Background: The role of pyruvate phosphate dikinase (PPDK), which catalyzes a reversible reaction, is unknown in many eukaryotes.
Results: Deletion of the trypanosomal PPDK gene affects glycolysis.
Conclusion: In trypanosomes, PPDK works in the glycolytic direction and participates in the maintenance of the glycosomal ATP/ADP balance.
Significance: The glycosomal PPDK provides a metabolic flexibility by producing 2 ATP per phosphoenolpyruvate consumed.
Keywords: Gene Knockout, Glucose Metabolism, Parasite Metabolism, Peroxisome, Trypanosoma brucei
Abstract
Trypanosoma brucei belongs to a group of protists that sequester the first six or seven glycolytic steps inside specialized peroxisomes, named glycosomes. Because of the glycosomal membrane impermeability to nucleotides, ATP molecules consumed by the first glycolytic steps need to be regenerated in the glycosomes by kinases, such as phosphoenolpyruvate carboxykinase (PEPCK). The glycosomal pyruvate phosphate dikinase (PPDK), which reversibly converts phosphoenolpyruvate into pyruvate, could also be involved in this process. To address this question, we analyzed the metabolism of the main carbon sources used by the procyclic trypanosomes (glucose, proline, and threonine) after deletion of the PPDK gene in the wild-type (Δppdk) and PEPCK null (Δppdk/Δpepck) backgrounds. The rate of acetate production from glucose is 30% reduced in the Δppdk mutant, whereas threonine-derived acetate production is not affected, showing that PPDK function in the glycolytic direction with production of ATP in the glycosomes. The Δppdk/Δpepck mutant incubated in glucose as the only carbon source showed a 3.8-fold reduction of the glycolytic rate compared with the Δpepck mutant, as a consequence of the imbalanced glycosomal ATP/ADP ratio. The role of PPDK in maintenance of the ATP/ADP balance was confirmed by expressing the glycosomal phosphoglycerate kinase (PGKC) in the Δppdk/Δpepck cell line, which restored the glycolytic flux. We also observed that expression of PGKC is lethal for procyclic trypanosomes, as a consequence of ATP depletion, due to glycosomal relocation of cytosolic ATP production. This illustrates the key roles played by glycosomal and cytosolic kinases, including PPDK, to maintain the cellular ATP/ADP homeostasis.
Introduction
Pyruvate phosphate dikinase (PPDK)3 (EC 2.7.9.1) is an inorganic pyrophosphate (PPi)-dependent enzyme, which reversibly catalyzes conversion of phosphoenolpyruvate (P-enolpyruvate), PPi, and AMP into pyruvate, inorganic phosphate, and ATP. In C4 plants, PPDK operates in the gluconeogenic direction (production of P-enolpyruvate from pyruvate) and contributes to CO2 fixation through photosynthesis. Involvement of PPDK in glycolysis (production of pyruvate from P-enolpyruvate) has been suggested in a number of eukaryotes, including Phytophthora (1), Giardia (2, 3), Entamoeba (4), and Trypanosoma (5–7), however, its coexistence with the ATP-dependent glycolytic pyruvate kinase (EC 2.7.1.40) makes it difficult to address its role. Here we address this question in Trypanosoma brucei, using very powerful reverse genetic approaches developed in this eukaryotic model.
T. brucei is a unicellular eukaryote, belonging to the protozoan order Kinetoplastida that causes sleeping sickness in humans (8). This parasite possesses a complex life cycle during transmission from the bloodstream of a mammalian host (bloodstream stages of the parasite) to the alimentary tract (procyclic stage) and salivary glands (epimastigote and metacyclic stages) of a blood-feeding insect vector, the tsetse fly. The procyclic insect stage of T. brucei, our experimental model in this analysis, develops an elaborate energy metabolism based on different carbon sources, including glucose, proline, and threonine (9–11). Although proline is the major component of the hemolymph of the fly (12), the parasite prefers glucose when this carbon source is available (13, 14).
The procyclic trypanosomes convert glucose by aerobic fermentation into partially oxidized end products, mainly succinate and acetate (for review, see Refs. 15 and 16) (Fig. 1A). Most of the glycolysis takes place in specialized peroxisomes, called glycosomes (steps 1–6) (17). In the course of glycolysis, P-enolpyruvate is produced in the cytosol (steps 10–12), where it is located at a branching point. It can re-enter the glycosomes to be converted to succinate within this compartment (steps 13–16) or in the mitochondrion (not shown in Fig. 1) (18, 19). P-enolpyruvate can also be converted into pyruvate (steps 17–18), which enters the mitochondrion to produce acetate (steps 19–22) (20, 21). Acetate is also produced in the mitochondrion from threonine, by the action of four enzymes, i.e. threonine dehydrogenase (TDH) (EC 1.1.1.103, step 23), whose expression is under metabolic control, 2,2-amino-3-ketobutyrate coenzyme A ligase (EC 2.3.1.29, step 24), acetate:succinate CoA-transferase (EC 2.8.3.8, step 20), and acetyl-CoA thioesterase (ACH, EC 3.1.2.1, steps 22) (11). The two later steps are shared with glucose metabolism (11).
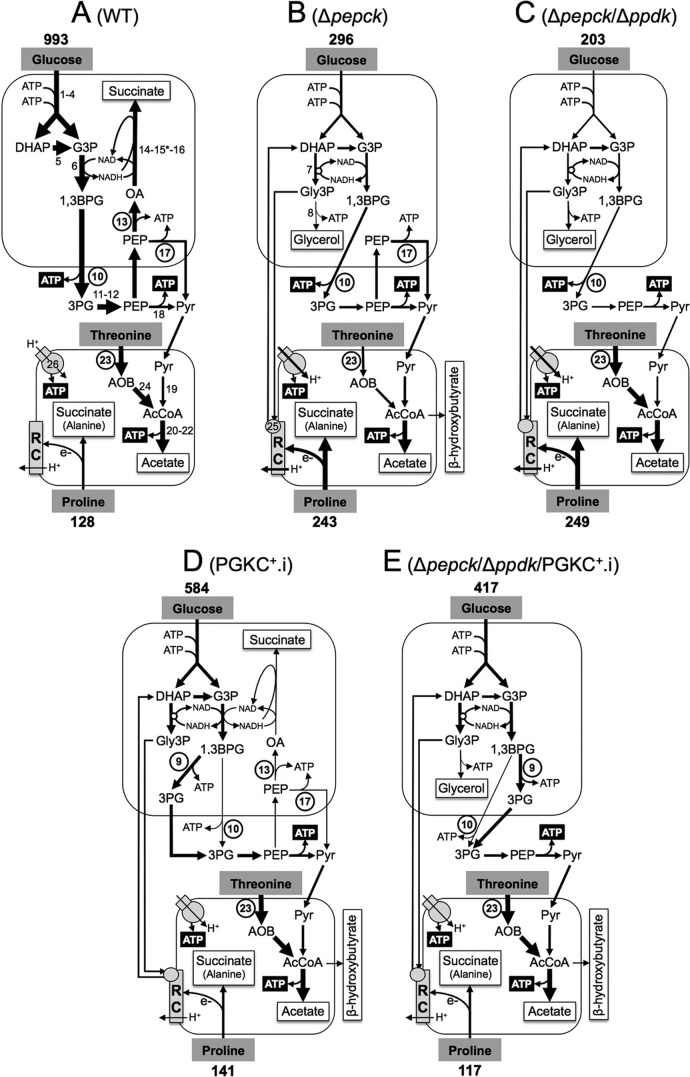
FIGURE 1.
Schematic representation of the intermediary metabolism of procyclic wild-type and mutant cell lines. This figure highlights steps from the glucose metabolism involved in the maintenance of the glycosomal NAD+/NADH and ATP/ADP balances, as well as steps involved in ATP production in the cytosol and mitochondrion from the three main carbon sources used by the procyclic trypanosomes (glucose, proline, and threonine). For simplification, mitochondrial production of succinate from glucose is not shown, as well as the steps of proline degradation and the cytosolic localization of fumarase (step 15*). For enzymatic steps involved in net production of ATP molecules, ATP is represented by white characters on a black background, and the circled step numbers represent enzymes analyzed here. Excreted end products from degradation of glucose, proline and threonine are boxed (succinate and alanine are the main end products excreted from proline metabolism, in the presence or absence of glucose, respectively (14)). The arrow thickness is representative of the measured or estimated metabolic flux through the corresponding branches. The rate of glucose and proline consumption (nmol/h/mg of protein) is indicated above and below the carbon source name, respectively (values from Fig. 3). Abbreviations: AcCoA, acetyl-CoA; AOB, amino oxobutyrate; 1,3BPG, 1,3-biphosphoglycerate; DHAP, dihydroxyacetone phosphate; e−, electrons; G3P, glyceraldehyde 3-phosphate; Gly3P, glycerol 3-phosphate; OA, oxaloacetate; 3PG, 3-phosphoglycerate; PEP, phosphoenolpyruvate; Pyr, pyruvate; RC, respiratory chain. Enzymes of glucose of threonine degradation are indicated in panel A, or when mentioned below, in panels B or D: 1, hexokinase; 2, glucose-6-phosphate isomerase; 3, phosphofructokinase; 4, aldolase; 5, triose-phosphate isomerase; 6, glyceraldehyde-3-phosphate dehydrogenase; 7, NADH-dependent glycerol-3-phosphate dehydrogenase (panel B); 8, glycerol kinase (panel B); 9, glycosomal phosphoglycerate kinase (PGKC) (panel D); 10, cytosolic phosphoglycerate kinase (PGKB); 11, phosphoglycerate mutase; 12, enolase; 13, PEPCK; 14, malate dehydrogenase; 15*, cytosolic fumarase (located in the glycosomes for simplification); 16, glycosomal NADH-dependent fumarate reductase; 17, pyruvate phosphate dikinase (PPDK); 18, pyruvate kinase; 19, pyruvate dehydrogenase complex; 20, acetate:succinate CoA-transferase; 21, succinyl-CoA synthetase; 22, acetyl-CoA thioesterase; 23, threonine dehydrogenase (TDH); 24, 2,2-amino-3-ketobutyrate coenzyme A ligase; 25, FAD-dependent glycerol-3-phosphate dehydrogenase (panel B); 26, F0F1-ATP synthase.
No exchanges of nucleotides or cofactors have been described so far between the glycosomal and cytosolic compartments in T. brucei. Consequently, consumption and production of ATP and NAD+ by glycolysis are tightly balanced within the organelle. In the wild-type procyclic trypanosomes NADH resulting from the reaction catalyzed by glyceraldehyde-3-phosphate dehydrogenase (EC 1.2.1.13, step 6) is re-oxidized inside the organelle by the glycosomal succinic fermentation pathway (steps 14 and 16) (18). In the absence of the succinate branch, such as in the P-enolpyruvate carboxykinase (PEPCK: EC 4.1.1.49, step 13) null mutant (Δpepck), NADH is re-oxidized by glycerol-3-phosphate dehydrogenase (EC 1.1.1.8) with the contribution of the glycerol 3-phosphate/dihydroxyacetone phosphate shuttle and the mitochondrial respiratory chain (steps 7 and 25, in Fig. 1B) (22).
ATP molecules consumed in the upper part of glycolysis (steps 1 and 3) also need to be regenerated inside the glycosomes by the lower part of the pathway. The T. brucei genome encodes five glycosomal kinases directly linked to glycolysis, which could theoretically produce ATP from conversion of triose phosphates inside the organelle (see Fig. 1) (16, 23), i.e. phosphoglycerate kinase isoform C (PGKC: Tb927.1.700) (EC 2.7.2.3, step 9 in Fig. 1D) and isoform A (PGKA: Tb927.1.720), PEPCK (Tb927.2.4210, step 13), glycerol kinase (GK: Tb927.9.12550) (EC 2.7.1.30, step 8 in Fig. 1B), and PPDK functioning in the glycolytic direction (Tb927.11.6280, step 17). PGKC is only expressed in bloodstream forms of T. brucei, PGKA shows a very low activity in both forms (24, 25) and wild-type procyclic cells do not excrete glycerol from glucose metabolism, implying that PGK isoforms and GK do not contribute significantly to glycosomal ATP production (18, 22). Consequently, PEPCK and PPDK are the only known possible enzymes playing a role in maintaining the glycosomal ATP/ADP balance in the wild-type procyclic cells. The role of PEPCK in this process has clearly been established, since the metabolic flux through the succinate branch represents up to 70% of the glycolytic flux in the wild-type parasite (18, 19, 26). However, the impact of PPDK on glycosomal ATP production is debatable, because metabolic fluxes through this step, in one direction or the other, has not been demonstrated so far.
