Abstract
Objective:
To evaluate the therapy effects of 125I implantation combined with chemoradiotherapy on pancreatic cancer patients.
Methods:
30 patients with Stage III or IV pancreatic cancer were equally divided into two groups (control and treatment group). The patients in the treatment group (nine males, six females) received chemotherapy in the first week and 125I implantation in the third week, followed by combined chemoradiotherapy in the fifth week. The patients in the control group (10 males, 5 females) received the same treatment except 125I implantation. The therapy in the control group and treatment group was repeated every 4 weeks.
Results:
The median conformal radiotherapy dose in the treatment group (30.62 Gy) was significantly lower than that in the control group (47.86 Gy). The total radiation dose was 88.71 ± 27.39 Gy, and the surface activity was 0.6 mCi in the treatment group. After treatment, the average tumour size decreased both in the treatment group [9.17 cm2, 95% confidence interval (CI): 5.60–12.74, p < 0.001] and in the control group (4.54 cm2, 95% CI: 2.74–6.35, p < 0.001). The median survival time in the treatment group was 14 months (95% CI: 12.215–14.785) and in the control group was 12 months (95% CI: 10.884–13.116). There was no statistical significance in survival rates between the two groups (χ2 = 0.908, p = 0.341).
Conclusion:
125I implanted into tumour combined with chemoradiotherapy has higher local control rate of advanced pancreatic cancer than chemoradiotherapy.
Advances in knowledge:
We combined chemoradiotherapy with 125I implantation to treat advanced pancreatic cancer and obtained a higher local control rate and better quality of life than when using chemoradiatherapy alone.
Pancreatic cancer is currently one of the most intractable cancers with high and continually rising mortality in China.1 The main risk factors are smoking, age and some genetic disorders, although the primary causes are poorly understood.2 Pancreatic cancer causes no early symptoms, so the majority of patients are diagnosed as having advanced cancer with rapid progression when they come to the hospital.3 Thus, patients miss the opportunity for tumour resection when first diagnosed. Even if the cancer is discovered early, only 20% of patients can undergo surgical excision, whereas the other 80% cannot.2 For patients who have undergone radical excision, the 5-year survival rate is just 20–25%.4–9
Advanced pancreatic cancer, according to the TNM stage of pancreatic carcinoma by the American Joint Committee on Cancer (AJCC),10 includes Stages III and IV, and pancreatectomy is not well accepted.11 It is reported that approximately 40% of pancreatic cancer patients present with locally advanced, non-metastatic disease.12 Local lesions play a vital role in a patient's survival.13–16 The aim of advanced pancreatic cancer treatment is to enhance local lesion control and improve the quality of life (QOL).17,18
Gemcitabine is a type of pyrimidine analogue, which acts as a ribonucleoside reductase inhibitor and destroys cells and terminates the DNA chains. It has been approved by the US Food and Drug Administration as a gold standard agent in chemotherapy19 for the treatment of cancer, especially for advanced pancreatic cancer.20 Currently, the major therapy is comprehensive treatment, namely chemoradiotherapy, which is superior to either radiotherapy21 or chemotherapy.22 But the overall survival time is not prolonged by chemoradiotherapy in advanced pancreatic cancer compared with single-agent gemcitabine.23 The 5-year survival rate is still <5%.24 However, interstitial implantation of radioactive seeds (brachytherapy) combined with conformal radiotherapy (external beam radiation therapy) has a good effect for local control of pancreatic cancer.25,26 125I particles are reported to be the most commonly used for brachytherapy because of their long half-life and short radiation distance.27 Therefore, we infer that 125I implantation combined with chemoradiation may obtain better curative effects.
In this study, we compared the local control rate, pain relief and survival rate of 30 patients with advanced pancreatic cancer who were treated with or without 125I implantation combined with chemoradiotherapy in our hospital during October 2006 to January 2012. We expected that the implantation of 125I particles could be an efficient therapy for patients with advanced pancreatic cancer.
METHODS AND MATERIALS
Inclusion and exclusion criteria
Informed consent about the basic process of radiotherapy, chemotherapy and 125I implantation and possible complications was obtained from all participating individuals. The protocol was approved by the Zhejiang Cancer Hospital Medical Research Ethics Committee, Zhejiang, China.
The inclusion criteria were as follows: (1) pathology-diagnosed pancreatic cancer or (2) two kinds of image examination combined with laboratory and clinical examination leading to a diagnosis of pancreatic cancer; (3) patients with unresectable pancreatic carcinoma, includes Stage III (cancer has spread to lymph nodes near the pancreas and may or may not spread to nearby organs) and Stage IV (although cancer has spread to places far away from the pancreas, such as the liver or lungs, the local lump was considered to be the major factor that caused pain or obstructive jaundice) according to TNM stage standards provided by AJCC.10
The exclusion criteria were as follows: (1) patients with mental disorders and those with other diseases and (2) patients with severe cardiopulmonary dysfunction, advanced cachexia, tumours with a diameter ≥7 cm or diffuse tumours.
All the patients' stages and inclusion criteria were decided by professors of the pancreatic surgery and abdominal radiotherapy department.
Patient information and groups
A total of 30 patients were included in this study, and the patients' data and characteristics are displayed in Table 1. They were randomly divided into a treatment group and a control group (n = 15). The treatment group received 125I implantation combined with chemoradiotherapy, whereas 125I implantation was absent in the control group. The average age was 61.2 years in the treatment group, and 59.5 in the control group with no statistical significance between these two groups. In the treatment group, there were 14 patients diagnosed with pancreatic cancer by pathology, whereas 1 patient who underwent CT-guided puncture biopsy had no obvious cancer cells in the pathological report but was diagnosed with Stage IV pancreatic cancer combined with intrahepatic multiple transfer and retroperitoneal lymph node metastasis with carbohydrate antigen 19-9 (CA19-9) >12 000 U ml−1 (reference value was 0–37 U ml). In the control group, 12 cases were pathologically diagnosed with pancreatic cancer, whereas the remaining 3 cases were diagnosed as malignant pancreatic cancer by imaging and CA19-9 > 370 U ml−1.
