Abstract
Background
Previous studies report that motor recovery after partial destruction of the primary motor cortex (M1) may be associated with adaptive functional reorganization within spared M1.
Objective
To test feasible methodologies for evaluating relationships between behavioral gains facilitated by rehabilitative training and functional adaptations in perilesional M1 and the cerebellum.
Methods
Four patients with hemiparesis for more than 3 months after a cortical lesion partially within M1 and 12 healthy volunteers participated. Functional magnetic resonance imaging (fMRI) using a finger-tapping task and concurrent behavioral assessments, including the Fugl-Meyer Motor Assessment of the upper extremity and the Wolf Motor Function Test, were conducted before and after 2 weeks of arm-focused training; 2 patients were further examined 6 and 12 months later to evaluate long-term persistence of brain-behavior adaptations.
Results
All patients showed higher activation magnitude in perilesional M1 than healthy controls before and after therapy. Further long-term functional gains paralleled the decrease of activation magnitude in perilesional M1 in the 2 more impaired cases.
Conclusion
The evolution of suggestive correlations between serial scans of fMRI adaptive activity within the primary motor cortex and the cerebellum in relation to relevant behavioral changes over the course of 2 weeks of task-specific therapy and then no formal therapy suggests that repeated assessments may be best for monitoring therapy-induced neuroplasticity. This approach may help develop optimal rehabilitation strategies to maximize poststroke motor recovery as well as improve the search for brain-behavior correlations in functional neuroimaging research.
Keywords: Stroke rehabilitation, fMRI, Wolf Motor Function Test, Primary motor cortex, Constraint-induced movement therapy, Adaptive reorganization
Poststroke recovery of motor function with and without specific rehabilitation training has been attributed in part to adaptive functional reorganization within the central nervous system.1,2 The underlying neurophysiological mechanisms may include changes in neuronal membrane excitability, synaptic strengthening, synaptogenesis, dendritic arborization, fiber sprouting from surviving neurons, and recruitment of nearby and remote neuronal ensembles after focal brain injury.3,4 Functional reorganization within the intact area surrounding an infarct restricted to the primary motor cortex (M1) was observed over the temporal course of recovery from stroke using functional magnetic resonance imaging (fMRI). The studies supported the notion that human M1 is capable of functional adaptation comparable to that seen in primate experiments.5,6 Growing evidence from both animal and human studies suggests the importance of perilesional adaptive reorganization and the potential modulative effects of focused, intensive rehabilitative training in facilitating this use-dependent reorganization.7,8
With the use of advanced neuroimaging technologies, rehabilitation therapy-induced adaptive reorganization within putative motor networks has been investigated in chronic stroke patients who received constraint-induced movement therapy (CIMT).9-12 The correlates between motor functional gains and changes in physiological signals have differed across studies, however. To best elucidate the mechanisms mediating cerebral adaptations after stroke, studies must account for intersubject variability in initial impairment level, lesion location and size, trajectory of behavioral gains associated with time and motor learning experience, and dose of rehabilitation therapy.13 This pilot study attempted to determine direct relationships between intensive training-related motor functional improvement and adaptive reorganization in perilesional M1 in patients with partial M1 damage. We performed consecutive fMRI scans with concurrent behavioral assessments in 4 patients who had a single stroke involving a portion of M1 before, immediately after 2 weeks of CIMT, and, in 2 willing subjects, 6 and 12 months later. The aims of the study were 2-fold: 1) to test the hypothesis that rehabilitative training-related behavioral gains are associated with specific functional adaptations within the intact perilesional M1 and the remotely connected cerebellum and 2) to test methods for evaluating direct brain-behavior correlates and generate preliminary data for larger scale studies.
METHODS
Subjects
Four patients (3 men and 1 woman; age range, 25-57 years) with hemiparetic stroke from a larger companion trial of CIMT participated.14 The 4 patients had had a single stroke event with a cortical lesion occupying part of M1 (Brodmann area 4). Inclusion criteria included the following: 1) ability to perform the fMRI activation task, 2) score >24 on the Folstein Mini-Mental State Exam, and 3) ability of voluntary movement of at least 10 degrees of wrist and finger extension. Exclusion criteria were inability to follow the fMRI instructions, claustrophobia, and MRI safety concerns (eg, pacemaker). Twelve healthy controls (5 men and 7 women; age range, 31-60 years, mean age = 48 ± 8 years) were recruited from the volunteer database at the UCLA Brain Mapping Center. The control subjects reported no history of neurological diseases or medical problems and took no medication. Patients with stroke were asked to maintain the same medication throughout the study period. All participants were right-handed, judged by the Edinburgh handedness questionnaire.15 An institutionally approved informed consent was obtained from all participants.
Rehabilitative Training
All patients participated in a standardized CIMT protocol for 2 weeks as defined in the multisite randomized controlled trial, Extremity Constraint-Induced Therapy Evaluation, EXCITE (refer to Winstein et al14 for details).
Behavioral Measures
The Wolf Motor Function Test (WMFT) and upper extremity Fugl-Meyer (FM) motor score were performed immediately before and after the intervention in all 4 patients and at 6 and 12 months later for patients 3 and 4, who returned for longitudinal follow-up.
WMFT
The WMFT has been successfully employed as a primary outcome measure, and its reliability has been established in stroke.16 The WMFT contains 15 timed and 2 strength items, and it also includes a hierarchy of single- and multiple-joint motions and functional tasks. All timed tasks were performed as quickly as possible within 120 seconds, and if a task was not completed, it was scored 121. In the current study, only the timed tasks were evaluated. The mean time for the 15 timed tasks of the WMFT (mWMFTT) and the percentage difference ((post − pre)/pre × 100) in the mWMFTT between pre- and posttherapy for the paretic hand were used for the comparisons across time.
FM motor assessment
The FM motor score for the upper extremity provides a quantifiable and commonly used assessment of selected upper extremity movements, including motor skill, coordination, and speed.17 The total score possible is 66. In the current study, the FM motor score was used as a measure of poststroke motor impairment.
Functional MRI Motor Activation Task
Sequential finger tapping was employed as the motor activation task. Participants were instructed and trained to tap their thumb, index finger, middle finger, and little finger sequentially on a wooden board secured on the stomach. To accommodate differences in tapping speed among the patients and our design of within-subject and between-session comparisons, patients were instructed to perform the task at a self-paced comfortable rate. In contrast, healthy controls performed the task at a fixed rate of 1 Hz. Before each fMRI scanning session, each participant practiced the task to isolate finger movements and limit any unwanted activity, such as mirror and associated movements of any other body parts.
Functional MRI Data Acquisition
Structural and functional MRI acquisition parameters were described previously.18 All patients practiced outside the scanner to reduce mirror movements from the less affected hand and associated movements from the wrist, elbow, and shoulder. During the functional run lasting 6.5 minutes, three 30-second epochs of repetitive left finger tapping were alternated with three 30-second epochs of repetitive right finger tapping. In between each 30-second tapping epoch was a 30-second rest period. Auditory cues were used for the “start” and “stop” of each tapping epoch through headphones worn during scanning. A total of 156 volumes were acquired. The entire fMRI session was videotaped for offline assessment of tapping performance. Eight of the 12 healthy controls were scanned twice, 2 to 3 weeks apart, to evaluate the reproducibility and stability of task-related motor cortical activation. Patients 1 and 2 were scanned before and immediately after the 2-week CIMT, and patients 3 and 4 were scanned before, immediately after, and 6 and 12 months after CIMT.
