. doi: 10.1016/S0140-6736(09)61255-1. ** Report of a clinical trial that resulted in the first FDA-approval of a PDE4 inhibitor, Roflumilast, for any indication.
- 82.Fabbri LM, Calverley PM, Izquierdo-Alonso JL, et al. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet. 2009;374:695–703. doi: 10.1016/S0140-6736(09)61252-6. [DOI] [PubMed] [Google Scholar]
- 83.Iona S, Cuomo M, Bushnik T, et al. Characterization of the rolipram-sensitive, cyclic AMP-specific phosphodiesterases: identification and differential expression of immunologically distinct forms in the rat brain. Mol Pharmacol. 1998;53:23–32. doi: 10.1124/mol.53.1.23. [DOI] [PubMed] [Google Scholar]
- 84.Johansson EM, Reyes-Irisarri E, Mengod G. Comparison of cAMP-specific phosphodiesterase mRNAs distribution in mouse and rat brain. Neurosci Lett. 2012;525:1–6. doi: 10.1016/j.neulet.2012.07.050. [DOI] [PubMed] [Google Scholar]
- 85.Perez-Torres S, Miro X, Palacios JM, et al. Phosphodiesterase type 4 isozymes expression in human brain examined by in situ hybridization histochemistry and[3H]rolipram binding autoradiography. Comparison with monkey and rat brain. J Chem Neuroanat. 2000;20:349–74. doi: 10.1016/s0891-0618(00)00097-1. [DOI] [PubMed] [Google Scholar]
- 86.Lobban M, Shakur Y, Beattie J, et al. Identification of two splice variant forms of type-IVB cyclic AMP phosphodiesterase, DPD (rPDE-IVB1) and PDE-4 (rPDEIVB2) in brain: selective localization in membrane and cytosolic compartments and differential expression in various brain regions. Biochem J. 1994;304(Pt 2):399–406. doi: 10.1042/bj3040399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McPhee I, Cochran S, Houslay MD. The novel long PDE4A10 cyclic AMP phosphodiesterase shows a pattern of expression within brain that is distinct from the long PDE4A5 and short PDE4A1 isoforms. Cell Signal. 2001;13:911–8. doi: 10.1016/s0898-6568(01)00217-0. [DOI] [PubMed] [Google Scholar]
- 88.McPhee I, Pooley L, Lobban M, et al. Identification, characterization and regional distribution in brain of RPDE-6 (RNPDE4A5), a novel splice variant of the PDE4A cyclic AMP phosphodiesterase family. Biochem J. 1995;310(Pt 3):965–74. doi: 10.1042/bj3100965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Miro X, Perez-Torres S, Puigdomenech P, et al. Differential distribution of PDE4D splice variant mRNAs in rat brain suggests association with specific pathways and presynaptical localization. Synapse. 2002;45:259–69. doi: 10.1002/syn.10100. [DOI] [PubMed] [Google Scholar]
- 90.Reyes-Irisarri E, Perez-Torres S, Miro X, et al. Differential distribution of PDE4B splice variant mRNAs in rat brain and the effects of systemic administration of LPS in their expression. Synapse. 2008;62:74–9. doi: 10.1002/syn.20459. [DOI] [PubMed] [Google Scholar]
- 91.Richter W, Jin SL, Conti M. Splice variants of the cyclic nucleotide phosphodiesterase PDE4D are differentially expressed and regulated in rat tissue. Biochem J. 2005;388:803–11. doi: 10.1042/BJ20050030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hansen G, Jin S, Umetsu DT, et al. Absence of muscarinic cholinergic airway responses in mice deficient in the cyclic nucleotide phosphodiesterase PDE4D. Proc Natl Acad Sci U S A. 2000;97:6751–6. doi: 10.1073/pnas.97.12.6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jin SL, Goya S, Nakae S, et al. Phosphodiesterase 4B is essential for T(H)2-cell function and development of airway hyperresponsiveness in allergic asthma. J Allergy Clin Immunol. 2010;126:1252–9. e12. doi: 10.1016/j.jaci.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mehats C, Jin SL, Wahlstrom J, et al. PDE4D plays a critical role in the control of airway smooth muscle contraction. FASEB J. 2003;17:1831–41. doi: 10.1096/fj.03-0274com. [DOI] [PubMed] [Google Scholar]