Table 1.
Patient information and treatment characteristics
| Data | Treatment group |
Control group |
χ2 | p-value | ||
|---|---|---|---|---|---|---|
| Cases | % | Cases | % | |||
| Age (mean ± standard deviation) | 61.20 ± 12.50 | 59.47 ± 10.62 | t = −0.410 | 0.685 | ||
| Sex | χ2 = 0.144 | 0.705 | ||||
| Male | 9 | 60.00 | 10 | 66.67 | ||
| Female | 6 | 40.00 | 5 | 33.33 | ||
| Diagnostic methods | χ2 = 1.154 | 0.283 | ||||
| Pathological diagnosis | 14 | 93.33 | 12 | 80.00 | ||
| Imaging and laboratory diagnosis | 1 | 6.67 | 3 | 20.00 | ||
| Clinical manifestations before treatment | χ2 = 0.304 | 0.859 | ||||
| Pain | 15 | 100.00 | 15 | 100.00 | ||
| Jaundice | 2 | 13.33 | 1 | 6.67 | ||
| Elevated carbohydrate antigen 19-9 | 14 | 93.33 | 13 | 86.67 | ||
| Diameter of nidus (largest) | 2.0–5.0 cm | 2.1–6.0 cm | ||||
| Location of pancreatic tumours | χ2 = 3.086 | 0.214 | ||||
| Head | 2 | 13.33 | 5 | 33.33 | ||
| Body | 9 | 60.00 | 9 | 60.00 | ||
| Tail | 4 | 26.67 | 1 | 6.67 | ||
| Clinical stage | χ2 = 1.540 | 0.215 | ||||
| Stage III | 11 | 73.33 | 14 | 93.33 | ||
| Stage IV | 4 | 26.67 | 1 | 6.67 | ||
All the patients' stages and inclusion criteria were decided by professors of the pancreatic surgery and abdominal radiotherapy department.
Scheme of treatment
The scheme of treatment is displayed in Table 2. Patients in the treatment group received chemotherapy on the first and eighth days, 125I particles implantation in the third week and conformal radiotherapy in the fifth week. Chemotherapy was simultaneously performed every 4 weeks as a cycle for a total of 4–6 cycles when treated with 125I particle implantation or external radiotherapy. Patients in the control group received the same treatment as the treatment group but without the implantation of 125I particles.
Table 2.
Scheme of treatment
| Cycle | Week | Treatment group | Control group |
|---|---|---|---|
| 1 | 1 | Chemotherapy (Day 1) | Chemotherapy (Day 1) |
| 2 | Chemotherapy (Day 8) | Chemotherapy (Day 8) | |
| 3 | 125I implantation | / | |
| 4 | / | / | |
| 2 | 5 | Chemotherapy (Day 1) + radiotherapya | Chemotherapy (Day 1) + radiotherapya |
| 6 | Chemotherapy (Day 8) | Chemotherapy (Day 8) | |
| 7 | / | / | |
| 8 | / | / |
/, no chemotherapy.
Treatment group: patients underwent chemotherapy (gemcitabine, 1000 mg m−2 once) on the first and eighth days, repeated every 4 weeks (Q4W); implantation was conducted on the third week (synchronous chemotherapy); three-dimensional (3D) conformal radiotherapy was performed in the fifth week (the beginning of the second cycle of chemotherapy).
Control group: patients underwent chemotherapy (gemcitabine, 1000 mg m−2 once) on the first day and eighth day, repeated every 4 weeks (Q4W); 3D conformal radiotherapy was performed in the fifth week (the beginning of the second cycle of chemotherapy).
Radiotherapy continued to the end of the course.
Chemotherapy regimens
Single-agent gemcitabine was given as 1000 mg m−2 once on the first day and eighth day.19 For patients with hepatic metastasis, interventional therapy (coeliac perfusion chemotherapy combined with transcatheter arterial chemoembolization) plus systematic venous chemotherapy on the eighth day was performed.
Radiotherapy regimens
Radiotherapy was performed as in previous reports.28 Pinnacle® 6.2b [three-dimensional (3D) radiation treatment planning system; Philips Medical Systems, Andover, MA] was used. The patients were in the supine position, fixed with a ventral thoracic covering and external radiation technology was used (10-MV linear accelerator, Elekta, Crawley, UK). A multileaf collimator was used to form a geometric conformal field. The planning target volume (PTV) was surrounded by a 95% isodose to make the conformal index close to 1. For the control group, a single dose was 1.8 Gy (5 times per week), and the accumulated dose reached 45.0–50.4 Gy within 4–6 weeks. In the treatment group, a single dose was 1.8 Gy (5 times per week), and the accumulated dose reached 30.6–39.6 Gy within 3–4 weeks. The maximum dose in the spinal cord was <42 Gy; in the small intestine was <45 Gy; in the liver, V5 was <86%, V2 <49%, V30 <28%, the average dose was <23 Gy; in the two kidneys, V12 was <50%, V22.5 was <30%, and the average dose was <16 Gy. Finally, the external radiation dose in the treatment group was 30.62 ± 10.18 Gy and 47.86 ± 32.11 Gy in the control group.
