Abstract
OBJECTIVE
This study evaluates the relationships between quantitative CT (QCT) and spirometric measurements of disease severity in cigarette smokers with and without chronic obstructive pulmonary disease (COPD).
MATERIALS AND METHODS
Inspiratory and expiratory CT scans of 4062 subjects in the Genetic Epidemiology of COPD (COPDGene) Study were evaluated. Measures examined included emphysema, defined as the percentage of low-attenuation areas ≤ −950 HU on inspiratory CT, which we refer to as “LAA-950I”; air trapping, defined as the percentage of low-attenuation areas ≤ −856 HU on expiratory CT, which we refer to as “LAA-856E”; and the inner diameter, inner and outer areas, wall area, airway wall thickness, and square root of the wall area of a hypothetical airway of 10-mm internal perimeter of segmental and subsegmental airways. Correlations were determined between spirometry and several QCT measures using statistics software (SAS, version 9.2).
RESULTS
QCT measurements of low-attenuation areas correlate strongly and significantly (p < 0.0001) with spirometry. The correlation between LAA-856E and forced expiratory volume in 1 second (FEV1) and the ratio of FEV1 to forced vital capacity (FVC) (r = −0.77 and −0.84, respectively) is stronger than the correlation between LAA-950I and FEV1 and FEV1/FVC (r = −0.67 and r = −0.76). Inspiratory and expiratory volume changes decreased with increasing disease severity, as measured by the Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD) staging system (p < 0.0001). When airway variables were included with low-attenuation area measures in a multiple regression model, the model accounted for a statistically greater proportion of variation in FEV1 and FEV1/FVC (R2 = 0.72 and 0.77, respectively). Airway measurements alone are less correlated with spirometric measures of FEV1 (r = 0.15 to −0.44) and FEV1/FVC (r = 0.19 to −0.34).
CONCLUSION
QCT measurements are strongly associated with spirometric results showing impairment in smokers. LAA-856E strongly correlates with physiologic measurements of airway obstruction. Airway measurements can be used concurrently with QCT measures of low-attenuation areas to accurately predict lung function.
Keywords: air trapping, airway measurements, chronic obstructive pulmonary disease, emphysema, quantitative CT
Chronic obstructive pulmonary disease (COPD) recently became the third leading cause of death in the United States [1], with an estimated 24 million affected individuals [2]. COPD is strongly associated with cigarette smoking but not all smokers will develop COPD, suggesting possible genetic differences in susceptibility to the adverse effects of cigarette smoke [3]. The Genetic Epidemiology of COPD (COPDGene) Study [4] is a multicenter observational study designed to identify genetic factors associated with COPD in a large cohort (10,000) of subjects including smokers with COPD across the range of disease severity, as measured by the Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD) staging system (GOLD stages 1–4), and smokers without COPD as control subjects. Inspiratory and expiratory chest CT examinations were acquired of all subjects in the COPDGene Study using a standardized protocol.
Quantitative CT (QCT) has the potential to quantify inspiratory and expiratory low-attenuation areas as distinct components of COPD [5], which may be important for phenotyping the disease and for devising individualized treatment. Phenotypic characterization of COPD subjects using QCT together with clinical and physiologic measures will enable the broad COPD syndrome to be classified into clinically significant subtypes. The evolution toward improved treatments for COPD including molecular (personalized) medicine requires quantitative test results [6]. The goal of QCT in the setting of COPD is to exploit quantitative imaging biomarkers as surrogate endpoints for disease characterization and to develop methods for accurate and reproducible measurements of biologically relevant processes [7]. This study shows relationships between QCT and spirometry for validation of quantitative imaging in the COPDGene Study. With renewal of the COPDGene Study, future work will include longitudinal studies assessing phenotypic subtypes, genetics, and lung cancer.
Quantitative imaging serves as a biomarker by representing disease features seen on CT images. Lung segmentation and densitometry measurements have been used extensively to quantify COPD [8–11]. The 3D airway tree has been used for lung lobe identification and airway measurements [12–14]. Multiple studies have shown substantial progress in using CT to quantify emphysema (defined as the percentage of low-attenuation areas ≤ −950 HU on inspiratory CT, which we refer to as “LAA-950I”) [15–20] and air trapping (defined as the percentage of low-attenuation areas ≤ −856 HU on expiratory CT, which we refer to as “LAA-856E”) as measures of small airways disease. However, to our knowledge, there has been no large-scale study evaluating the relationship between physiologic impairment and QCT indexes of both inspiratory and expiratory relative areas (including lobar data) and no large-scale study linking QCT airway measurements to spirometric measures.
This study evaluates the relationships between several QCT measures, including density, volume, and airway measurements, against spirometric measures of disease severity in cigarette smokers with and without COPD. We hypothesized that QCT density measures can be used as an accurate predictor of COPD and functional impairment by accurately predicting and confirming spirometric measurements. We further believe that airway measures used in combination with QCT density measures will provide an increase in correlation with functional measures of obstructive lung disease.
Materials and Methods
The COPDGene Study design article [4] describes the overall methods, population, and procedures used to analyze 10,000 subjects in the COPDGene Study, a prospective study approved by the institutional review board of each of the 21 participating clinical study centers. Oral consent and written consent were obtained from each subject before inclusion in the study.
Inclusion and Exclusion Criteria
Eligible subjects were between the ages of 45 and 80 years and had a smoking history of at least 10 pack-years. All subjects were non-Hispanic whites or non-Hispanic African Americans. Subjects did not have concomitant respiratory disorders other than asthma or COPD.
Subjects
Of the 10,000-subject cohort, 4542 subjects had complete airway and inspiratory lobe-by-lobe volumetric data available for analysis; 30 did not have expiratory CT. QCT data were suppressed from the cohort if scans showed extreme motion or had other technical inadequacies (e.g., nonprotocol reconstruction kernel, low exposure, or a value of > 1 for the ratio of functional residual capacity [FRC] to total lung capacity [TLC]). Also excluded were 450 smokers with a reduced value for forced expiratory volume in 1 second (FEV1) who did not meet the criteria for COPD because of a normal FEV1-to–forced vital capacity (FVC) ratio. The resultant dataset consists of a total of 4062 subjects: 1917 smokers without COPD (no spirometric evidence of airway obstruction) who served as control subjects, 363 subjects with GOLD stage 1 disease, 867 subjects with GOLD stage 2 disease, 575 subjects with GOLD stage 3 disease, and 340 subjects with GOLD stage 4 disease [21] (Table 1). The cohort consists of 2247 men (55%) and 1815 women (45%) and 3054 non-Hispanic whites (75%) and 1008 African-Americans (25%). The average age of the subjects is 60.8 years (SD, 9.2). The average number of pack-years, which the American Thoracic Society defines as the number of packs of cigarettes smoked every day multiplied by the total number of smoking years, for the subjects was 45.8 pack-years (SD, 25.7).
TABLE 1.
Demographic Characteristics and Spirometry Results of Study Subjects
| Characteristic | Control Subjects (Smokers Without COPD) |
Mild COPDa (GOLD Stage 1) |
Moderate COPDa (GOLD Stage 2) |
Severe COPDa (GOLD Stage 3) |
Very Severe COPDa (GOLD Stage 4) |
|---|---|---|---|---|---|
| No. of subjects | 1917 | 363 | 867 | 575 | 340 |
| Demographic characteristics | |||||
| Age (y), mean (SD) | 57.8 (9.0) | 61.9 (9.1) | 63.4 (8.9) | 64.4 (8.2) | 64.0 (7.7) |
| Sex, no. (%) of patients | |||||
| Male | 1025 (53) | 212 (58) | 475 (55) | 335 (58) | 200 (59) |
| Female | 892 (47) | 151 (42) | 392 (45) | 240 (42) | 140 (41) |
| Pack-yearsb smoking, mean (SD) | 37.8 (20.0) | 45.6 (25.3) | 52.2 (27.5) | 56.6 (28.3) | 56.5 (29.6) |
| Race, no. (%) of patients | |||||
| White | 1248 (65) | 294 (81) | 722 (83) | 496 (86) | 294 (86) |
| African American | 669 (35) | 69 (19) | 145 (17) | 79 (14) | 46 (14) |
| Body mass index, mean (SD) | 28.8 (5.7) | 27.4 (5.2) | 28.5 (5.8) | 28.0 (5.9) | 25.4 (5.5) |
| Spirometry (postbronchodilator) results, mean (SD) | |||||
| FEV1/FVCc | 0.79 (0.05) | 0.65 (0.04) | 0.57 (0.08) | 0.43 (0.09) | 0.31 (0.07) |
| FEV1 (% predicted)c | 97.8 (11.6) | 91.1 (9.1) | 65.0 (8.5) | 40.3 (5.7) | 22.6 (4.9) |
Note—All subjects had a smoking history of at least 10 pack-years. COPD = chronic obstructive pulmonary disease, GOLD = Global Initiative for Chronic Obstructive Pulmonary Disease, FEV1 = forced expiratory volume in 1 second, FVC = forced vital capacity.
