Structured Abstract
Background
The Occluded Artery Trial (OAT) randomized stable patients (n=2,201) >24 hours (calendar days 3–28) after myocardial infarction (MI) with totally occluded infarct-related arteries (IRA), to percutaneous coronary intervention (PCI) with optimal medical therapy, or optimal medical therapy alone (MED). PCI had no impact on the composite of death, reinfarction, or class IV heart failure over extended follow-up of up to 9 years. We evaluated the impact of early and late reinfarction and definition of MI on subsequent mortality.
Methods and Results
Reinfarction was adjudicated according to an adaptation of the 2007 universal definition of MI and the OAT definition (≥2 of the following - symptoms, EKG and biomarkers). Cox regression models were used to analyze the effect of post-randomization reinfarction and baseline variables on time to death.
After adjustment for baseline characteristics the 169 (PCI: n=95; MED: n=74) patients who developed reinfarction by the universal definition had a 4.15-fold (95% CI 3.03–5.69, p<0.001) increased risk of death compared to patients without reinfarction. This risk was similar for both treatment groups (interaction p=0.26) and when MI was defined by the stricter OAT criteria. Reinfarctions occurring within 6 months of randomization had similar impact on mortality as reinfarctions occurring later, and the impact of reinfarction due to the same IRA and a different epicardial vessel was similar.
Conclusions
For stable post-MI patients with totally occluded infarct arteries, reinfarction significantly independently increased the risk of death regardless of the initial management strategy (PCI vs. MED), reinfarction definition, location and early or late occurrence.
Keywords: Reinfarction, late revascularization, myocardial infarction, mortality
Introduction
The Occluded Artery Trial (OAT) 1 compared the clinical outcome of stable patients with totally occluded infarct-related arteries (IRA) after myocardial infarction (MI) re-canalized by percutaneous coronary intervention (PCI) versus conservative treatment with optimal medical therapy (MED) alone. PCI of occluded arteries had no impact on the composite of death, reinfarction and class IV heart failure (HF) over the initial or extended follow-up periods,2,3 or on quality of life.4 Most reinfarctions were spontaneous (type 1), and occurred at a statistically similar frequency in both treatment groups.5 There was a higher rate of reinfarction due to stent thrombosis in the PCI group (2.7% PCI vs 0.6% MED, P <0.001).
Reinfarction following fibrinolysis has been shown to be associated with a marked increase in mortality.6 The impact of reinfarction based on the definition (i.e., universal vs OAT definition) and based on timing of early vs. late reinfarction and reocclusion of the infarct vs. another artery in patients with prior total occlusion is unknown. Therefore, we analyzed long-term follow up data on OAT patients to study the consequences of reinfarction in stable patients initially randomized to late percutaneous IRA revascularization of total occlusions with optimal medical therapy or conservative initial optimal medical therapy alone in the subacute phase after an index MI.
Methods
This analysis of the 2201 patient OAT cohort2 was prospectively predefined as an aim in conjunction with the NHLBI/NIH supported long-term follow-up phase.
OAT study protocol and definition of reinfarction
The OAT protocol has previously been published.1 Briefly, stable patients who had total occlusion of the IRA >24 hours (on calendar days 3–28) after MI were randomly assigned to receive optimal medical therapy alone (n=1,100) or with PCI (n=1,101). Patients were followed via bi-annual telephone calls for up to 9 years (mean of 6 years). The combined primary endpoint was death, MI or hospitalization for New York Heart Association (NYHA) class IV HF. The OAT definition of reinfarction required 2 of the following 3 criteria: Ischemic symptoms for at least 30 minutes, electrocardiographic changes, and elevation of cardiac serum markers, with different threshold levels for MI peri-PCI.1 The OAT definition of elevation of markers required a creatine kinase (CK)-MB fraction that was greater than the upper limit of the normal (ULN) range at the local laboratory or, if unavailable, troponin I or T ≥ 2 times ULN or CK > 2 times ULN for spontaneous reinfarction. For peri-procedural reinfarction, marker elevation was defined as ≥ 3 times ULN after PCI and ≥ 5 times ULN after coronary artery bypass grafting. Troponin levels were not used to diagnose reinfarction within 10 days after the index MI.
An independent Morbidity and Mortality Classification Committee (MMCC) reviewed patient data on reinfarctions according to the original protocol definition of MI.1 In conjunction with the long term follow-up phase of OAT, reinfarctions during the entire follow-up period were also reviewed centrally by a group of 5 investigators to permit classification according to the universal definition of MI.3,5,7 This definition is an adapted, practical application of the universal definition of MI. This is necessary because most institutions use a local upper limit of normal for troponin and do not use the universal definition of MI recommended 99 percentile for troponin, as we have previously reported.8
Two reviewers, blinded to treatment assignment, reviewed hospital records and case report forms for each event; the group adjudicated disagreements. The universal definition of reinfarction required symptoms, EKG changes and an elevation of biomarkers (troponin preferred) to any level above the ULN for spontaneous or type 2 infarction (supply-demand), or ≥ 3× ULN after PCI, or ≥ 5× ULN after CABG. We used laboratory reported upper reference limit values according to the individual study site laboratories. This review also designated the IRA associated with the reinfarction.