Radioactive 125I particle implantation plan
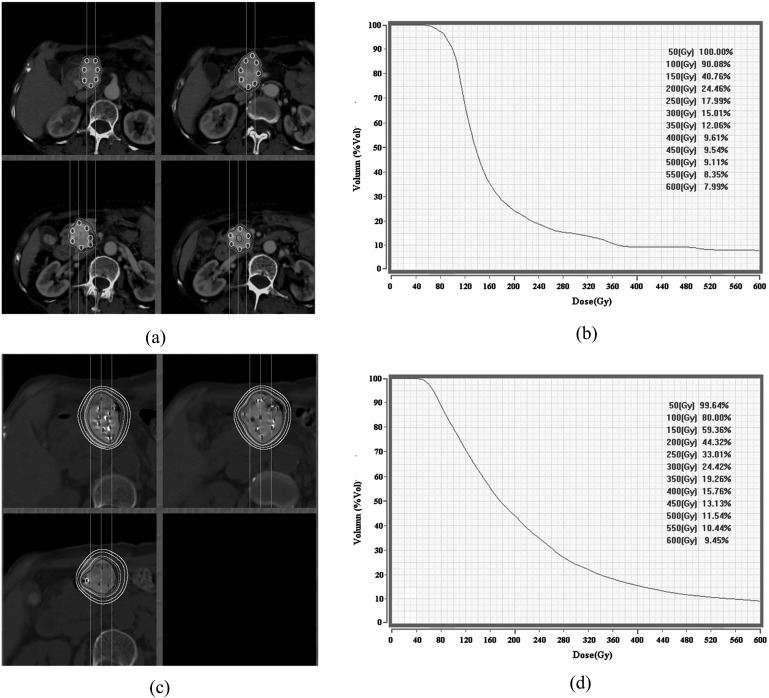
Patients underwent CT or minimal remission (MR) examination (HiSpeed CT/i; General Electric Company, New York, NY), and the images were entered into a treatment plan system (TPS; Radiological Institute of Fudan University, Shanghai, China). Gross tumour volume (GTV) was confirmed by the radiation oncologist or intervention practitioner. The PTV adds 0.5–1.0 cm to the boundary of the GTV. 125I radiological particles (radioactivity, 0.55–0.65 mCi; dose rate, 0.05–0.10 Gy h−1; half-life, 59.6 days; effective radius, 1.72 cm; Junan Pharmaceutical Company, Ningbo, China) were implanted at a dose of 120–140 Gy in the periphery of tumours. The radioactive dose was evaluated 1 week after the implantation. D90 (the dose to 90% of the volume) should have reached 60–140 Gy. If the actual dose was less than the reference dose, the evaluated images (Figure 1) were analysed carefully, and then it was decided whether supplementary particles should be implanted.
Figure 1.
CT scanning images and dose–volume histogram (DVH) curves before and after 125I implantation. (a) Particle isodose curve before implantation; (b) DVH figures before implantation; (c) particle isodose curve after implantation; (d) DVH after implantation.
Radioactive particle implantation method
Intraoperative implantation or CT-guided percutaneous puncture implantation was used.
Preoperative preparation
Patients were given laxatives (enema) to clean the intestine and were asked to have fluids for dinner on the day before the operation. The routine blood examination, coagulation function and abdominal enhanced CT examination were completed before the 125I particle implantation. Karnofsky performance status (KPS) scores29 were no less than 70.
Based on the prescription dose (140 Gy) and the total activity the number of treatment particles required was calculated. Particles and the implantation instruments were disinfected by high-pressure dry steam sterilization before use for more than 8 min, 0.21 MPa and 135 °C.
Intraoperative implantation
Open intraoperative implantation was performed according to the radioactive 125I particle implantation plan. When the pancreatic tumour area was exposed after conventional pancreatic tumour surgery, the radioactive particle implantation needle (18 G, 100–150 mm; Doctor Japan Co., Ltd, Tokyo, Japan) was placed in the PTV, and the particles were implanted into the tumour. One patient who was pre-operatively diagnosed with pancreatic cancer accompanied by hepatic metastasis and for whom pathological diagnosis was obtained during exploratory laparotomy underwent intraoperative implantation. Pancreatic CT scanning was performed 1 week after surgery, and the images were entered into the TPS again for dose verification.
CT-guided implantation
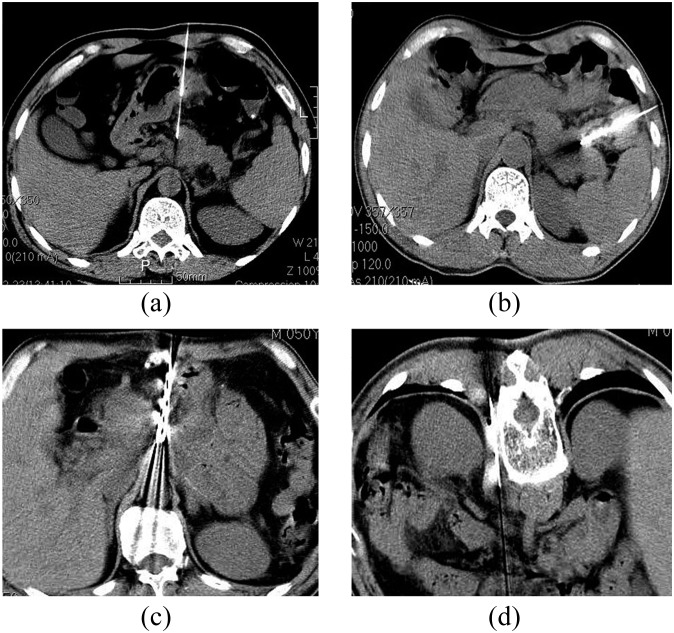
Patients adopted a supine position (needle inserted from the anterior and lateral path) or prostrate position (needle inserted from the posterior path). For those patients undergoing the lateral path, a lateral position, left front, right anterior oblique position was also available. The location device was stuck on the skin surface of the pathological position. Then the needle's entry point was marked. After regular disinfection, spreading towels and local anaesthesia (2% lidocaine, 3 ml), the radioactive particle implantation needles (18 G) were inserted into the nidus under CT guidance. The needles were arranged at intervals of 10 mm. After the needles were verified as being in the right position, the stylet was extracted and the 125I particle (0.8 m × 4.5 mm) was implanted into the nidus. The distance between every two particles was about 10–15 mm. After implantation, dose verification was performed based on CT scanning (section thickness, 5 mm). If the result was not consistent with the original plan or there appeared to be a “cold spot”, implantation was repeated. In this study, there were 15 patients who accepted 18-G puncture implantation under CT guidance. The anterior path (Figure 2a) was used for 10 cases, the lateral path (Figure 2b) for 3 cases and the anterior–posterior path (Figure 2c,d) for 2 cases.
