Abstract
Rationale
Transendocardial Stem Cell Injection (TESI) with mesenchymal stem cells improves remodeling in chronic ischemic cardiomyopathy, but the impact of the injection site remains unknown.
Objective
To address whether TESI exerts its effects at the site of injection only or also in remote areas, we hypothesized that segmental myocardial scar and segmental ejection fraction improve to a greater extent in injected than in non-injected segments.
Methods and Results
Biplane ventriculographic and endocardial tracings were recorded. TESI was guided to 10 sites in infarct-border zones. Sites were mapped according to the 17-myocardial segment model. As a result, 510 segments were analyzed in 30 patients before and 13-months after TESI. Segmental early enhancement defect (SEED, a measure of scar size) was reduced by TESI in both injected (−43.7±4.4%, n=95, p<0.01) and non-injected segments (−25.1±7.8%, n=148, p<0.001; between group comparison p<0.05). Conversely, segmental ejection fraction (SEF, a measure of contractility) improved in injected scar segments (19.9±3.3 to 26.3±3.5%, p=0.003) but not in non-injected scar segments (21.3±2.6 to 23.5±3.2%, p=0.20, between group comparison p<0.05). In the subgroup of scar segments with baseline SEF<20%, the SEF improvement was even greater in injected segments (12.1±1.2% to 19.9±2.7%, n=18, p=0.003) vs. non-injected segments (13.3±1.3% to 16.1±2.1%, n=15, p=0.05; between group comparison p<0.05).
Conclusions
These findings illustrate a dichotomy in regional responses to TESI. Although scar reduction was evident at the site of TESI and remotely, ventricular functional responses occurred preferentially at the sites of TESI. Furthermore, improvement was greatest when segmental left ventricular dysfunction was severe.
Keywords: Myocardial infarction, imaging, tomography, cells
INTRODUCTION
Although there are accumulating preclinical1, 2 and clinical trial3, 4 data supporting the use of transendocardial stem cell injection (TESI)5-9 to produce reverse remodeling in chronic heart failure, the impact of injection site is unknown. In the Percutaneous Stem Cell Injection Delivery Effects on Neomyogenesis (POSEIDON) trial4, TESI with autologous or allogeneic mesenchymal stem cells (MSCs) delivered to 10 sites around the infarct border zone improved left ventricular (LV) structure and function globally and resulted in reduced scar size, reduced LV volumes, and restored LV sphericity index toward normal4. The mechanistic basis underlying the myocardial regenerative effect(s) of MSCs include both direct2, 10, 11 and paracrine actions8, 12. Whether these actions are exerted locally, at a distance, or globally, and the impact of precisely localizing cell injections remain unclear. The POSEIDON trial4 results indicated improved global ventricular structure and function, but regional effects might be obscured by conventional LV imaging analysis13.
Here we combined the imaging advantages of Multidetector computed tomography (MDCT) and biplane left ventriculography to perform a myocardial segmental analysis of the POSEIDON clinical trial so as to test the hypothesis that sites of cell injection respond more favorably in terms of cardiac repair than sites not receiving cell injections. We investigated whether injected myocardial segments have greater reduction of segmental early enhancement defect (SEED, an indicator of myocardial scar) and improved ventricular performance, measured by segmental ejection fraction (SEF, a measure of regional myocardial contraction) in comparison to infarcted non-injected myocardial segments. The findings of this study have important implications for implementing stem cell therapy delivered by transendocardial injection.
METHODS
A full description of the study protocol, inclusion and exclusion criteria has been published4. All patients provided written informed consent for the University of Miami Institutional Review Board–approved protocol; enrollment and exclusions are shown in Online Figure I. In summary, POSEIDON was a phase I/II randomized, open-label clinical trial, designed (1) to explore the safety of allogeneic MSCs and (2) to compare the long-term safety and efficacy of allogeneic MSCs with autologous MSCs in patients with chronic LV dysfunction secondary to myocardial infarction (Baseline characteristics shown in Online Table I). Our earlier publication4 reported clinical safety and efficacy. Here, we used the POSEIDON imaging data and the total population of POSEIDON, to explore mechanistic insights related to the site of the injection.
MDCT analysis
MDCT provides detailed and accurate information that is useful in the preparation of an injection strategy, and in follow-up examination permits quantifying the response to stem cell therapy14. Cineangiographic MDCT was used for reconstruction of images in 30 patients at screening and at 13-months after TESI for analysis of scar size, segmental ejection fraction and wall thickening. Acquisition of images was performed using 128 slice (Siemens AS+, Siemens Medical Solutions) or 320-slice (Aquillion One-Toshiba) CT scanning systems with a spatial resolution of 0.30mm and 350 microns respectively, and analyzed using iNtuition version 4.4.7.47 (TeraRecon, Inc., Foster City, Calif., USA) as described previously4.
We evaluated the regional response to MSCs by measuring myocardial SEED as an indicator of myocardial scar size and SEF as a measure of regional myocardial contractility. Within regards to scar size, delayed enhancement (DE) obtained with cardiac magnetic resonance (CMR) imaging is often used to measure scar size; however, MDCT derived DE and early enhacement defect (EED) has also been used in studies to quantify scar size4, 15-17. Schulelri et al.14 described cardiac MDCT-DE in a swine model and concluded that it can accurately quantify scar tissue for preclinical and clinical studies for novel myocardial therapies. Mahnken et al.17 compared DE-CMR, DE-MDCT, and EED-MDCT in 28 patients within 2 weeks after MI and reported strong correlation between DE-CMR and DE-MDCT. DE-CMR and EED-MDCT were tightly correlated, they found that EED-MDCT underestimates the size of MI (mean size of MI was 31.2g on DE-CMR, 33g on DE-MDCT, and 24.5g on EED-MDCT)17. Nevertheless, MDCT-derived SEED analysis, as described in our earlier publication4, was preferred as a marker of infarct size since it provided 1) discernible difference in densities between normal and abnormal (infarcted) myocardium, 2) better demarcation of the endocardial border of abnormal myocardium in comparison to DE-MDCT, and 3) better image quality and less susceptibility to beam hardening artifacts secondary to automatic implantable cardioverter-defibrillator leads, which were implanted in all but one of our patients.
For regional analysis of myocardial function, previous studies18, using electron beam computed tomography (EBCT), demonstrated that SEF can detect abnormalities and provide accurate identification of ischemic myocardium in patients with varying degrees of coronary artery occlusion. Similarly, by applying the principle of SEF to regenerative myocardial therapy, we have been able to analyze regional myocardial performance and scarring following TESI in patients with chronic ischemic cardiomyopathy (Online Figure II).
