Background: Trigeminal sensations can arise from the direct stimulation of intraepithelial free nerve endings or indirectly through information transmission from adjacent cells.
Results: Exposure to the odorant chemicals induces a chemical communication between human HaCaT keratinocytes and mouse trigeminal neurons.
Conclusion: Keratinocytes act as chemosensors linking the environment and the trigeminal system via ATP signaling.
Significance: This work contributes to our knowledge of how chemical cues indirectly activate the trigeminal system.
Keywords: ATP, Calcium Imaging, Cell Signaling, Keratinocyte, Olfaction, ATP Signaling, Chemosensation, Odorants, Trigeminal Neurons
Abstract
Trigeminal fibers terminate within the facial mucosa and skin and transmit tactile, proprioceptive, chemical, and nociceptive sensations. Trigeminal sensations can arise from the direct stimulation of intraepithelial free nerve endings or indirectly through information transmission from adjacent cells at the peripheral innervation area. For mechanical and thermal cues, communication processes between skin cells and somatosensory neurons have already been suggested. High concentrations of most odors typically provoke trigeminal sensations in vivo but surprisingly fail to activate trigeminal neuron monocultures. This fact favors the hypothesis that epithelial cells may participate in chemodetection and subsequently transmit signals to neighboring trigeminal fibers. Keratinocytes, the major cell type of the epidermis, express various receptors that enable reactions to multiple environmental stimuli. Here, using a co-culture approach, we show for the first time that exposure to the odorant chemicals induces a chemical communication between human HaCaT keratinocytes and mouse trigeminal neurons. Moreover, a supernatant analysis of stimulated keratinocytes and subsequent blocking experiments with pyrodoxalphosphate-6-azophenyl-2′,4′-disulfonate revealed that ATP serves as the mediating transmitter molecule released from skin cells after odor stimulation. We show that the ATP release resulting from Javanol® stimulation of keratinocytes was mediated by pannexins. Consequently, keratinocytes act as chemosensors linking the environment and the trigeminal system via ATP signaling.
Introduction
The ability of the body to sense harmful environmental thermal, physical, and chemical cues is essential for survival. For the head, the principal sensory system is represented by the trigeminal nerve. Free trigeminal nerve endings innervate the facial mucosa and the skin and transmit sensory information regarding oral or respiratory substance intake and contact with the facial skin (1–4). The polymodal neurons express numerous sensory receptors that enable these cells to respond to a broad range of stimuli (5–16). However, their location and accessibility raise the question of whether trigeminal chemesthesis arises either from the direct stimulation of intraepithelial free nerve endings or indirectly through information transmission from adjacent cells of the peripheral innervation area (17–21). Anosmic patients, who have lost their sense of smell, can still discriminate between several odorants in higher concentrations, supporting the hypothesis that trigeminal neurons can essentially contribute to odor detection and discrimination (22, 23).
In the facial epidermis and mucosae, trigeminal free nerve endings are located in close proximity to epithelial cells. Keratinocytes, constituting the major epidermal cell type, express several thermo-, chemo-, osmo-, and mechano-sensitive receptors initially identified in sensory neurons (24–26). Previous studies have revealed that keratinocytes represent the frontier sensory system of the skin, transmitting mechanical and thermal information to sensory neurons (27, 28). In both cases, transient calcium elevations result in the secretion of the transmitter molecule ATP, subsequently activating P2 receptors of adjacent keratinocytes and neurons.
However, the question regarding chemical cues remains. Do trigeminal neurons also receive information regarding chemical stimuli from adjacent cells in their peripheral innervation area? The skin is permanently exposed to environmental chemical stimuli. For example, odorants are widely used as ingredients of cosmetics, perfumes, detergents, cigarettes, and aliment. The odorants can exert a strong physiological impact on skin cells, as shown for eugenol and isoeugenol, causing antiproliferative effects by affecting the intracellular aryl hydrocarbon receptor (29). Most odorants have a trigeminal component at high concentrations (22, 30) inducing typical trigeminal sensations, such as cooling, warming, burning, stinging, or prickling.
However, we discovered that high concentrations of odors that typically provoke trigeminal sensations in vivo surprisingly fail to activate monocultured trigeminal neurons. According to our hypothesis, “epithelial cells participate in chemodetection by transmitting signals to neighboring trigeminal fibers,” we demonstrated that Sandalore® and Javanol® induced signal transmission from human keratinocytes to mouse trigeminal neurons using a co-culture approach. This process was mediated by ATP release from keratinocytes, most likely via hemichannels rather than exocytotic events. Keratinocytes thus represent a functional link between the environment and the body's chemosensory system.