Here we have investigated the maintenance of the ATP/ADP balance in the organelle by generating and analyzing PPDK (Δppdk) and PPDK/PEPCK (Δppdk/Δpepck) null mutants. Our data show for the first time that PPDK is involved in the maintenance of the glycosomal ATP/ADP balance by functioning in the glycolytic direction.
EXPERIMENTAL PROCEDURES
Growth and Maintenance of Trypanosomes
The procyclic form of T. brucei EATRO1125.T7T (TetR-HYG T7RNAPOL-NEO), which constitutively expresses the T7 RNA polymerase (T7RNAPOL) gene and the tetracycline repressor (TetR) gene under control of a T7 RNA polymerase promoter for tetracycline inducible expression (27), as well as mutant cell lines, were cultured at 27 °C in SDM79 medium containing 10% (v/v) heat-inactivated fetal calf serum and 3.5 mg/ml of hemin (28). The bloodstream forms of T. brucei 427 90-13 were cultured at 37 °C in Iscove's modified Dulbecco's medium (Invitrogen) supplemented with 10% (v/v) heat-inactivated fetal calf serum, 0.25 mm β-mercaptoethanol, 36 mm NaHCO3, 1 mm hypoxanthine, 0.16 mm thymidine, 1 mm sodium pyruvate, 0.05 mm bathocuprone, and 2 mm l-cysteine (29).
Gene Knock-out
Replacement of the PEPCK gene (Tb927.2.4210, GeneDB) by the blasticidin (BSD) and puromycin (PAC) resistance markers via homologous recombination was described before (Δpepck cell line, TetR-HYG T7RNAPOL-NEO Δpepck::BSD/Δpepck::PAC) (22). Here, the same plasmid constructs were used to delete the PEPCK alleles in the Δppdk::TetR-HYG/Δppdk::T7RNAPOL-NEO (Δppdk) mutant cell line, in which both PPDK alleles have been replaced by TetR-HYG and T7RNAPOL-NEO genes, respectively (10, 30). The resulting cell line, Δppdk::TetR-HYG/Δppdk::T7RNAPOL-NEO Δpepck::BSD/Δpepck::PAC (Δppdk/Δpepck), was generated by transfection and selection of drug-resistant clones as previously reported (31). The first and second PEPCK alleles were replaced by BSD- and PAC-resistant genes, respectively. Transfected cells were selected in SDM79 medium containing hygromycin B (25 μg/ml), neomycin (10 μg/ml), blasticidin (10 μg/ml), and puromycin (1 μg/ml).
Inhibition of PGKB Gene Expression by RNAi
Inhibition of gene expression by RNAi in procyclic trypanosomes (32) was performed by expression of stem-loop “sense/antisense” RNA molecules of the targeted sequences introduced in the pHD1336 expression vector (kindly provided by C. Clayton) as previously described (27). To specifically down-regulate expression of the gene encoding the cytosolic phosphoglycerate kinase isoform (PGKB: Tb927.1.710), the pHD-PGKB-SAS plasmid was constructed to target a 352-bp fragment corresponding to the PGKB and PGKC (Tb927.1.700) intergenic regions. Briefly, a PCR-amplified 445-bp fragment, containing the antisense PGKB-PGKC intergenic sequence, the first 60 bp of the PGKC gene, and the appropriate restriction sites added to the primers was inserted into HindIII and BamHI restriction sites of the pLew100 plasmid. Then a PCR-amplified fragment containing the sense PGKB-PGKC intergenic sequence (372 bp) was inserted upstream of the antisense sequence, using HindIII and XhoI restriction sites (XhoI was introduced at the 3′-extremity of the antisense PCR fragment). Finally, the sense-antisense HindIII/BamHI cassette was inserted in the HindIII/BamHI-digested pHD1336 vector. The resulting plasmid (pHD-PGKB-SAS) contains a sense and antisense version of the targeted gene fragment, separated by a 60-bp fragment (PGKC coding sequence), under control of the procyclic acidic repetitive protein (PARP) promoter linked to a prokaryotic tetracycline (Tet) operator. The RNAiPGKB cell line (TetR-HYG T7RNAPOL-NEO RNAiPGKB-BSD) was produced by introducing the pHD-PGKB-SAS plasmid in the EATRO1125.T7T parental cell line. Transfected cells were selected in SDM79 medium containing hygromycin B (25 μg/ml), neomycin (10 μg/ml), and blasticidin (10 μg/ml).
Expression of the PGKC and PEPCK Genes
The pLew100 vector (kindly provided by E. Wirtz and G. Cross) (33) was used to express full-length PGKC and PEPCK glycosomal proteins in procyclic trypanosomes by inserting a 1323- or 1578-bp PCR fragment, respectively, in the HindIII and BamHI restriction sites of the plasmid. The resulting pLew-PEPCK+ plasmid was introduced in the Δppdk/Δpepck mutant to produce the Δppdk/Δpepck/PEPCK+ cell line (Δppdk::TetR-HYG/Δppdk::T7RNAPOL-NEO Δpepck::BSD/Δpepck::PAC PEPCK-BLE) and the pLew-PGKC+ plasmid was introduced in the EATRO1125.T7T, Δppdk/Δpepck, and RNAiPGKB cells to generate the PGKC+ (TetR-HYG T7RNAPOL-NEO PGKC-BLE), Δppdk/Δpepck/PGKC+ (Δppdk::TetR-HYG/Δppdk::T7RNAPOL-NEO Δpepck::BSD/Δpepck::PAC PGKC-BLE), and RNAiPGKB/PGKC+ (TetR-HYG T7RNAPOL-NEO RNAiPGKB-BSD PGKC-BLE) cell lines. Transfected cells were selected in SDM79 medium containing hygromycin B (25 μg/ml) and neomycin (10 μg/ml), in addition to 2.5 μg/ml of pheomycin (Δppdk/Δpepck/PGKC+, RNAiPGKB/PGKC+ and PGKC+), 10 μg/ml of blasticidin (Δppdk/Δpepck/PGKC+ and RNAiPGKB/PGKC+) and/or 1 μg/ml of puromycin (Δppdk/Δpepck/PGKC+).
Enzyme Assays
Threonine 3-dehydrogenase (EC 1.1.1.103) enzyme activity was adapted from Linstead et al. (34). Briefly, cells were washed in PBS, resuspended in hypotonic lysis buffer (5 mm Na2HPO4, 0.3 mm KH2PO4), and sonicated (5 s at 4 °C). Enzyme assay contained 0.1 mm NAD+, 200 mm Tris-HCl, pH 8.6, and 30 mm threonine. As a control, sonicated crude extracts of trypanosomes resuspended in the same hypotonic buffer were tested for the pyruvate dehydrogenase activity (35, 36).
Western Blot Analyses
Total protein extracts of wild-type or mutant procyclic or bloodstream forms of T. brucei (5 × 106 cells) were size-fractionated by SDS-PAGE (10%) or isoelectric focusing gel electrophoresis (Bio-Rad) and immunoblotted on Immobilon-P filters (Millipore) (37). Immunodetection was performed as described (37, 38) using as primary antibodies rabbit anti-PGK (diluted 1:500; gift from P. Michels, Edinburgh, UK, and M. Parsons, Seattle, WA) (39, 40), rat anti-PEPCK (diluted 1:1000; gift from T. Seebeck, Bern, Switzerland) (22), rabbit anti-PPDK (diluted 1:500) (5), rabbit anti-TDH (diluted 1:500) (11), rabbit anti-GPDH (glycerol-3-phosphate dehydrogenase, EC 1.1.1.8; diluted 1:100) (41), mouse anti-hsp60 (diluted 1:10,000) (42), and mouse anti-AMP-dependent acetyl-CoA synthetase (EC 6.2.1.1; diluted 1:100) (43) and as secondary antibodies, anti-mouse, anti-rat, or anti-rabbit IgG conjugated to horseradish peroxidase (Bio-Rad, 1:5,000 dilution). Revelation was performed using the SuperSignal® West Pico Chemiluminescent Substrate as described by the manufacturer (Thermo Scientific). Alternatively, for quantitative analyses, revelation was performed using the LuminataTM Crescendo Western HRP Substrate (Millipore). Images were acquired and analyzed with a KODAK Image Station 4000MM and quantitative analyses were performed with the KODAK MI application.
Digitonin Permeabilization
Trypanosomes were washed two times in cold PBS and resuspended at 6.5 × 108 cells/ml (corresponding to 3.3 mg of protein/ml) in STE buffer (250 mm sucrose, 25 mm Tris, pH 7.4, 1 mm EDTA) supplemented with 150 mm NaCl and the CompleteTM Mini EDTA-free protease inhibitor mixture (Roche Applied Bioscience). Cell aliquots (200 μl) were incubated with increasing quantities of digitonin (Sigma) for 4 min at 25 °C, before centrifugation at 14,000 × g for 2 min to collect the cellular pellet.
Immunofluorescence Analyses
Log phase cells were fixed with formaldehyde as described before (5). Slides were incubated with rabbit anti-PGK (diluted 1:800) (39) and H112 monoclonal antibodies anti-PPDK (undiluted) (5) followed by Alexa Fluor® 594-conjugated goat anti-mouse secondary antibody (diluted 1:100) and/or Alexa Fluor® 488-conjugated goat anti-rabbit secondary antibody (diluted 1:100) (Molecular Probes). Cells were viewed with a Leica DM5500B microscope and images were captured by an ORCA®-R2 camera (Hamamatsu) and Leica MM AF Imaging System software (MetaMorph®) and merged in Adobe Photoshop on a Macintosh iMac computer.
Determination of Glucose and Proline Consumption
To determine the rate of glucose consumption, procyclic cells (inoculated at 107 cells/ml) were grown in 10 ml of SDM79 medium containing 2.5 mm glucose and 6 mm proline. To determine the rate of proline consumption, the same incubations were done in SDM79 medium containing 10 mm glucose and 6 mm proline. Aliquots of each growth medium (500 μl) were collected periodically during the 24-h incubation at 27 °C. The quantity of glucose present in the medium was determined using the “Glucose GOD-PAP” kit (Biolabo SA). Proline concentration was determined with a colorimetric assay as previously described (44) after deproteinization of the samples by perchloric acid treatment. The amounts of glucose and proline consumed at a given time of incubation (Tx) was calculated by subtracting the remaining amounts in the spent medium at Tx from the initial amounts at T0. Then, the rate of glucose/proline consumed per hour was calculated from the equation of the linear curve deduced from plotting glucose consumption as a function of time of incubation. Finally, to express the consumption rate per hour and per mg of protein, the mean of the total cellular proteins measured at the beginning (T0) and end (T24) of the incubation was considered to minimize possible growth rate differences. Importantly, we considered only experiments with 100% of alive and motile cells at the end of the 24-h incubation.
Determination of Intracellular ATP Concentrations
The intracellular ATP concentrations were determined on established procyclic cells in mid-log growth phase. Cell pellets (1–2 × 108 procyclic cells) were washed in cold phosphate-buffered saline (PBS) and frozen in liquid nitrogen. Lysis and deproteinization of the cellular pellets involved homogenization in 500 μl of cold perchloric acid (0.9 m) and neutralization, pH 6.5, by addition of KOH/MOPS (2/0.5 m). Then ATP concentrations were determined with the firefly luciferase bioluminescence assay (“ATPlite 1 step Luminescence Assay,” PerkinElmer Life Sciences) (45).
