Abstract
Post-mortem MR (PMMR) imaging is a powerful diagnostic tool with a wide scope in forensic radiology. In the past 20 years, PMMR has been used as both an adjunct and an alternative to autopsy. The role of PMMR in forensic death investigations largely depends on the rules and habits of local jurisdictions, availability of experts, financial resources, and individual case circumstances. PMMR images are affected by post-mortem changes, including position-dependent sedimentation, variable body temperature and decomposition. Investigators must be familiar with the appearance of normal findings on PMMR to distinguish them from disease or injury. Coronal whole-body images provide a comprehensive overview. Notably, short tau inversion–recovery (STIR) images enable investigators to screen for pathological fluid accumulation, to which we refer as “forensic sentinel sign”. If scan time is short, subsequent PMMR imaging may be focussed on regions with a positive forensic sentinel sign. PMMR offers excellent anatomical detail and is especially useful to visualize pathologies of the brain, heart, subcutaneous fat tissue and abdominal organs. PMMR may also be used to document skeletal injury. Cardiovascular imaging is a core area of PMMR imaging and growing evidence indicates that PMMR is able to detect ischaemic injury at an earlier stage than traditional autopsy and routine histology. The aim of this review is to present an overview of normal findings on forensic PMMR, provide general advice on the application of PMMR and summarise the current literature on PMMR imaging of the head and neck, cardiovascular system, abdomen and musculoskeletal system.
“MRI may be an alternate method in restricted or denied autopsies”1
In 1990, Ros et al1 investigated the potential of pre-autopsy post-mortem MR (PMMR) imaging. Using a 0.15-T MR scanner they imaged six human cadavers prior to autopsy and found that “MRI was equal to autopsy in detecting gross cranial, pulmonary, abdominal and vascular pathologies” and even “superior to autopsy in detecting air and fluid”.1 The authors conclude their study with the visionary statement that PMMR may be an alternative to autopsy.
Approximately 10 years later, Bisset et al2,3 published two reports in the British Medical Journal to recount their experience with forensic PMMR imaging as alternative to autopsy in non-suspicious deaths. These reports caused a veritable furore in the medical community. Bisset's2 claim that MRI was “a credible alternative to invasive autopsy” was assailed by pathologists who criticized the lack of autopsy correlation and questioned both the qualification of clinical radiologists to correctly diagnose a cause of death and the technical ability of PMMR to demonstrate relevant pathologies as accurately as traditional necropsy.4
Within a few years after Bisset's first article, several additional studies on PMMR were published in the USA Switzerland, the UK and Japan.5–8 Although these studies reach somewhat discrepant conclusions, there is agreement that PMMR is a useful complement to traditional autopsy. In retrospect, some of the discrepancies of these early studies seem to be related to insufficient experience in performing and interpreting PMMR.
Over the past decade, both MR technology and post-mortem forensic radiology have significantly evolved.9,10 Today, pre-autopsy post-mortem cross-sectional imaging is a standard procedure in many forensic institutes worldwide.11 A recent analysis of the literature revealed that post-mortem CT (PMCT) enjoys a more widespread use in forensic radiology than PMMR.10 This finding is supported by a survey of the International Society of Forensic Radiology and Imaging (ISFRI) conducted in March 2013.12 Only 5% of all survey participants consider themselves to be familiar with PMMR (compared with 55% for PMCT) and only 12% are routinely using PMMR (compared with 42% for PMCT). Limited access to MR scanners, time constraints and the complexity of MR technology are thought to be the principal reasons why PMMR is used less frequently than PMCT.10
In spite of this, PMMR is a powerful tool in forensic death investigations and has the ability to enhance autopsy and uncover otherwise undetectable findings. The aim of this review article is to present an overview of normal findings on PMMR, provide general advice on the implementation of forensic PMMR and summarise the current literature on PMMR imaging of the head and neck, cardiovascular system, abdomen and musculoskeletal system.
STEP 1: NORMAL FINDINGS ON POST-MORTEM MR IMAGES
Clinical radiologists spend thousands of hours looking at radiographs, ultrasound, CT and MR images, searching for significant findings. To achieve this task they must have a thorough understanding of normal findings on any of these radiological images.13 Research on visual perception revealed that radiologists develop an ability to distinguish normal from abnormal findings at a single look.13,14 According to Drew et al,13 a short glance at an image will tell an experienced radiologist that something is “wrong” based on the gestalt of the image before he or she has actually identified the pathology. The differentiation between normal findings and true pathology is more difficult for inexperienced radiologists who lack internal reference standards for normal and abnormal. This principle also applies to post-mortem imaging; radiologists or pathologists who read PMMR images must first learn to distinguish normal from abnormal. This task remains a perpetual challenge in PMMR and forensic medicine in general.15–17
There is a wide range of normal post-mortem findings, including position-dependent sedimentation, post-mortem clotting and decomposition.18,19 The appearance of these normal findings will vary from case to case and depends on internal and external factors, such as body temperature, pre-existing conditions, underlying disease or injury and the post-mortem interval.19,20
The absence of motion artefacts
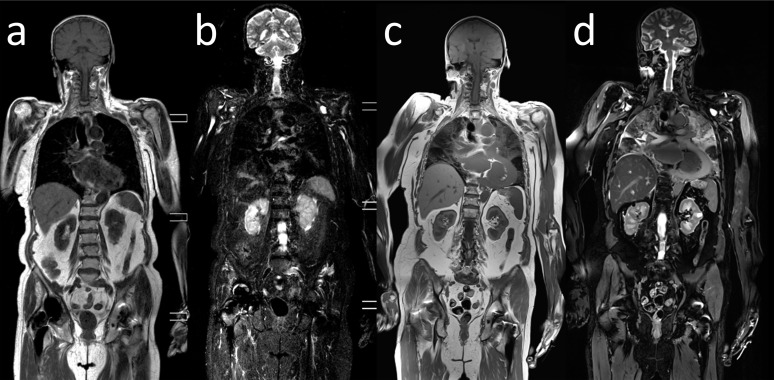
The first and most striking difference between clinical MR images and PMMR images is the absence of motion artefacts on PMMR. As a result, PMMR images provide substantially greater anatomical detail than clinical images (Figure 1).18,21
Figure 1.
Comparison between antemortem and post-mortem MR images: antemortem coronal whole-body T1 weighted (a) and short tau inversion–recovery (STIR) (b) images of an elderly patient suffering from aneurysm of the ascending aorta (not visualized on this image). (c, d) Post-mortem coronal whole-body T1 weighted (c) and STIR (d) images of the same patient after fatal rupture of the aneurysm with hemopericardium and pulmonary fluid accumulation. Note the absence of motion artefacts and the anatomical detail on the post-mortem images in comparison to the ante-mortem images.
Position-dependent sedimentation
Immediately after cessation of circulation, position-dependent fluid sedimentation develops.22,23 This results in a distinctive fluid–fluid level on T2 weighted PMMR images: cellular components of blood settle in the dependent areas of vascular structures or haemorrhagic collections as a dark hypointense layer, whereas the bright hyperintense fluid components are seen in a non-dependent position (Figure 2a).6,19 This appearance may be disturbed by the presence of post-mortem clots, which often are of mixed to intermediate signal intensity on T2 weighted images (Figure 2b).5,19,23 Position-dependent sedimentation is also visible in the lungs6,18,19 and can obscure or be confounded by the presence of underlying pulmonary pathology (Figure 2c).
Figure 2.
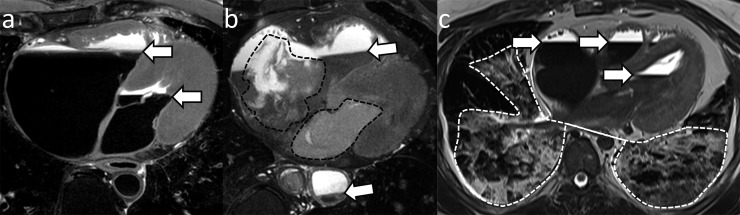
Position-dependent sedimentation on axial T2 weighted post-mortem MR images: (a) intravascular sedimentation typically exhibits fluid–fluid levels (arrows). Cellular components of blood settle in the dependent areas as a dark hypointense layer, whereas bright hyperintense fluid components are seen in a non-dependent position. (b) Fluid–fluid levels (arrows) may be disturbed by the presence of post-mortem clots (area within the dotted lines in the right and left atrium). (c) Position-dependent sedimentation (arrows) is also visible in the lungs (area within the dotted lines), but the differentiation between sedimentation and other coexisting fluid accumulations, such as pulmonary oedema, is challenging.