Figure 2.
The puncture paths used in this study. (a) Anterior path puncture; (b) lateral path puncture; (c) two puncture path—the anterior path; (d) two puncture path—the posterior path.
Finally, a total of 374 seeds of 125I were implanted in 15 patients in the treatment group with an average number of 24.93 (16–43) seeds. The dose test results after 125I implantation indicated that the D90 was 80–130 Gy (median, 100 Gy), and the average dose was 88.71 Gy.
Curative evaluation indicators
The local control rate
According to the World Health Organization response evaluation criteria in solid tumours guideline, the local control rate was investigated in our study. Complete remission (CR): tumour lesions disappeared for at least 4 months; partial remission (PR): the product of the maximum horizontal diameter and the maximum vertical diameter of the lesion was reduced by >50%; MR: the product of the maximum horizontal diameter and the maximum vertical diameter was reduced by 25–50%; no change (NC): the product of the maximum horizontal diameter and the maximum vertical diameter of the lesion was reduced by <25%. The total effective rate was the sum of CR and PR.
Carbohydrate antigen 19-9 evaluation
The reference value of CA19-9 evaluation was 0–37 U ml−1.
Visual analogue scale pain scoring
The pain scores before and after treatment were recorded by patients according to the visual analogue scale (VAS) scoring system, which divided pain into 10 grades (Grades 0–10), and the results were collected and analysed by one experienced doctor. Grade 0 indicated no pain, Grades 1–3 mild pain (discomfort, pressing sensation, passive pain, inflammatory pain and sleep not being affected), Grades 4–6 moderate pain (such as pain and cramps, burning sensation, squeezing feeling, stabbing pain, haphalgesia, pressing pain and sleep being affected), Grades 7–9 severe pain (such as preventing normal activities and sleep being significantly affected) and Grade 10 extreme pain (could not be controlled).
Statistical analysis
Data were analysed by the SPSS® v. 16.0 software (SPSS Inc., Chicago, IL). The comparison of the size of the tumour was performed using Student's t-test, and the reductions in the two groups were compared by paired-sample t-test. Local control rates between the two groups were compared by χ2 test. VAS pain scores between the two groups were compared using the t-test before surgery and the Mann–Whitney U-test after surgery (non-normal distribution). The pain scoring differences before and after treatment in the two groups were compared with the paired-sample t-test. The Kaplan–Meier method was used to estimate survival as a function of time, and survival differences were analysed by the log-rank test. p < 0.05 was considered significantly significant.
RESULT
Local control rate comparison
There was no statistical significance in tumour size between the two groups before treatment (Table 3). After treatment, the size of the tumours in the treatment group was significantly smaller than that of those in the control group (t = 2.088, p = 0.047). The size of the tumours was reduced by 4.54 cm2 [95% confidence interval (CI): 2.74–6.35] in the control group, and by 9.17 cm2 (95% CI: 5.60–12.74) in the treatment group. Paired t-test showed that the local control rate in the treatment group was better than that in the control group (t = 5.494, p < 0.01). Table 4 shows that the overall remission (CR + PR) in the treatment group was 73.33% (11/15), and 26.67% (4/15) in the control group with a significant difference (χ2 = 4.821, p = 0.028), and there were six cases in the treatment group that reached CR (Figures 3 and 4).
Table 3.
Comparison of tumour size in the two groups before and after treatment
| Group | Tumour size (cm2) |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before treatment | After treatment | |||||||||||||||||||
| Control group (mean ± standard deviation) | 12.85 ± 4.09 | 7.65 ± 4.16 | ||||||||||||||||||
| Treatment group (mean ± standard deviation) | 12.74 ± 4.99 | 4.09 ± 4.66 | ||||||||||||||||||
| t | 0.065 | 2.088 | ||||||||||||||||||
| P | 0.949 | 0.047 | ||||||||||||||||||
The comparison of tumour size was performed 2 weeks before treatment and 1 month after treatment based on CT images.
Table 4.
Comparison of short-term effects in the two groups
| Local control effects | Treatment group |
Control group |
||
|---|---|---|---|---|
| Cases | % | Cases | % | |
| CR | 6 | 40.00 | 0 | 0.00 |
| PR | 5 | 33.33 | 4 | 26.67 |
| MR | 1 | 6.67 | 6 | 40.00 |
| NC | 3 | 20.00 | 5 | 33.33 |
| CR + PR | 11 | 73.33 | 4 | 26.67 |
CR, complete remission; MR, minimal remission; NC, no change; PR, partial remission.
The comparison was performed 2 weeks before treatment and 1 month after treatment based on CT images (for CR patients, the comparison was between 2 weeks before treatment and 2 months after treatment).
