Abstract
Objective:
This study evaluated and quantified the feasibility of submandibular gland (SMG) sparing in intensity-modulated radiotherapy (IMRT) for N0-stage nasopharyngeal carcinoma (NPC).
Methods:
Ten patients with N0-stage NPC were enrolled in the study. Four IMRT plans were produced for each, with different limiting conditions. In plan A, SMG sparing was ignored; in plans B, C and D, the mean dose to SMGs was restricted to 39 Gy. In addition, at least 95% of planning target volume (PTV)-IIa (PTV of clinical target volume involving level IIa lymph node) in plan C and 90% of PTV-IIa in plan D were required to have a 60 Gy covering.
Results:
The average mean dose to SMGs was 54.6 ± 3.6 Gy in plan A and was lower 39.3 ± 0.3, 49.3 ± 1.9 and 46.7 ± 2.8 Gy in plans B, C and D, respectively. The volume of PTV-IIa covered by 60 Gy was 98.9%, 81.6%, 95.2% and 90.8% in plans A, B, C and D, respectively, and showed a parallel association between dose reduction to SMGs and the covering deficit of PTV-IIa.
Conclusion:
Reducing the mean dose received by SMG to 39 Gy or less in IMRT for N0-stage NPC is feasible.
Xerostomia is the most prevalent sequela following radiotherapy of nasopharyngeal carcinoma (NPC),1 but can be reduced by parotid gland sparing using intensity-modulated radiotherapy (IMRT) techniques. In recent years, the submandibular gland (SMG) was found to play an important role in the secretion of saliva,2,3 contributing up to 90% of unstimulated salivary output as well as contributing to a patient's subjective sense of moisture. Therefore, sparing the SMGs from high-dose irradiation would be useful in reducing the symptoms of xerostomia.
SMGs are located inside the area of neck node level Ib and anterior to the level II region. Level II neck nodes are generally elected to receive prophylactic irradiation. It has been reported that with three-dimensional conformal radiotherapy for head and neck cancers,4 the SMGs would receive an unplanned dose of 62 Gy on average. Preservation of SMG function was reported for head and neck cancer treated with IMRT.5–7 However, data from our institution showed that the average unplanned dose received by SMGs was 58 Gy in IMRT of N0-stage NPC, although level I neck nodes were omitted for selected irradiations.
To our knowledge, the abovementioned studies focused only on the contralateral SMG (cSMG).5–7 However, is it possible to spare the bilateral SMGs in N0-stage NPC patients but what will be the subsequent trade-off of dose distribution to planning target volume 1 (PTV1) around the SMG area? This study was designed to address this question, and the results will be a valuable reference in planning the IMRT of NPC.
METHODS AND MATERIALS
Patient selection
Between May 2011 and May 2012, a total of 530 NPC patients underwent radiotherapy with IMRT in our department. Ten patients were randomly selected for this dosimetric study. There were four stage T2N0M0, four stage T3N0M0 and two stage T4N0M0 tumours, according to the American Joint Committee on Cancer staging system (7th edition).
Definition of target volumes and volumes of interest
The target volumes were delineated in accordance with the International Commission on Radiation Units and Measurements, Reports 83.8 MRI was required to provide a delineation reference for targets.
GTVnx refers to gross tumour volume of primary nasopharyngeal lesions, the clinical target volume 1 (CTV1) was defined as regions at higher risk of subclinical spread, including the neck nodes level II, level III and level Va. The lower neck lymph nodes were omitted for stage N0 patients at our institution. From our long-term results, the off-field lymph node recurrence rate was 1.4% only in N0-stage NPC without selected irradiation to the lower neck.9
To evaluate the relationship between SMG sparing and the dose coverage trade-off to CTV1 and PTV1, a subvolume of CTV1 involving the level IIa lymph node area was defined as CTV-IIa.
All the PTVs were expanded from CTVs with a margin of 5 mm cephalocaudally and 3 mm transversally, based on our own data for set-up error.