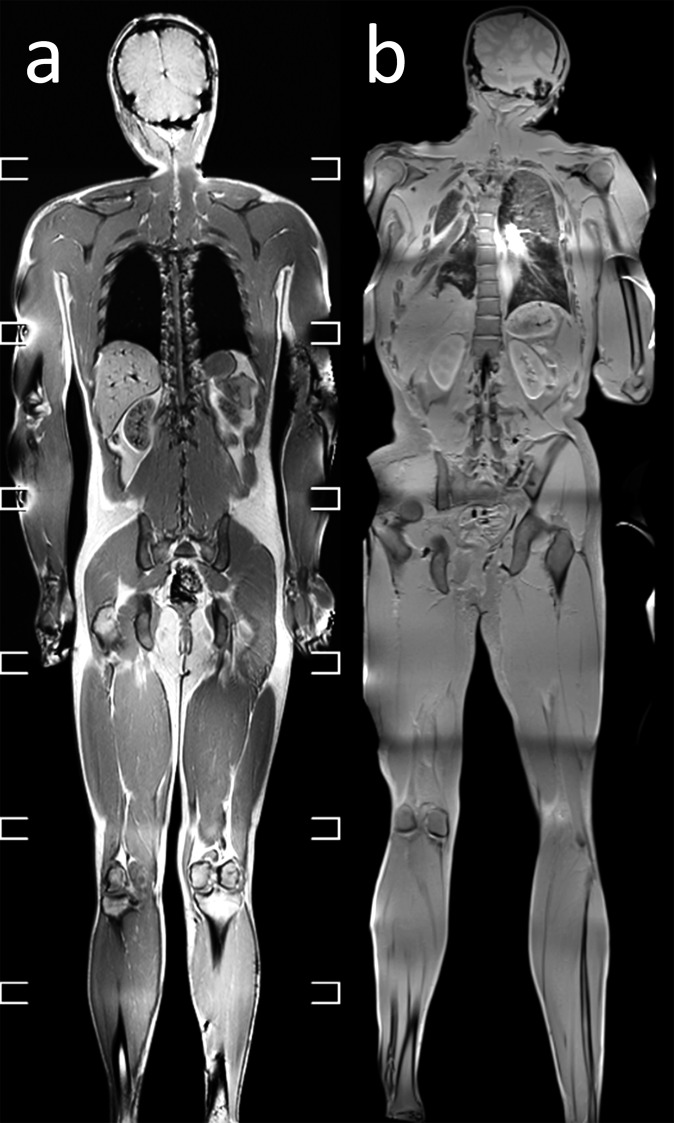
Temperature dependence of post-mortem MR image contrast
T1 and T2 relaxation times are temperature-dependent parameters.24,25 Because of post-mortem cooling, the temperature of cadavers is usually lower than in living patients. Notably low temperatures can alter image contrast on PMMR (Figure 3).20,26–28 Ruder et al20 found that low body temperatures result in low contrast between fat tissue and muscle tissue on T2 weighted images, whereas the contrast between fat tissue and fluids increases. Below 20 °C, the contrast between fat tissue and muscle tissue is annihilated and T2 weighted images resemble short tau inversion–recovery (STIR) images.20 On T1 weighted images, low body temperatures result in overall low image contrast.20 Below 10 °C, the image contrast deteriorates,20 which may confound the detection of pathology or injury.29 These results suggest that the influence of temperature on image quality is less problematic on T2 weighted than on T1 weighted images. Over the past years, several authors proposed to develop optimized scan parameters for PMMR.28–31 However, this topic is still being investigated,29 and to this date, there are no generally applicable dedicated PMMR scan protocols available.32
Figure 3.
Temperature dependence of post-mortem MR images: coronal whole-body T1 weighted images of two different cadavers (a) with body temperature of 24 °C and (b) with a body temperature of 4 °C. On T1 weighted images, image contrast deteriorates at body temperatures of 10 °C or lower.
It is our recommendation that radiographers, radiologists and pathologists working with PMMR should always measure the temperature of a cadaver prior to PMMR and carefully assess image quality.
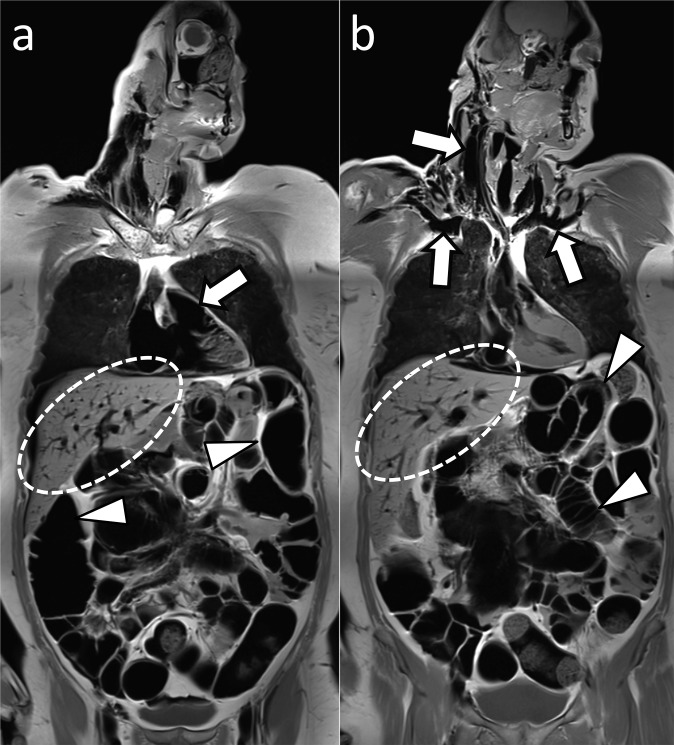
Gas
The presence of gas within vessels or organs is a frequent finding on post-mortem imaging (Figure 4). Certain patterns of gas collections may provide information regarding their source. However, gas formation and distribution depend on numerous factors, and one should be cautious to not overinterpret the meaning of post-mortem gas distribution.33–35 Intrahepatic gas, for example, may be the result of cardiopulmonary resuscitation, air embolism, penetrating liver injury or putrefaction.21 The effect of gas on image quality is less disturbing on PMCT than on PMMR, where it can cause artefacts.
Figure 4.
Post-mortem gas (a, b) coronal whole-body T1 weighted post-mortem MR images at two different levels in a case with significant intracardiac (a, arrow), intravascular (b, arrows), intrahepatic (circled by dotted line) and intestinal gas (arrowheads).
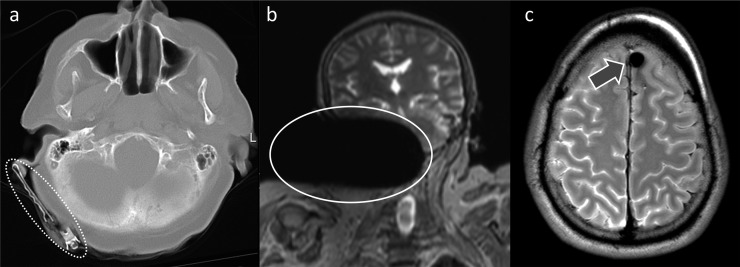
Metal artefacts
Image artefacts from metallic objects are a well-known phenomenon in both clinical and PMMR imaging. They typically consist of a zero signal zone and may induce geometric distortion36 (Figure 5). The extent of these artefacts may be reduced through special MR sequences.37 It is important to remember that any ferromagnetic object brought into an MR suite represents a potential hazard to staff and equipment.32 Although the rules and regulations regarding implanted medical devices may not necessarily apply to MRI of cadavers, it is our opinion that general MR safety guidelines38 should be observed.
Figure 5.
Metal artefacts on post-mortem MR. (a) Axial CT image at the level of the base of the skull with a metallic hair clip (circled by the white dotted line) behind the right ear. (b) Detailed view of a coronal whole-body short tau inversion–recovery image of the same case with extensive signal loss and distortion (circled by the white line) on the right side of the head and neck induced by the same hair clip. (c) Axial T2 weighted PMMR image of the skull with a small metal artefact in the left frontal lobe (arrow) caused by a non-ferromagnetic ballistic projectile.
It is the recommendation of these authors to perform a whole-body PMCT scan prior to PMMR to screen for metallic objects. In post-mortem forensic imaging, metallic objects may include debris from motor vehicle accidents, shrapnel from explosion, jewellery such as finger rings or projectiles from firearms. However, in our experience, prosthetic joints are the most frequent cause of metal artefacts on PMMR images.
We wish to emphasize that ballistic projectiles are not ferromagnetic unless they contain steel (i.e. iron). Projectiles made of lead or brass, for example, are not ferromagnetic. This means that gunshot victims with retained metal fragments may be safely scanned if the composition of the projectile is known prior to PMMR and does not contain iron (Figure 5c).
STEP 2: BASIC APPLICATION OF FORENSIC PMMR
Look out for the forensic sentinel sign
The perception of limited access and long scanning times are two principal limitations of forensic PMMR.10 Therefore, it may be practical to focus PMMR scan protocols to the most essential sequences.
The following suggestions regarding PMMR imaging are based on our personal experience and represent general advice to inexperienced investigators rather than a ready-to-use scan protocol. They also reflect the authors' belief that forensic imaging should be full body imaging, whenever possible. The literature provides strong evidence that T2 weighted MR images are of paramount importance in post-mortem imaging: their ability to highlight fluid accumulations makes them an ideal diagnostic tool for a wide range of pathologies, including subcutaneous haematoma, bone contusion, organ laceration, internal haemorrhage and fluid collections, ischaemic injury of the heart, brain oedema, pericardial or pleural effusion and pulmonary oedema.6,19,23,31,39–44 In our experience, STIR sequences are most suitable for screening purposes because they emphasize the signal from tissues with long T2 relaxation times45 and fluid accumulations literally flash like light bulbs when scrolling through images on STIR sequences. Thus, we refer to this phenomenon as the “forensic sentinel sign” (Figure 6).
Figure 6.
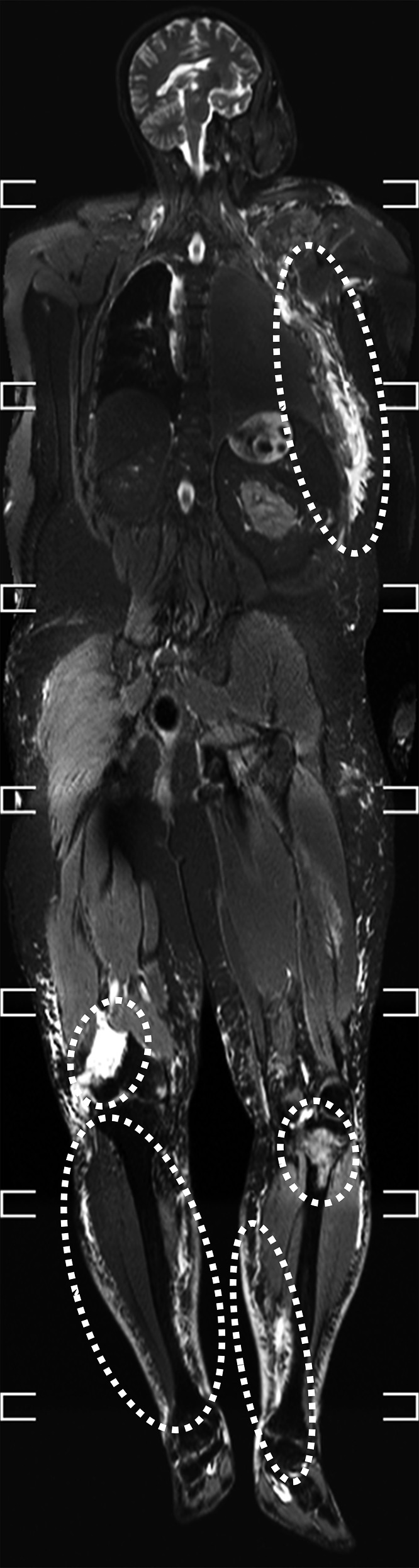
The forensic sentinel sign: coronal whole-body short tau inversion–recovery (STIR) image in a case of blunt force trauma featuring several pathological fluid accumulations, which are also referred to as “forensic sentinel sign” (circled by white dotted lines). Fluid accumulations are highly conspicuous on STIR sequences and may be used as an indicator of pathology.