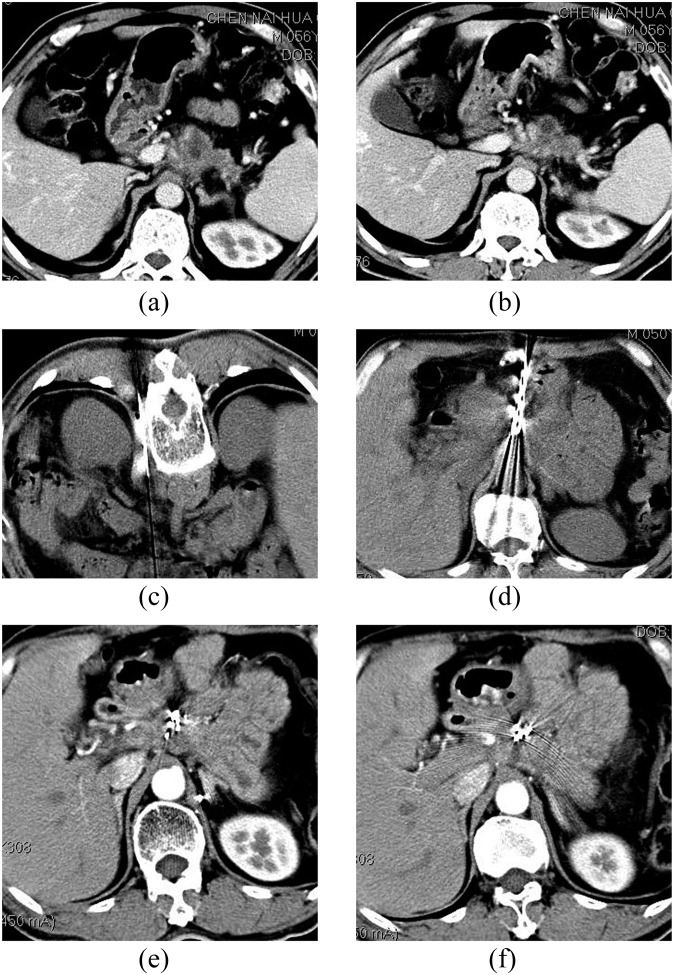
Figure 3.
(a,b) CT of a male patient (aged 50 years) 3 months after pancreatic cancer treatment. CT shows 3.8 × 2.7 cm occupying lesions at the junction of the body and pancreas head, with para-aortic lymph node enlargement and carbohydrate antigen 19-9 elevation and biopsy-confirmed tumour recurrence; (c) five 125I seeds were implanted into the para-aortic lymph nodes using a posterior pathway. The minimum peripheral dose was 120 Gy, and the particle surface activity was 0.6 mCi; (d) 20 125I seeds were implanted into the lesion using an anterior pathway. The minimum peripheral dose was 120 Gy, and the particle surface activity was 0.6 mCi; (e,f) treatment after 6 months, complete remission of the lesion and the patient has survived 63 months.
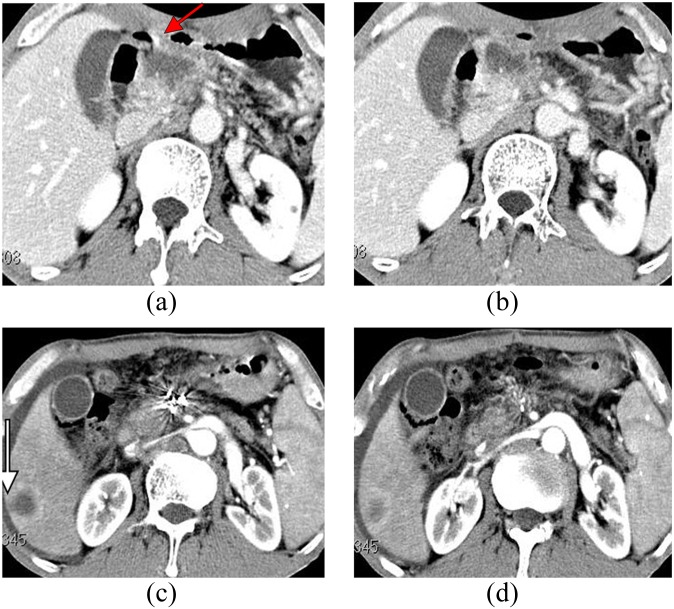
Figure 4.
(a,b) CT of a male patient (62 years old) with upper abdominal pain lasting 1 year. CT examination showed pancreatic mass lesions invading the gastric wall (arrow); the carbohydrate antigen carbohydrate antigen 19-9 was 301 U ml−1; and biopsy verified pancreatic adenocarcinoma. (c,d) On the third day after implantation, upper gastrointestinal bleeding occurred. 2 months later, lesions achieved CR, but hepatic metastasis occurred (see arrows).
Changes in tumour maker carbohydrate antigen 19-9
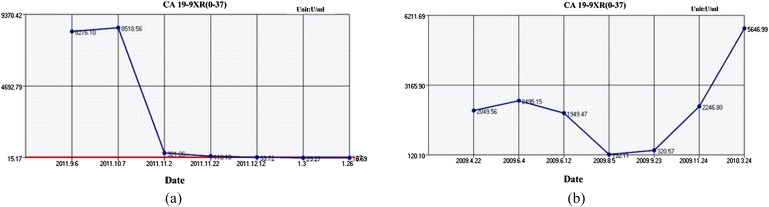
The CA19-9 curve reflected the progress of the disease (Figure 5). Before treatment, CA19-9 was negative in one case in the treatment group and two cases in the control group, and it was higher than the reference value (37 U ml−1) in the rest of the cases. CA19-9 in the two groups had different degrees of reduction after treatment, but there was no significant difference. Both the groups showed no statistical significance before treatment and after treatment.
Figure 5.
Patients' carbohydrate antigen 19-9 (CA19-9) curves during treatment progress. If the course was stable, the CA19-9 curve dropped from the high value at the beginning to a lower value and stayed low (a). If the disease progressed, the curve moved towards a high value (b).
Pain scoring
Pain scoring in the control group (5.20 ± 1.47) was significantly higher than that in the treatment group before treatment (4.27 ± 1.67, p < 0.05), whereas, after treatment, no statistical difference was found (U = 85, p = 0.102). The pain scoring in the control group and the treatment group were both significantly reduced after treatment (p < 0.001).