- 95.Jin SL, Conti M. Induction of the cyclic nucleotide phosphodiesterase PDE4B is essential for LPS-activated TNF-alpha responses. Proc Natl Acad Sci U S A. 2002;99:7628–33. doi: 10.1073/pnas.122041599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mackenzie KF, Topping EC, Bugaj-Gaweda B, et al. Human PDE4A8, a novel brain-expressed PDE4 cAMP-specific phosphodiesterase that has undergone rapid evolutionary change. Biochem J. 2008;411:361–9. doi: 10.1042/BJ20071251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Braun NN, Reutiman TJ, Lee S, et al. Expression of phosphodiesterase 4 is altered in the brains of subjects with autism. Neuroreport. 2007;18:1841–4. doi: 10.1097/WNR.0b013e3282f16dca. [DOI] [PubMed] [Google Scholar]
- 98.Fatemi SH, Reutiman TJ, Folsom TD, et al. Phosphodiesterase-4A expression is reduced in cerebella of patients with bipolar disorder. Psychiatr Genet. 2008;18:282–8. doi: 10.1097/YPG.0b013e3283060fb8. [DOI] [PubMed] [Google Scholar]
- 99.Vecsey CG, Baillie GS, Jaganath D, et al. Sleep deprivation impairs cAMP signalling in the hippocampus. Nature. 2009;461:1122–5. doi: 10.1038/nature08488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hajjhussein H, Suvarna NU, Gremillion C, et al. Changes in NMDA receptor-induced cyclic nucleotide synthesis regulate the age-dependent increase in PDE4A expression in primary cortical cultures. Brain Res. 2007;1149:58–68. doi: 10.1016/j.brainres.2007.02.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ahmed T, Frey JU. Expression of the specific type IV phosphodiesterase gene PDE4B3 during different phases of long-term potentiation in single hippocampal slices of rats in vitro. Neuroscience. 2003;117:627–38. doi: 10.1016/s0306-4522(02)00838-2. [DOI] [PubMed] [Google Scholar]
- 102.Ahmed T, Frey JU. Phosphodiesterase 4B (PDE4B) and cAMP-level regulation within different tissue fractions of rat hippocampal slices during long-term potentiation in vitro. Brain Res. 2005;1041:212–22. doi: 10.1016/j.brainres.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 103.Ahmed T, Frey S, Frey JU. Regulation of the phosphodiesterase PDE4B3-isotype during long-term potentiation in the area dentata in vivo. Neuroscience. 2004;124:857–67. doi: 10.1016/j.neuroscience.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 104.Navakkode S, Sajikumar S, Frey JU. Mitogen-activated protein kinase-mediated reinforcement of hippocampal early long-term depression by the type IV-specific phosphodiesterase inhibitor rolipram and its effect on synaptic tagging. J Neurosci. 2005;25:10664–70. doi: 10.1523/JNEUROSCI.2443-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Siuciak JA, McCarthy SA, Chapin DS, et al. Behavioral and neurochemical characterization of mice deficient in the phosphodiesterase-4B (PDE4B) enzyme. Psychopharmacology (Berl) 2008;197:115–26. doi: 10.1007/s00213-007-1014-6. [DOI] [PubMed] [Google Scholar]
- 106.Zhang HT, Huang Y, Masood A, et al. Anxiogenic-like behavioral phenotype of mice deficient in phosphodiesterase 4B (PDE4B) Neuropsychopharmacology. 2008;33:1611–23. doi: 10.1038/sj.npp.1301537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rubio-Perez JM, Morillas-Ruiz JM. A review: inflammatory process in Alzheimer’s disease, role of cytokines. ScientificWorldJournal. 2012;2012:756357. doi: 10.1100/2012/756357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Millar JK, Pickard BS, Mackie S, et al. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science. 2005;310:1187–91. doi: 10.1126/science.1112915. * First association of PDE4B with schizophrenia.