It is our suggestion to start any PMMR protocol with a coronal whole-body STIR sequence to screen for the forensic sentinel sign. Coronal imaging should be completed with a T1 weighted, and if time permits, a turbo spin echo T2 weighted sequence. Coronal whole-body imaging enables investigators to gain a comprehensive overview and tailor subsequent axial, sagittal or oblique images according to the forensic sentinel sign. Ideally, T2 weighted and T1 weighted axial imaging should cover the entire head, chest and abdomen. However, if scan time is short, imaging may be focussed on regions with a positive forensic sentinel sign. It is beyond the scope of this article to discuss the span of application of individual MR sequences, and we wish to refer to the manual by McRobbie et al46 who provide an excellent introduction to (clinical) MRI for further reading.
STEP 3: POST-MORTEM MR FROM HEAD TO TOE
Head and neck imaging
There are a number of publications on PMCT and PMMR of the head and/or the neck,47–50 but relatively few are dedicated solely to forensic PMMR.26,27,50,51 Perhaps, the first article on this topic was published in 1991 by Harris,52 who recounts the enduring effect of presenting PMMR images as evidence in a homicide case in court. He concludes that blunt force injures and penetrating trauma “are particularly well documented” by PMMR and, in retrospect, his reasoning is most clear sighted.52
It is our opinion that the article by Kobayashi et al27 is a must-read for investigators performing PMMR of the brain; it provides a concize summary of frequent normal findings on PMMR of the brain, which include high signal intensity of the basal ganglia and thalamus on T1 weighted images (Figure 7) and insufficient suppression of cerebrospinal fluid signal on standard fluid attenuated inversion recovery images, a problem also noted by other authors.26,27 In addition, Kobayashi et al27 noted a significant decrease of the apparent diffusion coefficient (ADC) value. Scheurer et al53 confirmed this finding and observed a correlation between decreasing ADC values and increasing post-mortem intervals. They also found that the ADC values were generally lower in cases with traumatic and hypoxic brain injuries than in cases of heart failure.53 Further research is currently underway to investigate and characterize how normal post-mortem changes, such as decomposition and changes in body temperature, affect the quality of PMMR and various MR parameters, including ADC values.29
Figure 7.
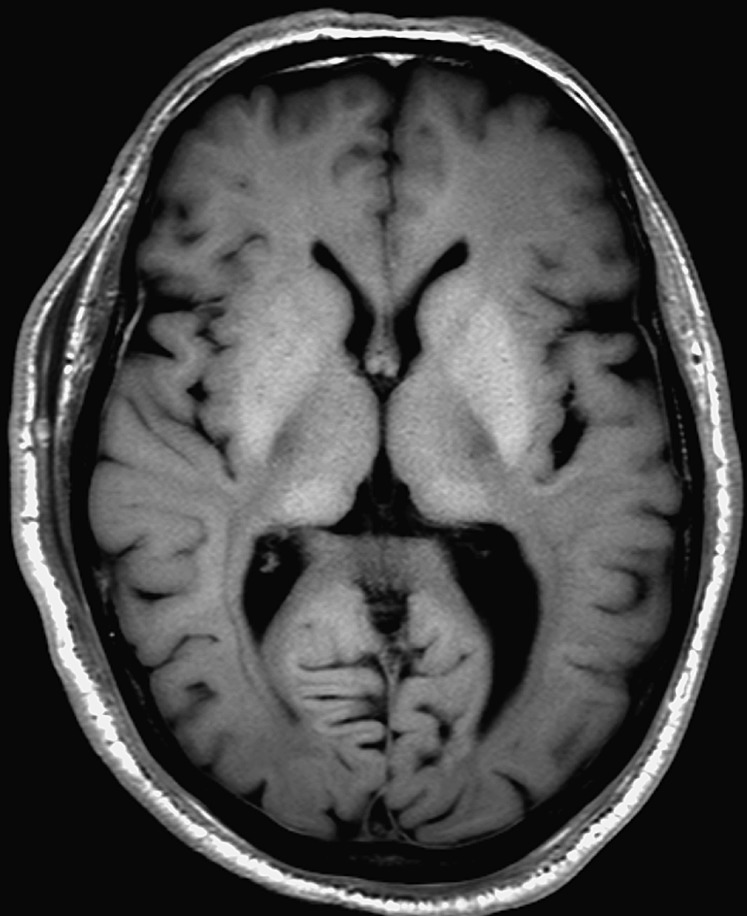
Post-mortem imaging of the brain: axial T1 weighted post-mortem MR image of the brain with typical hyperintensity of the basal ganglia.
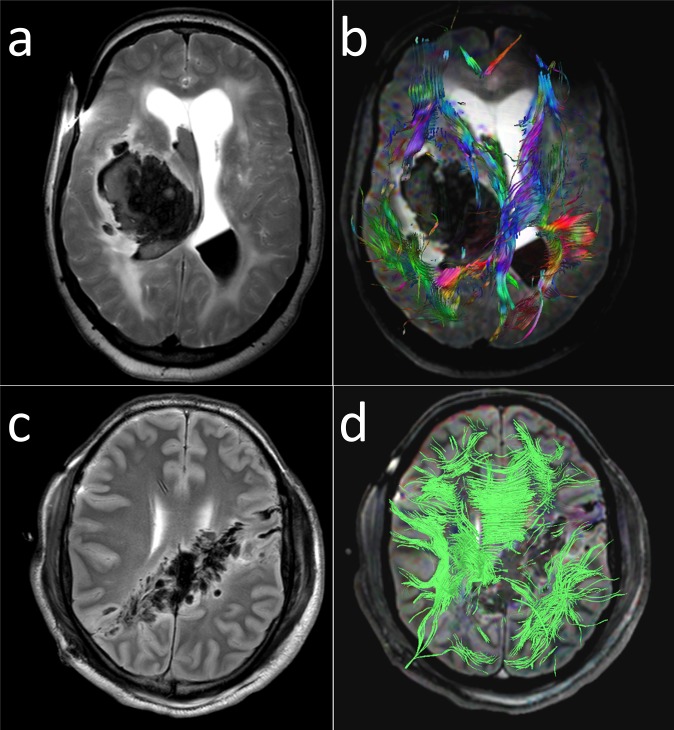
Both Añon et al49 and Yen et al48 compared PMMR (and PMCT) of the head to autopsy. In their study, Añon et al found that extra-axial haemorrhages were visible on both PMMR and PMCT in approximately 90% of all cases. Nevertheless, it is important to note that thin layers of blood may be invisible on cross-sectional imaging. The study by Yen et al revealed surprisingly heterogeneous results regarding the radiological detection of a wide range of pathologies (including injuries to the scalp, skull fractures, intracranial haemorrhage, intracranial pressure and gas collections). Sensitivity of PMMR and PMCT ranged from 100% (for gas collections) to 0% (for mediobasal impression marks, a typical autopsy finding of elevated intracranial pressure).48 The authors offer two reasons for the heterogeneity of their results: insufficiently standardized autopsy protocols and inadequate training in forensic medicine for radiologists. Imaging findings of elevated intracranial pressure or herniation were also investigated by Aghayev et al47 who report the presence of tonsillar herniation on imaging in three cases. As early as 2006, Yen et al51 tested the feasibility of diffusion tensor imaging (DTI) in the post-mortem setting to assess traumatic injury of the brain. DTI fibre tractography provides an effective means to visualize brain injury and is an integral element of post-mortem neuroimaging at the Institute of Forensic Medicine at the University of Zurich, Switzerland (Figure 8).
Figure 8.
Diffusion tensor imaging (DTI) fibre tractography provides an effective means to visualize brain injury. (a) Axial T2 weighted post-mortem MR (PMMR) image of a brain with acute hypertensive intracranial haemorrhage (note fluid–fluid level in the right posterior ventricle). (b) Same image complemented by DTI fibre tractography to visualize the effect of the massive cerebral haemorrhage with displacement and disruption of fibre tracts. (c) Axial T2 weighted PMMR image of a brain with a gunshot injury. (d) Same image complemented by DTI fibre tractography illustrating the extensive destructive power of a ballistic projectile.
Yen et al50 have also investigated the potential of PMMR of the neck in a small number of cases with cervical injury. The US National Institute of Justice recently funded an investigation of PMMR in the detection of intraneural trauma, a study that will also better elucidate the appearance of haemorrhage on PMMR at various ages and states of decomposition. In addition, PMMR has also proved useful to visualize lesions of the skin, the subcutaneous tissue and muscles of the neck from strangulation and hanging.54
The accurate estimation of the post-mortem interval (i.e. the time of death) represents a perpetual challenge to forensic investigators.55 Ith et al55–57 investigated the potential of MR spectroscopy to determine the post-mortem interval based on the changing profile of brain metabolites during decomposition in a sheep model. Although fascinating, this approach is still limited to the realm of research because of the complexity of MR spectroscopy and the significant logistical challenges related to using MR spectroscopy on a routine basis in forensic death investigations.
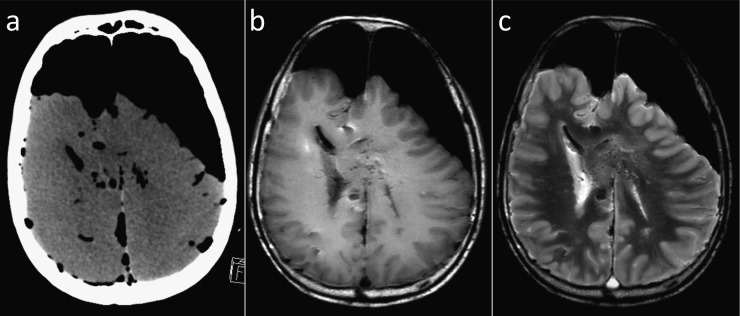
In our experience, PMMR of the brain provides detailed in situ information about the extra-axial space before it is disturbed by autopsy or lost in the process of fixation for formal brain dissection. In addition, PMMR displays anatomical details and relationships well into the process of decomposition, beyond the time when liquefaction limits the detail obtained at autopsy and with tissue contrast that is superior to PMCT (Figure 9).
