Abstract
Acute effects of methylenedioxymethamphetamine (MDMA), methamphetamine (MA) and methylphenidate (MPD) were studied using a within-subject, repeated acquisition/performance procedure adapted to the Morris Swim Task. To investigate place learning, the acquisition component consisted of a hidden platform that varied in location across experimental sessions. As a control for drug effects not specific to acquisition, a performance component was included in which the hidden platform was in the same pool location in every experimental session. All three drugs increased escape latencies and swim distances in dose-dependent fashion. However, impairment in the acquisition component was generally observed only at doses that also produced impairment in the performance component, suggesting that effects were not selective to place learning. None of the drugs produced enhancement of learning or performance at any dose. Taken together, the results suggest that acute exposure to these psychomotor stimulants produce global impairment of performance in the Morris task, rather than specific deficits in place learning.
Keywords: Spatial Learning, Morris Swim Task, MDMA, Methamphetamine, Methylphenidate
Psychomotor stimulants such as methylenedioxymethamphetamine (MDMA), methamphetamine (MA) and methylphenidate (MPD) are associated with high rates of abuse worldwide, and there is increasing concern about both the acute and long-term effects of these drugs on psychological functioning in users. In particular, use of both MDMA and MA has been associated with neurotoxicity and clinical studies have raised the possibility of adverse effects on cognition, learning and memory (e.g., Baicy & London, 2007; Homer, et al., 2008; McCann, et al., 2008; Nulsen, et al., 2010; Scott, et al., 2007; Stough, et al., 2012). In contrast however, some stimulant drugs are used to improve cognitive functioning in disorders such as ADHD and have been shown to produce cognitive enhancement following acute administration to healthy humans in laboratory settings (Barch & Carter, 2005; Hart et al., 2008; Kirkpatrick et al., 2012).
Ethical and practical issues limit determination of the effects of potentially hazardous drugs in humans, and point to the importance of research with nonhumans to characterize the effects of such drugs on cognitive function. Here the problem is the development of reliable and valid animal models of learning and memory that are sensitive to impairment and enhancement by psychoactive drugs. Several studies have assessed acute effects of psychomotor stimulants using various models of learning in nonhumans with somewhat mixed results. Although most studies have found only impairment of learning across dose-response functions for MDMA (e.g., Braida et al., 2002; Byrne et al., 2000), MA (e.g., Mayorga et al., 2000) and MPD (e.g., Chuhan & Taukulis, 2006; Mayorga et al., 2000), cognitive enhancements by these drugs have also been reported (e.g., Calhoun & Jones, 1974; Handley & Calhoun, 1978; Quinteros-Munoz et al., 2010; Tian et al., 2009; Zhu et al., 2007).
These studies differ in many aspects of methodology, making it difficult to determine the basis for their differing outcomes. One pervasive problem is in determining the specificity of drug effects on processes involving learning and memory as separated from effects on more general determinants of performance such as motivation, perceptual or motor processes. Historically, the preparation in behavioral pharmacology that has been most successful in separating learning processes from more general performance processes is the repeated acquisition/performance (RAP) procedure (Thompson & Moerschbaecher, 1979a). RAP procedures typically involve a multiple schedule in which two components alternate within a session. In both components, a particular sequence of responses is required to produce reinforcement, but in one component (acquisition) the sequence changes each session, and thus the animal must learn the correct sequence during the session. In the other component (performance), the same sequence is in operation every session and the animal simply executes a previously well-learned pattern of behavior. The inclusion of this performance component thus provides a within-session control for drug effects, which are specific to acquisition (learning) processes.
A few studies have evaluated the acute effects of psychomotor stimulants using such RAP procedures. Thompson and Moerschbaecher (1979b) found that d-amphetamine impaired acquisition of response chains in monkeys at doses that spared performance. Similarly, effects of d-amphetamine and MPD that were selective to learning were also found in pigeons (Moerschbaecher et al., 1979; Thompson, 1976). However, MDMA did not produce selective effects on acquisition of repeated chains in monkeys, as learning was impaired only at doses that also produced performance impairments (Thompson et al., 1987). In contrast, Galizio et al. (2009) found selective effects of MDMA, but only non-selective effects of MPD and MA using a RAP procedure in rats. These findings were precisely the opposite of those reviewed above in monkeys and pigeons in which MPD and MA both produced effects that were selective to acquisition and MDMA had only non-selective effects. One explanation for the discrepancies among these studies could be that the effects of these particular drugs on rats are different than in other species such as pigeons and monkeys; however, there are other, and perhaps more plausible accounts related to methodological differences across the previous RAP studies.
Specifically, the RAP procedure used by Galizio et al. (2009) required rats to make a single nose-poke response to a particular location on a touchscreen to produce food reinforcement rather than a sequence of responses. In this procedure, the correct location changed from session to session in the acquisition component (without an accompanying discriminative stimulus), but remained constant in the performance component. The baseline in the Galizio et al. study differed from previous RAP studies in reinforcement schedule, response topography and response rates; any of which can be important determinants of drug effects. However, because selection of the correct response location in the Galizio et al. procedure required a place discrimination, it could also be argued that the different outcomes obtained across RAP studies might be due to pharmacological differences associated with learning spatial (touchscreen RAP) versus non-spatial tasks (response sequence RAP).