EXPERIMENTAL PROCEDURES
Cell Culture
The methods for culturing trigeminal primary sensory neurons were similar to those of Spehr et al. (13). Briefly, male and female newborn mice (postnatal days 1–4) were decapitated, and the two trigeminal ganglia (ganglion grassi) were excised. The trigeminal ganglia (TG)6 were washed in PBS (Invitrogen) and collected in Leibovitz medium (L15; Invitrogen). The TG were incubated for 45 min in warm DMEM (Invitrogen) containing 0.024% collagenase (type IA; Sigma) at 37 °C (95% air and 5% CO2). Afterward, the TG were gently triturated with a file-polished glass pipette, and this solution was centrifuged at 1,000 rpm for 4 min. The pellet was resuspended in TG medium (DMEM/F-12 + GlutaMax) supplemented with 10% fetal bovine serum (Invitrogen), 100 units/ml penicillin, and 100 μg/ml streptomycin. The cells were plated on poly-l-lysine (0.01%)-coated Petri dishes (35 mm; Falcon, BD Biosciences) and maintained in a humidified atmosphere (37 °C; 95% air, 5% CO2). One hour after plating, 2 ml of culture medium was added to each dish. Trigeminal neurons were grown for 1–4 days before experimentation.
The human keratinocyte cell line HaCaT (31) was maintained in DMEM with 5% fetal bovine serum and 1% (100×) penicillin/streptomycin at 37 °C (95% air and 5% CO2). The cells were passaged twice a week. For experiments, the cells were grown in Petri dishes (35 mm) for a minimum of 2 days. For the ATP assay, the cells were grown in 24-well plates until confluence. The primary human keratinocytes cultures were kindly provided by the Beiersdorf AG (Hamburg, Germany) and maintained under the culture conditions described by Jacob et al. (32).
For the co-culture approach, 2-day-old HaCaT cells in Petri dishes were used. After removal of the HaCaT medium, fresh primed TG were diluted with TG culture medium and added to the HaCaT cell dishes. Co-cultures were used after 2–4 days for measurements when HaCaT cells reached a confluence of 70–80%. Under typical co-culture conditions, the relative proportion between neurons and HaCaT cells was about 2–5 per 100.
Calcium Imaging
For the calcium imaging experiments, the cells were treated with the calcium-sensitive dye Fura-2 AM (3 μm; Molecular Probes, Leiden, Netherlands) for 60 min (37 °C; 95% air, 5% CO2) (13). The cells were transferred to the stage of an inverted microscope equipped for ratiometric live cell imaging (Olympus IX71) with a 150-watt xenon arc lamp, a motorized fast change filter wheel illumination system (MT20, Olympus) for multiwavelength excitation, a 12-bit 1376 × 1032-pixel charge-coupled device camera (F-View II, Olympus), and Cell® imaging software (Olympus). The cells were viewed at ×10 and ×20 magnification and illuminated sequentially at 340 and 380 nm. The average pixel intensity within user-selected regions of interest was stored on a computer. Calcium-dependent fluorescence signals at 510 nm were calculated as the intensity ratio of f340/f380. Stimuli were applied using a custom-made, pressure-driven, multibarrel perfusion system that allows instantaneous solution change and focal application. For the calculation of the response frequency of trigeminal cells in experiments with three repetitive odor stimulations, a cell had to respond to at least one application. The cells were maintained in an extracellular bath solution consisting of 140 mm NaCl, 5 mm KCl, 2 mm CaCl2, 1 mm MgCl2, and 10 mm HEPES (pH 7.3). At the end of each measurement, a high potassium solution (K+; 45 mm) was applied as a positive control for neuronal cells. ATP (100 μm) application served as a control for cell viability of HaCaT cells.