Figure 9.
Post-mortem images of the decomposed brain: comparison between an axial post-mortem CT (PMCT) image (a) and axial T1 weighted (b) and T2 weighted (c) PMMR images of a brain in a moderate stage of decomposition. PMMR displays anatomical details and relationships well into the process of decomposition and with tissue contrast that is superior to PMCT.
Cardiovascular imaging
Cardiovascular imaging is certainly a core area of PMMR. Cardiovascular disease is a frequent cause of death in forensic death investigations and cases of sudden cardiac death can be especially difficult to recognize during autopsy.9,58 The definitions of sudden cardiac death vary between authors and range from death within 1–24 h after the onset of symptoms.59,60 Macroscopic evidence of ischaemic injury is often absent if death occurs within the first 12 h.59 On routine histology examination, ischaemia-induced microscopic changes will be detectable no sooner than 4 h after the onset of ischaemia.59 In 2005, Shiotani et al61 reported a case of sudden cardiac death where ischaemia-induced oedema was visible on PMMR. Autopsy revealed acute occlusion of the afferent coronary artery but no signs of myocardial infarction. This case raised hopes among forensic pathologists that PMMR might be able to close the diagnostic gap in sudden cardiac death.
To understand the challenges of cardiac PMMR, it is important to be aware of the principles of T2 weighted cardiac MR. In clinical cardiac MR, T2 weighted sequences are routinely used to detect myocardial oedema.45,62 Myocardial oedema represents a rapid but non-specific tissue response to ischaemic injury (and other cardiac conditions) and causes a prolongation of T2 relaxation times in the affected area.45,63,64 Regions of long T2 relaxation times are highlighted by increased signal intensity on T2 weighted MR images.45 Intracellular oedema (and consequentially prolongation of T2 times) develops within minutes of ischaemia.45,62,64,65 Recently, Abdel-Aty et al66 demonstrated increased signal intensity from ischaemic myocardial injury after 28 ± 4 min on T2 weighted images of live dogs. The extent of ischaemia-induced oedema depends heavily on the occurrence of vascular reperfusion.42,65 Combined ischaemia/reperfusion injury results in more extensive oedema (with both intracellular and interstitial fluid accumulation) than ischaemic injury without vascular reperfusion (where fluid accumulation is often limited to the intracellular space).65 A recent study by Ruder et al42 revealed that oedema from ischaemia/reperfusion injury can be detected on PMMR within 3 h after the onset of vascular occlusion.
Over the past years, Jackowski et al31,67,68 have repeatedly compared cardiac PMMR images with macroscopic and microscopic findings of the heart in cases of suspected cardiac death. They found that acute infarction [survival time: day(s)], subacute infarction [survival time: week(s)], and chronic infarction or scars [survival time: month(s)] can be identified on PMMR.31,67,68 The post-mortem imaging findings of acute myocardial infarction are comparable to those found in clinical cardiac MR and consist of focal necrosis surrounded by perifocal myocardial oedema with increased signal intensity on T2 weighted images (Figure 10).31,45 In a number of cases where circumstantial evidence was suggestive for sudden cardiac death, Jackowski et al31,67,68 noted a focally decreased signal intensity within the myocardium on T2 weighted images without perifocal oedema. This finding was interpreted as a sign of early acute myocardial infarction (survival time: minutes to hours), and recently, Jackowski et al68 published a new study which supports this interpretation. Immunohistochemical staining might allow for a comparison between imaging findings and cellular changes in early ischaemia and might support the ability of PMMR to detect early acute ischaemic injury.69 However, there are no generally accepted reference values regarding the interpretation of immunohistochemical staining, and there is only limited literature on this subject.
Figure 10.
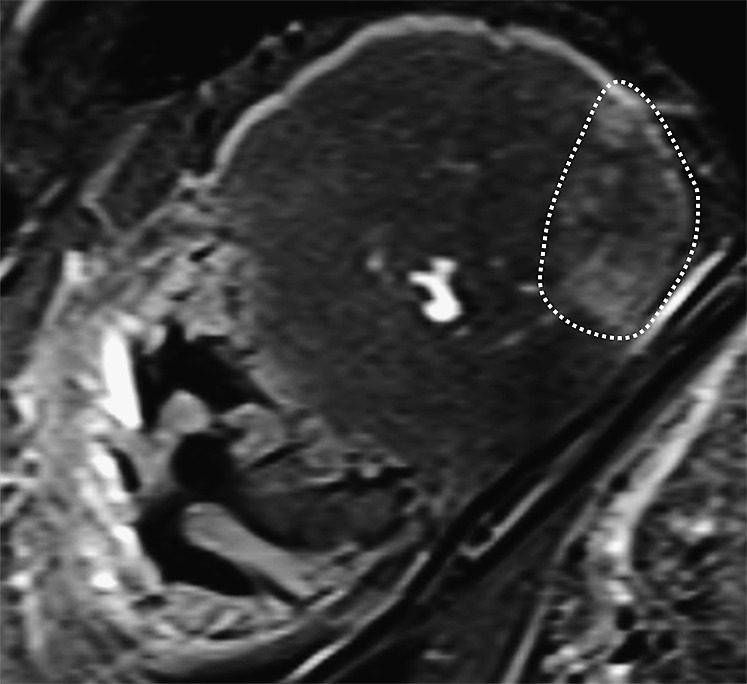
Cardiac post-mortem MR (PMMR) image of an acute myocardial infarction of the posterior wall: short axis T2 weighted PMMR image of the heart (near the apex). The post-mortem imaging findings of acute myocardial infarction (circled by white dotted line) are comparable to those in clinical cardiac MR and consist of focal necrosis surrounded by perifocal myocardial oedema with increased signal intensity on T2 weighted images.
In our experience, the detection of myocardial injury in an actual case of sudden cardiac death is often challenging. Therefore, we would like to offer the following advice to inexperienced investigators: if oedema is visible on cardiac PMMR, ischaemic injury is the first and most likely differential diagnosis.31,42,45,61–64,66–68 If oedema is not present, but PMMR features one or several small hypointense myocardial lesions, it is reasonable to include very early ischaemic injury into the differential diagnosis.6,31,67,68 However, these findings are often very subtle, and their interpretation depends on the subjective judgment of the investigator.45,70 In addition, several critical parameters such as post-mortem interval, duration of ischemia, degree of occlusion, extent of collateral circulation and occurrence of vascular reperfusion are often unknown in post-mortem investigations, and their impact on the appearance of ischaemic injury on PMMR is unaccounted for. To make matters more complex, post-mortem changes such as gas formation and low body temperature may further alter or degrade the PMMR image.
To overcome these limitations, several investigators are currently evaluating the potential of quantitative PMMR analysis.70,71 It is hoped that quantitative evaluation of PMMR will decrease observer variability and better differentiate pathology from normal post-mortem changes, thereby improving the often challenging comparison between PMMR and autopsy findings in cases of sudden cardiac death.
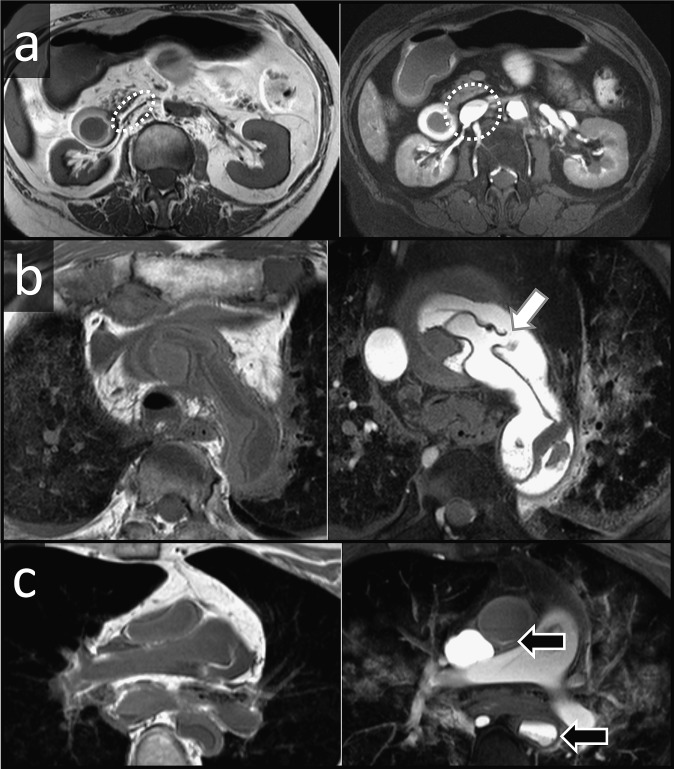
If death occurs before signs of ischemia are visible in the myocardium, the assessment of the coronary arteries is of paramount importance.19,72 In living patients, the presence and extent of coronary artery disease (CAD) is usually investigated by angiography.73 Angiography is also feasible in post-mortem imaging, and PMCT-angiography has become a valuable tool in forensic radiology.74–76 Ruder et al77 recently demonstrated the feasibility of whole-body PMMR angiography. Fat-saturated T1 weighted images offer good image contrast (Figure 11). However, because of the relatively long scanning times, PMMR angiography is susceptible to position-dependent sedimentation of contrast medium, which degrades the image quality (Figure 11c). Current research efforts are dedicated to developing new mixtures of PMMR contrast media to overcome this technical limitation.
Figure 11.
Post-mortem MR (PMMR) angiography: left column features non-contrast axial T1 weighted images of the abdomen (a), the aortic arch (b) and the pulmonary arteries (c), the right column features post-contrast T1 weighted fat-saturated images of the same levels. (a) Note the striking expansion of the inferior cava vein on the post-contrast image. (b) PMMR angiography clearly displays the intimal rupture (arrow) in this case of aortic dissection. (c) Position-dependent sedimentation of contrast medium is a current limitation of PMMR angiography. Note contrast-fluid levels in both ascending and descending aorta (arrows). This artefact is also visible (but to a lesser degree) in the inferior cava vein in (a).