Structural and Functional Image Analysis
Lesion location was identified on high-resolution T1-weighted structural images in reference to the atlas of Duvernoy.19 Lesion volume was calculated using MRIcro (http://www.psychology.nottingham.ac.uk/staff/cr1/mricro.html). Briefly, the boundary of the lesion was manually outlined on each slice of each individual patient’s high-resolution T1-weighted image to create a 3-dimensional mask. Then the volume within the mask was computed through a semiautomatic procedure within MRIcro. To compare perilesional M1 integrity across patients, the relative M1 structural integrity was calculated according to the following steps. First, the volume of the intact M1 from the nonlesioned hemisphere was measured, including the cortex lying within the anterior bank of the central sulcus and the posterior half of the precentral gyrus superiorly from the dorsal surface of the lateral ventricle. Second, volume of the intact perilesional M1 (ie, area within the M1 but sparing the portion occupied by the lesion) was computed. Third, the relative M1 structural integrity (integrity_M1) was calculated by dividing the volume of the perilesional M1 by the volume of the intact M1 in the nonlesioned hemisphere. Similarly, the volume of the hand representation (hand knob area) was measured by a neurologist (YD) familiar with neuroanatomy. The relative integrity of the hand representation (integrity_hand knob) was calculated by dividing the volume of the perilesional hand knob by the volume of the intact hand knob in the nonlesioned hemisphere.
FSL (FMRIB Software Library, Version 3.1; http://www.fmrib.ox.ac.uk/fsl) was used for fMRI data analysis.20 First, MELODIC (Multivariate Exploratory Linear Optimized Decomposition into Independent Components) was applied to decompose the fMRI time-series data. The components obviously related to motion artifacts were excluded from further analysis.21 Second, a series of preprocessing steps were applied to the functional data: 1) motion correction using MCFLIRT (Linear Image Registration Tool), 2) all functional volumes realigned to the middle volume to correct for misalignment and residual head motion, 3) spatial smoothness using a Gaussian kernel of 6 mm full-width-half-maximum, and 4) coregistration of functional scans, first to the high-resolution structural scan and then to the standard template in the Montreal Neurological Institute (MNI) space using 7- and 12-parameter rigid-body transformation, respectively. Third, a task-specific effect was estimated using FILM (FMRIB’s Improved Linear Model) with local autocor-relation correction. A t test was performed to construct a task-specific voxel-by-voxel activation map for each individual. The statistical threshold was set at cluster level Z > 3.1, with spatial extent at P < .05 (corrected for multiple comparisons according to random field theory).22,23 Moreover, second-level analysis (random effects model) was performed to generate a group mean activation map from the 12 healthy controls.
A region-of-interest (ROI) analysis was conducted to quantify the dynamic changes in bilateral M1 and cerebellum (CB) across time. The M1 ROI was defined in the same way as that applied for M1 volume calculation. The CB ROI covered each cerebellar hemisphere, excluding the vermis. For stroke patients, only the intact spared portion of M1 was included for M1 ROI calculation in the lesioned hemisphere. The ROIs were drawn on the individual patient’s high-resolution structural image in native space and then converted into MNI space using the FLIRT function within FSL. The ROIs for healthy controls were drawn on the standard template in MNI space. Functional MRI activation, including mean percentage signal change (%SC), absolute voxel counts (VC), and the geometric center (x, y, z coordinates) within each ROI, was computed using in-house software modified from FSL. A laterality index for M1 (LI_M1) was calculated from VC contralateral versus ipsilateral to the hand movements as LI = (contralateral − ipsilateral)/(contralateral + ipsilateral). The lateralization of CB activation was characterized using the ratio of ipsilateral CB activation (Ra_ipsiCB = ipsilateral/(ipsilateral + contralateral), ipslateral/contralateral to the hand movements). A between-session effect was initially tested at the group level using a voxel-wise whole-brain analysis (fixed effects model) in the 8 healthy controls who were scanned twice; then, a paired t test was performed on the ROI data to further test reproducibility and stability of the M1 and the CB activation (%SC, VC, LI_M1, and Ra_ipsiCB) in the same participants. Moreover, relative intersession variations were used to characterize between-session variability of fMRI measures. The relative intersession variation was defined as the difference in fMRI measures between the 2 sessions divided by their summation. Both descriptive and nonparametric correlation analyses (Spearman correlation coefficient by rank) were applied to assess for relationships between pre- to posttherapy changes in fMRI activation in perilesional M1 and cerebellum and behavioral gains, as well as between fMRI activation in the same areas and motor performance across time. The Mann-Whitney U-test was used to compare fMRI variables between controls (n = 8) and patients (n = 4). The fMRI variables from healthy controls used for group comparisons were the average of 8 subjects during dominant hand movements from scan session 1.
RESULTS
Clinical and Behavioral Outcomes
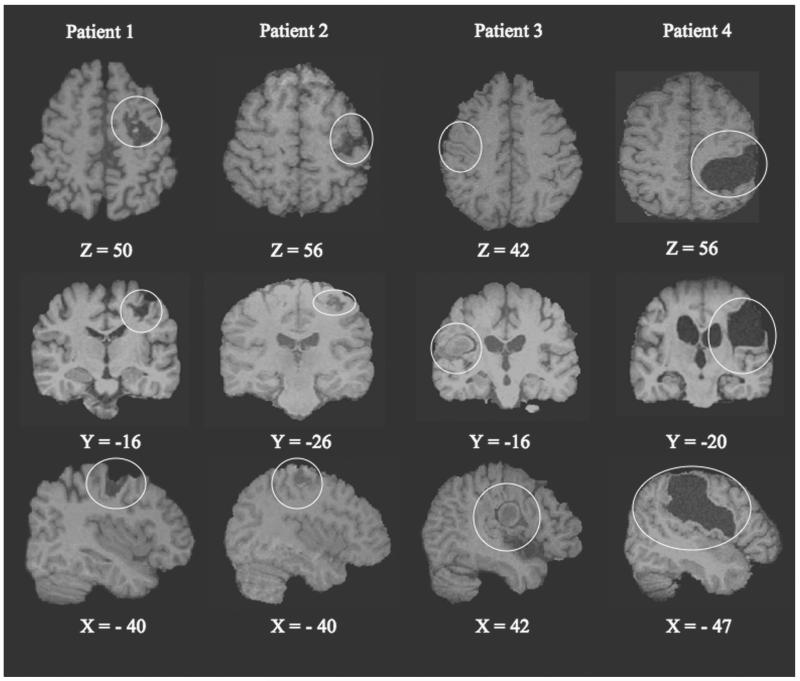
Patient characteristics are listed in Table 1. Three patients had a right and 1 had a left hemiparesis (Fig. 1). The total lesion volume (3.1–72.6 mm3) varied, but the relative structural integrity of perilesional M1 was comparable across patients, ranging from 45% to 75% (Table 1). Individual tapping performance was assessed offline from the videotapes recorded during each fMRI session and is summarized in Table 2. In general, tapping performance (amplitude and coordination of individual finger movements) of the paretic hand was less skilled in the patients than with the unaffected hand, as well as with the dominant or nondominant hand of healthy controls.
Table 1.
Characteristics of the Patients
| Patient ID | Age (Years) |
Sex | Paretic Hand |
Time From Onset (Months) |
Initial FM Score |
Lesion Location |
Lesion Volume (mm3) |
Relative Hand Knob Integrity |
Relative M1 Integrity |
Lesion Type |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 28 | M | R | 6.1 | 60 | L BA 4 and part of the premotor cortex (BA 6) |
10.4 | 0.54 | 0.64 | Hemorrhage |
| 2 | 25 | F | R | 15.5 | 63 | L BA 4 | 3.1 | 0.50 | 0.74 | Ischemic |
| 3 | 37 | M | L | 5.2 | 51 | R BA 4 and part of the premotor cortex (BA 6) |
14.5 | 0.50 | 0.45 | Hemorrhage |
| 4 | 57 | M | R | 8.0 | 50 | L fronto-temporo-parietal cortices; part of BA 4 |
72.6 | 0.67 | 0.59 | Hemorrhage |
FM = Fugl-Meyer motor score of the upper extremity; M1 = primary motor cortex; BA = Brodmann area. Relative M1 integrity = volume of the perilesional M1 divided by volume of the intact M1 in the nonlesioned hemisphere. Relative hand knob integrity = volume of the perilesional hand knob area divided by volume of the intact M1 in the nonlesioned hemisphere.
