Abstract
Background.
Few longitudinal studies compare changes in instrumental activities of daily living (IADLs) among stroke-free adults to prospectively document IADL changes among adults who experience stroke. We contrast annual declines in IADL independence for older individuals who remain stroke free to those for individuals who experienced stroke. We also assess whether these patterns differ by sex, race, or Southern birthplace.
Methods.
Health and Retirement Study participants who were stroke free in 1998 (n = 17,741) were followed through 2010 (average follow-up = 8.9 years) for self- or proxy-reported stroke. We used logistic regressions to compare annual changes in odds of self-reported independence in six IADLs among those who remained stroke free throughout follow-up (n = 15,888), those who survived a stroke (n = 1,412), and those who had a stroke and did not survive to participate in another interview (n = 442). We present models adjusted for demographic and socioeconomic covariates and also stratified on sex, race, and Southern birthplace.
Results.
Compared with similar cohort members who remained stroke free, participants who developed stroke had faster declines in IADL independence and lower probability of IADL independence prior to stroke. After stroke, independence declined at an annual rate similar to those who did not have stroke. The black-white disparity in IADL independence narrowed poststroke.
Conclusion.
Racial differences in IADL independence are apparent long before stroke onset. Poststroke differences in IADL independence largely reflect prestroke disparities.
Key Words: Minority aging, Disablement process, Stroke, Cardiovascular, Epidemiology.
Independence in instrumental activities of daily living (IADLs) is an essential component of health in middle and older age. People with more independence in carrying out IADLs—tasks like managing money, grocery shopping, using a map, talking on the telephone, preparing meals, and managing medications—have better self-reported subjective health measures including quality of life and self-rated health (1–3) as well as more objective health outcomes like lower mortality risk (4–6). IADL dependence also imposes a substantial burden on caregivers and health service providers: individuals with IADL dependence often require help from others (7) and those with IADL limitations incur roughly $2,000 more annually to Medicare Part A compared with individuals with no disability (8).
Although IADL independence typically declines with age (9), such declines may be accelerated by onset of acute medical events such as stroke (10,11). Studies of rehabilitation and health poststroke very commonly use IADLs as an outcome measure since strokes may affect one’s ability to carry out physically and cognitively demanding tasks like IADLs (11–13). However, studies that only consider IADLs poststroke, without describing the trajectory of IADL changes prior to stroke, may be inadequate to describe the effect of stroke onset on a person’s IADLs. Several studies have found pre-event disability associated with onset and mortality from acute health events, such as myocardial infarction (14,15) or stroke (16–20). However, these prior studies have looked only at basic activities of daily living. To our knowledge, no study has prospectively described changes in IADL independence prior to and following stroke onset. Such evidence is essential to understand how the consequences of stroke differ by patient characteristics such as sex, race, or geographic area of residence. Although there are noted differences in stroke outcomes by demographic characteristics (21–26), it is yet unknown if these differences merely reflect patterns that prevailed prior to stroke or result from differences in the consequences of stroke per se.
We used data from the U.S. Health and Retirement Study (HRS), a nationally representative sample of adults aged 50+, to assess trends in independence in six IADLs by stroke status over 12 years of follow-up. We compared IADL trends between respondents who did not have a stroke during follow-up to those who reported a stroke during follow-up. We also described IADL trends among those who had a stroke by their mortality status poststroke. We hypothesized that (i) even before stroke, declines in IADL independence accelerate as the date of stroke approaches; (ii) among stroke survivors, independence is lower after stroke compared with before the stroke; (iii) among stroke survivors, IADL independence continues to decline during the years after stroke; and (iv) the effects of stroke on IADL disability will be larger for individuals at higher risk of stroke onset (men compared with women; blacks compared with whites; those born in the South compared with non-Southerners).
Methods
HRS was initiated with enrollments in 1992, 1993, and 1998 to create a nationally representative cohort of community-residing U.S. adults born 1947 or earlier. Spouses of age-eligible respondents were also enrolled, regardless of age. Details of the study are provided elsewhere (27,28). Biennial interviews, typically via phone, are ongoing, with retention rates, through 2008, more than 80%. Respondents are followed even if they became institutionalized. After death, proxies are contacted to provide “exit interviews,” which include major health events that preceded the participants’ death (eg, stroke). HRS was approved by the University of Michigan Health Sciences Human Subjects Committee, and these analyses were determined exempt by Harvard School of Public Health Office of Human Research Administration.
In the current analyses, we included white and black HRS participants born between 1900 and 1947 who participated in the 1998 interview wave. There were 19,991 age-eligible respondents in 1998. We excluded 1,422 (7.1%) respondents who reported a diagnosis of stroke at the baseline (1998) interview, 644 (3.2%) who reported a race other than white or black, and 184 (0.9%) individuals whose covariate information was incomplete. In our final analyses, 17,741 individuals contributed person-time; respondents were followed through 2010.
Assessment of Stroke
Onset of stroke between 1998 and 2010 was assessed biennially by the respondent’s self-report of a doctor’s diagnosis of stroke (“Has a doctor ever told you that you had a stroke?”). Prior analyses of self-reported strokes in HRS found that the self-reported stroke incidence was similar to other studies of similar aged individuals that used physician-verified strokes, and major stroke risk factors also predicted similarly (29); this evidence suggests that self-reported stroke in HRS may be a sensitive measure, although we acknowledge it does not fully address the limitations of self-reported stroke. For participants who had died and those unavailable for a direct interview, interviews were conducted with proxy informants, typically spouses. No information on stroke subtypes was obtained; transient ischemic attacks were not coded as strokes. We used the month and year of participants’ first stroke to characterize respondents with respect to months-until-stroke and months-since-stroke trajectories; we did not “restart the clock” for respondents who reported a subsequent stroke. We classed people into three categories: never stroke (no event recorded during the HRS follow-up period), stroke survivors (stroke reported during follow-up and respondent survived to participate in a subsequent interview), and stroke decedents (stroke reported during follow-up, but respondent did not survive to participate in a subsequent interview; decedents include people who died from any cause, including but not restricted to stroke).
Participants or their proxies were asked the month and year of their stroke. For each biennial interview, we calculated the months-until-the-stroke date and months-since-the-stroke date. We estimated the association between IADL independence and the months-until and months-since-stroke variables. All coefficients were converted from months into years, in order to facilitate comparison with rate of change in IADL independence among stroke-free participants per year of age.