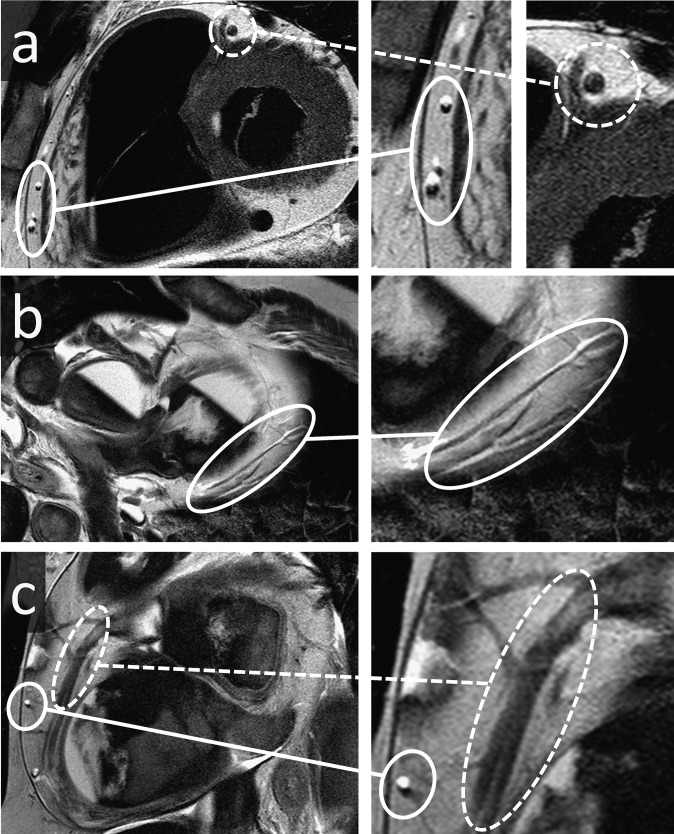
Post-mortem angiography is a relatively time consuming procedure, requires dedicated equipment and may not always be feasible. Therefore, the assessment of coronary artery disease is often limited to non-contrast post-mortem imaging. Calcified coronary artery plaques can be assessed by non-contrast CT and are helpful to estimate the risk of underlying stenosis, but provide no direct evidence of stenosis.73 The assessment of CAD on non-contrast PMMR was considered to be problematic.7,9 Recently, a novel approach was presented to detect coronary artery disease on PMMR.78 This approach is based on the occurrence or the absence of chemical shift artefacts along coronary arteries. Chemical shift artefacts are caused by the difference in resonance frequency of fat and water and appear as light and dark bands on opposite sides of an affected structure on T2 weighted images.79 Ruder et al78 found that chemical shift artefacts on cardiac PMMR occur only in the absence of coronary artery disease and may, therefore, be used as a marker for vessel patency (Figure 12). In addition, the presence of so called “paired dark bands” is linked to arteriosclerosis and an indicator of coronary artery disease. The evaluation of these two signs permits a basic evaluation of the coronary arteries on non-contrast T2 weigthed PMMR imaging. One final word of caution: investigators with no formal training in radiology must be very careful not to mistake MR image artefacts, such as the chemical shift, for position-dependent sedimentation (Figure 12).
Figure 12.
Assessment of coronary artery disease on non-contrast post-mortem MR: three sets of T2 weighted images of a heart with full field images and detailed images. Chemical shift artefacts (circled by continuous white line on all images) appear as light and dark signals on opposite sides of vascular structures within the epicardial fat, and their presence indicates vessel patency. These artefacts must not be confused with position-dependent sedimentation. Chemical shift artefacts are not present if the vascular lumen is filled by erythrocytes (a, dotted line) or in the presence of arteriosclerotic plaques, which may be visible as paired dark bands (c, dotted line).
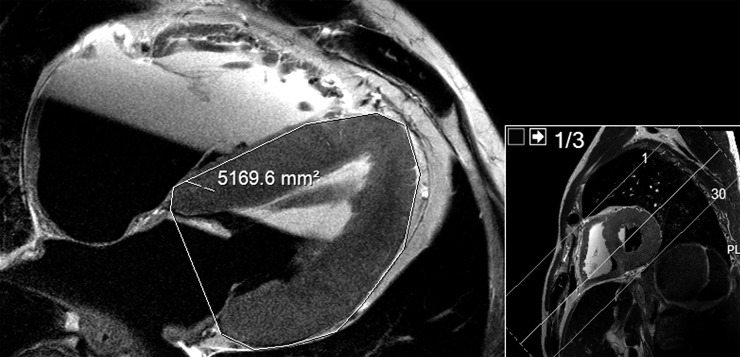
In cases where both the myocardium and the coronary arteries appear normal, but circumstantial evidence is strongly suggestive of sudden cardiac death, forensic pathologists are occasionally forced to refer to the weight and size of a heart to diagnose a case of sudden cardiac death.59 Left ventricular hypertrophy is an indicator of cardiac disease and related to sudden cardiac death.80 Heart weight can also be estimated prospectively by PMMR: Ruder et al81 found that single area measurements of the left ventricle on four-chamber views of the heart correlate closely to heart weight as measured at autopsy (Figure 13).
Figure 13.
Assessment of heart weight: four-chamber view post-mortem T2 weighted MR image and short-axis view topogram of the heart. Heart weight can be estimated by single area measurements on four-chamber view of the heart. The circumferential area (in squared centimetres) of the left ventricle at mid-level corresponds to approximately one-tenth of the heart weight, as measured at autopsy (caveat: area measurements on the figure are in squared millimetres).
In comparison to the comprehensive literature on cardiac imaging, there is very little literature on PMMR imaging of the vascular system. Nevertheless, there is strong evidence that PMMR is able to accurately depict cases of ruptured thoracic or abdominal aortic dissection.9,17,82,83 It is our opinion, that in these cases, imaging represents a valid alternative to autopsy. Meanwhile, the detection of pulmonary embolism is very challenging.9 Roberts et al9 reported in their study that pulmonary embolism was missed by imaging in every single case. The differentiation between post-mortem clot and true pulmonary embolism proves to be a difficult task. Recently, a first attempt was made to define imaging criteria for pulmonary embolism based on a series of eight autopsy-confirmed cases of pulmonary embolism, using a 3.0-T MR.84 However, the prospective diagnosis of pulmonary embolism by post-mortem imaging remains difficult and should be confirmed by targeted biopsy or autopsy. In cases where circumstantial evidence is suggestive of pulmonary embolism, it is certainly wise to acquire axial images of the lower extremities to screen for evidence of deep venous thrombosis.84
The existing literature on thoracic PMMR imaging is primarily focused on natural causes of death. However, PMMR may also be used in cases of thoracic trauma. Aghayev et al85–87 published several articles on the potential of PMMR and PMCT in thoracic trauma. A more recent study by Ross et al,40 dedicated solely to PMMR, found higher overall sensitivity and specificity rates regarding the detection of traumatic findings in the chest than the prior studies. The discrepancy between these studies indirectly indicates the relevance of dedicated training in forensic imaging and reflects how the understanding of PMMR improved in recent years.
Abdominal imaging
There is general agreement that non-contrast PMMR reveals better soft-tissue detail than non-contrast PMCT, and MR is therefore considered to be more useful than CT to assess the abdominal organs.6,9,10,88,89 High soft-tissue contrast and the ability of MR to visualize soft-tissue pathology are also the principal reason why PMMR is the modality of choice in post-mortem neonatal and paediatric imaging.90–93 However, in post-mortem imaging of the adult, abdominal imaging plays a marginal role and according to Baglivo et al,10 only 2% of all published articles on forensic post-mortem cross-sectional imaging are dedicated to abdominal imaging.
In their illustrative study from 2003, Thali et al6 reported that a significant portion of traumatic abdominal injuries were not detectable on either PMCT or PMMR. A few years later, Christe et al41 confirmed this observation in their comparative study on post-mortem imaging of abdominal trauma. Their research revealed that sensitivity and specificity of PMMR regarding the detection of abdominal injuries were substantially lower than expected (e.g. <60% and 50% for liver lacerations).41 In a follow-up study, Ross et al40 reported a marked higher sensitivity and specificity regarding liver lacerations (80% and 100%, respectively). Sensitivity levels for injuries of the spleen, pancreas and kidneys remained at about 60%, whereas overall sensitivity was >90%.40 As is the case of thoracic imaging, this significant improvement from the first to the second study demonstrates the importance of dedicated training and experience in forensic radiology to ensure high diagnostic accuracy.
The gastrointestinal tract remains somewhat of a blind spot on PMMR. In our personal experience, detection of gastrointestinal pathologies is hindered by both intraluminal and intramural post-mortem gas formation and the inability to introduce intraluminal contrast. This impression is supported by literature.7,9
PMMR imaging of the abdomen and the gastrointestinal tract remains underinvestigated, and more research is needed to deepen our understanding of this forensically relevant topic. In our experience, the most practical approach is to screen the abdominal organs for the forensic sentinel sign on T2 weighted images. This allows for the detection of a majority of traumatic injuries of the abdominal organs.
Musculoskeletal imaging
PMCT is the modality of choice to assess and visualize skeletal injury in forensic death investigations.6,9,10,88 However, the ability of PMMR to highlight bone marrow oedema on STIR sequences offers a more profound insight into the sequence of peri-mortem events than PMCT alone.43,94,95 Buck et al94 were the first to note the potential and occasional superiority of PMMR over PMCT in forensic case reconstruction of skeletal injury.93 Their publication reports on a series of five traffic fatalities, where PMMR enabled the detection of bone contusions unseen on PMCT. In these cases, PMMR was crucial for accident reconstruction. Furthermore, there is evidence that PMMR allows a distinction between antemortem and post-mortem fractures based on the presence or the absence of bone marrow oedema.94
In addition to these reports, Ross et al40 provided concrete evidence that PMMR is a valuable tool in forensic death investigations of trauma. In their analysis of 40 whole-body PMMR data sets, the overall sensitivity of PMMR to detect skeletal injuries was nearly 70% and reached a mean specificity of >90%.40 Fractures of the upper extremities were missed most frequently because of the limited field of view. The authors also reported that haematomas of the subcutaneous fat tissue were detected in 90% of all cases. This topic was further investigated by Yen et al39 who transferred an autopsy-rooted classification to grade traumatic injuries of the subcutaneous fat tissue to cross-sectional imaging.