Treatment planning and optimization
A static IMRT technique with nine equiangular beams (from 0°) with 6 Mv X-ray was used. All plans were generated using the Pinnacle™ 8.0 treatment planning system (Philips, Fitchburg, WI) with a direct machine parameter optimization algorithm. The goal was to prescribe 70.4 Gy (2.2 Gy daily) to the PTV of GTVnx (PTVnx) and 60 Gy (1.8 Gy daily) to the PTV1 in 32 fractions. The constraint dose to critical organs was maximum point dose (Dmax) ≤45 Gy for the planning organ at risk volume (PRV)-spinal cord, Dmax ≤66 Gy for the PRV-stem, Dmax ≤54 Gy for the optic chiasma, Dmax ≤5 Gy for the lens, dose covering 50% of the organ (D50) ≤50 Gy for the temporal lobe and D50 ≤30–32 Gy for the parotid glands.
Four different IMRT plans were created for each patient, of which the characteristics can be found in Table 1. Plan A was the one already being used for treatment, and SMG sparing was ignored. Plans B, C and D were designed for the purpose of the study. Taking the result of Murdoch-Kinch et al6 as a reference, a limitation of Dmean ≤39 Gy was added to SMGs in plans B, C and D, but the dose to PTVnx and PTV1 took priority over the limitation to SMGs. In addition, at least 95% of PTV-IIa in plan C and 90% of PTV-IIa in plan D had to have a 60 Gy covering.
Table 1.
The characteristics of the different intensity-modulated radiotherapy plans
| Plan | Prescription goal |
Limitations to SMG (Gy) | ||
|---|---|---|---|---|
| PTVnx (Gy) | PTV1 (Gy) | PTV-IIa (Gy) | ||
| A | D95 ≥70.4 | D95 ≥60 | NR | NR |
| B | D95 ≥70.4 | D95 ≥60 | NR | Dmean ≤39 |
| C | D95 ≥70.4 | D95 ≥60 | D95 ≥60 | Dmean ≤39a |
| D | D95 ≥70.4 | D95 ≥60 | D90 ≥60 | Dmean ≤39a |
D90, dose covering 90% of the volume; D95, dose covering 95% of the volume; Dmean, mean dose; NR, not required; PTV, planning target volume; PTVnx, planning target volume of the gross tumour volume of primary nasopharyngeal tumours; SMG, submandibular gland.
The dose to PTVnx, PTV1 and PTV-IIa took priority over the limitation to SMG.
Non-parametric testing was used to compare the values of the four plans, and significance was set at p < 0.05. The test power was calculated using G*Power 3.1 (Faul, Erdfelder, Lang, & Buchner, 2007). It showed that for a sample size of ten per group, the power would be in excess of 90% for a paired two-tailed Wilcoxon signed-rank test with α set at 0.05.
RESULTS
General dose–volume specifications
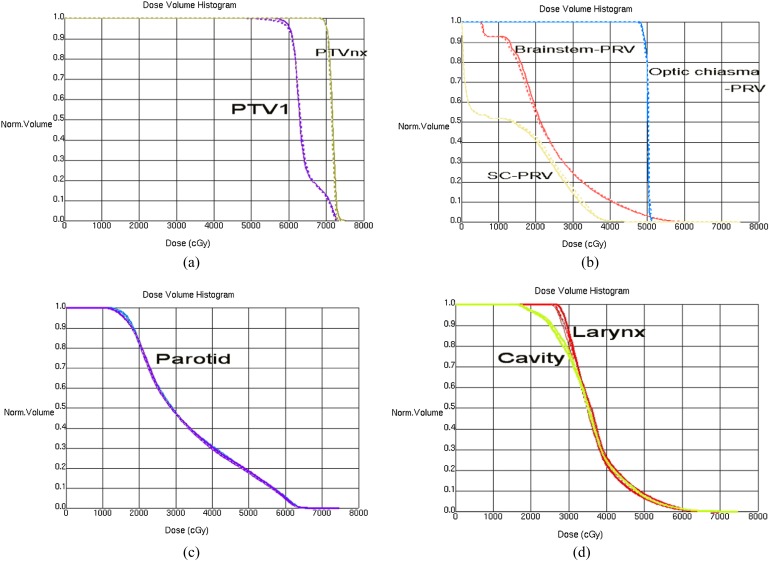
The dose–volume metrics and curves of PTVnx and PTV1 are shown in Table 2 and Figure 1. There was no difference between plans. A slight difference could be found between the dose–volume histogram (DVH) curves of PTV1 at the shoulder from Figure 1, which demonstrated the difference in the low-dose areas.
Table 2.