- 109.Murdoch H, Mackie S, Collins DM, et al. Isoform-selective susceptibility of DISC1/phosphodiesterase-4 complexes to dissociation by elevated intracellular cAMP levels. J Neurosci. 2007;27:9513–24. doi: 10.1523/JNEUROSCI.1493-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fatemi SH, King DP, Reutiman TJ, et al. PDE4B polymorphisms and decreased PDE4B expression are associated with schizophrenia. Schizophr Res. 2008;101:36–49. doi: 10.1016/j.schres.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 111.Numata S, Ueno S, Iga J, et al. Positive association of the PDE4B (phosphodiesterase 4B) gene with schizophrenia in the Japanese population. J Psychiatr Res. 2008;43:7–12. doi: 10.1016/j.jpsychires.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 112.Pickard BS, Thomson PA, Christoforou A, et al. The PDE4B gene confers sex-specific protection against schizophrenia. Psychiatr Genet. 2007;17:129–33. doi: 10.1097/YPG.0b013e328014492b. [DOI] [PubMed] [Google Scholar]
- 113.Tomppo L, Hennah W, Lahermo P, et al. Association between genes of Disrupted in schizophrenia 1 (DISC1) interactors and schizophrenia supports the role of the DISC1 pathway in the etiology of major mental illnesses. Biol Psychiatry. 2009;65:1055–62. doi: 10.1016/j.biopsych.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kahler AK, Otnaess MK, Wirgenes KV, et al. Association study of PDE4B gene variants in Scandinavian schizophrenia and bipolar disorder multicenter case-control samples. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:86–96. doi: 10.1002/ajmg.b.30958. [DOI] [PubMed] [Google Scholar]
- 115.Rastogi A, Zai C, Likhodi O, et al. Genetic association and post-mortem brain mRNA analysis of DISC1 and related genes in schizophrenia. Schizophr Res. 2009;114:39–49. doi: 10.1016/j.schres.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 116.Fadda F, Rossetti ZL. Chronic ethanol consumption: from neuroadaptation to neurodegeneration. Prog Neurobiol. 1998;56:385–431. doi: 10.1016/s0301-0082(98)00032-x. [DOI] [PubMed] [Google Scholar]
- 117.Hu W, Lu T, Chen A, et al. Inhibition of phosphodiesterase-4 decreases ethanol intake in mice. Psychopharmacology (Berl) 2011;218:331–9. doi: 10.1007/s00213-011-2290-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wen RT, Zhang M, Qin WJ, et al. The phosphodiesterase-4 (PDE4) inhibitor rolipram decreases ethanol seeking and consumption in alcohol-preferring fawn-hooded rats. Alcohol Clin Exp Res. 2012;36:2157–67. doi: 10.1111/j.1530-0277.2012.01845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Robbins TW, Ersche KD, Everitt BJ. Drug addiction and the memory systems of the brain. Ann N Y Acad Sci. 2008;1141:1–21. doi: 10.1196/annals.1441.020. [DOI] [PubMed] [Google Scholar]
- 120.Wang ZZ, Zhang Y, Liu YQ, et al. RNA interference-mediated phosphodiesterase 4D splice variants knock-down in the prefrontal cortex produces antidepressant-like and cognition-enhancing effects. Br J Pharmacol. 2013;168:1001–14. doi: 10.1111/j.1476-5381.2012.02225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schaefer TL, Braun AA, Amos-Kroohs RM, et al. A new model of Pde4d deficiency: genetic knock-down of PDE4D enzyme in rats produces an antidepressant phenotype without spatial cognitive effects. Genes Brain Behav. 2012;11:614–22. doi: 10.1111/j.1601-183X.2012.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang HT, Huang Y, Jin SL, et al. Antidepressant-like profile and reduced sensitivity to rolipram in mice deficient in the PDE4D phosphodiesterase enzyme. Neuropsychopharmacology. 2002;27:587–95. doi: 10.1016/S0893-133X(02)00344-5. ** A first report elucidating the role of individual PDE4 subtypes in the brain.
