Abstract
The water-reactive tissue adhesive 2-octyl cyanoacrylate (OCA) was microencapsulated in polyurethane shells and incorporated into Palacos R bone cement. The tensile and compressive properties of the composite material were investigated in accordance with commercial standards, and fracture toughness of the capsule-embedded bone cement was measured using the tapered double-cantilever beam geometry. Viability and proliferation of MG63 human osteosarcoma cells after culture with extracts from Palacos R bone cement, capsule-embedded Palacos R bone cement, and OCA were also analyzed. Incorporating up to 5 wt % capsules had little effect on the compressive and tensile properties of the composite, but greater than 5 wt % capsules reduced these values below commercial standards. Fracture toughness was increased by 13% through the incorporation of 3 wt % capsules and eventually decreased below the toughness of the capsule-free controls at capsule contents of 15 wt % and higher. The effect on cell proliferation and viability in response to extracts prepared from capsule-embedded and commercial bone cements were not significantly different from each other, whereas extracts from OCA were moderately toxic to cells. Overall, the addition of lower wt % of OCA-containing microcapsules to commercial bone cement was found to moderately increase static mechanical properties without increasing the toxicity of the material.
Keywords: self-healing, cytotoxicity, bone cement, mechanical properties, biomaterial
INTRODUCTION
Self-healing materials (SHM) are a rapidly emerging class of materials that prevent failure through the autonomous repair of microdamage in situ. One of the most broadly reported SHMs is the matrix repolymerization scheme pioneered by White, Sottos, and coworkers in which a polymer matrix is co-embedded with catalyst and microcapsules containing a reactive healing agent.1–7 On encountering a propagating microcrack, the microcapsule shell ruptures and releases the healing agent into the crack plane, exposing it to the catalyst embedded in the matrix. In situ curing of the healing agent ensues halting crack propagation.
Numerous implants fail because of fatigue, wear, and environmental cracking after the accumulation of microdamage, 6,8–12 marking these biomaterials potential candidates for the introduction of self-healing biomaterials; however, there has been little discussion of extending SHM into biomaterials. Consequently, none of the existing SHM systems currently under development use materials and reagents acceptable for in vivo load-bearing applications. Furthermore, no SHM have been tested under conditions that simulate the in vivo environment.
Because of its long history of use, ease of fabrication, and susceptibility to fatigue, acrylic bone cement is an attractive candidate for the first self-healing biomaterial based on the matrix repolymerization approach to self-healing. 6,7,13,14 Acrylic bone cement is a space-filling matrix that forms mechanical interlocks between the metallic stem of a total joint replacement and the surrounding bony tissue,15 serving to transfer loads from the prosthesis to the bone.16 Bone cement is a two-component thermoset consisting of a low-molecular-weight poly(methyl methacrylate) (PMMA) powder containing an initiator (e.g. benzoyl peroxide) and liquid methyl methacrylate (MMA) monomer. In situ mixing the two components initiates polymerization to yield a workable dough that is applied to the implant and hardens into a solid mass after the stem is inserted.7,17
The total loading experienced by bone cement in vivo is a mixture of compressive, bending, tensile, shear, and torsional forces,18 and as such, procedures to determine the mechanical properties involve the application of various static or dynamic stresses. Bone cements must adhere to static mechanical standards for tensile, compression, and fracture toughness testing before it is deemed suitable to investigate more biomimetic cyclic testing.18
Bone cements are also subject to cytotoxicity and tissue compatibility standards. Primary toxicity concerns associated with PMMA-based bone cements include localized cell death because of the exothermic reaction of the MMA and leaching of residual monomer from the matrix.19 An estimated 3–5% of the MMA remains 15 min post-polymerization and is reduced to about 1–2% over time as the residues are eliminated through the bloodstream.20,21 N,N-dimethyl-p-toluidine, which is present in small amounts (≤2% of the liquid component) and initiates MMA polymerization when it reacts with benzoyl peroxide present in the powder component is toxic at low concentrations and is able to inhibit protein synthesis and cause chromosomal mutations.20
Although the overall aim is to develop a bone cement capable of self-repair in response to damage, any new material proposed must also meet the standards required for the static mechanical properties of commercial formulations. Recently, we reported the microencapsulation of 2-octyl cyanoacrylate (OCA) in polyurethane shells and the distribution and fracture of the capsules embedded in a commercial bone cement matrix.7 Among candidate cyanoacrylates, OCA is an attractive healing agent for a self-healing biomaterial because it is widely used clinically22,23 and because its use would eliminate the need for the incorporation of a potentially toxic catalyst into the matrix; the catalyst for OCA polymerization is ambient moisture from the surrounding tissue.
The current study presents the first mechanical and cytotoxicity characterization of a biomaterial formulation consisting of OCA-containing microcapsules embedded in commercial PMMA bone cement. Incorporating up to 5 wt % capsules had little effect on the compressive and tensile properties of the composite, but greater than 5 wt % capsules reduced these values below commercial standards. Fracture toughness was increased by 13% through the incorporation of 3 wt % capsules and eventually decreased below the toughness of the capsule-free controls at capsule contents of 15 wt % and higher. Extracts prepared from capsule-embedded and commercial bone cements had little effect on cell proliferation and viability and were not significantly different from each other, whereas extracts from OCA alone were moderately toxic to cells. Overall, the addition of lower wt % of OCA-containing microcapsules to commercial bone cement was found to moderately increase static mechanical properties without increasing the toxicity of the material.
EXPERIMENTAL
Reagents
Unless otherwise specified, materials were obtained from commercial suppliers and used without further purification. OCA was generously donated by Ethicon, Raleigh, NC 27616. Methyl ethyl ketone (MEK), methyl isobutyl ketone (MIBK), and cyclohexanone (Sigma Aldrich) were used as solvents, and 2,4-toluene diisocyanate (TDI) and 1,4-butanediol (1,4-BD) (Sigma Aldrich) were used to synthesize the polyurethane prepolymer (pPUR) following the protocol outlined by Yang et al. and reported by the authors previously.7,24 Pluronic F-68 (Sigma Aldrich) was used as a surfactant. Para-toluenesulfonic acid (PTSA; Sigma Aldrich) was added to the organic phase as a stabilizer for the OCA monomer. Commercially available Palacos R PMMA bone cement (Zimmer) was used for all experiments reported herein.