Figure 1.
Representative axial, coronal, and sagittal slices with Montreal Neurological Institute (MNI) coordinates illustrating the cortical lesion in relation to the primary motor cortex in each of the 4 patients with chronic hemiparesis (lesion outlined by fine circle). The z coordinates (mm) in MNI space that cover the hypointensity areas on T1-weighted magnetic resonance imaging (MRI) are 55 to 68 (patient 1), 62 to 69 (patient 2), 42 to 60 (patient 3), and 38 to 74 (patient 4). Slices are in radiological convention (right side of the brain to viewer’s left for top 2 rows).
Table 2.
Summary of Individual Patients Tapping Performance With the Paretic Hand in Each fMRI Session
| Patient ID | Time Point | Description of the Movements | Individual Finger Isolation | Mirror and Associated Movements | Tapping Rate (Hz) |
|---|---|---|---|---|---|
| 1 | Pre | Separated thumb and index finger movements; low tapping amplitude |
Difficulty in isolating middle, ring, and little fingers |
Some wrist movements due to instability |
0.5 |
| Post | Good separated thumb and index finger movements; high tapping amplitude |
Same as “Pre” | None observed | 0.5 | |
| 2 | Pre | Near-normal tapping performance; high amplitude of finger movements but less stable |
Good | Some synergistic movements from the wrist |
1.8 |
| Post | No noticeable difference from “Pre” | Good | Same as “Pre” | 1.5 | |
| 3 | Pre | Fingers moving together; small tapping amplitude |
Poor | Synkinesia of the fingers | ~0.5 |
| Post | Same as “Pre” | Poor | Same as “Pre” | −0.5 | |
| 6-mo | Some individual finger movements; low tapping amplitude |
Moderate | None observed | 0.5 | |
| 12-mo | Same as “6-mo”; relatively high tapping amplitude |
Moderate; more individual finger isolation than “Post” |
None observed | 0.7 | |
| 4 | Pre | Observable individual finger movements; low tapping amplitude |
Moderate | Little finger moving together with others |
0.7 |
| Post | Some individual finger movements; high tapping amplitude |
Moderate | None observed | 0.7 | |
| 6-mo | Good individual finger movements; high tapping amplitude |
Good | None observed | 0.7 | |
| 12-mo | No obvious difference from “6-mo” | Good | None observed | 0.7 |
fMRI = functional magnetic resonance imaging; Pre = before; Post = after; 6-mo = 6 months after; 12-mo = 12 months after therapeutic training.
Initial motor impairment of the upper limb was mild across the 4 patients. The FM score ranged from 50 to 63 (Table 1). As expected, pre- to posttherapy behavioral gains (mWMFTT) varied across the patients, from a decrease of 19% to 69% in mWFMTT. Specifically, patient 4, who had a lower FM score, showed the most improvement (69% decrease for task time), whereas patient 2, who had a higher FM score, had the least improvement (19%), reflecting a ceiling effect. Patients 1 and 3 made intermediate improvements (25% and 27% decreases). Further improvements were observed at 6 and 12 months for patient 3, whereas patient 4 showed no change 6 months later compared to posttherapy along with a decline in performance (increased mWMFTT) at 12 months (Table 3). Longitudinal changes in the mWMFTT for patients 3 and 4 were of particular interest because of parallel changes in fMRI signals described below.
Table 3.
Behavioral Outcome Measure (WMFT) Across Time for Paretic and Less Affected Hand
| Mean Time of the WMFT Total 15 items (s) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Paretic Hand |
Less Affected Hand |
|||||||||
| ID | Pre | Post | 6-mo | 12-mo | Time Diff (Post-Pre) | % Time Diff | Pre | Post | 6-mo | 12-mo |
| 1 | 6.11 | 4.60 | NA | NA | −1.51 | −24.71 | 1.82 | 1.94 | NA | NA |
| 2 | 2.27 | 1.83 | NA | NA | −0.44 | −19.38 | 1.24 | 1.27 | NA | NA |
| 3 | 30.693 | 22.552 | 16.081 | 6.89 | −8.13 | −26.50 | 2.27 | 1.69 | 2.28 | 1.69 |
| 4 | 23.221 | 7.18 | 7.18 | 18.58 | −16.04 | −69.08 | 2.92 | 1.87 | 1.66 | 1.62 |
| Mean (SD) | 15.58*,** (13.58) |
9.04* (9.27) |
NA | NA | −6.53 (7.19) |
−34.91 (22.97) |
2.06** (0.71) |
1.69 (0.30) |
NA | NA |
% Time diff = [(post − pre)/pre] × 100. WMFT = Wolf Motor Function Test; NA = not available. Superscript for time shows the number of items incomplete within 120 seconds.
P= .07 between pre- and posttherapy for the paretic hand (Wilcoxon signed-rank test).
P= .06 between paretic and less affected hand before therapy (Mann-Whitney U-test).
Cerebral Activation Pattern for Healthy Controls and Stroke Patients
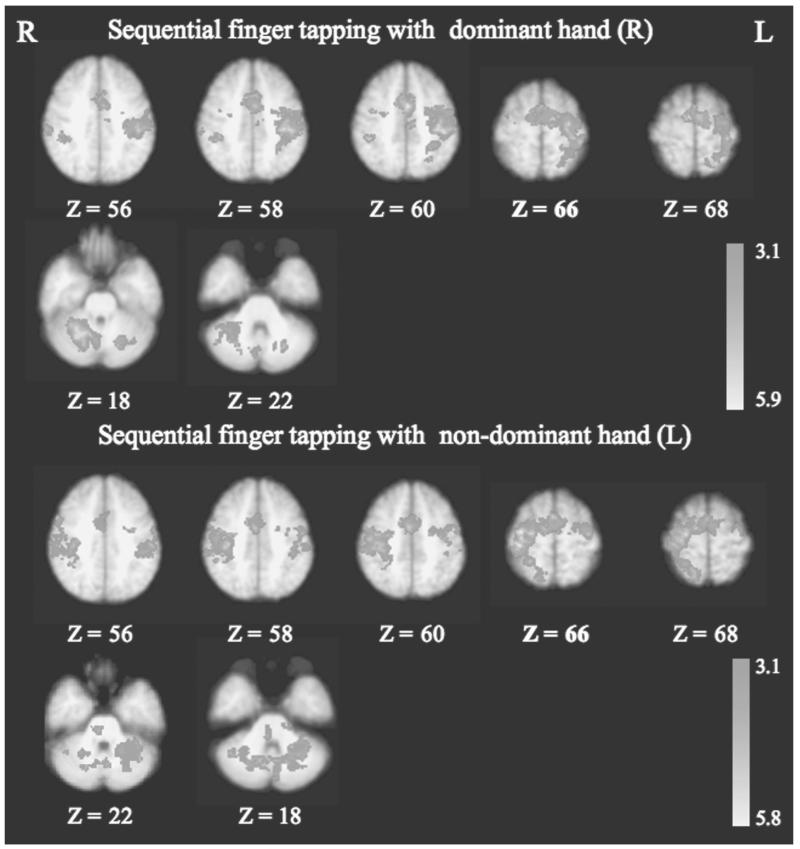
The brain activation pattern during sequential finger tapping (random effects model) from 12 healthy controls is illustrated in Figure 2. For dominant (right) hand tapping, the most lateralized activation was seen in the contralateral (left) primary sensorimotor cortex and ipsilateral (right) CB. Compared with that from the dominant (right) hand movements, finger tapping with the nondominant hand revealed less lateralized activation in the sensorimotor network and more bilaterally distributed CB activation (Fig. 2, lower half). Voxel-wise comparison (fixed effects model) revealed no difference in activation above the statistical threshold between the 2 repeated fMRI sessions in the 8 healthy controls. Moreover, between-session comparisons of single-subject ROI data revealed no significant difference in fMRI activation (%SC, VC, and LI_M1) in contralateral M1 for both dominant and nondominant hands (paired t; P > .1; Table 4). The geometric center (x, y, z coordinates in MNI space) of contralateral M1 activation was highly reproducible for both dominant and nondominant hands (<10% intersession variation; Table 4).