SUMMARY AND CONCLUSIONS
PMMR is a powerful diagnostic tool with a wide scope in forensic radiology. In the past 20 years, PMMR was used both as an adjunct and alternative to autopsy. Its role in forensic death investigation largely depends on the rules and habits of local jurisdictions, availability of experts, financial resources and individual case circumstances. PMMR images are affected by post-mortem changes, such as position-dependent sedimentation, variable body temperature and decomposition. Investigators must be familiar with the appearance of normal findings on PMMR to distinguish them from disease and injury. It is our recommendation to routinely document body temperature before PMMR imaging. Coronal whole-body images provide a comprehensive overview. Notably, STIR images enable investigators to screen for pathological fluid accumulation also known as “forensic sentinel sign”. If scan time is short, subsequent PMMR imaging may be focussed on regions with a positive forensic sentinel sign. PMMR offers excellent anatomical detail and is especially useful to visualize pathologies of the brain, heart, subcutaneous fat tissue and abdominal organs. PMMR may also be used to document skeletal injury. Cardiovascular imaging is a core area of PMMR; post-mortem cardiac MR is able to detect ischaemic injury at an earlier stage than traditional autopsy and routine histology. However, further research is needed to elucidate the effects of post-mortem changes on the PMMR appearance of forensically relevant pathologies and to optimize PMMR scan protocols.
In our opinion, PMMR remains underused in forensic death investigations. We hope that this review will raise the awareness of the potential of forensic PMMR in adults and will contribute to effective interdisciplinary collaborations between radiologists and forensic pathologists, which is in the best interest of medical sciences and the general public.
REFERENCES
- 1.Ros PR, Li KC, Vo P, Baer H, Staab EV. Preautopsy magnetic resonance imaging: initial experience. Magn Reson Imaging 1990; 8: 303–8. [DOI] [PubMed] [Google Scholar]
- 2.Bisset R. Magnetic resonance imaging may be alternative to necropsy. BMJ 1998; 317: 1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bisset RA, Thomas NB, Turnbull IW, Lee S. Postmortem examinations using magnetic resonance imaging: four year review of a working service. BMJ 2002; 324: 1423–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.bmj.com [homepage on the internet]. http://www.bmj.com/content/324/7351/1423?tab=responses London, UK: BMJ Publishing Group Ltd; c2013 [updated Sep 2013; cited Sep 2013]. Available from: [Google Scholar]
- 5.Patriquin L, Kassarjian A, Barish M, Casserley L, O'Brien M, Andry C, et al. Postmortem whole-body magnetic resonance imaging as an adjunct to autopsy: preliminary clinical experience. J Magn Reson Imaging 2001; 13: 277–87. [DOI] [PubMed] [Google Scholar]
- 6.Thali MJ, Yen K, Schweitzer W, Vock P, Boesch C, Ozdoba C, et al. Virtopsy, a new imaging horizon in forensic pathology: virtual autopsy by postmortem multisclice computed tomography (MSCT) and magnetic resonance imaging (MRI)—a feasibility study. J Forensic Sci 2003; 48: 386–403. [PubMed] [Google Scholar]
- 7.Roberts IS, Benbow EW, Bisset R, Jenkins JP, Lee SH, Reid H, et al. Accuracy of magnetic resonance imaging in determining cause of sudden death in adults: comparison with conventional autopsy. Histopathology 2003; 42: 424–30. [DOI] [PubMed] [Google Scholar]
- 8.Ezawa H, Yoneyama R, Kandatsu S, Yoshikawa K, Tsujii H, Harigaya K. Introduction of autopsy imaging redefines the concept of autopsy: 37 cases of clinical experience. Pathol Int 2003; 53: 865–73. [DOI] [PubMed] [Google Scholar]
- 9.Roberts IS, Benamore RE, Benbow EW, Lee SH, Harris JN, Jackson A, et al. Post-mortem imaging as an alternative to autopsy in the diagnosis of adult deaths: a validation study. Lancet 2012; 379: 136–42. doi: 10.1016/S0140-6736(11)61483-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baglivo M, Winklhofer S, Hatch GM, Ampanozi G, Thali MJ, Ruder TD. The rise of forensic and post-mortem radiology—analysis of the literature between the years 2000 and 2011. J Forensic Radiol Imaging 2013; 1: 3–9. [Google Scholar]
- 11.Rutty GN, Morgan B, O'Donnell C, Leth PM, Thali M. Forensic institutes across the world place CT or MRI scanners or both into their mortuaries. J Trauma 2008; 65: 493–4. doi: 10.1016/j.nicl.2012.12.005 [DOI] [PubMed] [Google Scholar]
- 12.Ruder TD. What are the key objectives of the ISFRI?—evaluation of the ISFRI member survey. J Forensic Radiol Imaging 2013; 3: 142–5. [Google Scholar]
- 13.Drew T, Evans K, Võ ML, Jacobson FL, Wolfe JM. Informatics in radiology: what can you see in a single glance and how might this guide visual search in medical images? Radiographics 2013; 33: 263–74. doi: 10.1148/rg.331125023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kundel HL, Nodine CF. Interpreting chest radiographs without visual search. Radiology 1975; 116: 527–32. doi: 10.1148/116.3.527 [DOI] [PubMed] [Google Scholar]
- 15.Sauvageau A, Racette S. Postmortem changes mistaken for traumatic lesions: a highly prevalent reason for coroner's autopsy request. Am J Forensic Med Pathol 2008; 29: 145–7. doi: 10.1097/PAF.0b013e318174f0d0 [DOI] [PubMed] [Google Scholar]
- 16.Ruder TD, Hatch GM, Thali MJ. ‘Horrible, most horrible’: Hamlet and forensic medicine. Med Humanit 2010; 36: 35. doi: 10.1136/jmh.2010.004846 [DOI] [PubMed] [Google Scholar]
- 17.Kluschke F, Ross S, Flach PM, Schweitzer W, Ampanozi G, Gascho D, et al. To see or not to see—ambiguous findings on post-mortem cross-sectional imaging in a case of ruptured abdominal aortic aneurysm. Leg Med (Tokyo) 2013; 15: 256–9. doi: 10.1016/j.legalmed.2013.03.001 [DOI] [PubMed] [Google Scholar]
- 18.Christe A, Flach P, Ross S, Spendlove D, Bolliger S, Vock P, et al. Clinical radiology and postmortem imaging (virtopsy) are not the same: specific and unspecific postmortem signs. Leg Med (Tokyo) 2010; 12: 215–22. doi: 10.1016/j.legalmed.2010.05.005 [DOI] [PubMed] [Google Scholar]
- 19.Jackowski C, Schweitzer W, Thali M, Yen K, Aghayev E, Sonnenschein M, et al. Virtopsy: postmortem imaging of the human heart in situ using MSCT and MRI. Forensic Sci Int 2005; 149: 11–23. doi: 10.1016/j.forsciint.2004.05.019 [DOI] [PubMed] [Google Scholar]
- 20.Ruder TD, Hatch GM, Siegenthaler L, Ampanozi G, Mathier S, Thali MJ, et al. The influence of body temperature on image contrast in post mortem MRI. Eur J Radiol 2012; 81: 1366–70. doi: 10.1016/j.ejrad.2011.02.062 [DOI] [PubMed] [Google Scholar]
- 21.Vock P. Intravital versus postmortem imaging. In: Thali MJ, Vock P, Dirnhofer R, eds. The virtopsy approach. Boca Raton, FL: CRC Press; 2009. pp. 145–6. [Google Scholar]
- 22.Shiotani S, Kohno M, Ohashi N, Yamazaki K, Itai Y. Postmortem intravascular high-density fluid level (hypostasis): CT findings. J Comput Assist Tomogr 2002; 26: 892–3. [DOI] [PubMed] [Google Scholar]
- 23.Jackowski C, Thali M, Aghayev E, Yen K, Sonnenschein M, Zwygart K, et al. Postmortem imaging of blood and its characteristics using MSCT and MRI. Int J Legal Med 2006; 120: 233–40. doi: 10.1007/s00414-005-0023-4 [DOI] [PubMed] [Google Scholar]
- 24.Nelson TR, Tung SM. Temperature dependence of proton relaxation times in vitro. Magn Reson Imaging 1987; 5: 189–99. [DOI] [PubMed] [Google Scholar]
- 25.Daniel BL, Butts K, Block WF. Magnetic resonance imaging of frozen tissues: temperature-dependent MR signal characteristics and relevance for MR monitoring of cryosurgery. Magn Reson Med 1999; 41: 627–30. [DOI] [PubMed] [Google Scholar]
- 26.Tofts PS, Jackson JS, Tozer DJ, Cercignani M, Keir G, MacManus DG, et al. Imaging cadavers: cold FLAIR and noninvasive brain thermometry using CSF diffusion. Magn Reson Med 2008; 59: 190–5. doi: 10.1002/mrm.21456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobayashi T, Shiotani S, Kaga K, Saito H, Saotome K, Miyamoto K, et al. Characteristic signal intensity changes on postmortem magnetic resonance imaging of the brain. Jpn J Radiol 2010; 28: 8–14. doi: 10.1007/s11604-009-0373-9 [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi T, Isobe T, Shiotani S, Saito H, Saotome K, Kaga K, et al. Postmortem magnetic resonance imaging dealing with low temperature objects. Magn Reson Med Sci 2010; 9: 101–8. [DOI] [PubMed] [Google Scholar]