COPD is defined as postbronchodilator FEV1/FVC ratio of < 0.7 by pulmonary function tests (spirometry).
Defined by the American Thoracic Society as the number of packs of cigarettes smoked every day multiplied by the total number of smoking years.
FEV1 is the amount of air that can be forcibly exhaled from the lungs in the first second of a forced exhalation. FVC is the amount of air that can be forcibly exhaled from the lungs after taking the deepest breath possible. The FEV1/FVC ratio is the percentage of the total amount of air exhaled from the lungs during the first second of forced exhalation.
Image Acquisition
The subjects underwent two volumetric chest CT examinations: one at full inspiration (TLC) and one at the end of a normal expiration (FRC). These scans were reconstructed with a slice thickness of 0.625, 0.75, or 0.9 mm depending on the manufacturer of the CT unit; corresponding slice intervals were 0.625, 0.5, or 0.45 mm, respectively, to achieve near-isotropic voxels. Three manufacturers and 11 different CT scanner models were used in the study: 1083 subjects were scanned on 16-detector scanners; 12, on 40-detector; 1667, on 64-detector; and 1300, on 128-detector. Inspiratory scans were acquired at 200 mAs and expiratory scans, at 50 mAs; all scans were acquired at 120 kVp. Standard B31f or B reconstruction kernels, depending on the manufacturer, were used to achieve medium smooth images. CT dose modulation and IV contrast agents were not used for this study. The average effective tube current–exposure time product was 45.33 mAseff for expiratory scans and 180 mAseff for inspiratory scans, and pitch values ranged from 0.923 to 1.375 depending on the manufacturer and scanner model. The complete CT protocols for the COPDGene Study are provided in the supplementary material of [22].
Imaging Core
Patient-identifying information was removed from the scans at each study site in a HIPAA-compliant fashion. Each examination (DICOM images submitted on DVD) underwent quality assurance by a trained research analyst to ensure compliance with rigorous study protocols.
Image Processing
Image analysis of all CT examinations was performed using specialized software (Pulmonary Workstation, version 2, VIDA Diagnostics). Automated segmentation of the right and left lungs from the chest wall and mediastinum was performed. Lung lobe segmentation was usually automated; however, manual edits were performed when necessary. Airway tree growth and analyses were usually automated using the Pulmonary Workstation software, but trained analysts intervened when necessary. Automated segmentation was performed using methods that have been validated with manual techniques [23]. Measurements were performed at the segmental (fourth-generation) and subsegmental (fifth-generation) airways, with the trachea defined as the first generation. The airways measurements included the following: outer and inner areas (measured in millimeters squared); inner diameter (measured in millimeters); inner perimeter (measured in millimeters); airway wall thickness (measured in millimeters); wall area (measured in millimeters squared and measured as a percentage); and square root of the wall area of a hypothetical airway of 10-mm internal perimeter, which we refer to as “Pi10” (measured in millimeters). With the exception of Pi10 measurements, all airway measurements were obtained along the centerline of the lumen and in the middle third of the airway segment. These airway measures were determined by taking the average of all airway segments at the fourth or fifth generation, respectively. Pi10 was calculated by performing a linear regression of all airways with an inner perimeter of 8–20 mm and interpolating a value at 10 mm [24].
For feature extraction on QCT, emphysema was defined as the percentage of lung pixels with an attenuation of −950 HU or less on inspiratory CT (i.e., LAA-950I) and air trapping was defined as the percentage of lung pixels with an attenuation of −856 HU or less on expiratory CT (i.e., LAA-856E). CT total lung capacity (TLCCT) was defined as the segmented lung volume on inspiratory CT, and CT functional residual capacity (FRCCT) was defined as the segmented lung volume on expiratory CT. Mean lung attenuation values were recorded for both inspiratory CT and expiratory CT. Additional CT density measures were also examined including the mean lung attenuation value at the 15th percentile, the percentage of lung pixels with an attenuation of −910 HU or less and of −856 HU or less on inspiratory CT, and the percentage of lung pixels with an attenuation of −950 HU or less and of −910 HU or less on expiratory CT. Measures of LAA-950I and LAA-856E and volumes were determined for the upper lobes (right upper lobe [RUL] + right middle lobe [RML] + left upper lobe [LUL]) and the lower lobes (right lower lobe [RLL] + left lower lobe [LLL]).
Lung segmentation, lobe segmentation, and airway tree growth were performed by multiple analysts, all of whom received standardized instructions and training in thoracic anatomy and specific aspects of the software. During this training period, these measures were examined and evaluated by a senior analyst. To determine the reproducibility of measures between and within analysts, we compared measures of a cohort of 32 subjects independently analyzed by two trained analysts. All compared measures were shown to have very high correlation between analysts, with r values ranging from 0.85 for airway measures to 0.99 for density and volume measures.
Pulmonary Function Tests
All spirometry data were collected using an EasyOne spirometer (ndd Medical Technologies) and were reviewed centrally by the pulmonary function test quality assurance core analyst of the COPDGene Study to ensure quality control. Spirometric data were typically collected the same day as the acquired CT studies (mean time between spirometry and CT, 0.31 hours).
Statistical Analysis
Linear regressions of relative area and airway measures, along with correlations between QCT variables and GOLD stage, were performed using statistics software (SAS/STAT software package, version 9.2, SAS Institute) for Microsoft Windows 7. A one-way analysis of variance between QCT variables and GOLD stage and multiple regressions to estimate functional measures from QCT measures were also performed using SAS software. For this model, functional measures were log10-transformed to ensure a distribution of residuals closer to normal. QCT variables included in this model were confirmed to be significant by performing both stepwise and backward elimination methods; variables remained in the model as long as p < 0.10. For multiple regression results, correlation is reported in R2 because R2 is the proportion of variation in the response accounted for by the regression model. For the univariate correlations between QCT variables and FEV1 (measured as the percent predicted) and FEV1/FVC ratio, 95% CIs were estimated using 10,000 bootstrap replications using R software (version 2.15.0, R Project for Statistical Computing) [25–27]. Bootstrap techniques are useful when the assumptions for a common normal-theory method such as the calculation of a CI for a correlation may not be satisfied [25, 26].
Results
Whole-Lung Analysis
Table 2 summarizes the QCT parameters of inspiratory and expiratory low-attenuation areas for whole-lung analysis by GOLD stage. For whole-lung analysis, Table 2 shows progressively increasing LAA-950I and LAA-856E for increasing GOLD stage and COPD disease severity. Mean LAA-950I and LAA-856E values progressively increased with increasing GOLD stage (p < 0.001).
TABLE 2.
