Abstract
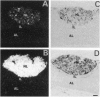
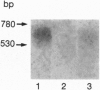
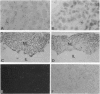
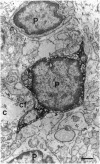
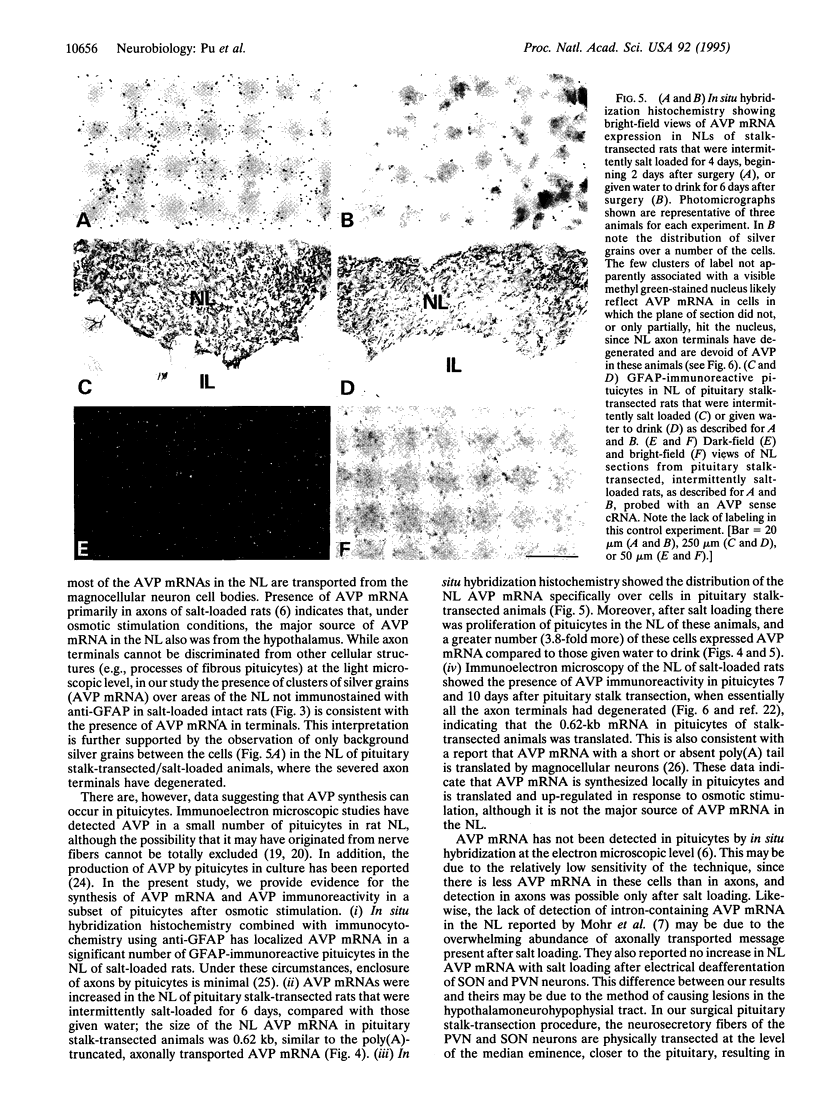
The presence of [arginine] vasopressin (AVP) mRNA and AVP immunoreactivity in pituicytes of the neural lobe (NL) of intact and pituitary stalk-transected rats, with and without osmotic stimulation, was examined. AVP mRNA was analyzed by Northern blotting, as well as by in situ hybridization in combination with immunocytochemistry using anti-glial fibrillary acidic protein (GFAP) as a marker for pituicytes. In intact rats, a poly(A) tail-truncated 0.62-kb AVP mRNA was detected in the NL and was found to increase 10-fold with 7 days of continuous salt loading. Morphological analysis of the NL of 7-day salt-loaded rats revealed the presence of AVP mRNA in a significant number of GFAP-positive pituicytes in the NL and in areas most probably containing nerve fibers. Eight days after pituitary stalk transection the NL AVP mRNA diminished in animals given water to drink, whereas in those given 2% saline for 18 h followed by 6 h of water, a treatment repeated on 6 successive days beginning 2 days after surgery, the 0.62-kb AVP mRNA was present. The AVP mRNA in the pituitary stalk-transected, salt-loaded rats showed an exclusive cellular distribution in the NL, indicative of localization in pituicytes. Immunoelectron microscopy showed the presence of AVP immunoreactivity in a subpopulation of pituicytes 7 and 10 days after pituitary stalk transection in salt-loaded animals, when almost all AVP fibers had disappeared from the NL. These data show that a subset of pituicytes in the NL is activated to synthesize AVP mRNA and AVP in response to osmotic stimulation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birch N. P., Rodriguez C., Dixon J. E., Mezey E. Distribution of carboxypeptidase H messenger RNA in rat brain using in situ hybridization histochemistry: implications for neuropeptide biosynthesis. Brain Res Mol Brain Res. 1990 Jan;7(1):53–59. doi: 10.1016/0169-328x(90)90073-m. [DOI] [PubMed] [Google Scholar]
- Birch N. P., Tracer H. L., Hakes D. J., Loh Y. P. Coordinate regulation of mRNA levels of pro-opiomelanocortin and the candidate processing enzymes PC2 and PC3, but not furin, in rat pituitary intermediate lobe. Biochem Biophys Res Commun. 1991 Sep 30;179(3):1311–1319. doi: 10.1016/0006-291x(91)91716-p. [DOI] [PubMed] [Google Scholar]
- Boersma C. J., Sonnemans M. A., Van Leeuwen F. W. Immunoelectron microscopic demonstration of oxytocin and vasopressin in pituicytes and in nerve terminals forming synaptoid contacts with pituicytes in the rat neural lobe. Brain Res. 1993 May 14;611(1):117–129. doi: 10.1016/0006-8993(93)91783-o. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dahl D., Bignami A. Immunogenic properties of the glial fibrillary acidic protein. Brain Res. 1976 Oct 29;116(1):150–157. doi: 10.1016/0006-8993(76)90257-2. [DOI] [PubMed] [Google Scholar]
- Dellmann H. D. Degeneration and regeneration of neurosecretory systems. Int Rev Cytol. 1973;36:215–315. doi: 10.1016/s0074-7696(08)60219-3. [DOI] [PubMed] [Google Scholar]