Of the stroke date information, only month was missing for 16.6% of events; an additional 6.7% of events were missing both month and year. For these events, we used the midpoint of the last known stroke-free date and the date when the stroke was first reported, based on all available biennial interviews and any date information provided, as the date of stroke. The extent of missing information on the date of event was greater among stroke survivors than decedents (% imputed: decedents = 9.9%, survivors = 18.6%; p ≤ .01). We repeated primary analyses excluding individuals with uncertain stroke dates; results were very similar in magnitude and significance (Supplementary Appendix Table A).
Instrumental Activities of Daily Living
Respondents were asked at each wave of interviews if, because of a health or memory problem, they had difficulties with each of six IADLs (taking medications as prescribed, grocery shopping, using the telephone, using maps, preparing meals, managing money) in the prior 30 days. Respondents reported whether they had difficulty with each IADL by answering: yes, no, cannot do, or do not do. For comparability with prior research, we used the RAND version of these variables, which dichotomized the response categories to no difficulty (“no”) versus difficulty (“yes” or “cannot do”) for each IADL; “do not do” responses (<0.05% per wave) were considered missing. We also created an indicator variable for any IADL difficulties (ie, 0 difficulties vs 1+ difficulties), based on the RAND summary item. These questions were asked consistently at each wave of survey administration from 1998 to 2010.
Covariates
We considered demographic and socioeconomic covariates that potentially confound the relationship between IADLs and stroke. Demographic characteristics included patients’ age, race (white/black; white as reference), Hispanic ethnicity (yes or no), sex (male or female), marital status (married, never married, divorced/separated, or widowed), height (30,31), and Southern birthplace (defined as Census region and categorized yes or no) (32). Socioeconomic variables included socioeconomic position currently (years of education and natural log of per capita household wealth) and in childhood (height (31) and maternal education unknown or <8 vs ≥8 years). We set mother’s education to the reference group (<8 years) for the 10.6% of respondents who did not report it and included an indicator of this imputation in regression models. All measures were assessed at baseline. Continuous variables were centered at the group mean; for categorical variables, the most prevalent group was used as the reference value.
Methods of Analysis
We used repeated-measures logistic regression to model independence trajectories. Changes in IADL independence were estimated by time-updated age among those who did not develop stroke during follow-up, and using the calculated time until and time since stroke for those who developed stroke during follow-up. The estimated equation is presented in Supplementary Appendix B with specific attention to the terms of the model that test our four hypotheses. Briefly, the “Years Until Stroke” terms test Hypothesis 1; the “Stroke” term tests Hypothesis 2; and the “Years Since Stroke” term tests Hypothesis 3. The time terms (e.g., time-until-stroke, time-since-stroke) are included as linear terms; we tested alternative functional forms (quadratic and cubic) and found linear forms best fit these data. Primary analyses were conducted in SAS 9.3 with PROC GENMOD (SAS, Cary, NC) using a logit link, specifying repeated measures on individuals (ie, generalized estimating equation model); these models estimate the change in probability status over time and not time-to-incident disability. Results reflect robust variance estimates and the 1998 (baseline) sampling weights. HRS used a multistage, clustered sample design, but the estimated design effects for the association between IADLs and stroke timing are very small. Thus, our analytic models account for repeated measures on the same individual (ie, generalized estimating equation estimates) but not for sample design clustering. The HRS sampling weights were applied to make the population representative of the 1998 U.S. population born 1947 or earlier.
We present adjusted odds ratios (ORs) for odds of independence in IADLs; for interpretation, ORs <1 reflect lower odds of independence (ie, worse health, more disability), and ORs >1 reflect higher odds of IADL independence (ie, better health, less disability). ORs may be misleading when the outcome is common (as is independence in IADLs). To illustrate patterns visually, predicted probabilities of independence for each IADL (defined as predicted odds/[1 + predicted odds]) are estimated from this model and plotted for a reference category: a 75-year-old, white, married woman who was not born in the South, whose mother had ≤8 years of education, who herself had 12 years of education, was 1.7 m tall, with per capita household wealth of $172,407. We consider how demographic factors may modify IADL trajectories using an indicator of difficulty with any of the IADLs as the outcome measure. We show models for the odds of any IADL limitation stratified by sex, race, and Southern birth; these test Hypothesis 4. Specifically, we estimated the probabilities with bootstrapped standard errors using 2,000 replications of a normal bootstrap procedure and tested the null hypothesis that the difference in the probabilities between strata was 0. The similarity of patterns between the individual IADL activities suggested it was reasonable to collapse the individual IADL activities into a single, dichotomous indicator. In sensitivity analyses to address the possibility that selected survival poststroke biased our estimates of IADL independence poststroke, we also estimated similar models stratified by number of waves of follow-up (Supplementary Appendix C).
Results
Respondents were followed for an average of 8.9 years. Participant characteristics at baseline are summarized in Table 1 by stroke status during follow-up: those who survived their stroke (“survivors,” n = 1,412); those who had a stroke and died before the next follow-up (“decedents,” n = 441); and those who remained stroke free (n = 15,888) during the course of this study.
Table 1.