Electrophysiological Recordings
Recordings were performed using the whole-cell patch clamp technique. The cells were maintained in an extracellular bath solution consisting of 140 mm NaCl, 5 mm KCl, 2 mm CaCl2, 1 mm MgCl2, and 20 mm HEPES (pH 7.4). Patch electrodes were pulled from borosilicate glass (1.2-mm OD × 1.17-mm ID; Harvard Apparatus, Edenbridge, Kent, UK) and fire-polished to 4–6-megaohm tip resistance using a horizontal pipette puller (Zeitz Instruments, Munich, Germany). The pipette solution contained 140 mm KCl, 5 mm EGTA, 1 mm MgCl2, 0.1 mm CaCl2, 10 mm HEPES, 2 mm ATP, and 1 mm GTP (pH 7.4). The recordings were performed at room temperature using a HEKA EPC7 amplifier. The membrane potential was held at −60 mV. The data were acquired using Pulse software.
ATP Assay
The ATP concentration of the supernatant of stimulated HaCaT cells was measured using the CellTiter-Glo® luminescent cell viability assay (Promega) according to the manufacturer's instructions. The HaCaT medium of a 24-well plate was exchanged with Ringer's solution with the tested substance. After incubation with the odorants or chemicals for 1–2 min, the supernatant was collected and transferred to a 96-well plate. For the blocker experiments, carbenoxolone (10 μm) or probenecid (10 μm) were also applied to the Javanol® stimulation. Brefeldin A (5 μg/ml) was added to the medium of the HaCaT cells 1 h before the experiment. The stimulation with Javanol® was also performed with 5 μg/ml brefeldin A. The relative luminescence was measured with a Mithras LB 940 multiplate scanner. Every substance was measured in at least three independent experiments.
Propidium Iodide Staining
Dishes with cultured keratinocytes were incubated with 1 mm Sandalore®, Javanol®, or Ringer's solution for 24 h. Then 25 μg/μl propidium iodide was added for 5 min. The dead cells were detected with a fluorescence microscope (Axioskop, Zeiss).
Substances
Unless otherwise noted, all substances were purchased from Sigma-Aldrich. The sandalwood derivatives were kindly provided by Symrise AG (Holzminden, Germany).
Statistical Analyses
Unless otherwise noted, the data were analyzed using a two-tailed unpaired t test, and p < 0.05 values were regarded as significant.
RESULTS
The Stimulation of Monocultured Mouse Trigeminal Neurons, Keratinocytes, and HaCaT Cells with Odorant Substances
To answer the question of whether trigeminal neurons directly react to chemical stimulation or receive their information from adjacent cells, we aimed to identify substances that activate human keratinocytes but fail to affect monocultured trigeminal neurons. Either monocultured human primary keratinocytes or HaCaT cells and mouse trigeminal neurons were subjected to chemical stimulation, and the reaction was measured using the calcium imaging technique. For chemical stimulation, several natural and synthetic substances from different substance classes with clear odorous and trigeminal impact were selected, diluted in Ringer's solution, and tested in comparison with the application of Ringer's solution only. Unexpectedly, in calcium imaging experiments, sandalwood oil derivatives did not elicit calcium signals in mouse trigeminal monocultures over Ringer's controls in concentrations up to 1 mm (selection shown in Table 1). Approximately 4% of the neurons showed spontaneous activity that did not correlate with the application of stimulus. The application of substances such as citronellol as a control, which was previously described as activating transient receptor potential (Trp) channels on sensory neurons (33–36), induced calcium transients, as expected. In contrast, the sandalwood derivatives Javanol® and Sandalore®, which elicit moderate trigeminal sensations, such as stinging or prickling, when inhaled, did not induce calcium signals in monocultured trigeminal neurons, indicating an indirect activation of the trigeminal system.
TABLE 1.
Substance screening for the ability to stimulate monocultured human primary keratinocytes, HaCaT cells, or trigeminal neurons
Several substances (odorants, 1 mm; histamine, 100 μm; LPA, 200 nm) induced calcium transients in human keratinocytes. Sandalore® and Javanol® evoked repetitive calcium signals in a large number of cells. Experiments were conducted on three different days, with the confluence of keratinocytes or HaCaT cells at 80% and neurons at 50% per dish. NA, not applicable.
| Substance | Keratinocytes | HaCaT | Trigeminal neurons |
|---|---|---|---|
| % | % | % | |
| Citronellol | 46 | 45 | 33 |
| Javanol® | 90 | 90 | 5 |
| Lyral | 80 | 80 | 80 |
| Sandalore® | 80 | 86 | 4 |
| Helional | NA | 27 | 28 |
| Histamine | 93 | 99 | 4 |
| LPA | 91 | 96 | 2 |
| Ringer's | 0 | 0 | 4 |
Surprisingly, all five tested substances induced a transient increase in intracellular calcium in human primary keratinocytes or HaCaT cells. Moreover, Javanol® and Sandalore® activated keratinocytes and HaCaT cells but not TG neurons (cf. Table 1).