Microcapsule preparation
Microcapsules were prepared and characterized as described previously.7 Briefly, at room temperature (RT), Pluronic F-68 surfactant (1.84 g) was dissolved in deionized water (90 mL) in a 250-mL beaker. The solution was agitated for 1 h with a digital mixer (VWR PowerMax Elite Dual-Speed Mixer) before beginning the encapsulation procedure. The aqueous phase was suspended in a hot water bath and heated to 50°C before the addition of the organic phase and chain extender.
OCA (4 mL) was dissolved in MIBK (8 mL), and pPUR (3 g) was dissolved in MEK (10 mL) separately at RT. PTSA (1%) was added to the OCA-MIBK solution to further stabilize the OCA monomer. The pPUR- and OCA-containing solutions were then added simultaneously to the aqueous phase but not mixed before this addition. After the organic and aqueous phases were combined, the 1,4-BD (3 mL) chain extender was added dropwise to the stirring mixture through a syringe to form the segmented PUR shell material consisting of hard TDI-based segments and soft 1,4-BD segments. After a reaction time of 2 h at an agitation rate of 700 rpm, the mixer was switched off and the suspension of microcapsules rinsed with deionized water and vacuum filtered. Capsules were air dried before use in the following experiments.7
Preparation of capsule-containing bone cement samples
The powder component was mixed with the liquid component according to the manufacturer’s instructions,25 OCA-containing capsules were added to the slurry, and the resultant material was added to molds to form the desired specimen shape. Samples were cured in the molds for at least 1 h, released, and smoothed with 240 mesh silicon carbide to conform to the specified geometrical requirements. The wt % of OCA-containing capsules in the bone cement composites ranged from 0 to 40 wt % where the wt % of total particulates (OCA capsules plus PMMA powder) was held constant at 67 wt % in all specimens.
Tensile testing of capsule-embedded bone cement
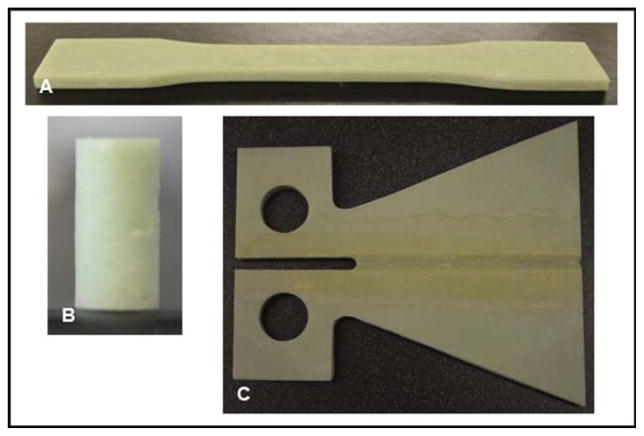
Following ASTM D638, bone cement specimens were cured in “dog bone”–shaped silicone rubber molds for 1 h and then smoothed, producing specimens with midsection dimensions of 13 ± 0.5 mm by 3.2 ± 0.4 mm [Figure 1(A)]. Twenty-four hours post-manufacturing, specimens were subjected to uniaxial tensile testing at a cross-head speed of 5 ± 1 mm/min until failure using a Tinius Olsen 1000 Ultimate Testing Machine. All tests were performed in air at RT. The ultimate tensile strength (UTS) was calculated as the force at failure divided by the cross-sectional area measured for each sample.
FIGURE 1.
PMMA samples for (A) tension, (B) compression, and (C) fracture toughness testing. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Compression testing of capsule-embedded bone cement
Following ASTM F451, bone cement specimens were cured in a polytetrafluoroethylene mold for 1 h and smoothed, yielding cylindrical specimens with a diameter of 6 ± 0.1 mm and height of 12 ± 0.1 mm [Figure 1(B)]. After 24 h post-fabrication, specimens were subjected to uniaxial compression testing at a cross-head speed of 20 mm/min using a Tinius Olsen 1000 Ultimate Testing Machine. All tests were performed in air at RT. The ultimate compressive strength (UCS) was calculated as the peak load divided by the pretest cross-sectional area measured for the specimen.
Fracture toughness testing of capsule-embedded bone cement
Fracture toughness, K, of capsule-embedded PMMA samples was determined using tapered double-cantilever beam (TDCB) specimens as described previously for other self-healing systems1,26–28 [Figure 1(C)]. Before mechanical testing, a razor blade was used to create a precrack in the grooved centerline region of the TDCB specimen, and specimen was loaded into a Test Resources Q series machine. The specimen was subjected to vertical displacement at 5 μm/s until the peak critical load, Pc, was reached and crack propagation was initiated.
Biocompatibility of capsule-containing bone cement
Preparation of bone cement and OCA extracts
Rectangular specimens of bone cement containing 0 or 10 wt % OCA-containing capsules were prepared in silicone rubber molds; the total particulates (OCA-containing capsules plus PMMA powder) in the composites were held constant at 67 wt %. The bone cement samples were cured at RT for 24 h and then autoclaved at 120°C for 30 min. Each sample was immersed in a 50-mL conical tube containing sterile complete culture medium and incubated for 24 h at 37°C.19 A ratio of 0.2 g bone cement to 1 mL extract medium was maintained.29
To prepare OCA extracts, 15 μL OCA was added to 5 mL culture medium and incubated at 37°C for 24 h in a 15-mL conical tube.30,31 As the OCA was not received sterile, the media was then filtered before use in these experiments. Loctite® Super Glue (ethyl cyanoacrylate) and copper sheets19 were selected as positive controls for this study. To prepare the Super Glue extracts, 15 μL of Super Glue was added to 5 mL culture medium and incubated at 37°C for 24 h in a 15-mL conical tube. Copper samples were immersed in a 15-mL conical tube and incubated at 37°C for 72 h; a ratio of 0.2 g copper to 1 mL extract medium was maintained.29 Extracts from the Super Glue and copper were both filtered before addition to cells.
