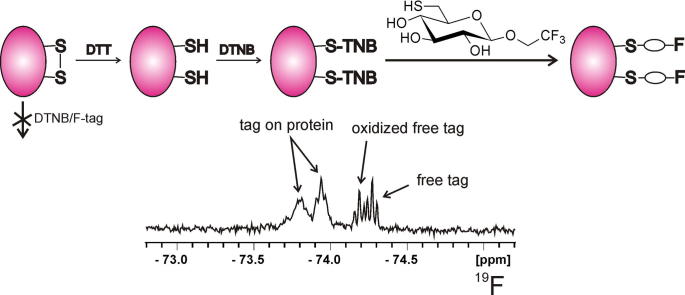
Graphical abstract
Keywords: Fluorinated glucose, NMR spectroscopy, Fischer glycosylation, Microwave irradiation, Cysteine redox state
Abstract
A synthetic route to a trifluoromethyl and thiol containing glucose derivative (2,2,2-trifluoroethyl 6-thio-β-d-glucopyranoside) is presented, which is based on microwave-assisted Fischer glycosylation under increased pressure. This water-soluble, neutral thiol-compound can be used to selectively introduce a fluorine probe for 19F NMR spectroscopy on cysteines in proteins. It can be attached under mild conditions in an aqueous environment without the risk of denaturing the protein. This tag has been applied to determine the redox-state of two cysteine residues in a bacterial transcription activator. Qualitative information about the solvent accessibility can be obtained from F-19 solvent PREs.
1. Introduction
The only naturally occurring fluorine isotope 19F constitutes one of the most versatile NMR detectable nuclei.1 Due to its high sensitivity (second most NMR sensitive element after hydrogen), virtual absence in natural organic and biological samples, and its very large chemical shift range, fluorine is often used to provide a unique perspective on structural and dynamical molecular features by solution1,2 and solid-state NMR spectroscopy.3 In particular, 19F NMR has been used for biomolecules to answer questions related to, for example, protein–protein and protein–ligand interactions,4,5 protein unfolding,6 enzymatic reactions,7 or membrane-binding.8 In order to incorporate the fluorine nucleus into the protein of interest it can be inserted biosynthetically by using fluorinated amino acid analogs or chemically attached, typically via protein –SH, –NH, or –OH groups.1 Fluorine tags in use are often rather hydrophobic and show low water solubility. Here we describe the synthesis of a fluorine tag based on glucose, containing a trifluoromethyl- and a thiol group. Glucose was the carrier substance of choice for being a water soluble, neutral, nontoxic, and cheap compound. The thiol can be used to selectively attach the compound to cysteine SH groups. The polarity due to the large number of OH groups makes it water soluble and allows the attachment to any solvent accessible, reduced cysteine in a protein. As a prerequisite to be useful for biomolecules, the tag can be attached under mild reaction conditions in aqueous environment to avoid protein denaturation. We used this tag to determine the redox state of two functionally important cysteine residues of a bacterial transcription activator protein. To get information about the solvent accessibility, 19F relaxation enhancements in a paramagnetic environment (solvent PREs)9,10 can be used. So far, solvent PREs have been used for 1H and 13C only.9 However, the large gyromagnetic ratio makes fluorine a very convenient nucleus for PREs. Their size is comparable to proton solvent PREs. For higher 19F NMR sensitivity a trifluoro group on the tag is advantageous.
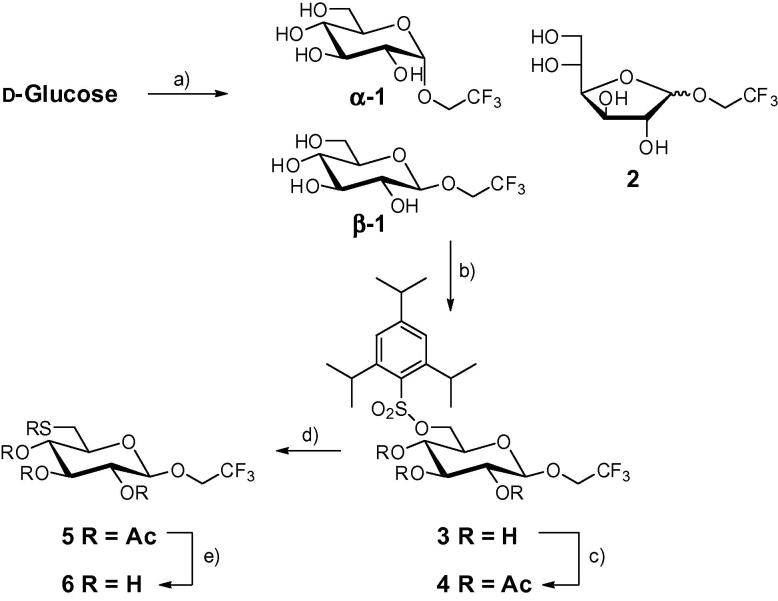
So far sugars bearing the 2,2,2-trifluoroethyl moiety as aglycone were accessible on preparative scale under Mitsunobo reaction conditions from 2,3,4,6-tetra-O-benzyl-d-glucopyranose,11 via excessive Lewis-acid promoted glycosylation on peracetylated d-glucose12,13 or 1-O-acetyl-2,3,4,6-tetra-O-benzyl-β-d-glucopyranoside,14 or via transglycosylation employing an α-d-galactosidase on 4-nitrophenyl α-d-galactopyranoside.15 Under classic Fischer glycosylation conditions—catalytic amounts of in situ formed HCl, reflux in the respective alcohol serving as reaction solvent at ambient pressure—we were unsuccessful in our efforts to react 2,2,2-trifluoroethanol (TFE) with d-glucose. This is presumably due to the fact that the sugar is insoluble in TFE, and the latter’s low nucleophilicity and boiling point. Therefore, the presented glycosylation was performed under increased pressure and microwave irradiation16 yielding the desired product.
