Abstract
Background
Older adults’ peak performance on memory and cognitive inhibition tasks tends to be in the morning while younger adults’ peak performance tends to be in the afternoon. Although these tasks require efficient attentional processes for optimal performance, previous research examining age differences in the effects of time of day has not measured the distinct aspects of attention quantified by the attentional network test (ANT; Fan, McCandliss, Sommer, & Posner, 2002).
Methods
We examined the relationship between time of testing and the efficiency of alerting, orienting and executive attention networks by randomly assigning younger (18–28 years; N = 27, M = 21.37 years, SD = 2.39) and older adults (65–85 years; N = 32, M = 73.34 years, SD = 5.18) to AM or PM testing of alerting, orienting and executive attention. Mean reaction times for each network was analyzed with a univariate ANOVA with age (younger, older) and time of day (AM, PM) as between-subjects factors.
Results
Consistent with our hypotheses, while time of day had little effect on orienting or executive attention, it affected alerting in opposite ways for younger and older adults, with alerting cues benefitting performance most at participants’ off-peak times of day. A larger benefit from alerting cues was observed when participants’ were tested at their off-peak (M = 30.11 ± 15.66) relative to their peak time (M = 2.18 ± 15.97).
Conclusion
Our findings show that age-related circadian patterns influence the alerting component of attention, with both age groups showing the largest benefit from alerting cues when testing occurs at non-optimal times of day. Overall, our findings underscore the importance of controlling for time of day in investigations of attention and add to our understanding of how age differences in circadian patterns impact attention.
Without a filtering system to prioritize some aspects of the sensory environment over others, we would be overwhelmed by the staggering amount of information that bombards our senses. The attentional system plays a key role in healthy cognitive functioning by allowing for efficient processing of information most relevant to our goals. Advances in cognitive neuroscience have allowed for the localization of anatomically distinct networks that support three major subprocesses of attention: alerting, orienting, and executive attention (Fan, McCandliss, Sommer, & Posner, 2002; Posner & Peterson, 1990). The alerting network, which prepares for a stimulus by establishing and maintaining a state of vigilance, is associated with frontal and parietal activity (Coull, Frith, Frackowiak, & Grasby, 1996). The orienting network, which guides the selection of information from specific locations in visual space, is associated with activity in the parietal lobes and activity in subcortical regions that support visual reflexes (Corbetta, Kincade, & Shulman, 2002). The executive attention network is responsible for monitoring and resolving conflict among competing sources of information (e.g., color versus semantic information in the Stroop task) and is associated with activity in anterior cingulate, medial frontal and lateral prefrontal cortices (Bush, Lu, & Posner, 2000; Fan, Fossella, Sommer, Wu, & Posner, 2003).
Age Differences in Attention
Given attention’s critical role in effective cognitive functioning, it is important to gain a fuller understanding of how functioning in different component processes may change across the lifespan. The Attention Network Test (ANT; Fan et al., 2002) combines a flankers task with a cued reaction time task to provide separate behavioral measures of alerting, orienting and executive control network efficiency. On each trial, a target stimulus (e.g., an arrow pointing to the left or right) appears on the computer screen, along with distracting stimuli to the left and right (flankers). Participants are required to respond to the target stimulus, while ignoring the flankers. On congruent trials, the flankers are associated with the same response as the target (e.g., all arrows point in the same direction). On incongruent trials, the flankers are associated with the opposite response (e.g., flanking arrows point left, target arrow points right). Executive attention efficiency is measured by subtracting average response times on congruent trials from those on incongruent trials. Three warning cue conditions (no, alerting, orienting) determine the nature of the information provided in anticipation of the upcoming target. Alerting cues carry temporal information, signaling that a target is about to appear, but do not specify the target’s location. Response times on trials with alerting cues are compared with those on trials in which the target appears without any warning to measure alerting efficiency. Spatial cues are single cues that signal an upcoming target and always predict the target location. Response times on trials with orienting cues are compared with trials with alerting cues to measure orienting efficiency.
Among the limited number of studies that have used the ANT to investigate age differences in attentional networks, there is general agreement for the preservation of orienting across the life span (Gamboz, Zamarian, & Cavallero, 2010; Jennings, Dagenback, Engle & Funke, 2007; Zhou, Fan, Li, Wang, & Wang, 2011). With respect to alerting, most studies have shown reduced alerting efficiency in older relative to younger adults (Festa-Marino, Ott, & Heidel, 2004; Gamboz et al., 2010; Jennings et al., 2007; Zhou et al., 2011)1.
Relative to the age-related patterns observed for orienting and alerting network efficiency, the findings for the executive attention network have been less consistent. Some studies suggest preserved performance among older adults as compared to their younger counterparts (e.g., Fernandez-Duque & Black, 2006; Gamboz et al., 2010; Jennings et al., 2007), whereas others have shown evidence of age-related decline (e.g., Zhou et al., 2011). One possible source of inconsistent findings is methodological differences across studies that have used the ANT, such as variability in size, perceptual salience and timing of cue and target stimuli. Systematic differences in the age and cognitive status of participants across studies and whether age-related slowing is distinguished from task-specific declines may also contribute to mixed results.