Baseline Characteristics of Study Population by Stroke Status During Follow-up: Health and Retirement Study, 1998
| Variable | Ever Stroke (n = 1,853) | Never Stroke (n = 15,888) | ||||||
|---|---|---|---|---|---|---|---|---|
| Decedents (n = 441) | Survivors (n = 1,412) | |||||||
| Mean/n | SD/% | Mean/n | SD/% | p Value (decedents vs survivors) | Mean/n | SD/% | p Value (ever vs never stroke) | |
| Age at stroke | 80.9 | 9.5 | 75.2 | 9.6 | <.0001 | |||
| Age at baseline | 74.6 | 9.7 | 68.9 | 9.5 | <.0001 | 65.7 | 9.9 | <.0001 |
| Male | 167 | 37.9 | 609 | 43.1 | .05 | 6,883 | 43.3 | .24 |
| Height | 1.7 | 0.1 | 1.7 | 0.1 | .02 | 1.7 | 0.1 | <.0001 |
| Race | ||||||||
| White | 364 | 82.5 | 1,160 | 82.2 | .85 | 13,717 | 86.3 | <.0001 |
| Black | 77 | 17.5 | 252 | 17.9 | .85 | 2,171 | 13.7 | <.0001 |
| Marital status | ||||||||
| Married | 208 | 47.2 | 902 | 63.9 | <.0001 | 11,104 | 69.9 | <.0001 |
| Divorced/separated | 41 | 9.3 | 117 | 8.3 | .51 | 1,571 | 9.9 | .06 |
| Widowed | 177 | 40.1 | 354 | 25.1 | <.0001 | 2,763 | 17.4 | <.0001 |
| Never married | 15 | 3.4 | 39 | 2.8 | .49 | 450 | 2.8 | .84 |
| Wealth, per capita, median (IQR) | 65,000.00 | 149,735.42 | 79,105.05 | 194,219.01 | .00 | 98,997.47 | 212,350.61 | <.0001 |
| Years of education | 10.9 | 3.7 | 11.8 | 3.2 | <.0001 | 12.1 | 3.2 | <.0001 |
| Southern birthplace | 85 | 19.3 | 267 | 18.9 | .86 | 2,510 | 15.8 | .0004 |
| Mother’s education | ||||||||
| <8 y | 673 | 47.7 | 739 | 52.3 | 0.05 | 7,669 | 48.3 | <.0001 |
| ≥8 y | 187 | 42.4 | 673 | 47.7 | 0.05 | 8,219 | 51.7 | <.0001 |
| Missing | 57 | 12.9 | 169 | 12.0 | 0.59 | 1,608 | 10.1 | 0.01 |
Note: Numbers were similar but not identical for all IADL outcomes. IADL = instrumental activity of daily living; IQR = interquartile range.
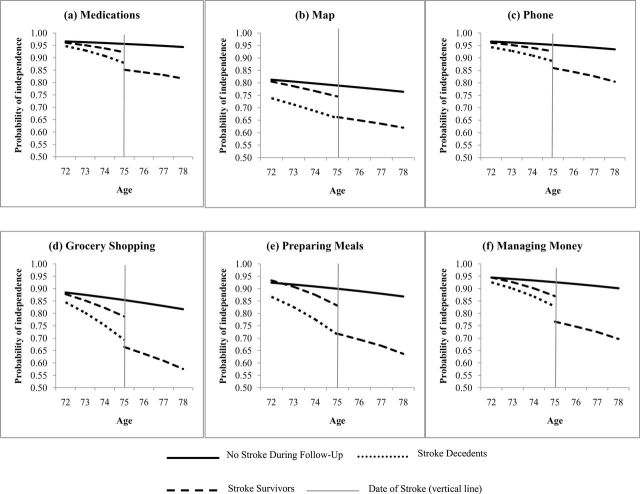
Overall, women, non-whites, and Southern-born individuals had lower odds of IADL independence (Table 2). Comparison of the rates of change in IADL independence among those who remain stroke free, stroke survivors, and stroke decedents are presented for each IADL in two ways: probabilities of independence plotted for the reference category (defined above) in Figure 1 and precise coefficients (ORs and confidence intervals [CIs]) in Table 2.
Table 2.
Odds Ratios (ORs) and Confidence Intervals (CIs) for Independence in Activities of Daily Living by Stroke Status and Timing
| Medications | Reading Maps | Talking on the Telephone | Shopping | Cooking Meals | Managing Money | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |||||||
| (obs = 157,693) | (obs = 142,003) | (obs = 161,683) | (obs = 156,768) | (obs = 153,586) | (obs = 156,722) | |||||||||||||
| Intercept | 21.80 | 19.21 | 24.74 | 3.75 | 3.43 | 4.09 | 20.12 | 17.80 | 22.75 | 5.84 | 5.30 | 6.44 | 8.96 | 8.04 | 9.99 | 12.52 | 11.23 | 13.97 |
| Age, centered at 75 | ||||||||||||||||||
| Never stroke | 0.91 | 0.91 | 0.92 | 0.95 | 0.95 | 0.96 | 0.89 | 0.89 | 0.90 | 0.91 | 0.91 | 0.92 | 0.90 | 0.90 | 0.91 | 0.90 | 0.89 | 0.91 |
| Age at stroke, survivor | 0.94 | 0.93 | 0.96 | 0.97 | 0.95 | 0.98 | 0.92 | 0.91 | 0.94 | 0.94 | 0.92 | 0.95 | 0.93 | 0.91 | 0.94 | 0.93 | 0.91 | 0.94 |
| Age at stroke, decedent | 0.97 | 0.94 | 1.00 | 1.00 | 0.98 | 1.02 | 0.95 | 0.92 | 0.98 | 0.94 | 0.92 | 0.97 | 0.96 | 0.94 | 0.99 | 0.94 | 0.91 | 0.97 |
| Ever stroke survivor | 0.54 | 0.43 | 0.69 | 0.78 | 0.67 | 0.92 | 0.63 | 0.50 | 0.79 | 0.63 | 0.53 | 0.75 | 0.55 | 0.46 | 0.66 | 0.53 | 0.44 | 0.65 |
| Years until stroke | 0.78 | 0.72 | 0.86 | 0.89 | 0.86 | 0.92 | 0.80 | 0.73 | 0.87 | 0.80 | 0.76 | 0.84 | 0.71 | 0.66 | 0.75 | 0.73 | 0.68 | 0.78 |
| Stroke | 0.48 | 0.39 | 0.61 | 0.67 | 0.58 | 0.78 | 0.49 | 0.40 | 0.59 | 0.53 | 0.46 | 0.63 | 0.52 | 0.44 | 0.61 | 0.49 | 0.42 | 0.58 |
| Years since stroke | 0.92 | 0.90 | 0.95 | 0.94 | 0.91 | 0.97 | 0.88 | 0.85 | 0.91 | 0.89 | 0.86 | 0.91 | 0.89 | 0.86 | 0.92 | 0.90 | 0.87 | 0.92 |
| Ever stroke decedent | 0.33 | 0.22 | 0.50 | 0.51 | 0.37 | 0.72 | 0.39 | 0.26 | 0.59 | 0.39 | 0.28 | 0.53 | 0.28 | 0.20 | 0.39 | 0.38 | 0.27 | 0.55 |
| Years until stroke | 0.74 | 0.66 | 0.83 | 0.88 | 0.82 | 0.95 | 0.78 | 0.72 | 0.85 | 0.75 | 0.70 | 0.79 | 0.73 | 0.67 | 0.79 | 0.73 | 0.67 | 0.80 |
| Male | 1.06 | 0.89 | 1.25 | 2.52 | 2.24 | 2.84 | 0.73 | 0.62 | 0.85 | 1.84 | 1.61 | 2.10 | 1.46 | 1.26 | 1.69 | 1.11 | 0.96 | 1.27 |
| Black | 0.89 | 0.75 | 1.05 | 0.63 | 0.56 | 0.71 | 0.91 | 0.78 | 1.08 | 0.93 | 0.81 | 1.06 | 0.90 | 0.77 | 1.04 | 0.82 | 0.72 | 0.95 |
| Southern birth | 0.84 | 0.72 | 0.98 | 0.79 | 0.71 | 0.88 | 1.02 | 0.88 | 1.18 | 0.88 | 0.78 | 0.99 | 0.86 | 0.75 | 0.98 | 0.85 | 0.75 | 0.98 |