Cell viability and proliferation
MG63 human osteosarcoma cells have been used in numerous biocompatibility studies19,20,32– 35 because they share several features with normal human osteoblasts, including secretion of insulin-like growth factor, binding proteins, matrix metalloproteinases, and osteocalcin. Similar to undifferentiated osteoblasts, MG63 cells also synthesize collagen types I and III and have a low basal expression of alkaline phosphatase that is increased in response to 1,25-dihydroxyvitamin D3.35,36
MG63 human osteosarcoma cells were cultured in minimum essential medium eagle (Sigma) supplemented with 10% heat-inactivated fetal bovine serum, and 1% each of sodium pyruvate (100 mM), nonessential amino acids (100×), and penicillin–streptomycin (10,000 units/mL penicillin and 10,000 μg/mL streptomycin; Gibco). Cells were routinely passaged and incubated at 37°C in a humidified environment of 5% CO2 in air.
Cells were passaged and plated in 96-well plates at a density of 2000 cells/well and allowed to attach for 24 h. After 24 h, the growth media was replaced by the same volume of treatment media. Media was also changed in the control wells so that cells in all wells were exposed to a media change 24 h after plating. For all experiments, cells in three different wells received each treatment, and the experiments were performed four times with new extract media prepared from different material samples for each trial.
To investigate the effects of bone cement extract, four treatment groups were studied: an undiluted capsule-free PMMA extract; undiluted capsule-embedded PMMA extract (10 wt % capsules); and capsule-embedded PMMA extract (10 wt % capsules) diluted to 50 and 25% using fresh media.19 To investigate the effects of OCA extract, three treatment groups were studied: OCA extract diluted to 50, 25, and 10% using fresh media.30 A negative control of cells not treated with any material extract and positive controls of cells treated with extract prepared from Loctite® Super Glue and copper sheets were also investigated.
Cell viability was assessed by a combination of staining with calcein AM and Hoechst 33342 (Invitrogen). After 72 h culture, the various treatment media were removed and cells rinsed twice with Dulbecco’s phosphate-buffered saline (DPBS) containing calcium chloride and magnesium chloride (1×; Gibco) and then incubated at 37°C in calcein AM in DPBS (0.75 μL/1.5 mL) for 20 min. The DPBS containing calcein AM was removed, and the cells were rinsed twice with DPBS and incubated an additional 20 min at 37°C in DPBS containing Hoechst 33342 (1.5 μL/1.5 mL). The DPBS containing Hoechst 33342 was removed, and the cells were rinsed twice with DPBS and then imaged using a Nikon Eclipse TE2000-U inverted fluorescence microscope and NIS Elements software (Nikon). Images were analyzed with ImageJ.
Cell proliferation was investigated with EdU staining following the manufacturer’s instructions included with the Click-iT EdU Alexa Fluor 488 kit (Invitrogen). The stain was performed after 24, 48, and 72 h of culture to examine potential effects of extract dilution and exposure time on proliferation. The plates were imaged using a Nikon Eclipse TE2000-U inverted fluorescence microscope and NIS Elements software (Nikon). Images were analyzed with ImageJ.
Statistical analysis
Statistical analyses were performed to compare the results of treatment cultures with respect to the negative and positive controls using a one-way analysis of variance with the significance of individual differences established by Tukey post hoc test. In all cases, the level of statistical significance was set at p < 0.05.
RESULTS
Capsule morphology
Capsules fabricated at an agitation rate of 700 rpm were shown to have uniformly spherical morphology with average diameter and shell thickness of 121 ± 24 μm and 3 ± 0.9 μm, respectively, consistent with measurements obtained previously.7 Capsules made at this agitation rate were used for all experiments.
Tensile testing of capsule-embedded bone cement
Capsule-free (unfilled) Palacos R bone cement used in the current study had a UTS of 41.2 ± 1.5 MPa (average ± SEM, n = 5) and a Young’s modulus of 1.85 ± 0.04 GPa (average ± SEM, n = 5), both of which fall within the reported literature ranges of 25–70 MPa16,18,37 and 1.36– 3.07 GPa,16,37–39 respectively. The UTS of the capsule-embedded (filled) specimens decreased monotonically with increasing wt % capsules [Figure 2(A)], whereas the Young’s modulus of capsule-containing samples seemed to drop more sharply up to 5 wt % and then decreased more slowly thereafter [Figure 2(B)]. The UTS of cements filled with 5 wt % was 29.1 ± 1.1 MPa (average ± SEM, n = 5), which lies above the lower limit of the literature-reported values; however, at this capsule content, the Young’s modulus for the material (1.13 ± 0.04 GPa, average ± SEM, n = 5) was outside the range of values reported in the literature.
FIGURE 2.
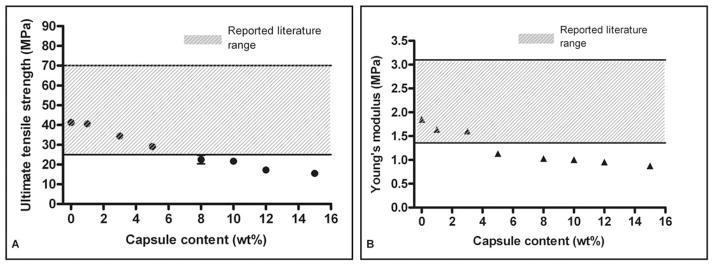
Relationship between capsule content and (A) ultimate tensile strength and (B) Young’s modulus (average ± SEM, n = 5).
Compression testing of capsule-embedded bone cement
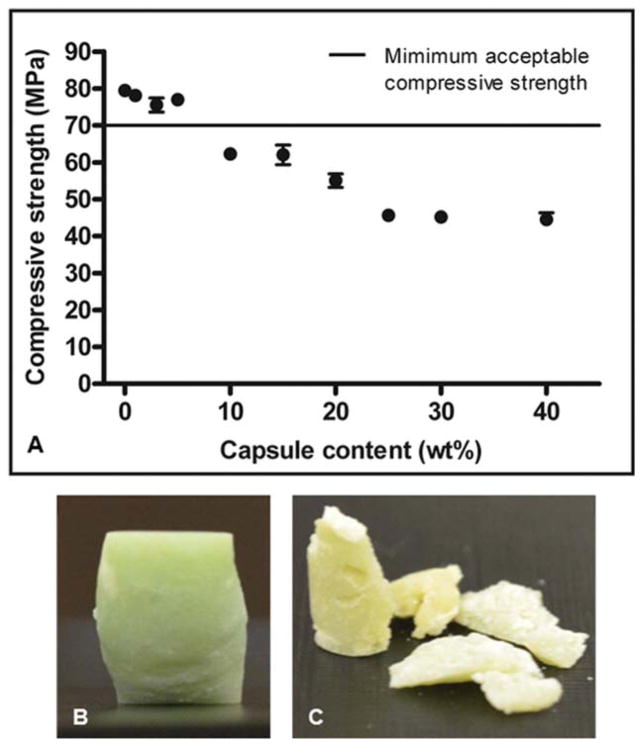
Compressive tests of bone cement specimens filled with 5 wt % or fewer capsules all exceeded the minimum standard for the compressive strength of 70 MPa18 [Figure 3(A)], whereas specimens filled with 10 wt % or higher capsules all had compressive strengths that declined steadily below this standard. In addition, specimens containing 10 wt % or fewer capsules deformed plastically on failure, whereas specimens with higher capsule content fragmented on failure [Figure 3(B,C)].