Quantitative CT (QCT) Results
| QCT Results | Control Subjects (Smokers Without COPD) |
Mild COPDa (GOLD Stage 1) |
Moderate COPDa (GOLD Stage 2) |
Severe COPDa (GOLD Stage 3) |
Very Severe COPDa (GOLD Stage 4) |
|---|---|---|---|---|---|
| No. of subjects | 1917 | 363 | 867 | 575 | 340 |
| Inspiratory CT | |||||
| % Lung pixels ≤ −950 HUb | 2.5 (2.8) | 6.0 (6.0) | 8.4 (8.6) | 18.2 (12.7) | 28.1 (14.0) |
| % Lung pixels ≤ −910 HU | 19.9 (14.3) | 29.9 (15.5) | 31.2 (16.3) | 43.8 (17.0) | 54.3 (13.9) |
| % Lung pixels ≤ −856 HU | 58.9 (18.4) | 67.2 (14.2) | 66.0 (14.3) | 71.5 (13.1) | 77.1 (8.3) |
| Mean lung attenuation at 15th percentile (HU) | −909.3 (23.7) | −925.2 (20.9) | −929.3 (24.1) | −949.1 (26.1) | −964.8 (19.4) |
| Mean lung attenuation (HU) | −833.1 (31.7) | −849.7 (25.3) | −849.3 (27.4) | −865.1 (29.7) | −881.6 (23.2) |
| TLCCT (L)c | 5.4 (1.3) | 6.1 (1.4) | 5.8 (1.4) | 6.2 (1.4) | 6.8 (1.5) |
| Expiratory CT | |||||
| % Lung pixels ≤ −856 HUd | 11.7 (9.6) | 20.6 (12.0) | 28.9 (15.2) | 48.2 (16.9) | 63.3 (12.8) |
| % Lung pixels ≤ −950 HU | 0.9 (1.3) | 2.2 (2.5) | 4.3 (5.0) | 11.8 (9.3) | 21.0 (11.7) |
| % Lung pixels ≤ −910 HU | 3.0 (3.5) | 6.4 (5.6) | 11.2 (9.4) | 25.7 (14.0) | 40.4 (14.7) |
| Mean lung attenuation at 15th percentile (HU) | −830.1 (41.9) | −864.5 (36.1) | −886.8 (38.6) | −928.9 (38.2) | −956.4 (25.7) |
| Mean lung attenuation (HU) | −691.5 (54.4) | −730.4 (46.0) | −758.8 (47.0) | −808.8 (46.4) | −848.5 (33.1) |
| FRCCT (L)e | 2.8 (0.7) | 3.3 (0.8) | 3.5 (0.9) | 4.3 (1.1) | 5.2 (1.3) |
| Upper lobes (RUL + RML + LUL) minus lower lobes (RLL + LLL) | |||||
| Difference in LAA-950I (%) | 0.6 (1.9) | 2.4 (4.9) | 3.0 (7.8) | 5.3 (12.4.) | 4.9 (15.8 |
| Difference in LAA-856E (%) | 8.6 (8.1) | 13.5 (10.8) | 11.7 (12.2) | 10.9 (16.9) | 8.1 (17.4) |
| Difference in inspiratory CT-expiratory CT volume change (%) | −10.2 (7.0) | −9.9 (8.4) | −6.9 (8.2) | −4.7 (9.3) | −3.3 (9.7) |
| Segmental airway (fourth generation) | |||||
| Inner diameter (mm) | 5.5 (0.7) | 5.4 (0.7) | 5.0 (0.6) | 4.9 (0.6) | 4.8 (0.6) |
| Airway wall thickness (mm) | 1.5 (0.1) | 1.5 (0.1) | 1.5 (0.1) | 1.5 (0.1) | 1.4 (0.1) |
| Outer area (mm2) | 59.5 (11.8) | 57.3 (11.9) | 52.2 (10.9) | 50.7 (10.0) | 49.4 (10.2) |
| Inner area (mm2) | 24.7 (6.6) | 23.7 (6.5) | 20.7 (5.7) | 19.7 (5.1) | 19.1 (5.2) |
| Inner perimeter (mm) | 17.8 (2.2) | 17.5 (2.3) | 16.3 (2.1) | 16.0 (2.0) | 15.8 (2.0) |
| Wall area (%)f | 59.8 (2.8) | 60.1 (2.8) | 61.8 (2.8) | 62.6 (2.6) | 62.6 (2.6) |
| Wall area (mm2)f | 34.7 (5.7) | 33.6 (5.7) | 31.6 (5.6) | 31.1 (5.3) | 30.3 (5.4) |
| Subsegmental airway (fifth generation) | |||||
| Inner diameter (mm) | 4.2 (0.5) | 4.0 (0.5) | 3.9 (0.4) | 3.8 (0.4) | 3.7 (0.4) |
| Airway wall thickness (mm) | 1.2 (0.1) | 1.2 (0.1) | 1.2 (0.1) | 1.2 (0.1) | 1.2 (0.1) |
| Outer area (mm2)f | 36.9 (7.6) | 34.9 (6.6) | 32.8 (6.6) | 32.1 (6.2) | 31.8 (6.0) |
| Inner area (mm2)f | 14.4 (4.3) | 13.5 (3.4) | 12.2 (3.4) | 11.7 (3.2) | 11.6 (3.0) |
| Inner perimeter (mm)f | 13.7 (1.5) | 13.3 (1.5) | 12.7 (1.5) | 12.5 (1.4) | 12.5 (1.3) |
| Wall area (%)f | 62.2 (1.9) | 62.4 (2.0) | 63.7 (2.1) | 64.4 (2.1) | 64.7 (2.0) |
| Wall area (mm2)f | 22.6 (3.6) | 21.5 (3.4) | 20.5 (3.5) | 20.4 (3.3) | 20.3 (3.2) |
| Pi10 (mm) | 3.6 (0.1) | 3.6 (0.1) | 3.7 (0.1) | 3.7 (0.1) | 3.8 (0.1) |
Note—All subjects had a smoking history of at least 10 pack-years. Unless noted otherwise, each CT result differed across the five groups (analysis of variance, p< 0.05). COPD = chronic obstructive pulmonary disease, GOLD = Global Initiative for Chronic Obstructive Pulmonary Disease, TLCCT = CT total lung capacity, FRCCT= CT functional residual capacity, RUL = right upper lobe, RML = right middle lobe, LUL = left upper lobe, RLL = right lower lobe, LLL = left lower lobe, LAA-950I = percentage of low-attenuation areas ≤ −950 HU on inspiratory CT, LAA-856E = percentage of low-attenuation areas ≤ −856 HU on expiratory CT, Pi10 = square root of the wall area of a hypothetical airway of 10-mm internal perimeter.
COPD is defined as postbronchodilator ratio of forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) of < 0.7 by pulmonary function tests (spirometry).
Referred to as “LAA-950I” in this article.
TLCCT is the amount of air in the lungs after inhaling as deep as possible (inspiratory CT segmented lung volume).
Referred to as “LAA-856E” in this article.
FRCCT is the amount of air in the lungs at the end of a normal exhaled breath (expiratory CT segmented lung volume).
Each CT result did not differ across the five groups.
Inspiratory and expiratory lung densitometry metrics of LAA-950I, LAA-856E, and mean lung attenuation at the 15th percentile, as well as the difference in CT volume (TLC – FRC) and difference in mean lung attenuation (i.e., mean lung attenuation on inspiratory CT minus mean lung attenuation on expiratory CT), were compared with functional measures to determine the extent of emphysema and air trapping. For air trapping, LAA-856E correlation for both FEV1 and FEV1/FVC (r = −0.77 and −0.84, respectively). Emphysema showed similar results, with LAA-950I showing the highest correlation for FEV1 and FEV1/FVC (r = −0.67 and −0.76, respectively). Other measures, such as the difference in mean lung attenuation, showed very high correlation with spirometric measures also. Table 3 shows these results compared with the entire cohort. To determine the effect of asthma on expiratory air trapping measures, we excluded subjects with self-reported asthma (n = 1949) and repeated the analyses. The results were very similar, with LAA-856E again showing the greatest correlation for both FEV1 and FEV1/FVC (r = −0.73 and −0.82, respectively).
TABLE 3.