In order to test the spatial vs. non-spatial hypothesis, the present study investigated the effects of MDMA, MA and MPD on a RAP adaptation of a more traditional spatial learning procedure: the Morris Swim Task (MST--Morris, 1981). In the performance component (defined by one set of distal cues surrounding a pool), rats were trained to swim to a hidden platform that remained in same location throughout the experiment. In the acquisition component (defined by a different set of extra-pool stimuli), the location of the hidden platform varied from session to session. This procedure has previously been demonstrated to result in rapid and direct swims to the platform on virtually all trials in the performance component and in steep learning curves within each session in the acquisition component (Keith & Galizio, 1997). The RAP version of the MST is also a sensitive baseline for behavioral pharmacology as some drugs (e.g., benzodiazepines) produce selective effects on learning, while other drugs (e.g., NMDA antagonists) generally produce only non-selective effects (Galizio et al., 2003; Keith & Galizio, 1997; Keith et al., 2003). If the effects of psychomotor stimulants observed in the Galizio et al. (2009) touchscreen RAP study indicate differential pharmacological actions on spatial vs. non-spatial learning, then it would be predicted that MA and MPD would impair acquisition only at doses which also impaired performance in the spatial MST procedure, whereas MDMA would be expected to selectively impair learning.
Method
Subjects
Subjects were 13 Holtzman Sprague-Dawley male rats with six animals tested with each drug (some rats were tested in two drug conditions-see Table 1). The animals were between 90 and 120 days old at the beginning of the study. They were housed individually under a 12/12 hr light/dark environment.
Table 1.
Order of drug experiments.
| Rat # | MA | MDMA | MPD |
|---|---|---|---|
| 1 | 2nd | − | 1st |
| 2 | 1st | − | 2nd |
| 3 | 1st | − | 2nd |
| 4 | 2nd | − | 1st |
| 5 | 1st | 2nd | − |
| 6 | 2nd | 1st | − |
| 7 | − | 1st | − |
| 8 | − | 1st | − |
| 9 | − | 2nd | 1st |
| 10 | − | 1st | − |
| 11 | − | − | 1st |
Apparatus
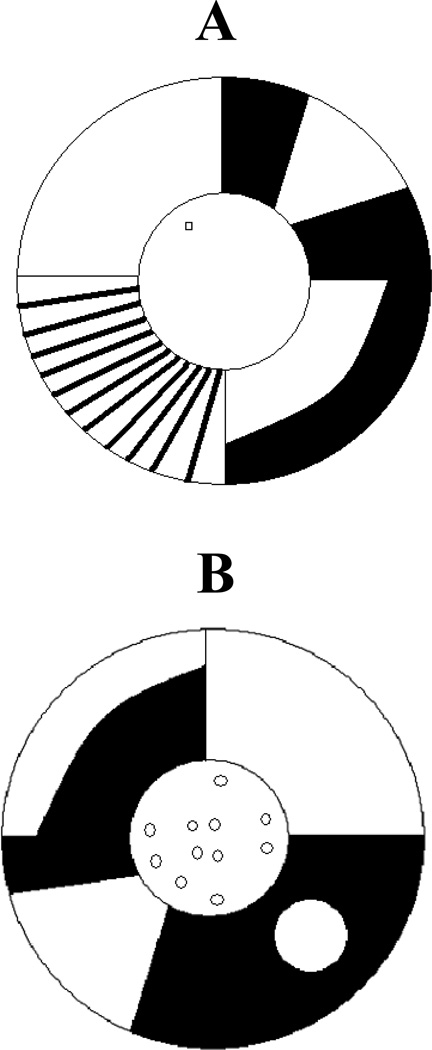
Subjects were trained in one of two nearly identical gray circular fiberglass pools (c. 1.5 m in diameter). A clear plastic cylindrical platform (10 cm in diameter) was placed in the pool, submerged so that the lip was 2.5 cm below the surface of the water. Black non-toxic paint was used to color the water and keep the platform location hidden. A digital video camera was mounted above the center of the pool to record the subjects’ movements using a video-tracking system (Noldus) attached to a microcomputer running data acquisition software. The temperature of the water was maintained at 30° (± 2°C). The pool was enclosed by patterned plastic shower curtains, forming the distinct stimulus configurations used to signal a component change as well as providing distal cues during navigation training (Figure 1).
Figure 1.
The two curtain configurations are shown. One was used to define the acquisition component and the other to define the performance component for each rat (randomly assigned). Configuration A represents the performance component with a single platform placement shown in the circular maze. Configuration B depicts the acquisition component curtain arrangement and the various escape platform locations.
Procedure
Preliminary training
Initial training began with six trials per session with the escape platform in a fixed location and the performance curtain configuration in place; this comprised the performance component. The experimenter placed the subject facing the wall in the water at one of the four designated start positions (North, South, East, or West—determined randomly). If the rat failed to locate the platform within 60 seconds, the experimenter placed the rat on the platform by hand. Once reaching the platform, the rat was permitted to remain there for 15 seconds and was then returned to the home cage for 2.5 m intertrial interval. After three consecutive sessions with an average escape latency of less than 10 s, conditions were changed to include the multiple-component training.