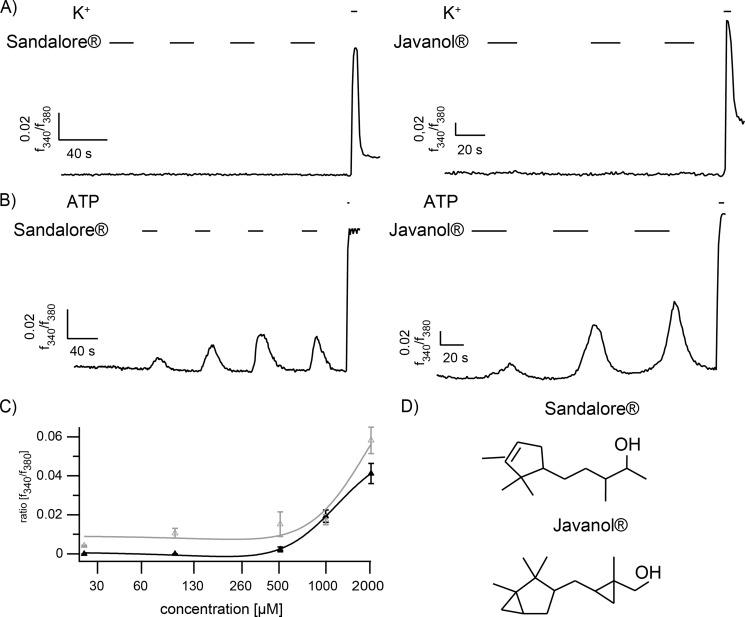
Depending on the tested substance, the kinetic and amplitude of the evoked calcium signals varied, and several substances, such as helional, affected the cells only once, even when applied with longer interstimulus intervals of approximately 1 min (data not shown). For subsequent experiments, we required odorants that evoke stable, repetitive calcium signals in HaCaT cells. We selected Javanol® and Sandalore® because neither sandalwood derivative activated trigeminal neurons more strongly than the Ringer's control, but they induced robust calcium signals in ∼80% of either primary keratinocytes or HaCaT cells. Furthermore, the cells responded to each stimulus when applied repetitively (cf. Fig. 1, A and B). The dose-response curves showed that Sandalore® acted at a minimum concentration of 500 μm (Fig. 1C), whereas the cells were slightly more sensitive to Javanol® (∼300 μm).
FIGURE 1.
A, representative calcium imaging experiments of monocultured trigeminal neurons that fail to react to Sandalore® and Javanol® stimulation (n = 413 and 85, respectively; 1 mm, 20 s). B, HaCaT keratinocytes in monoculture show repetitive calcium signals at each application of Sandalore® and Javanol® (n = 168 and 242, respectively; 1 mm, 20 s). ATP (100 μm) application served as a positive control. High potassium (K+; 45 mm) served as a positive control for neuronal cells. C, the dose-response curve of measurements of HaCaT cells after stimulation with 25, 100, 500, 1000, and 2000 μm Sandalore® (gray) or Javanol® (black). The mean amplitudes of stimulated HaCaT cells at the first application are shown. At 500 μm, the intracellular calcium was notably increased at the first application. D, the chemical structure of Sandalore® and Javanol®.