NMR Analysis of Excreted End Products from Glucose and Threonine Metabolism
For 13C NMR analyses of glucose metabolism, 5–8 × 108 T. brucei procyclic cells were collected by centrifugation at 1,400 × g for 10 min, washed once with PBS, and incubated in 30 ml of PBS. The cells were maintained for 6 h at 27 °C in incubation buffer containing 110 mmol of d-[1-13C]glucose and 2 g/liter of NaHCO3, pH 7.4. The supernatant was lyophilized and 13C NMR spectra were collected at 125.77 MHz with a Bruker DPX500 spectrometer, as described (19). For 1H NMR analyses of glucose and threonine metabolism, 108 T. brucei procyclic cells were collected by centrifugation at 1,400 × g for 10 min, washed once with PBS, and incubated for 6 h at 27 °C in 5 ml of incubation buffer (PBS supplemented with 5 g/liter of NaHCO3, pH 7.4) containing d-[U-13C]glucose (4 mm) and threonine (4 mm). 50 μl of maleate (20 mm) were added as an internal reference to a 500-μl aliquot of the collected supernatant, and 1H NMR spectra were performed at 500.13 MHz on a Bruker DPX500 spectrometer equipped with a 5-mm broadband probe head as described before (11, 46). Acquisition conditions were as follows: 90° flip angle, 5,000 Hz spectral width, 32 K memory size, and 9.3-s total recycle time. Measurements were performed with 256 scans for a total time close to 40 min. Protons linked to acetate carbon C2 generate by 1H NMR five resonances, a single peak ([12C]acetate) flanked by two doublets ([13C]acetate). In both NMR analyses, the integrity of the cells during the incubation was checked by microscopic observation. Measurements were recorded at 25 °C with an ERETIC method, which provides an electronically synthesized reference signal (47). Before each experiment, phase of ERETIC peak was precisely adjusted and, after acquisition, resonances of obtained spectra were integrated and results were expressed relative to ERETIC peak integration.
RESULTS
PPDK Gene Knock-out Affects Acetate Production from Glucose Metabolism
The PPDK null mutant (Δppdk) was previously generated in the wild-type procyclic trypanosomes by replacing both PPDK alleles by neomycin (NEO) and hygromycin (HYG) markers, together with the T7 RNA polymerase (T7RNAPOL) and the tetracycline repressor (TetR) under control of the T7RNAPOL promoter, respectively (10). Western blot analyses confirmed the absence of PPDK expression in the Δppdk mutant (Fig. 2A). Doubling times of Δppdk and wild-type cells are identical (Fig. 2B), however, glucose and proline metabolism of the mutant grown in SDM79 medium is affected (Fig. 3). Indeed the rate of glucose consumption is reduced by 22% in the Δppdk cell line, which is compensated by a 35% increase of the rate of proline consumption compared with wild-type cells (Student's t test values <0.05). Induction of proline metabolism caused by reduction or abolition of the glycolytic flux was previously described in procyclic trypanosomes (13, 14, 22). Quantitative analyses of 13C-enriched end products excreted from [1-13C]glucose metabolism were performed by 13C NMR. When the wild-type parasite was incubated in PBS/NaHCO3 containing 4 mm [1-13C]glucose, a total of 743 nmol of 13C-labeled metabolites/h/mg of protein were recovered in the supernatant, with most of the label being detected in succinate (63.3%) and acetate (25%), and small amounts of malate, lactate, fumarate, and alanine are also detected (Table 1). We observed that the rate of acetate production decreased in the Δppdk mutant (19.9% of the excreted end products), whereas this value was increased for succinate (70.6% of the excreted end products) (Table 1).
FIGURE 2.
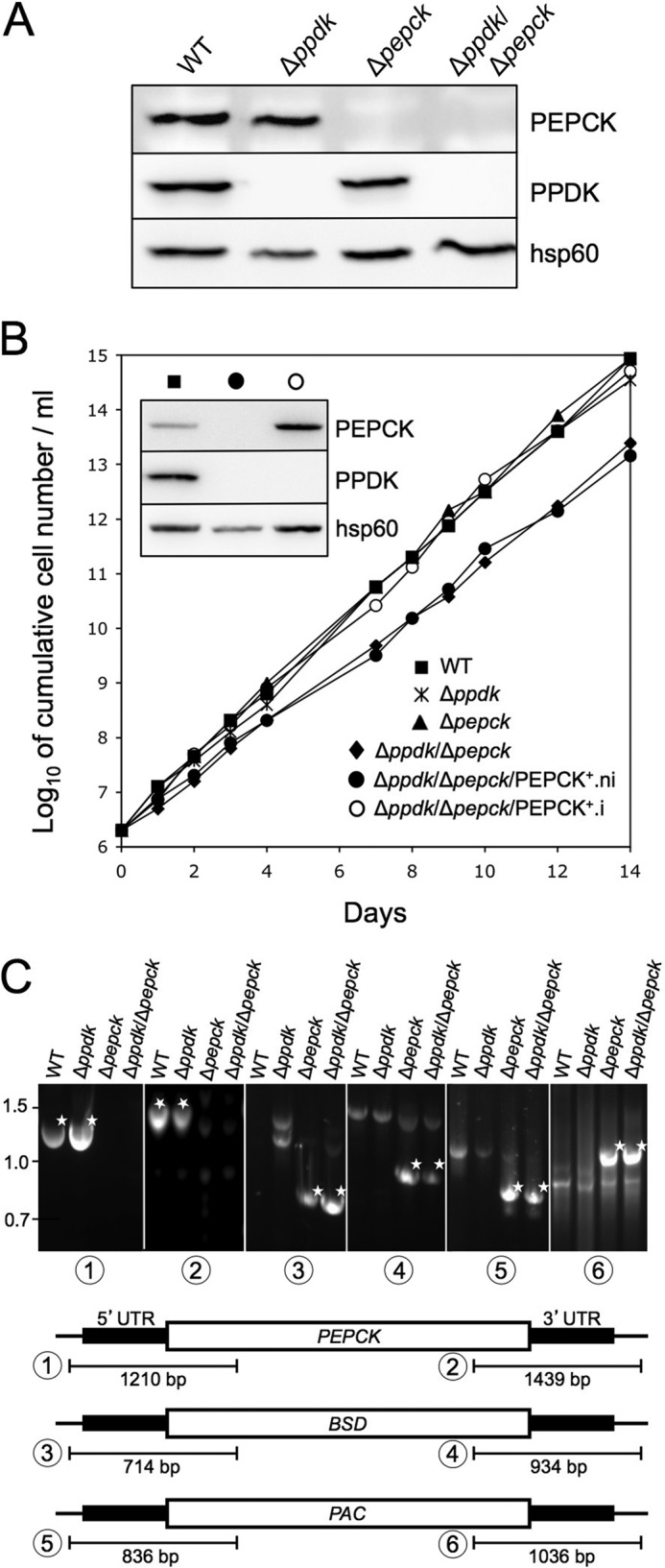
Production of Δppdk, Δppdk/Δpepck, and rescue cell lines. Panel A shows a Western blot analysis of the parental procyclic (WT) and mutant cell lines with the immune sera indicated in the right margin. Panel B shows growth curves of the wild-type (WT), Δppdk, Δpepck, Δppdk/Δpepck, and tetracycline-induced (.i) and uninduced (.ni) Δppdk/Δpepck/PEPCK+ cell lines, as well as a Western blot analysis of the wild-type (■), Δppdk/Δpepck/PEPCK+.ni (●), and Δppdk/Δpepck/PEPCK+.i (○) cell lines with the immune sera indicated in the right margin of the inset. Panel C shows a PCR analysis of genomic DNA isolated from the parental wild-type and Δppdk, Δpepck, and ΔppdkΔpepck cell lines. Amplifications were performed with primers based on sequences that flank the 5′ UTR and 3′ UTR fragments used to target the PEPCK gene depletion (black boxes) and internal sequences of the blasticidin (BSD, PCR products 3 and 4), puromycin (PAC, PCR products 5 and 6) resistance genes and, as controls, the PEPCK gene (products 1 and 2). As expected, PCR amplification of the PEPCK gene was only observed in wild-type and Δppdk cell lines, whereas BSD and PAC PCR products were observed only in Δpepck and ΔppdkΔpepck cell lines. White stars indicate the expected PCR fragment.
FIGURE 3.
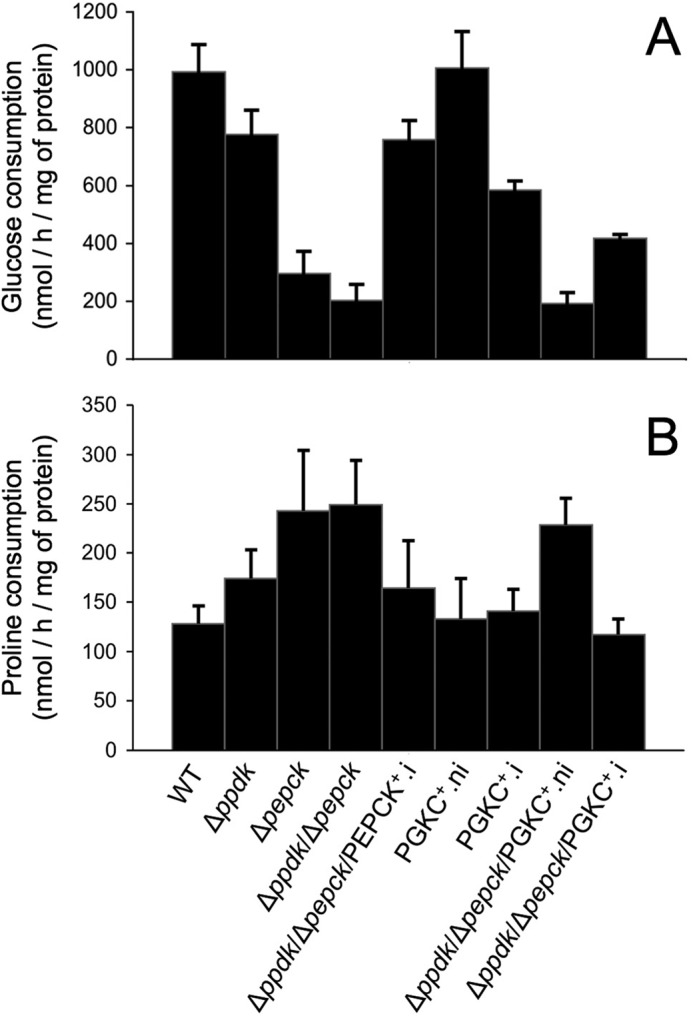
Glucose and proline consumption by wild-type (WT) and mutant cell lines. The rate of glucose (panel A) and proline (panel B) consumed by the indicated cell lines incubated in SDM79 medium is expressed as nanomole consumed per h and per mg of protein. Mean of at least 3 biological replicates are presented.
TABLE 1.