Analysis of survival rate
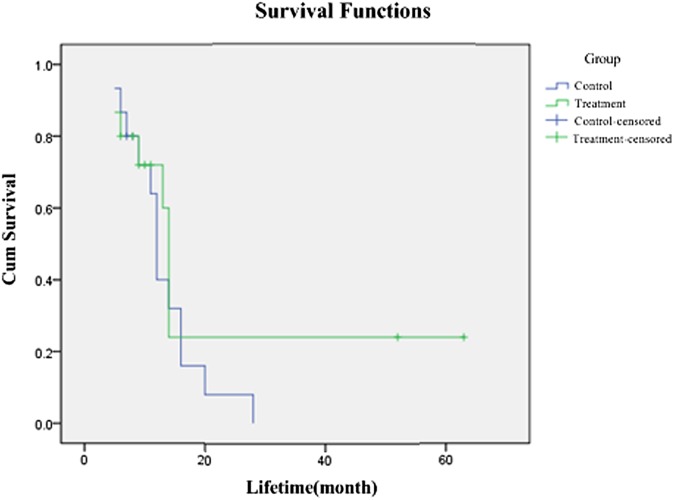
The median survival time in the treatment group was 14 months, whereas it was 12 months in the control group (Figure 6). No statistical difference was found by log-rank test in the survival rate at 6 months, 1 year, 2 years and 5 years between the two groups (χ2 = 0.908, p = 0.314).
Figure 6.
Survival curves of the two groups.
Comparison of toxic effects and side effects of the two groups after treatment
There were five patients in the treatment group and seven patients in the control group who suffered acute radiation side effects, such as mild abdominal pain, abdominal distension, nausea and vomiting in the 2 months after treatment. These side effects were relieved by antinausea therapy and a low dose of hormone. Bleeding, cancerous ulcer and hepatic metastases occurred in a patient (Figure 4) who died 5 months after the treatment. In the control group, one patient suffered incomplete ileus, and the condition improved after receiving conservative treatment. The remaining patients had no obvious side effects.
The complications of 125I implantation
Among 15 patients in the treatment group, 14 patients underwent percutaneous pancreatic puncture 125I implantation guided by CT with 18 G implanting needles for 65 times, among whom 4 patients had intra-abdominal haemorrhage (occurrence rate 6.15%, 4/65). Massive haemorrhage of the upper gastrointestinal tract occurred in one patient and was stopped by operation. Another three patients suffered a small amount of bleeding (<5 ml estimated) between bowel clearance, and the patients accepted no specific treatment since there were no any uncomforted.
DISCUSSION
In this study, we evaluated the curative effects of 125I particle implantation combined with chemoradiotherapy for patients with unresectable advanced pancreatic cancer. As previous reports have indicated, for unresectable advanced pancreatic cancer, uncontrolled local lesions and treatment failure were the main factors influencing survival time and QOL.14 Thus, strengthening the local control of the lesion is the key to scientific and efficient treatment15,16 and is also our main purpose in the study. A high local control rate of 73.33% (CR + PR) was achieved, and 6 (40%) patients in the treatment group achieved CR, which was significantly higher than in the control group.
3D conformal radiotherapy has been widely used in the treatment of unresectable advanced pancreatic cancer, especially when combined with chemotherapy.30 Since pancreatic cancer is not sensitive to radiotherapy, the cumulative dose of radiotherapy needed to be <60 Gy to obtain satisfactory curative effect.15 In Stage I of the clinical trials reported by Koong et al,31 a single segment illumination dose was 25 Gy and the local control rate could reach 100%. Chang et al13 have reported a total 6-month local control rate of 91%, and a total 12-month local control rate of 84%, with a combination of 25 Gy single segment illumination and 45–54 Gy fractional integral exposure. All these studies were expected to improve the local control rate and to obtain the greatest clinical benefit by increasing the feasible exposure dose. Expanding the target region is necessary to avoid the influence of respiration. But because the pancreatic lesions are closely related to the surrounding enteric cavity, the surrounding organs, especially the small intestine, are affected by the high irradiation dose, which furthermore limits the improvement of the radiation dose rate in the pancreas. Recently, 125I interstitial implantation for treating tumour lesions has been used in clinics because of its long half-life and short radiation distance, so that the dosage distribution can fit well with the tumour target. The incessant and short radioactive rays produced by the miniature radioactive source can continue to work and the cumulative dosage to the target can reach to 160 Gy.27 This can damage the tumour tissues to a great extent, while the surrounding normal tissue has no or only minor damage. Additionally, there is little influence from external radiotherapy. In the present study, implantation of 125I particles effectively improved the feasible dose where the total radiation dose in the cancer lesion reached 88.71 Gy. This was mainly contributed by 125I particles, whereas the conformal radiotherapy dose of the treatment group (30.62 Gy) was significantly lower than that of the control group (47.86 Gy). This indicated that 125I particles combined with chemoradiotherapy result in a lower external radiation dose.
From the above, 125I particle implantation and chemoradiotherapy can be complementary and combined theoretically. Their combination can reduce the dose of 3D conformal radiotherapy and make up for the dose “cold spot” produced by implanted 125I particle radiotherapy. So the total radiotherapy dose rate and the distributive uniformity of the radiotherapy dose can be ensured, and the exposure dose of the surrounding normal tissues can be reduced as well. Their combination can improve the local control rate and reduce the occurrence of complications, thus improving the short-term and long-term curative effect. There are many studies26,32–34 reporting the outcomes of combined therapy of 125I particle implantation and chemotherapy or radiotherapy for pancreatic cancer. Among which, the highest control rate (CR + PR) was 61.3% with 125I implantation (51.5 Gy) + chemotherapy [gemcitabine, 1.0 g m−2 + 5-fluorouracil (5-FU) 300 mg m−2],33 and the highest 1-year survival rate was 63.1% with 125I implantation (51.5 Gy) + cryosurgery + adjuvant regional chemotherapy (5-FU 500 mg m−2 + mitomycin C 8.5 mg m−2 + gemcitabine 500 mg m−2)34. We performed 125I particle implantation (88.71 Gy) combined with chemotherapy (gemcitabine, 1000 mg m−2) and radiotherapy (30.62 Gy) and obtained a higher local control rate (73.33%) and higher survival rates (1 year, 72.0%; 2 years, 60.0%; 5 years, 24.0%).