Figure 2.
Functional magnetic resonance imaging (fMRI) activation patterns during sequential finger tapping with dominant (R) and nondominant (L) hands for the healthy control group (n = 12). Dominant (R) hand movements activated predominantly contralateral sensorimotor networks; nondominant (L) hand movements activated bilaterally distributed sensorimotor areas. Finger tapping with both dominant and nondominant hands activated predominantly the cerebellum ipsilateral to the hand movements (Ra_ipsiCB: 0.88 for dominant hand and 0.59 for nondominant hand). Statistical images were thresholded at Z > 3.1, and significant clusters were defined according to spatial extent (P < .01). M1 = primary motor cortex; Ra_ipsiCB = ratio of the ipsilateral CB activation.
Table 4.
Between-Session Comparison of fMRI Variables in the 8 Healthy Controls During Sequential Finger Tapping
| Dominant (R) Hand |
Non-dominant (L) Hand |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 |
S2 |
ISV |
S1 |
S2 |
ISV |
|||||||
| fMRI Variables | Contra M1 | Ipsi CB | Contra M1 | Ipsi CB | Contra M1 | Ipsi CB | Contra M1 | Ipsi CB | Contra M1 | Ipsi CB | Contra M1 | Ipsi CB |
| VC | 543 (110) |
903 (221) |
548 (88) |
845 (102) |
0.24 (0.09) |
0.30 (0.11) |
536 (65) |
869 (213) |
539 (119) |
1336 (420) |
0.26 (0.09) |
0.29 (0.06) |
| %SC | 1.00 (0.11) |
0.75 (0.05) |
0.95 (0.08) |
0.71 (0.05) |
0.06 (0.01) |
0.07 (0.03) |
1.64 (0.08) |
1.25 (0.09) |
1.46 (0.14) |
1.27 (0.14) |
0.10 (0.02) |
0.07 (0.01) |
| GC x | −37 (1) |
−36 (1) |
0.02 (0.01) |
38 (1) |
38 (1) |
0.03 (0.01) |
||||||
| y | −20 (1) |
NA | −20 (1) |
NA | 0.04 (0.01) |
NA | −17 (2) |
NA | −18 (1) |
NA | 0.07 (0.04) |
NA |
| Z | 57 (1) |
59 (1) |
0.03 (0.01) |
56 (2) |
56 (1) |
0.03 (0.01) |
||||||
| LI_MI | 0.86 (0.06) |
0.90 (0.04) |
0.07 (0.03) |
0.73 (0.08) |
0.57 (0.13) |
0.24 (0.09) |
||||||
| Ra_ipsiCB | 0.88 (0.06) |
0.89 (0.03) |
0.09 (0.03) |
0.63 (0.07) |
0.70 (0.06) |
0.10 (0.03) |
||||||
VC: voxel counts; % SC: percentage signal change; LI_M1: laterality index of M1 from VC; Ra_ipsiCB: ratio of ipsilateral cerebellar activation from VC; Ra_ipsiCB = ipsilateral / (ipsilateral + contralateral); M1: primary motor cortex; CB: cerebellum; S1: session 1; S2: session 2; GC: geometric center of peri-lesional M1 activation; ISV: inter-session variation; ISV was the absolute difference in fMRI variables between the two sessions divided by their summation. NA: not available. Numbers are mean and se (in parenthesis)
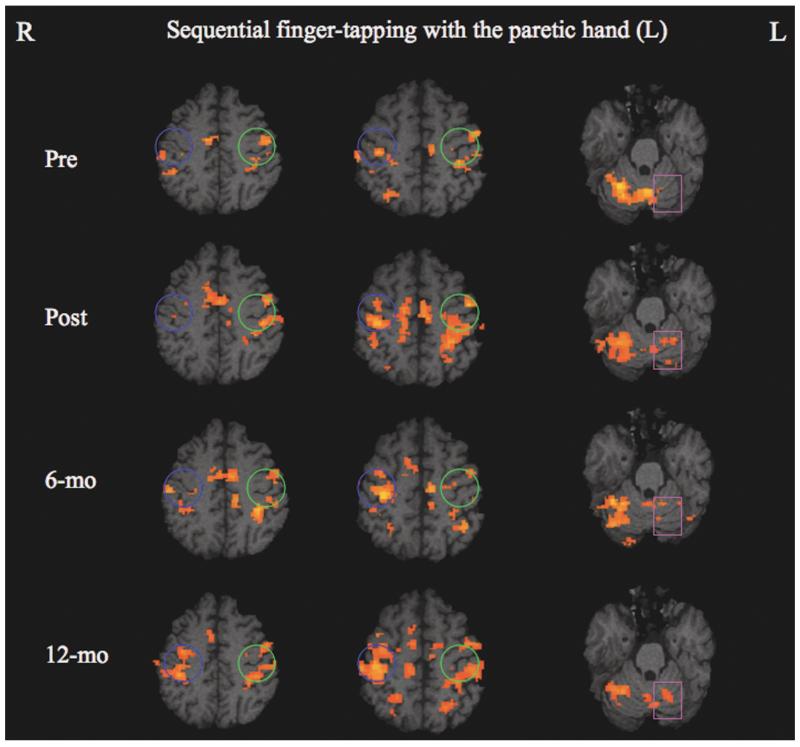
In general, patients with stroke and healthy controls differed in their pattern of cortical motor activation. Task-related activation for patient 2 (youngest, smallest lesion volume, highest relative M1 integrity) was focused in the contralateral primary sensorimotor cortex and the CB ipsilateral to the paretic hand. The other patients exhibited bilaterally distributed activation within the sensorimotor network, including M1, premotor, supplementary motor area (SMA), and CB. The evolution of motor cortical and cerebellar activation patterns across time in patient 3, who had the least integrity of M1 (45%) and the slowest pretherapy mean WMFT time, is illustrated in Figure 3. Before therapy, a relatively small amount of activation was detected in the bilateral primary sensorimotor cortices and more ipsilateral than contralateral, resulting in a negative LI_M1 (−0.57). Shortly after therapy, there was an increase in bilateral sensorimotor activation and a shift in lateralization of M1 activation toward a more contralateral (ipsilesional) recruitment pattern (LI_M1 = − 0.34). A focusing of this bilateral sensorimotor activation pattern, but with no meaningful shift in M1 lateralization (LI_M1 = −0.32), was observed 6 months later. At the 12-month follow-up, a large increase in perilesional M1 activation was detected, resulting in a shift of the M1 activation more contralaterally (ipsilesionally) with less ipsilateral activity (LI_M1 = 0.04). The cerebellar activation was predominantly contralateral (ipsilesional) to the paretic hand movements before therapy but evolved progressively toward more ipsilateral (contralesional) recruitment across time (Ra_ipsiCB = 0.10 before therapy and 0.13 immediately after therapy, then 0.29 at 6 months and 0.37 at 12 months).