Notes: Dependent variable is a binary indicator of reporting independence with the specific instrumental activity of daily living (IADL) tasks listed as the column heading: medication, maps, phone, money, shopping, and cooking meals, respectively. Stroke-free respondents are the reference group. In other words, lower ORs reflect a lower odds of reported ability to take medications independently associated with a 1-unit increase in the respective independent variable. These estimates are adjusted for covariates: height (m), marital status (divorced, widowed, never married; married reference), wealth (mean centered), own education (y), mother’s education (>8/≤8 y), missing indicator for mother’s education. Age for never stroke reflects the annual age-related change in IADL independence; age at stroke is fixed at the age of stroke and reflects the differential in IADL independence between a stroke/survivor decedent just prior to stroke and a similar aged, never stroke respondent. In this model, “Years After Stroke” is set to zero for assessments that occurred before stroke and ranges from 0.04 to 10.4 y for assessments after stroke. The variable “Years Before Stroke” is set to zero for assessments that occurred after stroke and ranges from −9.5 to −0.001 for assessments prior to stroke. The coefficient for “Years After Stroke” describes the annual change in log odds of disability after stroke. The coefficient for “Years Before Stroke” describes the change in log odds of disability in each year leading up to stroke. obs = the total number of person-observations in the analysis.
Figure 1.
Predicted probabilities of independence in each activity of daily living (ADL): (a) dressing, (b) eating, (c) walking, (d) transferring to/from bed, (e) bathing, (f) managing money, by stroke status over follow-up (never/survivor/decedent)—Health and Retirement Study, 1998–2010. Reference group is defined as 75-year-old, white, married women who were born in the South, whose mothers had ≤8 years of education, who themselves had 12 years of education, who were 1.7 m tall, and with household wealth of $172,407. Vertical line represents the transition at the time of stroke for stroke patients, with predicted probabilities of independence modeled for someone whose stroke occurred at age 75.
Those who remained stroke free during follow-up had a 9% decreased odds of independence in managing medications for each additional year of age (OR = 0.91, 95% CI: 0.91, 0.92) (Table 2). Individuals who subsequently had a stroke averaged significantly steeper annual declines in independence compared with those who remained stroke free, both among those who survived the stroke (OR = 0.78, 95% CI: 0.72, 0.88) and those who died after stroke (OR = 0.74, 95% CI: 0.66, 0.83) (supporting Hypothesis 1). In other words, stroke decedents had a 26% annual decline in odds of independence managing medications associated with each year leading up to stroke, compared with an 8% annual decline in odds of independence in similar aged individuals who remained stroke free. As a result of this steeper decline, those who had a stroke had lower levels of IADL independence just prior to stroke compared with comparable aged individuals who remained stroke free; for example, a 75-year-old person on the brink of stroke had 46% lower odds of independence managing medications than an otherwise similar participant who remained stroke free throughout follow-up (OR = 0.54, 95% CI: 0.43, 0.69). Among those who died after stroke, the odds of independence in managing medications just prior to stroke was 77% lower than the odds of independence among those who remained stroke free. As shown in Figure 1, although the differences in independence were quite far from the null when expressed as ORs, all participants had high levels of independence in managing medications regardless of stroke status. For example, the absolute difference in the predicted probability of independence between stroke survivors (probability = .922) and those who remained stroke free (probability = .956) of reference age and characteristics is 3.5 percentage points. For those who survived the stroke, the stroke itself was associated with a 52% decline in odds of independence in managing medications (OR = 0.48, 95% CI: 0.39, 0.61) (supporting Hypothesis 2). After the stroke, the odds of independence continued to decline annually (OR = 0.92, 95% CI: 0.90, 0.95) (supporting Hypothesis 3). The rate of the decline in independence poststroke was significantly slower (in multiplicative terms) than the rate of decline prior to stroke and similar to the average annual age-related decline in IADL independence seen in those who remained stroke free throughout follow-up (OR = 0.91, 95% CI: 0.91, 0.92).
The pattern was similar for other IADL outcomes, with accelerated decline prior to stroke, especially among those who did not survive stroke; substantial decline at the time of stroke; and relatively slower poststroke declines. For example, the estimated decrements in IADL independence associated with the stroke itself were very consistent across the six outcomes (eg, with absolute declines ranging from 6.7 to 11.8 percentage points), as were the annual rates of change (Table 2). However, the absolute levels of independence were lower for reading maps: on average, 3 years after stroke, someone who survived a stroke had an estimated probability of independence using a map of .62, compared with a probability of .82 for managing money (Figure 1).