- 123.Duplantier AJ, Biggers MS, Chambers RJ, et al. Biarylcarboxylic acids and -amides: inhibition of phosphodiesterase type IV versus [3H]rolipram binding activity and their relationship to emetic behavior in the ferret. J Med Chem. 1996;39:120–5. doi: 10.1021/jm9505066. [DOI] [PubMed] [Google Scholar]
- 124.Souness JE, Rao S. Proposal for pharmacologically distinct conformers of PDE4 cyclic AMP phosphodiesterases. Cell Signal. 1997;9:227–36. doi: 10.1016/s0898-6568(96)00173-8. [DOI] [PubMed] [Google Scholar]
- 125.Zhang HT, Zhao Y, Huang Y, et al. Antidepressant-like effects of PDE4 inhibitors mediated by the high-affinity rolipram binding state (HARBS) of the phosphodiesterase-4 enzyme (PDE4) in rats. Psychopharmacology (Berl) 2006;186:209–17. doi: 10.1007/s00213-006-0369-4. [DOI] [PubMed] [Google Scholar]
- 126.Jin S-LC, Richard F, Kuo W-P, et al. Impaired growth and fertility of cAMP-specific phosphodiesterase PDE4D-deficient mice. Proc Natl Acad Sci U S A. 1999;96 doi: 10.1073/pnas.96.21.11998. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Donnell AF, Dollings PJ, Butera JA, et al. Identification of pyridazino[4,5-b]indolizines as selective PDE4B inhibitors. Bioorg Med Chem Lett. 2010;20:2163–7. doi: 10.1016/j.bmcl.2010.02.044. [DOI] [PubMed] [Google Scholar]
- 128.Kranz M, Wall M, Evans B, et al. Identification of PDE4B Over 4D subtype-selective inhibitors revealing an unprecedented binding mode. Bioorg Med Chem. 2009;17:5336–41. doi: 10.1016/j.bmc.2009.03.061. [DOI] [PubMed] [Google Scholar]
- 129.Mitchell CJ, Ballantine SP, Coe DM, et al. Pyrazolopyridines as potent PDE4B inhibitors: 5-heterocycle SAR. Bioorg Med Chem Lett. 2010;20:5803–6. doi: 10.1016/j.bmcl.2010.07.136. [DOI] [PubMed] [Google Scholar]
- 130.Naganuma K, Omura A, Maekawara N, et al. Discovery of selective PDE4B inhibitors. Bioorg Med Chem Lett. 2009;19:3174–6. doi: 10.1016/j.bmcl.2009.04.121. [DOI] [PubMed] [Google Scholar]
- 131.Aspiotis R, Deschenes D, Dube D, et al. The discovery and synthesis of highly potent subtype selective phosphodiesterase 4D inhibitors. Bioorg Med Chem Lett. 2010;20:5502–5. doi: 10.1016/j.bmcl.2010.07.076. [DOI] [PubMed] [Google Scholar]
- 132. Robichaud A, Stamatiou PB, Jin SL, et al. Deletion of phosphodiesterase 4D in mice shortens alpha(2)-adrenoceptor-mediated anesthesia, a behavioral correlate of emesis. J Clin Invest. 2002;110:1045–52. doi: 10.1172/JCI15506. ** Report suggesting that inactivation of PDE4D, but not PDE4B, induces a correlate of emesis in the mouse.
- 133.Baillie GS, Sood A, McPhee I, et al. beta-Arrestin-mediated PDE4 cAMP phosphodiesterase recruitment regulates beta-adrenoceptor switching from Gs to Gi. Proc Natl Acad Sci U S A. 2003;100:940–5. doi: 10.1073/pnas.262787199. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 134.De Arcangelis V, Liu R, Soto D, et al. Differential association of phosphodiesterase 4D isoforms with beta2-adrenoceptor in cardiac myocytes. J Biol Chem. 2009;284:33824–32. doi: 10.1074/jbc.M109.020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.McCahill A, McSorley T, Huston E, et al. In resting COS1 cells a dominant negative approach shows that specific, anchored PDE4 cAMP phosphodiesterase isoforms gate the activation, by basal cyclic AMP production, of AKAP-tethered protein kinase A type II located in the centrosomal region. Cell Signal. 2005;17:1158–73. doi: 10.1016/j.cellsig.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 136.Richter W, Day P, Agrawal R, et al. Signaling from beta1- and beta2-adrenergic receptors is defined by differential interactions with PDE4. EMBO J. 2008;27:384–93. doi: 10.1038/sj.emboj.7601968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bolger GB, Baillie GS, Li X, et al. Scanning peptide array analyses identify overlapping binding sites for the signalling scaffold proteins, beta-arrestin and RACK1, in cAMP-specific phosphodiesterase PDE4D5. Biochem J. 2006;398:23–36. doi: 10.1042/BJ20060423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Murdoch H, Vadrevu S, Prinz A, et al. Interaction between LIS1 and PDE4, and its role in cytoplasmic dynein function. J Cell Sci. 2011;124:2253–66. doi: 10.1242/jcs.082982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Beduneau A, Saulnier P, Benoit JP. Active targeting of brain tumors using nanocarriers. Biomaterials. 2007;28:4947–67. doi: 10.1016/j.biomaterials.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 140.Patel MM, Goyal BR, Bhadada SV, et al. Getting into the brain: approaches to enhance brain drug delivery. CNS Drugs. 2009;23:35–58. doi: 10.2165/0023210-200923010-00003. [DOI] [PubMed] [Google Scholar]