Myocardial infarct scar assessment: SEED
Assessment of myocardial SEED was previously described4, 15, 16. Briefly, contiguous 8 mm thick short-axis reconstructions were made for the end diastolic, end systolic, as well as mid diastolic (usually 70% of the R-R interval) cardiac phases encompassing the entire left ventricular myocardial volume. Normal reference CT based myocardial density was measured (Hounsfield units – HU) from regions of interest of approximately 10-15 mm2 in a myocardial segment that demonstrated normal regional function, i.e. normal myocardial wall motion and thickening. For optimal evaluation of myocardial SEED, the CT window level was adjusted so that the center of the scale corresponded to 20 HU below the normal myocardial density reference value17, 19. This approach was chosen to define a visual threshold of approximately 2 standard deviations below the mean of normal myocardium enhancement, allowing optimal delineation of hypoenhancement. A narrow window width was used (100–150 HU) to emphasize the threshold between normal and abnormal myocardium and still allow the surrounding anatomy to be visualized. SEEDs were identified by their crescentic shape, subendocardial and/or transmural location, and presence in at least two of the three examined cardiac phases. Total myocardial SEED mass per patient was calculated by summing all segmental defect areas and multiplying the total by section thickness (0.8 cm) and then by 1.05 g/cm3 (myocardial specific gravity)19. Each SEED identified in each short-axis section (4 slices at base, 4 slices at mid, and distal 3-4 slices at apical LV regions) was allocated into either six (base and mid LV regions) or four (apical LV region) myocardial segments according to the AHA 17-myocardial segment model20.
EF Analysis: Global EF analysis and SEF analysis
Cardiac volumes and global LVEF ((LV End Diastolic Volume - LV End Systolic Volume) * 100) / LV End Diastolic Volume) were calculated, and 17 segment polar maps were generated. SEF is an expression of regional function, reflecting the volume of blood ejected under a given myocardial segment, contributing to the global LVEF18. The left anterior descending (LAD) artery in the interventricular sulcus between the basal anterior (AHA seg #1) and the basal anteroseptal (AHA seg #2) segments in the short axis view was used as an anatomical landmark for segmentation. The basal edge of the LV was identified by the mitral valve annulus in 4 and 2 chamber views. Definition of endocardial borders of the LV chamber in end-systolic and end-diastolic phases were manually assessed with contour corrections, when necessary, following the software protocol recommendations.
For SEF calculation, the software assumes that each one of the 17-AHA segments is composed from numerous fan shaped micro-segments (Online Figure II). The volume of all micro-segments is obtained in end-diastole and end-systole while maintaining an automated-independent center of the LV in the endsystolic phase, so called “fixed axis”. The individual SEF value presented in the polar map for all 17-AHA segments represents the average SEF of all fan shaped micro-segments within the same particular segment ((Segmental LV End Diastolic Volume – Segmental LV End Systolic Volume)*100) / Segmental LV End Diastolic Volume)..
Wall thickening
Segmental wall thickening ( Systolic wall thickness- Diastolic wall thickness) was calculated following the 17-segments model for each patient.
Topographic analysis
We further evaluated the regional response to MSCs by conducting topographic analyses using MDCTEED images. To address whether there was a similar response (SEED and SEF) in transmural vs. nontransmural scars all scars were grouped based on baseline transmurality (>50% and <50%).
Strategy of cell delivery in the POSEIDON trial
Catheter left ventriculography guided the sequence of injections, and was compared with MDCT for procedural planning and selection of target sites. Biplane left ventriculography was performed in the 60° LAO and 30° RAO views5. MDCT global and regional cardiac function permitted for the assessment of myocardial viability and wall motion defects. Together these orthogonal projections describe the position and contractility of each myocardial segment. End-diastolic endocardial contour tracings were traced from these images; hypokinetic and akinetic segments were marked as infarct zone and the adjacent area to it was identified as the border zone. During TESI each site of injection was recorded onto these ventriculographic maps in two projections.
17-segment model reconstruction: Biplane left ventriculography- Endocardial tracings and use of the 17-segment model for recognition of areas injected
Injection sites were manually translated onto 17-segment polar maps by two independent cardiologists (JPZ and KN), who assessed if a segment received at least one injection. Overall agreement between the two readers resulted in a Kappa coefficient of 0.8896 (95% CI, 0.8447-0.9346; p<0.0001). Disagreement was resolved by a third reader (VK). All readers were blinded to the SEED and SEF values. Each injection site was allocated to one of the 17 segments (Figure 1).
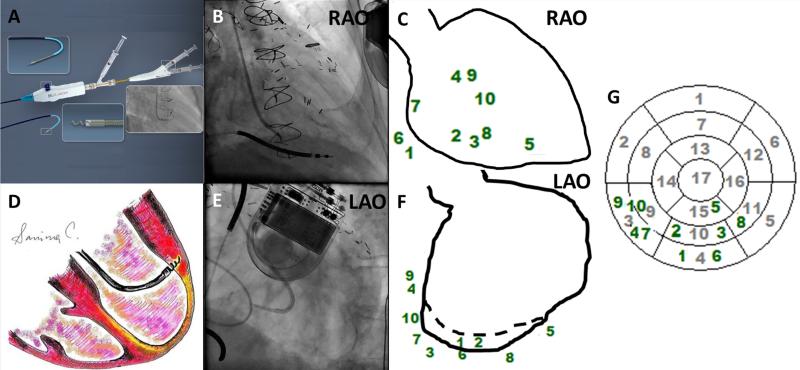
Figure 1. Allocation of 10 injections using biplane ventriculography during Transendocardial Stem Cell Injection.
(Panels A and D) Biocardia helical infusion catheter and schematic depiction of an intraendocardial injection, respectively. (Panels B and E) Biplane fluoroscopy was used for navigation. (Panels C and F) Every injection site was marked in two projections using ventriculographic diastolic contours and translated onto a 17-segment polar map (Panel G) to identify all segments that received intramyocardial injections of MSCs.
Definition of subgroups of myocardial segments for analysis
For segmental analysis, myocardial segments were categorized as follows; “Scar- injected” included all myocardial segments with SEED treated with TESI. “Scar-non-injected” included all myocardial segments with SEED not treated with TESI. “Non-scar-injected” included all myocardial segments without SEED treated with TESI. And “non-scar-non-injected” included all myocardial segments without SEED not treated with TESI. Additionaly, for radius of activity analysis purposes, the segments around and in the opposite side of the “scar-injected” were categorized as “adjacent” and “remote” myocardial segments respectively. Within each patient, we compared the accumulated mass value of all SEED treated and non treated with TESI before and after TESI. Finally, to elucidate the changes in each subgroup of segments, we correlated the polar maps obtained from MDCT with those marking injection sites to perform a myocardial segmental analysis of the POSEIDON clinical trial.
Statistical analysis
For analysis purposes, all 30 patients who received either autologous or allogeneic MSCs were assessed. All data are presented as mean ± standard error of mean. GraphPad Prism (Version 4.03, La Jolla, CA) was used to analyze all data points. To compare the change in SEF and SEED at 13-month follow-up, paired t-tests were applied. For between group comparisons, one and two way ANOVA were applied with a Bonferroni/Dunn's Multiple comparison test when applicable. A p-value of less than 0.05 was considered statistically significant.