Among individuals who remained stroke free, the rates of change in all IADL independence (ie, odds of independence in all 6 IADLs) were similar by sex, race and Southern birth status: coefficients are presented in Table 3 and predicted probabilities for reference groups are plotted in Figure 2. For example, the annual decline in odds of independence in all IADLs was 8% for stroke-free women (OR = 0.92, 95% CI: 0.91, 0.92) and 7% for stroke-free men (OR = 0.93, 95% CI: 0.92, 0.93). However, the decrement associated with the stroke was a 9.5 percentage point decrease in probability of IADL independence for men (Figure 2a) but 15.7% decrease for women (Figure 2b), although this difference could be due to chance (test of difference: p > .1). Compared with those who remained stroke free, both white and black stroke survivors had lower levels of independence prior to stroke. However, the magnitude of the gap between those who were about to have a stroke from those who remained stroke free was larger for blacks than whites: prior to stroke, blacks had a 47% lower odds of IADL independence than blacks who remained stroke free (OR = 0.53, 95% CI: 0.37, 0.71). In contrast, prior to stroke, whites who survived a stroke had 33% lower (OR = 0.67, 95% CI: 0.57, 0.79) odds of IADL independence just prior to stroke compared with whites who remained stroke free. In absolute terms, the difference in probability of IADL independence between stroke-free and stroke survivors was 13 percentage points for non-whites (Figure 2c) and 6.7 percentage points for whites just prior to stroke (Figure 2d) (test of difference: p < .05). The magnitude of these prestroke differences transmitted to the poststroke population: though non-white stroke survivors averaged lower poststroke independence than white stroke survivors, this difference was not attributable to the effects of stroke. Rather, this difference emerged prior to stroke and was actually slightly smaller after stroke: 3 years prior to stroke, whites who later experienced stroke had 0.102 percentage point higher independence than blacks (p < .001), whereas 3 years after stroke, this gap was only 0.091 percentage points (p > .1; test of difference, p < .1). On the other hand, the gap in IADL independence between Southern and non-Southern born was wider after stroke (Figure 2e and f), although this change was not statistically significantly different from zero (test of difference: p > .1).
Table 3.
Odds Ratios (ORs) and Confidence Intervals (CIs) for Independence in all IADLs by Stroke Status and Timing, Stratified by Demographic Characteristics (sex, race, Southern birthplace)
| Female | Male | Non-White | White | Non-Southern Born | Southern Born | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |||||||
| (obs = 94,495) | (obs = 70,461) | (obs = 22,349) | (obs = 140,146) | (obs = 137,034) | (obs = 25,461) | |||||||||||||
| Intercept | 4.15 | 3.75 | 4.59 | 5.29 | 4.70 | 5.96 | 3.49 | 2.82 | 4.31 | 4.32 | 3.93 | 4.74 | 4.26 | 3.88 | 4.67 | 3.68 | 3.03 | 4.47 |
| Age, centered at 75 | ||||||||||||||||||
| Never stroke | 0.92 | 0.91 | 0.92 | 0.93 | 0.92 | 0.93 | 0.95 | 0.94 | 0.96 | 0.92 | 0.91 | 0.92 | 0.92 | 0.91 | 0.92 | 0.93 | 0.92 | 0.94 |
| Age at stroke, survivor | 0.95 | 0.93 | 0.96 | 0.94 | 0.93 | 0.96 | 0.97 | 0.95 | 0.99 | 0.94 | 0.93 | 0.95 | 0.95 | 0.93 | 0.96 | 0.94 | 0.92 | 0.97 |
| Age at stroke, decedent | 0.96 | 0.93 | 0.98 | 0.96 | 0.92 | 1.00 | 0.95 | 0.91 | 0.99 | 0.96 | 0.94 | 0.98 | 0.96 | 0.93 | 0.98 | 0.97 | 0.91 | 1.03 |
| Ever stroke survivor | 0.60 | 0.49 | 0.72 | 0.71 | 0.56 | 0.89 | 0.53 | 0.37 | 0.77 | 0.67 | 0.57 | 0.79 | 0.65 | 0.55 | 0.76 | 0.60 | 0.42 | 0.85 |
| Years until stroke | 0.84 | 0.80 | 0.88 | 0.81 | 0.75 | 0.88 | 0.87 | 0.80 | 0.95 | 0.82 | 0.78 | 0.86 | 0.84 | 0.80 | 0.87 | 0.80 | 0.73 | 0.88 |
| Stroke | 0.50 | 0.41 | 0.61 | 0.61 | 0.49 | 0.75 | 0.54 | 0.37 | 0.76 | 0.54 | 0.46 | 0.64 | 0.55 | 0.47 | 0.64 | 0.52 | 0.36 | 0.75 |
| Years since stroke | 0.91 | 0.88 | 0.95 | 0.89 | 0.85 | 0.93 | 0.92 | 0.86 | 0.99 | 0.89 | 0.87 | 0.92 | 0.90 | 0.87 | 0.92 | 0.92 | 0.86 | 0.98 |
| Ever stroke decedent | 0.35 | 0.25 | 0.50 | 0.43 | 0.27 | 0.70 | 0.48 | 0.27 | 0.86 | 0.36 | 0.26 | 0.49 | 0.37 | 0.27 | 0.50 | 0.42 | 0.21 | 0.83 |
| Years until stroke | 0.78 | 0.72 | 0.84 | 0.79 | 0.71 | 0.88 | 0.82 | 0.73 | 0.92 | 0.78 | 0.73 | 0.83 | 0.79 | 0.74 | 0.84 | 0.78 | 0.69 | 0.87 |
Notes: Dependent variable is a binary indicator of reporting difficulty with any of the six IADL tasks (medication, maps, phone, money, shopping, and cooking meals). Stroke-free respondents are the reference. ORs reflect odds of IADL disability, adjusted for covariates: race (whites reference), sex (female reference), Southern birth (non-Southern as reference), height (m), marital status (divorced, widowed, never married; married reference), wealth (mean centered), own education (years), mother’s education (>8/≤8 years), missing indicator for mother’s education. Age for never stroke reflects the annual age-related change in IADL independence; age at stroke is fixed at the age of stroke and reflects the differential in IADL independence between a stroke/survivor decedent just prior to stroke and a similar aged, never stroke respondent. In this model, “Years After Stroke” is set to zero for assessments that occurred before stroke and ranges from 0.04 to 10.4 y for assessments after stroke. The variable “Years Before Stroke” is set to zero for assessments that occurred after stroke and ranges from −9.5 to −0.001 for assessments prior to stroke. The coefficient for “Years After Stroke” describes the annual change in log odds of disability after stroke. The coefficient for “Years Before Stroke” describes the change in log odds of disability in each year leading up to stroke. IADLs = instrumental activities of daily living; obs = the total number of person-observations in the analysis.
Figure 2.
