Abstract
Objective
Two types of sleep preference have been supported in the literature. Morning types awaken early and are refreshed upon waking whereas Evening types rise later and have more erratic sleep schedules. Sleep affects menstrual functioning in adult women. However, there is scant research on the association between sleep preference and menstrual functioning in adolescents. Thus, the present study examined the association between sleep preference and menstrual functioning in 210 adolescent girls (11–17 years).
Methods
Data represent baseline measures from a longitudinal study examining the association of psychological functioning and smoking with reproductive and bone health. Measures included the Menstrual Symptom Questionnaire (MSQ), regularity and duration of menstrual cycles, and the Morningness/Eveningness scale (measuring sleep preference). MSQ factor scores were used in analyses: abdominal pain, negative affect/somatic complaints, back pain, and anxiety/fatigue.
Results
The results from hierarchical linear regression analyses showed significant associations between Evening preference and more symptoms of abdominal pain (p<.01), negative affect/somatic complaints (p<.01), anxiety/fatigue (p<.01), and shorter menses (p<.05).
Conclusion
Adolescent girls with Evening preference experience more menstrual symptoms than those with Morning preference. Future research should include sleep preference in studies of health and behavior particularly in adolescence when there is a normative shift toward Evening preference.
Keywords: adolescent females, dysmenorrhea, menstrual symptoms, morningness/eveningness, sleep, chronobiology
Introduction
Sleep preference (Morningness vs. Eveningness) is based on chronobiology and circadian rhythms [1, 2]. Two sleep types are typically referred to in the literature. Morning types awaken early, are refreshed upon awakening, and go to bed early, whereas Evening types have difficulty rising in the morning, are tired when awakening, and stay up late. Evening types have irregular sleep-wake schedules; they go to bed later, wake up later, sleep more on weekends than on school nights, and complain of sleep debt compared to Morning types [3].
As children enter adolescence, they begin going to sleep later and will stay asleep later when permitted [4]. Younger children tend to be Morning types, although around age 13 the preference shifts to Evening type, and then changes back to Morning type in adulthood [5]. Overall, adult men are more likely to be Evening types whereas women show greater Morningness tendencies [6–9]. Sleep preference may impact quality of life. Evening types have been found to less emotionally stable than Morning types [1] and in adolescence, Evening types tended to have more behavioral problems; [5] particularly for boys [10]. Additionally, sleep preference affects school performance due to the shift towards eveningness in early adolescence [11].
Although a number of studies have examined personality and demographic characteristics associated with sleep preference [8, 12–15], there is little evidence regarding the association between sleep preference and menstrual functioning. Sleep affects regularity and functioning of the reproductive system. For example, night shift nurses were found to have changes in menstrual function and a higher incidence of painful menstruation [16]. Additionally, Eveningness was associated with menstrual pain and depression in university students [17].
Around the time that sleep preference shifts to Evening type, most girls are menarcheal and may experience dysmenorrhea or other menstrual symptoms. Dysmenorrhea is prevalent in adolescents and is identified as the leading cause of school and work absences among female adolescents and young adults [18, 19]. In primary dysmenorrhea (pain during menses without an identifiable pathologic lesion) [20], lower abdominal cramping is the most common symptom. However nausea, vomiting, headaches, backaches, and dizziness also occur during menses [19, 21]. Studies have reported a school absenteeism rate of 14 to 52% among adolescents with dysmenorrhea [22, 23]. Such absences may have an important impact on psychosocial and cognitive development. Although dysmenorrhea is less common during the first 2–3 years post-menarche, its prevalence increases during mid and late adolescence [19]. Coupled with changing sleep preference, dysmenorrhea may profoundly affect development in adolescence.
This study seeks to fill a gap in the understanding of sleep preference and menstrual functioning in adolescence. Although dysmenorrhea clearly affects many adolescents, multiple factors that may impact such symptoms are understudied. In particular, sleep disturbances are associated with menstrual irregularity and pain in adult women [24]. Additionally Evening preference is associated with more menstrual pain in young adulthood [17]. However, the relationship between sleep preference and menstrual functioning has not been examined in adolescents. We hypothesized that Evening type adolescents would experience more menstrual dysfunction (irregularity, pain, negative affect) than Morning types.