Aging and Time of Day
Beyond methodological differences, previous work suggests that the time of day during which testing occurs may also contribute variability to cognitive performance when comparing younger and older adults (for a review see Yoon, May, & Hasher, 1999). Several studies have shown reliable cross-cultural similarities in age-related circadian patterns, with older adults’ peak time of functioning occurring in the morning and younger adults’ peak time of functioning tending to be later in the day (e.g., Adan & Almirall, 1990; Mecacci, Zani, Rocchetti, & Lucioli,1986; Wilson, 1990). Moreover, previous work has shown that older adults tend to prefer to carry out most daily activities in the morning, whereas younger adults prefer the afternoon (Hasher, Zacks, & May, 1999; May, Hasher, & Stoltzfus, 1993).
Age differences in the timing of optimal performance on cognitive tasks (with optimal functioning occurring at peak relative to off-peak times) are seen in judgments of timing (Lustig & Meck, 2001), implicit and explicit memory, and inhibitory control (Zacks, Hasher, & Li, 2000). Although these tasks require efficient attentional control processes for optimal performance, previous research examining age differences in the effects of time of day has not measured the anatomically and functionally distinct aspects of attention quantified by the ANT. In addition, previous investigations that have used the ANT to examine the relationship between aging and attentional processes have not controlled for time of testing. In the present study, we examined the relationship between time of testing and the efficiency of alerting, orienting and executive attention networks in younger and older adults. We examined age differences in circadian patterns of alerting, orienting and executive attention network efficiency by randomly assigning half of our younger and half of our older participants to morning and afternoon testing times.
In a study in which undergraduates completed the ANT at several times throughout the day (Matchock & Mordkoff, 2009), alerting and orienting showed no overall main effect of time of day, while executive attention showed an inverse-u-shape function, with performance lowest at 8 AM and 8 PM compared with more midday testing times. The investigators also compared those younger adults who were evening types to those who were neither morning nor evening types (there were almost no morning types among this cohort, as is typical of younger adults). Alerting was the only attention network with different trajectories for these two chronotype groups, with the neither-morning-nor-evening types showing a larger benefit in reaction times when they received a cue compared to no cue in the late afternoon into the evening. Thus, alerting cues were more beneficial when they coincided with times of day during which alertness in this group is likely to be low.
Given that younger and older adults differ in their peak times of day, we anticipated that we would see an age by time-of-day interaction in the effect of alerting cues. The alerting component of the ANT involves cueing participants to be vigilant for an upcoming target. As such, it should help performance most when tonic alertness is low, or when people are having trouble maintaining alertness in the absence of environmental input. Thus, we expected that older adults would show the greatest benefit from alerting cues in the afternoon rather than in the morning, whereas the reverse would be the case for younger adults.
As chronotype did not influence circadian rhythms for the orienting or executive attention networks in Matchock and Mordkoff’s (2009) study, it seemed less likely that we would see age by time-of-day interactions for these two networks. However, the executive attention network has been associated with several higher-order cognitive processes (Fan et al., 2002) and previous studies have found age by time-of-day interactions in other higher-order cognitive processes (Lustig & Meck, 2001; May & Hasher, 1998; Zacks et al., 2000). In particular, tasks involving cognitive control, such as the acquisition of new information, implicit and explicit use of newly acquired information and inhibitory control over irrelevant and distracting material are sensitive to age-related circadian arousal patterns (see Zacks et al., 2000, for a review). Thus, it is possible that we would see an age by time-of-day interaction for executive attention, as well as alerting.
Method
Participants
Participants are from a previously reported study that reported only the overall executive attention measure of the ANT (Mather & Knight, 2005, Experiment 2). All those who completed the ANT were included in the current study. Older adults were recruited through local radio and newspaper announcements and flyers (65–85 years; N = 32, M = 73.34 years, SD = 5.18). All older individuals were screened in a phone interview for dementia using a version of the Mini-Mental State Examination (Folstein, Folstein, & McHugh, 1975).2 The younger participants were either undergraduates or members of the community who received payment for their participation (18–28 years; N = 27, M = 21.37 years, SD = 2.39).3 Participants who traveled to campus for the study received $40 and those already on campus received $30. Older adults reported having had more years of education (M = 16.50, SD = 2.11) than younger adults did (M = 14.07, SD = 1.11), t(57) = 5.32, p < .001.
Apparatus
Stimuli were presented on a 15-in. monitor of a Power Macintosh G4 using PsyScope experimental software (Cohen, MacWhinney, Flatt, & Provost, 1993).
Stimuli
The target stimulus was a central arrow pointing to the right or left. Two flankers were presented to the left and right of the target and were one of three types: neutral (dashes), congruent (arrows pointing in the same direction), or incongruent (arrows point in the opposite direction). An asterisk (*) that served as a warning cue was presented before each target stimulus. There were four types of warning cues. Center cues were presented in the center of the screen. Double cues appeared 1.06° above and below central fixation. Spatial cues appeared either 1.06° above or below central fixation. On no cue trials, a central fixation cross was displayed, then replaced by the target stimulus. The size, location and appearance of cue, flanker and target stimulus matched the dimensions used in Fan et al. (2002).