Study report forms collected information on whether cardiac markers were designated by sites to be re-elevated within 48 hours of the initial randomization in OAT to ascertain PCI-related marker release, and comparable rates in the MED group. Laboratory data for these cases of asymptomatic marker re-elevation were not centrally confirmed and this information alone did not constitute MI by either the OAT or universal MI definition.
Study sites submitted clinical records of HF-related hospitalizations for review. Whether HF was the primary cause for these hospitalizations was centrally confirmed according to pre-specified criteria. The impact of reinfarction on the subsequent risk of NYHA class III or IV HF was a secondary aim of this analysis.
Statistical Methods
Statistical analysis was performed on baseline variables using the t-test, Wilcoxon, chi-square or Fisher exact test as appropriate. Kaplan Meier product-limit estimates were used to show survival curves for patients with and without reinfarction.9,10 Cox regression models were used to analyze the effect of post-randomization reinfarction on time to death adjusting for baseline variables and interactions with the study treatment.11 Reinfarction was fit as a time-dependent variable in the Cox regression models. Results are presented as hazard ratio (HR) for mortality compared to patients with no post-randomization reinfarction and 95% confidence interval (CI). Two different cutoff times (30 days, 6 months) for early or late reinfarction were examined. Patients experiencing a fatal reinfarction were included in all analyses.
The 7-year event rates are presented because the number of patients followed for more than 7 years was small. Data for the patients lost to follow-up were censored as of the last contact. This last contact occurred at 5 years from randomization for patients who declined consent for extension of follow up. Only 1.4% of patients (14 in PCI and 16 in MED group) were lost to follow–up before the occurrence of a primary end-point event or 12 months of follow-up. Average follow-up time for survivors was 6 years and was similar in the two treatment groups.
Analyses were performed according to the intention-to-treat principle. To control for the Type I error rate, it was pre-specified in the study protocol that a p-value of ≤ 0.01 would be considered as showing evidence of differences in secondary analysis. Therefore, a variable with p-value ≤ 0.01 in the final multivariate model would be presented as having independent impact on death. In this analysis a variable with p-value between 0.05 and 0.01 in the final multivariate model would be considered as showing trend toward the impact on death.
All analyses were performed using SAS V9.2 (SAS Institute, Cary, NC).
Results
Patient characteristics
Mean age of the 2,201 randomized patients was 58.6±11 years, 78% were male, ejection fraction was 47.7±11.1% and prevalence of Killip Class 2–4 during index MI was 18.9%. The time interval between MI and randomization was a median of 8 days (IQR 5–16). Among 2201 total patients, 303 patients died (PCI vs. MED HR=0.98, 95% CI 0.78–1.22), and 142 and 169 had reinfarction according to the OAT and universal definition, respectively, over 6 year mean follow-up. 29 events were identified by the universal definition but not by the OAT study definition. The 7-year reinfarction event rate by the OAT definition was 7.4% (PCI vs. MED HR=1.20, 95% CI 0.86–1.67, p=0.27) and by the universal definition was 8.7% (PCI vs. MED HR=1.31, 95% CI 0.97–1.77 p=0.08)3,5. Details of baseline and angiographic characteristics of patients with and without reinfarction are presented in Table 1a for patients who died and in Table 1b for patients who survived the follow-up period, respectively. Medical therapy in hospital and at discharge is presented in Table 2. Statins, beta blockers and angiotensin converting enzyme inhibitors or angiotensin receptor blockers were used at high rates during follow-up, with no difference between patients with or without reinfarction, or by treatment group.
Table 1a.
Baseline characteristics by reinfarction versus no reinfarction for patients who died during the follow-up period.