Dose–volume metrics of the planning target volume of the gross tumour volume of primary nasopharyngeal tumours (PTVnx) and planning target volume 1 (PTV1) in all plans
| Plans | PTVnx |
PTV1 |
||||
|---|---|---|---|---|---|---|
| Dmean (Gy) | Dmax (Gy) | Dmin (Gy) | Dmean (Gy) | Dmax (Gy) | Dmin (Gy) | |
| A | 71.8 ± 0.3 | 74.2 ± 0.3 | 69.5 ± 0.6 | 64.5 ± 0.5 | 74.1 ± 0.2 | 54.3 ± 3.6 |
| B | 71.8 ± 0.3 | 74.0 ± 0.3 | 69.5 ± 0.7 | 64.4 ± 0.5 | 74.0 ± 0.3 | 39.1 ± 3.4a |
| C | 71.9 ± 0.5 | 74.1 ± 0.4 | 69.3 ± 0.5 | 64.2 ± 0.3 | 74.1 ± 0.1 | 52.0 ± 2.7 |
| D | 71.8 ± 0.4 | 74.1 ± 0.2 | 69.5 ± 0.5 | 64.2 ± 0.4 | 74.0 ± 0.2 | 50.3 ± 2.7a |
Dmax, maximum point dose; Dmean, mean dose; Dmin, minimum point dose.
Dmin of plan B and D differed significantly from that of plan A (p < 0.01).
Data shown as ± standard deviation.
Figure 1.
Dose–volume histogram of planning target volumes (PTVs), organs at risk and normal structures. (a) PTV of the gross tumour volume of primary nasopharyngeal tumours (PTVnx) and PTV1; (b) brainstem-planning volume organ at risk (PRV), spinal cord (SC)-PRV and optic chiasma (c) parotid-right and parotid-left; (d) oral cavity and larynx. Norm, normal.
The DVH curves of all depicted organs at risk and normal structures, except SMGs, were almost the same in all plans and are presented in Figure 1.
Dose–volume parameters of submandibular glands
The dosimetric parameters of SMGs and the DVHs are shown in Table 3. The planned doses to SMG ranged from high to low in the order A, C, D and B. The mean dose and Dmin of plans B, C and D were significantly lower than those of plan A. Adding the dose limitation of dose covering 95% of the volume and dose covering 90% of the volume to PTV-IIa, the mean dose to SMGs could be reduced to 49.3 ± 1.9 and 46.7 ± 2.8 Gy in plans C and D, respectively.
Table 3.
Dosimetric parameters of submandibular glands in all plans
| Plans | Dmean (Gy) | Dmin (Gy) | Dmax (Gy) | p1 | p2 | p3 |
|---|---|---|---|---|---|---|
| A | 54.6 ± 3.6 | 36.9 ± 4.5 | 65.2 ± 1.5 | – | – | – |
| B | 39.3 ± 0.3 | 26.7 ± 5.2 | 61.8 ± 3.2 | <0.01 | <0.01 | 0.03 |
| C | 49.3 ± 1.9 | 33.6 ± 1.9 | 64.9 ± 1.3 | 0.02 | 0.03 | 0.89 |
| D | 46.7 ± 2.8 | 31.9 ± 1.9 | 65.0 ± 1.2 | <0.01 | 0.03 | 0.30 |
Dmax, maximum point dose; Dmean, mean dose; Dmin, minimum point dose; p1, Dmean compared to plan A; p2, Dmin compared to plan A; p3, Dmax compared to plan A.
Data shown as ± standard deviation.