Multiple Regression Models for Log10 Values of Forced Expiratory Volume in 1 Second (FEV1) and Ratio of FEV1 to Forced Vital Capacity (FVC)
| Multiple Regression Results |
||||
|---|---|---|---|---|
| Quantitative CT Results | Estimate | Error | t Valuea | Increase in R2 |
| Log10 of FEV1b: (R2 = 0.72) | ||||
| Intercept | 2.911 | 0.064 | 45.76 | — |
| % LAA-950I | −0.005 | 0.000 | −15.22 | 0.51 |
| % LAA-856E | −0.005 | 0.000 | −33.31 | 0.13 |
| Inner diameter of segmental airway, fourth generation (mm) | 0.071 | 0.004 | 19.84 | 0.04 |
| Airway wall thickness of segmental airway, fourth generation (mm) | −0.228 | 0.019 | −11.88 | 0.02 |
| Pi10 | −0.252 | 0.016 | −15.96 | 0.02 |
| Log10 of FEV1/FVCc: (R2 = 0.77) | ||||
| Intercept | 0.151 | 0.040 | 3.81 | — |
| % LAA-950I | −0.004 | 0.000 | −20.45 | 0.62 |
| % LAA-856E | −0.004 | 0.000 | −37.94 | 0.12 |
| Inner diameter of segmental airway, fourth generation (mm) | 0.035 | 0.002 | 15.87 | 0.02 |
| Airway wall thickness of segmental airway, fourth generation (mm) | −0.082 | 0.012 | −6.88 | 0.01 |
| Pi10 | −0.081 | 0.010 | −8.26 | 0.00 |
Note—Dash (—) indicates not applicable. LAA-950I = percentage of low-attenuation areas ≤ −950 HU on inspiratory CT, LAA-856E = percentage of low-attenuation areas ≤ −856 HU on expiratory CT, Pi10 = square root of the wall area of a hypothetical airway of 10-mm internal perimeter.
All corresponding p values are ≤ 0.0001.
FEV1 is the amount of air that can be forcibly exhaled from the lungs in the first second of a forced exhalation.
The FEV1/FVC ratio is the percentage of the total amount of air exhaled from the lungs during the first second of forced exhalation.
Compared with other lung densitometry metrics summarized in Table 2 for inspiratory CT, the definition of emphysema as the percentage of lung pixels ≤ −950 HU (i.e., LAA-950I) shows the most consistent progressive change across GOLD stage. For expiratory CT, the lung densitometry metrics summarized in Table 2 also change across GOLD stage; the percentage of lung pixels ≤ −856 HU (i.e., LAA-856E) shows the greatest change. Both TLCCT and FRCCT increase across GOLD stage (Table 2).
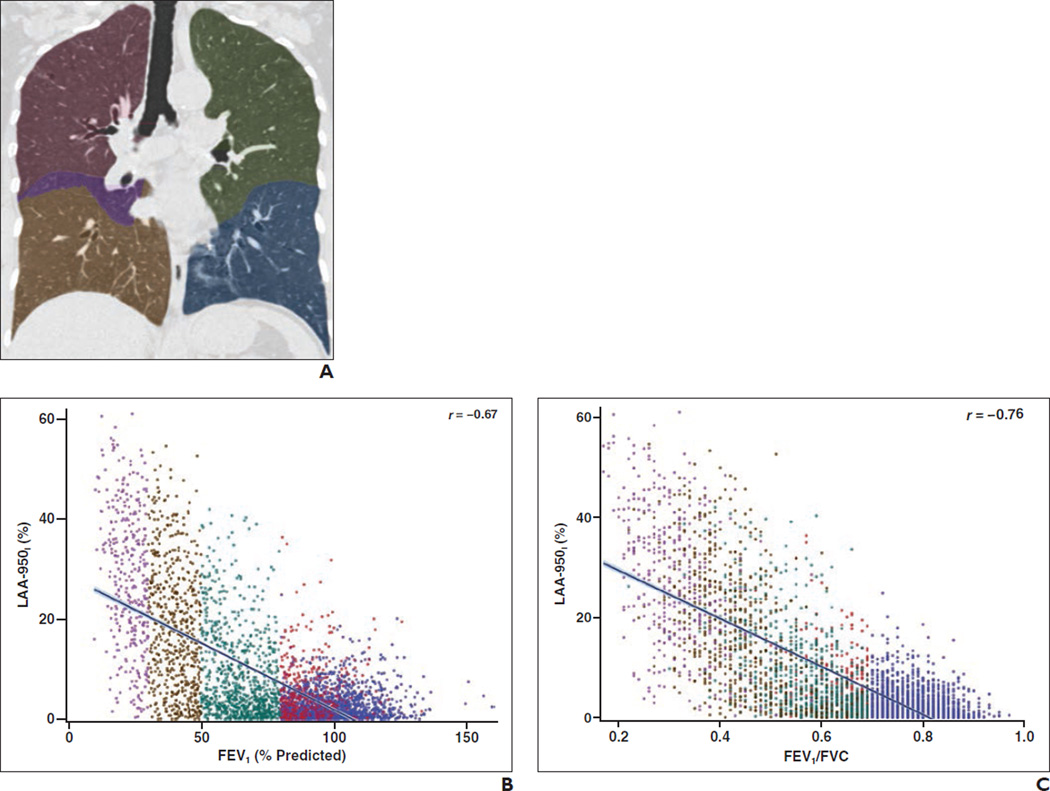
Figure 1 provides the correlations between whole-lung QCT parameters and FEV1 and FEV1/FVC ratio. Both inspiratory and expiratory low-attenuation area measurements correlate strongly with physiologic measures of disease severity from pulmonary function tests. The correlations between whole-lung LAA-856E and FEV1 and FEV1/FVC are stronger than the corresponding correlations for whole-lung LAA-950I.
Fig. 1.
Correlation of quantitative CT parameters with physiologic measurements from spirometry grouped by disease severity as measured by Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD) staging system for 4062 subjects.
A, Sample inspiratory CT scan shows lobar segmentation in a subject with a smoking history of at least 10 pack-years. Red = right upper lobe, purple = right middle lobe, brown = right lower lobe, green = left upper lobe, blue = left lower lobe.
B, Scatterplot shows percentage of low-attenuation areas ≤ −950 HU on inspiratory CT, which we refer to as “LAA-950I,” and forced expiratory volume in 1 second (FEV1). Line shows best-fit linear correlation. Blue = control subjects (smokers without COPD), red = subjects with GOLD stage 1 disease, green = subjects with GOLD stage 2 disease, brown = subjects with GOLD stage 3 disease, purple = subjects with GOLD stage 4 disease.
C, Scatterplot shows LAA-950I and ratio of FEV1 to forced vital capacity (FVC). Line shows best-fit linear correlation. Blue = control subjects (smokers without COPD), red = subjects with GOLD stage 1 disease, green = subjects with GOLD stage 2 disease, brown = subjects with GOLD stage 3 disease, purple = subjects with GOLD stage 4 disease.
D, Sample expiratory CT scan shows lobar segmentation in a subject with a smoking history of at least 10 pack-years. Red = right upper lobe, purple = right middle lobe, brown = right lower lobe, green = left upper lobe, blue = left lower lobe.
E, Scatterplot shows percentage of low-attenuation areas ≤ −856 HU on expiratory CT, which we refer to as “LAA-856E,” and FEV1. Line shows best-fit linear correlation. Blue = control subjects (smokers without COPD), red = subjects with GOLD stage 1 disease, green = subjects with GOLD stage 2 disease, brown = subjects with GOLD stage 3 disease, purple = subjects with GOLD stage 4 disease.
F, Scatterplot shows LAA-856E and FEV1/FVC ratio. Line shows best-fit linear correlation. Blue = control subjects (smokers without COPD), red = subjects with GOLD stage 1 disease, green = subjects with GOLD stage 2 disease, brown = subjects with GOLD stage 3 disease, purple = subjects with GOLD stage 4 disease.