| Clinical characteristics | Reinfarction (N=50) | No Reinfarction (N=253) | p-value | ||
|---|---|---|---|---|---|
| n | % (mean ± sd) median{IQR} | n | % (mean ± sd) median{IQR} | ||
| Age (Years) | 50 | (59.9±12.9) | 253 | (64.8±11) | 0.005 |
|
| |||||
| Sex | 0.90 | ||||
| Male | 36 | 72.0 | 180 | 71.1 | |
| Female | 14 | 28.0 | 73 | 28.9 | |
|
| |||||
| Prior History of | |||||
| Angina | 16 | 32.0 | 81 | 32.0 | 1.00 |
| MI | 11 | 22.0 | 31 | 12.3 | 0.07 |
| Cerebrovascular Disease | 3 | 6.0 | 24 | 9.5 | 0.43 |
| Peripheral Vessel Disease | 4 | 8.0 | 22 | 8.7 | 0.87 |
| Congestive Heart Failure | 8 | 16.0 | 18 | 7.1 | 0.04 |
| PCI | 7 | 14.0 | 20 | 7.9 | 0.17 |
| CABG | 1 | 2.0 | 2 | 0.8 | 0.43 |
| Diabetes | 14 | 28.0 | 84 | 33.2 | 0.47 |
| Hypertension | 23 | 46.0 | 155 | 61.3 | 0.05 |
|
| |||||
| Current Smoker | 19 | 38.0 | 83 | 32.8 | 0.48 |
|
| |||||
| CHF at Baseline | 27 | 54.0 | 126 | 49.8 | 0.59 |
| Highest Killip Class II–IV During Index MI | 15 | 30.6 | 91 | 36.0 | 0.47 |
| Highest NYHA Classification II–IV | 17 | 34.0 | 85 | 33.6 | 0.96 |
|
| |||||
| ECG with index MI | |||||
| ST-segment Elevation | 36 | 72.0 | 178 | 71.5 | 0.94 |
| New Q-Waves | 20 | 40.0 | 169 | 66.8 | <0.001 |
| ST-segment elevation /new Q waves/R wave loss | 40 | 80.0 | 218 | 86.2 | 0.26 |
|
| |||||
| Rales Present | 9 | 18.0 | 41 | 16.2 | 0.76 |
| Collateral Vessels Present | 42 | 84.0 | 208 | 82.5 | 0.80 |
| Ejection Fraction | 49 | (43.6±12.6) | 250 | (43.1±13) | 0.82 |
| Systolic Blood Pressure | 50 | (118.3±22) | 253 | (122.6±20.2) | 0.18 |
| Diastolic Blood Pressure | 50 | (69±11.8) | 253 | (72.7±12) | 0.05 |
| BMI | 50 | (28.9±6.8) | 253 | (29.1±6.3) | 0.80 |
| Glomerular filtration*rate (ml/min/1.72m^2) | 50 | (79.4±27.4) | 248 | (73±22.8) | 0.08 |
| Fasting Glucose (mg/dl) | 48 | (133±54.8) | 234 | (127.2±47.8) | 0.46 |
|
| |||||
| Interval between MI and randomization (days) | 50 | 7{4–13} | 253 | 8{4–15} | 0.31 |
| Infarct-related Artery | 0.40 | ||||
| LAD | 23 | 46 | 110 | 43.5 | |
| LCX | 11 | 22 | 40 | 15.8 | |
| RCA | 16 | 32 | 103 | 40.7 | |
| Ischemia in Infarct-Related Artery Territory | 2 | 28.6 | 21 | 44.7 | 0.42 |
|
| |||||
| Thrombolytic Therapy During 1st 24 hours | 12 | 24.0 | 48 | 19.0 | 0.42 |
PCI: Percutaneous coronary intervention, CABG: Coronary artery bypass graft, CHF: Congestive heart failure, NYHA: New York Heart Association, ECG: Electrocardiogramm, MI: Myocardial infarction, BMI: Body mass index, LAD: Left anterior descending coronary artery, LCX: Left circumflex coronary artery, RCA: Right coronary artery.
Table 1b.
Baseline characteristics by reinfarction versus no reinfarction for survivors over the complete follow-up period.
| Clinical characteristics | Reinfarction (N=119) | No Reinfarction (N=1779) | p-value | ||
|---|---|---|---|---|---|
|
| |||||
| n | % (mean ± sd) median{IQR} | n | % (mean ± sd) median{IQR} | ||
| Age (Years) | 119 | (56.2±12.4) | 1779 | (57.8±10.5) | 0.11 |
|
| |||||
| Sex | 0.62 | ||||
| Male | 92 | 77.3 | 1409 | 79.2 | |
| Female | 27 | 22.7 | 370 | 20.8 | |
|
| |||||
| Prior History of | |||||
| Angina | 31 | 26.1 | 367 | 20.6 | 0.16 |
| MI | 24 | 20.2 | 181 | 10.2 | <0.001 |
| Cerebrovascular Disease | 5 | 4.2 | 50 | 2.8 | 0.38 |
| Peripheral Vessel Disease | 8 | 6.7 | 49 | 2.8 | 0.01 |
| Congestive Heart Failure | 3 | 2.5 | 23 | 1.3 | 0.27 |
| PCI | 15 | 12.6 | 63 | 3.5 | <0.001 |
| CABG | 2 | 1.7 | 4 | 0.2 | 0.006 |
| Diabetes | 35 | 29.4 | 321 | 18.0 | 0.002 |