Excreted end products of glucose metabolism by procyclic T. brucei cell lines
The spent PBS medium of trypanosome cell lines incubated in the presence of 4 mm [1-13C]glucose, was analyzed by 13C NMR spectrometry to detect and quantify 13C-enriched excreted end products.
| Cell linea | nb |
13C-enriched excreted molecules |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Succinate | Acetate | Lactate | Malate | Fumarate | Alanine | β-hydroxybutyrate | Pyruvate | Glycerol | Total | ||
| nmol/h/mg of protein | |||||||||||
| WT (EATRO1125.T7T)c | 5 | 470 ± 88 | 186 ± 21 | 55.0 ± 15 | 20.0 ± 1.7 | 4.0 ± 1.7 | 8.0 ± 2.1 | NDd | ND | <2e | 743 ± 124 |
| Δppdk | 3 | 500 ± 61 | 141 ± 30 | 45.1 ± 10 | 13.7 ± 6.4 | 2.2 ± 1.1 | 6.2 ± 3.9 | ND | ND | <2 | 708 ± 53 |
| Δpepcke | 3 | 1.2 ± 1.4 | 154 ± 54 | 18.0 ± 3.7 | ND | ND | 14.0 ± 8.7 | 8.5 ± 4.4 | ND | 6.3 ± 4.6 | 208 ± 58 |
| Δppdk/Δpepck | 3 | ND | 40 ± 6.5 | 7.0 ± 1.0 | ND | ND | 9.3 ± 2.6 | ND | ND | 17.4 ± 3.7 | 74 ± 4 |
| Δppdk/Δpepck/PEPCK+.i | 2 | 493 | 168 | 15.8 | 26.6 | 5.0 | 1.4 | ND | ND | <2 | 712 |
| PGKC+.ni | 1 | 353 | 151 | 11.6 | 30.5 | 5.3 | 2.4 | ND | ND | <2 | 554 |
| PGKC+.i (1 day) | 1 | 210 | 141 | 38.5 | 11.5 | 1.5 | 2.4 | 2.9 | 2.0 | <2 | 414 |
| PGKC+.i (2 days) | 3 | 91 ± 31 | 118 ± 33 | 39.8 ± 9.2 | 4.2 ± 3.3 | ND | 3.4 ± 1.2 | 2.2 ± 0.8 | 1.7 ± 3.0 | <2 | 261 ± 68 |
| Δppdk/Δpepck/PGKC+.i (3 days) | 3 | 2.9 ± 0.5 | 181 ± 14 | 108.1 ± 9.6 | ND | ND | 7.8 ± 1.1 | 9.2 ± 1.6 | ND | 16.4 ± 2.0 | 325 ± 28 |
a .i: RNAi cell lines tetracycline-induced during 1 to 3 days depending on the cell line and the experiments; .ni: non-induced RNAi cell lines.
b Number of biological replicates.
c From Ebikeme et al. (22).
d Non detectable.
e Since the glycerol peak is embedded in the acylglycerol massif, the detection limit of glycerol was considered as 2 nmol/h/mg of protein.
To confirm the role of PPDK in acetate production, we compared threonine and glucose contributions to acetate production in wild-type and Δppdk cell lines. Indeed, a recently developed metabolite profiling assay showed that threonine is the main acetate source of procyclic trypanosomes (11, 46). This approach is based on the ability of 1H NMR spectrometry to distinguish 13C-enriched from 12C molecules, such as [13C]acetate and [12C]acetate derived from uniformly 13C-enriched [U-13C]glucose (4 mm) and unenriched threonine (4 mm), respectively. [13C]Acetate molecules derived from [U-13C]glucose (annotated A13 in Fig. 4A) are represented by two doublets, with chemical shifts at around 2.0 and 1.75 ppm, respectively, whereas the central resonance (1.88 ppm) corresponds to threonine-derived [12C]acetate (annotated A12 in Fig. 4A). The rate of acetate production from glucose is reduced by 30% in Δppdk compared with wild-type cells (Student's t test values <0.05), whereas acetate production from threonine remains in the same range (Table 2 and Fig. 4A). It is noteworthy that PPDK contribution to acetate production may be underestimated, because pyruvate can also be generated from malate produced in the glycosomes by the action of the cytosolic and mitochondrial malic enzymes (steps not indicated in Fig. 1A) (48). In the absence of PPDK, contribution of this pathway, as well as pyruvate kinase (step 18), to acetate production could increase. Altogether, these data show that PPDK contributes to acetate production from glucose in wild-type cells, implying that the enzyme function in the glycolytic direction (see Fig. 1A). In this context, the role of PPDK is probably to participate in the maintenance of the glycosomal ATP/ADP balance, as proposed before (6, 7).
FIGURE 4.
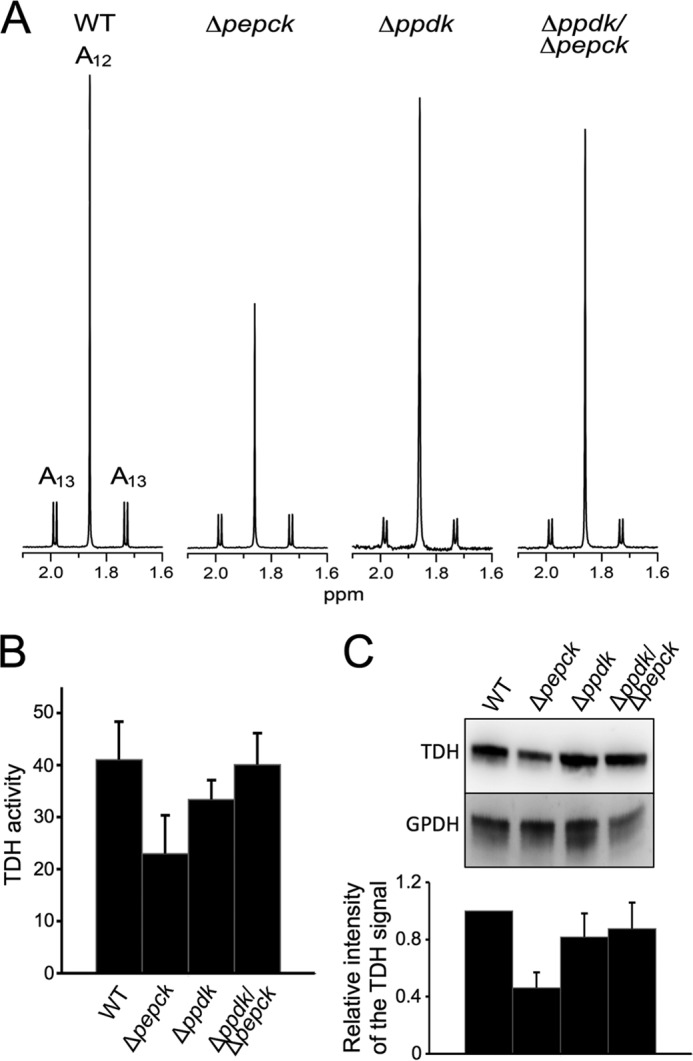
TDH expression and activity are not reduced in the Δppdk/Δpepck cell line. In panel A, contribution of threonine and glucose to acetate production was determined by 1H NMR analysis. Acetate excreted by the procyclic wild-type (WT), Δppdk, Δpepck, and Δppdk/Δpepck cell lines from 4 mm d-[U-13C]glucose and 4 mm threonine was determined by 1H NMR. Each spectrum corresponds to one representative experiment from a set of at least 3. A part of each spectrum ranging from 1.6 to 2.1 ppm is shown. The resonances were assigned as indicated: A12, threonine-derived acetate; A13, 13C-enriched glucose-derived acetate. Panel B shows the TDH activity (milliunits/mg of protein), normalized with the pyruvate dehydrogenase activity measured in the same samples. In panel C, expression of TDH and glycerol-3-phosphate dehydrogenase (GPDH) was analyzed by Western blotting with specific immune sera. The ratio between the TDH and GPDH signals, indicated below the blot, represents a mean ± S.D. of 4 different experimental duplicates, with an arbitrary value of 1 for the parental cells (WT).
TABLE 2.
Excreted acetate from glucose and threonine metabolism by procyclic T. brucei cell lines
The spent PBS medium of trypanosome cell lines incubated in the presence of 4 mm threonine and 4 mm [U-13C]glucose was analyzed by 1H NMR spectrometry to detect and quantify excreted acetate.
| Cell lines | na | Acetate production from threonine | Acetate production from glucose | Student's t test (compared with WT)b |
|---|---|---|---|---|
| nmol/h/mg protein | ||||
| WT | 38 | 3155 ± 561 | 1240 ± 250 | / |
| Δpepck | 11 | 1714 ± 215 | 1030 ± 136 | 0.01042 |
| Δppdk | 3 | 3074 ± 316 | 863 ± 39 | 0.01370 |
| Δppdk/Δpepck | 6 | 2631 ± 295 | 836 ± 123 | 0.00038 |
a Number of biological replicates.
b Statistical significance for the difference in the acetate production levels from glucose between the EATRO1125.T7T (WT) cells and the other cell lines was determined using the Student's t test (bilateral and equal variance parameters). Statistical differences are significant for values <0.05.
Glucose Metabolism Is Strongly Impaired in the Δppdk/Δpepck Mutant
According to the current model, PEPCK and PPDK are the main glycosomal kinase candidates involved in the maintenance of the glycosomal ATP/ADP balance of the procyclic trypanosomes (Fig. 1A), however, this has not been experimentally investigated so far (7, 49). To address this question, we generated a double Δppdk/Δpepck knock-out mutant by deleting the PEPCK gene in the PPDK null background (Δppdk). Both PEPCK alleles were replaced by puromycin (PAC) and blasticidin (BSD) markers to generate the Δppdk/Δpepck cell line. Deletion of both PEPCK alleles in the PPDK null background was confirmed by Western blot (Fig. 2A) and PCR analyses (Fig. 2C). The resulting Δppdk/Δpepck cell line is viable although it shows an increased doubling time (14.5 h) compared with the wild-type (11.9 h), Δppdk (12 h), and Δpepck (11.8 h) cell lines (Fig. 2B). A conditional re-expression of an ectopic copy of the PEPCK gene in the PPDK/PEPCK null background was performed to produce the Δppdk/Δpepck/PEPCK+ cell line. Addition of tetracycline restored the wild-type growth phenotype of the Δppdk/Δpepck/PEPCK+.i mutant (.i and .ni stand for tetracycline-induced and uninduced, respectively) (Fig. 2B) upon re-expression of PEPCK as shown by Western blot analysis (Fig. 2B, inset).
To determine the effect of PPDK and/or PEPCK gene depletion on the glycosomal metabolism, the rate of glucose and proline consumption was measured for all these cell lines grown in SDM79 medium (Fig. 3). As previously observed, the Δpepck mutant showed a strong reduction of glucose consumption (70%) compensated by a 90% increase in proline consumption (22). The Δppdk/Δpepck mutant still consumes glucose, although with a moderately reduced rate compared with the Δpepck mutant (1.5-fold), whereas the rate of proline consumption is not affected. The absence of compensation toward proline metabolism may explain the reduced growth rate of the Δppdk/Δpepck cell line. As expected, re-expression of PEPCK in the Δppdk/Δpepck cell line (Δppdk/Δpepck/PEPCK+.i) restored the rate of glucose and proline consumption observed in the Δppdk cell line (Fig. 3). To confirm these data, a 13C NMR quantitative analysis of 13C-enriched end products excreted from [1-13C]glucose metabolism was performed. Similar results have been obtained for the Δppdk and Δppdk/Δpepck/PEPCK+.i cell lines (Table 1). In contrast, succinate production is abolished in the Δpepck mutant, whereas acetate production is not affected, with a 3.6-fold reduction of end product excretion from the glucose metabolism (Table 1) (22). Interestingly, the Δppdk/Δpepck mutant excreted 2.8-fold less end products from glucose metabolism compared with the Δpepck mutant (Table 1), suggesting that glycolysis of the double mutant is strongly impaired when glucose is the only carbon source. In other words, the PPDK activity appears critical to maintain glycolytic activity in the PEPCK null background.
Comparison of Glucose and Threonine Metabolism in the Δpepck and Δppdk/Δpepck Mutants
To determine why glycolysis is more affected in the Δppdk/Δpepck double mutant than in the Δpepck single mutant, we compared acetate production from glucose and threonine in both cell lines. We previously observed that deletion of the PEPCK gene induced a down-regulation of expression of the TDH gene and activity, leading to a reduction of threonine contribution to acetate production (Fig. 4) (11). We have interpreted this TDH down-regulation as a consequence of an increased metabolic flux from glucose metabolism toward the acetate branch, due to abolition of the succinate branch in the Δpepck mutant (see Fig. 1B). In addition, the Δpepck mutant, but not the wild-type cells, excretes detectable amounts of β-hydroxybutyrate from glucose metabolism, which was probably derived from accumulation of acetyl-CoA (22) (Fig. 5 and Table 1). Altogether, these data suggest that redirection of the glycolytic flux toward acetate production induces accumulation of acetyl-CoA, which directly or indirectly induces down-regulation of TDH expression to reduce contribution of threonine degradation to acetyl-CoA production (see Fig. 1B). This is probably due to the limited capacity of acetate production, as previously proposed (50). The same analyses revealed that the Δppdk/Δpepck cell line behaves differently. First, analysis of [1-13C]glucose metabolism, as the only carbon source, showed no evidence of β-hydroxybutyrate production (Fig. 5). Second, TDH activities are in the same range in Δppdk/Δpepck and wild-type cells (40.1 and 41 milliunits/mg of proteins, respectively), ∼2-fold higher compared with the Δpepck mutant (Fig. 4B). This is in agreement with Western blot analyses of TDH expression (Fig. 4C). Third, to confirm the absence of TDH down-regulation in the Δppdk/Δpepck mutant, the contribution of threonine and glucose metabolism to acetate production was compared by 1H NMR spectrometry. The wild-type procyclic cells produce 2.5-fold more acetate from threonine than from glucose, whereas this ratio is 1.7 for the Δpepck mutants (Fig. 4A and Table 2). As previously reported, decreased threonine contribution in acetate production in the Δpepck cell line is correlated with down-regulation of TDH (11). Interestingly, high threonine contribution to acetate production is restored in Δppdk/Δpepck cells, which correlates with the wild-type expression level of TDH (Fig. 4A and Table 2).