2. Results and discussion
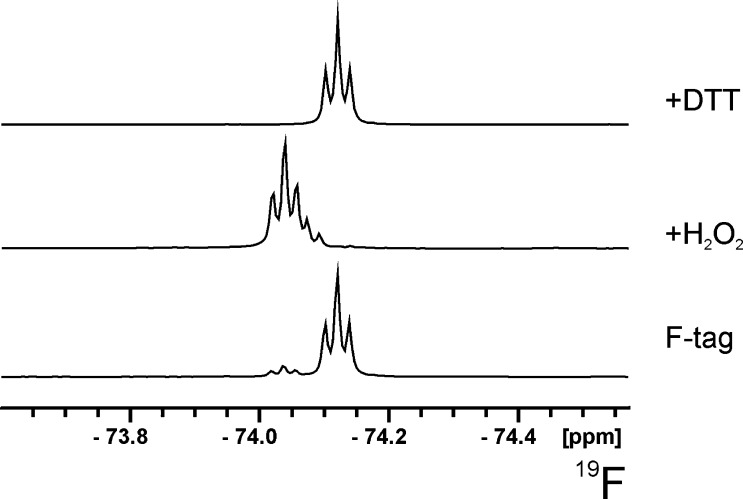
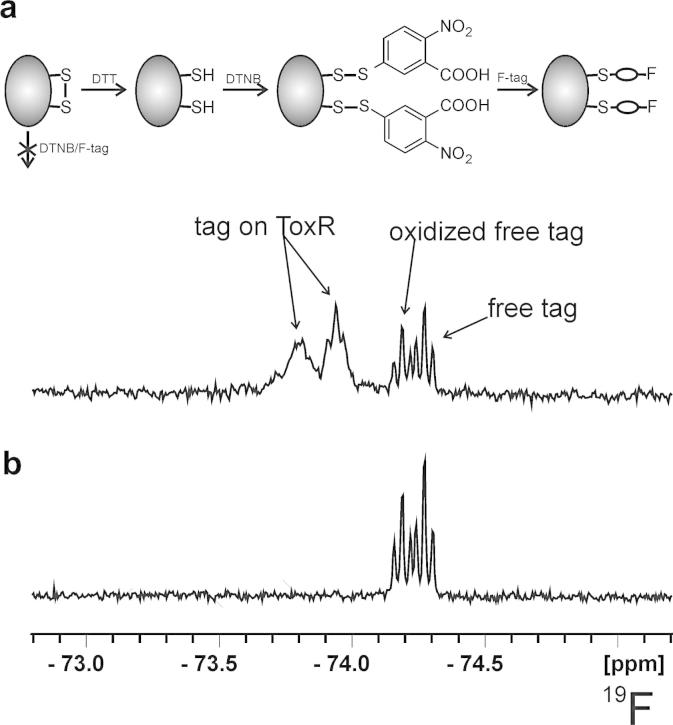
The reaction rate acceleration by means of microwave irradiation is known for the Fischer glycosylation.17 Therefore, we performed the synthesis in a sealed pressurized tube in a microwave reactor at temperatures above the melting point of d-glucose and obtained a conversion to the expected mixture of four trifluoroethyl glucosides (Scheme 1) with the anomeric pair of pyranosides 1 being the predominant product (∼75%). Although 2′,2′,2′-trifluoroethyl α-d-glucopyranoside α-1 was the major product (60%) the only isomer that could be isolated with satisfactory purity was found to be the β-anomer β-1. The other isomers could not be separated by silica-gel flash chromatography. The stereochemistry of α-1, β-1 and its subsequent derivatives was supported by HMBC and NOESY NMR experiments. The primary hydroxy group in β-1 was selectively converted with 2,4,6-trisisopropyl-benzenesulfonic chloride into sulfonate 3 in 73% yield. Although a successful substitution of the leaving group on carbon-6 with potassium thioacetate has been published by Ramström and co-workers for methyl 6-tosyl-α-d-mannopyranoside,18 applying this method to the 6-O-tosyl analog of 3 led to the same difficulty, which was also reported by the aforementioned group, where the conversion of methyl β-d-galactopyranoside had failed.19 As described, this problem was circumvented by acetylation of the remaining three hydroxy groups prior to the substitution reaction with potassium thioacetate. Acetylation of 3 in pyridine and acetic anhydride gave 4 in quantitative yield. The displacement with thioacetate afforded 5 in very good yield (92%). Finally, the global deprotection with sodium methoxide in methanol yielded thiol compound 6 as a white crystalline powder in 71% yield. When stored at air for several days, the 19F NMR spectrum of 6 contains two signals (see Fig. 1) where the larger one at −74.12 ppm corresponds to the tag in the reduced state and the smaller peak at −74.04 ppm to the oxidized tag. This was confirmed by reaction with DTT or H2O2 which results in spectra containing only the reduced or oxidized tag, respectively (Fig. 1). Compound 6 was attached to free thiol groups of reduced cysteines of the Vibrio cholerae transcription activator ToxR. Due to very inefficient direct reaction of 6 with free thiol groups, the latter were first activated with Ellman’s reagent 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB).20,21 The better leaving group 5-thio-2-nitrobenzoic acid allows much higher yields by disulfide interchange. Without activation, tagging with 6 gave less than 5% yield on the model compound glutathione (not shown), while after activation with DTNB the reaction gave 90–95% of the desired tagged compound. This procedure was applied to investigate the redox state of ToxR. It has been speculated that ToxR forms dimers through disulfide bond formation in its initial activation step, which finally leads to cholera toxin production.22 However, recently a preformed intramolecular disulfide bond