Multiple-component training
Twelve trials were conducted in each session of training in this phase. Sessions were conducted five days per week (M-F) and only one session was conducted per day. Six of these trials were conducted under conditions identical to those described above and thus involved swimming to a previously learned location (performance component—Figure 1A). For the additional six trials (acquisition component), the curtain configuration was changed and the platform was moved to a different location. In the acquisition component, the platform remained in a constant location for all trials in a particular session, but was changed to a randomly selected location before each new session (Figure 1B). In this way, the rate of learning a new platform location could be determined within each session (acquisition component) while assessing navigation to a well-learned location in the same session (performance component). Sessions always began with a performance trial and alternated between performance and acquisition throughout the session. Curtain configurations were changed during the ITI between each successive trial. One curtain configuration was always associated with the performance component and the other with the acquisition component with rats randomly assigned to particular configurations. Generally, rats were tested once each day, Monday through Friday. The following criteria had to be met across 10 consecutive sessions before drug treatments began: a) mean escape latencies for each session were below 10 s in the performance component; b) mean escape latencies for each session were below 20 s in the acquisition component; and c) the difference between the mean escape latencies of the most recent five sessions and the immediately preceding five sessions did not exceed 15% of the 10 session mean in either component.
Drug Preparation and Administration
Drug solutions were prepared by dissolving each compound in an isotonic (0.9%) sodium chloride solution. Drug and saline injections (ip.) were administered in a volume of 1 ml/kg twice per week (Tuesday and Friday) 15 min before behavioral testing began. Dose-effect functions were designed to test a range of doses from one that produced no effect to one that substantially disrupted swimming (animals were removed from the apparatus by the experimenter in cases where disruption of swimming was severe): 0.01–1.0 methamphetamine HCL (MA--Sigma); 1.0–30.0 mg/kg methylphenidate HCL (MPD--Sigma); and 0.3–5.6 (+/−) 3, 4 methylenedioxymethamphetamine HCL (MDMA--National Institute on Drug Abuse). The first determination of drug doses was generally administered in an ascending order with subsequent determinations administered psuedorandomly with the constraint that no dose was administered on successive drug days and that a full cycle (one exposure to each dose, including a saline injection) of the drug regimen was completed before beginning the next cycle. Each dose was determined at least twice with additional determinations performed when variability across determinations was observed. In several cases, a second drug experiment was conducted following completion of the first. A minimum of 10 days with no injections intervened between experiments and there was no evidence of carry-over effects in any of these cases (see Table 1 for order of the drug experiments).
Dependent Variables
There were three dependent measures used in this study: escape latency, swim path ratio, and swim speed. The escape latency was the time from releasing the animal in the drop location until it climbed onto the platform. The swim path ratio was another measure of the directness of the swim: the actual swim path distance minus the optimal distance (straight line) between the platform and drop locations, divided by the optimal distance. Thus, a higher ratio reflected a more indirect swim. Finally, as an index of performance impairment, swim speed was computed (cm traveled/latency). Session means were computed for the dependent variables for each subject. Because learning could not be manifest until the platform position was encountered on the first trial, only Trials 2–6 were used to compute mean latency and path ratio in the acquisition component. A single mean for each subject was then computed at each dose level for statistical analysis. Within-subject factorial ANOVAs were performed for each dependent measure using component (performance vs. acquisition) and dose as main factors (SPSS). Post hoc Tukey tests were performed when indicated.
Results
Completion of the pre-drug training phases required 21–57 sessions. At this point, all rats showed rapid, direct swims to the fixed platform position in the performance component characterized by short latencies and low path ratios. Because platform position varied from session to session in the acquisition component, latencies and path ratios were typically high on the first trial of the session, but declined rapidly on subsequent trials. Thus, the procedure was effective in demonstrating relatively direct swims to the familiar platform location in the performance component and rapid place learning in the acquisition component.
MA effects
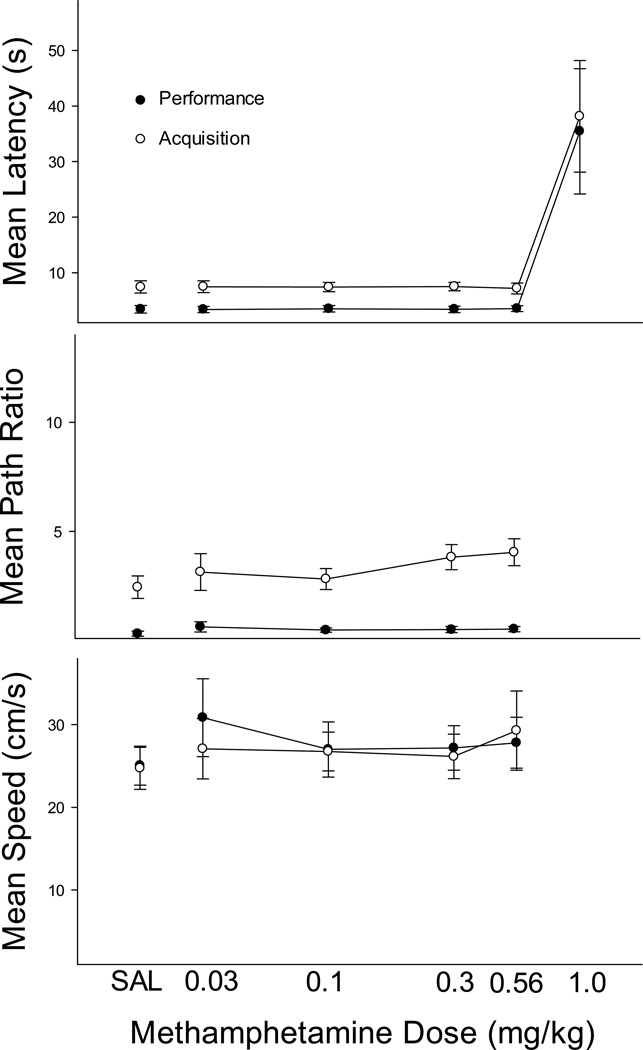
Figure 2 shows the effects of MA on escape latency (top panel), path ratio (middle panel) and swim speed (bottom panel) for the performance (black circles) and acquisition (white circles) components. The leftmost points represent data obtained following saline injections and characterize baseline performances. As would be expected, latencies and path ratios were consistently higher in the acquisition component, but swimming speed was nearly constant. MA did not affect escape latency until the 1.0 mg/kg dose was reached and at this dose, latencies in both performance and acquisition components were sharply elevated, indicating a non-selective effect of MA. These observations were confirmed statistically by significant main effects for component, F(1, 5) = 45.3, p < .01, and dose, F(5, 25) = 8.4, p < .01. The dose X component interaction was not significant (F < 1). Post hoc tests indicated that the only dose differing from saline was 1.0 mg/kg. The striking increase in latency observed at the 1.0-mg/kg dose was in part based on very erratic swimming behavior generated at this dose. This included swimming around the edge of the water maze (thigmotaxis), failure to climb onto the platform after reaching it, and difficulties in swimming that were severe enough to cause termination of the session in three of the six rats (in cases in which the animal failed to reach the platform with 60 s, a 60-s escape latency was recorded).