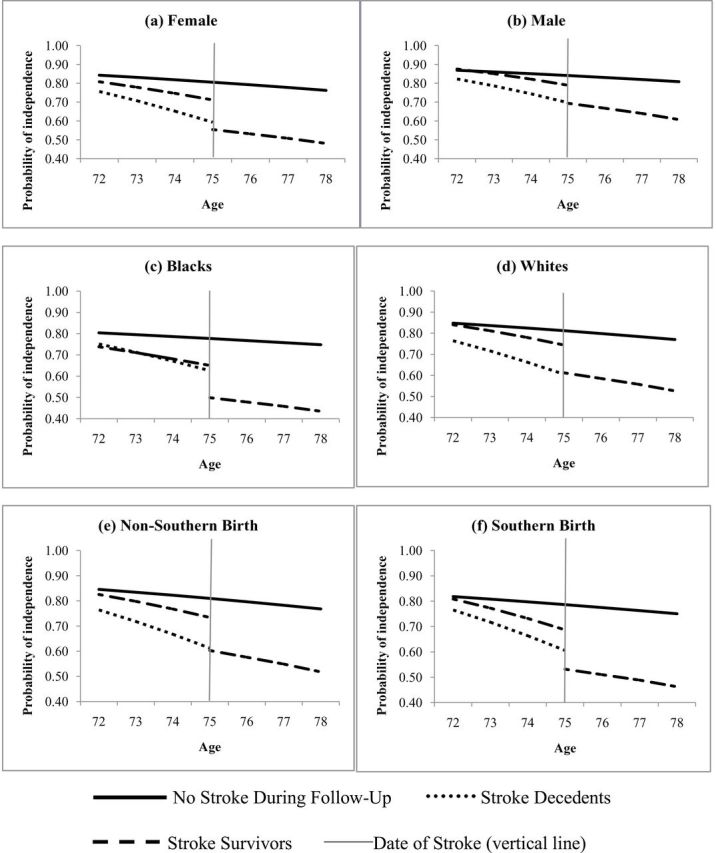
Predicted probabilities of independence in all activities of daily living (ADLs; an indicator of self-reported difficulty with any of the six individual instrumental activities of daily living [IADLs]) stratified by demographic factors (sex, race, Southern birth): (a) female, (b) male, (c) blacks, (d) whites, (e) non-Southern birth, (f) Southern birth, by stroke status over follow-up (never/survivor/decedent)—Health and Retirement Study, 1998–2010. Reference group is defined as 75-year-old, white, married women who were born in the South, whose mothers had ≤8 years of education, who themselves had 12 years of education, who were 1.7 m tall, and with household wealth of $172,407. Vertical line represents the transition at the time of stroke for stroke patients, with predicted probabilities of independence modeled for someone whose stroke occurred at age 75.
Discussion
This large, prospective, observational study offers novel evidence indicating differences in IADL independence are apparent years before onset of stroke. We found that those people who experienced stroke had more notable IADL limitations years prior to their stroke, compared with similarly aged respondents who remained stroke free during follow-up. Prestroke differences were especially marked for non-whites, among whom odds of independence was much lower than among similar individuals who remained stroke free. Among stroke survivors, IADL independence declined dramatically at the time of stroke and continued to decline after the stroke; poststroke decline occurred at a rate similar to the age-related decline among stroke-free participants.
Study Limitations
Our study relies on self- or proxy-reported strokes, lacks information on stroke subtype, and has missing data on date of stroke for some respondents. Prior research shows similar stroke incidence rates in HRS compared with cohorts using physician-verified stroke (29). Our sensitivity analyses based on complete cases (see Supplementary Appendix Table A for missing data analyses) suggested missing stroke date data did not substantially affect our findings. IADL data were either self- or proxy reported. The number of proxy reports of IADLs was small (8%) and not likely to introduce considerable bias. Also, measuring IADLs as a dichotomous variable may obscure subtle differences in disability that might be detected with an ordinal Likert scale. We focus here only on IADLs, as we are limited by measures in the HRS to examine for more complex physical functioning phenomena like frailty and timing of stroke. Finally, there may be selection bias in comparisons of stroke-free to stroke survivors across demographic characteristics because all of the characteristics we examined are themselves associated with stroke (33). To the extent that there are unobserved causes of stroke, which also influence IADL independence, the patterns we observe will not correspond to the causal effects of each demographic factor on independence among stroke patients. This potential bias may be even more complex when considering stroke survivors, if there are any factors that influence survival and, among survivors, influence disability. However, this limitation is unavoidable in research on determinants of stroke outcomes. Plausible unmeasured factors that may affect both stroke and IADL independence include both genetic and environmental exposures.
In spite of these limitations, this study has many strengths and extends the existing research on the effects of cerebrovascular disease on IADL independence. In particular, the longitudinal design enabled prospective assessment of IADLs prior to stroke; this is in contrast to many studies of stroke that are limited to either retrospective measures or no measures of prestroke characteristics. Because of the large HRS sample, we had sufficient events to estimate associations of interest within major demographic strata. Finally, a nationally representative cohort such as HRS enhances generalizability of these findings; the majority of prior stroke research has focused on regional or geographically constrained samples.
Comparisons to Prior Research
Extensive prior research indicates that dependence in IADLs predicts adverse future health outcomes and quality of life. We note that IADLs are a single component of a larger, complex process of ability and physical function, of which many measures have been associated with cardiovascular disease risks and outcomes. For example, frailty is commonly associated with presence of cardiovascular disease risk factors (34–37). However, to our knowledge, our study is the first study regarding IADL independence and before and after stroke, and we discuss our results in comparison to other work in IADLs to the extent that there is relevant literature to do so. We found a faster IADL decline and lower level of independence associated with onset stroke. We also add to the literature by estimating associations for individual IADLs rather than an index of IADL independence that aggregates differences among the IADL outcomes. The study fits into a small but growing literature challenging the conventional view that disability emerges after acute onset conditions such as myocardial infarction, as in other work, and stroke, as in this analysis. We find that what is often thought of as stroke-related disability actually precedes stroke onset, requiring further interpretation. This evidence suggests that covert cerebrovascular disease may be compromising independence even prior to diagnosis of stroke. Considerations include the possibility that people who suffer strokes may have prior IADLs limitations related to other comorbidities such as hypertension or cardiac disease, which in turn may make them more susceptible to suffering a stroke. We tested whether such chronic conditions affected our primary results and we found they did not change the patterns significantly (Supplementary Appendix E). Additionally, lower literacy levels, which may differentially affect black and Southern-born elderly persons, given historical differences in school quality (38,39), may negatively impact people’s ability to perform some of the measured IADL such as reading a map.