Dose–volume specification of planning target volume IIa and clinical target volume IIa
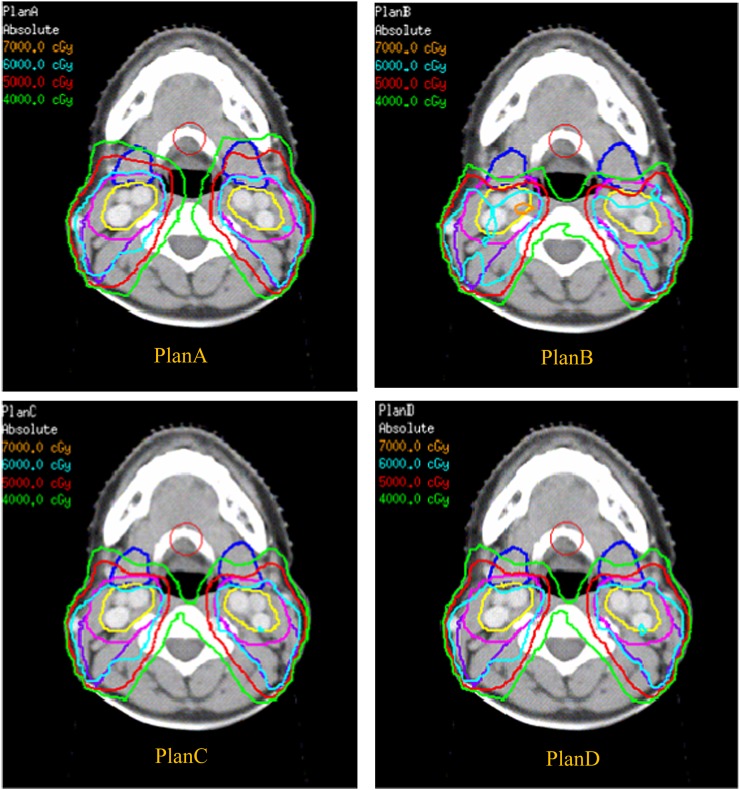
Figure 2 shows that the isodose curves shifted between SMG, PTV-IIa and CTV-IIa, corresponding to different combinations of dose constraint and prescription goal. The reduced dose in the SMGs correlated with dose reduction in the anterior parts of PTV-IIa and CTV-IIa. These trends are also shown quantitatively in Table 4 and Table 5. The Dmin to the PTV-IIa and CTV-IIa of plan B was obviously lower than in plan A (39.1 and 44.4 Gy, respectively). The percentages of the volume covered by 60 Gy to PTV-IIa and CTV-IIa were all >95% for plans A and C but were 81.6% and 88.2% for plan B, respectively, and 90.8% and 95.3% for plan D, respectively.
Figure 2.
Typical dose distributions of submandibular gland (SMG) for different intensity-modulated radiotherapy plans in one patient. The dose curves move backwards and generate a low-dose area in the level IIa region with the declining dose to SMGs. This trend is most obvious in plan B. The mean dose received by SMGs decreases from A to C, D and B in that order. The blue lines are the submandibular glands, the clinical target volume (CTV)-IIa is delineated in yellow and the planning target volume IIa is delineated in pink with a 3 mm margin from CTV-IIa. For colour images please see www.birpublications.org/doi/abs/10.1259/bjr.20130651
Table 4.
Dosimetric parameters of planning target volume-IIa in all plans
| Plans | Dmean (Gy) | Dmax (Gy) | Dmin (Gy) | V60 | p1 | p2 | p3 | p4 |
|---|---|---|---|---|---|---|---|---|
| A | 63.3 ± 0.4 | 67.9 ± 1.9 | 54.3 ± 3.6 | 98.9 | – | – | – | – |
| B | 62.2 ± 0.9 | 71.1 ± 3.6 | 39.1 ± 3.4 | 81.6 | 0.82 | 0.03 | <0.01 | <0.01 |
| C | 63.0 ± 0.4 | 68.0 ± 1.7 | 52.0 ± 2.7 | 95.2 | 0.97 | 0.24 | 0.55 | 0.03 |
| D | 63.0 ± 0.3 | 68.0 ± 1.8 | 50.3 ± 2.7 | 90.8 | 0.11 | 0.87 | 0.43 | <0.01 |
Dmax, maximum point dose; Dmean, mean dose; Dmin, minimum point dose; p1, Dmean compared to plan A; p2, Dmin compared to plan A; p3, Dmax compared to plan A; p4, V60 compared to plan A; V60, percentage of the volume covered by the dose of 60 Gy.
Data shown as ± standard deviation.
Table 5.