RESULTS
Baseline scar characteristics
The baseline scar characteristics in the “scar-injected” and “scar-non-injected” groups were not significantly different with respect to scar size or thickness of segment with scar; 83.3% (n=25) and 96.7% (n=29) of myocardial segments were transmural in the “scar-injected” and “scar-non-injected,” respectively (Online Table II).
Greater scar size reduction at sites of stem cell injection
Similar scar size reduction was observed in the “scar-injected” segments with autologous (from 10.2±1.8g to 5.5±0.8g, n=15, p=0.001) or allogeneic MSCs (from 9.6±1.7g to 5.3±1.1g, n=15, p=0.001). The “scar-non-injected” segments had similar reduction in both groups (from 13.2±2.5g to 9.5±1.7g, p<0.01 for the autologous and from 10.1±1.5g to 7.3±1.2g p=0.002 for the allogeneic group). When considering the autologous and allogeneic groups combined, the scar size was reduced by −43.7±4.4% (from 9.8±1.2g to 5.4±0.7g, n=30, p<0.01) in the “scar-injected” vs by −25.1±7.8% in the “scar-non-injected” segments (from 11.7±1.4g to 8.6±1.0g, n=30, p<0.001; between group comparison “scar-injected” vs. “scar-non-injected” p<0.05; Figures 2, 3, and Online Video II). When considering cell type and cell dose, the greatest reduction was found in the injected segments that received 20 million autologous MSCs (from 7.1±1.4g to 3.7±0.8g p=0.02, between group comparison p<0.01, Online figure III).
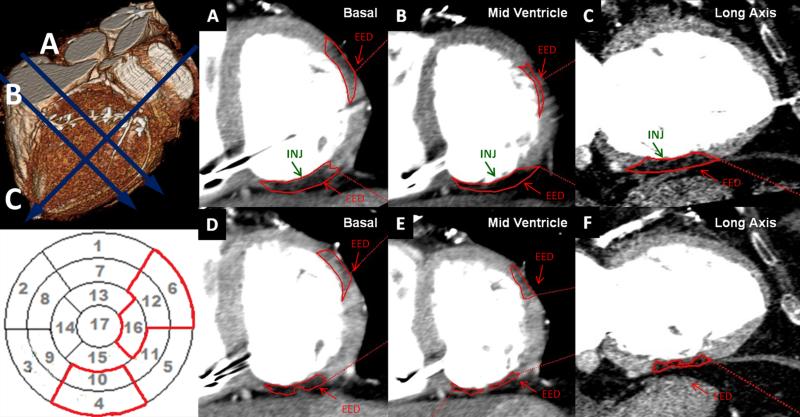
Figure 2. Greater reduction in scar injected segments.
(Upper left Panel) 3D reconstruction at baseline and MDCT images with 20HU below the normal myocardium density at (Panel A) basal short-axis, (Panel B) mid-ventricle short-axis and (Panel C) long-axis representing EED areas delineated in red. (Lower left Panel) Polar map reconstruction with EED delineated in red. Scar mass (EED) at baseline (Panels A-C) has a greater reduction in the injected segments (marked by INJ) located in inferior segments at basal, mid-ventricle and long-axis, from 5.9g to 1.4g compared to (Panels D-F) non-injected lateral segments at basal, mid-ventricle and long-axis images, from 4.6g to 1.7g thirteen months after MSCs.
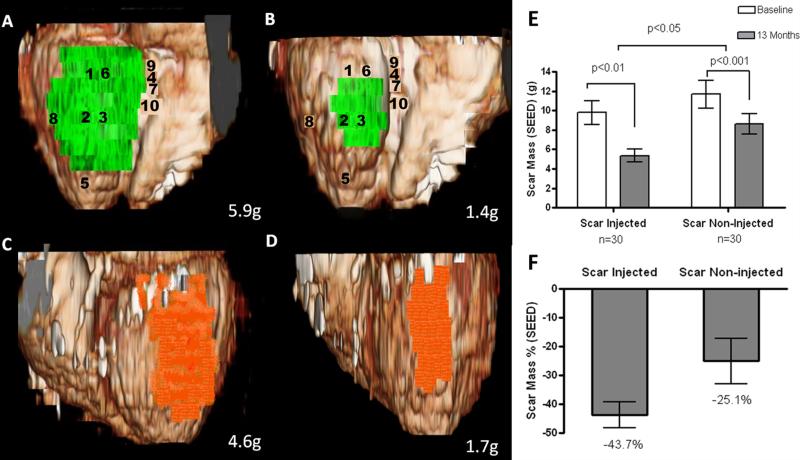
Figure 3. Volume rendered 3D reformats of left ventricle with color encoding of scar tissue.
(Panels A and B) Scar mass (green) of inferior segments treated by TESI at baseline and 13-months post TESI, respectively (numbers represent sites of injection). (Panels C and D) Scar mass (orange) of lateral segments not treated by TESI at baseline and at 13-month follow up, respectively. Actual scar mass (grams) is depicted in the lower right corner of each panel. 3D-recontructions in this figure correspond to SEED measurements of the same patient as in Figure 2 and Online Video I. (Panels E and F) Absolute values and %changes of scar mass obtained by segmental imaging analysis approach. When considering the autologous and allogeneic groups combined, there is greater scar size reduction in the “scar-injected” segments (−43.7±4.4%, from 9.8±1.2 to 5.4±0.7g, n=30, p<0.01), compared to the “scar-non-injected” segments (−25.1±7.8%, from 11.7±1.4 to 8.6±1.0g, n=30, p<0.001; between group comparison “scar-injected” vs. “scar-non-injected” p<0.05).
Segmental EF improves in injected segments, but is unchanged in non-injected segments
A total of 510 myocardial segments were analyzed in a total of 30 patients. Improvement in myocardial function was observed in “scar-injected” segments in both the autologous (from 19.0±3.0% to 25.25±3.2%, n=15, p=0.03) and allogeneic groups (from 20.9±3.7% to 27.22±3.9%, n=15, p=0.02; between group comparison p=NS). When considering the autologous and allogeneic groups combined, 95 myocardial “scar-injected” segments were evaluated in a total of 30 patients; SEF improved by +44.3%±11.2% (from 19.9±3.3% at screening to 26.3±3.5%) at 13-months after TESI (p=0.003). Conversely, SEF did not significantly improve when 148 “scar-non-injected” segments were evaluated alone or combined. The “non-scar-injected” (n=63) and “non-scar-non-injected” (n=204 ) myocardial segments did not demonstrate changes in SEF at 13-months (Figure 4). There was no difference between groups when considering cell type and cell dose. Wall thickening significantly improved in the “scar-injected” segments (1.6±0.34mm at baseline to 2.2±0.38mm at 13-months, n=30, p=0.02) but not in the “non-injected segments”(1.5±0.24mm at baseline to 2.0±0.37mm at 13-months, n=30, p=0.2), between groups p>0.05. In addition, the Pearson correlation coefficient between the number of “scar-injected” segments and the improvement of myocardial SEF was r=0.57 (95% CI, 0.23 to 0.78; p=0.002; Figure 6). There was no difference between groups when considering cell type and cell dose.