Methods
Participants
Girls were enrolled in a longitudinal study examining the impact of smoking and mood on bone and reproductive health in adolescence (N = 264). From the full sample, 210 girls (80%) were menarcheal and thus completed the Menstrual Symptom Questionnaire (MSQ)[25]. Girls were primarily Caucasian (57%) or African American (40%). The remaining 3% represented mixed-race or other races. Girls were enrolled in the Institutional Review Board approved study by an initial eligibility questionnaire in age cohorts of 11, 13, 15, and 17 years old and based on five previously defined levels of smoking. Participants were recruited from an urban teen health clinic and surrounding community. Exclusion criteria were: 1) pregnancy or breast feeding within 6 months, 2) primary (> age 16) or secondary (< 6 cycles/year) amenorrhea, 4) body mass index less than the 1st percentile; body weight above 300 pounds, 5) medication/medical disorder influencing bone health, and 6) psychological disabilities impairing comprehension or compliance.
Procedures
Participants came to a children’s hospital for their visit. After parental consent and adolescent assent were obtained, a physician or nurse practitioner obtained a standardized menstrual history and use of medications. The adolescent then completed the Menstrual Symptom Questionnaire [25] and the Morningness/Eveningness scale (M/E) [26]1. Measures are further described in Table 1.
Table 1.
Descriptive statistics for 210 adolescent females
| Reliability (α) | Mean | Standard deviation | Range | |
|---|---|---|---|---|
| Age (years) | 15.69 | 1.74 | 11.07 – 17.99 | |
| Socioeconomic status (SES) | 35.90 | 13.25 | 14 – 66 | |
| Gynecological age (years) | 3.41 | 1.91 | .01 – 10.07 | |
| Age at menarche (years) | 12.28 | 1.30 | 7.42 – 16.08 | |
| Morningness/Eveningness (10 items) | 0.78 | 25.93 | 5.01 | 12 – 38 |
| MSQ abdominal pain (7 items) | 0.87 | 16.15 | 6.21 | 6 – 29 |
| MSQ negative affect/somatic complaints (7 items) | 0.82 | 16.47 | 6.17 | 7 – 35 |
| MSQ back pain (3 items) | 0.84 | 6.28 | 3.41 | 3 – 15 |
| MSQ anxiety/fatigue (3 items) | 0.72 | 5.60 | 2.67 | 3 – 15 |
| Duration of typical menses (days) | 5.47 | 2.27 | 0 – 21 | |
| Regularity of cycle | 3.20 | 1.35 | 1 – 5 | |
Note: SES = Hollingshead socioeconomic status; the Morningness/Eveningness scale [26] assesses preferred time for sleep/wake activities (e.g. waking up, bedtime, test taking). Lower scores indicate Evening preference. Menstrual Symptom Questionnaire (MSQ)[25] is a 24 item self-report measure assessing menstrual pain and symptoms (e.g. “I have cramps that begin on the first day of my period”; “I feel depressed for several days before my period”). Item scores range from 1 (never) to 5 (always) with a higher composite score indicating more symptoms. Factor analysis indicated 4 factors shown in table. Regularity of cycle 1 = highly irregular, 5 = highly regular; written definitions were provided for each level.
Data Analyses
The associations between M/E and the dependent variables of MSQ factors, cycle regularity, and duration of menses were examined using six separate hierarchical linear regression models for each dependent variable. Covariates were entered (Step 1) followed by the M/E total score (Step 2). Level of significance was set to p< .05. Covariates included age, race (Caucasian/non-Caucasian), socioeconomic status (SES) [27], hormone contraception (any type), and gynecologic age (years and months post-menarche).
Results
Descriptive Statistics
Table 1 reports means and standard deviations. In reporting history of hormone contraceptives, 48% of the sample had taken some type of hormone contraception in their lifetime and of those, 27% had taken oral contraceptives. No group differences were noted on menstrual symptoms for those on hormonal contraceptives versus not (F (1, 202) = .09, p > .05).
Morningness/Eveningness and Menstrual Symptoms
After controlling for covariates, there were significant associations between M/E and the MSQ factors of abdominal pain (β = −.22, p < .01) (Figure 1), negative affect/somatic complaints (β = −.26, p < .01), and anxiety/fatigue (β = −.20, p < .01), as well as duration of menses (β = .15, p < .05). No significant associations were noted between M/E and the MSQ factor score of back pain (β = −.10, p = .14) or regularity of cycle (β = .06, p = .36).