Figure 2.
Mean escape latency (top), path ratio (middle) and swimming speed (bottom) for performance (black symbols) and acquisition (white symbols) trials in the MA study. Error bars represent SEM.
A similar pattern is evident in Figure 2 for path ratio with swim paths, which were nearly optimal in the performance component and substantially longer in the acquisition component. MA produced no effects until the 1.0 mg/kg dose was reached, and because three of the six animals failed to swim to the platform at this dose, it was excluded from the statistical analysis. The ANOVA revealed a significant main effect for component, F(1, 5) = 66.9, p < .01, but no significant dose or interaction effects were obtained (p > .05). Similarly, swimming speed was relatively constant across components and was unaffected by MA up to the highest dose at which some animals stopped responding. No statistically significant effects were obtained (p > .05). In sum, MA affected neither learning a new platform location nor navigation to a familiar location until a dose was reached that disrupted swimming in both components.
MPD effects
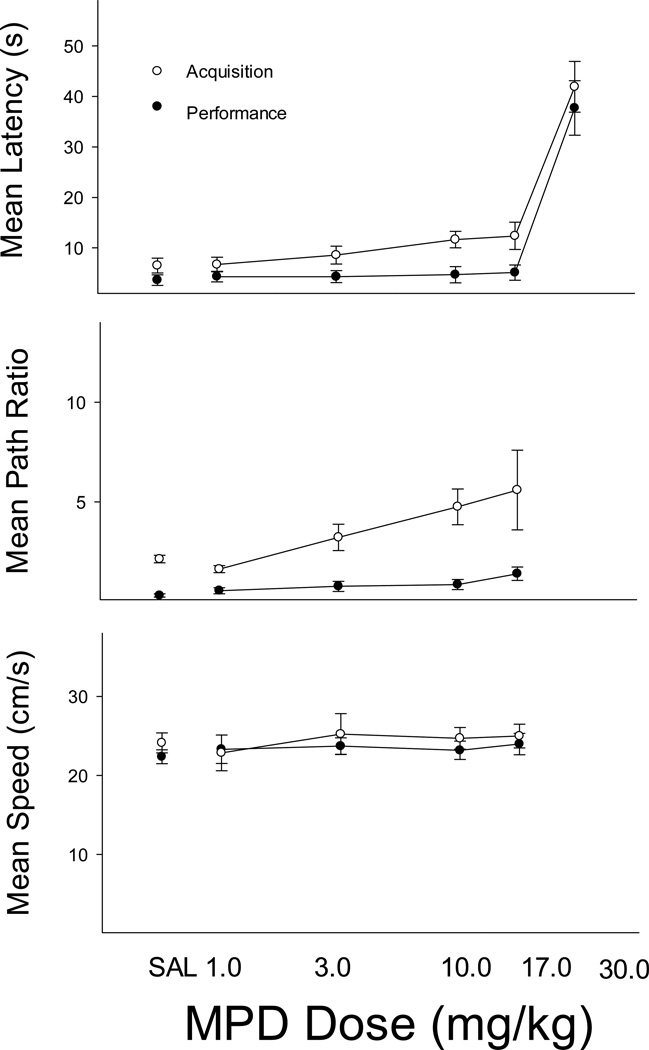
One animal was highly sensitive to the effects of MPD and showed escape failures at all but the lowest dose. This animal was dropped from further analyses. For the remaining five rats the effects of MPD were quite similar to those of MA. As Figure 3 shows, latencies (top panel) were not significantly affected until the highest dose of 30.0 mg/kg MPD was given. At this dose, latencies in both acquisition and performance components were greatly elevated and, as with MA, the increase was largely due to escape failures caused by swimming difficulty. There were main effects of component, F(1, 4) = 20.23, p < .05, and dose, F(5, 20) = 7.15, p < .01, but no significant interaction. The only MPD dose which produced latencies differing from saline was the 30.0 mg/kg dose (p < .05). The middle panel of Figure 3 shows path ratio and indicates a somewhat more selective effect of MPD at the 10.0 and 17.0 mg/kg doses in that ratios appeared more elevated in the acquisition than in the performance component (path ratios could not be computed for all animals at the 30.0 mg/kg dose due to session terminations and this dose was not included in the analysis). As with latency, there was a significant main effect for component, F(1, 4) = 34.44, p < .01, and dose, F(4, 16) = 4.26, p < .05, but the trend toward selective effects in the acquisition component was not supported as the component X dose interaction was not significant, F(4, 16) = 1.35, p > .05. Post hoc tests revealed that only the 17.0-mg/kg dose differed significantly from the saline control condition (p < .05). Swimming speed did not differ across components and was not affected at any MPD dose (all Fs < 1).