FIGURE 5.
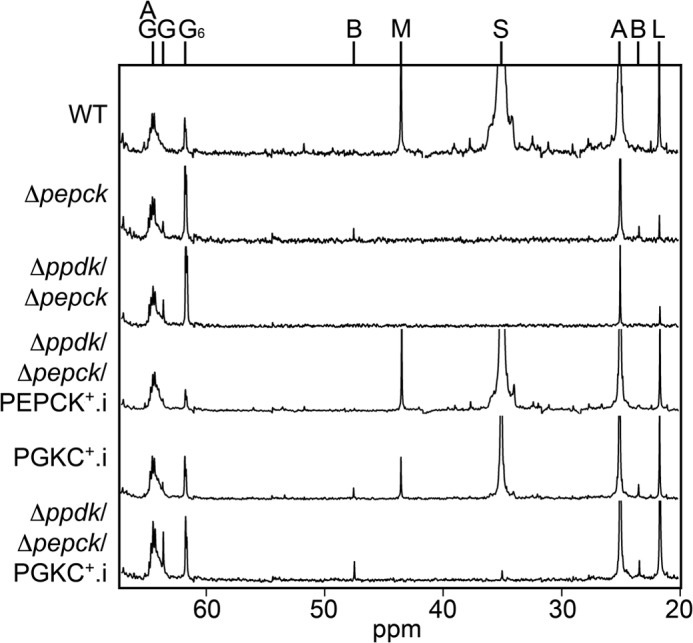
Analysis of β-hydroxybutyrate and glycerol production from glucose. Supernatant of wild-type (WT) and mutant cell lines incubated with 4 mm [1-13C]glucose were analyzed by 13C NMR. The resonances were assigned as follows: A, acetate; B, β-hydroxybutyrate; G, glycerol; G6, carbon C6 of glucose; L, lactate; M, malate; AG, acylglycerol; S, succinate.
Altogether these data suggest that the reasons for glycolytic down-regulation differ in the Δpepck and Δppdk/Δpepck cell lines. As mentioned above, deletion of the PEPCK gene induces a redistribution of the metabolic flux toward the acetate branch, with as a consequence accumulation of intermediary metabolites (such as β-hydroxybutyrate), reduction of the glycolytic flux, and down-regulation of the TDH activity. In the Δppdk/Δpepck mutant, TDH activity remains high and production of β-hydroxybutyrate from glucose is not detectable, suggesting that the acetate branch is not overloaded. Consequently, the reason for glycolysis down-regulation may reside inside the glycosomes, such as the imbalanced ATP/ADP ratio. This hypothesis is strengthened by the important increase of glycerol production in the Δppdk/Δpepck mutant. Indeed, 13C NMR analysis of the [1-13C]glucose metabolism showed that [13C]glycerol represents 23 and 3% of the 13C-enriched end products excreted from [1-13C]glucose in the Δppdk/Δpepck and Δpepck mutants, respectively, whereas it is not detectable in wild-type cells (Table 1 and Fig. 5). Increase of glycerol production in the Δppdk/Δpepck mutant by the ATP generating glycerol kinase is certainly induced to compensate for the simultaneous loss of PPDK and PEPCK kinase activities (see Fig. 1C), which strongly supports the role of PPDK in intraglycosomal ATP production.
Expression of the Glycosomal rPGKC Is Lethal for the Procyclic Trypanosomes
To restore glycosomal ATP production, the recombinant glycosomal phosphoglycerate kinase (rPGKC), which has never been detected in procyclic cells, was conditionally overexpressed in the Δppdk/Δpepck mutant, as well as in wild-type cells as a control. To our surprise, growth of PGKC+.i and Δppdk/Δpepck/PGKC+.i cells stopped after 2 days of tetracycline induction (Fig. 6A). Western blot analyses with anti-PGK immune sera, which recognize all of the trypanosomal PGK isoforms, showed an increase of PGK expression in PGKC+.i cells, compared with PGKC+.ni and wild-type cells (Fig. 6B). The PGK activity measured in these cell lines is in agreement with Western blot analysis, i.e. 88.6 ± 9.3, 92.8 ± 2.5, and 130.3 ± 14.3 milliunits/mg of proteins in the wild-type, PGKC+.ni, and PGKC+.i cells, respectively. Because PGKB, the PGK isoform expressed in the cytosol of procyclic trypanosomes, and PGKC have the same apparent electrophoretic mobility in SDS-PAGE, but different pKi (PGKC, 9.25; PGKB, 7.11), both proteins have been separated by isoelectric focusing gel electrophoresis. As expected, the bloodstream and procyclic forms of T. brucei total extracts contain a single PGK band with a high (PGKC) and low (PGKB) pKi, respectively (Fig. 6C). The PGKC+.i cell line expresses similar amounts of both PGK isoforms, whereas the recombinant PGKC isoform (rPGKC) is not detectable in the PGKC+.ni cells (Fig. 6C).
FIGURE 6.
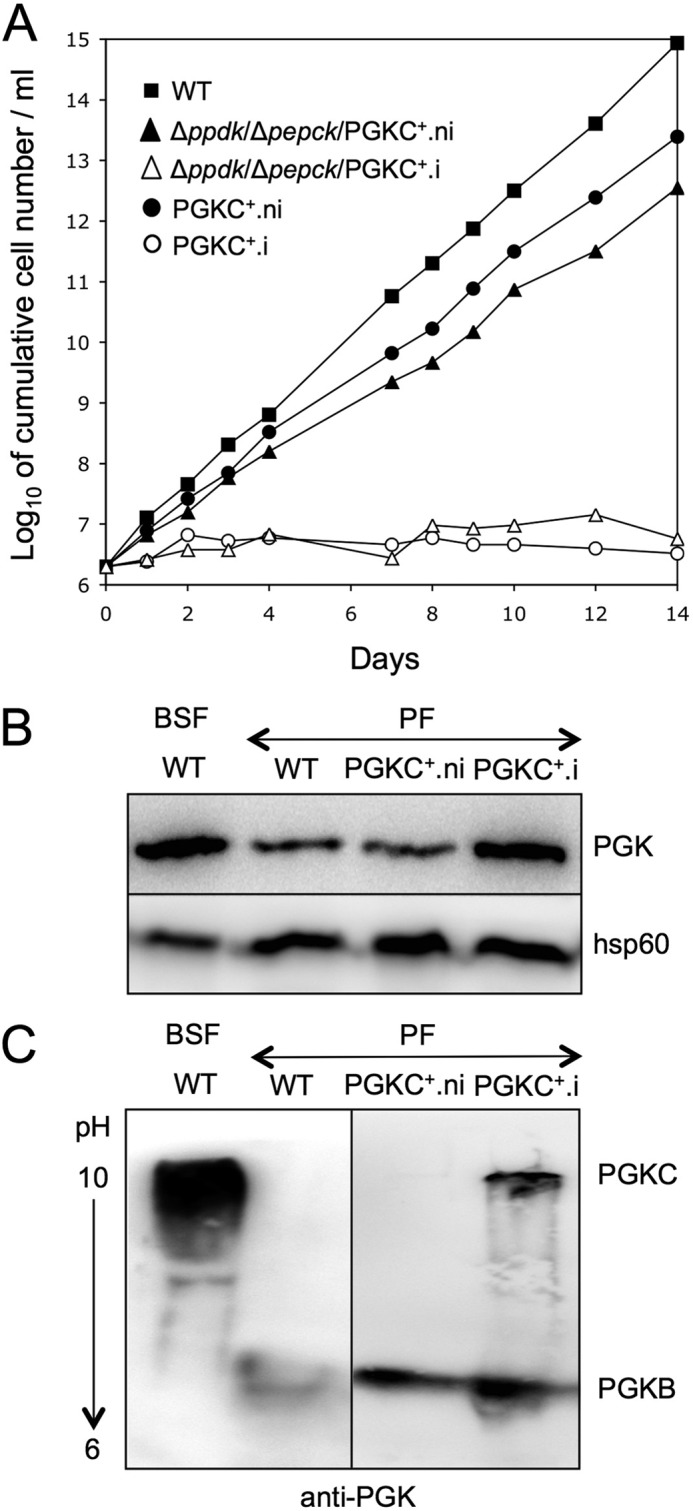
Expression of PGKC in procyclic (PF) and bloodstream (BSF) forms of T. brucei. The growth curves of the parental (WT) and tetracycline-induced (.i) and uninduced (.ni) mutant procyclic cell lines expressing rPGKC in the wild-type or Δppdk/Δpepck backgrounds is shown in panel A. Expression of the PGK isoforms was analyzed by Western blot on total cellular protein extracts separated by SDS-PAGE (panel B) or isoelectric focusing gel electrophoresis (panel C). The anti-hps60 immune serum was used as a loading control.
Because of the presence of high amounts of PGKB in the cytosol of the procyclic cells, we determined the cellular localization of rPGKC in procyclic cells down-regulated for expression of PGKB by RNAi (RNAiPGKB/PGKC+.i cell line). Down-regulation of PGKB to a very low level of expression (Fig. 7B) does not affect growth of the RNAiPGKB.i parasites (Fig. 7A), whereas the RNAiPGKB/PGKC+.i mutant declined 1 day post-induction and died within the next 2 weeks (Fig. 7). Immunofluorescence analyses of the RNAiPGKB/PGKC+.i cell line with the anti-PGK antibodies showed a punctuate glycosomal-like pattern overlapping with the anti-PPDK glycosomal marker, whereas wild-type cells showed a diffuse cytosolic-like pattern (Fig. 7C). The glycosomal localization of rPGKC was confirmed by a cellular fractionation experiment wherein the different membranes of the procyclic trypanosomes were differentially permeabilized by increasing concentrations of the detergent digitonin. A Western blot analysis of the pellet fractions indicates that the PGK isoform detected in the wild-type parasites (PGKB) is released together with the cytosolic marker, acetyl-CoA synthetase (43), at 40 μg of digitonin/mg of protein, whereas the PPDK glycosomal marker is released at much higher digitonin concentrations (300 μg of digitonin/mg of protein) (Fig. 7D, upper panel). However, the PGK isoform expressed in the RNAiPGKB/PGKC+.i cell line is released together with PPDK, confirming that rPGKC is expressed in the glycosomes (Fig. 7D, lower panel).
FIGURE 7.