has been postulated.23 To determine the redox state and solvent accessibility of the two cysteines of ToxR the protein was incubated with DTNB and then 6 both with and without prior treatment with d,l-dithiothreitol (DTT) to reduce any cysteine disulfide bonds. After chromatographic separation from the majority of excess tag 6, 19F NMR spectra were recorded. The fluorine spectrum of ToxR without prior DTT treatment (Fig. 2) only shows signals from the free tag and oxidized free tag which is always present and obviously not separated completely by the short gel filtration chromatography step. After reduction by DTT two additional broad signals appear downfield (Fig. 2) which belong to 6 attached to the two cysteine residues at positions 236 and 293 in ToxR. The additional two fluorine signals in the 19F NMR spectrum of ToxR previously treated with DTT are much broader than the one from the free tag and no scalar coupling to the neighboring CH2-group can be seen. This is a result of the slow tumbling of the protein and therefore faster T2 relaxation. In contrast, the signals of free tag and oxidized tag are much sharper and the scalar coupling to the neighboring CH2-group of 6 leads to triplets in the 19F NMR spectra. To assign the two broad protein-bound fluorine signals, single cysteine mutants (C236S and C293S) were constructed. Both mutants were much less soluble than the wild-type protein and only C293S was stable at a concentration that allowed the acquisition of a 19F spectrum. The 19F NMR spectrum of this tagged mutant is shown in the Supplementary data and contains beside free tag only the low-field signal at −73.80 ppm. Therefore, the peak at −73.95 ppm has to belong to cysteine 236. Treatment of ToxR with DTT is necessary to enable the attachment of DTNB and subsequently the fluorine tag. Therefore, both cysteines exist in the oxidized state in native ToxR, which is in accordance with the recently proposed intramolecular disulfide bond of ToxR23 and in contradiction with the previously hypothesized disulfide bond formation after interaction with ToxS.22 Considering that the two cysteines of ToxR are located in the oxidative environment of the periplasm, the formation of a disulfide seems even more realistic. To check if oxidation by oxygen during protein expression and/or purification could have produced the cysteine disulfide in ToxR, we artificially reduced ToxR by DTT and let it stand at air. Even after several weeks only very minor amounts of oxidized ToxR were found.
Scheme 1.
Reagents and conditions: (a) TFE, H+ cat., 160 °C mw, 10 min, 51%; (b) N2, 2,4,6-trisisopropylbenzene-sulfonyl chloride, pyridine, 71%; (c) Ac2O, pyridine, quant.; (d) N2, KSAc, DMF, 78 °C, 20 h, 92%; (e) N2, NaOMe, MeOH, 71%.
Figure 1.
Fluorine-19 NMR spectra of compound 6 stored under aerobic conditions and treated with H2O2 or with DTT.
Figure 2.
Schematic description of protein fluor-tagging of reduced cysteines and 19F NMR spectra of ToxR tagged with 2,2,2-trifluoroethyl 6-thio-α-d-glucopyranoside after activation with DTNB and previous DTT treatment (a) and without DTT treatment (b).