In our study, obvious clinical benefit and long-term curative effects were obtained. Pains were eased in both groups. In the treatment group, the total remission rate reached 80%, significantly higher than that in the control group (20%). Data showed that the median lifetime in the treatment group was 14 months, and 12 months in the control group, but the comparison was not significant (χ2 = 1.5400, p = 0.215). From the treatment group, two patients are still alive and have lived for 52 months and 63 months, respectively, and they are both Stage III with no distant metastasis. Therefore, we consider that it is a superior therapeutic method for Stage III pancreatic cancer with no distant metastasis.
CT-guided percutaneous puncture for 125I particle implantation should be adopted first. In principle, a fine needle should be used for puncture to avoid great vessels and the main pancreatic ducts; if not, intraoperative implantation should be used by surgical operation. In this study, 14 patients underwent CT-guided percutaneous puncture, and one patient underwent intraoperative implantation because hepatic metastasis was found by preoperative imaging examinations and laboratory CA19-9 tests.
However, the complications and side effects and toxic effects should be noted. On the third day after implantation, upper gastrointestinal bleeding occurred in a patient and was stopped by operation. Cancerous ulcer caused by tumours invading the posterior wall of the stomach was found (Figure 4), which might be the main reason for cancerous ulcer bleeding, and the radioactive side effects and puncture implantation were secondary. This patient recovered after surgery. However, because of hepatic metastases, severe anaemia and dyscrasia, the patient died 5 months after treatment.
The low number of samples is the major limitation in this study, which was due to pancreatic cancer being diagnosed relatively late and most patients not wanting to take the risk of pancreatic puncture implantation or intraoperative implantation and giving up treatment. Another limitation of our study is the lack of the QOL assessment after surgery, e.g. KPS scores, to which attention should be paid in future research. The factors that limited the application of this technique are complex operations and researchers lacking experience.
Consequently, we conclude that 125I implanted into tumours combined with radiotherapy and chemotherapy has a higher local control rate in patients with unresectable advanced pancreatic cancer than chemoradiotherapy, especially for Stage III pancreatic cancer with no distant metastasis. More attention should be focused on the percutaneous pancreatic puncture path and the improvement of long-term survival rate in future studies.
FUNDING
The research fund of Zhejiang Province for Medical Sciences, no. 2009B021.
Acknowledgments
ACKNOWLEDGMENTS
We would like to thank Dr Qing-Hua Deng for his help with radiotherapy. We also appreciate the contributions and support of all nurses and technicians at the Department of Intervention, Zhejiang Cancer Hospital, Zhejiang, China.
REFERENCES
- 1.Jie H, Ping Z, Qing CW. Chinese cancer registry annual report. Beijing, China: Military Medical Science Press; 2011. [Google Scholar]
- 2.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet 2004; 363: 1049–57. doi: 10.1016/S0140-6736(04)15841-8 [DOI] [PubMed] [Google Scholar]
- 3.Brentnall TA, Bronner MP, Byrd DR, Haggitt RC, Kimmey MB. Early diagnosis and treatment of pancreatic dysplasia in patients with a family history of pancreatic cancer. Ann Intern Med 1999; 131: 247–55. [DOI] [PubMed] [Google Scholar]
- 4.Nitecki SS, Sarr MG, Colby TV, van Heerden JA. Long-term survival after resection for ductal adenocarcinoma of the pancreas. Is it really improving? Ann Surg 1995; 221: 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Group GTS. Comparative therapeutic trial of radiation with or without chemotherapy in pancreatic carcinoma. Int J Radiat Oncol 1979; 5: 1643–47. [DOI] [PubMed] [Google Scholar]
- 6.Allema JH, Reinders ME, van Gulik TM, Koelemay MJ, van Leeuwen DJ, de Wit LT, et al. Prognostic factors for survival after pancreaticoduodenectomy for patients with carcinoma of the pancreatic head region. Cancer 1995; 75: 2069–76. [DOI] [PubMed] [Google Scholar]
- 7.Yeo CJ, Abrams RA, Grochow LB, Sohn TA, Ord SE, Hruban RH, et al. Pancreaticoduodenectomy for pancreatic adenocarcinoma: postoperative adjuvant chemoradiation improves survival. A prospective, single-institution experience. Ann Surg 1997; 225: 621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conlon KC, Klimstra DS, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Ann Surg 1996; 223: 273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeo CJ, Cameron JL, Sohn TA, Lillemoe KD, Pitt HA, Talamini MA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg 1997; 226: 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010; 17: 1471–4. doi: 10.1245/s10434-010-0985-4 [DOI] [PubMed] [Google Scholar]
- 11.Eosewicz S, Wiedenmann B. Pancreatic carcinoma. Lancet 1997; 349: 485–9. [DOI] [PubMed] [Google Scholar]
- 12.Russo S, Butler J, Ove R, Blackstock AW. Locally advanced pancreatic cancer: a review. Semin Oncol 2007; 34: 327–34. doi: 10.1053/j.seminoncol.2007.05.007 [DOI] [PubMed] [Google Scholar]