Procedure
Participants were randomly assigned to a morning (8 AM – 10 AM) or an afternoon (2 PM – 5 PM) testing time. After giving informed consent, participants completed the Attentional Network Test (Fan et al., 2002) as part of a larger set of measures. On each trial, a centrally located fixation cross was followed by a variable 400–1600 ms delay during which a cue appeared (for 100 ms) or did not appear. At the end of the delay a row of stimuli (arrows or lines) appeared. Participants had 1700 ms to press a key to indicate which direction a central arrow pointed (right or left) before the trial timed out. On congruent trials, all of the arrows pointed in the same direction. On incongruent trials the centrally located arrow pointed in a different direction than the flanker arrows. Participants completed 24 practice trials with feedback on speed and accuracy, followed by two experimental blocks of 96 trials each (4 cues × 2 target locations × 3 flanker conditions × 2 repetitions). Individual reaction times were calculated for each age group, cue and distractor type. Consistent with other studies (e.g., Fan et al., 2002; Zhou et al., 2011), only correct responses were included in statistical analyses. To control for the possible influence of age-related slowing on the size of the effects observed, proportion scores were computed for each participant (average RT in each condition/overall RT). The same pattern of results was obtained with proportion scores, unless otherwise specified. We quantified alerting, orienting and executive attention efficiency with the cognitive subtractions specified by Fan et al. (2002). Alerting efficiency was calculated by subtracting the average double cue reaction time from the average no-cue reaction time. Orienting efficiency was calculated by subtracting the average spatial-cue reaction time from the average center-cue reaction time. Executive attention efficiency was determined by subtracting the average congruent trial reaction time from the average incongruent trial reaction time.
Results
Mean response times for each participant were calculated for the 12 different conditions (4 cue types × 3 flanker types) and are presented in Table 1. As expected, there were significant benefits of double over no cue trials (alerting: t(58) = 2.32, p < .05), of spatial over central cue trials (orienting: t(58) = 13.14, p < .001) and of congruent over incongruent trials (executive attention: t(58) = 14.37, p < .001). Replicating previous findings showing independence of the networks (e.g., Fan et al., 2002; Matchock & Mordkoff, 2009), none of the correlations between alerting and orienting (r(59) = −.20), alerting and executive attention (r(59) =.03), and orienting and executive attention (r(59) = −.08), were significant (all ps > .12).
Table 1.
Mean reaction times (and standard errors) of younger and older adults according to cue and flanker type
| Cue Type | ||||||
|---|---|---|---|---|---|---|
| Flanker | No cue | Double cue | Center cue | Spatial cue | ||
| Younger | ||||||
| AM | Neutral | 580.30 (32.74) | 532.34 (33.30) | 550.03 (31.22) | 487.22 (32.28) | |
| Congruent | 628.04 (33.65) | 588.33 (41.21) | 584.25 (33.93) | 531.05 (33.89) | ||