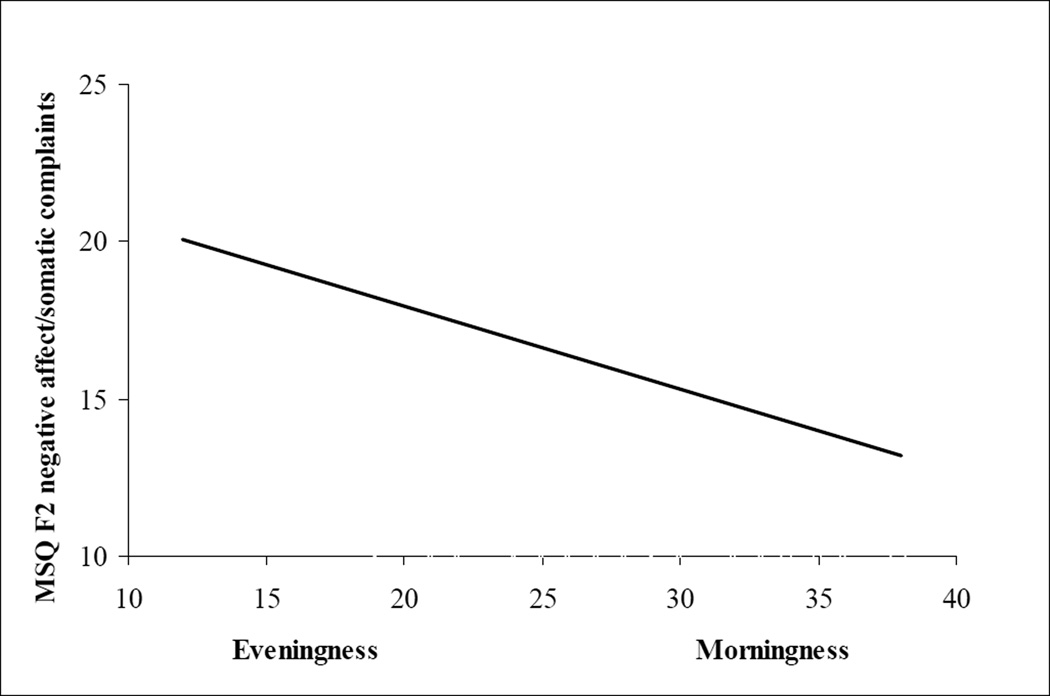
Figure 1.
Regression line showing the association between Evening preference and more menstrual symptoms using the factor score of negative affect/somatic complaints (F2) from the Menstrual Cycle Questionnaire (MSQ)[25]. Similar significant regression lines were evident for three of the four factor scores including (F1) abdominal pain and (F4) anxiety/fatigue.
Discussion
In adolescent females, Eveningness was associated with more abdominal pain, negative affect/somatic complaints, anxiety/fatigue, and shorter duration of menses. However, Eveningness was not related to back pain or menstrual regularity. Studies have shown that abnormal sleep schedules affect reproductive functioning [16, 28, 29]; however this is one of the first studies to show associations between sleep preference and menstrual symptoms. Previous research found Evening preference was associated with “stable” menstrual cycles in adolescents and menstrual pain in young adults [17]. However, we found no association between Eveningness and menstrual irregularity, which may be due in part to the young gynecologic age of our sample. Adolescents may not yet have a well developed menstrual history to determine regularity. Further, regularity often does not occur until several years post-menarche.
Of particular interest is the emergence of these associations in adolescents who are just beginning to develop ovulatory cycles. Analyses controlled for gynecological age, thus partialling out possible effects of being at different developmental stages for ovulation. Additionally, as per the study design most participants completed their visit during the follicular phase, precluding symptomatic differences that may vary by menstrual phase. Once ovulation becomes more regular, menstrual symptoms should decrease; thus this period of development is an important time to understand the effects of sleep on menstrual functioning. Additionally, adolescence is a time of varied sleep; entry into adolescence is accompanied by a delayed phase preference for sleep [30] when most individuals shift from Morning to Evening preference [5, 11, 12]. However, there is still variation in sleep preference and the implication for the effect of sleep timing on menstrual symptoms is important to understand.
Studies report that Morning types have higher sleep efficiency than Evening types [8], and Evening types report higher scores on the Sleep/Wake Behavior Scale indicating more sleep problems [3]. Thus, menstrual disturbances experienced by Evening types may be related to the disruption in normal circadian and sleep-regulated 24-hour rhythms [16, 24, 29]. Biological rhythms play an important role in reproductive regulation. Specifically the 24-hour clock is key in regulating the production, release, synthesis, and action of reproductive hormones. The altered sleep timing of Evening types may be partially responsible for increased menstrual symptoms. In addition, to counteract their sleepiness Evening types may more frequently use psychoactive substances such as caffeine and tobacco, which have also been linked to increased menstrual pain [31–33]. Sleep problems, sleep disruption, or psychoactive substance use were not assessed in this study, therefore our results cannot support these inferences. However they are interesting directions for future research.