Lung Lobar Analysis by Upper Lobes (Including the Right Middle Lobe) and Lower Lobes
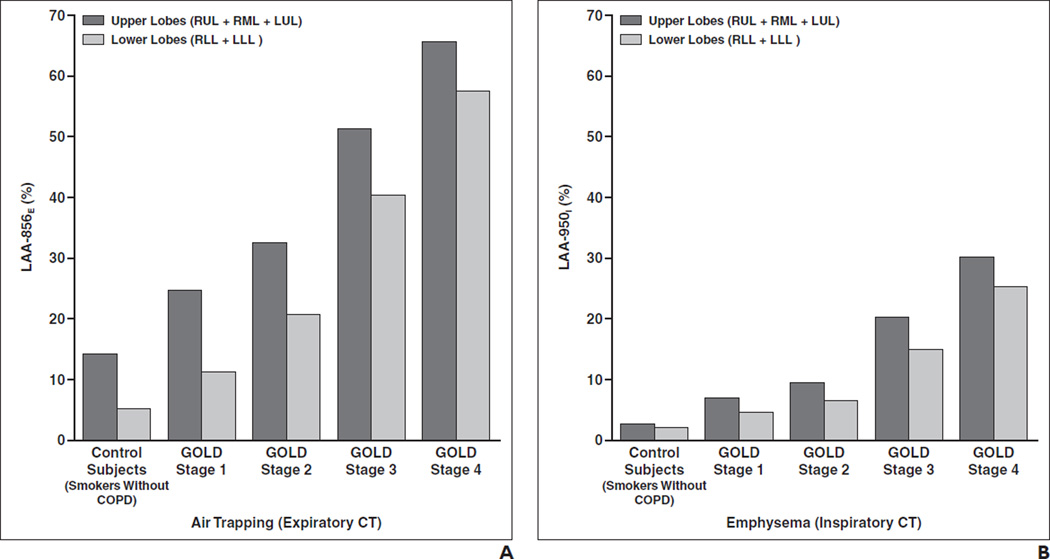
To simplify analysis of lobar differences, we categorized lobar parameters into upper lobes and lower lobes. We included the RML with the upper lobes because it is homologous with the LUL. Table 2 summarizes QCT results categorized by the upper lobes, defined to include the RML, and the lower lobes. Both inspiratory and expiratory low-attenuation area measures are higher in the upper lobes (RUL + RML + LUL) compared with the lower lobes (RLL + LLL) and progressively increase with increases in disease severity (GOLD stages 1–4) (Fig. 2). The same analyses were also performed excluding the RML with similar results (data not shown).
Fig. 2.
Percentage of low-attenuation areas ≤ −856 HU on expiratory CT, which we refer to as “LAA-856E,” and percentage of low-attenuation areas ≤ −950 HU on inspiratory CT, which we refer to as “LAA-950I.” Values are shown for upper lobes (right upper lobe [RUL] + right middle lobe [RML] + left upper lobe [LUL]) and lower lobes (right lower lobe [RLL] + left lower lobe [LLL]). LAA-856E and LAA-950I values increase progressively with Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD) stage.
A and B, Bar graphs show LAA-856E (A) and LAA-950I (B) values for upper and lower lobes grouped by GOLD stage.
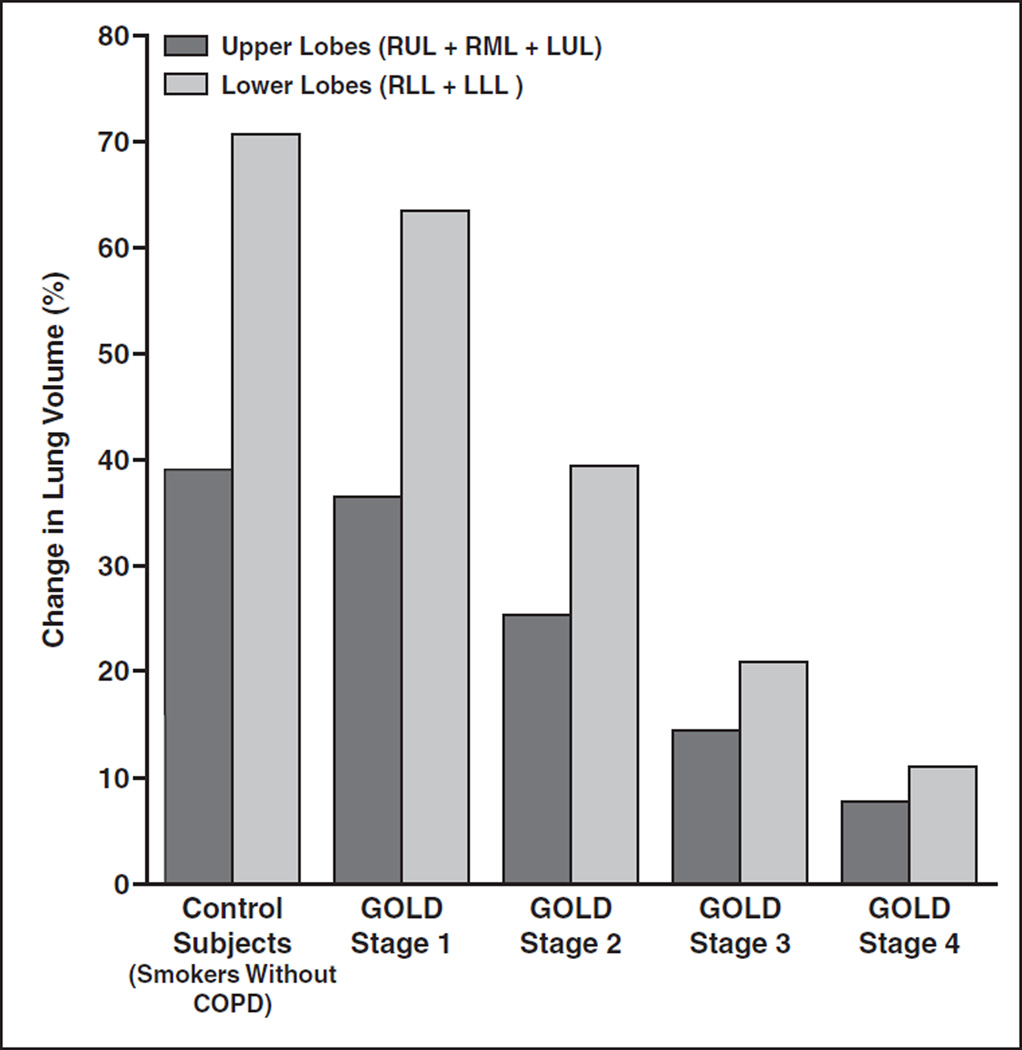
For smoker control subjects and subjects with COPD (GOLD stages 1–4), most of the volume change between inspiratory CT and expiratory CT is in the lower lobes (Fig. 3). Both upper lobe (RUL + RML + LUL) and lower lobe (RLL + LLL) inspiratory-expiratory volume changes (measured as a percentage) decrease progressively as GOLD stage increases (p < 0.001) (Table 2). For subjects with greater disease severity by GOLD stage, there is a decreased difference (measured as a percentage) in volume change between inspiration CT and expiration CT and there is relatively greater loss in emptying of the lower lobes compared with the upper lobes (Fig. 3). For very severe COPD (GOLD stage 4), the difference in lobe volume change (measured as a percentage) among the upper lobes narrows to −3.3% compared with −10.2% for smoker control subjects (Table 2), with the negative sign indicating a greater volume change in the lower lobes compared with the upper lobes. Upper lobe–lower lobe differences in LAA-856E were determined to be statistically significant between all GOLD stages (p < 0.0001); however, the differences were largest for GOLD stages 1 and 2.
Fig. 3.
Change in lung volume (inspiratory CT– expiratory CT) in upper lobes (right upper lobe [RUL] + right middle lobe [RML] + left upper lobe [LUL]) and lower lobes (right lower lobe [RLL] + left lower lobe [LLL]). Quantitative CT parameters are shown by Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD) stage. Inspiratory-expiratory change in lung volume is normally substantially greater in lower lungs than in upper lungs, and this upper lobe–lower lobe difference is much smaller in subjects with more advanced chronic obstructive lung disease.
Airway Measures
Measures of inner diameter, inner perimeter, inner area, outer area, airway wall thickness, wall area, and Pi10 were determined. Each of these values alone showed very poor correlation to both FEV1 (r = 0.15 to −0.44) and FEV1/FVC (r = 0.19 to −0.34) for all subjects in the cohort (Table 4). We performed similar analysis for subsegmental airways with similar results for both FEV1 (r = 0.12– 0.38) and FEV1/FVC (r = 0.16–0.33).
TABLE 4.