Figure 3.
Evolution of motor cortical and cerebellar activation patterns during sequential finger tapping with the paretic left hand in patient 3. A progressive shift in M1 activation toward more contralateral (ipsilesional) involvement was observed across time (blue circle, ipsilesional; green circle, contralesional). The cerebellum was activated predominantly contralateral to the paretic hand movements for all 4 time points, but a continuous increase of ipsilateral cerebellar activation across time was observed (square).
Functional MRI activation (%SC and VC) in M1 for the paretic hand in individual patients and for dominant and nondominant hands in healthy controls is listed in Table 5. As a group, the patients had a higher %SC in perilesional M1 for the paretic hand both pre-and posttherapy (mean ± SE = 2.05 ± 0.35 pretherapy, P = .004; 1.46 ± 0.12 posttherapy, P = .008) than the %SC in the contralateral M1 for the dominant hand of the healthy controls (0.98 ± 0.10). In addition, patients had a lower pretherapy LI_M1 (0.15 ± 0.28) than controls for the dominant hand (0.88 ± 0.05, P = .004), but no difference was found when comparing mean posttherapy LI_M1 (0.34 ± 0.29, P > .1) to controls. Patients showed different CB activation patterns compared to controls—that is, lower Ra_ipsiCB both before (0.40 ± 0.17, P = .02) and immediately after therapy (0.55 ± 0.15, P = .05) than found for dominant hand movements of controls (0.88 ± 0.05). In addition, %SC in the CB ipsilateral to paretic hand movements in patients was higher (1.64 ± 0.35 pretherapy, P = .008; 1.25 ± 0.19 posttherapy, P = .004) than the mean %SC ipsilateral to dominant hand movements in controls (0.74 ± 0.05).
Table 5.
FMRI Activation (%SC and VC) and Laterality Index in the Primary Motor Cortex for Paretic Hand Movements in Individual Patients and for Dominant (R) and Nondominant (L) Hand Tapping in the Healthy Control Group
| Contralateral M1 |
Ipsilateral M1 |
||||||
|---|---|---|---|---|---|---|---|
| Subjects | Task Hand | Time Points | %SC | VC | %SC | VC | LI_M1 |
| Patient 1 | R | Pre | 1.87 | 142 | 0.94 | 144 | −0.01 |
| Post | 1.48 | 318 | 1.65 | 269 | 0.08 | ||
| Patient 2 | R | Pre | 1.33 | 37 | 1.76 | 9 | 0.61 |
| Post | 1.12 | 53 | 0 | 0 | 1 | ||
| Patient 3 | L | Pre | 1.98 | 107 | 1.61 | 394 | −0.57 |
| Post | 1.55 | 242 | 1.79 | 490 | −0.34 | ||
| 6-mo | 1.39 | 180 | 1.41 | 353 | −0.32 | ||
| 12-mo | 1.20 | 421 | 1.38 | 387 | 0.04 | ||
| Patient 4 | R | Pre | 3.01 | 491 | 3.66 | 138 | 0.56 |
| Post | 1.70 | 103 | 1.31 | 25 | 0.61 | ||
| 6-mo | 1.21 | 308 | 0.92 | 22 | 0.87 | ||
| 12-mo | 4.72 | 610 | 4.27 | 264 | 0.40 | ||
| HC (mean ± SE) |
R | Scan 1 | 1.00 (0.11) |
543 (110) |
0.39 (0.16) |
63 (32) |
0.86 (0.06) |
| Scan 2 | 0.95 (0.08) |
548 (88) |
0.60 (0.16) |
39 (17) |
0.90 (0.04) |
||
| L | Scan 1 | 1.64 (0.08) |
536 (65) |
1.27 (0.74) |
114 (47) |
0.73 (0.08) |
|
| Scan 2 | 1.46 (0.14) |
539 (119) |
1.13 (0.28) |
252 (93) |
0.57 (0.13) |
||
fMRI = functional magnetic resonance imaging; %SC =percent signal change; VC = voxel counts; M1 = primary motor cortex; LI_M1= laterality indexof M1 activation from voxel counts; LI_M1 = (contralateral_M1 − ipsilateral_M1)/(contralateral_M1 + ipsilateral_M1); HC = healthy control; Pre = before therapy; Post = immediately after therapy; 6-mo = 6 months after therapy; 12-mo = 12 months after therapy. Data for healthy controls were the average from the 8 subjects who were scanned twice 2 to 3 weeks apart. The ipsilateral M1 activation (%SC and VC) for dominant hands was the average from 3 HC, and that for nondominant hands was the average from 5 HC who showed ipsilateral activation in both fMRI sessions.
Brain-Behavior Relationships
Several correlations were suggested between adaptive reorganization in perilesional M1 and in the cerebellum with training-related functional improvements of the upper extremity. Among the 4 patients, relative M1_integrity was closely related to the pretherap mWMFTT—that is, greater M1 integrity corresponded to a shorter mWMFTT (Spearman correlation coefficient by rank, P = .08). However, no similar relationship was found either between pretherapy mWMFTT and total lesion volume or between pretherapy mWMFTT and relative hand knob integrity.
All 4 patients (Fig. 4A-B) demonstrated a decrease in %SC in perilesional M1 and an increase in LI_M1 for paretic hand tapping from pre- to posttherapy. The pre- to posttherapy decrease in %SC for paretic hand movements was significantly greater than the between-session difference for dominant hand movements of the controls (mean ± SE = −0.59 ± 0.25 for patients and −0.05 ± 0.04 for controls; P = .004). No group difference (P = .11) was found between the change in LI_M1 in patients (0.21 ± 0.07) and the between-session difference in LI_M1 in controls (0.05 ± 0.06). Figure 4A suggests a tendency for a correlation between a pre- to posttherapy perilesional M1 decrease in %SC and behavioral gains (percent decrease in mWMFTT). Figure 4D suggests that a greater increase in Ra_ipsiCB corresponded to better posttherapy improvement in the mWMFTT. No difference was found in the geometric center (x, y, z coordinates) of perilesional M1 activation from pre- to posttherapy at the group level (P > .1) in patients. However, an individual patient’s pre- to posttherapy shift in the geometric center of activation suggests a close relation with the initial impairment level and the degree of posttherapy behavioral gains (Table 6). Specifically, before therapy, patient 1 had a geometric center of perilesional M1 activation in an area identified as face motor representation but shifted superiorly back to the hand representation after therapy. Patient 2 exhibited the geometric center of M1 activation within the finger and hand motor area identified in controls both before and after therapy. For patient 3, before therapy, the center of perilesional M1 activation was in the area for wrist and elbow representation and remained unchanged immediately after therapy but evolved 6 months later, making it closer to the finger and hand area in controls. Patient 4 demonstrated a perilesional M1 activation center near the wrist and elbow representation before therapy and immediately after therapy. However, a reemergence of the geometric center of perilesional M1 activation in the finger and hand motor area occurred 6 and 12 months after therapy.
Figure 4.