- Hauser K. F., Osborne J. G., Stiene-Martin A., Melner M. H. Cellular localization of proenkephalin mRNA and enkephalin peptide products in cultured astrocytes. Brain Res. 1990 Jul 9;522(2):347–353. doi: 10.1016/0006-8993(90)91482-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- KENNEDY G. C., LIPSCOMB H. S., HAGUE P. PLASMA CORTICOSTERONE IN RATS WITH EXPERIMENTAL DIABETES INSIPIDUS. J Endocrinol. 1963 Nov;27:345–353. doi: 10.1677/joe.0.0270345. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Javedan S., Bondy C. A. Coordinate expression of insulin-like growth factor system components by neurons and neuroglia during retinal and cerebellar development. J Neurosci. 1992 Dec;12(12):4737–4744. doi: 10.1523/JNEUROSCI.12-12-04737.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann E., Hänze J., Pauschinger M., Ganten D., Lang R. E. Vasopressin mRNA in the neurolobe of the rat pituitary. Neurosci Lett. 1990 Mar 26;111(1-2):170–175. doi: 10.1016/0304-3940(90)90363-e. [DOI] [PubMed] [Google Scholar]
- Lightman S. L., Ninkovic M., Hunt S. P., Iversen L. L. Evidence for opiate receptors on pituicytes. Nature. 1983 Sep 15;305(5931):235–237. doi: 10.1038/305235a0. [DOI] [PubMed] [Google Scholar]
- Maciejewski-Lenoir D., Jirikowski G. F., Sanna P. P., Bloom F. E. Reduction of exogenous vasopressin RNA poly(A) tail length increases its effectiveness in transiently correcting diabetes insipidus in the Brattleboro rat. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1435–1439. doi: 10.1073/pnas.90.4.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe J. T., Lehmann E., Chastrette N., Hänze J., Lang R. E., Ganten D., Pfaff D. W. Detection of vasopressin mRNA in the neurointermediate lobe of the rat pituitary. Brain Res Mol Brain Res. 1990 Oct;8(4):325–329. doi: 10.1016/0169-328x(90)90046-g. [DOI] [PubMed] [Google Scholar]
- Mohr E., Fehr S., Richter D. Axonal transport of neuropeptide encoding mRNAs within the hypothalamo-hypophyseal tract of rats. EMBO J. 1991 Sep;10(9):2419–2424. doi: 10.1002/j.1460-2075.1991.tb07781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr E., Zhou A., Thorn N. A., Richter D. Rats with physically disconnected hypothalamo-pituitary tracts no longer contain vasopressin-oxytocin gene transcripts in the posterior pituitary lobe. FEBS Lett. 1990 Apr 24;263(2):332–336. doi: 10.1016/0014-5793(90)81407-f. [DOI] [PubMed] [Google Scholar]
- Murphy D., Levy A., Lightman S., Carter D. Vasopressin RNA in the neural lobe of the pituitary: dramatic accumulation in response to salt loading. Proc Natl Acad Sci U S A. 1989 Nov;86(22):9002–9005. doi: 10.1073/pnas.86.22.9002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu L. P., Hayes W. P., Mill J. F., Ghose S., Friedman T. C., Loh Y. P. Frog prohormone convertase PC2 mRNA has a mammalian-like expression pattern in the central nervous system and is colocalized with a subset of thyrotropin-releasing hormone-expressing neurons. J Comp Neurol. 1995 Mar 27;354(1):71–86. doi: 10.1002/cne.903540107. [DOI] [PubMed] [Google Scholar]
- Salm A. K., Hatton G. I., Nilaver G. Immunoreactive glial fibrillary acidic protein in pituicytes of the rat neurohypophysis. Brain Res. 1982 Mar 25;236(2):471–476. doi: 10.1016/0006-8993(82)90729-6. [DOI] [PubMed] [Google Scholar]