FIGURE 3.
(A) Relationship between capsule content and the ultimate compressive strength of bone cement (average ± SEM, n = 3 with five replicates per group). Photographs of samples containing (B) 0 wt % and (C) 40 wt % capsules postcompression testing. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Fracture toughness testing of capsule-embedded bone cement
Figure 4(A) compares the load–vertical displacement curves for unfilled bone cement (dashed line) and bone cement filled with 3 wt % capsules (solid line). The unfilled specimen exhibited instantaneous crack growth to complete failure on reaching the peak critical load (~197 N), whereas the specimen filled with 3 wt % capsules exhibited slow and progressive crack growth beyond the peak critical load (~211 N) before the initiation of rapid propagation leading to complete failure.
FIGURE 4.
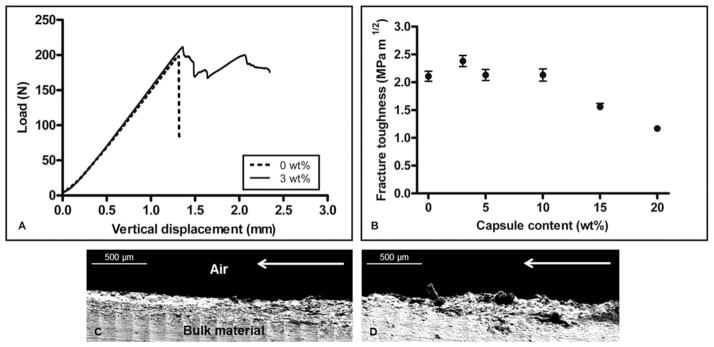
Load with increasing vertical displacement is shown in (A) for samples containing 0 and 3 wt % capsules. Effect of capsule content on fracture toughness is presented in (B) (average ± SEM, n = 5). Fracture plane roughness and subsurface microcracking are observed in SEM images of the side views of samples containing (C) 0 wt % and (D) 3 wt % capsules. Direction of crack propagation is indicated by arrows.
Fracture toughness, K, varies based on numerous factors, including the molecular weight of the PMMA, sterilization methods, mixing techniques, storage conditions, and specimen geometry. The fracture toughness of unfilled specimens was found to be 2.11 ± 0.09 MPa m1/2, and the effects of capsule inclusion on K are shown in Figure 4(B). The addition of 3 wt % capsules resulted in a modest 13% increase in average K, whereas samples filled with 5 and 10 wt % capsules yielded K values approximately equal to that of unfilled control samples. Increasing capsule content to 15 and 20 wt % resulted in decreases of 26 and 45% in K, respectively, when compared with unfilled controls, but remained within the literature-reported range of 0.88–2.58 MPa m1/2.16,40–43
Scanning electron microscopy (SEM) images of the crack planes of unfilled [Figure 4(C)] and filled [3 wt %; Figure 4(D)] specimens revealed a marked increase in the roughness of the crack plane with increasing capsule content. In capsule-containing specimens, regions of slow, progressive crack growth exhibited subfracture plane microcracking that was less evident in capsule-free control specimens.
Cytotoxicity testing of OCA and capsule-embedded bone cement
MG63 viability
The cytotoxic effects of leachates extracted from unfilled bone cement, bone cement filled with 10 wt % capsules, and OCA tissue adhesive were assessed after 72 h exposure using calcein AM staining to detect live cells and Hoechst 33342 staining to detect all cells. Cells cultured in media containing no material extract served as negative controls. Loctite® Super Glue (ethyl cyanoacrylate) and copper sheets19 were used as positive controls. Viability was assessed as the total area covered by live cells in the treatment groups compared with coverage by live cells observed in control samples.
Extracts from all bone cement specimens exhibited significantly reduced cell viability compared with the negative control but not with respect to each other; a slight but not significant dose-dependent response was also observed [Figure 5(A)]. In contrast, extracts from OCA-containing medium showed a significant reduction in coverage by live cells for all dilutions with respect to control samples and a significant dose-dependent dilution effect was observed [Figure 5(B)]. No live cells were present after 72 h culture with extract from either the copper or Super Glue positive controls.
FIGURE 5.
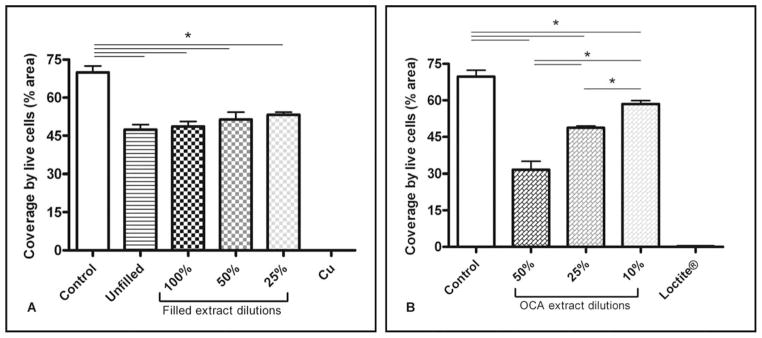
Viability of MG63 human osteosarcoma cells after 72 h exposure to (A) PMMA bone cement and (B) OCA extracts (average ± SEM, n = 4). Coverage by live cells in the positive controls, Cu and Loctite®, was significantly different from all treatment groups, although significance is not indicated on the figures.