Relationships between pre- to posttherapy changes in functional magnetic resonance imaging (fMRI) activation (%SC, LI_M1, and Ra_ipsiCB; left y-axis) and behavioral gains (percent decrease in mean WMFT time; right y-axis) in the 4 patients. Functional MRI activation patterns versus percent decrease in mean WMFT time for (A) posttherapy decrease in %SC in perilesional M1, (B) pre- to posttherapy increase in LI_M1, (C) decrease in %SC in the cerebellum ipsilateral to hand movement, and (D) posttherapy increase in Ra_ipsiCB. The increase in LI_M1 was inversely related to the percent decrease in the mean WMFT time. The pre- to posttherapy decrease in %SC in perilesional M1 and in Ra_ipsiCB paralleled the percent decrease in mean WMFT time (nonparametric correlation analysis; P = .08). No clear relationship was observed between the decrease in %SC in ipsilateral CB and the percent decrease in the mean WMFT time. Dashed bar = pre- to posttherapy changes in fMRI activation in stroke patients. Solid bar = intersession difference of fMRI activation in healthy controls. Diamond symbol = percent decrease in the mean WMFT time. Pt = patient; HC = healthy control; Ra_ipsiCB = ratio of ipsilateral cerebellar activation; LI_M1 = laterality index of perilesional M1 activation; %SC = percent signal change; CB = cerebellum; WMFT = Wolf Motor Function Test.
Table 6.
Coordinates in MNI Space for the Geometric Center of the Contralateral M1 Activation During Paretic Hand Movements in the Patients and During Dominant (R) and Nondominant (L) Hand Movements in the Healthy Control Group
| Coordinates in MNI Space |
|||||
|---|---|---|---|---|---|
| Subjects | Movement Hand | Time Point | x | y | z |
| Patient 1 | R | Pre | −44 | −9 | 40 |
| Post | −36 | −18 | 57 | ||
| Patient 2 | R | Pre | −33 | −12 | 55 |
| Post | −29 | −13 | 56 | ||
| Patient 3 | L | Pre | 39 | −7 | 65 |
| Post | 36 | −10 | 63 | ||
| 6-mo | 38 | −10 | 56 | ||
| 12-mo | 37 | −10 | 59 | ||
| Patient 4 | R | Pre | −35 | −22 | 64 |
| Post | −36 | −19 | 64 | ||
| 6-mo | −34 | −18 | 58 | ||
| 12-mo | −36 | −16 | 57 | ||
| Healthy control (n = 8) |
Dominant (R) | Session 1 | −37 ± 3 | −20 ± 3 | 57 ± 4 |
| Session 2 | −36 ± 2 | −20 ± 3 | 58 ± 3 | ||
| Nondominant (L) | Session 1 | 38 ± 3 | −17 ± 5 | 56 ± 4 | |
| Session 2 | 38 ± 3 | −17 ± 4 | 56 ± 4 | ||
MNI = Montreal Neurological Institute; Pre = before therapy; Post = after therapy; 6-mo = 6 months after therapy; 12-mo = 12 months after therapy. Numbers in bold represent the coordinates shifted away from normal values observed in healthy controls. Numbers for healthy controls are mean ± SE.
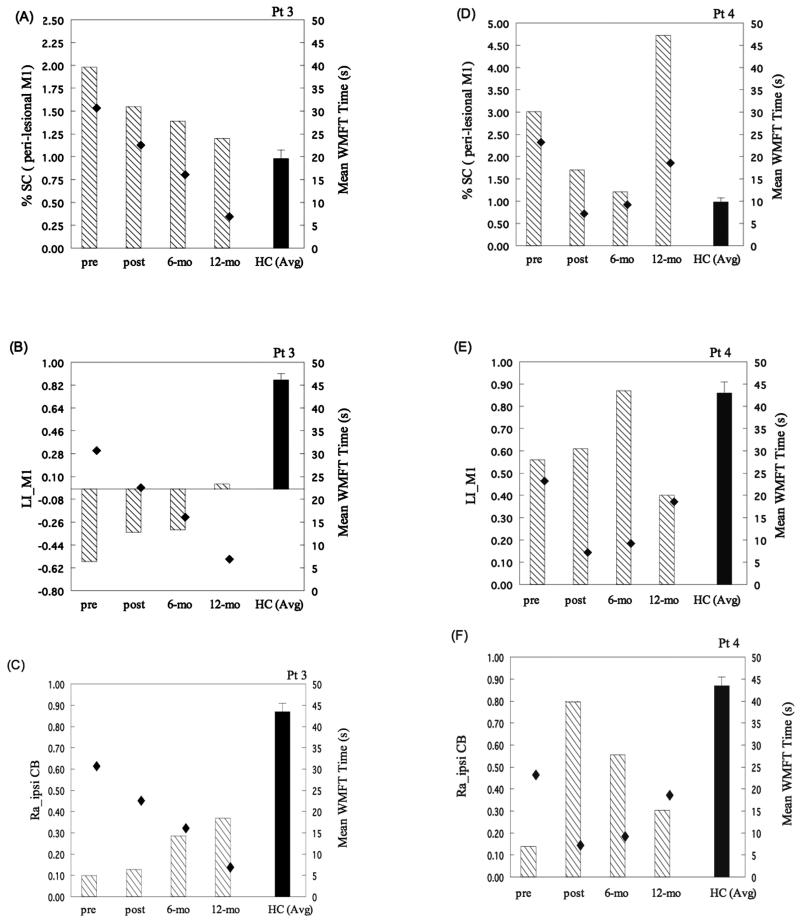
Longitudinal observations in patients 3 and 4 revealed that perilesional M1 activation (%SC and LI_M1) and lateralization of ipsilateral CB activation (Ra_ipsiCB) paralleled the degree of change in motor performance (mWMFTT), despite different recovery trajectories (Fig. 5). Specifically, patient 3 showed a continuous decrease in mean WMFT time across the 4 time points, which corresponded to a progressive increase in LI_M1 and Ra_ipsi_CB, as well as a decrease in %SC of perilesional M1 activation (Fig. 5A-C). For patient 4, a robust decrease in mean WMFT time at 6 months was associated with a decrease in %SC, an increase in LI_M1, and an increase in Ra_ipsi_CB. However, a decline in motor performance (increase in mWMFTT) at 12 months corresponded to an increase in %SC, a decrease in LI_M1, and a decrease in Ra_ipsi_CB (Fig. 5D-F).
Figure 5.
Relationships between functional magnetic resonance imaging (fMRI) activation (%SC, LI_M1, and Ra_ipsiCB; left y-axis) and motor performance (mean WMFT time; right y-axis) across time in patients 3 and 4. Functional MRI activation patterns, including (A, D) %SC of perilesional M1 activation, (B, E) LI_M1, and (C, F) Ra_ipsiCB plotted versus mean WMFT time across time. For patient 3, across the 4 time points, a close relationship between fMRI activation and motor performance was observed—namely, a lower %SC in perilesional M1, a higher LI_M1, and a greater Ra_ipsiCB corresponded to a better motor performance, as indexed by shorter WMFT time. For patient 4, across the 4 time points, only Ra_ipsiCB paralleled the degree of motor improvement (ie, a greater Ra_ipsiCB corresponded to a better motor performance). Dashed bar, fMRI activation in perilesional M1 and cerebellum in patients. Solid bar = fMRI activation in perilesional M1 and cerebellum in healthy controls. Diamond symbol = mean WMFT time. Pre = before therapy; post = immediately after therapy; 6-mo = 6 months after therapy; 12-mo = 12 months after therapy; %SC = percent signal change; Ra_ipsiCB = ratio of the ipsilateral cerebellar activation; CB = cerebellum; WMFT = Wolf Motor Function Test.
DISCUSSION
Our approach of capturing serial changes in brain activation in relation to behavioral gains after a short course of task-oriented upper extremity rehabilitation in stable patients with partial sparing of the M1 hand representation did support a feasible methodology for examining brain-behavior correlates during poststroke motor recovery. We observed an association between short- and long-term behavioral changes in the time needed to perform standard upper extremity tasks and the functional activity in perilesional M1 measured by fMRI. Despite the small number of patients, our results suggest that future larger rehabilitation trials may be able to determine therapy-related improvements or lack of meaningful gains in motor function over time of treatment in relation to functional adaptations in the intact perilesional M1 and possibly in the cerebellum. The potential utility of this approach is to learn more about the ongoing efficacy of a specific training strategy than a behavioral or neurological examination can reveal.