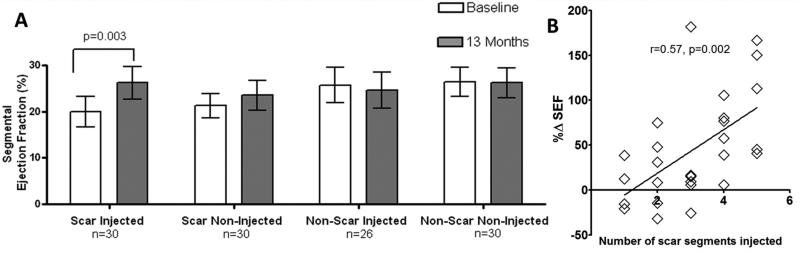
Figure 4. Restoration in contractility (SEF) and association between number of injected segments vs. SEF.
(Panel A) Greater improvement in SEF was observed in patients with more scar (SEED) injected segments, when considering the autologous and allogeneic groups combined, “scar-injected” segments SEF improved from 19.9±3.3% to 26.3±3.5% at 13-months after TESI (p=0.003). Conversely, SEF did not significantly improve when “scar-non-injected” segments were evaluated. The “non-scar-injected” and “non-scar-non-injected”segments did not demonstrate changes in SEF at 13-months. (Panel B) Pearson correlation coefficient r=0.57, 95% CI, 0.23 to 0.78; two-tailed p value=0.002.
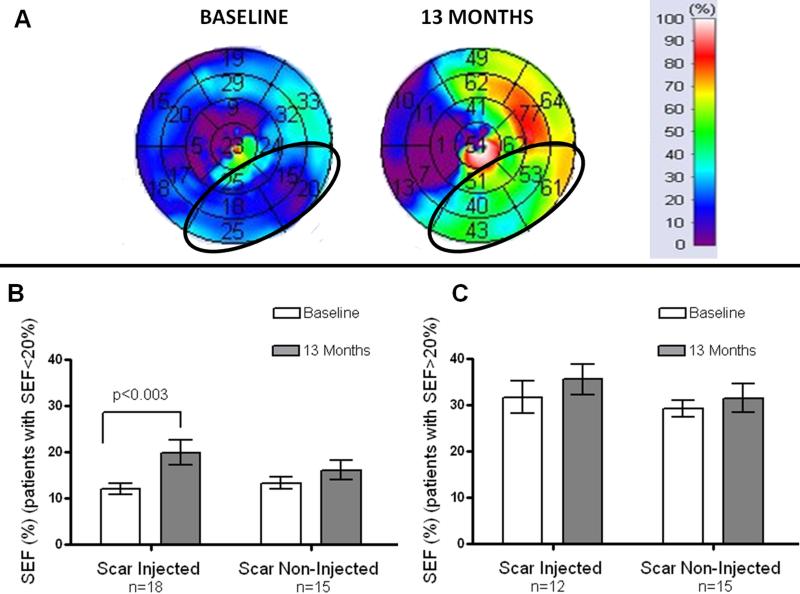
Figure 6. Improvement in contractility is particularly evident in the highly dysfunctional segments.
(Panel A) SEF<20%, scar-injected segments (baseline) which are encircled with an ellipse in the 17-segment polar map with their corresponding improvement after injection of mesenchymal stem cells. (Panel B) Represents the changes in SEF with highly dysfunctional segments (SEF<20%), the “scar- injected” SEF was 12.1±1.23% at baseline and improved to 19.9±2.69% (p=0.003) at 13-months after TESI (n=18 patients). In this subgroup analysis, the “scar-non-injected” SEF (n=15 patients) showed a trend of increase from 13.3±1.3% to 16.1±2.13%, (p=0.05).(Panel C) Shows changes in segments with SEF>20%.
Radius of activity as measured by SEF
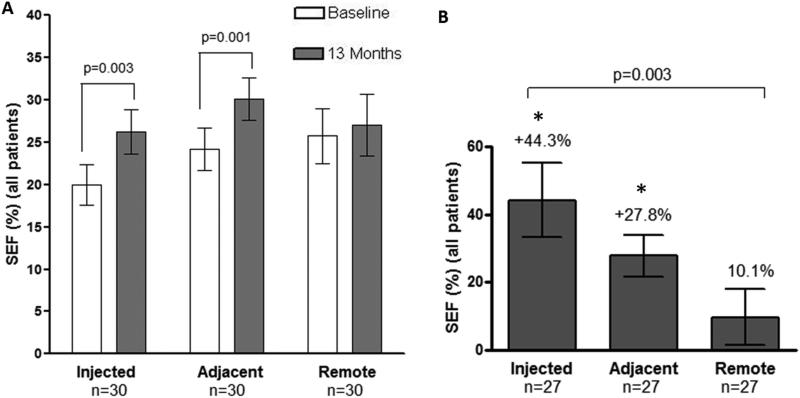
From our earlier analysis, SEF improved by +44.3±11.2% (from 19.9±3.3% at screening to 26.3±3.5%) at 13-months after TESI (p=0.003) in all “scar-injected” segments. To analyze the radius of activity, segments adjacent to and remore from “scar-injected” segments were compared. We observed that SEF improved in “adjacent” segments in both the autologous (from 18.9±1.7% to 26.0±2.8%, n=15, p=0.005) and allogeneic groups (from 29.4±4.3% to 34.0±4.0%, n=15, p=0.02; between group comparison p>0.05). When autologous and allogeneic groups were combined, SEF % change from baseline improvement in “adjacent” segments was +27.8±6.4% (from 24.2±2.6% to 30.1±2.6%, n=30, p=0.001). On the other hand, the “remote” segments did not demonstrate changes in contractility when considering cell type and cell dose, nor when combining autologous and allogeneic groups (% change in SEF from baseline was 10.1±8.9% from 25.8±3.2% to 27.0±3.6%, n=30, p=0.4). Importantly, SEF in “scar-injected” and “adjacent” segments improved more than “remote” segments (one way analysis of variance p=0.003. Dunn's post test p<0.05 in “scar-injected” vs. “remote” and “adjacent” vs. “remote”; Figure 5)
Figure 5. Radius of Activity for Transendocardial Stem Cell Injections (TESI) with Mesenchymal Stem Cells in Segmental Ejection Fraction (SEF%).