- 29.Adolphi N, Gerrard Ch, Hatch G, Takacs N, Nolte K. Determining the temperature-dependence of tissue relaxation times (T1 and T2) for prospective optimization of post-mortem magnetic resonance (PMMR) image contrast. J Forensic Radiol Imaging 2013; 1: 80. [Google Scholar]
- 30.Ozdoba C, Weis J, Plattner T, Dirnhofer R, Yen K. Fatal scuba diving incident with massive gas embolism in cerebral and spinal arteries. Neuroradiology 2005; 47: 411–6. doi: 10.1007/s00234-004-1322-z [DOI] [PubMed] [Google Scholar]
- 31.Jackowski C, Christe A, Sonnenschein M, Aghayev E, Thali MJ. Postmortem unenhanced magnetic resonance imaging of myocardial infarction in correlation to histological infarction age characterization. Eur Heart J 2006; 27: 2459–67. doi: 10.1093/eurheartj/ehl255 [DOI] [PubMed] [Google Scholar]
- 32.Hatch GM, Ebert LC, Näther S, Ruder TD, Thali MJ. The evidence provided by New Imaging Technologies. In: Wecht CH, ed. Forensic Sciences. New Providence, NJ: Matthew Bender & Company, Inc., a member of LexisNexis; 2012. [Google Scholar]
- 33.Yokota H, Yamamoto S, Horikoshi T, Shimofusa R, Ito H. What is the origin of intravascular gas on postmortem computed tomography? Leg Med (Tokyo) 2009; 11: S252–5. doi: 10.1016/j.legalmed.2009.02.051 [DOI] [PubMed] [Google Scholar]
- 34.Gebhart FT, Brogdon BG, Zech WD, Thali MJ, Germerott T. Gas at postmortem computed tomography—an evaluation of 73 non-putrefied trauma and non-trauma cases. Forensic Sci Int 2012; 222: 162–9. doi: 10.1016/j.forsciint.2012.05.020 [DOI] [PubMed] [Google Scholar]
- 35.Egger C, Bize P, Vaucher P, Mosimann P, Schneider B, Dominguez A, et al. Distribution of artifactual gas on post-mortem multidetector computed tomography (MDCT). Int J Legal Med 2012; 126: 3–12. doi: 10.1007/s00414-010-0542-5 [DOI] [PubMed] [Google Scholar]
- 36.McRobbie DW, Moore EA, Graves MJ, Prince MR. Improving your image: how to avoid artefacts. In: McRobbie DW, Moore EA, Graves MJ, Prince MR, eds. MRI from picture to proton. Cambridge, UK: Cambridge University Press, 2007: 79–107. [Google Scholar]
- 37.Ulbrich EJ, Sutter R, Aguiar RF, Nittka M, Pfirrmann CW. STIR sequence with increased receiver bandwidth of the inversion pulse for reduction of metallic artifacts. AJR Am J Roentgenol 2012; 199: W735–42. doi: 10.2214/AJR.11.8233 [DOI] [PubMed] [Google Scholar]
- 38.Expert Panel on MR Safety, Kanal E, Barkovich AJ, Bell C, Borgstede JP, Bradley WG Jr, Froelich JW, et al. ACR guidance document on MR safe practices: 2013. J Magn Reson Imaging 2013; 37: 501–30. doi: 10.1002/jmri.24011 [DOI] [PubMed] [Google Scholar]
- 39.Yen K, Vock P, Tiefenthaler B, Ranner G, Scheurer E, Thali MJ, et al. Virtopsy: forensic traumatology of the subcutaneous fatty tissue; multislice computed tomography (MSCT) and magnetic resonance imaging (MRI) as diagnostic tool. J Forensic Sci 2004; 49: 799–806. [PubMed] [Google Scholar]
- 40.Ross S, Ebner L, Flach P, Brodhage R, Bolliger SA, Christe A, et al. Postmortem whole-body MRI in traumatic causes of death. AJR Am J Roentgenol 2012; 199: 1186–92. doi: 10.2214/AJR.12.8767 [DOI] [PubMed] [Google Scholar]
- 41.Christe A, Ross S, Oesterhelweg L, Spendlove D, Bolliger S, Vock P, et al. Abdominal trauma—sensitivity and specificity of postmortem noncontrast imaging findings compared with autopsy findings. J Trauma 2009; 66: 1302–7. doi: 10.1097/TA.0b013e31818c1441 [DOI] [PubMed] [Google Scholar]
- 42.Ruder TD, Ebert LC, Khattab AA, Rieben R, Thali MJ, Kamat P. Edema is a sign of early acute myocardial infarction on post-mortem magnetic resonance imaging. Forensic Sci Med Pathol 2013; 9: 501–5. doi: 10.1007/s12024-013-9459-x [DOI] [PubMed] [Google Scholar]
- 43.Cha JG, Kim DH, Kim DH, Paik SH, Park JS, Park SJ, et al. Utility of postmortem autopsy via whole-body imaging: initial observations comparing MDCT and 3.0 T MRI findings with autopsy findings. Korean J Radiol 2010; 11: 395–406. doi: 10.3348/kjr.2010.11.4.395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ampanozi G, Preiss U, Hatch GM, Zech WD, Ketterer T, Bolliger S, et al. Fatal lower extremity varicose vein rupture. Leg Med (Tokyo) 2011; 13: 87–90. doi: 10.1016/j.legalmed.2010.11.002 [DOI] [PubMed] [Google Scholar]
- 45.Mirakhur A, Anca N, Mikami Y, Merchant N. T2-weighted imaging of the heart—a pictorial review. Eur J Radiol 2013; 82: 1755–62. doi: 10.1016/j.ejrad.2013.06.005 [DOI] [PubMed] [Google Scholar]
- 46.McRobbie DW, Moore EA, Graves MJ, Prince MR, eds. MRI from picture to proton. Cambridge, UK: Cambridge University Press; 2007. [Google Scholar]
- 47.Aghayev E, Yen K, Sonnenschein M, Ozdoba C, Thali M, Jackowski C, et al. Virtopsy post-mortem multi-slice computed tomography (MSCT) and magnetic resonance imaging (MRI) demonstrating descending tonsillar herniation: comparison to clinical studies. Neuroradiology 2004; 46: 559–64. [DOI] [PubMed] [Google Scholar]
- 48.Yen K, Lövblad KO, Scheurer E, Ozdoba C, Thali MJ, Aghayev E, et al. Post-mortem forensic neuroimaging: correlation of MSCT and MRI findings with autopsy results. Forensic Sci Int 2007; 173: 21–35. doi: 10.1016/j.forsciint.2007.01.027 [DOI] [PubMed] [Google Scholar]
- 49.Añon J, Remonda L, Spreng A, Scheurer E, Schroth G, Boesch C, et al. Traumatic extra-axial hemorrhage: correlation of postmortem MSCT, MRI, and forensic-pathological findings. J Magn Reson Imaging 2008; 28: 823–36. doi: 10.1002/jmri.21495 [DOI] [PubMed] [Google Scholar]
- 50.Yen K, Sonnenschein M, Thali MJ, Ozdoba C, Weis J, Zwygart K, et al. Postmortem multislice computed tomography and magnetic resonance imaging of odontoid fractures, atlantoaxial distractions and ascending medullary edema. Int J Legal Med 2005; 119: 129–36. [DOI] [PubMed] [Google Scholar]
- 51.Yen K, Weis J, Kreis R, Aghayev E, Jackowski C, Thali M, et al. Line-scan diffusion tensor imaging of the posttraumatic brain stem: changes with neuropathologic correlation. AJNR Am J Neuroradiol 2006; 27: 70–3. [PMC free article] [PubMed] [Google Scholar]
- 52.Harris LS. Postmortem magnetic resonance images of the injured brain: effective evidence in the courtroom. Forensic Sci Int 1991; 50:179–85. [DOI] [PubMed] [Google Scholar]
- 53.Scheurer E, Lovblad KO, Kreis R, Maier SE, Boesch C, Dirnhofer R, et al. Forensic application of postmortem diffusion-weighted and diffusion tensor MR imaging of the human brain in situ. AJNR Am J Neuroradiol 2011; 32: 1518–24. doi: 10.3174/ajnr.A2508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yen K, Thali MJ, Aghayev E, Jackowski C, Schweitzer W, Boesch C, et al. Strangulation signs: initial correlation of MRI, MSCT, and forensic neck findings. J Magn Reson Imaging 2005; 22: 501–10. doi: 10.1002/jmri.20396 [DOI] [PubMed] [Google Scholar]
- 55.Ith M, Scheurer E, Kreis R, Thali M, Dirnhofer R, Boesch C. Estimation of the postmortem interval by means of ¹H MRS of decomposing brain tissue: influence of ambient temperature. NMR Biomed 2011; 24: 791–8. doi: 10.1002/nbm.1623 [DOI] [PubMed] [Google Scholar]
- 56.Ith M, Bigler P, Scheurer E, Kreis R, Hofmann L, Dirnhofer R, et al. Observation and identification of metabolites emerging during postmortem decomposition of brain tissue by means of in situ 1H-magnetic resonance spectroscopy. Magn Reson Med 2002; 48: 915–20. doi: 10.1002/mrm.10294 [DOI] [PubMed] [Google Scholar]
- 57.Scheurer E, Ith M, Dietrich D, Kreis R, Hüsler J, Dirnhofer R, et al. Statistical evaluation of time-dependent metabolite concentrations: estimation of post-mortem intervals based on in situ 1H-MRS of the brain. NMR Biomed 2005; 18: 163–72. doi: 10.1002/nbm.934 [DOI] [PubMed] [Google Scholar]
- 58.Schoen FJ. The heart. In: Cotran RS, Kumar V, Collins T, eds. Robbins pathologic basis of disease. Philadelphia, PA: WB Saunders Company; 1999. pp. 543–600. [Google Scholar]
- 59.Saukko P, Knight B, eds. Knight's forensic pathology. London, UK: Hodder Arnold (part of Hachett Livre UK); 2004. pp. 492–526. [Google Scholar]
- 60.Michaud K, Grabherr S, Jackowski C, Bollmann MD, Doenz F, Mangin P. Postmortem imaging of sudden cardiac death. Int J Legal Med Jan 2013. Epub ahead of print. doi: 10.1007/s00414-013-0819-6 [DOI] [PubMed] [Google Scholar]
- 61.Shiotani S, Yamazaki K, Kikuchi K, Nagata C, Morimoto T, Noguchi Y, et al. Postmortem magnetic resonance imaging (PMMRI) demonstration of reversible injury phase myocardium in a case of sudden death from acute coronary plaque change. Radiat Med 2005; 23: 563–5. [PubMed] [Google Scholar]