Mobility disability has been associated with risk of stroke (16–19) onset; other studies with similar designs have found similar results with myocardial infarction (14,15). This finding is especially important when considering disparities in the burden of stroke. Although blacks in general have worse stroke outcomes than whites, our results suggest that this primarily reflects prestroke disparities. The black-white gap in disability was actually slightly narrowed after stroke. Interpretation of this result should acknowledge that the burden of stroke in black populations is nonetheless differentially high because blacks consistently experience elevated incidence rates. Critically, blacks who subsequently had stroke had much higher levels of disability than blacks who did not experience stroke during our follow-up. This suggests that higher comorbidity burdens, lower levels of literacy, and other factors not identified in this study may be having especially adverse consequences for blacks. Approximately 40% of black men aged 20+ in the United States are hypertensive, and only 25% of black male hypertensives have blood pressure within recommended levels of control (40). Our results reemphasize the urgent need for more aggressive attention to indicators of stroke risk factors and subtle cerebrovascular disease in blacks without diagnosed stroke.
Future Directions and Implications
There is some evidence that the changes between IADLs before and after stroke varied by age. Future studies should extend these results by testing whether these patterns are similar when stratified by age. Other measures of ability and function, such as frailty, may be important to consider with respect to before and after stroke. Study designs that are able to disentangle the temporality of ADLs and IADLs to estimate these associations independently may also be important to identify the precise disability trajectories prior to and after stroke. This is especially true with respect to health disparities, disability and stroke. Moreover, it remains to be seen whether other characteristics that are associated with stroke, such as socioeconomic and health characteristics (17), may modify the patterns we report here. This study looked at first strokes; future work might consider how these trajectories are affected by a second stroke, as multiple strokes are common among survivors. This is especially relevant for understanding inequalities in poststroke disability. These results are descriptive and not causal; however, the clinical implications of these results suggest that much of the poststroke IADL limitation burden is less a function of the stroke and was instead apparent long prior to the stroke onset. Clinically, our findings emphasize the important role of prevention and treatment of cerebrovascular disease risk factors among individuals never diagnosed with stroke. To eliminate the disability burden of cerebrovascular disease, we must begin well before stroke diagnosis.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
This work was supported by the National Institutes of Health (R21 AG034385, T32 HD007168, R24 HD050924) and American Heart Association (10SDG2640243). The U.S. Health and Retirement Study (HRS), conducted by the University of Michigan, is sponsored by the National Institute on Aging (NIA U01AG009740).
Acknowledgements
We thank Amy Ehntholt for editorial assistance and two anonymous reviewers for comments on a previous version of this article.
References
- 1. Shooshtari S, Menec V, Tate R. Comparing predictors of positive and negative self-rated health between younger (25–54) and older (55+) Canadian adults. Res Aging. 2007;29(6):512–554 doi:10.1177/0164027507305729 [Google Scholar]
- 2. Bailis DS, Segall A, Chipperfield JG. Two views of self-rated general health status. Soc Sci Med. 2003;56(2):203–217 doi:10.1016/s0277-9536(02)00020-5 [DOI] [PubMed] [Google Scholar]
- 3. Seidel D, Jagger C, Brayne C, Matthews FE, Cfas M. Recovery in instrumental activities of daily living (IADLs): findings from the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS). Age Ageing. 2009;38(6):663–668 doi:10.1093/ageing/afp128 [DOI] [PubMed] [Google Scholar]
- 4. Cesari M, Onder G, Zamboni V, et al. Physical function and self-rated health status as predictors of mortality: results from longitudinal analysis in the ilSIRENTE study. BMC Geriatr. 2008;8(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scott WK, Macera CA, Cornman CB, Sharpe PA. Functional health status as a predictor of mortality in men and women over 65. J Clin Epidemiol. 1997;50(3):291–296 doi: 10.1016/s0895-4356(96)00365-4 [DOI] [PubMed] [Google Scholar]
- 6. Reuben DB, Rubenstein LV, Hirsch SH, Hays RD. Value of functional status as a predictor of mortality: results of a prospective study. Am J Med. 1992;93(6):663–669 [DOI] [PubMed] [Google Scholar]
- 7. Dudgeon BJ, Hoffman JM, Ciol MA, Shumway-Cook A, Yorkston KM, Chan L. Managing activity difficulties at home: a survey of Medicare beneficiaries. Arch Phys Med Rehabil. 2008;89(7):1256–1261 doi:10.1016/j.apmr.2007.11.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Manton KG, Gu X, Lamb VL. Change in chronic disability from 1982 to 2004/2005 as measured by long-term changes in function and health in the U.S. elderly population. Proc Nat Acad Sci U S A. 2006;103(48):18374–18379 doi:10.1073/pnas.0608483103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stuck AE, Walthert JM, Nikolaus T, Büla CJ, Hohmann C, Beck JC. Risk factors for functional status decline in community-living elderly people: a systematic literature review. Soc Sci Med. 1999;48(4):445–469 doi:10.1016/s0277-9536(98)00370-0 [DOI] [PubMed] [Google Scholar]
- 10. Roger VL, Go AS, Lloyd-Jones DM, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–e220 doi:10.1161/CIR.0b013e31823ac046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chong DK. Measurement of instrumental activities of daily living in stroke. Stroke. 1995;26(6):1119–1122 [DOI] [PubMed] [Google Scholar]
- 12. Wu CY, Chuang LL, Lin KC, Horng YS. Responsiveness and validity of two outcome measures of instrumental activities of daily living in stroke survivors receiving rehabilitative therapies. Clin Rehabil. 2011;25(2):175–183 doi:10.1177/0269215510385482 [DOI] [PubMed] [Google Scholar]