(Panel A) Improvement in contractility was observed in “scar-injected” (from 19.9±3.3% at screening to 26.3±3.5% at 13-month after TESI (p=0.003), and “adjacent” segments (from 24.2±2.6% to 30.1±2.6%, n=30, p=0.001). Conversely, the “remote” segments did not have changes in contractility (15.8 ±12.4% from 25.8±3.3% to 27.1±3.7%, n=30, p=0.4); between group comparison p>0.05). (Panel B) Percent changes from baseline in SEF showed changes by +44.3%±11.2% in “scar-injected”, by +27.8±6.4% in “adjacent” and by 10.1±8.9% in the “remote” segments (one way analysis of variance p=0.003. Dunn's posttest *p<0.05 in “scar-injected” vs. “remote” and “adjacent” vs. “remote”).
Improvement in contractility is particularly evident in the highly dysfunctional segments
In a subgroup analysis of all segments with a SEF<20%, the mean SEF in “scar injected” myocardial segments was 12.1±1.23% at baseline and improved to 19.9±2.69% (p=0.003) at 13-months after TESI (n=18 patients). In this subgroup analysis, the “scar non-injected” myocardial segments (n=15 patients) showed a trend of increase SEF from 13.3±1.3% to 16.1±2.13%, (p=0.05; Figure 7 and Online Video II)
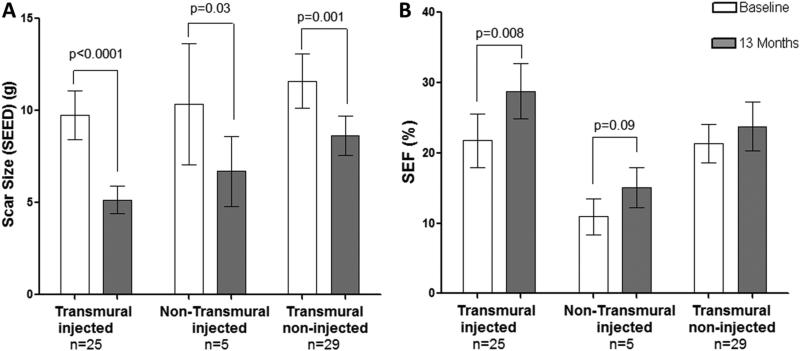
Figure 7. Dual effects of MSCs, global scar size reduction but restoration of contractility only in scar-injected segments, regardless of transmurality.
(Panel A) Similar magnitude of change in SEED of the “scar-injected” and “non-injected” with a transmural extent of >50% (from 9.8±1.3 to 5.2±0.7g, n=25, p<0.0001), and <50% (from 10.4±3.3 to 6.7±5.8g, n= 5, p=0.03). (Panel B) Similar magnitude of change in SEF only in the “scar-injected” with a transmural extent of >50% (from 21.8±3.8% to 28.8±4.0%, n=25, p=0.008) and <50% (from 11.0±2.6 to 15.1±2.9, n=5, p=0.09).
Similar magnitude of change of the “scar-injected and non-injected” with a transmural extent of >50% and <50%
“Scar-injected” and “Scar non-injected” were grouped based on baseline transmurality of >50% and <50%. For the “scar-injected”, there was a similar magnitude of change in SEED and SEF regardless of the transmurality (between group comparison p>0.05 for all analyses): SEED decreased for the “scar-injected” segments with a transmural extent of >50% (from 9.8±1.3g to 5.2±0.7, n=25, p<0.0001), and transmural extent of <50% (from 10.4±3.3 to 6.7±5.8, n= 5, p=0.03). For SEF, the “scar-injected” segments SEF with a transmural extent of >50% increased from 21.8±3.8% to 28.8±4.0%, n=25, p=0.008), and showed a trend of improvement in segments with a transmural extent of <50% (from 11.0±2.6 to 15.1±2.9, n=5, p=0.09; Figure 7).
Transmural infarct size reduces also in “scar non-injected” segments
For “scar non-injected” in patients treated with MSCs, we observed that the scar with a baseline transmurality of >50% had a similar magnitude of change in SEED with the “scar-injected” segments. SEED decreased from 11.6±1.5g to 8.6±1.1, n=29, p=0.001. For SEF, the “scar non-injected” with a transmural extent of >50% did not show any change (from 21.3±2.8 to 23.8±3.5, n=29, p>0.05) (Figure 7).
DISCUSSION
The current study has three new major findings: First, the actual site of injection of MSCs plays a fundamental role in the response to cell therapy for chronic ischemic heart disease. Injected sites respond with a reduction in scar tissue and improvement in wall thickening accompanied by functional recovery. Second, while remote segments also exhibit reduction in scar, these segments do not exhibit comparable improvement in myocardial contractility. Finally, non-scarred segments do not improve functional contraction even when cell injections are delivered. These three major findings were similar when considering transmural vs. non transmural injected scarred segments. Together these findings offer both mechanistic and practical insights regarding this new and potentially important form of therapy for chronic heart disease.
These findings can best be viewed in the context of mechanistic findings from preclinal models2, 10, 11, 21 and clinical trials 3, 4, 22-25 and are in agreement with both the direct and indirect mechanisms that underly these MSC actions26. Directly, MSCs are known to engraft and differentiate preferentially in the infarct / border zone 2, 10, 11 producing both reduction of scar size and contractile restoration2, 11, 27. In addition, MSCs can release paracrine factors 8, 12, 28 which can have local and remote effects; for example the release of matrix metaloproteases8, 12, 28 can contribute to scar size reduction, an effect that could occur remotely. Thus, the improvement in ventricular architecture is driven by a global reduction in scar size which is accompanied by improved contraction that occur preferentially in the injected myocardial segments. These data show that the site of injection matters and that MSCs generate scar size reduction and functional restoration regardless of the transmurality.
The direct and indirect mechanisms underlying these MSC actions have been previously explored in a robust and representative preclinical swine model of ischemic cardiomyopathy29, 30. Using this model, our group and others2, 10, 31 demonstrated that MSCs engraft into chronically scarred myocardium11, undergo trilineage differentiation10, 11, promote neovascularization21, 31, 32, and enhance the proliferation and differentiation of endogenous cardiac stem cells33 in the infarct territory,10, 21 especially at the interface between scar tissue and bordering viable myocardium2, 10, 11. As a result MSCs lead to reduction of scar size, and to improvement in LVEF34. Moreover, direct hemodynamic assessments of contractility, lusitropy, and myocardial energetics have shown global ventricular improvement including preload recruitable stroke work (PRSW), an integrated measure of hemodynamic studies27.