- 62.Friedrich MG. Myocardial edema—a new clinical entity? Nat Rev Cardiol 2010; 7: 292–6. doi: 10.1038/nrcardio.2010.28 [DOI] [PubMed] [Google Scholar]
- 63.Higgins CB, Herfkens R, Lipton MJ, Sievers R, Sheldon P, Kaufman L, et al. Nuclear magnetic resonance imaging of acute myocardial infarction in dogs: alterations in magnetic relaxation times. Am J Cardiol 1983; 52: 184–8. [DOI] [PubMed] [Google Scholar]
- 64.Tscholakoff D, Higgins CB, McNamara MT, Derugin N. Early-phase myocardial infarction: evaluation by MR imaging. Radiology 1986; 159: 667–72. doi: 10.1148/radiology.159.3.3704148 [DOI] [PubMed] [Google Scholar]
- 65.Willerson JT, Scales F, Mukherjee A, Platt M, Templeton GH, Fink GS, et al. Abnormal myocardial fluid retention as an early manifestation of ischemic injury. Am J Pathol 1977; 87: 159–88. [PMC free article] [PubMed] [Google Scholar]
- 66.Abdel-Aty H, Cocker M, Meek C, Tyberg JV, Friedrich MG. Edema as a very early marker for acute myocardial ischemia: a cardiovascular magnetic resonance study J Am Coll Cardiol 2009; 53: 1194–201. doi: 10.1016/j.jacc.2008.10.065 [DOI] [PubMed] [Google Scholar]
- 67.Jackowski C, Warntjes MJ, Berge J, Bär W, Persson A. Magnetic resonance imaging goes postmortem: noninvasive detection and assessment of myocardial infarction by postmortem MRI. Eur Radiol 2011; 21: 70–8. doi: 10.1007/s00330-010-1884-6 [DOI] [PubMed] [Google Scholar]
- 68.Jackowski C, Schwendener N, Grabherr S, Persson A. Post-mortem cardiac 3-T magnetic resonance imaging: visualization of sudden cardiac death? J Am Coll Cardiol 2013; 62: 617–29. doi: 10.1016/j.jacc.2013.01.089 [DOI] [PubMed] [Google Scholar]
- 69.Ortmann C, Pfeiffer H, Brinkmann B. A comparative study on the immunohistochemical detection of early myocardial damage. Int J Legal Med 2000; 113: 215–20. [DOI] [PubMed] [Google Scholar]
- 70.Croojimans HJA, Ruder TD, Zech WD, Somaini S, Scheffeler K, Thali MJ, et al. Feasibility of quantitative diffusion imaging of the heart in post-mortem MR. J Forensic Radiol Imaging 2013; 1: 124–8. [Google Scholar]
- 71.Jackowski C, Warntjes MJ, Kihlberg J, Berge J, Thali MJ, Persson A. Quantitative MRI in isotropic spatial resolution for forensic soft tissue documentation. Why and how? J Forensic Sci 2011; 56: 208–15. doi: 10.1111/j.1556-4029.2010.01547.x [DOI] [PubMed] [Google Scholar]
- 72.Jackowski C, Hofmann K, Schwendener N, Schweitzer W, Keller-Sutter M. Coronary thrombus and peracute myocardial infarction visualized by unenhanced postmortem MRI prior to autopsy. Forensic Sci Int 2012; 214: e16–9. doi: 10.1016/j.forsciint.2011.07.010 [DOI] [PubMed] [Google Scholar]
- 73.Shelton KD. Cardiac anatomy physiology and imaging methods. In: Brant WE, Helms CA, eds. Fundamentals of diagnostic radiology. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. pp. 603–28. [Google Scholar]
- 74.Christine C, Francesco D, Paul V, Cristian P, Alejandro D, Stefano B, et al. Postmortem computed tomography angiography vs. conventional autopsy: advantages and inconveniences of each method. Int J Legal Med 2013; 127: 981–9. doi: 10.1007/s00414-012-0814-3 [DOI] [PubMed] [Google Scholar]
- 75.Ross SG, Thali MJ, Bolliger S, Germerott T, Ruder TD, Flach PM. Sudden death after chest pain: feasibility of virtual autopsy with postmortem CT angiography and biopsy. Radiology 2012; 264: 250–9. doi: 10.1148/radiol.12092415 [DOI] [PubMed] [Google Scholar]
- 76.Palmiere C, Lobrinus JA, Mangin P, Grabherr S. Detection of coronary thrombosis after multi-phase postmortem CT-angiography. Leg Med (Tokyo) 2013; 15: 12–8. doi: 10.1016/j.legalmed.2012.08.005 [DOI] [PubMed] [Google Scholar]
- 77.Ruder TD, Hatch GM, Ebert LC, Flach PM, Ross S, Ampanozi G, et al. Whole body postmortem magnetic resonance angiography. J Forensic Sci 2012; 57: 778–82. doi: 10.1111/j.1556-4029.2011.02037.x [DOI] [PubMed] [Google Scholar]
- 78.Ruder TD, Bauer-Kreutz R, Ampanozi G, Rosskopf AB, Pilgrim TM, Weber OM, et al. Assessment of coronary artery disease by post-mortem cardiac MR. Eur J Radiol 2012; 81: 2208–14. doi: 10.1016/j.ejrad.2011.06.042 [DOI] [PubMed] [Google Scholar]
- 79.Huda W, Slone R. Magnetic resonance. In: Huda W, Slone R, eds. Review of radiologic physics. Philadelphia, PA: Lippincott Williams & Wilkins; 2003. pp. 192–221. [Google Scholar]
- 80.Ghali JK, Liao Y, Simmons B, Castaner A, Cao G, Cooper RS. The prognostic role of left ventricular hypertrophy in patients with or without coronary artery disease. Ann Intern Med 1992; 117: 831–6. [DOI] [PubMed] [Google Scholar]
- 81.Ruder TD, Stolzmann P, Thali YA, Hatch GM, Somaini S, Bucher M, et al. Estimation of heart weight by post-mortem cardiac magnetic resonance imaging. J Forensic Radiol Imaging 2013; 1: 15–8. [Google Scholar]
- 82.Filograna L, Hatch G, Ruder T, Ross SG, Bolliger SA, Thali MJ. The role of post-mortem imaging in a case of sudden death due to ascending aorta aneurysm rupture. Forensic Sci Int 2013; 228: e76–80. doi: 10.1016/j.forsciint.2013.01.039 [DOI] [PubMed] [Google Scholar]
- 83.Schwendener N, Mund M, Jackowski C. Type II DeBakey dissection with complete aortic rupture visualized by unenhanced postmortem imaging. Forensic Sci Int 2013; 225: 67–70. doi: 10.1016/j.forsciint.2012.09.002 [DOI] [PubMed] [Google Scholar]
- 84.Jackowski C, Grabherr S, Schwendener N. Pulmonary thrombembolism as cause of death on unenhanced postmortem 3T MRI. Eur Radiol 2013; 23: 1266–70. doi: 10.1007/s00330-012-2728-3 [DOI] [PubMed] [Google Scholar]
- 85.Aghayev E, Thali MJ, Sonnenschein M, Hurlimann J, Jackowski C, Kilchoer T, et al. Fatal steamer accident; blunt force injuries and drowning in post-mortem MSCT and MRI. Forensic Sci Int 2005; 152: 65–71. doi: 10.1016/j.forsciint.2005.02.034 [DOI] [PubMed] [Google Scholar]
- 86.Aghayev E, Jackowski C, Thali MJ, Yen K, Dirnhofer R, Sonnenschein M. Heart luxation and myocardium rupture in postmortem multislice computed tomography and magnetic resonance imaging. Am J Forensic Med Pathol 2008; 29: 86–8. doi: 10.1097/PAF.0b013e318165c0d8 [DOI] [PubMed] [Google Scholar]
- 87.Aghayev E, Christe A, Sonnenschein M, Yen K, Jackowski C, Thali MJ, et al. Postmortem imaging of blunt chest trauma using CT and MRI: comparison with autopsy. J Thorac Imaging 2008; 23: 20–7. doi: 10.1097/RTI.0b013e31815c85d6 [DOI] [PubMed] [Google Scholar]
- 88.Dirnhofer R, Jackowski C, Vock P, Potter K, Thali MJ. Virtopsy: minimally invasive, imaging-guided virtual autopsy. Radiographics 2006; 26: 1305–33. doi: 10.1148/rg.265065001 [DOI] [PubMed] [Google Scholar]
- 89.Ruder TD, Hatch GM, Thali MJ, Fischer N. One small scan for radiology, one giant leap for forensic medicine—post-mortem imaging replaces autopsy in a case of traumatic aortic rupture. Leg Med (Tokyo) 2011; 13: 41–3. doi: 10.1016/j.legalmed.2010.10.003 [DOI] [PubMed] [Google Scholar]
- 90.Brookes JA, Hall-Craggs MA, Sams VR, Lees WR. Non-invasive perinatal necropsy by magnetic resonance imaging. Lancet 1996; 348: 1139–41. doi: 10.1016/S0140-6736(96)02287-8 [DOI] [PubMed] [Google Scholar]
- 91.Rutty GN, Swift B. Accuracy of magnetic resonance imaging in determining cause of sudden death in adults: comparison with conventional autopsy. Histopathology 2004; 44: 187–9. [DOI] [PubMed] [Google Scholar]
- 92.Thayyil S, Sebire NJ, Chitty LS, Wade A, Olsen O, Gunny RS, et al. Post mortem magnetic resonance imaging in the fetus, infant and child: a comparative study with conventional autopsy (MaRIAS Protocol). BMC Pediatr 2011; 11: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Thayyil S, Sebire NJ, Chitty LS, Wade A, Chong W, Olsen O, et al. Post-mortem MRI versus conventional autopsy in fetuses and children: a prospective validation study. Lancet 2013; 382: 223–33. [DOI] [PubMed] [Google Scholar]
- 94.Buck U, Christe A, Naether S, Ross S, Thali MJ. Virtopsy—noninvasive detection of occult bone lesions in postmortem MRI: additional information for traffic accident reconstruction. Int J Legal Med 2009; 123: 221–6. doi: 10.1007/s00414-008-0296-5 [DOI] [PubMed] [Google Scholar]
- 95.Ruder TD, Germerott T, Thali MJ, Hatch GM. Differentiation of ante-mortem and post-mortem fractures with MRI: a case report. Br J Radiol 2011; 84: e75–8. doi: 10.1259/bjr/10214495 [DOI] [PMC free article] [PubMed] [Google Scholar]