MG63 proliferation
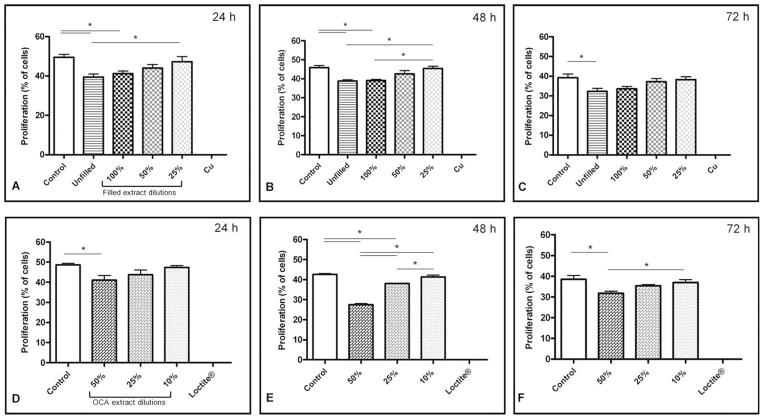
The effects of bone cement and OCA extracts on MG63 cell proliferation over 24, 48, and 72 h are shown in Figure 6(A–F). Compared with extract-free negative controls, cell proliferation was significantly reduced on exposure to undiluted extracts from unfilled and filled bone cement and to 50% diluted extracts from OCA at all time points, but proliferation trended upward with increased dilution of the extracts.
FIGURE 6.
Proliferation of MG63 human osteosarcoma cells in response to growth in extract from (A, B, C) bone cement and (D, E, F) OCA after (A, D) 24, (B, E) 48, and (C, F) 72 h (average ± SEM, n = 4). Proliferation of cells in the positive controls, Cu and Loctite®, was significantly different from all treatment groups, although significance is not indicated on the figures.
Overall, cell proliferation in undiluted extracts from unfilled and capsule filled bone cements was nearly indistinguishable, indicating that the addition of capsules to the bone cement did not significantly affect proliferation of cells at 24, 48, or 72 h [Figure 6(A–C)]. However, the dose-dependent dilution effect for capsule-filled bone cement was significant only at 48 h between undiluted extract and extract diluted to 25%.
In contrast, extract obtained from OCA had a more substantial effect on curtailing cell proliferation, thus requiring that 50% dilution be the least diluted extract tested [Figure 6(D–F)]. A significant decrease in the proliferation of cells exposed to OCA extract-containing medium diluted to 50% was observed at each time point with respect to the negative control, with the largest decrease observed after 48 h. The dose-dependent dilution effect for OCA extract was significant between all dilutions at 48 h and between 50 and 10% at 72 h. Treatment with extract from Super Glue and copper sheets eliminated proliferation at every time point. In addition, cells in all groups exhibited decreased proliferation over time.
DISCUSSION
This work describes the static mechanical testing of an acrylic bone cement matrix embedded with OCA-containing microcapsules using well-established ASTM and ISO mechanical and cytotoxicity standards for acrylic bone cements. Each test was designed to examine how capsule incorporation affected the integrity and cytotoxicity of the bone cement relative to the unfilled control.
Overall, composites containing up to 5 wt % capsules had compressive strengths above the commercially acceptable minimum and tensile strengths and stiffnesses within the reported ranges for commercial acrylic bone cements. Incorporation of higher wt % capsules did however result in mechanical weakening. Under compression, the composite began to fragment at high capsule contents [>25 wt %; Figure 3(C)] and could be attributed to the increased void space introduced by the capsules or possibly by altering the PMMA powder:MMA monomer ratio that is well known to influence polymerization rate, setting time, and mechanical properties of PMMA.44 The decrease in UTS could be attributed to a reduction in the cross-sectional area occupied by bone cement matrix in samples containing higher capsule contents or to poor shell/matrix interfacial bonding.
The weakening of a composite matrix through the addition of particulates typically occurs when the particulate–matrix interfacial adhesion is poor and increasing the particulate content provides a path for crack propagation. These observations are consistent with the general trend of limiting the incorporation of encapsulated healing agent to 5–10 wt % in self-healing polymeric composites.28 Evidence of capsule pull-out after tensile tests, and the fragmenting of the matrix under compression observed at high capsule contents suggests poor interfacial bonding between the polyurethane shell material and the PMMA matrix. In addition to chemical modification of the shell, alterations to the physical properties of the capsule, such as changing the shell material45 or adding a coating,46,47 could be investigated to further promote adhesion through increased surface roughness.
The fracture toughness was relatively unaffected by capsule incorporation up to 10 wt % capsules, above which it also declined. For a specimen containing 3 wt % capsules [solid line in Figure 4(A)], the load increased linearly with increased displacement identically to the unfilled specimen [dashed line in Figure 4(A)] until reaching a load of approximately 200 N, after which the crack in the unfilled specimen propagated immediately to failure, whereas the crack in the 3 wt % sample advanced in short unstable jumps, as reflected by the jagged load–displacement curve. This difference is also reflected in the distinctly different morphologies of the crack planes of the two specimens [Figure 4(C,D)]. Previous examinations of self-healing composites observed similar stick-slip crack propagation in virgin load–displacement curves.48,49
The addition of 3 wt % OCA-containing capsules only slightly increased the fracture toughness when compared with capsule-free bone cement. In contrast, the addition of microencapsulated dicyclopentadiene to an epoxy matrix was previously demonstrated to increase fracture toughness up to 127%.28 As the acrylic bone cement is already a highly filled composite of 60–70 wt % PMMA particles in a PMMA matrix, the fracture toughness of the bone cement here (2.11 ± 0.09 MPa m1/2) was already nearly four times higher than that of neat EPON 828 epoxy28 (0.55 ± 0.04 MPa m1/2). Consequently, the addition of 3 wt % capsules does not seem to have a significant toughening effect on the bone cement composite.
Numerous investigations of the cytotoxicity of various bone cement formulations have been reported.19,20,32,50–52 As the toxicity associated with bone cement has been primarily attributed to the MMA monomer and its activator,19–21 both of which are unchanged in the capsule-embedded formulation, the similar cellular responses observed between the filled and unfilled bone cements were anticipated [Figures 5 and 6(A–C)].
Similar to the results presented here, previous studies found the proliferation of MG63 cells to be reduced as the exposure time to bone cement extracts was increased.20 However, these results also indicate the addition of capsules did not significantly affect cell proliferation or viability in response to PMMA bone cement. It is also understood that the toxic effect of bone cement on cells and tissue primarily occurs before and during polymerization in vivo20,53; therefore, in vitro analyses of fully cured specimens do not fully reflect what happens to the cells as the PMMA cures in vivo but do support the potential promise of the system.