| Hypertension | 60 | 50.4 | 833 | 46.8 | 0.45 |
|
| |||||
| Current Smoker | 55 | 46.2 | 702 | 39.5 | 0.15 |
|
| |||||
| CHF at Baseline | 40 | 33.6 | 504 | 28.3 | 0.22 |
| Highest Killip Class II–IV During Index MI | 20 | 16.8 | 291 | 16.4 | 0.92 |
| Highest NYHA Classification II–IV | 24 | 20.2 | 333 | 18.7 | 0.70 |
|
| |||||
| ECG with index MI | |||||
| ST-segment Elevation | 71 | 61.2 | 1131 | 65.7 | 0.32 |
| New Q-Waves | 74 | 62.2 | 1212 | 68.1 | 0.18 |
| ST-segment elevation /new Q waves/R wave loss | 108 | 90.8 | 1541 | 86.6 | 0.20 |
|
| |||||
| Rales Present | 14 | 11.9 | 73 | 4.1 | <0.001 |
| Collateral Vessels Present | 104 | 87.4 | 1568 | 89.5 | 0.47 |
| Ejection Fraction | 119 | (47.4±10.4) | 1767 | (48.5±10.6) | 0.28 |
| Systolic Blood Pressure | 119 | (121.4±19.5) | 1777 | (120.6±17.4) | 0.61 |
| Diastolic Blood Pressure | 119 | (71.5±12.5) | 1777 | (72.4±11.1) | 0.40 |
| BMI | 119 | (29±5.6) | 1765 | (28.4±4.7) | 0.17 |
| Glomerular filtration*rate (ml/min/1.72m^2) | 119 | (80.3±22.7) | 1743 | (81.8±20.8) | 0.47 |
| Fasting Glucose (mg/dl) | 100 | (123±43.7) | 1619 | (118.2±40.4) | 0.25 |
|
| |||||
| Interval between MI and randomization (days) | 119 | 7{4–14} | 1779 | 9{5–17} | 0.02 |
| Infarct-related Artery | 0.23 | ||||
| LAD | 33 | 27.7 | 627 | 35.2 | |
| LCX | 21 | 17.6 | 263 | 14.8 | |
| RCA | 65 | 54.6 | 889 | 50.0 | |
| Ischemia in Infarct-Related Artery Territory | 22 | 61.1 | 195 | 38.4 | 0.007 |
|
| |||||
| Thrombolytic Therapy During 1st 24 hours | 24 | 20.2 | 340 | 19.1 | 0.78 |
PCI: Percutaneous coronary intervention, CABG: Coronary artery bypass graft, CHF: Congestive heart failure, NYHA: New York Heart Association, ECG: Electrocardiogramm, MI: Myocardial infarction, BMI: Body mass index, LAD: Left anterior descending coronary artery, LCX: Left circumflex coronary artery, RCA: Right coronary artery.
Table 2.
In hospital medical therapy and medication prescribed at discharge for patients with and without reinfarction.
| Discharge or in hospital medication | Reinfarction | No reinfarction | p-value | ||
|---|---|---|---|---|---|
|
| |||||
| n | % | n | % | ||
| Aspirin | 165 | 97.6 | 1940 | 95.5 | 0.19 |
| Ticlopidine or clopidogrel | 117 | 69.2 | 1212 | 59.6 | 0.01 |
| Ticlopidine | 16 | 9.5 | 243 | 12.0 | 0.33 |
| Clopidogrel | 101 | 59.8 | 974 | 47.9 | 0.003 |
| Beta blocker | 149 | 88.2 | 1783 | 87.7 | 0.87 |
| Lipid lowering drug | 136 | 80.5 | 1652 | 81.3 | 0.79 |
| ACE inhibitor or AT-1 blocker | 143 | 84.6 | 1628 | 80.1 | 0.16 |
| ACE inhibitor or ARB | 138 | 81.7 | 1576 | 77.6 | 0.22 |
| ARB | 7 | 4.1 | 63 | 3.1 | 0.46 |
| Warfarin | 13 | 7.7 | 202 | 9.9 | 0.34 |
| Glycoprotein IIb/IIIa inhibitor | 3 | 1.8 | 15 | 0.7 | 0.15 |
| Diuretics | 38 | 22.5 | 333 | 16.4 | 0.04 |
| Spironolactone | 8 | 4.7 | 116 | 5.7 | 0.60 |
| Calcium channel blocker | 12 | 7.1 | 117 | 5.8 | 0.48 |
| Long acting nitrate | 30 | 17.8 | 468 | 23.0 | 0.12 |
| Sublingual nitrate | 58 | 34.3 | 594 | 29.2 | 0.16 |
| Antiarrhythmic agent | 6 | 3.6 | 78 | 3.8 | 0.85 |
| Digoxin | 5 | 3.0 | 56 | 2.8 | 0.88 |
| Insulin | 19 | 11.2 | 118 | 5.8 | 0.005 |
| Oral antidiabetic | 31 | 18.3 | 267 | 13.1 | 0.06 |
ACE inhibitor: Angiotensin converting enzyme inhibitor, ARB: Angiotensin receptor 1 blocker
Impact of reinfarction on mortality
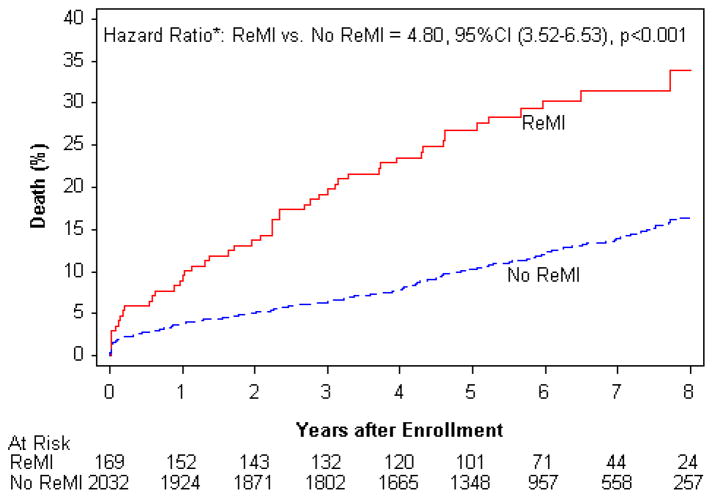