Another clinical study of intramyocardial MSC therapy that used cardiac magnetic resonance (CMR) analysis, also demonstrated a relationship between regional restoration of myocardial contractility, assessed as peak Eulerian circumferential strain (Ecc)8, and reverse ventricular remodeling. In that study, improved Ecc in the scar border zone occurred at 3-months after cell injection and correlated with a reduction in ventricular volumes at 12-months after cell therapy. The results of the Cardiopoietic stem Cell Therapy in heart failure (C-CURE) study also showed that MSCs targeted to viable but dysfunctional LV segments resulted in reduction in scar size and increased LVEF in chronic ischemic cardiomyopathy patients treated with autologous cardiopoietic MSCs22.
The imaging approach used in the POSEIDON study provided the means to test the differential effects of local vs. remote (paracrine) effects on function and scar size. In the POSEIDON trial not all scarred myocardial segments were injected allowing a comparison of TESI-treated and non treated myocardial segments. Indeed, we found a divergent response regarding functional restoration and scar reduction. While myocardial scar segments treated with TESI showed a greater reduction in scar size than those not treated with TESI, the latter also had a significant reduction in scar size. However, only TESI-treated myocardial scar segments exhibited a significant improvement in SEF, indicating that direct cell effects preferentially augment functional restoration. Furthermore, treated non-scarred myocardial segments did not show changes in contractility, deriving that the changes happening in the infarcted myocardium favor the mechanisms underlying MSCs therapy. These data also demonstrate that there is a radius of activity for these phenotypic responses: MSCs lead to scar size reduction and restoration in SEF also in “adjacent” myocardial segments (around “scar-injected” sites), while remote segments only showed reduction in scar size. Collectively, these findings demonstrate a dichotomy in the mechanisms driving the functional (local) and reverse remodeling (local and remote) responses to MSC therapy. Clinically, this suggests that new therapeutic strategies for TESI should be anatomically personalized, with an individualized patient-specific approach based not only on location and infarct size but also number of injections, dose, and concentration of cells.
In the POSEIDON study4, a paradoxical response to the cell dose was found. When comparing 20 vs. 100 vs. 200 million MSCs, the greatest benefit in total scar size reduction was seen in the group that received 20 million cells. Similar observation was seen in the current results, with a greater reduction of scar size in the scar-injected segments receiving 20M of MSCs. Among all the groups, 20M of autologous MSCs showed a greater reduction of scar size which is consistent. Variables which may affect the response to treatment thus include cell number (total dose), cell concentration and the number and distribution of injections. The small sample size of our study limits the possibility of making definitive conclusions but brings the basis for better strategies in stem cell thjerapy. Optimization of the response to TESI is likely to include a number and pattern of injections specific to a given infarction. In this segmental analysis, a linear positive correlation was noted between the number of myocardial segments with scar treated by TESI and the improvement of SEF. A higher number of myocardial segments with scar treated by TESI could promote a greater improvement; nonetheless, other factors also need to be explored. In the SCIPIO clinical trial23, 35, patients received cardiac stem cells by intracoronary infusion; both the infusion site and the cell dose were customized according to the infarct location and size. Patients who were followed up with CMR were analyzed for regional EF. The SEF showed improvement to 24.5% at 4-months and to 28.2% at 12-months in the infarct-related segments. This improvement was even greater when the dysfunction was severe. Similarly, we observed improvement in contractility in the infarcted segments, and that the lower the SEF at baseline the greater the improvement at 13-months after MSC therapy. In light of the above, we can argue that indivualized tailoring including the number of stem cell injection sites and the total cell dose may offer an optimal strategy compared with other fixed approaches which do not account for different locations and extent and severity of the infarction.
Together these findings support the biological activity of mesenchymal stem cell therapy to reverse pathologic remodeling through a process driven by infarct size reduction. These changes are associated with improvement in myocardial contraction that is preferentially found in the scar tissue zones. These data suggests the dualilty of the functional benefits of MSCs, involving both direct local and also diffusible, potentially paracrine mechanisms. What remains to be determined is whether the local effect of the injected MSCs is specific for structural and/or functional repair and whether it is more effective or even more enduring than the paracrine effects. Moreover, it is unknown whether the local cellular and paracrine effects happen in synchrony or even in synergy.
Conclusions
The study of segmental myocardial dynamics can expand the current understanding of local changes that guide cardiac remodeling following TESI. This study revealed that significant improvement in SEF occurred in myocardial scar segments treated with TESI but not in myocardial scar segments without TESI, whereas scar size reduction was evident in TESI treated and non-treated segments. We also demonstrated that myocardial segments with severe regional dysfunction, SEF<20%, showed even more significant improvement in SEF, suggesting that more impaired myocardial segments have better response to TESI. Collectively, these findings suggest that local and paracrine factors differentially influence functional restoration and reverse remodeling. Although this study does not answer the mechanistic issues underlying the functional and structural responses to MSC therapy, it lends support to the transformation of cell therapy into a more customized, targeted therapy. Myocardial regional assessment tools, such as SEF and myocardial SEED, should be considered as potential measures of efficacy for cardiac stem cell therapy.
Supplementary Material
Novelty and Significance.
What Is Known?
Transendocardial Stem Cell Injection (TESI) with mesenchymal stem cells (MSCs) improves remodeling, decrease infarct size, and improves function and quality of life in chronic ischemic heart disease.
MSCs mechanisms include anti-fibrotic effects, neovascularization, neomyogenesis and stimulation of endogenous cardiac cells.
Whether these actions are exerted locally, at a distance, or globally, and the impact of precisely localizing cell injections, remain unclear.
What New Information Does This Article Contribute?
The injection site for cardiac cell therapy has an important impact on the phenotypic response, such that cardiac function improved preferentially at injection sites whereas scar reduction was evident both locally and remotely to sites of cell delivery.
Cell injections enhanced ventricular performance to a greater extent at sites that had greater baseline dysfunction.
Cell therapy reduced fibrosis in infarct scars that were both transmural and non-transmural.
We performed a regional analysis after TESI, to test the hypothesis that sites of cell injection respond more favorably in terms of cardiac repair than sites not receiving cell injections. We report three new major findings. First, injected scarred areas respond with a reduction in fibrotic tissue and improvement in wall motion. Second, while segments remote to the injected area also exhibit reduction in scar, they do not exhibit comparable improvement in performance. Finally, non-scarred segments do not improve performance with TESI. These findings elucidate a dichotomy in the phenotypic response to MSC injections, demonstrating that local and paracrine factors differentially influence functional restoration and scar size reduction. Locally, there is evidence of reduction of scar size associated with significant improvement in performance, whereas remotely only scar size reduction is seen. Collectively, these findings can guide a personalized approach for optimizing the response to cell therapy in humans by tailoring the injection strategy to patient anatomy.
ACKNOWLEDGMENTS
The authors would like to thank Sha He, MD and Hongwei Tang, MD from TeraRecon Inc. for their assistance regarding MDCT imaging analysis. Drs. He and Tang did not receive any compensation for their contribution.