Figure 3.
Mean escape latency (top), path ratio (middle) and swimming speed (bottom) for performance (black symbols) and acquisition (white symbols) trials in the MPD study. Error bars represent SEM.
MDMA effects
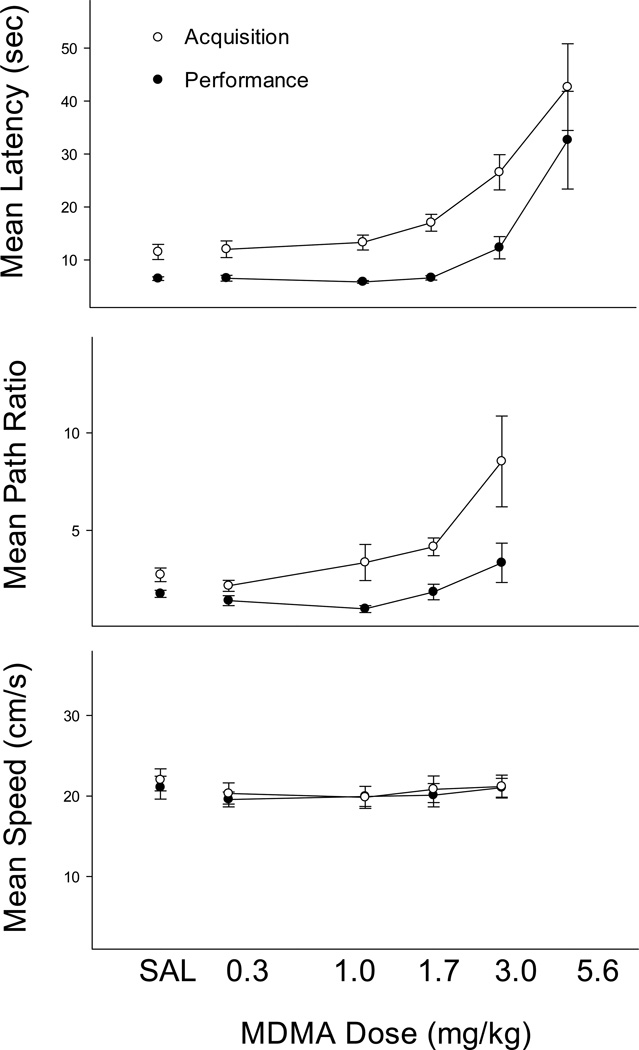
Figure 4 shows the effects of MDMA and once again the effects were non-selective: the doses that impaired place acquisition also impaired performance. Latencies in both components increased in a dose-dependent fashion with some impairment at the 3.0 mg/kg MDMA dose and more striking increases at the 5.6 mg/kg dose, F(5, 25) = 11.42, p < .01. As with the other drugs, this highest dose (5.6 mg/kg) resulted in non-selective effects such that in both components, some subjects displayed both swimming difficulty and escape failures. Once again, latencies were longer in the acquisition component than in the performance component, F(1, 5) = 56.54, p < .01, and there was no significant interaction, F(5, 25) = 2.29, p > .05. Post hoc tests revealed latencies that were significantly higher than saline controls at both the 3.0 and 5.6 mg/kg doses of MDMA. Outcomes with path ratio were similar with significant main effects of component, F(1, 5) = 8.56, p < .05, and dose, F(4, 20) = 6.29, p < .01, but no significant interaction, F(4, 20) = 2.80, p > .05. Only the 3.0 mg/kg dose produced path ratios that were significantly higher than those obtained after saline (p < .05—note that path ratios could not be calculated for all rats at the 5.6 mg/kg dose due to experimenter intervention). Finally, swimming speeds were unaffected at any dose (p > .05).
Figure 4.
Mean escape latency (top), path ratio (middle) and swimming speed (bottom) for performance (black symbols) and acquisition (white symbols) trials in the MDMA study. Error bars represent SEM.
Discussion
In sum, the effects of MA, MPD and MDMA on navigation were strikingly similar. In each case, no effects on any of the dependent measures were observed until doses were reached which produced impairments both in learning a new platform location (acquisition component) and in navigating to the well-learned location (performance component). Thus, none of these drugs produced effects that appeared to be selective to place learning. Indeed, the effects at these relatively high doses often appeared to involve difficulties in swimming and climbing onto the platform, rather than involving learning or memory processes at all. The absence of selective effects of MA and MPD in the current study is consistent with the findings of Galizio et al. (2009) using the spatial touch-screen task and may be viewed as providing support for the hypothesis that selective effects of MA and MPD psychomotor stimulants on acquisition observed with more traditional operant procedures (e.g., Thompson, 1976; Thompson & Moerschbaecher, 1979b) are not present in spatial learning. However, the finding of nonselective effects of MDMA in the present study contrasts with Galizio, et al., and suggests that MDMA effects are not modulated by the spatial or non-spatial features of the task. Rather it appears that some other feature of the touchscreen RAP task used by Galizio, et al. (e.g., escape vs. food reinforcement) may have been critical to the finding of MDMA effects selective to learning.