- 141.Alam MI, Beg S, Samad A, et al. Strategy for effective brain drug delivery. Eur J Pharm Sci. 2010;40:385–403. doi: 10.1016/j.ejps.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 142.Joshi S, Ornstein E, Bruce JN. Targeting the brain: rationalizing the novel methods of drug delivery to the central nervous system. Neurocrit Care. 2007;6:200–12. doi: 10.1007/s12028-007-0034-8. [DOI] [PubMed] [Google Scholar]
- 143.Pasha S, Gupta K. Various drug delivery approaches to the central nervous system. Expert Opin Drug Deliv. 2010;7:113–35. doi: 10.1517/17425240903405581. [DOI] [PubMed] [Google Scholar]
- 144.Potschka H. Targeting the brain--surmounting or bypassing the blood-brain barrier. Handb Exp Pharmacol. 2010;411:31. doi: 10.1007/978-3-642-00477-3_14. [DOI] [PubMed] [Google Scholar]
- 145.Kalgutkar AS, Choo E, Taylor TJ, et al. Disposition of CP-671, 305, a selective phosphodiesterase 4 inhibitor in preclinical species. Xenobiotica. 2004;34:755–70. doi: 10.1080/00498250400005682. [DOI] [PubMed] [Google Scholar]
- 146.Yeadon M, Clarke N, Ward J. Phosphodiesterase type 4 (PDE4) inhibition: The search for effective therapy with minimal side effects. In: HTaB JP, editor. New Drugs and Targets for Asthma and COPD. Prog Respir Res. Karger; Basel: 2010. pp. 269–278. [Google Scholar]
- 147.Gekel I, Neher E. Application of an Epac activator enhances neurotransmitter release at excitatory central synapses. J Neurosci. 2008;28:7991–8002. doi: 10.1523/JNEUROSCI.0268-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gelinas JN, Banko JL, Peters MM, et al. Activation of exchange protein activated by cyclic-AMP enhances long-lasting synaptic potentiation in the hippocampus. Learn Mem. 2008;15:403–11. doi: 10.1101/lm.830008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Laurent AC, Breckler M, Berthouze M, et al. Role of Epac in brain and heart. Biochem Soc Trans. 2012;40:51–7. doi: 10.1042/BST20110642. [DOI] [PubMed] [Google Scholar]
- 150.Ma N, Abel T, Hernandez PJ. Exchange protein activated by cAMP enhances long-term memory formation independent of protein kinase A. Learn Mem. 2009;16:367–70. doi: 10.1101/lm.1231009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mironov SL, Skorova EY. Stimulation of bursting in pre-Botzinger neurons by Epac through calcium release and modulation of TRPM4 and K-ATP channels. J Neurochem. 2011;117:295–308. doi: 10.1111/j.1471-4159.2011.07202.x. [DOI] [PubMed] [Google Scholar]
- 152.Ostroveanu A, van der Zee EA, Eisel UL, et al. Exchange protein activated by cyclic AMP 2 (Epac2) plays a specific and time-limited role in memory retrieval. Hippocampus. 2010;20:1018–26. doi: 10.1002/hipo.20700. [DOI] [PubMed] [Google Scholar]
- 153.Peace AG, Shewan DA. New perspectives in cyclic AMP-mediated axon growth and guidance: The emerging epoch of Epac. Brain Res Bull. 2011;84:280–8. doi: 10.1016/j.brainresbull.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 154.Ster J, De Bock F, Guerineau NC, et al. Exchange protein activated by cAMP (Epac) mediates cAMP activation of p38 MAPK and modulation of Ca2+-dependent K+ channels in cerebellar neurons. Proc Natl Acad Sci U S A. 2007;104:2519–24. doi: 10.1073/pnas.0611031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Benarroch EE. HCN channels: function and clinical implications. Neurology. 2013;80:304–10. doi: 10.1212/WNL.0b013e31827dec42. [DOI] [PubMed] [Google Scholar]
- 156.Craven KB, Zagotta WN. CNG and HCN channels: two peas, one pod. Annu Rev Physiol. 2006;68:375–401. doi: 10.1146/annurev.physiol.68.040104.134728. [DOI] [PubMed] [Google Scholar]