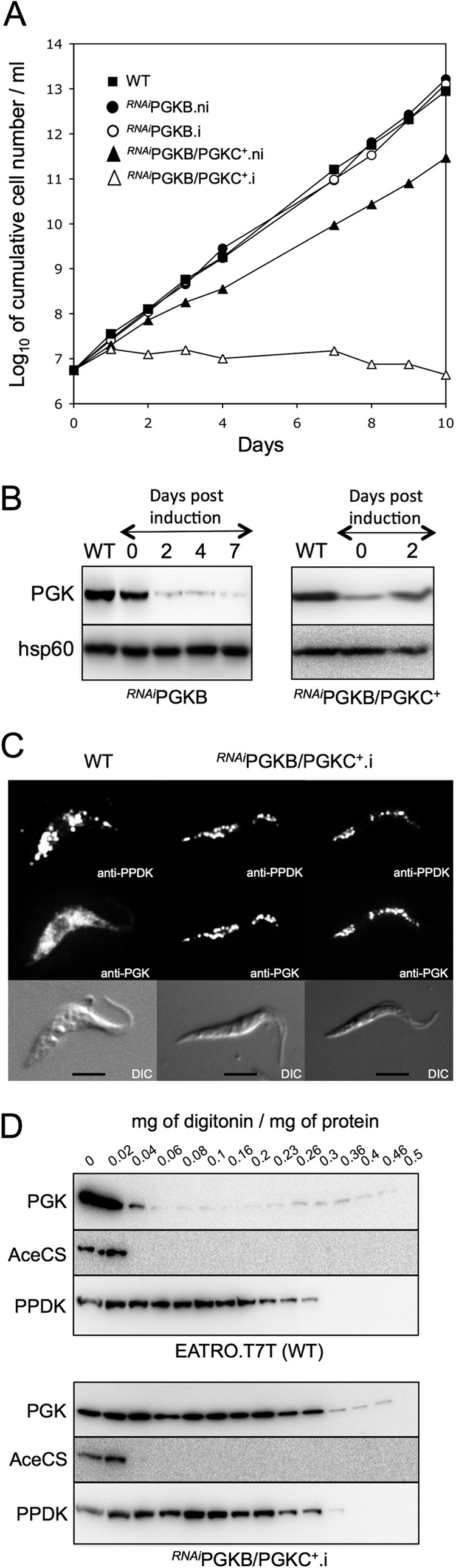
Subcellular localization of rPGKC. Panel A shows growth curves of the parental procyclic cells (WT) and tetracycline-induced (.i) and uninduced (.ni) RNAiPGKB and RNAiPGKB/PGKC+ cell lines. Expression of PGK isoforms upon induction in the RNAiPGKB and RNAiPGKB/PGKC+ mutants is shown by Western blotting analyses in panel B, with hsp60 immune serum serving as internal control. Panel C shows immunofluorescence analyses of procyclic cells stained with the rabbit anti-PGK (Alexa Fluor 488 channel green) and the monoclonal mouse anti-PPDK (Alexa Fluor 594 channel red) as glycosomal control. Differential interference contrast of cells is shown at the bottom of each panel. Scale bar, 5 μm. In panel D, the presence of the PGK isoforms, the cytosolic acetyl-CoA synthetase (AceCS, cytosolic marker), and PPDK (glycosomal marker), in the pellet fractions from wild-type (upper panel) and RNAiPGKB/PGKC+.i (lower panel) cells incubated with 0.02–0.5 mg of digitonin/mg of protein in STE buffer containing 150 mm NaCl was determined by Western blot analyses.
Expression of rPGKC Affects Succinate and ATP Production
To determine why expression of rPGKc is lethal for the procyclic trypanosomes, the fate of the carbon sources metabolism was analyzed 1 and 2 days post-induction, when the cells were still highly mobile. After 3 days post-induction, cell motility and viability were affected, with possible secondary effects on the intermediate and energy metabolism. The rate of glucose and proline consumption was measured for the PGKC+ cell line grown in the SDM79 medium (Fig. 3). Two days post-induction, the rate of glucose consumption is reduced by 42% with no increase of the proline metabolism to compensate for the reduced glycolysis. The reduced glycolytic flux was confirmed by the 25 and 53% reduction of glucose-derived excreted end products after 1 and 2 days of induction, respectively, compared with uninduced cells (Table 1). Interestingly, the rate of acetate production is only moderately affected (7 and 22% of reduction) compared with succinate (41 and 74% of reduction) after 1 and 2 days of induction, respectively (Table 1 and Fig. 8). The ∼4-fold reduction of succinate production, although acetate production is poorly affected, is probably related to the maintenance of the glycosomal ATP/ADP balance. Indeed, one may expect that, to prevent accumulation of ATP in the organelle, reduction of ATP production by PEPCK and PPDK should compensate for glycosomal ATP production by rPGKC, with as a consequence a reduced metabolic flux in the succinate branch (see Fig. 1D). Another consequence of rPGKC expression in the glycosomes of the procyclic cells is the reduced net production of ATP from glycolysis, due to reduction of PGKB contribution to cytosolic ATP production (see Fig. 1D). In agreement with this hypothesis, the intracellular ATP concentration was reduced by 38 and 51% after 1 and 2 days of rPGKc induction, respectively, compared with the wild-type cells (Table 3), although amounts of intracellular ATP remain high in the Δppdk, Δpepck, Δppdk/Δpepck, and RNAiPGKB.i cell lines. This strongly supports the interpretation that death of the PGKC+.i mutant is due to a great reduction of cytosolic ATP production, which is not compensated by an increase of mitochondrial ATP production, because the rate of proline consumption is not up-regulated in this mutant (Fig. 3B).
FIGURE 8.
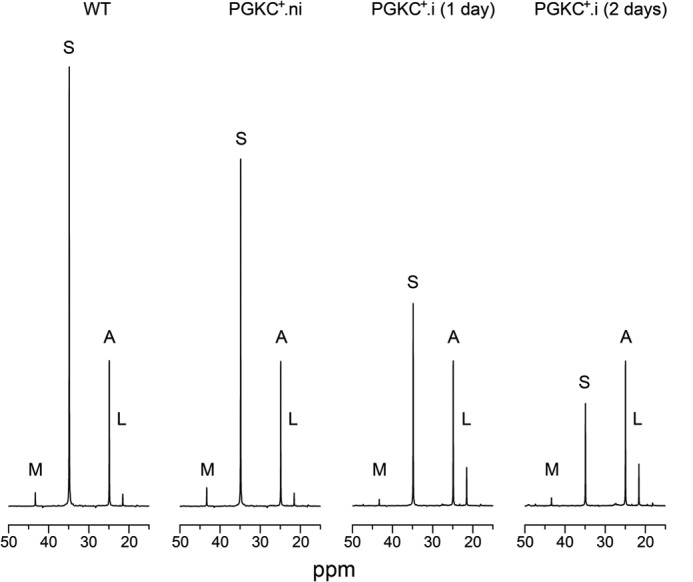
Carbon-13 NMR spectra of metabolic end products excreted by procyclic cell lines incubated with [1-13C]glucose. For these 13C NMR analyses, the parental EATRO1125.T7T (WT) and uninduced (.ni) and tetracycline-induced (.i, 1 and 2 days) PGKC+ mutant cell lines were incubated with 4 mm d-[1-13C]glucose in PBS/NaHCO3 buffer. The NMR spectra of the incubation medium were obtained after addition of 15 μl of dioxane, to quantify 13C-enriched excreted end products from glucose metabolism (Table 1). The size of the spectra is normalized on the acetate peaks. The resonances were assigned as follows: A, acetate; L, lactate; M, malate; S, succinate.
TABLE 3.
Intracellular ATP concentration
ATP concentrations (nmol/mg of protein) were determined on trypanosome extracts using the firefly luciferase bioluminescence assay.
| Cell lines | na | Nmol of ATP/mg of protein | Student's t test (compared with WT)b |
|---|---|---|---|
| WT | 18 | 4.10 ± 0.41 | |
| Δppdk | 12 | 3.53 ± 0.38 | 0.00009 |
| Δpepck | 12 | 3.98 ± 0.55 | 0.54389c |
| Δppdk/Δpepck | 13 | 3.55 ± 0.91 | 0.03776 |
| PGKC+.ni | 8 | 3.74 ± 1.32 | 0.33238c |
| PGKC+.i (1 day) | 5 | 2.55 ± 0.69 | 0.00001 |
| PGKC+.i (2 days) | 6 | 1.84 ± 0.30 | 5.56 10−11 |
| Δppdk/Δpepck/PGKC+.ni | 7 | 2.09 ± 0.56 | 2.53 10−9 |
| Δppdk/Δpepck/PGKC+.i (1 day) | 8 | 1.72 ± 0.24 | 2.29 10−13 |
| Δppdk/Δpepck/PGKC+.i (2 days) | 8 | 1.51 ± 0.19 | 1.95 10−14 |
| RNAiPGKB.ni | 8 | 4.07 ± 0.71 | 0.89509c |
| RNAiPGKB.i | 7 | 3.81 ± 0.58 | 0.19717c |
a Number of biological replicates.
b Statistical significance for the difference in the ATP levels between the EATRO1125.T7T (WT) cells and the other cell lines was determined using the student's t test (bilateral and equal variance parameters). Statistical differences are significant for values <0.05.
c Not significant.
Expression of rPGKC Restores Glycolysis in the Δppdk/Δpepck Mutant
The same analyses were conducted on the Δppdk/Δpepck/RNAiPGKC cell line. Two days post-induction, the rate of glucose consumption is 2.2-fold increased with a ∼2-fold decrease of proline consumption, compared with the uninduced cells (Fig. 3). The increased glycolytic flux was confirmed by 1H NMR analyses because the rate of 13C-enriched end product excretion from [1-13C]glucose metabolism, when glucose is the only carbon source, is 4.5-fold increased compared with the parental Δppdk/Δpepck cell line (Table 1). It is noteworthy that, in these incubation conditions, the rate of acetate production is fully restored in the Δppdk/Δpepck/RNAiPGKC.i mutant compared with wild-type cells (181 ± 14 versus 186 ± 21 nmol/h/mg of protein). Because the succinate branch is abolished in the Δppdk/Δpepck/RNAiPGKC.i cell line, restoration of the flux through the acetate branch suggests that the maximal glycolytic capacity in the PPDK/PEPCK null background has been restored by rPGKC expression. Consequently, providing additional ATP molecules in the glycosomes, through the rPGKC activity, restores glycolytic flux in the PPDK/PEPCK null background. This further confirms that the dramatic reduction of glycosomal ATP production is the main reason of glycolytic flux down-regulation in the Δppdk/Δpepck mutant.
The reduced rate of proline consumption is probably the consequence of the increased glycolytic flux, as we previously observed for wild-type cells (13, 14). However, as observed for the RNAiPGKC.i cell line, the intracellular ATP concentration was ∼2-fold reduced after 2 days of rPGKc induction compared with wild-type cells (Table 3). This was probably due, as proposed above for the RNAiPGKC.i mutant, to the glycosomal redistribution of PGK-dependent ATP production leading to cellular ATP depletion and cell death (see Fig. 1E). It is noteworthy that the leaky expression of rPGKC in the Δppdk/Δpepck/RNAiPGKC.ni cell line (data not shown) is probably responsible for the reduced intracellular ATP concentration (Table 3) and the reduced growth rate (Fig. 6A) of the uninduced cells.
DISCUSSION
According to the current model, the glycosomal ATP/ADP balance has to be maintained by glycosomal kinase activities to regenerate ATP molecules consumed in the upper part of glycolysis. These kinase activities are essential, because compartmentalization of glycolysis inside the glycosomes is required to prevent a lethal turbo-explosion of glycolysis in trypanosomes (51, 52). This function is ensured by PGKC in the bloodstream trypanosomes (53). In the procyclic trypanosomes, the succinate branch plays this role with ATP being produced by PEPCK, whereas the role of PPDK in ATP production was proposed but not experimentally addressed (6, 7). Here we show that in the absence of PEPCK, PPDK participates in the maintenance of glycolytic flux by providing ATP. The evidence comes from the reduction of glucose-derived acetate production in the Δppdk mutant, which can only be interpreted as a consequence of PPDK functioning in the glycolytic direction with production of ATP inside the glycosomes (see Fig. 1A). Comparison of Δppdk/Δpepck and Δpepck metabolism and restoration of glycosomal ATP production by expression of the glycosomal PGKC isoform in the Δppdk/Δpepck background further supports the role of PPDK in maintaining the ATP/ADP balance. We previously demonstrated that in the absence of PEPCK the reduced glycolytic flux results from abolition of the succinate branch associated with the limited capacity of the acetate branch (11, 50) (see Fig. 1B). When glucose is the only carbon source available, glycolytic flux is strongly affected in the Δppdk/Δpepck cell line, with a ∼3-fold reduction of acetate production, whereas glycerol production is ∼3-fold increased, compared with the Δpepck cell line. Consequently, the rates of glycerol and acetate production are in the same range in the double mutant (40 versus 17 nmol/h/mg of protein) (Table 1). These data are in agreement with (i) glycerol kinase activity substituting PPDK activity to produce ATP, in the PEPCK null background and (ii) PPDK being a more successful step, versus glycerol kinase, to balance the ATP/ADP ratio in the procyclic trypanosomes. The role of PPDK in glycosomal ATP production was confirmed by a functional rescue. Indeed, expression of the glycosomal PGKC isoform restored the glycolytic rate, with a 4.4-fold increase compared with the Δppdk/Δpepck cell line (Table 1).