Correlation of Inspiratory and Expiratory Quantitative CT (QCT) Results With Functional Measures
| Pulmonary Function Tests (n = 4062) |
||||
|---|---|---|---|---|
| Correlation Coefficient (r) | 95% CIs of Correlation Coefficients With 10,000 Bootstrap Replications |
|||
| QCT Results | FEV1a | FEV1/FVCb | FEV1a | FEV1/FVCb |
| Inspiratory CT | ||||
| % LAA-950I | −0.67 | −0.76 | −0.68 to −0.65 | −0.77 to −0.74 |
| Attenuation, 15th percentile | 0.55 | 0.69 | 0.53–0.58 | 0.68–0.71 |
| Expiratory CT | ||||
| % LAA-856E | −0.77 | −0.84 | −078 to −0.75 | −0.85 to −0.83 |
| Attenuation, 15th percentile | 0.71 | 0.80 | 0.69–0.72 | 0.79–0.81 |
| ΔVolume (TLCc–FRCd) | 0.41 | 0.27 | 0.39–0.44 | 0.24–0.30 |
| Δ Mean lung attenuation | 0.69 | 0.71 | 0.67–0.70 | 0.70–0.73 |
| Airway variables (segmental, fourth generation) | ||||
| Inner diameter (mm) | 0.41 | 0.34 | 0.38–0.43 | 0.31–0.37 |
| Airway wall thickness (mm) | 0.15 | 0.19 | 0.12–0.19 | 0.16–0.22 |
| Outer area (mm2) | 0.35 | 0.31 | 0.33–0.38 | 0.28–0.33 |
| Inner area (mm2) | 0.37 | 0.31 | 0.35–0.40 | 0.28–0.33 |
| Inner perimeter (mm) | 0.39 | 0.32 | 0.36–0.41 | 0.29–0.35 |
| Wall area (%) | −0.44 | −0.34 | −0.46 to −0.41 | −0.36 to −0.31 |
| Wall area (mm2) | 0.30 | 0.28 | 0.27–0.33 | 0.25–0.31 |
| Pi10 | −0.33 | −0.22 | −0.36 to −0.30 | −0.25 to −0.19 |
Note—FEV1 = forced expiratory volume in 1 second, FVC = forced vital capacity, LAA-950I = percentage of low-attenuation areas ≤ −950 HU on inspiratory CT, LAA-856E = percentage of low-attenuation areas ≤ −856 HU on expiratory CT, TLC = total lung capacity, FRC = functional residual capacity, Pi10 = square root of the wall area of a hypothetical airway of 10-mm internal perimeter.
FEV1 is the amount of air that can be forcibly exhaled from the lungs in the first second of a forced exhalation.
The FEV1/FVC ratio is the percentage of the total amount of air exhaled from the lungs during the first second of forced exhalation.
Total lung capacity: the amount of air in the lungs after inhaling as deep as possible (inspiratory CT segmented lung volume).
Functional residual capacity: the amount of air in the lungs at the end of a normal exhaled breath (expiratory CT segmented lung volume)
Multiple Regression Model
Six segmental airway variables (outer area, inner area, inner diameter, inner perimeter, airway wall thickness, and Pi10) were combined into a multiple regression model along with change in CT volume, change in mean lung attenuation value, LAA-950I, and LAA-865E to predict values of FEV1 and FEV1/FVC. Of these variables, three were determined to be statistically useful to the model: inner diameter, airway wall thickness, and Pi10; these three variables gave a strong correlation to FEV1 and FEV1/FVC (R2 = 0.72 and 0.77, respectively). Table 3 gives the results of these models and the partial R2 provided by each included variable. When these models were repeated using subsegmental airway data in place of segmental airway data, correlations to both FEV1 and FEV1/FVC were unaffected (R2 = 0.71 and 0.76, respectively). When Pi10 was removed from the model that included fourth-generation airway measures, correlations to both FEV1 and FEV1/FVC did not noticeably change (R2 = 0.70 and 0.76, respectively). Because of the large number of subjects in this study, we can be sure of a small effect on the overall R2 values for variables included in the model; however, all were considered to be statistically significant (p < 0.0001).
Discussion
The results of this investigation strongly support our hypothesis that QCT assessments of inspiratory and expiratory low-attenuation areas correlate with airflow obstruction assessed by measures of FEV1 and FEV1/FVC and that these parameters increase in severity with increasing GOLD stage. Statistical analysis suggests that CT-determined LAA-856E is most strongly associated with decline in airflow in patients with COPD. Lobe-by-lobe analysis suggests that low-attenuation area measures on both inspiratory and expiratory CT in all lung lobes contribute to physiologic impairment. Differences between the upper lobes and lower lobes are greatest for LAA-856E in patients with mild GOLD stage 1 and stage 2 COPD; these findings suggest that there are regional differences in early small airways disease, with small airways disease being predominant in the upper lobes. There is less of a difference between upper lobe and lower lobe LAA-856E values in patients with severe GOLD stage 4 COPD than in those with the lower GOLD stages of COPD.
CT is uniquely able to detect, classify, and quantify LAA-950I in adults. Quantitative assessment of low-attenuation areas is most often based on determining the percentage of lung pixels below a specific threshold, such as −910 or −950 HU (density mask technique) [28–32]. The relationship between the extent of LAA-950I on QCT and the presence of pathologic emphysema is well established [28]. Bankier et al. [30] reported that QCT measurements correlated better with macroscopic measurements of emphysema than visual CT scoring. Madani et al. [32] reported that the best correlation between QCT and macroscopic and microscopic measures of emphysema was obtained using a density mask technique with a threshold level of −960 or −970 HU, although the correlation for a threshold of −950 HU was almost as high. Because of concerns that a threshold of −960 or −970 HU might exclude milder degrees of emphysema, we selected a threshold of −950 HU for the purposes of this study. As in our study, several previous QCT studies have shown moderate correlations between CT measures of LAA-950I and FEV1 and FEV1/FVC ratio [15–18].
Multiple studies of individuals with asthma have shown that there is a strong relationship between QCT evidence of expiratory air trapping and spirometric abnormality [33–36]. Most of these studies have used a threshold-based density mask technique similar to that used in our study, with threshold values ranging between −856 and −910 HU. In subjects with severe asthma, Busacker et al. [34] used a threshold for assessing air trapping of −850 HU on chest CT scans obtained at FRC. The selection of −856 HU as the threshold for air trapping on expiratory CT is based on the fact that −856 HU is the mean attenuation of normally inflated lung (≈ 6 mL air per gram of lung) on inspiration [36]: Therefore, in a normal lung, the attenuation should be higher than −856 HU on expiration. There have been relatively few studies that have evaluated the relationship between expiratory CT and airflow limitation in cigarette smokers with or without COPD [19, 20]. The results of our study confirm that there is a strong relationship between air trapping measured on expiratory CT and expiratory airflow obstruction assessed by FEV1 and FEV1/FVC ratio; these findings suggest that this measure provides a robust independent measure of airflow obstruction.
Revel et al. [37] have documented that lobar segmentation of LAA-950I correlates with visual assessment of emphysema, but we are unaware of other studies that have evaluated the quantitative lobar extent of emphysema and air trapping and their relationship to COPD severity. Our study suggests that smokers with normal spirometry results and those with mild COPD are characterized by air trapping and emphysema that is more marked in the upper lungs. In patients with more severe COPD, emphysema and air trapping are more diffuse and the difference between the upper and lower lobes decreases. For subjects with greater disease severity as measured by GOLD stage, the percentage difference in volume change between inspiratory CT and expiratory CT is less compared with the lower GOLD stages and there is loss in the ability to empty the lower lobes compared with the upper lobes on expiratory imaging.
Coxson [38] suggested that airways that are responsible for airflow limitations (< 2 mm internal diameter) are smaller than available QCT resolution. Our findings agree with that theory. At either the segmental (fourth-generation) or subsegmental (fifth-generation) airway level, there is modest correlation between functional or density measures and available airway measures. Measurement beyond the subsegmental level, however, is likely to be limited by CT spatial resolution and is highly dependent on CT scanner and protocol parameters. Although Hasegawa et al. [39] described improved correlation coefficients (r) with FEV1 for airways becoming smaller from segmental (fourth generation) to subsegmental (fifth generation) and successively smaller sixth and seventh generation airways in selected pathways, our analysis shows little difference in correlation coefficients with FEV1 for segmental (r = 0.15–0.41) and subsegmental (r = 0.12–0.38) airway levels (data not shown for subsegmental airways). Tight clustering and similar measurements of airway luminal area across FEV1 at the subsegmental level (Fig. 4), for example, may indicate airway measurements approaching a spatial limitation constraint due to CT voxel size. Although our analyses indicate that currently available airway measures may not be useful to independently predict spirometric values, we have shown that including them in a multiple regression model along with density measures will better predict functional measures of the lungs [40, 41]. It is important to point out that these limitations of airway measurements are specific only to correlation between QCT airway measures and spirometry. Han et al. [22] have recently shown that airway wall measurements may be useful for predicting acute exacerbations of COPD.