There are numerous studies dedicated to analyses of toxicity of cyanoacrylate adhesives for clinical applications,30,31,54,55 albeit not in the context of a SHM. Cyanoacrylate adhesives degrade into formaldehyde and cyanoacetate, which may cause tissue irritation.31,54–58 Shorter chain cyanoacrylate adhesives such as the Loctite® positive control used here are known to be more toxic than longer alkyl chain adhesives such as OCA.31,54–59
Proliferation and viability testing of OCA extracts [Figures 5B and 6(D–F)] clearly showed the OCA was toxic to the MG63 cells in culture, and extracts from the Loctite® positive control killed virtually all of the cells. Similar to the work of others with various cyanoacrylates, these results showed both time- and dose-dependent effects on cell proliferation and viability.31,55
Although 50 and 25% OCA extract significantly decreased proliferation with respect to the negative control, there was no significant decrease in proliferation of cells treated with 10% OCA extract, suggesting the effects of the extract were mediated through the dilution of the extract. Therefore, it is hypothesized that some effects of both PMMA and OCA on surrounding cells may be mediated through clearance (e.g. dilution) of the released leachates in vivo. Furthermore, as the OCA is intended to polymerize within the PMMA matrix on contact with moisture, limited exposure to cells is anticipated.
Finally, it is important to note that the testing described here examines OCA-containing capsule-filled acrylic bone cement strictly within the context of ASTM and ISO commercial standards for the static mechanical properties of acrylic bone cement. Therefore, these results do not speak to the self-healing capacity of the system. Dynamic testing of the self-healing capacity of the system will be presented in a subsequent manuscript.
CONCLUSIONS
The effects of capsule incorporation on the compressive, tensile, and fracture toughness properties of bone cement showed that inclusion of greater than 5 wt % capsules resulted in the decrease of UCS and UTS below the commercially required levels; the fracture toughness was improved with the incorporation of 3 wt % capsules but declined as content was increased above 15 wt %. The effects of extract from a capsule-embedded bone cement on the proliferation and viability of MG63 human osteosarcoma cells indicated the addition of capsules did not significantly affect the viability and proliferation of the cells in response to the PMMA. The effects of both capsule-embedded bone cement and OCA extracts were found to be mediated somewhat through dilution of the extract. These findings support the future promise of this system and indicate that its toxicity is similar to existing commercial formulations.
Acknowledgments
Contract grant sponsor: National Institutes of Health; contract grant numbers: T32-GM8555 (to A.B.W.B.); R21 EB 013874-01 (to W.M.R.)
The authors thank Ethicon Inc. for the generous donation of 2-octyl cyanoacrylate. The authors also gratefully recognize the contributions of Duke University colleagues Dr. Marcus Henderson, Steven Owen, and Sonia George with respect to TDCB testing protocols and specimen mold fabrication in addition to Dr. Stephen Craig and Zachary Kean for polymer synthesis and Matthew Novak for assistance with statistical analyses.
References
- 1.White SR, Sottos NR, Geubelle PH, Moore JS, Kessler MR, Sriram SR, Brown EN, Viswanathan S. Autonomic healing of polymer composites. Nature. 2001;409:794–797. doi: 10.1038/35057232. [DOI] [PubMed] [Google Scholar]
- 2.Kessler MR. Self-healing: A new paradigm in materials design. Proc Inst Mech Eng G - J Aerosp Eng. 2007;221:479–495. [Google Scholar]
- 3.Kessler MR, White SR. Self-activated healing of delamination damage in woven composites. Compos A Appl Sci Manuf. 2001;32:683–699. [Google Scholar]
- 4.Andersson HM, Keller MW, Moore JS, Sottos NR, White SR. Self healing polymers and composites. In: Zwaag SVD, editor. Self Healing Materials: An Alternative Approach to 20 Centuries of Materials Science. AA Dordrecht, The Netherlands: Springer; 2007. pp. 19–44. [Google Scholar]
- 5.Bergman SD, Wuld F. Re-mendable polymers. In: Zwaag SVD, editor. Self Healing Materials: An Alternative Approach to 20 Centuries of Materials Science. AA Dordrecht, The Netherlands: Springer; 2007. pp. 45–68. [Google Scholar]
- 6.Brochu ABW, Craig SL, Reichert WM. Self-healing biomaterials. J Biomed Mater Res A. 2011;96:492–506. doi: 10.1002/jbm.a.32987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brochu ABW, Chyan WJ, Reichert WM. Microencapsulation of 2-octylcyanoacrylate tissue adhesive for self-healing acrylic bone cement. J Biomed Mater Res B. 2012;100:1764–1772. doi: 10.1002/jbm.b.32743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edidin AA, Kurtz SM. Influence of mechanical behavior on the wear of 4 clinically relevant polymeric biomaterials in a hip simulator. J Arthroplasty. 2000;15:321–331. doi: 10.1016/s0883-5403(00)90647-8. [DOI] [PubMed] [Google Scholar]
- 9.Dabagh M, Abdekhodaie MJ, Khorasani MT. Effects of polydime-thylsiloxane grafting on the calcification, physical properties, and biocompatibility of polyurethane in a heart valve. J Appl Polym Sci. 2005;98:758–766. [Google Scholar]
- 10.Kidane AG, Burriesci G, Cornejo P, Dooley A, Sarkar S, Bonhoeffer P, Edirisinghe M, Seifalian AM. Current developments and future prospects for heart valve replacement therapy. J Biomed Mater Res B. 2009;88:290–303. doi: 10.1002/jbm.b.31151. [DOI] [PubMed] [Google Scholar]