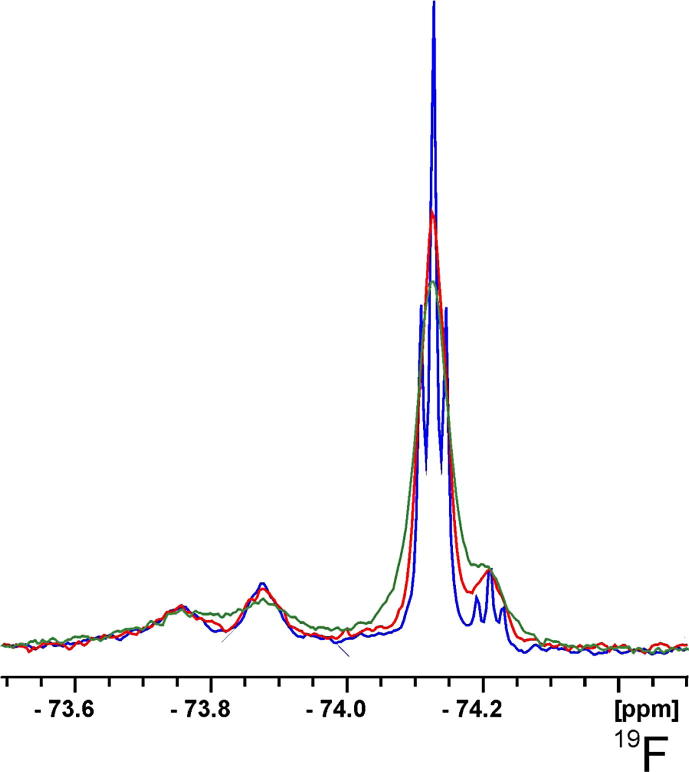
To the best of our knowledge this study is the first example of using a fluorine tag to determine the oxidation state of cysteines in proteins. The large fluorine chemical shift range and its superb sensitivity to small changes in the chemical environment allow the separation of fluorine signals due to changes in the protein environment of the individual cysteines and thus simply determining the number of reduced cysteines by counting the peaks in the 19F NMR spectrum. Information about the solvent accessibility or distance to the molecular surface of a fluorine nucleus can be obtained by monitoring 19F NMR relaxation times or line widths upon the addition of the water soluble inert paramagnetic molecule gadolinium-diethylenetriamine pentaacetic acid-bismethylamide (Gd(DTPA-BMA)). The resulting solvent PREs are larger for solvent exposed atoms and smaller the more protected the fluorine atom is. This effect has been used on proteins,9,24 membrane-bound peptides,25–28 and also small organic molecules bound to large molecular assemblies.29 Addition of Gd(DTPA-BMA) to the fluorine-tagged ToxR sample even at relatively low concentration (2 mM) of the paramagnetic agent led to significant broadening of the signals of free and oxidized tag, but almost no influence on the signals from the protein (Fig. 3). Because of its size the free tag is accessible to the paramagnetic solvent from each side, while the fluorine tag on the protein is more protected. Only at a concentration of 9 mM Gd(DTPA-BMA) more significant changes can be seen on the protein fluorine signals. When compared to solvent PREs found on proteins,9 this indicates that the tags on ToxR are somewhat protected from the solvent but are certainly not deeply buried within the protein. Solvent PREs are proportional to the observed nucleus gyromagnetic moment. Since fluorine and protons have very similar values their solvent PREs should be comparable. A very rough estimation of the insertion depth can be given comparing the influence of Gd(DTPA-BMA) on the observed fluorine signals to proton immersion depths on proteins and micelle-bound peptides.9,10,28 The distance from the paramagnetically accessible surface of the fluorine tags has to be between 3 and 15 Å. However, it should be mentioned that so far the theory of solvent PREs has not been developed for 19F and any quantitative interpretation should be treated with caution. While the oxidation state of cysteines can be determined by UV/Vis or MS measurements of proteins reacted with DTNB,20,30 the use of fluorine tagging and 19F NMR not only allows the determination of surface accessibility by solvent PREs, but could for example also be used to monitor protein–protein or protein–ligand interactions, structural changes of the protein upon changing environmental conditions, or follow chemical reactions. In contrast to the traditional UV/Vis based determination of cysteine redox states by DTNB, the exact protein concentration does not need to be known for the presented NMR approach and it also works if the reaction with DTNB would not proceed to completion for whatever reason.
Figure 3.
Fluorine-19 NMR spectra of tagged ToxR before (blue) and after the addition of 2 mM (red) and 9 mM (green) Gd(DTPA-BMA).
In conclusion, we have synthesized a water-soluble glucose based fluorine-tag, which can easily be attached selectively to cysteines in proteins. This compound can be covalently bound under very mild conditions at neutral pH values, which makes it a favorable tag for biomolecules. As an example it has been attached to a bacterial transcription activator to determine the redox state of its two cysteines by 19F NMR spectroscopy. Qualitative information about the solvent accessibility can be obtained from solvent PREs on the fluorine NMR signals.
3. Experimental
3.1. General methods
All chemicals were purchased from Sigma Aldrich (St. Louis, MO, USA). A microwave synthesis reactor monowave 300 by Anton Paar (Graz, Austria) was used. Analytical TLC was performed on precoated aluminum plates Silica Gel 60 F254 (Merck), detected with UV light (254 nm), dipped either in p-anisaldehyde (6 vol % p-anisaldehyde and 1 vol % H2SO4 conc. in EtOH), or ceric ammonium molybdate (100 g ammonium molybdate tetrahydrate and 4 g ceric sulfate dihydrate in 1 L H2SO4 10%) and developed by a heatgun. For flash chromatography Silica Gel 60 220–440 mesh (Merck) was used. All 1H, 13C, and most 19F NMR spectra were recorded on a Bruker Avance III 300 MHz spectrometer at 298 K. They were referenced to the residual non-deuterated solvent peak. Qualitative 19F solvent PREs were acquired at 300 K on a Bruker Avance III 500 MHz NMR spectrometer, using a 5 mm SEF probe operating at a F-19 frequency of 470 MHz. They were obtained by addition of a 60 mM stock solution of Gd(DTPA-BMA) to final concentrations of 2 and 9 mM. Gd(DTPA-BMA) was purified from the commercially available contrast agent Omniscan® as described previously.10 Optical rotations were measured on a Perkin Elmer 341 polarimeter at 589 nm and a path length of 10 cm. High resolution mass spectra were recorded on a Waters GCT Premier equipped with Electron impact (EI, 70 eV) and direct insertion.