Interestingly, PPDK is required to maintain a high glycolytic flux, as shown by the 22 and 24% reduction of the rate of glucose consumption in the Δppdk and Δppdk/Δpepck/PEPCK+.i cell lines, respectively. This may be due to intraglycosomal accumulation of PPi, which could affect glycolysis, because no pyrophosphatase activity has been detectable in glycosomal extracts of trypanosomes so far (6). This hypothesis is not supported by the recent discovery of glycosomal pores large enough for diffusion of PPi (49), which can be hydrolyzed by the cytosolic or acidocalcisomal pyrophosphatase activities (54, 55). Alternatively, the role of PPDK in the maintenance of a high glycolytic flux may be related in its PPi dependence. The PPi-dependent glycolytic enzymes, PPDK and phosphofructokinase (PPi-PFK), are expressed in a number of parasites to increase the energy efficiency of glycolysis. Indeed, the use of PPi as energy substrate increases the rate of ATP production from glucose 2.5-fold, assuming that PPi is a by-product of the biosynthetic reaction that is commonly wastefully hydrolyzed by pyrophosphatase activity (56). Thus, the trypanosome PPDK can theoretically produce 2 molecules of ATP/molecule of P-enolpyruvate consumed, if adenylate kinase (2 ADP → ATP + AMP) and the absence of a glycosomal pyrophosphatase activity are integrated in the equation. Indeed, in the presence of a glycosomal adenylate kinase activity, which was well described in trypanosomes (57), the PPDK reaction (P-enolpyruvate + AMP + PPi → ATP + Pi + pyruvate) can be written as follows: P-enolpyruvate + 2 ADP + PPi → 2 ATP + Pi + pyruvate. Considering that PPDK produces 2 times more ATP per P-enolpyruvate consumed than PEPCK, higher is the contribution of PPDK compared with PEPCK, lower is the need to metabolize P-enolpyruvate in the glycosomes to maintain the organellar ATP/ADP balance. This implies that the PPDK contribution favors a metabolic flux redistribution toward pyruvate kinase, with an associated increase of cytosolic ATP production (see Fig. 1A). We propose that the main role of PPDK in the procyclic trypanosomes grown in glucose-rich conditions is to increase the rate of intraglycosomal ATP production by taking advantage of the PPi high-energy bond produced by glycosomal biosynthetic pathways (49, 58), to increase the net production of ATP in the cytosol. Thus, the net yield of ATP production per molecule of glucose consumed may depend on the involvement of PPDK versus PEPCK.
Altogether, the reduced glycolytic flux combined with the reduced efficiency of glycosomal ATP production, in the absence of the PPDK gene, may induce a reduction of cytosolic ATP production, which is compensated by an increase of proline consumption (36 and 28% in the Δppdk and Δppdk/Δpepck/PEPCK+.i cell lines, respectively). Induction of proline metabolism has previously been observed in trypanosomes, in response to glucose depletion (13, 14) or reduction of the glycolytic flux caused by PEPCK gene deletion (22) or down-regulation of the glycosomal fumarate reductase (18, 19). Indeed, proline can substitute for glucose depletion to feed the central metabolism and produce ATP molecules. Proline is metabolized in the mitochondrion where it produces ATP by substrate-level phosphorylation (succinyl-CoA synthetase) and oxidative phosphorylation as a consequence of respiratory chain-mediated oxidation of proline-derived reducing equivalents (59).
Expression of the rPGKC in the PEPCK/PPDK null and wild-type backgrounds is lethal 2–3 days post-induction because of intracellular ATP depletion (Table 3). The PGK substrate (1,3-biphosphoglycerate) is synthesized in the glycosomes where it can be metabolized by rPGKC before reaching the cytosol where PGKB is located. Thus, we propose that relocation of the PGK activity in the glycosomes leads to ATP deprivation in the cytosol, which is not compensated by an increase of the rate of proline consumption to increase ATP production in the mitochondrion. The absence of a switch toward proline metabolism to increase ATP production is probably related to the remaining high glycolytic flux in PGKC+.i and Δppdk/Δpepck/PGKC+.i cell lines. Indeed, expression of PGKC does not have a direct effect on the wild-type glycolytic flux, because its expression in the PEPCK/PPDK null background stimulates glycolysis. The observed 1.7-fold reduction of glycolytic flux in the PGKC+.i mutant, although the cells start to decline after 2 days of induction, is probably the consequence of ATP deprivation (see Table 3). This strongly supports the view that a high glycolytic flux prevents a switch toward proline metabolism, even if ATP production from glycolysis is impaired. The same phenotype was also reported before for the pyruvate kinase mutant (step 13 in Fig. 1A) (10). This implies that intracellular amounts of ATP are not the driven force leading to metabolic switch to proline metabolism, observed in the absence of glucose or in mutants showing a reduced glycolytic flux.
Manipulating PGK expression is also detrimental for bloodstream forms of T. brucei, because expression of the cytosolic PGKB isoform, whereas the glycosomal PGKC isoform is endogenously expressed, is lethal for the parasite (53). It was proposed that cell death is caused by the reduced glycolytic flux consecutive of imbalance in the glycosomal ADP/ATP ratio. This highlights the central and different role of PGK in the metabolism of these two forms, maintaining the glycosomal ATP/ADP balance in the bloodstream forms and net production of cytosolic ATP in the procyclic cells.
Acknowledgments
We thank Paul A. Michels and Marilyn Parsons for providing the immune serum against the phosphoglycerate kinase and Thomas Seebeck (Bern, Switzerland) for providing the anti-PEPCK antibody.
This work was supported by the Centre National de la Recherche Scientifique (CNRS), the Université de Bordeaux, the Conseil Régional d'Aquitaine, the Laboratoire d'Excellence (LabEx) ParaFrap ANR-11-LABX-0024, Agence Nationale de la Recherche (ANR) Grant ACETOTRYP of the ANR-BLANC-2010 call, and the ParaMet PhD programme of the Marie Curie Initial Training Network (FP7).
- PPDK
- pyruvate phosphate dikinase
- PEPCK
- phosphoenolpyruvate carboxykinase
- PGKB
- cytosolic phosphoglycerate kinase isoform
- PGKC
- glycosomal phosphoglycerate kinase isoform
- TDH
- threonine 3-dehydrogenase
- .i and .ni
- tetracycline-induced and uninduced, respectively
- rPGKC
- recombinant glycosomal PGKC isoform
- GK
- glycerol kinase
- BSD
- blasticidin
- PAC
- puromycin.
REFERENCES
- 1. Marshall J. S., Ashton A. R., Govers F., Hardham A. R. (2001) Isolation and characterization of four genes encoding pyruvate, phosphate dikinase in the oomycete plant pathogen Phytophthora cinnamomi. Curr. Genet. 40, 73–81 [DOI] [PubMed] [Google Scholar]
- 2. Nevalainen L., Hrdý I., Müller M. (1996) Sequence of a Giardia lamblia gene coding for the glycolytic enzyme, pyruvate, phosphate dikinase. Mol. Biochem. Parasitol. 77, 217–223 [DOI] [PubMed] [Google Scholar]
- 3. Feng X. M., Cao L. J., Adam R. D., Zhang X. C., Lu S. Q. (2008) The catalyzing role of PPDK in Giardia lamblia. Biochem. Biophys. Res. Commun. 367, 394–398 [DOI] [PubMed] [Google Scholar]
- 4. Saavedra E., Encalada R., Pineda E., Jasso-Chávez R., Moreno-Sánchez R. (2005) Glycolysis in Entamoeba histolytica. Biochemical characterization of recombinant glycolytic enzymes and flux control analysis. FEBS J. 272, 1767–1783 [DOI] [PubMed] [Google Scholar]
- 5. Bringaud F., Baltz D., Baltz T. (1998) Functional and molecular characterization of a glycosomal PPi-dependent enzyme in trypanosomatids: pyruvate, phosphate dikinase. Proc. Natl. Acad. Sci. U.S.A. 95, 7963–7968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Acosta H., Dubourdieu M., Quiñones W., Cáceres A., Bringaud F., Concepción J. L. (2004) Pyruvate, phosphate dikinase and pyrophosphate metabolism in the glycosome of Trypanosoma cruzi epimastigotes. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 138, 347–356 [DOI] [PubMed] [Google Scholar]
- 7. Ghozlane A., Bringaud F., Soueidan H., Dutour I., Jourdan F., Thébault P. (2012) Flux analysis of the Trypanosoma brucei glycolysis based on a multiobjective-criteria bioinformatic approach. Adv. Bioinformatics 2012, 159423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barrett M. P., Burchmore R. J., Stich A., Lazzari J. O., Frasch A. C., Cazzulo J. J., Krishna S. (2003) The trypanosomiases. Lancet 362, 1469–1480 [DOI] [PubMed] [Google Scholar]
- 9. Cross G. A., Klein R. A., Linstead D. J. (1975) Utilization of amino acids by Trypanosoma brucei in culture: L-threonine as a precursor for acetate. Parasitology 71, 311–326 [DOI] [PubMed] [Google Scholar]
- 10. Coustou V., Besteiro S., Biran M., Diolez P., Bouchaud V., Voisin P., Michels P. A., Canioni P., Baltz T., Bringaud F. (2003) ATP generation in the Trypanosoma brucei procyclic form: cytosolic substrate level phosphorylation is essential, but not oxidative phosphorylation. J. Biol. Chem. 278, 49625–49635 [DOI] [PubMed] [Google Scholar]
- 11. Millerioux Y., Ebikeme C., Biran M., Morand P., Bouyssou G., Vincent I. M., Mazet M., Riviere L., Franconi J. M., Burchmore R. J., Moreau P., Barrett M. P., Bringaud F. (2013) The threonine degradation pathway of the Trypanosoma brucei procyclic form: the main carbon source for lipid biosynthesis is under metabolic control. Mol. Microbiol. 90, 114–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fairlamb A. H., Opperdoes F. R. (1986) Carbohydrate metabolism in african trypanosomes, with special reference to the glycosome. in Carbohydrate Metabolism in Cultured Cells (Morgan M. J., ed) pp. 183–224, Plenum Publishing Corporation, New York [Google Scholar]
- 13. Lamour N., Rivière L., Coustou V., Coombs G. H., Barrett M. P., Bringaud F. (2005) Proline metabolism in procyclic Trypanosoma brucei is down-regulated in the presence of glucose. J. Biol. Chem. 280, 11902–11910 [DOI] [PubMed] [Google Scholar]
- 14. Coustou V., Biran M., Breton M., Guegan F., Rivière L., Plazolles N., Nolan D., Barrett M. P., Franconi J. M., Bringaud F. (2008) Glucose-induced remodelling of intermediary and energy metabolism in procyclic Trypanosoma brucei. J. Biol. Chem. 283, 16342–16354 [DOI] [PubMed] [Google Scholar]
- 15. Cazzulo J. J. (1992) Aerobic fermentation of glucose by trypanosomatids. FASEB J. 6, 3153–3161 [DOI] [PubMed] [Google Scholar]
- 16. Bringaud F., Rivière L., Coustou V. (2006) Energy metabolism of trypanosomatids: adaptation to available carbon sources. Mol. Biochem. Parasitol. 149, 1–9 [DOI] [PubMed] [Google Scholar]
- 17. Opperdoes F. R., Borst P., Spits H. (1977) Particle-bound enzymes in the bloodstream form of Trypanosoma brucei. Eur. J. Biochem. 76, 21–28 [DOI] [PubMed] [Google Scholar]