| Incongruent | 693.09 (41.15) | 676.65 (38.65) | 699.68 (37.50) | 659.80 (37.31) | ||
| PM | Neutral | 550.53 (33.94) | 511.37 (34.55) | 532.13 (32.40) | 476.54 (33.50) | |
| Congruent | 566.82 (34.93) | 549.80 (42.76) | 562.56 (35.21) | 510.21 (35.16) | ||
| Incongruent | 710.76 (42.70) | 701.07 (40.11) | 723.15 (38.92) | 657.81 (38.72) | ||
| Older | ||||||
| AM | Neutral | 777.21 (30.83) | 790.13 (31.42) | 815.83 (29.45) | 718.87 (30.29) | |
| Congruent | 818.53 (31.67) | 874.76 (38.88) | 828.14 (31.98) | 770.14 (31.87) | ||
| Incongruent | 980.08 (37.39) | 970.69 (34.79) | 946.19 (33.70) | 879.38 (32.65) | ||
| PM | Neutral | 790.39 (30.83) | 763.07 (31.42) | 789.10 (29.45) | 720.09 (30.29) | |
| Congruent | 845.69 (31.98) | 827.77 (38.88) | 820.10 (31.98) | 723.23 (31.87) | ||
| Incongruent | 977.31 (37.39) | 949.83 (34.79) | 954.51(33.70) | 876.36 (32.65) | ||
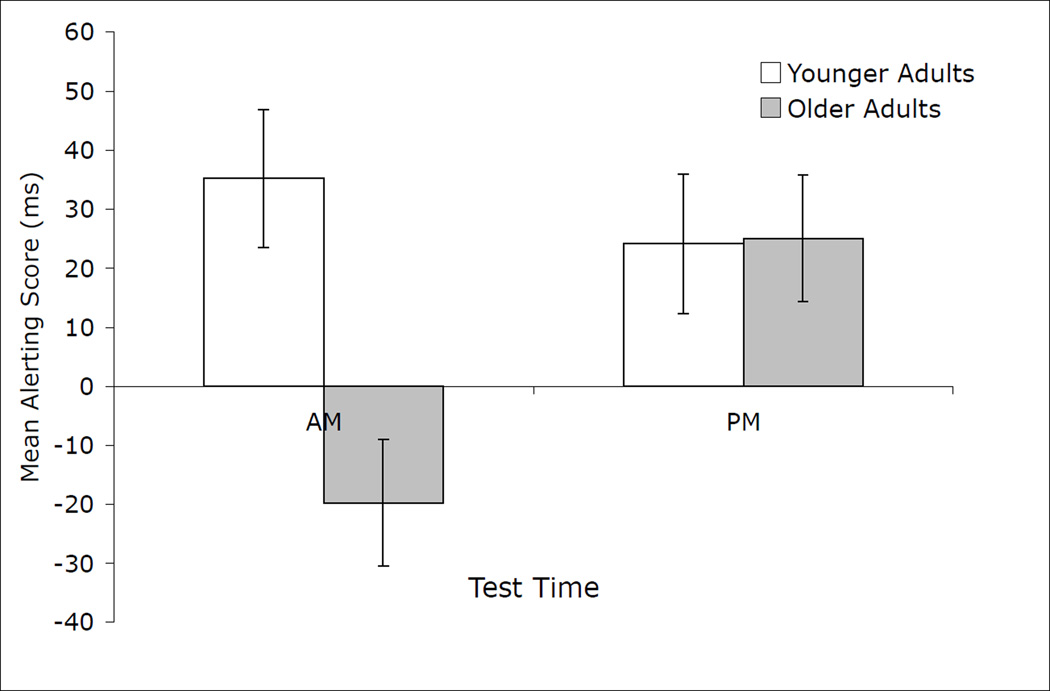
In order to see how time of day and age affected each separate attention network, we conducted separate ANOVAs on mean subtraction scores reflecting each of the three attentional processes. Mean reaction times in the alerting condition were submitted to a univariate ANOVA with age (younger, older) and time of day (AM, PM) as between-subjects factors. There was a significant main effect of age, F(1, 55) = 5.58, p < .05, ηp2 = .09. Younger adults derived a larger benefit from alerting cues (M = 29.63 ± 15.13) than older adults (M = 2.67 ± 14.95). In addition, the age by time of day interaction was significant, F(1, 55) = 5.98, p < .05, ηp2 = .10 (see Figure 1). Younger adults showed a significant alerting effect on the morning (M = 35.16 ± 22.95) and afternoon test (M = 24.10 ± 23.81). Although the alerting effect for younger adults in the morning was numerically larger than it was in the afternoon, this difference was not statistically significant, as indicated by the 95% confidence intervals. In contrast, older adults showed a significant alerting effect on the afternoon (M = 25.06 ± 21.40) but not on the morning test (M = −19.73 ± 21.40), with the confidence intervals indicating a significant difference between the two times. The same pattern of results was obtained with proportion scores to control for age-related slowing. Overall, the pattern of results suggests that both age groups benefited the most from alerting cues at their respective off-peak times of day. To test this interpretation, we conducted a univariate ANOVA with age (younger, older) and time of test (peak, off-peak) as between-subjects factors. The results showed a significant main effect of time of test, F(1, 55) = 5.98, p < .05, ηp2 = .10. A larger benefit from alerting cues was observed when participants’ were tested at their typical off-peak (M = 30.11 ± 15.66) relative to their typical peak time (M = 2.18 ± 15.97). Age did not interact with time of test (p =.15).
Figure 1.
Mean subtraction scores (ms) for the alerting network for younger and older adults according to time of testing. Alerting network efficiency was computed by subtracting mean reaction times on trials with double cues from mean reaction times on non-cued trials.