3.2. Cysteine tagging via disulfide bond formation
The attachment of 6 to reduced cysteines in proteins was tested on the Vibrio cholerae transcription activator ToxR. The purified protein was dialyzed extensively against 50 mM Tris buffer (pH 7.6) before applying 1 ml of the protein solution (2 mg/ml) to a PD MidiTrap G-25 Column (Life Technologies) equilibrated with the same buffer. The protein was eluted from the column with 1.5 ml of the Tris buffer. The fractions containing protein were pooled and DTNB (dissolved in Tris buffer and adjusted to approximately pH 7 with 1 M Tris buffer pH 8.0) was added to a final concentration of 10 mM. The resulting light yellow mixture was incubated at room temperature for 30 min prior to being applied to the newly equilibrated column. Again the protein solution was eluted with 1.5 ml of the Tris buffer, the protein fractions united and once more applied to the equilibrated column. This step was repeated twice to remove the excess DTNB, which could be monitored easily due to the yellowish color of the DTNB. In a next step, 6 dissolved in the same Tris buffer (colorless) was added to the protein solution in a final concentration of 5 mM and the resulting light yellow mixture was incubated at room temperature for another 30 min before being applied to the equilibrated column. Again the protein solution was eluted with 1.5 ml of the Tris buffer and the combined protein fractions once more applied to the column. This step was repeated twice. In parallel another ToxR sample of the same concentration but pre-treated with DTT was tagged. For this purpose DTT dissolved in the same Tris buffer was added to the protein solution to a final concentration of 10 mM. This mixture was incubated at room temperature for approximately 45 min prior to being applied to a PD MidiTrap G-25 Column. All subsequent steps were then carried out in the exact same way as described above for the untreated ToxR sample. For the NMR measurements D2O (10% v/v) was added for field/frequency locking.
3.3. 2,2,2-Trifluoroethyl β-d-glucopyranoside (β-1)
In a 30 ml microwave reactor vessel fine powdered d-glucose (1.35 g, 7.5 mmol) was suspended in 2,2,2-trifluoroethanol (15 ml). Acetic chloride (10 μL, 0.14 mmol) was added, the tube was sealed by a Teflon lid and the suspension was stirred (600 rpm) at 160 °C for 10 min. After cooling to rt the resulting yellowish solution was quenched with triethylamine (100 μL) and concentrated under reduced pressure to give a tan colored foam. The residue was purified by flash chromatography (200 g SiO2, eluted with 5% EtOH in EtOAc, 1.5 L, then 10% EtOH in EtOAc, 500 ml) which gave β-d-glucopyranoside β-1 (160 mg, 8%) as amorphous white solid in addition to a mixture of other isomers (835 mg, 43%). Fractions containing mainly α-1 were purified twice again to give the material sufficiently clean for NMR and optical rotation.
Compound β-1: Rf = 0.59 (20% EtOH in EtOAc), −20 (c 1.4, MeOH), 1H NMR (CD3OD) δ 4.40 (d, J = 7.7 Hz, 1H, H-1), 4.29 (dq, 3JH,F = 9.1 Hz, 2JH,H = 12.3 Hz, 1H), 4.11 (dq, 3JH,F = 8.9 Hz, 2JH,H = 12.3 Hz, 1H), 3.90 (dd, J = 1.1, 11.9 Hz, 1H), 3.74–3.63 (m, 1H), 3.43–3.28 (m, 3H), 3.24 (dd, J = 7.8, 8.8 Hz, 1H, H-2); 13C NMR (CD3OD) δ 125.4 (q, 1JC,F = 277 Hz, C-2′), 104.1 (C-1), 78.1, 77.8, 74.7, 71.4, 66.6 (q, 2JC,F = 35 Hz, C-1′), 62.6 (C-6); 19F NMR (CD3OD) δ −75.6 (t, 3JH,F = 9.0 Hz);
Compound α-1: Rf = 0.52 (20% EtOH in EtOAc), +120 (c 4.95, MeOH), 1H NMR (CD3OD) δ 4.90 (d, J = 3.7 Hz, 1H, H-1), 4.13 (dq, 3JH,F = 9.1 Hz, 2JH,H = 12.4 Hz, 1H, H-1′), 4.03 (dq, 3JH,F = 8.9 Hz, 2JH,H = 12.4 Hz, 1H, H-1′), 3.82 (dd, J = 2.2, 11.8 Hz, 1H, H-6), 3.72–3.61 (m, 2H), 3.58 (ddd, J = 2.2, 5.5, 9.8 Hz, 1H, H-5), 3.44 (dd, J = 3.7, 9.8 Hz, 1H, H-2), 3.35–3.26 (m, 1H, H-4); 13C NMR (CD3OD) δ 125.5 (q, 1JC,F = 278 Hz, C-2′), 100.8 (C-1), 74.5 (C-3), 74.3 (C-5), 73.1 (C-2), 71.4 (C-4), 65.5 (q, 2JC,F = 35 Hz, C-1′), 62.4 (C-6); 19F NMR (CD3OD) δ −75.4 (t, 3JH,F = 9.0 Hz).