Patients who developed reinfarction by the universal definition had a significantly higher mortality compared to the patients without reinfarction (31.5% vs. 13.9%, Figure 1) with an unadjusted risk of death that was 4.8-fold increased (95% CI 3.52–6.53, p<0.001). After adjustment for baseline characteristics, occurrence of reinfarction fit as a time-dependent variable was an independent predictor of death (HR 4.15; 95% CI 3.03–5.69, p<0.001) (Table 3). The risk of death following reinfarction was similar in the two treatment groups (PCI: 3.64; 95% CI 2.35–5.64, <0.001; MED: 4.90; 95% CI 3.09–7.75, p<0.001; PCI vs MED HR=0.91, 95% CI 0.73–1.14, p=0.42; reinfarction and treatment interaction p=0.26). 29 events were identified by the universal definition but not by the OAT study definition. Of these 29 subjects with events, 16 died during the follow-up period. The risk of death was similar and independent predictors of death were unchanged when the original OAT definition of reinfarction was assessed (HR=3.22 95% CI 2.24–4.65, p<0.001, reinfarction and treatment interaction p=0.28).
Figure 1.
Time to mortality of post-MI patients with totally occluded infarct arteries with and without reinfarction according to the 2007 universal definition of MI.
Table 3.
Hazard ratio and 95%CI of reinfarction on mortality and subsequent class III–IV heart failure (HF) using Cox proportional hazard model. Reinfarction was fit as a time-dependent variable.
| Sub-group and Comparison | Unadjusted | Adjusted* | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Hazard Ratio | 95% CI | p-value | Hazard Ratio | 95% CI | p-value | ||
| Reinfarction by Universal Definition | |||||||
| Reinfarction on Death | |||||||
| Overall | ReMI vs. noReMI** | 4.80 | 3.52–6.53 | <0.001 | 4.15 | 3.03–5.69 | <0.001 |
| PCI | ReMI vs. noReMI | 4.26 | 2.79–6.52 | <0.001 | 3.64 | 2.35–5.64 | <0.001 |
| MED | ReMI vs. noReMI | 5.80 | 3.69–9.10 | <0.001 | 4.90 | 3.09–7.75 | <0.001 |
| Early and Late Reinfarction on Death | |||||||
| Cutoff=6 months | Early ReMI vs. noReMI | 3.21 | 2.04–5.07 | <0.001 | 2.55 | 1.60–4.05 | <0.001 |
| Cutoff=30 days | Late ReMI vs. noReMI | 6.23 | 4.49–9.79 | <0.001 | 6.22 | 4.18–9.27 | <0.001 |
| Early ReMI vs. noReMI | 3.09 | 1.69–5.65 | <0.001 | 2.25 | 1.21–4.16 | 0.010 | |
| Late ReMI vs. noReMI | 5.56 | 3.93–7.87 | <0.001 | 5.08 | 3.56–7.25 | <0.001 | |
| Reinfarction on Class III–IV HF | |||||||
| Overall | ReMI vs. noReMI | 3.08 | 1.61–5.91 | <0.001 | 2.66 | 1.37–5.17 | 0.004 |
|
| |||||||
| Reinfarction by OAT Definition | |||||||
| Reinfarction on Death | |||||||
| Overall | ReMI vs. noReMI | 3.43 | 2.39–4.92 | <0.001 | 3.22 | 2.24–4.65 | <0.001 |
| PCI | ReMI vs. noReMI | 2.43 | 1.42–4.17 | 0.001 | 2.27 | 1.31–3.92 | 0.003 |
| MED | ReMI vs. noReMI | 5.10 | 3.13–8.30 | <0.001 | 4.35 | 2.65–7.15 | <0.001 |
| Early and Late Reinfarction on Death | |||||||
| Cutoff=6 months | Early ReMI vs. noReMI | 2.48 | 1.45–4.25 | 0.001 | 2.08 | 1.21–3.58 | 0.008 |
| Cutoff=30 days | Late ReMI vs. noReMI | 4.47 | 2.81–7.10 | <0.001 | 4.84 | 3.02–7.76 | <0.001 |
| Early ReMI vs. noReMI | 2.72 | 1.35–5.50 | 0.005 | 2.39 | 1.17–4.88 | 0.016 | |
| Late ReMI vs. noReMI | 3.69 | 2.45–5.56 | <0.001 | 3.57 | 2.35–5.41 | <0.001 | |
| Reinfarction on Class III–IV HF | |||||||
| Overall | ReMI vs. noReMI | 2.81 | 1.37–5.78 | 0.005 | 2.78 | 1.34–5.76 | 0.006 |
Adjusted for baseline factors that included heart failure at baseline, history of cerebrovascular disease, diabetes history, PCI history, age (10-years interval), ejection fraction (10% interval). For all the overall models, treatment group (PCI vs. MED) was included in the controlled factors.