Dosimetric parameters of clinical target volume-IIa in all plans
| Plans | Dmean (Gy) | Dmax (Gy) | Dmin (Gy) | V60 | p1 | p2 | p3 | p4 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 63.7 ± 0.8 | 67.7 ± 1.9 | 59.9 ± 1.5 | 99.9 | – | – | – | – | ||||||||||||
| B | 63.5 ± 0.2 | 71.1 ± 3.6 | 44.4 ± 4.3 | 88.2 | 0.24 | 0.03 | 0.02 | <0.01 | ||||||||||||
| C | 63.7 ± 0.7 | 67.9 ± 1.7 | 57.5 ± 2.9 | 99.5 | 0.17 | 0.80 | 0.35 | 0.82 | ||||||||||||
| D | 63.7 ± 0.6 | 67.9 ± 1.8 | 56.6 ± 2.4 | 95.3 | 0.11 | 0.87 | 0.48 | 0.04 |
Dmax, maximum point dose; Dmean, mean dose; Dmin, minimum point dose; p1, Dmean compared to plan A; p2, Dmin compared to plan A; p3, Dmax compared to plan A; p4, V60 compared to plan A; V60, percentage of the volume covered by the dose of 60 Gy.
Data shown as ± standard deviation.
DISCUSSION
In recent years, SMGs have been found to play an important role in the secretion of saliva2,3 and to contribute to a patient's subjective sense of moisture. In addition to parotid sparing, protecting the SMGs during radiotherapy of NPC is useful.
The feasibility of SMG sparing during IMRT has been reported. However, the dose–volume effect of irradiation on SMG function is not conclusive. In the study of 18 patients with head and neck cancer by Saarilahti et al,5 salivary flow at 1 year following IMRT was apparently higher in those whom the mean SMG dose was restricted to 26 Gy. A mean dose (Dmean) of 39 Gy is the highest threshold dose reported to date.6 The salivary flow was significantly reduced when the mean dose to the SMG was >39 Gy. Therefore, in this study, we set the restricted mean dose to the SMG at 39 Gy.
A dilemma of SMG sparing in IMRT of NPC is the subsequent negative effect on dose coverage of the adjacent target volumes. It is a basic rule of physics that restricting the dose to the SMGs will also reduce the dose to the surrounding structures.
The level II region, located adjacent to the SMG, is at high risk of NPC spread10,11 and is usually defined as part of CTV1 in an IMRT beam prescription of 60 Gy. There was concern about whether SMG sparing would lead to a low dose at level II and increase regional failure.
All plans in this study met the goal that at least 95% of PTVnx and PTV1 received 100% of the prescribed dose (70 and 60 Gy, respectively). However, when looking at the dose distribution slice by slice, we found a variation of dose coverage at neck node level II, which was represented by PTV-IIa and CTV-IIa in this study.
Plan A, the one without constraint for SMGs had the best PTV-IIa dose coverage and the highest SMG dose of 54.6 ± 3.6 Gy (percentage of the volume covered by the dose of 60 Gy of PTV-IIa >95%). By contrast, plan B reduced the mean dose to SMGs to 39.3 ± 0.3 Gy when a constraint of Dmean <39 Gy was added to the SMGs. However, the V60 of PTV-IIa also decreased to 81.6%. The dose to the SMGs in plans C and D ranged between those of plans A and B, with the V60 of PTV-IIa reduced from 95% to 90%. The Dmean of SMGs decreased from 49.3 ± 1.9 to 46.7 ± 2.8 Gy accordingly. The above results demonstrate the trade-off between SMG sparing and PTV-IIa dose coverage. Houweling et al7 reported a similar result in patients with oropharyngeal cancer. When the dose coverage of the adjacent target volume was reduced from 95% to 90% of the prescribed dose, the mean dose to cSMG could be limited to 40 Gy. Therefore, PTV-IIa should be used as an extra dose limitation and evaluation of the low-dose area at node level II during SMG sparing.
In contrast to PTV-IIa, the CTV-IIa had lower deficiency of dosimetric coverage owing to SMG sparing. Only in plan B, with the strictest SMG dose limitation of Dmean of 39 Gy, <95% of CTV-IIa was covered by the prescribed dose of 60 Gy, and the V60 was 88.2% instead. This means that the deficiency dose coverage of level II lymph nodes induced by SMG sparing was isolated to the front edge of level II in millimetres. Such a minor effect on level II coverage might be clinically meaningless. From our institution's historical data, the regional recurrence was as low as 3% for N0-stage NPC that received cervical irradiation with 50 Gy.12 For other head and neck cancers, Saarilahti et al5 suggested that cSMG sparing was safe and not associated with locoregional recurrence of cancer within the spared volume; however, the study was not randomized. Therefore, plan B is better for SMG sparing and should be used in the clinical practice for N0-stage NPC, but, it needs to be elucidated in the scenarios with positive cervical diseases.