- 13.Chang DT, Schellenberg D, Shen J, Kim J, Goodman KA, Fisher GA, et al. Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer 2009; 115: 665–72. doi: 10.1002/cncr.24059 [DOI] [PubMed] [Google Scholar]
- 14.Willett CG, Czito BG, Bendell JC, Ryan DP. Locally advanced pancreatic cancer. J Clin Oncol 2005; 23: 4538–44. doi: 10.1200/JCO.2005.23.911 [DOI] [PubMed] [Google Scholar]
- 15.Ceha HM, van Tienhoven G, Gouma DJ, Veenhof CH, Schneider CJ, Rauws EA, et al. Feasibility and efficacy of high dose conformal radiotherapy for patients with locally advanced pancreatic carcinoma. Cancer 2000; 89: 2222–9. [DOI] [PubMed] [Google Scholar]
- 16.Chang ST, Goodman KA, Yang G, Koong AC. Stereotactic body radiotherapy for unresectable pancreatic cancer. Front Radiat Ther Oncol 2007; 40: 386–94. doi: 10.1159/0000106048 [DOI] [PubMed] [Google Scholar]
- 17.Crane CH, Janjan NA. Conformal radiation therapy in pancreatic cancer. New York, NY:Springer; 2002. pp. 295–300. [Google Scholar]
- 18.Shi Y, Xu S, Zheng X, Yan W, Chen L. Therapeutic effect of three-dimensional conformal radiotherapy on locally advanced pancreatic carcinoma. Di Yi Jun Yi Da Xue Xue Bao 2004; 24: 213. [PubMed] [Google Scholar]
- 19.Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997; 15: 2403–13. [DOI] [PubMed] [Google Scholar]
- 20.Burris HA 3rd. Recent updates on the role of chemotherapy in pancreatic cancer. Semin Oncol 2005; 32(Suppl. 6): S1–3. doi: 10.1053/j.seminoncol.2005.06.022 [DOI] [PubMed] [Google Scholar]
- 21.Yeo CJ, Cameron JL, Lillemoe KD, Sitzmann JV, Hruban RH, Goodman SN, et al. Pancreaticoduodenectomy for cancer of the head of the pancreas. 201 patients. Ann Surg 1995; 221: 721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boz G. Treatment of locally unresectable carcinoma of the pancreas: comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. J Natl Cancer Inst 1988; 80: 751–5. [PubMed] [Google Scholar]
- 23.Van Cutsem E, Van De Velde H, Karasek P, Oettle H, Vervenne W, Szawlowski A, et al. Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. J Clin Oncol 2004; 22: 1430–8. doi: 10.1200/JCO.2004.10.112 [DOI] [PubMed] [Google Scholar]
- 24.Halloran CM, Ghaneh P, Neoptolemos JP. Chemotherapy and radiotherapy for pancreatic and periampullary cancer: adjuvant, neoadjuvant, and palliative. In: Jarnagin WR, ed. Blumgart's surgery of the liver, pancreas and biliary tract. 5th edn. Philadelphia, PA: WB Saunders; 2012. pp. 972–8. [Google Scholar]
- 25.DeNittis AS, Stambaugh MD, Lang P, Wallner PE, Lustig RA, Dillman RO, et al. Complete remission of nonresectable pancreatic cancer after infusional colloidal phosphorus-32 brachytherapy, external beam radiation therapy, and 5-fluorouracil: a preliminary report. Am J Clin Oncol 1999; 22: 355–60. [DOI] [PubMed] [Google Scholar]
- 26.Jin Z, Du Y, Li Z, Jiang Y, Chen J, Liu Y. Endoscopic ultrasonography-guided interstitial implantation of iodine 125-seeds combined with chemotherapy in the treatment of unresectable pancreatic carcinoma: a prospective pilot study. Endoscopy 2008; 40: 314–20. [DOI] [PubMed] [Google Scholar]
- 27.Popescu CC, Wise J, Sowards K, Meigooni AS, Ibbott GS. Dosimetric characteristics of the Pharma Seed model BT-125-I source. Med Phys 2000; 27: 2174–81. [DOI] [PubMed] [Google Scholar]
- 28.Patel AV, Rodriguez C, Bernstein L, Chao A, Thun MJ, Calle EE. Obesity, recreational physical activity, and risk of pancreatic cancer in a large US Cohort. Cancer Epidemiol Biomarkers Prev 2005; 14: 459–66. doi: 10.1158/1055-9965.EPI-04-0583 [DOI] [PubMed] [Google Scholar]
- 29.Picot J, Cooper K, Bryant J, Clegg AJ. The clinical effectiveness and cost-effectiveness of bortezomib and thalidomide in combination regimens with an alkylating agent and a corticosteroid for the first-line treatment of multiple myeloma: a systematic review and economic evaluation. Health Technol Assess 2011; 15: 1–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mendlovic S, Symon Z, Kundel Y, Rabin T, Catane R, Pfeffer R. Three-dimensional conformal radiation therapy concurrent with full dose gemcitabine for locally advanced inoperable pancreatic cancer. [In Hebrew.] Harefuah 2008; 147: 384–7. [PubMed] [Google Scholar]
- 31.Koong AC, Le QT, Ho A, Fong B, Fisher G, Cho C, et al. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int J Radiat Oncol 2004; 58: 1017–21. doi: 10.1016/j.ijrobp.2003.11.004 [DOI] [PubMed] [Google Scholar]
- 32.Montemaggi P, Dobelbower R, Crucitti F, Caracciolo F, Morganti AG, Smaniotto D, et al. Interstitial brachytherapy for pancreatic cancer: report of seven cases treated with 125I and a review of the literature. Int J Radiat Oncol 1991; 21: 451–7. [DOI] [PubMed] [Google Scholar]
- 33.Zhongmin W, Yu L, Fenju L, Kemin C, Gang H. Clinical efficacy of CT-guided iodine-125 seed implantation therapy in patients with advanced pancreatic cancer. Eur Radiol 2010; 20: 1786–91. doi: 10.1007/s00330-009-1703-0 [DOI] [PubMed] [Google Scholar]
- 34.Xu K-C, Niu L-Z, Hu Y-Z, He W-B, He Y-S, Li Y-F, et al. A pilot study on combination of cryosurgery and (125)iodine seed implantation for treatment of locally advanced pancreatic cancer. World J Gastroenterol 2008; 14: 1603. [DOI] [PMC free article] [PubMed] [Google Scholar]