The Reactivity of Trigeminal Neurons Increased in a Co-culture Approach with Human Keratinocytes or HaCaT Cells
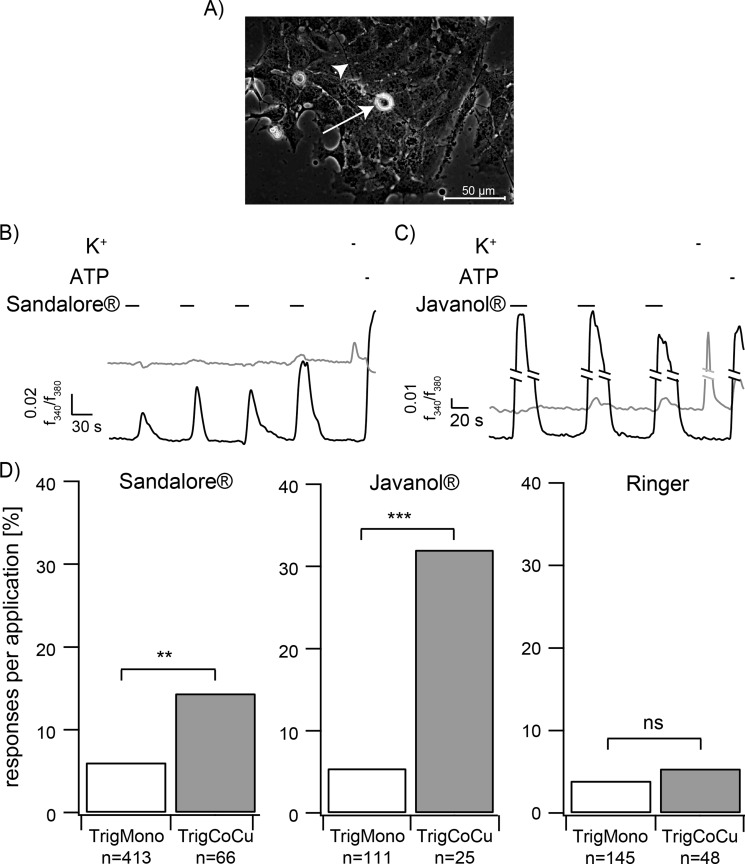
To prove the hypothesis of a chemically induced signal transmission from either keratinocytes or HaCaT cells to trigeminal neurons, a co-culture model was established. Mouse trigeminal neurons were prepared from newborn CD1 mice (postnatal days 1–4) and added to cultures of primary human keratinocytes or HaCaT cells. Both cell types survived in the co-culture, showed typical morphologies (Fig. 2A), and exhibited normal reactivity to ATP stimulation in calcium imaging experiments (Fig. 2, B and C). The application of high potassium provoked depolarization of trigeminal neurons, indicating normal reactivity (Fig. 2, B and C). After 2 days in co-culture, neurite outgrowth was observed from trigeminal neurons. Furthermore, HaCaT cells and keratinocytes retained the ability to respond to the tested substances in the co-culture approach (Fig. 2, B and C). Subsequent experiments were performed with HaCaT keratinocytes to ensure charge- and culture-independent conditions.
FIGURE 2.
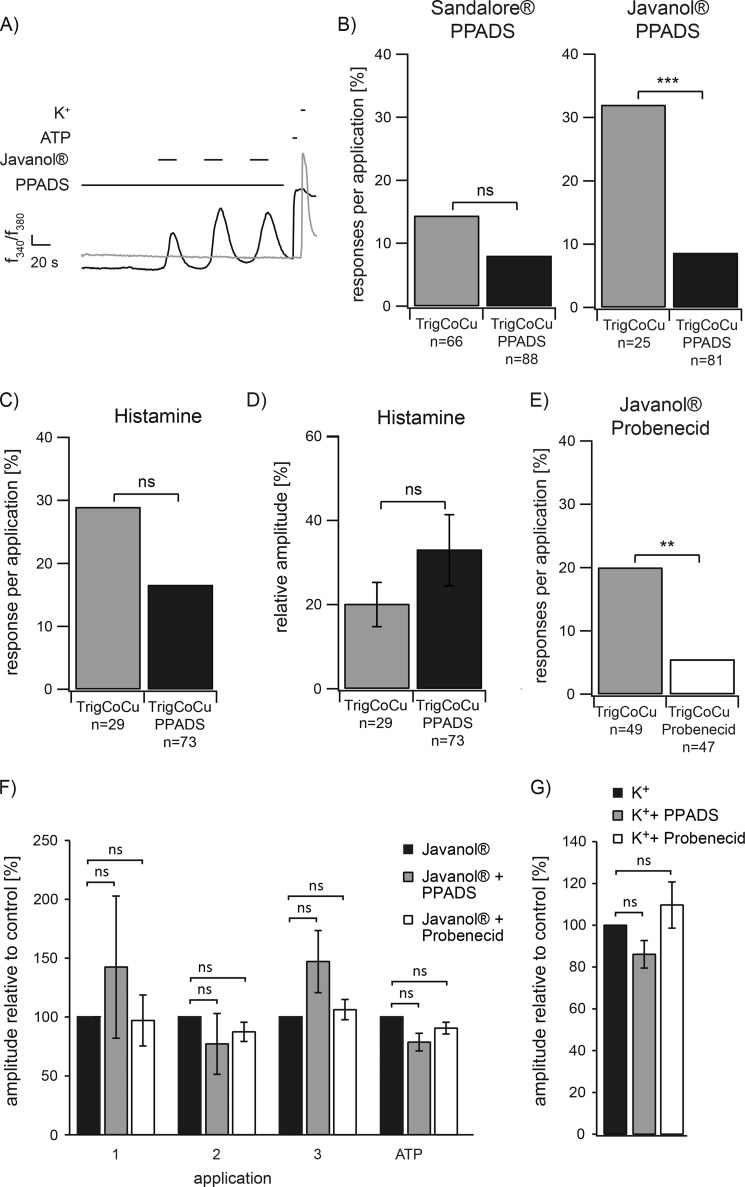
A, co-culture of HaCaT cells and trigeminal neurons (arrow). Arrowhead, neurite processes. B and C, representative calcium imaging experiments with co-cultured HaCaT cells (black lines) and trigeminal neurons (gray lines). Stimulation with the tested sandalwood derivative (1 mm) leads to an increase of trigeminal activity. D, quantitative analysis of the responses of trigeminal neurons in monocultures (TrigMono) compared with co-cultures (TrigCoCu). The activity of trigeminal neurons in co-cultures observed upon application of Sandalore® and Javanol® was significantly increased compared with the activity in monoculture (Sandalore®: TrigMono n = 413 neurons in 21 monocultures, TrigCoCu n = 66 neurons in 23 co-cultures; Javanol®: TrigMono n = 111 neurons in 3 monocultures, TrigCoCu n = 25 neurons in 7 co-cultures; Ringer: TrigMono n = 145 neurons in 5 monocultures, TrigCoCu n = 48 neurons in 7 co-cultures). **, p < 0.01; ***, p < 0.001; ns, not significant; Fisher's exact test.
In the co-culture system, the keratinocytes were activated by the repetitive application of 1 mm Javanol® or Sandalore® as in the monocultures. Strikingly, this activation induced calcium responses of co-cultured but not of monocultured trigeminal neurons that were significantly enhanced over the Ringer's solution control (Fig. 2, B–D). However, the majority of activated neurons reacted only once, independent of the number of preceding responses from HaCaT cells in the same dish. Therefore, to quantify the results, trigeminal neuron reactions were summarized for all three subsequent odorant applications and described as the average response rate per experiment (Fig. 2D). Bar graph analysis demonstrated a dramatic increase in the rate of trigeminal responses induced by Javanol® and Sandalore® in the co-culture. In contrast, in the monoculture, response rates to Sandalore® (6%) or Javanol® (5%) were not significantly different from the Ringer's control (4%); Sandalore® or Javanol® enhanced the neuronal activity by almost 6- or 2.4-fold in the co-culture approach. In the case of Javanol® application, 32% of the trigeminal neurons responded in the co-culture, but only 5% responded in the monoculture, which was indistinguishable from background activity (Ringer's solution). This stimulus-dependent effect can thus be clearly attributed to the presence of skin cells, as demonstrated by control experiments with monocultured trigeminal neurons.