ReMI: Reinfarction; noReMI: Without reinfarction.
The infarct-related artery (IRA) could be identified based on angiography, wall motion studies and/or ECG in 135 of 169 patients with reinfarction by the universal definition. Sixty-seven of these 135 patients (49.6%) had reinfarction due to the initial OAT IRA. Reinfarctions due to the qualifying IRA fit as a time-dependent variable independently increased mortality (HR 2.94, 95% CI 1.76–4.93, p<0.001). Reinfarctions occurring in an epicardial coronary artery different from the initial IRA also increased mortality (HR 3.77, 95% CI 2.22–6.44, p<0.001). The impact of reinfarction on death was similar when reinfarction was due to the OAT index IRA or a different epicardial vessel (HR 1.11, 95% CI 0.55–2.25, p=0.77).
Biomarkers were re-elevated above the upper limit of normal in 131/1964 patients with available data within 48 hours of the initial randomization. Isolated marker re-elevation within the first 48 hours following randomization, excluding 8 patients who had marker elevation in association with a confirmed MI was associated with a higher risk of death (n=14; 10 PCI, 4 MED; HR 1.83, 95% CI 1.23–2.72, p=0.003). This association of isolated biomarker elevation early after randomization and subsequent death was not statistically significant after adjustment for other baseline variables associated with death (i.e. ejection fraction, history of diabetes, cerebrovascular disease, angina pectoris, as well as age, body mass index and heart failure at baseline; HR 1.47, 95% CI 0.99–2.19, p=0.06).
The impact of early versus late reinfarction by universal definition on mortality
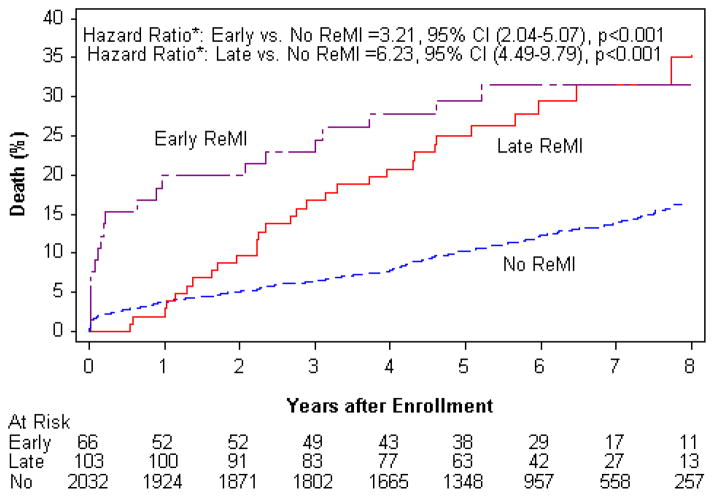
Of the 169 reinfarctions, 66 (39.1%) occurred within 6 months, while 103 (60.9%) occurred later. The median time to first reinfarction was 273 days (IQR 25–1002 days) in the PCI group and 438 days (IQR 66–1147 days) in the MED group (p=0.21). Early reinfarction was associated with higher mortality compared to no reinfarction (HR 3.21, 95% CI 2.04–5.07, p<0.001), as was late reinfarction compared to no reinfarction (HR 6.23, 95% CI 4.49–9.79, p<0.001). Reinfarctions occurring more than 6 months after randomization had similar impact on mortality as compared to early reinfarctions within 6 months after randomization (29.1% vs. 30.3%; HR 1.17, 95% CI 0.66–2.05, p=0.60). Kaplan Meier survival curves for the subgroups with early reinfarction, late reinfarction, or no reinfarction groups are depicted in Figure 2. The proportion of early and late reinfarction, as well as the impact on mortality were similar when the OAT definition of MI was used. Changing the cut-off for early reinfarction from 6 months to 30 days led to comparable results (HR=1.12, 95% CI 0.58–2.19, p=0.734), again were similar when the OAT study definition of MI was applied (Table 3).
Figure 2.
Time to mortality of post-MI patients with totally occluded infarct arteries with reinfarction occurring within 6 months (Early reinfarction) or after 6 months (Late reinfarction) of initial MI and without reinfarction (No reinfarction) according to the universal definition of MI.