3.4. 2′,2′,2′-Trifluoroethyl 6-O-(2,4,6-trisisopropybenzenesulfonyl)-β-d-glucopyranoside (3)
Under nitrogen protection β-1 (285 mg, 1.1 mmol) was dissolved in dry pyridine (4 ml) and cooled to 0 °C. 2,4,6-Trisisopropylbenzenesulfonyl chloride (97%, 440 mg, 1.4 mmol) was added, the ice bath was removed 50 min later and the clear slightly orange reaction mixture was stirred for 3 d at rt after which time the reaction was quenched with water (50 ml) and extracted with EtOAc (3 × 25 ml). The combined organic layers were washed with HCl (1 M, 2 × 30 ml) and saturated NaHCO3 (30 ml), dried (MgSO4), filtered, and concentrated under reduced pressure. The residue was purified by flash chromatography (30 g SiO2, eluted with 30% cyclohexane in EtOAc, 350 ml) which gave compound 3 as white solid (417 mg, 71%). Rf = 0.22 (30% cyclohexane in EtOAc), −23.8 (c 4.29, CHCl3); 1H NMR (CDCl3) δ 7.19 (s, 2H), 4.79 (br s, 3H, D2O-exchangeable), 4.44 (d, J = 7.5 Hz, 1H, H-1), 4.35 (dd, J = 4.0, 10.3 Hz, 1H, H-6), 4.23 (d, J = 10.3 Hz, 1H, H-6), 4.17–3.82 (m, 4H), 3.70–3.50 (m, 3H, H-3, H-4, H-5), 3.44 (dd, J = 8.2, 8.4 Hz, 1H, H-2), 2.91 (hept, J = 6.8 Hz, 1H), 1.34–1.14 (m, 18H); 13C NMR (CDCl3) δ 154.0, 151.0, 129.0, 123.9, 123.6 (q, 1JC,F = 278 Hz, C-2′), 102.3 (C-1), 75.9 (C-3), 73.7 (C-5), 73.1 (C-2), 69.4 (C-4), 68.0 (C-6), 65.8 (q, 2JC,F = 35 Hz, C-1′), 34.3, 29.8, 24.8, 24.6, 23.6; 19F NMR (CDCl3) δ −74.0 (t, 3JH,F = 8.6 Hz); HRMS (EI TOF): [M–CF3CH2OH]+ calcd for C21H32F3O7S: 428.1869, found: 428.1881.
3.5. 2′,2′,2′-Trifluoroethyl 2,3,4-tri-O-acetyl-6-O-(2,4,6-trisisopropybenzenesulfonyl)-β-d-glucopyranoside (4)
Under nitrogen protection 3 (387 mg, 0.73 mmol) was dissolved in dry pyridine (5 ml, 62 mmol) and acetic anhydride (2 ml, 21 mmol) was added. The resulting solution was stirred overnight at rt. The reaction was quenched with water (40 ml) and extracted with EtOAc (2 × 25 ml), washed with HCl (1 M, 2 × 30 ml) and saturated NaHCO3 (35 ml), dried (MgSO4), filtered, and concentrated under reduced pressure to give compound 4 as a colorless glass (479 mg, 100%), which was used without further purification in the following step. Rf = 0.61 (50% EtOAc in cyclohexane), +0.38 (c 2.9, CHCl3); 1H NMR (CDCl3) δ 7.19 (s, 2H), 5,21 (t, J = 9.5 Hz, 1H, H-3), 4.97 (dd, J = 7.9, 9.6 Hz, 1H, H-2), 4.92 (dd, J = 9.5, 9.8 Hz, 1H, H-4), 4.61 (d, J = 7.9 Hz, 1H, H-1), 4.17–3.80 (m, 7H), 2.91 (hept, J = 6.9 Hz, 1H), 2.03 (s, 3H), 2.00 (s, 3H), 1.99 (s, 3H), 1.28–1.22 (m, 18H); 13C NMR (CDCl3) δ 170.2, 169.5, 169.4, 154.3, 151.0, 129.1, 124.0, 123.4 (q, 1JC,F = 278 Hz, C-2′), 100.7 (C-1), 72.5 (C-5), 72.2 (C-3), 70.7 (C-2), 68.6 (C-4), 66.8 (C-6), 65.9 (q, 2JC,F = 35 Hz, C-1′), 34.4, 29.8, 24.7, 23.6, 20.7, 20.7, 20.6, 20.6, 20.5, 20.5; 19F NMR (CDCl3) δ −74.5 (t, 3JH,F = 8.5 Hz); HRMS (EI TOF) [M–H]+ calcd for C29H40F3O11S: 653.2244, found: 653.2266.