Very few other studies of the acute effects of psychomotor stimulants on spatial navigation in the MST are available for comparison. We were unable to find any studies of acute MA or even d-amphetamine effects in the MST. There exists a previous study of acute MDMA effects by Arias-Cavieres et al. (2010) which found that relatively low doses of MDMA (0.2 and 2.0 mg/kg) impaired acquisition in the MST and concluded that acute MDMA impairs spatial learning. Two features of the Arias-Cavieres et al. study may be responsible for the differences from the present outcomes, and consideration of these may permit reconciliation with the present findings. First, Arias-Cavieres et al. used a visible platform as a control condition. This is a popular control in the MST literature, but is not as stringent as the hidden platform RAP performance control used here because the visual platform cue eliminates the need for spatial navigation using distal cues (see Keith & Galizio, 1997). Second, Arias-Cavieres et al. administered MDMA on the first day that rats were exposed to the hidden platform condition, whereas in contrast, rats in our study had extensive experience in the MST prior to drug exposure. This is an important point because several researchers have noted that initial acquisition in the MST is complicated by interfering behaviors such as thigmotaxis (circling the circumference of pool) and failing to climb onto the platform when it is reached (Cain et al., 1996; Saucier et al., 1996). Initial acquisition requires inhibition of these interfering behaviors as well as the development of exploratory swimming patterns that will then permit successful place learning. Viewed in this way, it may be the inhibition of thigmotaxic tendencies and/or the development of appropriate swimming patterns that MDMA interfered with in the Arias-Cavieres, et al. study—rather than any effects that were selective to place learning per se.
No additional studies of acute effects of MDMA on MST performance are available, but there are studies with other spatial learning tasks. For example, Marston, et al. (1999) found that acute MDMA impaired delayed match-to-place performance and Braida, et al. (2002) showed that acute MDMA impaired performance in the radial arm maze. The conclusions of the above studies were that acute MDMA results in working memory impairments. However, it has been argued that these findings do not involve working memory effects, but rather reflect disruptions of reference memory involving the rules or strategies associated with a given task (Harper et al., 2005; 2006; Kay et al., 2010). As an illustration, Kay et al. (2010) used a version of the radial arm maze designed to separate working and reference memory and found that MDMA had no effect on the working memory task (arms were always baited for one visit), but impaired the reference memory task (arms that were never baited throughout the experiment).
The present findings can be interpreted within the Kay et al. (2010) framework, but first a point of terminological confusion must be addressed: the distinction between acquisition and performance in the RAP procedures of behavioral pharmacology is generally replaced by the working versus reference memory distinction in the cognitive neuroscience literature that informs most spatial learning studies (Dudchenko, 2004). Spatial procedures in which a place is remembered only within a session are generally referred to as working memory tasks, but those in which a place is to be remembered from one session to the next are said to involve reference memory. Using this terminology, the acquisition component of the present study can be described as a working memory version of the MST and the performance component as a reference memory task. In the present study, the acute effects of MDMA, MA and MPD in the MST were non-selective; acquisition (working memory) was affected only at doses that also impaired performance (reference memory). Although an account of the present findings in terms of non-cognitive variables seems most parsimonious, the Kay et al. (2010) study suggests that the possibility of reference memory impairment cannot be completely ruled out. For example, it could be argued that interference with reference memory processes resulted in a breakdown of the performance/acquisition discrimination at high doses. Such an account is particularly plausible as an explanation of the MDMA effects in the present study. Impairment induced by 3.0 mg/kg MDMA occurred without disruption in swimming speed (Figure 4), which makes interpretation in terms of motor impairment or reduced motivation less likely in this case. It should also be noted that the failure to observe selective effects on place learning with the present RAP task is not simply due to an inherent insensitivity of the procedure. On the contrary, several previous studies have observed selective effects of amnestic drugs (e.g., benzodiazepines) with this identical procedure in our laboratory (Keith & Galizio, 1997; Keith et al., 2003; Padlubnaya et al., 2005).
A final point is that no enhancement of learning or performance was observed at any dose with any of the three drugs. The absence of learning enhancement by MPD is of particular interest given that Tian et al. (2009) found improved place learning in the MST following injections of 10.0 mg/kg MPD in spontaneously hypertensive rats (SHR). As these SHR rats are often used as a model of ADHD, perhaps the failure to observe MPD enhancement of learning with normal rats in the present study is not unexpected, but other procedural differences may have been important as well. For example, Tian et al. administered MPD to rats naïve to the MST that were still learning to adjust to immersion in the pool as well as to navigate to the hidden platform. In the present study, rats were highly experienced with the MST and were learning only the platform location. The only other comparable study with normal rats (Zeise et al., 2007) found no effect of MPD on place learning in the MST, but they used only a single low dose (1.0 mg/kg). A replication of the present study with SHR rats would certainly be valuable in clarifying whether the Tian et al. findings involve enhancement of place learning or some more general effects on MST performance.
Acknowledgments
The research was supported in part by grants DA12879 and DA029252 to Mark Galizio. The authors thank the following UNCW students who assisted in data collection and analysis: Shanna Baird, Erica Blackwell, Laura Bullard, Carol Dwan, Mitch Ferguson, Miles Hulick, Patrick McKinney, Laurence Miller, Shayna Nesbitt, and Jessie Ramsey.