Impact of reinfarction by universal definition on subsequent class III or IV heart failure
Over the long-term follow-up, the 7-year life table rate of class III or IV HF was 6.3% for patients without reinfarction compared to 22.2% for patients with reinfarction (p<0.001). Reinfarction fit as a time-dependent variable was associated with an increased risk for subsequent hospitalization for class III–IV HF (HR 3.08, 95% CI 1.61–5.91, p<0.001). Reinfarction was independently associated with increased risk for class III or IV HF on multiple Cox regression, controlled for randomized treatment group and baseline variables. The results were comparable when the OAT study definition of MI was used (Table 3).
Discussion
For stable patients with persistent total occlusion of the IRA post MI, reinfarction had a major impact on mortality risk despite high rates of use of evidence-based secondary prevention measures in OAT. Importantly, the independent impact of reinfarction on mortality was not affected by location of the IRA in the previously occluded vessel, timing of reinfarction, definition of MI by more or less stringent criteria, or management of the index MI with PCI or MED alone. Overall, reinfarction was also a strong independent predictor of subsequent class III or IV HF.
Primary angioplasty is known to reduce the risk of reinfarction and the risk of death after reinfarction.12,13 In contrast to these older studies, the OAT study evaluated stable post-MI patients with totally occluded IRAs in the subacute phase. Furthermore, thienopyridines, stents and glycoprotein IIb/IIIa antagonists were used more frequently in the OAT study population.2
The first reinfarction event had significant impact on mortality regardless of the initially applied management strategy (PCI vs. MED) following the index event. This is in accordance with previous studies showing an effect of reinfarction on mortality after fibrinolysis6 and during short-term follow-up after PCI.14 Our previous findings indicating that mortality was no different between treatment arms3 despite a higher rate of type 4b (stent thrombosis) reinfarction in the PCI arm is consistent with the low rate of type 4b reinfarction, and the statistically similar overall rates of reinfarction between the groups.6
The annual reinfarction rates observed for our patients were higher compared to those published after primary PCI in acute MI using DES (1%) or bare metal stents (1.4%) in the acute phase of STEMI.15 On the other hand, the 3-year MI rate of 3.3% for PCI-treated ACS patients in the recently published PROSPECT trial is closer to our findings.16
Regardless of which MI definition (MMCC-adjudicated OAT or universal) was used, reinfarction remained a significant independent predictor of mortality and hospitalization for class III–IV HF. We found no differences with respect to the presence or absence of collaterals in patients randomized to PCI with and without subsequent reinfarction.
The prognostic importance of reinfarction in the initially qualifying totally occluded IRA is noteworthy. The clinical importance of reinfarction in the infarct zone is not surprising in light of the OAT viability results. An ancillary study using direct measurement of viability17 showed that most OAT patients had viable myocardium in the infarct zone.3 Indirect evidence based on a rise in EF over one year in 66% of 389 patients in whom it was measured also supports infarct zone viability in these patients.18 In the TOSCA-2 angiographic ancillary study, the presence of well-developed collaterals at baseline was associated with a greater magnitude of improvement in EF over time. 19
Our data show that early reinfarctions were associated with a similar risk of death compared to reinfarctions occurring later after the index MI. Published data on early compared to late reinfarction are scarce. Analysis of a large, unselected cohort experiencing index MI between 1985–2002 found a higher rate of reinfarction than in OAT and also indicated that later reinfarction had a greater adverse impact on mortality than earlier reinfarction. However, this analysis excluded deaths within the first 30 days and the population studied was likely not comparable to a clinical trial cohort. 18
Study Limitations
Core lab measurement of biomarkers was not performed and local upper reference limit values were used, which may or may not have corresponded to the 99th percentile reference limits in this large international clinical trial. There was no central review of site reported re-elevation of biomarkers to confirm that cardiac markers were normal or decreasing pre-PCI. The overall number of re-infarctions was small-to moderate, therefore the study had limited power to detect differences regarding the impact of re-infarction on mortality, including the effect of IRA location and MI timing.
Troponin plays a central role in the universal definition of MI. Use of new high-sensitivity troponin assays would have resulted in higher MI rates across all types, including also peri-procedural MIs. It is unclear what the prognostic significance of those very small MIs would have been in this population.
Conclusions
Reinfarction significantly and independently impacted mortality in post-MI patients with totally occluded infarct arteries regardless of whether the initial management strategy is PCI or medical therapy alone. Reinfarction was an independent predictor of hospitalization for class III or IV heart failure. The effect of reinfarction on mortality was independent of reinfarction IRA location, reinfarction definition and early or later occurrence.
Highlights.
Patients with totally occluded infarct related arteries late after myocardial infarction who developed reinfarction had a 4.15-fold risk of death compared to patients without reinfarction.
This risk was similar for both initially randomized treatment groups (PCI vs. MED).
Risk of death was independent from reinfarction definition, reinfarction due to the same infarct related artery and a different epicardial vessel, as well as early or late occurrence.
Acknowledgments
We thank the patients who enrolled in the study, their physicians, and the staff at the study sites for their important contributions; and Carole Russo and Anna Yick, for assistance in the preparation of the manuscript.