SOURCES OF FUNDING
This study was funded by the US National Heart, Lung, and Blood Institute (NHLBI) as part of the Specialized Centers for Cell-Based Therapy U54 grant (U54HL081028-01). Dr Hare is also supported by National Institutes of Health (NIH) grants RO1 HL094849, P20 HL101443, RO1 HL084275, RO1 HL107110, RO1 HL110737, and UM1HL113460. The NHLBI provided oversight of the clinical trial through the independent Gene and Cell Therapy Data and Safety Monitoring Board (DSMB). Biocardia Inc provided the Helical Infusion Catheters for the conduct of POSEIDON.
Nonstandard Abbreviations and Acronyms:
- TESI
Transendocardial Stem Cell Injection
- EED
Early Enhacement Defect
- SEED
Segmental Early Enhancement Defect
- SEF
Segmental Ejection Fraction
- LAO
Left anterior oblique
- RAO
Right anterior oblique
- EF
Ejection Fraction
- POSEIDON
Percutaneous Stem Cell Injection Delivery Effects on Neomyogenesis
- MSC
Mesenchymal Stem Cell
- LV
Left ventricular
- MDCT
Multidetector Computed Tomography
- AHA
American Heart Association
- HU
Hounsfield units
- LAD
Left anterior descending
- PRSW
Preload Recruitable Stroke Work
- Ecc
Eulerian circumferential strain
- CMR
Cardiac Magnetic resonance
- DE
Delayed enhancement
- C-CURE
Cardiopoietic stem cell therapy in heart failure
Footnotes
DISCLOSURES
Dr Hare reported having a patent for cardiac cell-based therapy, receiving research support from Biocardia, being a board-member, consultant, and having equity interest in Vestion Inc, and being a consultant for Kardia.
Dr. Joshua Hare holds equity in Vestion and maintains a professional relationship with Vestion as a consultant and member of the Board of Directors and Scientific Advisory Board. Dr George reported serving on the board of GE-Healthcare, consulting for ICON-Medical-Imaging, and receiving trademark royalties for fluoroperfusion-imaging. Mr Mendizabal is an employee of EMMES-Corporation. Dr Altman is employee of Biocardia-Inc. Dr McNiece reported being a consultant and board member of Proteonomic-Inc. Dr Heldman reported having a patent for cardiac cell-based therapy, receiving research support from Biocardia, and being a board-member, consultant, and having equity interest in Vestion Inc. No other authors reported any financial disclosures.
REFERENCES
- 1.Schuleri KH, Feigenbaum GS, Centola M, Weiss ES, Zimmet JM, Turney J, Kellner J, Zviman MM, Hatzistergos KE, Detrick B, Conte JV, McNiece I, Steenbergen C, Lardo AC, Hare JM. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. Eur Heart J. 2009;30:2722–2732. doi: 10.1093/eurheartj/ehp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amado LC, Saliaris AP, Schuleri KH, St John M, Xie JS, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart G, Lehrke S, Baumgartner WW, Martin BJ, Heldman AW, Hare JM. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci U S A. 2005;102:11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams AR, Trachtenberg B, Velazquez DL, McNiece I, Altman P, Rouy D, Mendizabal AM, Pattany PM, Lopera GA, Fishman J, Zambrano JP, Heldman AW, Hare JM. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: Functional recovery and reverse remodeling. Circ Res. 2011;108:792–796. doi: 10.1161/CIRCRESAHA.111.242610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis-Sproul J, Schulman IH, Byrnes J, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong Po Foo C, Ruiz P, Amador A, Da Silva J, McNiece IK, Heldman AW, George R, Lardo A. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: The poseidon randomized trial. JAMA : the journal of the American Medical Association. 2012;308:2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dib N, Menasche P, Bartunek JJ, Zeiher AM, Terzic A, Chronos NA, Henry TD, Peters NS, Fernandez-Aviles F, Yacoub M, Sanborn TA, Demaria A, Schatz RA, Taylor DA, Fuchs S, Itescu S, Miller LW, Dinsmore JH, Dangas GD, Popma JJ, Hall JL, Holmes DR., Jr. Recommendations for successful training on methods of delivery of biologics for cardiac regeneration: A report of the international society for cardiovascular translational research. JACC Cardiovasc Interv. 2010;3:265–275. doi: 10.1016/j.jcin.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 6.Gyongyosi M, Dib N. Diagnostic and prognostic value of 3d noga mapping in ischemic heart disease. Nat Rev Cardiol. 2011;8:393–404. doi: 10.1038/nrcardio.2011.64. [DOI] [PubMed] [Google Scholar]
- 7.Sherman W, Martens TP, Viles-Gonzalez JF, Siminiak T. Catheter-based delivery of cells to the heart. Nat Clin Pract Cardiovasc Med. 2006;3(Suppl 1):S57–64. doi: 10.1038/ncpcardio0446. [DOI] [PubMed] [Google Scholar]
- 8.Williams AR, Hare JM. Mesenchymal stem cells: Biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011;109:923–940. doi: 10.1161/CIRCRESAHA.111.243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarmento-Leite R, Silva GV, Dohman HF, Rocha RM, Dohman HJ, de Mattos ND, Carvalho LA, Gottechall CA, Perin EC. Comparison of left ventricular electromechanical mapping and left ventricular angiography: Defining practical standards for analysis of noga maps. Tex Heart Inst J. 2003;30:19–26. [PMC free article] [PubMed] [Google Scholar]
- 10.Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, Mazhari R, Boyle AJ, Zambrano JP, Rodriguez JE, Dulce R, Pattany PM, Valdes D, Revilla C, Heldman AW, McNiece I, Hare JM. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quevedo HC, Hatzistergos KE, Oskouei BN, Feigenbaum GS, Rodriguez JE, Valdes D, Pattany PM, Zambrano JP, Hu Q, McNiece I, Heldman AW, Hare JM. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci U S A. 2009;106:14022–14027. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suncion VY, Schulman IH, Hare JM. Concise review: The role of clinical trials in deciphering mechanisms of action of cardiac cell-based therapy. Stem Cells Transl Med. 2012;1:29–35. doi: 10.5966/sctm.2011-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schuleri KH, Centola M, Choi SH, Evers KS, Dawoud F, George RT, Lima JAC, Lardo AC. Ct for evaluation of myocardial cell therapy in heart failure a comparison with cmr imaging. Jacc-Cardiovasc Imag. 2011;4:1284–1293. doi: 10.1016/j.jcmg.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerber BL, Belge B, Legros GJ, Lim P, Poncelet A, Pasquet A, Gisellu G, Coche E, Vanoverschelde JL. Characterization of acute and chronic myocardial infarcts by multidetector computed tomography: Comparison with contrast-enhanced magnetic resonance. Circulation. 2006;113:823–833. doi: 10.1161/CIRCULATIONAHA.104.529511. [DOI] [PubMed] [Google Scholar]