References
- Arias-Cavieres A, Rozas C, Reyes-Parada M, Barrera N, Pancetti F, Loyola S, et al. MDMA (“ecstasy”) impairs learning in the Morris water maze and reduces hippocampal LTP in young rats. Neuroscience Letters. 2010;469:375–379. doi: 10.1016/j.neulet.2009.12.031. [DOI] [PubMed] [Google Scholar]
- Baicy K, London ED. Corticolimbic dysregulation and chronic methamphetamine abuse. Addiction. 2007;102(Suppl 1):5–15. doi: 10.1111/j.1360-0443.2006.01777.x. [DOI] [PubMed] [Google Scholar]
- Barch DM, Carter CS. Amphetamine improves cognitive function in medicated individuals with schizophrenia and in healthy volunteers. Schizophrenia Research. 2005;77:43–58. doi: 10.1016/j.schres.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Braida D, Pozzi M, Cavallini R, Sala M. 3,4 Methylenedioxymethamphetamine (Ecstasy) impairs eight-arm radial maze performance and arm entry pattern in rats. Behavioral Neuroscience. 2002;116:298–304. doi: 10.1037//0735-7044.116.2.298. [DOI] [PubMed] [Google Scholar]
- Byrne T, Baker LE, Poling A. MDMA and learning: effects of acute and neurotoxic exposure in the rat. Pharmacology Biochemistry & Behavior. 2000;66:501–508. doi: 10.1016/s0091-3057(00)00227-6. [DOI] [PubMed] [Google Scholar]
- Cain DP, Saucier D, Hall J, Hargreaves EL, Boon F. Detailed behavioral analysis of water maze acquisition under APV or CNQX: Contribution of sensorimotor disturbances to drug-induced acquisition deficits. Behavioral Neuroscience. 1996;110:86–102. doi: 10.1037//0735-7044.110.1.86. [DOI] [PubMed] [Google Scholar]
- Calhoun WH, Jones EA. Methamphetamine’s effect on repeated acquisitions with serial discrimination reversals. Psychopharmacology. 1974;39:303–308. doi: 10.1007/BF00422969. [DOI] [PubMed] [Google Scholar]
- Chuhan YS, Taukulis HK. Impairment of single-trial memory formation by oral methylphenidate in the rat. Neurobiology of Learning and Memory. 2006;85:125–131. doi: 10.1016/j.nlm.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Dudchenko PA. An overview of the tasks used to test working memory in rodents. Neuroscience & Biobehavioral Reviews. 2004;28:699–709. doi: 10.1016/j.neubiorev.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Galizio M, Keith JR, Mansfield W, Pitts RC. Repeated spatial acquisition: Effects of NMDA antagonists and morphine. Experimental and Clinical Psychopharmacology. 2003;11:79–90. doi: 10.1037//1064-1297.11.1.79. [DOI] [PubMed] [Google Scholar]
- Galizio M, McKinney P, Cerutti DT, Pitts RC. Effects of MDMA, methamphetamine and methylphenidate on repeated acquisition and performance in rats. Pharmacology, Biochemistry & Behavior. 2009;94:305–311. doi: 10.1016/j.pbb.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley GW, Calhoun WH. The effects of methylphenidate on repeated acquisition of serial discrimination reversals. Psychopharmacology. 1978;57:115–117. doi: 10.1007/BF00426967. [DOI] [PubMed] [Google Scholar]
- Harper DN, Hunt M, Schenk S. Attenuation of the disruptive effects of (+/−) 3,4-methylene dioxymethamphetamine on delayed matching-to-sample performance in rats. Behavioral Neuroscience. 2006;120:201–205. doi: 10.1037/0735-7044.120.1.201. [DOI] [PubMed] [Google Scholar]
- Harper DN, Wisniewski R, Hunt M, Schenk S. (+/−) 3,4-methylene dioxymethamphetamine, d-amphetamine and cocaine impair delayed matching-to-sample performance via an increase in susceptibility to proactive interference. Behavioral Neuroscience. 2005;119:455–463. doi: 10.1037/0735-7044.119.2.455. [DOI] [PubMed] [Google Scholar]
- Hart CL, Gunderson EW, Perez A, Kirkpatrick MG, Thurmond A, Comer SD, Foltin RW. Acute physiological and behavioral effects of intranasal methamphetamine in humans. Neuropsychopharmacology. 2008;33:1847–1855. doi: 10.1038/sj.npp.1301578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homer BD, Solomon TM, Moeller RW, Mascia A, DeRaleau L, Halkitis PN. Methamphetamine abuse and impairment of social functioning: a review of the underlying neurophysiological causes and behavioral implications. Psychological Bulletin. 2008;134:301–310. doi: 10.1037/0033-2909.134.2.301. [DOI] [PubMed] [Google Scholar]
- Kay C, Harper DN, Hunt M. Differential effects of MDMA and scopolamine on working versus reference memory in the radial arm maze task. Neurobiology of Learning and Memory. 2010;93:151–156. doi: 10.1016/j.nlm.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Keith JR, Galizio M. Acquisition in the Morris swim task is impaired by a benzodiazepine but not an NMDA antagonist: a new procedure for distinguishing acquisition and performance effects. Psychobiology. 1997;25:217–228. [Google Scholar]