- 18. Besteiro S., Biran M., Biteau N., Coustou V., Baltz T., Canioni P., Bringaud F. (2002) Succinate secreted by Trypanosoma brucei is produced by a novel and unique glycosomal enzyme, NADH-dependent fumarate reductase. J. Biol. Chem. 277, 38001–38012 [DOI] [PubMed] [Google Scholar]
- 19. Coustou V., Besteiro S., Rivière L., Biran M., Biteau N., Franconi J. M., Boshart M., Baltz T., Bringaud F. (2005) A mitochondrial NADH-dependent fumarate reductase involved in the production of succinate excreted by procyclic Trypanosoma brucei. J. Biol. Chem. 280, 16559–16570 [DOI] [PubMed] [Google Scholar]
- 20. Van Hellemond J. J., Opperdoes F. R., Tielens A. G. (1998) Trypanosomatidae produce acetate via a mitochondrial acetate:succinate CoA transferase. Proc. Natl. Acad. Sci. U.S.A. 95, 3036–3041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rivière L., van Weelden S. W., Glass P., Vegh P., Coustou V., Biran M., van Hellemond J. J., Bringaud F., Tielens A. G., Boshart M. (2004) Acetyl:succinate CoA-transferase in procyclic Trypanosoma brucei: gene identification and role in carbohydrate metabolism. J. Biol. Chem. 279, 45337–45346 [DOI] [PubMed] [Google Scholar]
- 22. Ebikeme C., Hubert J., Biran M., Gouspillou G., Morand P., Plazolles N., Guegan F., Diolez P., Franconi J. M., Portais J. C., Bringaud F. (2010) Ablation of succinate production from glucose metabolism in the procyclic trypanosomes induces metabolic switches to the glycerol 3-phosphate/dihydroxyacetone phosphate shuttle and to proline metabolism. J. Biol. Chem. 285, 32312–32324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Michels P. A., Bringaud F., Herman M., Hannaert V. (2006) Metabolic functions of glycosomes in trypanosomatids. Biochim. Biophys. Acta 1763, 1463–1477 [DOI] [PubMed] [Google Scholar]
- 24. Osinga K. A., Swinkels B. W., Gibson W. C., Borst P., Veeneman G. H., Van Boom J. H., Michels P. A., Opperdoes F. R. (1985) Topogenesis of microbody enzymes: a sequence comparison of the genes for the glycosomal (microbody) and cytosolic phosphoglycerate kinases of Trypanosoma brucei. EMBO J. 4, 3811–3817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Colasante C., Ellis M., Ruppert T., Voncken F. (2006) Comparative proteomics of glycosomes from bloodstream form and procyclic culture form Trypanosoma brucei brucei. Proteomics 6, 3275–3293 [DOI] [PubMed] [Google Scholar]
- 26. van Weelden S. W., van Hellemond J. J., Opperdoes F. R., Tielens A. G. (2005) New functions for parts of the Krebs cycle in procyclic Trypanosoma brucei, a cycle not operating as a cycle. J. Biol. Chem. 280, 12451–12460 [DOI] [PubMed] [Google Scholar]
- 27. Bringaud F., Robinson D. R., Barradeau S., Biteau N., Baltz D., Baltz T. (2000) Characterization and disruption of a new Trypanosoma brucei repetitive flagellum protein, using double-stranded RNA inhibition. Mol. Biochem. Parasitol. 111, 283–297 [DOI] [PubMed] [Google Scholar]
- 28. Brun R., Schönenberger M. (1979) Cultivation and in vitro cloning or procyclic culture forms of Trypanosoma brucei in a semi-defined medium. Acta Trop. 36, 289–292 [PubMed] [Google Scholar]
- 29. Hirumi H., Hirumi K. (1989) Continuous cultivation of Trypanosoma brucei bloodstream forms in a medium containing a low concentration of serum protein without feeder cell layers. J. Parasitol. 75, 985–989 [PubMed] [Google Scholar]
- 30. Estévez A. M., Kierszenbaum F., Wirtz E., Bringaud F., Grunstein J., Simpson L. (1999) Knockout of the glutamate dehydrogenase gene in bloodstream Trypanosoma brucei in culture has no effect on editing of mitochondrial mRNAs. Mol. Biochem. Parasitol. 100, 5–17 [DOI] [PubMed] [Google Scholar]
- 31. Coustou V., Biran M., Besteiro S., Rivière L., Baltz T., Franconi J. M., Bringaud F. (2006) Fumarate is an essential intermediary metabolite produced by the procyclic Trypanosoma brucei. J. Biol. Chem. 281, 26832–26846 [DOI] [PubMed] [Google Scholar]
- 32. Ngô H., Tschudi C., Gull K., Ullu E. (1998) Double-stranded RNA induces mRNA degradation in Trypanosoma brucei. Proc. Natl. Acad. Sci. U.S.A. 95, 14687–14692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wirtz E., Leal S., Ochatt C., Cross G. A. (1999) A tightly regulated inducible expression system for conditional gene knock-outs and dominant-negative genetics in Trypanosoma brucei. Mol. Biochem. Parasitol. 99, 89–101 [DOI] [PubMed] [Google Scholar]
- 34. Linstead D. J., Klein R. A., Cross G. A. (1977) Threonine catabolism in Trypanosoma brucei. J. Gen. Microbiol. 101, 243–251 [DOI] [PubMed] [Google Scholar]
- 35. Klein R. A., Linstead D. J., Wheeler M. V. (1975) Carbon dioxide fixation in trypanosomatids. Parasitology 71, 93–107 [DOI] [PubMed] [Google Scholar]
- 36. Else A. J., Clarke J. F., Willis A., Jackman S. A., Hough D. W., Danson M. J. (1994) Dihydrolipoamide dehydrogenase in the Trypanosoma subgenus, trypanozoon. Mol. Biochem. Parasitol. 64, 233–239 [DOI] [PubMed] [Google Scholar]
- 37. Harlow E., Lane D. (1988) Antibodies: A Laboratory Manual, pp. 469–527, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 38. Sambrook J., Russell D. W. (2001) Molecular Cloning: A Laboratory Manual, 3rd Ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 39. Misset O., Opperdoes F. R. (1987) The phosphoglycerate kinases from Trypanosoma brucei: a comparison of the glycosomal and the cytosolic isoenzymes and their sensitivity towards suramin. Eur. J. Biochem. 162, 493–500 [DOI] [PubMed] [Google Scholar]
- 40. Alexander K., Parsons M. (1991) A phosphoglycerate kinase-like molecule localized to glycosomal microbodies: evidence that the topogenic signal is not at the C-terminus. Mol. Biochem. Parasitol. 46, 1–10 [DOI] [PubMed] [Google Scholar]
- 41. Denise H., Giroud C., Barrett M. P., Baltz T. (1999) Affinity chromatography using trypanocidal arsenical drugs identifies a specific interaction between glycerol-3-phosphate dehydrogenase from Trypanosoma brucei and Cymelarsan. Eur. J. Biochem. 259, 339–346 [DOI] [PubMed] [Google Scholar]
- 42. Bringaud F., Peyruchaud S., Baltz D., Giroud C., Simpson L., Baltz T. (1995) Molecular characterization of the mitochondrial heat shock protein 60 gene from Trypanosoma brucei. Mol. Biochem. Parasitol. 74, 119–123 [DOI] [PubMed] [Google Scholar]
- 43. Rivière L., Moreau P., Allmann S., Hahn M., Biran M., Plazolles N., Franconi J. M., Boshart M., Bringaud F. (2009) Acetate produced in the mitochondrion is the essential precursor of lipid biosynthesis in procyclic trypanosomes. Proc. Natl. Acad. Sci. U.S.A. 106, 12694–12699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bates L. S., Waldren R. P., Teare I. D. (1973) Rapid determination of free proline for water-stress studies. Plant and Soil 39, 205–207 [Google Scholar]
- 45. Lemasters J. J., Hackenbrock C. R. (1979) Continuous measurement of adenosine triphosphate with firefly luciferase luminescence. Methods Enzymol. 56, 530–544 [DOI] [PubMed] [Google Scholar]
- 46. Mazet M., Morand P., Biran M., Bouyssou G., Courtois P., Daulouède S., Millerioux Y., Franconi J. M., Vincendeau P., Moreau P., Bringaud F. (2013) Revisiting the central metabolism of the bloodstream forms of Trypanosoma brucei: production of acetate in the mitochondrion is essential for parasite viability. PLoS Negl. Trop. Dis. 7, e2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Akoka S., Barantin L., Trierweiler M. (1999) Concentration measurement by proton NMR using the ERETIC method. Anal. Chem. 71, 2554–2557 [DOI] [PubMed] [Google Scholar]
- 48. Allmann S., Morand P., Ebikeme C., Gales L., Biran M., Hubert J., Brennand A., Mazet M., Franconi J. M., Michels P. A., Portais J. C., Boshart M., Bringaud F. (2013) Cytosolic NADPH homeostasis in glucose-starved procyclic Trypanosoma brucei relies on malic enzyme and the pentose phosphate pathway fed by gluconeogenic flux. J. Biol. Chem. 288, 18494–18505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gualdrón-López M., Brennand A., Hannaert V., Quiñones W., Cáceres A. J., Bringaud F., Concepción J. L., Michels P. A. (2012) When, how and why glycolysis became compartmentalised in the Kinetoplastea. A new look at an ancient organelle. Int. J. Parasitol. 42, 1–20 [DOI] [PubMed] [Google Scholar]
- 50. Millerioux Y., Morand P., Biran M., Mazet M., Moreau P., Wargnies M., Ebikeme C., Deramchia K., Gales L., Portais J. C., Boshart M., Franconi J. M., Bringaud F. (2012) ATP synthesis-coupled and -uncoupled acetate production from acetyl-CoA by the mitochondrial acetate:succinate CoA-transferase and acetyl-CoA thioesterase in Trypanosoma. J. Biol. Chem. 287, 17186–17197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Furuya T., Kessler P., Jardim A., Schnaufer A., Crudder C., Parsons M. (2002) Glucose is toxic to glycosome-deficient trypanosomes. Proc. Natl. Acad. Sci. U.S.A. 99, 14177–14182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Haanstra J. R., van Tuijl A., Kessler P., Reijnders W., Michels P. A., Westerhoff H. V., Parsons M., Bakker B. M. (2008) Compartmentation prevents a lethal turbo-explosion of glycolysis in trypanosomes. Proc. Natl. Acad. Sci. U.S.A. 105, 17718–17723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Blattner J., Helfert S., Michels P., Clayton C. (1998) Compartmentation of phosphoglycerate kinase in Trypanosoma brucei plays a critical role in parasite energy metabolism. Proc. Natl. Acad. Sci. U.S.A. 95, 11596–11600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lemercier G., Dutoya S., Luo S., Ruiz F. A., Rodrigues C. O., Baltz T., Docampo R., Bakalara N. (2002) A vacuolar-type H+-pyrophosphatase governs maintenance of functional acidocalcisomes and growth of the insect and mammalian forms of Trypanosoma brucei. J. Biol. Chem. 277, 37369–37376 [DOI] [PubMed] [Google Scholar]
- 55. Luginbuehl E., Kunz S., Wentzinger L., Freimoser F., Seebeck T. (2011) The exopolyphosphatase TbrPPX1 of Trypanosoma brucei. BMC Microbiol. 11, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mertens E. (1993) ATP versus pyrophosphate: glycolysis revisited in parasitic protists. Parasitol. Today 9, 122–126 [DOI] [PubMed] [Google Scholar]
- 57. Ginger M. L., Ngazoa E. S., Pereira C. A., Pullen T. J., Kabiri M., Becker K., Gull K., Steverding D. (2005) Intracellular positioning of isoforms explains an unusually large adenylate kinase gene family in the parasite Trypanosoma brucei. J. Biol. Chem. 280, 11781–11789 [DOI] [PubMed] [Google Scholar]
- 58. Michels P. A., Hannaert V., Bringaud F. (2000) Metabolic aspects of glycosomes in Trypanosomatidae: new data and views. Parasitol. Today 16, 482–489 [DOI] [PubMed] [Google Scholar]
- 59. Bringaud F., Barrett M. P., Zilberstein D. (2012) Multiple roles of proline transport and metabolism in trypanosomatids. Front. Biosci. 17, 349–374 [DOI] [PubMed] [Google Scholar]