In summary, for IMRT of N0-stage NPC, limiting the mean dose of SMG to 39 Gy should be feasible.
REFERENCES
- 1.Anand AK, Jain J, Negi PS, Chaudhoory AR, Sinha SN, Choudhury PS, et al. Can dose reduction to one parotid gland prevent xerostomia?—a feasibility study for locally advanced head and neck cancer patients treated with intensity-modulated radiotherapy. Clin Oncol R Coll Radiol 2006; 18: 497–504. [DOI] [PubMed] [Google Scholar]
- 2.Jellema AP, Doornaert P, Slotman BJ, Leemans CR, Langendijk JA. Does radiation dose to the salivary glands and oral cavity predict patient-rated xerostomia and sticky saliva in head and neck cancer patients treated with curative radiotherapy? Radiother Oncol 2005; 77: 164–71. [DOI] [PubMed] [Google Scholar]
- 3.Cheng SC, Wu VW, Kwong DL, Ying MT. Assessment of post-radiotherapy salivary glands. Br J Radiol 2011; 84: 393–402. doi: 10.1259/bjr/66754762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang ZH, Yan C, Zhang ZY, Zhang CP, Hu HS, Kirwan J, et al. Radiation-induced volume changes in parotid and submandibular glands in patients with head and neck cancer receiving postoperative radiotherapy: a longitudinal study. Laryngoscope 2009; 119: 1966–74. [DOI] [PubMed] [Google Scholar]
- 5.Saarilahti K, Kouri M, Collan J, Kangasmäki A, Atula T, Joensuu H, et al. Sparing of the submandibular glands by intensity modulated radiotherapy in the treatment of head and neck cancer. Radiother Oncol 2006; 78: 270–5. [DOI] [PubMed] [Google Scholar]
- 6.Murdoch-Kinch CA, Kim HM, Vineberg KA, Ship JA, Eisbruch A. Dose–effect relationships for the submandibular glands and implications for their sparing by intensity modulated radiotherapy. Int J Radiat Oncol Biol Phys 2008; 72: 373–82. doi: 10.1016/j.ijrobp.2007.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Houweling AC, Dijkema T, Roesink JM, Terhaard CH, Raaijmakers CP. Sparing the contralateral submandibular gland in oropharyngeal cancer patients: a planning study. Radiother Oncol 2008; 89: 64–70. doi: 10.1016/j.radonc.2008.04.008 [DOI] [PubMed] [Google Scholar]
- 8.The International Commission on Radiation Units and Measurements. Report 83: prescribing, recording, and reporting photon-beam intensity-modulated radiation therapy (IMRT). J ICRU 2010; 10: 1–106. doi: 10.1093/jicru/ndq001 [DOI] [Google Scholar]
- 9.Chen JZ, Le QT, Han F, Lu LX, Huang SM, Lin CG, et al. Results of a Phase 2 study examining the effects of omitting elective neck irradiation to nodal levels IV and Vb in patients with N (0–1) nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2013; 85: 929–34. doi: 10.1016/j.ijrobp.2012.07.2356 [DOI] [PubMed] [Google Scholar]
- 10.King AD, Ahuja AT, Leung SF, Lam WW, Teo P, Chan YL, et al. Neck node metastases from nasopharyngeal carcinoma: MRI of patterns of disease. Head Neck 2000; 22: 275–81. [DOI] [PubMed] [Google Scholar]
- 11.Liu LZ, Zhang GY, Xie CM, Liu XW, Cui CY, Li L. Magnetic resonance imaging of retropharyngeal lymph node metastasis in nasopharyngeal carcinoma: patterns of spread. Int J Radiat Oncol Biol Phys 2006; 66: 721–30. doi: 10.1016/j.ijrobp.2006.05.054 [DOI] [PubMed] [Google Scholar]
- 12.Chen CZ, Li DR, Chen ZJ, Li DS, Guo LJ, Guo H. Long-term efficacy of prophylactic upper neck irradiation for stage N0 nasopharyngeal carcinoma. [In Chinese.] Ai Zheng 2008; 27: 295–8. [PubMed] [Google Scholar]