Funding Sources: The project described was supported by Award Numbers U01 HL062509 and U01 HL062511 from the National Heart, Lung, And Blood Institute, Bethesda, Maryland, United States. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, And Blood Institute or the National Institutes of Health.
Footnotes
Conflict of Interest: none declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hochman JS, Lamas GA, Knatterud GL, et al. Design and methodology of the Occluded Artery Trial (OAT) American heart journal. 2005;150:627–42. doi: 10.1016/j.ahj.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Hochman JS, Lamas GA, Buller CE, et al. Coronary intervention for persistent occlusion after myocardial infarction. N Engl J Med. 2006;355:2395–407. doi: 10.1056/NEJMoa066139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hochman JS, Reynolds HR, Dzavik V, et al. Long-Term Effects of Percutaneous Coronary Intervention of the Totally Occluded Infarct-Related Artery in the Subacute Phase After Myocardial Infarction. Circulation. 2011;124:2320–8. doi: 10.1161/CIRCULATIONAHA.111.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mark DB, Pan W, Clapp-Channing NE, et al. Quality of life after late invasive therapy for occluded arteries. N Engl J Med. 2009;360:774–83. doi: 10.1056/NEJMoa0805151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White HD, Reynolds HR, Carvalho AC, et al. Reinfarction after percutaneous coronary intervention or medical management using the universal definition in patients with total occlusion after myocardial infarction: results from long-term follow-up of the Occluded Artery Trial (OAT) cohort. American Heart Journal. 2012;163:563–71. doi: 10.1016/j.ahj.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hudson MP, Granger CB, Topol EJ, et al. Early reinfarction after fibrinolysis: experience from the global utilization of streptokinase and tissue plasminogen activator (alteplase) for occluded coronary arteries (GUSTO I) and global use of strategies to open occluded coronary arteries (GUSTO III) trials. Circulation. 2001;104:1229–35. doi: 10.1161/hc3601.095717. [DOI] [PubMed] [Google Scholar]
- 7.Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Circulation. 2007;116:2634–53. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 8.Berger JS, Newman JD, Gregoire J, et al. Geographical Variation in Ischemia Severity in Patients Referred for Stress Imaging Studies: Screening Data from the ISCHEMIA Trial, Poster to be presented at the American College of Cardiology 2014 Scientific Sessions. J Am Coll Cardiol. 2014 (in press) [Google Scholar]
- 9.Kaplan EL, Meier P. Nonparametric-Estimation from Incomplete Observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 10.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox DR. Regression Models and Life-Tables. J Roy Stat Soc B. 1972;34:187–220. [Google Scholar]
- 12.Donges K, Schiele R, Gitt A, et al. Incidence, determinants, and clinical course of reinfarction in-hospital after index acute myocardial infarction (results from the pooled data of the maximal individual therapy in acute myocardial infarction [MITRA], and the myocardial infarction registry [MIR]) Am J Cardiol. 2001;87:1039–44. doi: 10.1016/s0002-9149(01)01458-8. [DOI] [PubMed] [Google Scholar]
- 13.Kornowski R, Goldbourt U, Zion M, et al. Predictors and long-term prognostic significance of recurrent infarction in the year after a first myocardial infarction. SPRINT Study Group. Am J Cardiol. 1993;72:883–8. doi: 10.1016/0002-9149(93)91100-v. [DOI] [PubMed] [Google Scholar]
- 14.Kernis SJ, Harjai KJ, Stone GW, et al. The incidence, predictors, and outcomes of early reinfarction after primary angioplasty for acute myocardial infarction. Journal of the American College of Cardiology. 2003;42:1173–7. doi: 10.1016/s0735-1097(03)00920-3. [DOI] [PubMed] [Google Scholar]
- 15.Spaulding C, Henry P, Teiger E, et al. Sirolimus-eluting versus uncoated stents in acute myocardial infarction. N Engl J Med. 2006;355:1093–104. doi: 10.1056/NEJMoa062006. [DOI] [PubMed] [Google Scholar]
- 16.Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–35. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]
- 17.Udelson JE, Pearte CA, Kimmelstiel CD, et al. The Occluded Artery Trial (OAT) Viability Ancillary Study (OAT-NUC): influence of infarct zone viability on left ventricular remodeling after percutaneous coronary intervention versus optimal medical therapy alone. American heart journal. 2011;161:611–21. doi: 10.1016/j.ahj.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buch P, Rasmussen S, Gislason GH, et al. Temporal decline in the prognostic impact of a recurrent acute myocardial infarction 1985 to 2002. Heart. 2007;93:210–5. doi: 10.1136/hrt.2006.092213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dzavik V, Carere RG, Mancini GB, et al. Predictors of improvement in left ventricular function after percutaneous revascularization of occluded coronary arteries: a report from the Total Occlusion Study of Canada (TOSCA) American heart journal. 2001;142:301–8. doi: 10.1067/mhj.2001.116960. [DOI] [PubMed] [Google Scholar]