- 16.Lardo AC, Cordeiro MA, Silva C, Amado LC, George RT, Saliaris AP, Schuleri KH, Fernandes VR, Zviman M, Nazarian S, Halperin HR, Wu KC, Hare JM, Lima JA. Contrast-enhanced multidetector computed tomography viability imaging after myocardial infarction: Characterization of myocyte death, microvascular obstruction, and chronic scar. Circulation. 2006;113:394–404. doi: 10.1161/CIRCULATIONAHA.105.521450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahnken AH, Koos R, Katoh M, Wildberger JE, Spuentrup E, Buecker A, Gunther RW, Kuhl HP. Assessment of myocardial viability in reperfused acute myocardial infarction using 16-slice computed tomography in comparison to magnetic resonance imaging. J Am Coll Cardiol. 2005;45:2042–2047. doi: 10.1016/j.jacc.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 18.Zeb I, Li D, Nasir K, Gupta M, Kadakia J, Gao Y, Ma E, Mao SS, Budoff M. Computerized left ventricular regional ejection fraction analysis for detection of ischemic coronary artery disease with multidetector ct angiography. The international journal of cardiovascular imaging. 2013;29:685–692. doi: 10.1007/s10554-012-0121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lessick J, Dragu R, Mutlak D, Rispler S, Beyar R, Litmanovich D, Engel A, Agmon Y, Kapeliovich M, Hammerman H, Ghersin E. Is functional improvement after myocardial infarction predicted with myocardial enhancement patterns at multidetector ct? Radiology. 2007;244:736–744. doi: 10.1148/radiol.2443061397. [DOI] [PubMed] [Google Scholar]
- 20.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the cardiac imaging committee of the council on clinical cardiology of the american heart association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 21.Gomes SA, Rangel EB, Premer C, Dulce RA, Cao Y, Florea V, Balkan W, Rodrigues CO, Schally AV, Hare JM. S-nitrosoglutathione reductase (gsnor) enhances vasculogenesis by mesenchymal stem cells. Proc Natl Acad Sci U S A. 2013;110:2834–2839. doi: 10.1073/pnas.1220185110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartunek J, Behfar A, Dolatabadi D, Vanderheyden M, Ostojic M, Dens J, El Nakadi B, Banovic M, Beleslin B, Vrolix M, Legrand V, Vrints C, Vanoverschelde JL, Crespo-Diaz R, Homsy C, Tendera M, Waldman S, Wijns W, Terzic A. Cardiopoietic stem cell therapy in heart failure: The c-cure (cardiopoietic stem cell therapy in heart failure) multicenter randomized trial with lineage-specified biologics. J Am Coll Cardiol. 2013;61:2329–2338. doi: 10.1016/j.jacc.2013.02.071. [DOI] [PubMed] [Google Scholar]
- 23.Bolli R, Chugh AR, D'Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (scipio): Initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Heldman AW, Difede DL, Fishman JE, Zambrano JP, Trachtenberg BH, Karantalis V, Mushtaq M, Williams AR, Suncion VY, McNiece IK, Ghersin E, Soto V, Lopera G, Miki R, Willens H, Hendel R, Mitrani R, Pattany P, Feigenbaum G, Oskouei B, Byrnes J, Lowery MH, Sierra J, Pujol MV, Delgado C, Gonzalez PJ, Rodriguez JE, Bagno LL, Rouy D, Altman P, Foo CW, da Silva J, Anderson E, Schwarz R, Mendizabal A, Hare JM. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: The tac-hft randomized trial. JAMA : the journal of the American Medical Association. 2014;311:62–73. doi: 10.1001/jama.2013.282909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karantalis V, Difede Velazquez DL, Gerstenblith G, Pham S, Symes J, Zambrano JP, Fishman J, Pattany PM, McNiece I, Conte JV, Schulman S, Wu K, Shah A, Breton E, Davis-Sproul J, Schwarz R, Feigenbaum GS, Mushtaq M, Suncion V, Lardo AC, George R, Borello I, Mendizabal AM, Karas TZ, Byrnes J, Lowery MH, Heldman AW, Hare JM. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting. Circulation. 2013:128. doi: 10.1161/CIRCRESAHA.114.303180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Telukuntla KS, Suncion VY, Schulman IH, Hare JM. The advancing field of cell-based therapy: Insights and lessons from clinical trials. Journal of the American Heart Association. 2013;2:e000338. doi: 10.1161/JAHA.113.000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams AR, Hatzistergos KE, Addicott B, McCall F, Carvalho D, Suncion V, Morales AR, Da Silva J, Sussman MA, Heldman AW, Hare JM. Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation. 2013;127:213–223. doi: 10.1161/CIRCULATIONAHA.112.131110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burchfield JS, Dimmeler S. Role of paracrine factors in stem and progenitor cell mediated cardiac repair and tissue fibrosis. Fibrogenesis & tissue repair. 2008;1:4. doi: 10.1186/1755-1536-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCall FC, Telukuntla KS, Karantalis V, Suncion VY, Heldman AW, Mushtaq M, Williams AR, Hare JM. Myocardial infarction and intramyocardial injection models in swine. Nature protocols. 2012;7:1479–1496. doi: 10.1038/nprot.2012.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schuleri KH, Boyle AJ, Centola M, Amado LC, Evers R, Zimmet JM, Evers KS, Ostbye KM, Scorpio DG, Hare JM, Lardo AC. The adult gottingen minipig as a model for chronic heart failure after myocardial infarction: Focus on cardiovascular imaging and regenerative therapies. Comparative medicine. 2008;58:568–579. [PMC free article] [PubMed] [Google Scholar]
- 31.Silva GV, Litovsky S, Assad JA, Sousa AL, Martin BJ, Vela D, Coulter SC, Lin J, Ober J, Vaughn WK, Branco RV, Oliveira EM, He R, Geng YJ, Willerson JT, Perin EC. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111:150–156. doi: 10.1161/01.CIR.0000151812.86142.45. [DOI] [PubMed] [Google Scholar]
- 32.Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nature medicine. 2001;7:430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 33.Weil BR, Canty JM., Jr. Stem cell stimulation of endogenous myocyte regeneration. Clinical science. 2013;125:109–119. doi: 10.1042/CS20120641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams AR, Suncion VY, McCall F, Guerra D, Mather J, Zambrano JP, Heldman AW, Hare JM. Durable scar size reduction due to allogeneic mesenchymal stem cell therapy regulates whole-chamber remodeling. Journal of the American Heart Association. 2013;2:e000140. doi: 10.1161/JAHA.113.000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chugh AR, Beache GM, Loughran JH, Mewton N, Elmore JB, Kajstura J, Pappas P, Tatooles A, Stoddard MF, Lima JA, Slaughter MS, Anversa P, Bolli R. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: The scipio trial: Surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation. 2012;126:S54–64. doi: 10.1161/CIRCULATIONAHA.112.092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.