Relationship Between M1 Integrity and Pretherapy Residual Motor Function
Crafton et al24 suggested that incorporation of normal functional anatomy (ie, fMRI activation maps generated from motor activation tasks in healthy controls) into lesion measurements may provide more precise insights into the behavioral effects of focal brain injury than measures of lesion volume alone. In our small sample, we did observe that an individual patient’s initial motor function, indicated by the Wolf Motor Function Test time (pretherapy mWMFTT), was closely related to M1 structural integrity compared to total lesion volume. The lack of association between hand knob integrity and pretherapy mWMFTT may be attributed to the small difference in hand knob volume among the patients and the nature of the WMFT, which relies on functional tasks of the hand, arm, and shoulder, requiring single-joint and multijoint complex movements of the entire upper extremity.
Correlates Between Cerebral Adaptive Reorganization and Motor Functional Gains
In general, the activation magnitude (%SC) in perilesional M1 in patients before and after therapy was higher than that in contralateral M1 in the control group. Furthermore, a posttherapy decrease in activation magnitude (%SC in Fig. 4A), which was accompanied by an increase in the laterality index (LI_M1), tended to be associated with posttherapy behavioral gains (percent decrease in mWMFTT). This brain-behavior correspondence, in which a lower magnitude of perilesional M1 activation (evolving closer to control level) was associated with greater behavioral improvement, persisted longitudinally at 6 and 12 months in the 2 most impaired patients (Fig. 5A, D).
We speculate that an initial recruitment of neuronal ensembles (increased %SC), followed by the posttherapy reduction in perilesional M1 activation magnitude, may reflect strengthened synaptic efficacy among the M1 motor neuron pools spared by the lesion and modulated by repetitive task-oriented training and motor skill learning.25,26 The correspondence between changes in perilesional M1 activation magnitude and motor behavioral changes suggests that the decrease in activation magnitude across time is not simply a fluctuation of perilesional blood oxygenation-dependent (BOLD) signals but rather represents a training-related functional adaptation. This brain-behavior association is well illustrated in the exemplary data of patients 3 and 4. At 12 months posttherapy, an increase in perilesional M1 activation magnitude corresponded to a decline in motor performance in patient 4, in contrast to the reduction in perilesional M1 magnitude in patient 3, whose motor performance continued to improve. The short- and long-term brain-behavior correspondence suggests that the motor recovery trajectory triggered by specific rehabilitation training can be monitored by functional adaptations in the perilesional M1 in patients who have a cortical lesion that partially spares M1. Therefore, if the brain-behavior correspondence observed in the current study can be replicated in a larger sample, the dynamic changes in functional activity in perilesional M1 may serve as a “central indicator” for the potential for continued rehabilitative training or practice to promote task-specific behavioral gains.13
Jaillard et al6 observed that in patients with a focal M1 infarct, the location of task-related motor activation in the perilesional area that represented the paretic hand was located more dorsal to that of controls. This dorsal shift progressively increased over 2 years, reflecting functional reorganization in the motor cortex adjacent to the lesion. Before CIMT, patients 3 and 4 showed a dorsal shift, and patient 1 showed a ventral shift in M1 activation, indicating the emergence of a newly expanded representation of finger movements in novel locations adjacent to the M1 lesion.27,28 Of particular interest, this pretherapy dorsal shift observed in the 2 more impaired patients was unchanged immediately after therapy, corresponding to relatively poor residual motor function, less skillful finger-tapping performance, and only moderate posttherapy motor performance gains on the WMFT (Table 3). However, concurrent with the reemergence of perilesional M1 activation, which became centered more closely to that of the healthy control group by 6 to 12 months after therapy, patients 3 and 4 progressively regained more motor function, especially for fine finger coordination and dexterity required for finger tapping. This longitudinal change supports a brain-behavior correspondence between cerebral adaptation and recovery of motor function that is optimized by specific training in the short term and that may change further over time as the hand is used in daily activities. The close to normal location of perilesional M1 activation observed in patient 2 before and after therapy was associated with the least initial motor impairment and nearly normal tapping performance at the onset of the study. Several potential confounders for shifts in the geometric center of perilesional M1 activation need to be addressed. The relatively large lesion in patients 1, 3, and 4 could have caused anatomical distortion during image processing, resulting in a “pseudo-shift” of activation in patients compared to controls. The dynamic shift in the location of perilesional M1 activation across time did not support this bias because training itself would not alter lesion size to diminish the effect of anatomical distortion. The dorsal shift toward the wrist/elbow representation of perilesional M1 activation in patients 3 and 4 with poor task performance raised the concern that compensatory use of proximal muscles during finger tapping resulted in activation dorsal to the hand motor area representing proximal arm somatotopy. Offline inspection of videotapes helped us rule out this possibility as no noticeable movements from the proximal arm were observed. More likely, this shift reflected training-induced incorporation of synaptic inputs from neuronal ensembles that represented the more proximal arm and hand.
We observed that functional gains after therapy were associated with the evolution of CB activity toward being more ipsilateral to the paretic hand. This correlation persisted up to 12 months after completion of therapy. Dynamic shift in the CB activation pattern in close relation to poststroke motor recovery was supported by previous findings.29 Just how the CB interacts with other nodes within the sensorimotor network to facilitate the recovery process is unresolved. Given its broad connections to the sensorimotor cortices, the CB may act synergistically with the intact perilesional M1 through multiple feedback loops known to be important for motor learning.30 The evolving CB activation pattern, in close relation to short- and long-term training-related motor functional improvement, supports the essential role of the CB in the performance and learning of motor skills.31,32
A major limitation of this pilot study is the small sample size. Future studies with a larger sample size of patients with focal M1 lesions are needed to verify and generalize these findings. The method of serial behavioral testing and scanning can also be applied to patients with subcortical lesions. A potential confounder was the stability and reproducibility of the BOLD signal across a longitudinal study. Our results from the healthy controls showed high between-session reproducibility for magnitude of activation (%SC) in M1 contralateral and CB ipsilateral to the tapping fingers, as well as for LI_M1 and Ra_ipsiCB (Table 4). Our results and others in healthy controls suggest that these highly reproducible fMRI variables can be used for monitoring dynamic changes in brain function in longitudinal studies.33,34
In conclusion, if the “brain-behavior correlates” from this pilot study can be replicated in larger studies of patients with partial M1 lesions or subcortical lesions that spare M1, the evolving fMRI signal in particular regions of interest may serve as a physiologic assay for predicting behavioral gains for a given type and duration of rehabilitation.13 If so, the approach of monitoring ongoing brain-behavior correlates may make it possible to customize rehabilitative programs to optimize poststroke motor recovery.
ACKNOWLEDGMENTS
This study was supported by the National Institutes of Health (NIH) grant NS 45485 to Dr Carolee Winstein and grants R24 HD 39629 and RO1 HD 046740 to Dr Bruce Dobkin. We thank Michelle Prettyman, Chris Hahn, Janice Lin, Jarugool Tretriluxana, and Indu Sattiraju at the University of Southern California. Imaging studies were made possible by the Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson-Lovelace Foundation, the Ahmanson Foundation, William M. and Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community Foundation, Tamkin Foundation, Jennifer Jones-Simon Foundation, Capital Group Companies Charitable Foundation, Robson Family and Northstar Fund, and RR12169, RR13642, and RR00865 from the National Center for Research Resources (NCRR). Analyses were also supported by the Adelson Program in Neural Repair and Rehabilitation of the Dr Miriam and Sheldon G. Adelson Medical Research Foundation.