- Sherman T. G., Day R., Civelli O., Douglass J., Herbert E., Akil H., Watson S. J. Regulation of hypothalamic magnocellular neuropeptides and their mRNAs in the Brattleboro rat: coordinate responses to further osmotic challenge. J Neurosci. 1988 Oct;8(10):3785–3796. doi: 10.1523/JNEUROSCI.08-10-03785.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda H., Marini A. M., Schwartz J. P. Developmental expression of the proenkephalin and prosomatostatin genes in cultured cortical and cerebellar astrocytes. Brain Res Dev Brain Res. 1992 Jun 19;67(2):205–210. doi: 10.1016/0165-3806(92)90220-q. [DOI] [PubMed] [Google Scholar]
- Stornetta R. L., Hawelu-Johnson C. L., Guyenet P. G., Lynch K. R. Astrocytes synthesize angiotensinogen in brain. Science. 1988 Dec 9;242(4884):1444–1446. doi: 10.1126/science.3201232. [DOI] [PubMed] [Google Scholar]
- Suess U., Pliska V. Identification of the pituicytes as astroglial cells by indirect immunofluorescence-staining for the glial fibrillary acidic protein. Brain Res. 1981 Sep 21;221(1):27–33. doi: 10.1016/0006-8993(81)91061-1. [DOI] [PubMed] [Google Scholar]
- THORN N. A., SMITH M. W., SKADHAUGE E. THE ANTIDIURETIC EFFECT OF INTRAVENOUS AND INTRACAROTID INFUSION OF CALCIUM CHLORIDE IN HYDRATED RATS. J Endocrinol. 1965 May;32:161–165. doi: 10.1677/joe.0.0320161. [DOI] [PubMed] [Google Scholar]
- Trembleau A., Morales M., Bloom F. E. Aggregation of vasopressin mRNA in a subset of axonal swellings of the median eminence and posterior pituitary: light and electron microscopic evidence. J Neurosci. 1994 Jan;14(1):39–53. doi: 10.1523/JNEUROSCI.14-01-00039.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweedle C. D., Hatton G. I. Evidence for dynamic interactions between pituicytes and neurosecretory axons in the rat. Neuroscience. 1980;5(3):661–671. doi: 10.1016/0306-4522(80)90063-9. [DOI] [PubMed] [Google Scholar]
- Tweedle C. D., Hatton G. I. Morphological adaptability at neurosecretory axonal endings on the neurovascular contact zone of the rat neurohypophysis. Neuroscience. 1987 Jan;20(1):241–246. doi: 10.1016/0306-4522(87)90016-9. [DOI] [PubMed] [Google Scholar]
- Vilijn M. H., Vaysse P. J., Zukin R. S., Kessler J. A. Expression of preproenkephalin mRNA by cultured astrocytes and neurons. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6551–6555. doi: 10.1073/pnas.85.17.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar M. J., Meister B., Hökfelt T. Reorganization of neural peptidergic systems in the median eminence after hypophysectomy. J Neurosci. 1994 Oct;14(10):5996–6012. doi: 10.1523/JNEUROSCI.14-10-05996.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young W. S., 3rd, Kovács K., Lolait S. J. The diurnal rhythm in vasopressin V1a receptor expression in the suprachiasmatic nucleus is not dependent on vasopressin. Endocrinology. 1993 Aug;133(2):585–590. doi: 10.1210/endo.133.2.8344200. [DOI] [PubMed] [Google Scholar]
- Zimmerman E. A., Robinson A. G., Husain M. K., Acosta M., Frantz A. G., Sawyer W. H. Neurohypophysial peptides in the bovine hypothalamus: the relationship of neurophysin I to oxytocin, and neurophysin II to vasopressin in supraoptic and paraventricular regions. Endocrinology. 1974 Oct;95(4):931–936. doi: 10.1210/endo-95-4-931. [DOI] [PubMed] [Google Scholar]