- 13. Sveen U, Thommessen B, Bautz-Holter E, Wyller TB, Laake K. Well-being and instrumental activities of daily living after stroke. Clin Rehabil. 2004;18(3):267–274 [DOI] [PubMed] [Google Scholar]
- 14. Mendes de Leon CF, Bang W, Bienias JL, Glass TA, Vaccarino V, Kasl SV. Changes in Disability Before and After Myocardial Infarction in Older Adults. Arch Intern Med. 2005;165(7):763–768 doi:10.1001/archinte.165.7.763 [DOI] [PubMed] [Google Scholar]
- 15. Vaccarino V, Berkman LF, Mendes de Leon CF, Seeman TE, Horwitz RI, Krumholz HM. Functional disability before myocardial infarction in the elderly as a determinant of infarction severity and postinfarction mortality. Arch Intern Med. 1997;157(19):2196–2204 doi:10.1001/archinte.1997.00440400046006 [PubMed] [Google Scholar]
- 16. Longstreth WT, Jr, Bernick C, Fitzpatrick A, et al. Frequency and predictors of stroke death in 5,888 participants in the Cardiovascular Health Study. Neurology. 2001;56(3):368–375 [DOI] [PubMed] [Google Scholar]
- 17. Colantonio A, Kasi SV, Ostfeld AM. Depressive symptoms and other psychosocial factors as predictors of stroke in the elderly. Am J Epidemiol. 1992;136(7):884–894 doi:10.1093/aje/136.7.884 [DOI] [PubMed] [Google Scholar]
- 18. Colantonio A, Kasl SV, Ostfeld AM, Berkman LF. Prestroke physical function predicts stroke outcomes in the elderly. Arch Phys Med Rehabil. 1996;77(6):562–566 doi:10.1016/s0003-9993(96)90295-6 [DOI] [PubMed] [Google Scholar]
- 19. Capistrant BD, Wang Q, Liu SL, Glymour MM. Differences in rate of ADL loss between stroke patients and stroke-free adults emerge years prior to stroke onset. J Am Geriatr Soc. 2013;6:931–938. doi:10.1111/jgs.12270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang Q, Capistrant BD, Ehntholt A, Glymour MM. Long-term rate of change in memory functioning before and after stroke onset. Stroke. 2012;43:2561–2566 doi:10.1161/strokeaha.112.661587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kelly-Hayes M, Beiser A, Kase CS, Scaramucci A, D’Agostino RB, Wolf PA. The influence of gender and age on disability following ischemic stroke: the Framingham study. J Stroke Cerebrovasc Dis. 2003;12(3):119–126 doi:10.1016/S1052-3057(03)00042-9 [DOI] [PubMed] [Google Scholar]
- 22. Petrea RE, Beiser AS, Seshadri S, Kelly-Hayes M, Kase CS, Wolf PA. Gender differences in stroke incidence and poststroke disability in the Framingham heart study. Stroke. 2009;40(4):1032–1037 doi:10.1161/STROKEAHA.108.542894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Horner RD, Swanson JW, Bosworth HB, Matchar DB; VA Acute Stroke (VAST) Study Team. Effects of race and poverty on the process and outcome of inpatient rehabilitation services among stroke patients. Stroke. 2003;34(4):1027–1031 doi:10.1161/01.STR.0000060028.60365.5D [DOI] [PubMed] [Google Scholar]
- 24. Lawrence ES, Coshall C, Dundas R, et al. Estimates of the prevalence of acute stroke impairments and disability in a multiethnic population. Stroke. 2001;32(6):1279–1284 [DOI] [PubMed] [Google Scholar]
- 25. Gillum RF, Kwagyan J, Obisesan TO. Ethnic and geographic variation in stroke mortality trends. Stroke. 2011;42(11):3294–3296 doi:10.1161/STROKEAHA.111.625343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Glymour MM, Kosheleva A, Boden-Albala B. Birth and adult residence in the Stroke Belt independently predict stroke mortality. Neurology. 2009;73(22):1858–1865 doi:10.1212/WNL.0b013e3181c47cad [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Juster FT, Suzman R. An overview of the health and retirement study. The J Hum Resour. 1995;30:S7–S56. [Google Scholar]
- 28. Heeringa SG. Technical Description of the Asset and Health Dynamics (AHEAD) Survey Sample. Ann Arbor, MI: Institute for Social Research, University of Michigan; 1995 [Google Scholar]
- 29. Glymour MM, Avendano M. Can self-reported strokes be used to study stroke incidence and risk factors?: evidence from the health and retirement study. Stroke. 2009;40(3):873–879 doi:10.1161/STROKEAHA.108.529479 [DOI] [PubMed] [Google Scholar]
- 30.Collaboration TERF. Adult height and the risk of cause-specific death and vascular morbidity in 1 million people: individual participant meta-analysis. Int J Epidemiol. 2012;41:1419–1433 doi:10.1093/ije/dys086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moon JR, Capistrant BD, Kawachi I, et al. Stroke incidence in older US Hispanics: is foreign birth protective? Stroke. 2012;43(5):1224–1229 doi:10.1161/STROKEAHA.111.643700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Glymour MM, Avendaño M, Berkman LF. Is the ‘stroke belt’ worn from childhood?: risk of first stroke and state of residence in childhood and adulthood. Stroke. 2007;38(9):2415–2421 [DOI] [PubMed] [Google Scholar]
- 33. Cole SR, Platt RW, Schisterman EF, et al. Illustrating bias due to conditioning on a collider. Int J Epidemiol. 2010;39:417–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Afilalo J. Frailty in patients with cardiovascular disease: why, when, and how to measure. Curr Cardiovasc Risk Rep. 2011;5(5):467–472 doi:10.1007/s12170-011-0186-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Afilalo J, Karunananthan S, Eisenberg MJ, Alexander KP, Bergman H. Role of frailty in patients with cardiovascular disease. Am J Cardiol. 2009;103(11):1616–1621 doi:10.1016/j.amjcard.2009.01.375 [DOI] [PubMed] [Google Scholar]
- 36. Phan HM, Alpert JS, Fain M. Frailty, inflammation, and cardiovascular disease: evidence of a connection. Am J Geriatr Cardiol. 2008;17(2):101–107 [PubMed] [Google Scholar]
- 37. Walston J, McBurnie MA, Newman A, et al. ; Cardiovascular Health Study. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002;162(20):2333–2341 [DOI] [PubMed] [Google Scholar]
- 38. Manly JJ. Deconstructing race and ethnicity: implications for measurement of health outcomes. Med Care. 2006;44(11 s uppl 3):S10–S16. 10.1097/1001.mlr.0000245427.0000222788.be [DOI] [PubMed] [Google Scholar]
- 39. Card D, Krueger A. Does School Quality Matter? Returns to Education and the Characteristics of Public Schools in the United States. National Bureau of Economic Research Working Paper Series; 1990; No. 3358. [Google Scholar]
- 40.Statistics NCFH. Health, United States, 2011: With Special Feature on Socioeconomic Status and Health. Hyattsville, MD: National Center for Health Statistics; 2012 [PubMed] [Google Scholar]