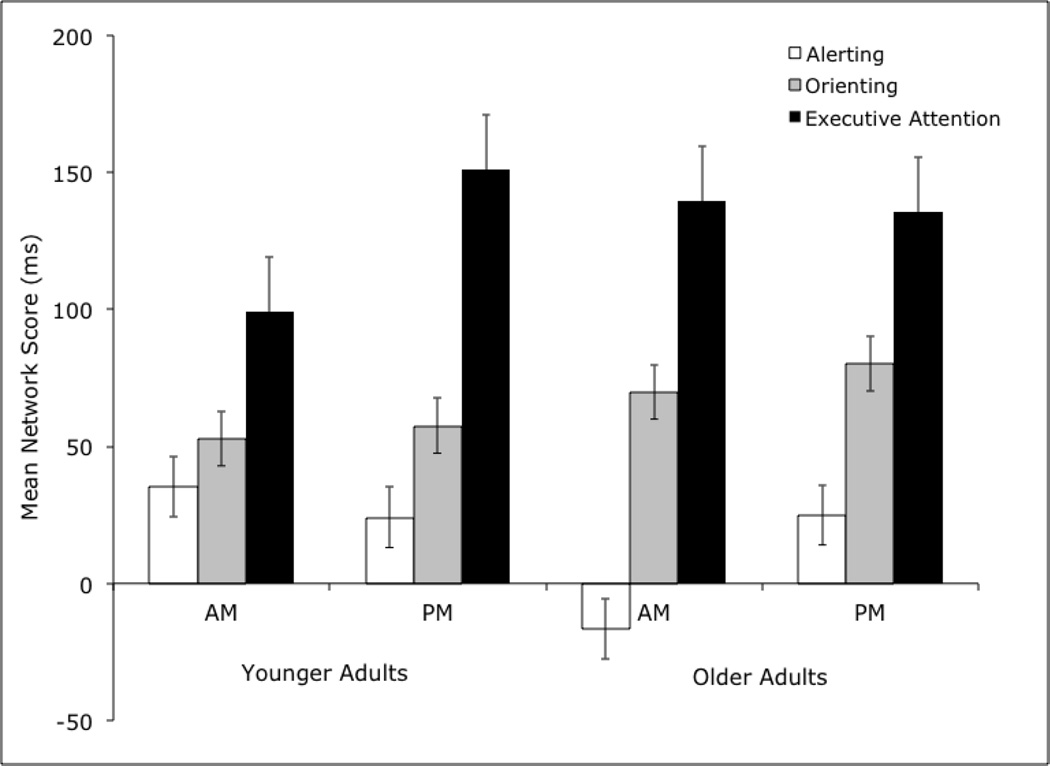
The results for orienting showed a significant main effect of age, F(1, 55) = 4.45, p < .05, ηp2 = .08. Older adults showed a larger benefit from orienting cues (M = 76.56 ± 13.41) than younger adults (M = 55.22 ± 14.68). However, the main effect of age was no longer significant after controlling for age-related slowing with proportion scores (p = .93), indicating that the age differences in orienting that emerged with the uncorrected scores may be attributable to generalized slowing. No significant main effects or interactions emerged in the executive attention analysis (see Figure 2).
Figure 2.
Mean subtraction scores (ms) for the alerting, orienting and executive attention networks for younger and older adults according to time of testing.
Discussion
Age differences in the relationship between time of day and cognitive performance have been observed across a variety of tasks that differ in their cognitive and behavioral demands (see Schmidt, Collette, Cajochen, & Peigneux, 2007; Zacks et al., 2000 for reviews). These studies have shown younger and older adults’ best cognitive performance occurring at their respective optimal time of circadian arousal (Hasher et al., 1999). Attentional processes are an important foundation for optimal cognitive functioning. This is the first study to examine how age and time of testing are related to alerting, orienting and executive control within a single experimental paradigm. As such, our study extends previous work by showing how age-related circadian patterns are associated with functioning in the anatomically and functionally distinct aspects of attention quantified by the ANT.
Our findings suggest that the efficiency of the alerting network is susceptible to modulation by age-related circadian patterns. With respect to alerting efficiency, we observed a significant interaction between age and time of day. Alerting cues were more beneficial at times of day during which subjective alertness tends to be lower rather than higher for each age group. Younger adults showed a significant alerting effect on both morning and afternoon tests. The benefit for alerting cues was larger in the morning than in the afternoon. However, this difference was not large enough to reach statistical significance. In contrast, older adults showed a significantly greater alerting effect in the afternoon than in the morning—and only showed a significant alerting effect on the afternoon test. The size of the alerting effect for older adults on the afternoon test did not significantly differ from the size of alerting effect for younger adults on the morning or afternoon tests. In a separate analysis in which older and younger adults were categorized according to whether their test time corresponded with the typical peak time for that age group, the results showed a larger alerting effect during age-typical off-peak versus peak times of testing. This main effect did not interact with age. The overall pattern of results suggests that both age groups experienced the greatest benefit from alerting cues at their off-peak time of cognitive functioning. Fan and Posner (2004) have indicated that higher alerting scores have been associated with difficulty in maintaining alertness without a cue. Our findings are consistent with this proposal in suggesting that younger and older adults benefit the most from cues that enhance alertness when age-related circadian patterns are most likely to produce low general levels of alertness. In addition, our findings suggest that age differences in alerting network efficiency are more likely to emerge when older and younger adults are tested in the morning rather than the afternoon.
After controlling for age-related slowing, orienting network efficiency did not differ as a function of age or time of test. This finding is in agreement with several other studies that have not found age differences in orienting using the ANT with younger and older adults (Fernandez-Duque & Black, 2006; Gamboz et al., 2010; Jennings et al. 2007; Zhou et al., 2011). Similarly, executive attention network efficiency was not significantly influenced by age or the time of testing manipulation. Our finding of overall age equivalence in executive attention performance is consistent with several other studies (Fernandez-Duque & Black, 2006; Gamboz et al., 2010; Jennings et al. 2007). Taken together, these findings suggest that the efficiency of the orienting and executive attention networks is not strongly modulated by age-related circadian patterns. Our study extends previous work by providing evidence consistent with the proposal that the anatomically and functionally distinct attentional networks that control alerting, orienting and executive attention may be differentially susceptible to circadian patterns.