- Keith JR, Pitts RC, Pezzuti T, Galizio M. GABA-A modulator effects on a multiple-component, repeated-acquisition test of spatial learning. Behavioral Pharmacology. 2003;14:67–76. doi: 10.1097/00008877-200302000-00007. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick MG, Gunderson EW, Perez AY, Haney M, Foltin RW, Hart CL. A direct comparison of the behavioral and physiological effect of methamphetamine and 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology. 2012;219:109–122. doi: 10.1007/s00213-011-2383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston HM, Reid ME, Lawrence JA, Olverman HJ, Butcher SP. Behavioural analysis of the acute and chronic effects of MDMA treatment in the rat. Psychopharmacology. 1999;144:67–76. doi: 10.1007/s002130050978. [DOI] [PubMed] [Google Scholar]
- Mayorga AJ, Popke EJ, Fogle CM, Paule MG. Similar effects of amphetamine and methylphenidate on the performance of complex operant tasks in rats. Behavioral Brain Research. 2000;109:59–68. doi: 10.1016/s0166-4328(99)00165-5. [DOI] [PubMed] [Google Scholar]
- McCann UD, Kuwabara H, Kumar A, Palermo M, Abbey R, Brasic J, et al. Persistent cognitive and dopamine transporter deficits in abstinent methamphetamine users. Synapse. 2008;62:91–100. doi: 10.1002/syn.20471. [DOI] [PubMed] [Google Scholar]
- Moerschbaecher JM, Boren JJ, Schrot J, Simoes-Fontes JC. Effects of cocaine and d-amphetamine on the repeated acquisition and performance of conditional discriminations. Journal of the Experimental Analysis of Behavior. 1979;31:127–140. doi: 10.1901/jeab.1979.31-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RGM. Spatial localization does not require the presence of local cues. Learning and Motivation. 1981;12:39–260. [Google Scholar]
- Nulsen CE, Fox AM, Hammond GR. Differential effects of ecstasy on short-term and working memory: A meta-analysis. Neuropsychology Review. 2010;20:21–32. doi: 10.1007/s11065-009-9124-z. [DOI] [PubMed] [Google Scholar]
- Padlubnaya D, Galizio M, Pitts RC, Keith J. Chlordiazepoxide interactions with scopolamine and dizocilpine: Novel cooperative and antagonistic effects on spatial learning. Behavioral Neuroscience. 2005;119:1331–1338. doi: 10.1037/0735-7044.119.5.1331. [DOI] [PubMed] [Google Scholar]
- Quintero-Munoz D, Saez-Briones P, Diaz-Veliz G, Mora-Gutierrez S, Rebolledo-Fuentes M, Cassels BK. Behavioral profiles in rats distinguish among “ecstasy,” methamphetamine and 2,5-Dimethoxy-4-iodoamphetamine: Mixed effects for “ecstasy” analogues. Behavioral Neuroscience. 2010;124:662–676. doi: 10.1037/a0020827. [DOI] [PubMed] [Google Scholar]
- Saucier D, Hargreaves EL, Boon F, Vanderwolf CH, Cain DP. Detailed behavioral analysis of water maze acquisition under systemic NMDA or muscarinic antagonism: Nonspatial pretraining eliminates spatial learning deficits. Behavioral Neuroscience. 1996;110:103–116. [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I. Neurocognitive effects of methamphetamine: A critical review and meta-analysis. Neuropsychology Review. 2007;17:275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Stough C, King R, Papafotiou K, Swann P, Ogden E, Wesnes K, Downey LA. The acute effects of 3,4-methlenedioxymethamphetamine and d-methamphetamine on human cognitive functioning. Psychopharmacology. 2012;220:799–807. doi: 10.1007/s00213-011-2532-9. [DOI] [PubMed] [Google Scholar]
- Thompson DM. Repeated acquisition of behavioral chains: effects of methylphenidate and imipramine. Pharmacology, Biochemistry & Behavior. 1976;6:671–677. doi: 10.1016/0091-3057(76)90218-5. [DOI] [PubMed] [Google Scholar]
- Thompson DM, Moerschbaecher JM. Drug effects on repeated acquisition. In: Thompson T, Dews PB, editors. Advances in behavioral pharmacology. Vol. 2. New York: Academic Press; 1979a. pp. 229–259. [Google Scholar]
- Thompson DM, Moerschbaecher JM. An experimental analysis of the effects of d-amphetamine and cocaine on the acquisition and performance of response chains in monkeys. Journal of the Experimental Analysis of Behavior. 1979b;32:433–444. doi: 10.1901/jeab.1979.32-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DM, Winsauer PJ, Mastropaolo J. Effects of phencyclidine, ketamine, and MDMA on complex operant behavior in monkeys. Pharmacology, Biochemistry & Behavior. 1987;26:401–405. doi: 10.1016/0091-3057(87)90136-5. [DOI] [PubMed] [Google Scholar]
- Tian Y, Wang Y, Deng Y, Maeda K. Methylphenidate improves spatial memory of spontaneously hypertensive rats: Evidence in behavioral and ultrastructural changes. Neuroscience Letters. 2009;461:106–109. doi: 10.1016/j.neulet.2009.05.080. [DOI] [PubMed] [Google Scholar]
- Zeise ML, Espinoza S, Gonzalez A, Cerda FS, Nacarate J, Yanez CG, Morales B. Methylphenidate improves cue navigation in the Morris water maze in rats. Neuroreports. 2007;18:1059–1062. doi: 10.1097/WNR.0b013e32819f8f3f. [DOI] [PubMed] [Google Scholar]
- Zhu N, Weedon J, Dow-Edwards DL. Oral methylphenidate improves spatial learning and memory in pre- and periadolescent rats. Behavioral Neuroscience. 2007;121:1272–1279. doi: 10.1037/0735-7044.121.6.1272. [DOI] [PubMed] [Google Scholar]