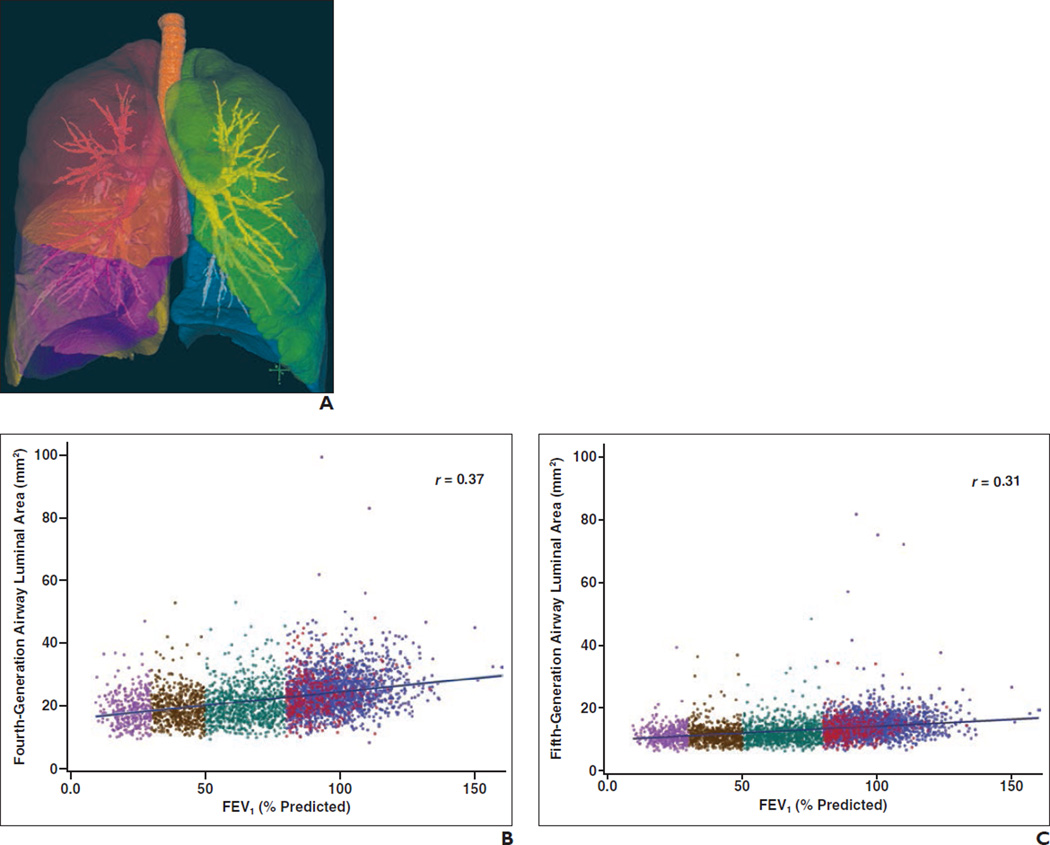
Fig. 4.
Inspiratory airway luminal area and forced expiratory volume in 1 second (FEV1) grouped by disease severity as measured by Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD) staging system for 4062 subjects.
A, Sample airway tree with lobar overlay in a subject with a smoking history of at least 10 pack-years. Red = right upper lobe, purple = right middle lobe, brown = right lower lobe, green = left upper lobe, blue = left lower lobe.
B and C, Scatterplots show inspiratory airway luminal area in segmental (fourth-generation) (B) and subsegmental (fifth-generation) (C) airways and FEV1 by GOLD stage. Line shows best-fit linear correlation.. Blue = control subjects (smokers without COPD), red = subjects with GOLD stage 1 disease, green = subjects with GOLD stage 2 disease, brown = subjects with GOLD stage 3 disease, purple = subjects with GOLD stage 4 disease.
QCT is associated with multiple limitations. There is substantial variation in measurement of CT attenuation of the lung by different scanner models; reconstruction algorithms; and CT protocol parameters including voxel size, tube voltage (kVp), and tube current–exposure time product (mAs); these variations support the importance of phantom studies [42]. Additionally, the CT attenuation values are affected by variation in inspiratory and expiratory lung volumes and acquisition techniques. However, the strong correlations identified in this study suggest that variation due to these technical factors may be relatively small.
There were several limitations to this study. Because of the size and number of associated clinical centers, a large variety of CT scanner manufacturers and models were represented in this study; however, the protocols were designed to minimize these differences. Furthermore, although phantom lung objects were scanned for this study, no corrections were performed to ensure consistent measures across scanners. Last, full-body plethysmography was not performed as part of the COPDGene Study; therefore, measures such as diffusion capacity of the lung for carbon monoxide (Dlco) and true TLC were not determined.
We conclude that QCT measurements of inspiratory and expiratory low-attenuation areas are strongly associated with spirometric impairment in cigarette smokers. Although univariate correlation between airway measures and spirometric impairment is less strong, inclusion of these measures in the multiple regression model strengthens the correlation. In particular, air trapping on expiratory imaging measured as LAA-856E strongly correlates with physiologic measurements of airway obstruction.
Acknowledgments
Supported by the National Institutes of Health (grants U01 HL089897 and U01 HL089856, to COPDGene Study), Siemens Healthcare, and the COPD Foundation.
D. A. Lynch is a consultant to Perceptive Informatics, InterMune, and Gilead and receives research support from Centocor and Siemens Healthcare.
J. D. Schroeder receives research support from Siemens Healthcare.
We gratefully acknowledge the help and expertise of the following members of the National Jewish Health Quantitative Imaging Laboratory: Alex Kluiber, Demitry Kazlouski, Deanna Richert, Mustafa Al Qaisi, Tom Gethin-Jones, and Tanya Mann. Additionally, we are grateful for assistance from James Crapo and Edwin Silverman and from the COPDGene Investigators.