In this and other studies that have measured executive attention network efficiency using the ANT, the absence of an age difference may seem to be at odds with previous work showing age-related deficits in measures of executive control functions and well-documented age related declines in frontal regions that mediate them (Hedden & Gabrieli, 2004; Raz, 2000). However, the tasks used to measure these functions are diverse and are not designed to measure separate components of attention that are distinctly quantified by the ANT. For example, age-related inhibitory deficits have been observed in the Stroop task (e.g., Brink & McDowd, 1999; Wurm, Labouvie-Vief, Aycock, Rebucal, & Koch, 2004), in which the conflicting information is combined in a single stimulus. Studies using a letters flankers paradigm - a closer analogue to the executive attention component of the ANT - suggest that the sensitivity of measures of age-related deficits in inhibitory processes may depend on whether conflicting information is combined versus spatially separated (e.g., Zeef, Sonke, Kok, Buiten & Kenemans, 1996) and the extent to which the perceptual load imposed by the distracting information encourages early versus late selective attention (Maylor & Lavie, 1998). Previous work has shown that older adults are more susceptible to flankers effects at smaller versus larger separations between targets and distracters (Zeef et al., 1996). Thus, it is possible that reducing the separation between target and distracting arrows in the ANT may yield age-related declines in performance. A study by Maylor and Lavie (1998) suggests that age differences in flankers effect are less likely to emerge when the perceptual load of target information consumes older adults’ more limited attentional resources, which can decrease their susceptibility to distracting information relative to younger adults. It is possible that the cognitive demands of the ANT, which requires different combinations of warning signals and target arrays to be processed from trial to trial may have paradoxically facilitated older adults performance on incongruent trials. Nevertheless, our finding of overall age equivalence in executive attention performance is in agreement with several other studies in suggesting that older adults can effectively ignore conflicting information when it is spatially separated from target information.
Our findings are also consistent with the proposal that tasks involving a diverse suite of high-level cognitive and behavioral demands (e.g., access, deletion, planning, response inhibition, task switching) may be more likely to reveal age-related performance declines relative to those designed to isolate single components of attention. Indeed, although the older adults in this study did as well as younger adults on executive attention, they did worse than younger adults on a sentence span task and a task measuring the ability to refresh the contents of working memory (see Mather & Knight, 2005). One possible reason for the apparent disagreement between the finding of no age differences on the executive component of ANT and age differences on working memory tasks (e.g., Darowski, Helder, Zacks, & Habrick, 2008) may be the increased demands placed upon executive control processes supporting working memory that are not present in the ANT. On trials measuring executive attention, participants must identify the target arrow, inhibit the distractors, and execute a motor response to indicate which direction the target arrow is pointing in. Complex span tasks often involve processes in addition to attention and discrepancies between studies may in part reflect differences in the effects of age and/or time of day on these nonattentional processes. Indeed, a recent review of the literature concluded that top-down attention (such as visual search tasks based on the observer’s expectations) is relatively well-maintained in normal aging (Madden, 2007), going against what would be expected based on executive-control theories of aging. We should also note that Zhou et al. (2011) observed a significant impairment in older adults’ executive attention performance using the ANT, a finding that does not match our results. Cross-cultural differences may offer one possible explanation for their results. Previous research shows that individuals from East Asian cultures are more likely to attend to the relationship between objects and their context, whereas Westerners are more likely to attend to objects independent of their context (e.g., Ko, Lee, Yoon, Kwon, & Mather, 2011). This cultural difference in attention may lead to higher interference on incongruent flanker trials for East Asians relative to Westerners and thus it may have been have been harder to override for older relative to younger Chinese participants in the Zhou et al. study.
Our study had some limitations. One was that we did not have a measure of individual differences in morningness or eveningness preferences. While previous literature indicates that there are large age differences in these preferences, it would have enriched our study to be able to separately examine the subgroup of younger adults who are not evening types and the subgroup of older adults who are not morning types. Another limitation is that we only included two times of day of testing, which meant we could not examine the full trajectory of circadian patterns in performance. For instance, our testing times may not have extended early and late enough in the day to detect the inverted-u shape seen for executive attention by Matchock and Mordkoff (2009) for younger adults.
In conclusion, we have extended previous work by specifying which aspects of attention are most and least susceptible to age-related circadian patterns. Our findings show that age-related circadian patterns influence the alerting component of attention, with both age groups showing the largest benefit from alerting cues when testing occurred at non-optimal times of day. In contrast with alerting, performance on measures of orienting and executive attention networks did not differ as a function of age or time of testing. This finding is intriguing in its suggestion that functions of attention that are more subject to voluntary strategy may also be less susceptible to circadian fluctuations and to other well-documented changes in neurophysiology and cognition linked to aging. In terms of complex tasks that draw heavily on these networks, older and younger adults may be able to strategically compensate for lowered efficiency at off-peak times. Overall, our findings underscore the importance of controlling for time of day in investigations of attention and add to our understanding of the relationship between age differences in circadian patterns and attention.