3.6. 2′,2′,2′-Trifluoroethyl 2,3,4-tri-O-acetyl-6-S-acetyl-β-d-glucopyranoside (5)
Under nitrogen protection 4 (448 mg, 0.68 mmol) was dissolved in anhydrous DMF (4.5 ml) and potassium thioacetate (98%, 400 mg, 3.43 mmol, 5 equiv) was added. The clear yellow solution was heated to 78 °C for 20 h. The reaction mixture was diluted with water (50 ml) and extracted with EtOAc (3 × 20 ml). The combined organic layers were washed with brine (20 ml), dried (MgSO4), filtered, and concentrated under reduced pressure. The residue was dissolved in CH2Cl2 (∼5 ml) and purified by flash chromatography (37 g SiO2, eluted with 25% EtOAc in cyclohexane, 400 ml). The chromatographic purification was repeated (28 g SiO2, eluted with 25% EtOAc in cyclohexane, 200 ml) and gave the title compound 5 as viscous oil (280 mg, 92%). Rf = 0.19 (33% EtOAc in cyclohexane), −18.1 (c 3.31, CHCl3); 1H NMR (CDCl3) δ 5.14 (dd, J = 9.3, 9.5 Hz, 1H, H-3), 5.00–4.86 (m, 2H), 4.56 (d, J = 7.9 Hz, 1H, H-1), 4.05 (dq, J = 8.8, 12.4 Hz, 1H, H-2′), 3.89 (dq, J = 8.3, 12.4 Hz, 1H, H-2′), 3.60 (m, 1H, H-5), 3.21 (dd, J = 1.5, 14.4 Hz, 1H, H-6), 3.02 (dd, J = 6.6, 14.4 Hz, 1H, H-6), 2.29 (s, 3H, SAc), 2.02 (s, 3H), 1.98 (s, 3H), 1.94 (s, 3H); 13C NMR (CDCl3) δ 194.5 (SAc), 170.1, 169.7, 169.3, 123.4 (q, 1JC,F = 279 Hz, C-2′), 100.5 (C-1), 73.3 (C-5), 72.3 (C-3), 70.8, 70.3, 65.7 (q, 2JC,F = 35 Hz, C-1′), 30.4, 30.4, 29.9 (C-6), 20.7, 20.6, 20.6, 20.5, 20.4, 20.4; 19F NMR (CDCl3) δ −74.5 (t, 3JH,F = 8.5 Hz); HRMS (EI TOF): [M–H]+ calcd for C16H20F3O9S: 445.0780, found: 445.0810.
3.7. 2′,2′,2′-Trifluoroethyl 6-thio-β-d-glucopyranoside (6)
Under nitrogen protection 5 (270 mg, 0.60 mmol) was suspended in dry MeOH (3 ml) and sodium methoxide (0.34 M in MeOH, 2 ml, 0.67 mmol) was added. The resulting homogeneous mixture was stirred for 2.5 h at rt. Silica (2 g) was added and the solvent was removed under reduced pressure. The dried residue was loaded onto a flash chromatography column (28 g SiO2, eluted with EtOAc, 250 ml) which gave title compound 6 as a hygroscopic white gum (120 mg, 71%). Rf = 0.32 (in EtOAc), −17 (c 0.56, MeOH); 1H NMR (D2O, referenced with acetone as internal standard at 2.22 ppm) δ 4.60 (d, J = 7.9 Hz, 1H, H-1), 4.41–4.15 (m, 2H, H-1′), 3.56–3.36 (m, 3H), 3.33 (t, J = 8.6 Hz, 1H, H-2), 3.01 (br d, J = 14.2 Hz, 1H, H-6), 2.74 (dd, J = 7.1, 14.2 Hz, 1H, H-6); 13C NMR (D2O, referenced with acetone as internal standard at 30.89 ppm) δ 124.3 (q, 1JH,F = 278 Hz, C-2′), 103.3 (C-1), 76.7, 75.8, 73.6, 72.3, 66.9 (q, 2JC,F = 34 Hz, C-1′), 25.7 (C-6); 19F NMR (D2O) δ −74.2 (t, 3JH,F = 8.8 Hz); HRMS (EI TOF): [M–SH]+ calcd for C8H12F3O5: 245.0637, found: 245.0666, [M–H2O]+ calcd for C8H11F3O4S: 260.0330, found: 260.0350.
Acknowledgements
Financial support to K.Z. by the Austrian Science Foundation (FWF) (project numbers P20020 and P22630) is gratefully acknowledged. E.S. thanks the Austrian Academy of Sciences for a DOCfFORTE fellowship.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.carres.2012.08.010.