REFERENCES
- 1.Ward NS, Cohen LG. Mechanisms underlying recovery of motor function after stroke. Arch Neurol. 2004;61:1844–1848. doi: 10.1001/archneur.61.12.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calautti C, Baron JC. Functional neuroimaging studies of motor recovery after stroke in adults: a review. Stroke. 2003;34:1553–1566. doi: 10.1161/01.STR.0000071761.36075.A6. [DOI] [PubMed] [Google Scholar]
- 3.Jones TA, Schallert T. Overgrowth and pruning of dendrites in adult rats recovering from neocortical damage. Brain Res. 1992;581:156–160. doi: 10.1016/0006-8993(92)90356-e. [DOI] [PubMed] [Google Scholar]
- 4.Carmichael ST. Plasticity of cortical projections after stroke. Neuroscientist. 2003;9:64–75. doi: 10.1177/1073858402239592. [DOI] [PubMed] [Google Scholar]
- 5.Dobkin BH. The Clinical Science of Neurologic Rehabilitation. Vol. 2003. Oxford University Press; Oxford, UK: p. 179. [Google Scholar]
- 6.Jaillard A, Martin CD, Garambois K, et al. Vicarious function within the human primary motor cortex? A longitudinal fMRI stroke study. Brain. 2005;128:1122–1138. doi: 10.1093/brain/awh456. [DOI] [PubMed] [Google Scholar]
- 7.Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J Neurophysiol. doi: 10.1152/jn.1996.75.5.2144. [DOI] [PubMed] [Google Scholar]
- 8.Xerri C, Merzenich MM, Peterson BE, Jenkins W. Plasticity of primary somatosensory cortex paralleling sensorimotor skill recovery from stroke in adult monkeys. J Neurophysiol. 1998;79:2119–2148. doi: 10.1152/jn.1998.79.4.2119. [DOI] [PubMed] [Google Scholar]
- 9.Schaechter JD, Kraft E, Hilliard TS, et al. Motor recovery and cortical reorganization after constraint-induced movement therapy in stroke patients: a preliminary study. Neurorehabil Neural Repair. 2002;16:326–338. doi: 10.1177/154596830201600403. [DOI] [PubMed] [Google Scholar]
- 10.Wittenberg GF, Chen R, Ishii K, et al. Constraint-induced therapy in stroke: magnetic-stimulation motor maps and cerebral activation. Neurorehabil Neural Repair. 2003;17:48–57. doi: 10.1177/0888439002250456. [DOI] [PubMed] [Google Scholar]
- 11.Johansen-Berg H, Dawes H, Guy C, et al. Correlation between motor improvements and altered fMRI activity after rehabilitative therapy. Brain. 2002;125:2731–2742. doi: 10.1093/brain/awf282. [DOI] [PubMed] [Google Scholar]
- 12.Dong Y, Dobkin BH, Cen SY, et al. Motor cortex activation during treatment may predict therapeutic gains in paretic hand function after stroke. Stroke. 2006;37:1552–1555. doi: 10.1161/01.STR.0000221281.69373.4e. [DOI] [PubMed] [Google Scholar]
- 13.Dobkin BH. Rehabilitation and functional neuroimaging dose-response trajectories for clinical trials. Neurorehabil Neural Repair. 2005;19:276–282. doi: 10.1177/1545968305281892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winstein CJ, Miller JP, Blanton S, et al. Methods for a multisite randomized trial to investigate the effect of constraint-induced movement therapy in improving upper extremity function among adults recovering from a cerebrovascular stroke. Neurorehabil Neural Repair. 2003;17:137–152. doi: 10.1177/0888439003255511. [DOI] [PubMed] [Google Scholar]
- 15.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 16.Wolf SL, Thompson PA, Morris DM, et al. The EXCITE trial attributes of the Wolf Motor Function Test in patients with subacute stroke. Neurorehabil Neural Repair. 2005;19:194–205. doi: 10.1177/1545968305276663. [DOI] [PubMed] [Google Scholar]
- 17.Gladstone DJ, Danells CJ, Black SE. The Fugl-Meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair. 2002;16:232–240. doi: 10.1177/154596802401105171. [DOI] [PubMed] [Google Scholar]
- 18.Dobkin BH, Firestine A, West M, et al. Ankle dorsiflexion as an fMRI paradigm to assay motor control for walking during rehabilitation. Neuroimage. 2004;23:370–381. doi: 10.1016/j.neuroimage.2004.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duvernoy HM. The Human Brain: Surface, Blood Supply, and Three-Dimensional Anatomy. Springer-Verlag; New York: 1991. [Google Scholar]
- 20.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 21.Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- 22.Worsley KJ, Evans AC, Marrett S, et al. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab. 1992;12:900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- 23.Friston KJ, Worsley KJ, Frackowiak RS, et al. Assessing the significance of focal activations using spatial extent. Human Brain Mapp. 1994;1:214–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- 24.Crafton KR, Mark AN, Cramer SC. Improved understanding of cortical injury by incorporating measures of functional anatomy. Brain. 2003;126:1650–1659. doi: 10.1093/brain/awg159. [DOI] [PubMed] [Google Scholar]
- 25.Logothetis NK, Pauls J, Augath M, et al. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 26.Buchel C, Coull JT, Friston KJ. The predictive value of changes in effective connectivity for human learning. Science. 1999;283:1538–1541. doi: 10.1126/science.283.5407.1538. [DOI] [PubMed] [Google Scholar]
- 27.Friel KM, Heddings AA, Nudo RJ. Effects of postlesion experience on behavioral recovery and neurophysiologic reorganization after cortical injury in primates. Neurorehabil Neural Repair. 2000;14:187–198. doi: 10.1177/154596830001400304. [DOI] [PubMed] [Google Scholar]
- 28.Nudo RJ, Plautz EJ, Frost SB. Role of adaptive plasticity in recovery of function after damage to motor cortex. Muscle Nerve. 2001;24:1000–1019. doi: 10.1002/mus.1104. [DOI] [PubMed] [Google Scholar]
- 29.Small SL, Hlustik P, Noll DC, et al. Cerebellar hemispheric activation ipsilateral to the paretic hand correlates with functional recovery after stroke. Brain. 2002;125:1544–1557. doi: 10.1093/brain/awf148. [DOI] [PubMed] [Google Scholar]
- 30.Middleton FA, Strick PL. The cerebellum: an overview. Trends Neurosci. 1998;21:367–369. doi: 10.1016/s0166-2236(98)01330-7. [DOI] [PubMed] [Google Scholar]
- 31.Sanes JN, Dimitrov B, Hallett M. Motor learning in patients with cerebellar dysfunction. Brain. 1990;113(pt 1):103–120. doi: 10.1093/brain/113.1.103. [DOI] [PubMed] [Google Scholar]
- 32.Jueptner M, Weiller C. A review of differences between basal ganglia and cerebellar control of movements as revealed by functional imaging studies. Brain. 1998;121:1437–1449. doi: 10.1093/brain/121.8.1437. [DOI] [PubMed] [Google Scholar]
- 33.Yoo SS, Wei X, Dickey CC, et al. Long-term reproducibility analysis of fMRI using hand motor task. Int J Neurosci. 2005;115:55–77. doi: 10.1080/00207450490512650. [DOI] [PubMed] [Google Scholar]
- 34.Liu JZ, Zhang L, Brown RW, et al. Reproducibility of fMRI at 1.5 T in a strictly controlled motor task. Magn Reson Med. 2004;52:751–760. doi: 10.1002/mrm.20211. [DOI] [PubMed] [Google Scholar]