Table 2.
Proportion scores (and standard errors) of younger and older adults according to flanker type
| Cue Type | |||||
|---|---|---|---|---|---|
| No cue | Double cue | Center cue | Spatial cue | ||
| Younger | |||||
| AM | 1.06 (.01) | 1.00 (.01) | 1.02 (.01) | 0.93 (.01) | |
| PM | 1.04 (.01) | 1.00 (.01) | 1.04 (.01) | 0.94 (.01) | |
| Older | |||||
| AM | 1.02 (.01) | 1.04 (.01) | 1.02 (.01) | 0.93 (.01) | |
| PM | 1.04 (.01) | 1.01 (.01) | 1.02 (.01) | 0.92 (.01) | |
Acknowledgments
This research was supported by National Institutes of Health grants R01 AG025340 and K02 AG032309.
Footnotes
In contrast, Fernandez-Duque and Black (2006) observed a larger alerting effect in older relative to younger adults. However, this study incorporated a modified version of the ANT with larger stimuli and a longer duration for alerting, which may have contributed to the enhanced alerting effect in older adults. In addition, the error data suggest a more conservative response criterion for older adults may have also contributed to the larger alerting effect (Fernandez-Duque & Black, 2006).
Answering the questions involved reporting today’s date, naming the current and previous presidents, counting backwards by threes, repeating and later recalling and then recognizing three words the interviewer said, and describing how a dog and a lion are alike and how sugar and vinegar are different. Most participants answered all questions correctly. The one question some participants missed was the delayed recall for the three words (the most missed was two words). However, perfect performance on the recognition test that followed was required for the participant to be invited to come in for the session.
All older adult participants completed the ANT. Of the 32 younger adult participants, data from 5 were excluded because of a computer malfunction that resulted in premature termination of the ANT.
Contributor Information
Marisa Knight, Department of Psychology, University of San Francisco.
Mara Mather, Davis School of Gerontology and Department of Psychology, University of Southern California.
References
- Adan A, Almirall H. Adaptation and standardization of a Spanish version of the Morningness-Eveningness Questionnaire: Individual differences. Personality and Individual Differences. 1990;11(11):1123–1130. [Google Scholar]
- Brink JM, McDowd JM. Aging and selective attention: An issue of complexity or multiple mechanisms? The Journal of Gerontology: Series B: Psychological Sciences and Social Sciences. 1999;54B(1):30–33. doi: 10.1093/geronb/54b.1.p30. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cohen JD, MacWhinney B, Flatt RR, Provost J. PsyScope: An interactive graphic system for designing and controlling experiments in the psychology laboratory using Macintosh computers. Behavioral Research Methods, Instruments, and Computers. 1993;25:257–271. [Google Scholar]
- Coull JT, Frith CD, Frackowiak RJ, Grasby PM. A fronto-parietal network for rapid visual information processing: A PET study of sustained attention and working memory. Neuropsychologia. 1996;34(11):1085–1095. doi: 10.1016/0028-3932(96)00029-2. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade J, Shulman GL. Neural systems for visual orienting and their relationships to spatial working memory. Journal of Cognitive Neuroscience. 2002;14(3):508–523. doi: 10.1162/089892902317362029. [DOI] [PubMed] [Google Scholar]
- Darowski ES, Helder E, Zacks RT, Hasher L, Hambrick DZ. Age-related differences in cognition: The role of distraction control. Neuropsychology. 2008;22(5):638–644. doi: 10.1037/0894-4105.22.5.638. [DOI] [PubMed] [Google Scholar]
- Fan J, Fossella J, Sommer T, Wu Y, Posner MI. Mapping the genetic variation of executive attention onto brain activity. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:7406–7411. doi: 10.1073/pnas.0732088100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. Journal of Cognitive Neuroscience. 2002;14(3):340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- Fan J, Posner MI. Human attentional networks. Psychiatrische Praxis. 2004;31:S210–S214. doi: 10.1055/s-2004-828484. [DOI] [PubMed] [Google Scholar]
- Fernandez-Duque D, Black SE. Attentional networks in normal aging and Alzheimer's disease. Neuropsychology. 2006;20(2):133–143. doi: 10.1037/0894-4105.20.2.133. [DOI] [PubMed] [Google Scholar]
- Festa-Martino E, Ott BR, Heindel WC. Interactions between phasic alerting and spatial orienting: Effects of normal aging and Alzheimer's disease. Neuropsychology. 2004;18(2):258–268. doi: 10.1037/0894-4105.18.2.258. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gamboz N, Zamarian S, Cavallero C. Age-related differences in the Attention Network Test (ANT) Experimental Aging Research. 2010;36(3):287–305. doi: 10.1080/0361073X.2010.484729. [DOI] [PubMed] [Google Scholar]