Supplementary data
Spectral data
References
- 1.Kitevski-LeBlanc J.L., Prosser R.S. Prog. NMR Spectrosc. 2012;62:1–33. doi: 10.1016/j.pnmrs.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Gerig J.T. Prog. NMR Spectrosc. 1994;26:293–370. [Google Scholar]
- 3.Ulrich A.S. Prog. NMR Spectrosc. 2005;46:1–21. [Google Scholar]
- 4.Papeo G., Giordano P., Brasca M.G., Buzzo F., Caronni D., Ciprandi F., Mongelli N., Veronesi M., Vulpetti A., Dalvit C. J. Am. Chem. Soc. 2007;129:5665–5672. doi: 10.1021/ja069128s. [DOI] [PubMed] [Google Scholar]
- 5.Quint P., Ayala I., Busby S.A., Chalmers M.J., Griffin P.R., Rocca J., Nick H.S., Silverman D.N. Biochemistry. 2006;45:8209–8215. doi: 10.1021/bi0606288. [DOI] [PubMed] [Google Scholar]
- 6.Bann J.G., Pinkner J., Hultgren S.J., Frieden C. Proc. Natl. Acad. Sci. U.S.A. 2002;99:709–714. doi: 10.1073/pnas.022649599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thorson J.S., Shin I., Chapman E., Stenberg G., Mannervik B., Schultz P.G. J. Am. Chem. Soc. 1998;120:451–452. [Google Scholar]
- 8.Oxenoid K., Sonnichsen F.D., Sanders C.R. Biochemistry. 2002;41:12876–12882. doi: 10.1021/bi020335o. [DOI] [PubMed] [Google Scholar]
- 9.Madl T., Bermel W., Zangger K. Angew. Chem., Int. Ed. 2009;48:8259–8262. doi: 10.1002/anie.200902561. [DOI] [PubMed] [Google Scholar]
- 10.Respondek M., Madl T., Göbl C., Golser R., Zangger K. J. Am. Chem. Soc. 2007;129:5228–5234. doi: 10.1021/ja069004f. [DOI] [PubMed] [Google Scholar]
- 11.Gueyrard D., Rollin P., Nga T.T.T., Ourevitch M., Begue J.P., Bonnet-Delpon D. Carbohydr. Res. 1999;318:171–179. [Google Scholar]
- 12.Beaver M.G., Woerpel K.A.J. Org. Chem. 2010;75:1107–1118. doi: 10.1021/jo902222a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xue J.L., Cecioni S., He L., Vidal S., Praly J.P. Carbohydr. Res. 2009;344:1646–1653. doi: 10.1016/j.carres.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Iori R., Bernardi R., Gueyrard D., Rollin P., Palmieri S. Bioorg. Med. Chem. Lett. 1999;9:1047–1048. doi: 10.1016/s0960-894x(99)00136-5. [DOI] [PubMed] [Google Scholar]
- 15.Simerska P., Kuzma M., Monti D., Riva S., Mackova M., Kren V. J. Mol. Catal. B. 2006;39:128–134. [Google Scholar]
- 16.Kappe C.O. Angew. Chem., Int. Ed. 2004;43:6250–6284. doi: 10.1002/anie.200400655. [DOI] [PubMed] [Google Scholar]
- 17.Bornaghi L.F., Poulsen S.A. Tetrahedron Lett. 2005;46:3485–3488. [Google Scholar]
- 18.Pei Z.C., Larsson R., Aastrup T., Anderson H., Lehn J.M., Ramström O. Biosens. Bioelectron. 2006;22:42–48. doi: 10.1016/j.bios.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 19.Pei Z., Dong H., Caraballo R., Ramström O. Eur. J. Org. Chem. 2007:4927–4934. [Google Scholar]
- 20.Ellman G.L. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 21.Hermanson G.T. 2nd ed. Academic Press; USA: 2008. Bioconjugate Techniques. Vol. 187. [Google Scholar]
- 22.Kolmar H., Hennecke F., Gotze K., Janzer B., Vogt B., Mayer F., Fritz H.J. EMBO J. 1995;14:3895–3904. doi: 10.1002/j.1460-2075.1995.tb00061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fengler, V. H. I.; E.C., B.; Tutz, S.; Seper, A.; Schild, S.; Reidl, PLoS One2012, submitted for publication. [DOI] [PMC free article] [PubMed]
- 24.Pintacuda G., Otting G. J. Am. Chem. Soc. 2002;124:372–373. doi: 10.1021/ja016985h. [DOI] [PubMed] [Google Scholar]
- 25.Franzmann M., Otzen D., Wimmer R. ChemBioChem. 2009;10:2339–2347. doi: 10.1002/cbic.200900347. [DOI] [PubMed] [Google Scholar]
- 26.Hohlweg W., Kosol S., Zangger K. Curr. Protein Pept. Sci. 2012;13(3):267–279. doi: 10.2174/138920312800785049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kosol S., Zangger K. J. Struct. Biol. 2010;170:172–179. doi: 10.1016/j.jsb.2009.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zangger K., Respondek M., Gobl C., Hohlweg W., Rasmussen K., Grampp G., Madl T. J. Phys. Chem. B. 2009;113:4400–4406. doi: 10.1021/jp808501x. [DOI] [PubMed] [Google Scholar]
- 29.Kosol S., Schrank E., Bukvić-Krajačić M., Wagner G.E., Meyer H., Göbl C., Zangger K., Novak P. J. Med. Chem. 2012;55(11):5632–5636. doi: 10.1021/jm300647f. [DOI] [PubMed] [Google Scholar]
- 30.Khatai L., Goessler W., Lorencova H., Zangger K. Eur. J. Biochem. 2004;271:2408–2416. doi: 10.1111/j.1432-1033.2004.04160.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Spectral data