- 11.Perez MA, Garcia-Aznar JM, Doblare M. Does increased bone-cement interface strength have negative consequences for bulk cement integrity? A finite element study. Ann Biomed Eng. 2009;37:454–466. doi: 10.1007/s10439-008-9616-7. [DOI] [PubMed] [Google Scholar]
- 12.Hoey D, Taylor D. Quantitative analysis of the effect of porosity on the fatigue strength of bone cement. Acta Biomater. 2009;5:719–726. doi: 10.1016/j.actbio.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 13.Biggs PL, Jones L, II, Wellborn B, Lewis G. In: A self-healing PMMA bone cement: Influence of crystal size of Grubbs’ catalyst. McGoron CL, Lin W-C, editors. 2009. pp. 147–150. [Google Scholar]
- 14.Lewis G, Wellborn B, Jones L, II, Biggs PL. A room-temperature autonomically-healing PMMA bone cement: Influence of composition on fatigue crack propagation rate. J Appl Biomater Biomech. 2009;7:90–96. [PubMed] [Google Scholar]
- 15.Giddings VL, Kurtz SM, Jewett CW, Foulds JR, Edidin AA. A small punch test technique for characterizing the elastic modulus and fracture behavior of PMMA bone cement used in total joint replacement. Biomaterials. 2001;22:1875–1881. doi: 10.1016/s0142-9612(00)00372-0. [DOI] [PubMed] [Google Scholar]
- 16.Saha S, Pal S. Mechanical properties of bone cement—A review. J Biomed Mater Res. 1984;18:435–462. doi: 10.1002/jbm.820180411. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed AM, Morrey BF. Polymethylmethacrylate. In: Morrey BF, editor. Joint Replacement Arthroplasty. Philadelphia: Elsevier Science; 2003. pp. 7–18. [Google Scholar]
- 18.Kuehn KD, Ege W, Gopp U. Acrylic bone cements: Mechanical and physical properties. Orthop Clin North Am. 2005;36:29–39. doi: 10.1016/j.ocl.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Almeida T, Ferreira BJML, Loureiro J, Correia RN, Santos C. Preliminary evaluation of the in vitro cytotoxicity of PMMA-co-EHA bone cement. Mater Sci Eng C. 2011;31:658–662. [Google Scholar]
- 20.Granchi D, Stea S, Ciapetti G, Savarino L, Cavedagna D, Pizzoferrato A. In vitro effects of bone cements on the cell cycle of osteoblast-like cells. Biomaterials. 1995;16:1187–1192. doi: 10.1016/0142-9612(95)93585-2. [DOI] [PubMed] [Google Scholar]
- 21.Harper EJ, Braden M, Bonfield W. Mechanical properties of hydroxyapatite reinforced poly(ethylmethacrylate) bone cement after immersion in a physiological solution: Influence of a silane coupling agent. J Mater Sci: Mater Med. 2000;11:491–497. doi: 10.1023/a:1013057724268. [DOI] [PubMed] [Google Scholar]
- 22.Coulthard P, Esposito M, Worthington HV, van der Elst M, van Waes OJF, Darcey J. Tissue adhesives for closure of surgical incisions. Cochrane Db Syst Rev. 2010;(5):Art No. CD004287. doi: 10.1002/14651858.CD004287.pub3. [DOI] [PubMed] [Google Scholar]
- 23.Singer AJ, Thode HC. A review of the literature on octylcyanoacrylate tissue adhesive. Am J Surg. 2004;187:238–248. doi: 10.1016/j.amjsurg.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 24.Yang JL, Keller MW, Moore JS, White SR, Sottos NR. Microen-capsulation of isocyanates for self-healing polymers. macromolecules. 2008;41:9650–9655. [Google Scholar]
- 25.Palacos R. Instructions for use. Zimmer Surgical, Inc; Dover: 2012. [Google Scholar]
- 26.Brown EN. Use of the tapered double-cantilever beam geometry for fracture toughness measurements and its application to the quantification of self-healing. J Strain Anal Eng. 2011;46:167–186. [Google Scholar]
- 27.Brown EN, White SR, Sottos NR. Fatigue crack propagation in microcapsule-toughened epoxy. J Mater Sci. 2006;41:6266–6273. [Google Scholar]
- 28.Brown EN, White SR, Sottos NR. Microcapsule induced toughening in a self-healing polymer composite. J Mater Sci. 2004;39:1703–1710. [Google Scholar]
- 29.International Standard 10993–12:2012. Biological evaluation of medical devices. Part 12: Sample preparation and reference materials. ISO; Switzerland: 2012. [Google Scholar]
- 30.Kaplan M, Baysal K. In vitro toxicity test of ethyl 2-cyanoacrylate, a tissue adhesive used in cardiovascular surgery, by fibroblast cell culture method. Heart Surg Forum. 2005;8:E169–E172. doi: 10.1532/HSF98.20041126. [DOI] [PubMed] [Google Scholar]
- 31.Ciapetti G, Stea S, Cenni E, Sudanese A, Marraro D, Toni A, Pizzoferrato A. Toxicity of cyanoacrylates in vitro using extract dilution assay on cell cultures. Biomaterials. 1994;15:92–96. doi: 10.1016/0142-9612(94)90256-9. [DOI] [PubMed] [Google Scholar]
- 32.Jyoti MA, Song H-Y. Initial in vitro biocompatibility of a bone cement composite containing a poly-epsilon-caprolactone microspheres. J Mater Sci Mater Med. 2011;22:1333–1342. doi: 10.1007/s10856-011-4311-x. [DOI] [PubMed] [Google Scholar]
- 33.Lopes MA, Knowles JC, Kuru L, Santos JD, Monteiro FJ, Olsen I. Flow cytometry for assessing biocompatibility. J Biomed Mater Res. 1998;41:649–656. doi: 10.1002/(sici)1097-4636(19980915)41:4<649::aid-jbm17>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 34.Nayab SN, Jones FH, Olsen I. Modulation of the human bone cell cycle by calcium ion-implantation of titanium. Biomaterials. 2007;28:38–44. doi: 10.1016/j.biomaterials.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 35.Martin JY, Schwartz Z, Hummert TW, Schraub DM, Simpson J, Lankford J, Dean DD, Cochran DL, Boyan BD. Effect of titanium surface roughness on proliferation, differentiation, and protein synthesis of human osteoblast-like cells (MG63) J Biomed Mater Res. 1995;29:389–401. doi: 10.1002/jbm.820290314. [DOI] [PubMed] [Google Scholar]