References
- 1.Miniño AM, Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2008. Natl Vital Stat Rep. 2011;59:1–126. [PubMed] [Google Scholar]
- 2.Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. Chronic obstructive pulmonary disease surveillance: United States, 1971–2000. MMWR Surveill Summ. 2002;51:1–16. [PubMed] [Google Scholar]
- 3.Løkke A, Lange P, Scharling H, Fabricius P, Vestbo J. Developing COPD: a 25 year follow up study of the general population. Thorax. 2006;61:935–939. doi: 10.1136/thx.2006.062802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Regan EA, Hokanson JE, Murphy JR, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7:32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedlander AL, Lynch D, Dyar LA, Bowler RP. Phenotypes of chronic obstructive pulmonary disease. COPD. 2007;4:355–384. doi: 10.1080/15412550701629663. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan DC. Imaging as a quantitative science. Radiology. 2008;248:328–332. doi: 10.1148/radiol.2482080242. [DOI] [PubMed] [Google Scholar]
- 7.Buckler AJ, Schwartz LH, Petrick N, et al. Data sets for the qualification of volumetric CT as a quantitative imaging biomarker in lung cancer. Opt Express. 2010;18:15267–15282. doi: 10.1364/OE.18.015267. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman EA, Simon BA, McLennan G. State of the art: a structural and functional assessment of the lung via multidetector-row computed tomography— phenotyping chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3:519–532. doi: 10.1513/pats.200603-086MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez FJ, Foster G, Curtis JL, et al. NETT Research Group. Predictors of mortality in patients with emphysema and severe airflow obstruction. Am J Respir Crit Care Med. 2006;173:1326–1334. doi: 10.1164/rccm.200510-1677OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chatila WM, Hoffman EA, Gaughan J, Robinswood GB, Criner GJ. Advanced emphysema in African-American and white patients: do differences exist? Chest. 2006;130:108–118. doi: 10.1378/chest.130.1.108. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman EA, Jiang R, Baumhauer H, et al. Reproducibility and validity of lung density measures from cardiac CT scans: the Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Acad Radiol. 2009;16:689–699. doi: 10.1016/j.acra.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tschirren J, Hoffman EA, McLennan G, Sonka M. Segmentation and quantitative analysis of intrathoracic airway trees from computed tomography images. Proc Am Thorac Soc. 2005;2:484–487. 503–504. doi: 10.1513/pats.200507-078DS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palágyi K, Tschirren J, Hoffman EA, Sonka M. Quantitative analysis of pulmonary airway tree structures. Comput Biol Med. 2006;36:974–996. doi: 10.1016/j.compbiomed.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Hoffman EA, Reinhardt JM. Atlas-driven lung lobe segmentation in volumetric X-ray CT images. IEEE Trans Med Imaging. 2006;25:1–16. doi: 10.1109/TMI.2005.859209. [DOI] [PubMed] [Google Scholar]
- 15.Arakawa A, Yamashita Y, Nakayama Y, et al. Assessment of lung volumes in pulmonary emphysema using multidetector helical CT: comparison with pulmonary function tests. Comput Med Imaging Graph. 2001;25:399–404. doi: 10.1016/s0895-6111(01)00004-0. [DOI] [PubMed] [Google Scholar]
- 16.Crausman RS, Ferguson G, Irvin CG, Make B, Newell JD., Jr Quantitative chest computed tomography as a means of predicting exercise performance in severe emphysema. Acad Radiol. 1995;2:463–469. doi: 10.1016/s1076-6332(05)80400-3. [Erratum in Acad Radiol 1995; 2:870] [DOI] [PubMed] [Google Scholar]
- 17.Park KJ, Bergin CJ, Clausen JL. Quantitation of emphysema with three-dimensional CT densitometry: comparison with two-dimensional analysis, visual emphysema scores, and pulmonary function test results. Radiology. 1999;211:541–547. doi: 10.1148/radiology.211.2.r99ma52541. [DOI] [PubMed] [Google Scholar]
- 18.Zompatori M, Fasano L, Mazzoli M, et al. Spiral CT evaluation of pulmonary emphysema using a low-dose technique. Radiol Med (Torino) 2002;104:13–24. [PubMed] [Google Scholar]
- 19.Lee YK, Oh YM, Lee JH, et al. Quantitative assessment of emphysema, air trapping, and airway thickening on computed tomography. Lung. 2008;186:157–165. doi: 10.1007/s00408-008-9071-0. [DOI] [PubMed] [Google Scholar]
- 20.Matsuoka S, Kurihara Y, Yagihashi K, Nakajima Y. Quantitative assessment of peripheral airway obstruction on paired expiratory/inspiratory thin-section computed tomography in chronic obstructive pulmonary disease with emphysema. J Comput Assist Tomogr. 2007;31:384–389. doi: 10.1097/01.rct.0000243457.00437.10. [DOI] [PubMed] [Google Scholar]
- 21.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 22.Han MK, Kazerooni EA, Lynch DA, et al. COPDGene Investigators. Chronic obstructive pulmonary disease exacerbations in the COPDGene Study: associated radiologic phenotypes. Radiology. 2011;261:274–282. doi: 10.1148/radiol.11110173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu S, Hoffman EA, Reinhardt JM. Automatic lung segmentation for accurate quantitation of volumetric x-ray CT images. IEEE Trans Med Imaging. 2001;20:490–498. doi: 10.1109/42.929615. [DOI] [PubMed] [Google Scholar]
- 24.Nakano Y, Wong JC, de Jong PA, et al. The prediction of small airway dimensions using computed tomography. Am J Respir Crit Care Med. 2005;171:142–146. doi: 10.1164/rccm.200407-874OC. [DOI] [PubMed] [Google Scholar]
- 25.Curran-Everett D. Explorations in statistics: the bootstrap. Adv Physiol Educ. 2009;33:286–292. doi: 10.1152/advan.00062.2009. [DOI] [PubMed] [Google Scholar]
- 26.Efron B, Tibshirani RJ. An introduction to the bootstrap. New York, NY: Chapman and Hall; 1993. [Google Scholar]
- 27. [Accessed July 16, 2012];The R Project for Statistical Computing website. www.r-project.org/
- 28.Müller NL, Staples CA, Miller RR, Abboud RT. “Density mask”: an objective method to quantitate emphysema using computed tomography. Chest. 1988;94:782–787. doi: 10.1378/chest.94.4.782. [DOI] [PubMed] [Google Scholar]
- 29.Roth MD, Connett JE, D’Armiento JM, et al. Feasibility of retinoids for the treatment of emphysema study. Chest. 2006;130:1334–1345. doi: 10.1378/chest.130.5.1334. [DOI] [PubMed] [Google Scholar]
- 30.Bankier AA, De Maertelaer V, Keyzer C, Gevenois PA. Pulmonary emphysema: subjective visual grading versus objective quantification with macroscopic morphometry and thin-section CT densitometry. Radiology. 1999;211:851–858. doi: 10.1148/radiology.211.3.r99jn05851. [DOI] [PubMed] [Google Scholar]
- 31.Coxson HO, Rogers RM, Whittall KP, et al. A quantification of the lung surface area in emphysema using computed tomography. Am J Respir Crit Care Med. 1999;159:851–856. doi: 10.1164/ajrccm.159.3.9805067. [DOI] [PubMed] [Google Scholar]
- 32.Madani A, Zanen J, de Maertelaer V, Gevenois PA. Pulmonary emphysema: objective quantification at multi-detector row CT—comparison with macroscopic and microscopic morphometry. Radiology. 2006;238:1036–1043. doi: 10.1148/radiol.2382042196. [DOI] [PubMed] [Google Scholar]
- 33.Jain N, Covar RA, Gleason MC, Newell JD, Jr, Gelfand EW, Spahn JD. Quantitative computed tomography detects peripheral airway disease in asthmatic children. Pediatr Pulmonol. 2005;40:211–218. doi: 10.1002/ppul.20215. [DOI] [PubMed] [Google Scholar]
- 34.Busacker A, Newell JD, Jr, Keefe T, et al. A multivariate analysis of risk factors for the air-trapping asthmatic phenotype as measured by quantitative CT analysis. Chest. 2009;135:48–56. doi: 10.1378/chest.08-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeidler MR, Kleerup EC, Goldin JG, et al. Montelukast improves regional air-trapping due to small airways obstruction in asthma. Eur Respir J. 2006;27:307–315. doi: 10.1183/09031936.06.00005605. [DOI] [PubMed] [Google Scholar]
- 36.Newman KB, Lynch DA, Newman LS, Ellegood D, Newell JD., Jr Quantitative computed tomography detects air trapping due to asthma. Chest. 1994;106:105–109. doi: 10.1378/chest.106.1.105. [DOI] [PubMed] [Google Scholar]
- 37.Revel MP, Faivre JB, Remy-Jardin M, et al. Automated lobar quantification of emphysema in patients with severe COPD. Eur Radiol. 2008;18:2723–2730. doi: 10.1007/s00330-008-1065-z. [DOI] [PubMed] [Google Scholar]
- 38.Coxson HO. Quantitative computed tomography assessment of airway wall dimensions. Proc Am Thorac Soc. 2008;5:940–945. doi: 10.1513/pats.200806-057QC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hasegawa M, Nasuhara Y, Onodera Y, et al. Airflow limitation and airway dimensions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173:1309–1315. doi: 10.1164/rccm.200601-037OC. [DOI] [PubMed] [Google Scholar]
- 40.Nakano Y, Muro S, Sakai H, et al. Computed tomographic measurements of airway dimensions and emphysema in smokers: correlation with lung function. Am J Respir Crit Care Med. 2000;162:1102–1108. doi: 10.1164/ajrccm.162.3.9907120. [DOI] [PubMed] [Google Scholar]
- 41.Kim W, Silverman EK, Hoffman E, et al. CT metrics of airway disease and emphysema in severe COPD. Chest. 2009;136:396–404. doi: 10.1378/chest.08-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sieren JP, Newell JD, Judy PF, et al. Reference standard and statistical model for intersite and temporal comparisons of CT attenuation in a multicenter quantitative lung study. Med Phys. 2012;39:5757–5767. doi: 10.1118/1.4747342. [DOI] [PMC free article] [PubMed] [Google Scholar]