There are several limitations of the current study. First, this study is cross-sectional, and cannot support causal mechanisms between sleep preference and menstrual symptoms. Additionally, depression and anxiety have been linked to dysmenorrhea [34], but these variables were not included in the current study. Lastly, because this was a normative sample there were few participants with severe menstrual dysfunction which limits reporting in the upper range of the MSQ.
In conclusion, this study provides important evidence for the association between sleep preference and menstrual functioning in adolescents. Evening types are more likely to have increased menstrual symptoms. Although this study cannot ascertain the causality it points to important considerations for examining reproductive function and dysmenorrhea in adolescence. Future research should investigate longitudinal associations between menstrual symptoms and sleep preference because sleep will tend to shift again as adolescents enter young adulthood.
Acknowledgements
This research was supported by Grant Number R01 DA 16402, National Institute of Drug Abuse, NIH. PI: Lorah D. Dorn, Ph.D., U.S.P.H.S. Grant Number M01 RR 08084, General Clinical Research Centers Program, National Center for Research Resources, NIH, and National Research Service Award Training Grant 1T32PE10027.
Footnotes
Only the measures pertinent to this study are reported here.
References
- 1.Tankova I, Adan A, Buela-Casal G. Circadian typology and individual differences: A review. Pers Ind Diff. 1994;16(5):671–684. [Google Scholar]
- 2.Young MW, Kay SA. Time zones: A comparative genetics of circadian clocks. National Review of Genetics. 2001;2:702–715. doi: 10.1038/35088576. [DOI] [PubMed] [Google Scholar]
- 3.Giannotti F, et al. Circadian preference, sleep and daytime behaviour in adolescence. J Sleep Res. 2002;11(3):191–199. doi: 10.1046/j.1365-2869.2002.00302.x. [DOI] [PubMed] [Google Scholar]
- 4.Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Dev. 1998;69(4):875–887. [PubMed] [Google Scholar]
- 5.Cavallera GM, Giudici S. Morningness and eveningness personality: A survey in literature from 1995 up till 2006. Pers Ind Diff. 2008;44(1):3–21. [Google Scholar]
- 6.Adan A, Natale V. Gender differences in morningness-eveningness preference. Chronobiol Int. 2002;19(4):709–720. doi: 10.1081/cbi-120005390. [DOI] [PubMed] [Google Scholar]
- 7.Randler C. Gender differences in morningness-eveningness assessed by self-report questionnaires: A meta-analysis. Pers Ind Diff. 2007;43(7):1667–1675. [Google Scholar]
- 8.Lehnkering H, Siegmund R. Influence of chronotype, season, and sex of subject on sleep behavior of young adults. Chronobiol Int. 2007;24(5):875–888. doi: 10.1080/07420520701648259. [DOI] [PubMed] [Google Scholar]
- 9.Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms. 2003;18(1):80–90. doi: 10.1177/0748730402239679. [DOI] [PubMed] [Google Scholar]
- 10.Susman EJ, et al. Morningness/eveningness, morning-to-afternoon cortisol ratio, and antisocial behavior problems during puberty. Dev Psychol. 2007;43(4):811–822. doi: 10.1037/0012-1649.43.4.811. [DOI] [PubMed] [Google Scholar]
- 11.Kim S, et al. Children's time of day preference: Age, gender and ethnic differences. Pers Ind Diff. 2002;33(7):1083–1090. [Google Scholar]
- 12.Gau SS, Soong WT. The transition of sleep-wake patterns in early adolescence. Sleep. 2003;26(4):449–454. doi: 10.1093/sleep/26.4.449. [DOI] [PubMed] [Google Scholar]
- 13.Chelminski I, et al. An analysis of the 'eveningness-morningness' dimension in 'depressive' college students. J Affect Disord. 1999;52(1–3):19–29. doi: 10.1016/s0165-0327(98)00051-2. [DOI] [PubMed] [Google Scholar]
- 14.Diaz-Morales JF, Sanchez-Lopez MP. Morningness-eveningness and anxiety among adults: A matter of sex/gender? Pers Ind Diff. 2008;44(6):1391–1401. [Google Scholar]
- 15.Song J, Stough C. The relationship between morningness-eveningness, time-of-day, speed of information processing, and intelligence. Pers Ind Diff. 2000;29(6):1179–1190. [Google Scholar]