- 36.Clover J, Gowen M. Are MG63 and HOS human osteosarcoma cell lines representative models of the osteoblastic phenotype. Bone. 1994;15:585–591. doi: 10.1016/8756-3282(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 37.Davies JP, Oconnor DO, Greer JA, Harris WH. Comparison of the mechanical properties of Simplex P, Zimmer Regular, and LVC bone cements. J Biomed Mater Res. 1987;21:719–730. doi: 10.1002/jbm.820210604. [DOI] [PubMed] [Google Scholar]
- 38.Kurtz SM, Villarraga ML, Zhao K, Edidin AA. Static and fatigue mechanical behavior of bone cement with elevated barium sulfate content for treatment of vertebral compression fractures. Biomaterials. 2005;26:3699–3712. doi: 10.1016/j.biomaterials.2004.09.055. [DOI] [PubMed] [Google Scholar]
- 39.Lewis G, Mladsi S. Effect of sterilization method on properties of Palacos (R) R acrylic bone cement. Biomaterials. 1998;19:117–124. doi: 10.1016/s0142-9612(97)00165-8. [DOI] [PubMed] [Google Scholar]
- 40.Perek J, Pilliar RM. Fracture toughness of composite acrylic bone cements. J Mater Sci Mater Med. 1992;3:333–344. [Google Scholar]
- 41.Graham J, Pruitt L, Ries M, Gundiah N. Fracture and fatigue properties of acrylic bone cement—The effects of mixing method, sterilization treatment, and molecular weight. J Arthroplasty. 2000;15:1028–1035. doi: 10.1054/arth.2000.8188. [DOI] [PubMed] [Google Scholar]
- 42.Lewis G. Apparent fracture toughness of acrylic bone cement: Effect of test specimen configuration and sterilization method. Biomaterials. 1999;20:69–78. doi: 10.1016/s0142-9612(98)00145-8. [DOI] [PubMed] [Google Scholar]
- 43.Lewis G, Mladsi S. Correlation between impact strength and fracture toughness of PMMA-based bone cements. Biomaterials. 2000;21:775–781. doi: 10.1016/s0142-9612(99)00226-4. [DOI] [PubMed] [Google Scholar]
- 44.Lewis G, Xu J, Madigan S, Towler MR. Influence of two changes in the composition of an acrylic bone cement on its handling, thermal, physical, and mechanical properties. J Mater Sci Mater Med. 2007;18:1649–1658. doi: 10.1007/s10856-007-3042-5. [DOI] [PubMed] [Google Scholar]
- 45.Brown EN, Kessler MR, Sottos NR, White SR. In situ poly(urea-formaldehyde) microencapsulation of dicyclopentadiene. J Microencapsul. 2003;20:719–730. doi: 10.1080/0265204031000154160. [DOI] [PubMed] [Google Scholar]
- 46.Jackson AC, Bartelt JA, Marczewski K, Sottos NR, Braun PV. Silica-protected micron and sub-micron capsules and particles for self-healing at the microscale. Macromol Rapid Commun. 2011;32:82–87. doi: 10.1002/marc.201000468. [DOI] [PubMed] [Google Scholar]
- 47.Caruso MM, Blaiszik BJ, Jin H, Schelkopf SR, Stradley DS, Sottos NR, White SR, Moore JS. Robust, double-walled microcapsules for self-healing polymeric materials. ACS Appl Mater Interface. 2010;2:1195–1199. doi: 10.1021/am100084k. [DOI] [PubMed] [Google Scholar]
- 48.Kessler MR, Sottos NR, White SR. Self-healing structural composite materials. Compos A Appl Sci Manuf. 2003;34:743–753. [Google Scholar]
- 49.Brown EN, Sottos NR, White SR. Fracture testing of a self-healing polymer composite. Exp Mech. 2002;42:372–379. [Google Scholar]
- 50.Alt V, Bechert T, Steinrucke P, Wagener M, Seidel P, Dingeldein E, Domann E, Schnettler R. An in vitro assessment of the antibacterial properties and cytotoxicity of nanoparticulate silver bone cement. Biomaterials. 2004;25:4383–4391. doi: 10.1016/j.biomaterials.2003.10.078. [DOI] [PubMed] [Google Scholar]
- 51.Kim SB, Kim YJ, Yoon TR, Park SA, Cho IH, Kim EJ, Kim IA, Shin JW. The characteristics of a hydroxyapatite-chitosan-PMMA bone cement. Biomaterials. 2004;25:5715–5723. doi: 10.1016/j.biomaterials.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 52.Kim HS, Park YB, Oh JH, Yoo KH, Lee SH. The cytotoxic effect of methotrexate loaded bone cement on osteosarcoma cell lines. Int Orthop. 2001;25:343–348. doi: 10.1007/s002640100293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Linder L. The tissue response to bone cement. In: Williams DF, editor. Biocompatibility of Orthopedic Implants. Boca Raton: CRC Press; 1982. pp. 1–23. [Google Scholar]
- 54.Thumwanit V, Kedjarune U. Cytotoxicity of polymerized commercial cyanoacrylate adhesive on cultured human oral fibroblasts. Aust Dent J. 1999;44:248–252. doi: 10.1111/j.1834-7819.1999.tb00228.x. [DOI] [PubMed] [Google Scholar]
- 55.Evans CE, Lees GC, Trail IA. Cytotoxicity of cyanoacrylate adhesives to cultured tendon cells. J Hand Surg-Br. 1999;24:658–661. doi: 10.1054/jhsb.1999.0279. [DOI] [PubMed] [Google Scholar]
- 56.Weber SC, Chapman MW. Adhesives in orthopaedic surgery: A review of the literature and in vitro bonding strengths of bone-bonding agents. Clin Orthop Relat Res. 1984;191:249–261. [PubMed] [Google Scholar]
- 57.Leonard F, Kulkarni RK, Nelson J, Brandes G. Tissue adhesives and hemostasis-inducing compounds: The alkyl cyanoacrylates. J Biomed Mat Res. 1967;1:3–9. doi: 10.1002/jbm.820010103. [DOI] [PubMed] [Google Scholar]
- 58.Vinters HV, Galil KA, Lundie MJ, Kaufmann JCE. The histotoxicity of cyanoacrylates—A selective review. Neuroradiology. 1985;27:279–291. doi: 10.1007/BF00339559. [DOI] [PubMed] [Google Scholar]
- 59.Matsumoto T, Hardaway RM, Heisterkamp CA, Pani KC, Leonard F. Higher homologous cyanoacrylate tissue adhesives in surgery of internal organs. Arch Surg. 1967;9:861–864. doi: 10.1001/archsurg.1967.01330120115022. [DOI] [PubMed] [Google Scholar]