- Hasher L, Zacks RT, May CP. Inhibitory control, circadian arousal, and age. In: Gopher D, Koriat A, Gopher D, Koriat A, editors. Attention and performance XVII: Cognitive regulation of performance: Interaction of theory and application. Cambridge, MA US: The MIT Press; 1999. pp. 653–675. [Google Scholar]
- Hedden T, Gabrieli JE. Insights into the ageing mind: A view from cognitive neuroscience. Nature Reviews Neuroscience. 2004;5(2):87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Jennings JM, Dagenbach D, Engle CM, Funke LJ. Age-related changes and the attention network task: An examination of alerting, orienting, and executive function. Aging, Neuropsychology, and Cognition. 2007;14(4):353–369. doi: 10.1080/13825580600788837. [DOI] [PubMed] [Google Scholar]
- Ko S, Lee T, Yoon H, Kwon J, Mather M. How does context affect assessments of facial emotion? The role of culture and age. Psychology and Aging. 2011;26(1):48–59. doi: 10.1037/a0020222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig C, Meck WH. Paying attention to time as one gets older. Psychological Science. 2001;12(6):478–484. doi: 10.1111/1467-9280.00389. [DOI] [PubMed] [Google Scholar]
- Madden DJ. Aging and visual attention. Current Directions In Psychological Science. 2007;16(2):70–74. doi: 10.1111/j.1467-8721.2007.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matchock RL, Mordkoff J. Chronotype and time-of-day influences on the alerting, orienting, and executive components of attention. Experimental Brain Research. 2009;192(2):189–198. doi: 10.1007/s00221-008-1567-6. [DOI] [PubMed] [Google Scholar]
- Mather M, Knight M. Goal-directed memory: The role of cognitive control in older adults' emotional memory. Psychology and Aging. 2005;20(4):554–570. doi: 10.1037/0882-7974.20.4.554. [DOI] [PubMed] [Google Scholar]
- May CP, Hasher L, Stoltzfus ER. Optimal time of day and the magnitude of age differences in memory. Psychological Science. 1993;4(5):326–330. [Google Scholar]
- May CP, Hasher L. Synchrony effects in inhibitory control over thought and action. Journal of Experimental Psychology: Human Perception And Performance. 1998;24(2):363–379. doi: 10.1037//0096-1523.24.2.363. [DOI] [PubMed] [Google Scholar]
- Maylor EA, Lavie N. The influence of perceptual load on age differences in selective attention. Psychology and Aging. 1998;13(4):563–573. doi: 10.1037//0882-7974.13.4.563. [DOI] [PubMed] [Google Scholar]
- Mecacci L, Zani A, Rocchetti G, Lucioli R. The relationships between morningness-eveningness, ageing and personality. Personality and Individual Differences. 1986;7(6):911–913. [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Raz N. Aging of the brain and its impact on cognitive performance: Integration of structural and functional findings. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. 2nd ed. Mahwah: NJ: Lawrence Erlbaum Associates; 2000. pp. 91–153. [Google Scholar]
- Schmidt C, Collette F, Cajochen C, Peigneux P. A time to think: Circadian rhythms in human cognition. Cognitive Neuropsychology. 2007;24(7):755–789. doi: 10.1080/02643290701754158. [DOI] [PubMed] [Google Scholar]
- Wilson GD. Personality, time of day and arousal. Personality and Individual Differences. 1990;11(2):153–168. [Google Scholar]
- Wurm LH, Labouvie-Vief G, Aycock J, Rebucal KA, Koch HE. Performance in auditory and visual emotional Stroop tasks: A comparison of older and younger adults. Psychology and Aging. 2004;19(3):523–535. doi: 10.1037/0882-7974.19.3.523. [DOI] [PubMed] [Google Scholar]
- Yoon C, May CP, Hasher L. Aging, circadian arousal patterns, and cognition. In: Schwarz N, Park DC, Knaüper B, Sudman S, editors. Cognition, aging, and self-reports. Hove England: Psychology Press/Erlbaum (UK) Taylor & Francis; 1999. pp. 117–143. [Google Scholar]
- Zacks RT, Hasher L, Li KH. Human memory. In: Craik FM, Salthouse TA, Craik FM, Salthouse TA, editors. The handbook of aging and cognition. 2nd ed. Mahwah, NJ US: Lawrence Erlbaum Associates Publishers; 2000. pp. 293–357. [Google Scholar]
- Zeef EJ, Sonke CJ, Kok A, Buiten MM, Kenemans J. Perceptual factors affecting age-related differences in focused attention: Performance and psychophysiological analyses. Psychophysiology. 1996;33(5):555–565. doi: 10.1111/j.1469-8986.1996.tb02432.x. [DOI] [PubMed] [Google Scholar]
- Zhou S, Fan J, Lee TC, Wang C, Wang K. Age-related differences in attentional networks of alerting and executive control in young, middle-aged, and older Chinese adults. Brain and Cognition. 2011;75(2):205–210. doi: 10.1016/j.bandc.2010.12.003. [DOI] [PubMed] [Google Scholar]