- 16.Labyak S, et al. Effects of shiftwork on sleep and menstrual function in nurses. Health Care Women Int. 2002;23(6–7):703–714. doi: 10.1080/07399330290107449. [DOI] [PubMed] [Google Scholar]
- 17.Takeuchi H, Oishi T, Harada T. Association between morningness-eveningness preference and mental/physical premenstrual symptoms in Japanese females 12 to 31 years of age. Chronobiol Int. 2005;22(6):1055–1068. doi: 10.1080/07420520500398007. [DOI] [PubMed] [Google Scholar]
- 18.Davis AR, Westhoff CL. Primary dysmenorrhea in adolescent girls and treatment with oral contraceptives. Journal of Pediatric Adolescent Gynecology. 2001;14(1):3–8. doi: 10.1016/s1083-3188(00)00076-0. [DOI] [PubMed] [Google Scholar]
- 19.Klein JR, Litt IF. Epidemiology of adolescent dysmenorrhea. Pediatrics. 1981;68(5):661–664. [PubMed] [Google Scholar]
- 20.Davis AR, et al. Oral contraceptives for dysmenorrhea in adolescent girls: a randomized trial. Obstet Gynecol. 2005;106:97–104. doi: 10.1097/01.AOG.0000165826.03915.65. [DOI] [PubMed] [Google Scholar]
- 21.Harel Z. Dysmenorrhea in adolescents and young adults: Etiology and management. J Pediatr Adolesc Gynecol. 2006;19:363–371. doi: 10.1016/j.jpag.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Banikarim C, Chacko MR, Kelder SH. Prevalence and impact of dysmenorrhea on Hispanic female adolescents. Arch Pediatr Adolesc Med. 2000;154(12):1226–1229. doi: 10.1001/archpedi.154.12.1226. [DOI] [PubMed] [Google Scholar]
- 23.Johnson J. Level of knowledge among adolescent girls regarding effective treatment for dysmenorrhea. J Adolesc Health Care. 1988;9(5):398–402. doi: 10.1016/0197-0070(88)90036-8. [DOI] [PubMed] [Google Scholar]
- 24.Baker FC, et al. High nocturnal body temperatures and disturbed sleep in women with primary dysmenorrhea. The American Journal of Physiology. 1999;277(6):E1013–E1021. doi: 10.1152/ajpendo.1999.277.6.E1013. [DOI] [PubMed] [Google Scholar]
- 25.Chesney MA, Tasto DL. The Development Of The Menstrual Symptom Questionnaire. Behavior Research & Therapy. 1975;13:237–244. doi: 10.1016/0005-7967(75)90028-5. [DOI] [PubMed] [Google Scholar]
- 26.Carskadon MA, Vieira C, Acebo C. Association between puberty and delayed phase preference. Sleep: Journal of Sleep Research & Sleep Medicine. 1993;16(3):258–262. doi: 10.1093/sleep/16.3.258. [DOI] [PubMed] [Google Scholar]
- 27.Hollingshead AF. Four Factor Index of Social Status: Manual. New Haven, CT: Department of Sociology, Yale University; 1976. [Google Scholar]
- 28.Axelsson G, Rylander R, Molin I. Outcome of pregnancy in relation to irregular and inconvenient work schedules. Br J Ind Med. 1989;46:393–398. doi: 10.1136/oem.46.6.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee KA, et al. Sleep patterns related to menstrual cycle phase and premenstrual affective symptoms. Sleep: Journal of Sleep Research & Sleep Medicine. 1990;13(5):403–409. [PubMed] [Google Scholar]
- 30.Carskadon MA. Patterns of sleep and sleepiness in adolescents. Pediatrician. 1990;17(1):5–12. [PubMed] [Google Scholar]
- 31.Harlow SD, Park M. A longitudinal study of risk factors for the occurrence, duration and severity of menstrual cramps in a cohort of college women. Br J Obstet Gynaecol. 1996;103(11):1134–1142. doi: 10.1111/j.1471-0528.1996.tb09597.x. [DOI] [PubMed] [Google Scholar]
- 32.Teperi J, Rimpela M. Menstrual Pain, Health and Behaviour in Girls. Soc Sci Med. 1989;29(2):163–169. doi: 10.1016/0277-9536(89)90164-0. [DOI] [PubMed] [Google Scholar]
- 33.Rossignol AM, Bonnlander H. Caffeine-containing beverages, total fluid consumption, and premenstrual syndrome. Am J Public Health. 1990;80(9):1106–1110. doi: 10.2105/ajph.80.9.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alonso C, Coe CL. Disruptions of social relationships accentuate the association between emotional distress and menstrual pain in young women. Health Psychol. 2001;20(6):411–416. [PubMed] [Google Scholar]