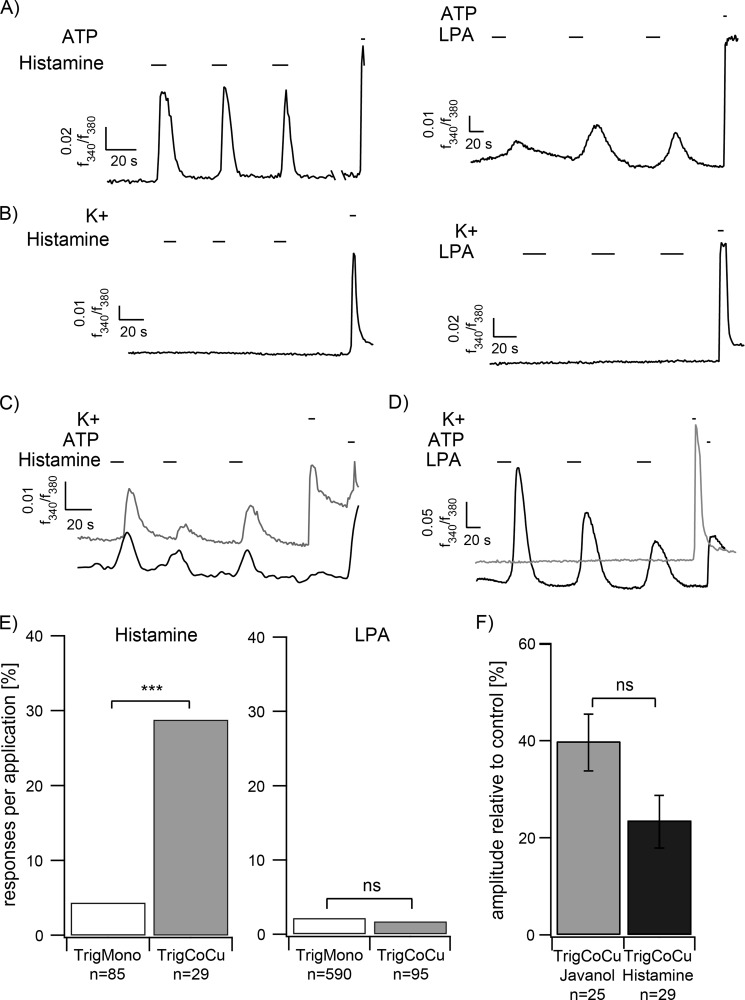
To further investigate whether other chemicals generally provoke communication events in the co-culture approach, the effects of histamine and lysophosphatidic acid (LPA) application were examined. Keratinocytes have previously been shown to respond to histamine and LPA application (37, 38). Indeed, repetitive calcium transients could be observed in calcium imaging experiments for HaCaT keratinocytes stimulated by either substance (Fig. 3A). However, monocultured mouse trigeminal neurons failed to react to histamine and LPA stimulation (Fig. 3B). In the co-culture, the number of reactive trigeminal neurons was significantly enhanced ∼6.7-fold upon histamine stimulation (Fig. 3, C–E). In contrast to histamine, Javanol®, and Sandalore®, LPA thus represents an exemplary activator of repetitive calcium signals in keratinocytes that does not lead to subsequent signal transmission of co-cultured trigeminal neurons (Fig. 3, D and E).
FIGURE 3.
A, HaCaT keratinocytes show strong repetitive calcium signals to histamine (n = 215; 100 μm, 10 s) and LPA (n = 165; 200 nm, 10 s) stimulation. ATP (100 μm) application serves as a positive control for cell viability. B, in monoculture, trigeminal neurons fail to react to histamine (n = 85) or LPA (n = 590) stimulation. C, trigeminal neurons react in a co-culture approach subsequent to keratinocytes. D, repetitive LPA application induced calcium signals in keratinocytes but failed to induce signal transmission to trigeminal neurons. E, quantification of the co-culture results. The number of responding cells at histamine stimulation was significantly increased in the co-cultures. In contrast, LPA application did not lead to a significant increase in the co-cultures (histamine: TrigMono n = 85 neurons in 3 monocultures, TrigCoCu n = 29 neurons in 7 co-cultures; histamine: TrigMono n = 590 neurons in 10 monocultures, TrigCoCu n = 95 neurons in 13 co-cultures). F, quantitative analysis of Ca2+ signals of trigeminal neurons in co-cultures induced by Javanol® (1 mm; n = 25 neurons in 7 co-cultures) or histamine (100 μm; n = 29 neurons in 7 co-cultures) stimulation. Shown are mean amplitudes normalized to high potassium (K+; 45 mm). ***, p < 0.001; Fisher's exact test; ns, not significant. Error bars, S.E.
Further, we analyzed whether histamine or Javanol® stimulation induced different calcium signals in co-cultured neurons. The quantification showed no statistically significant difference in amplitude levels upon Javanol® or histamine application (Fig. 3F).
Responding Trigeminal Neurons Were Not Distributed Randomly but Were Located in Close Proximity to Keratinocytes
Approximately 67% of the reactive neurons were located in close proximity to the responding keratinocytes. Although substantially more distantly located, the remaining 33% of reacting neurons were in obvious contact with keratinocytes via long neurite projections. Silent neurons were predominantly located quite distant from the reactive skin cells and did not show visible neurite outgrowth. These findings support the occurrence of communication events between the cell types that potentially require direct cell contact. However, the latencies between the induction of calcium signals in skin cells and the response of trigeminal neurons varied and did not appear to be linked to the distance between reacting cells.
Keratinocytes Release ATP upon Chemical Stimulation with Javanol® and Sandalore®
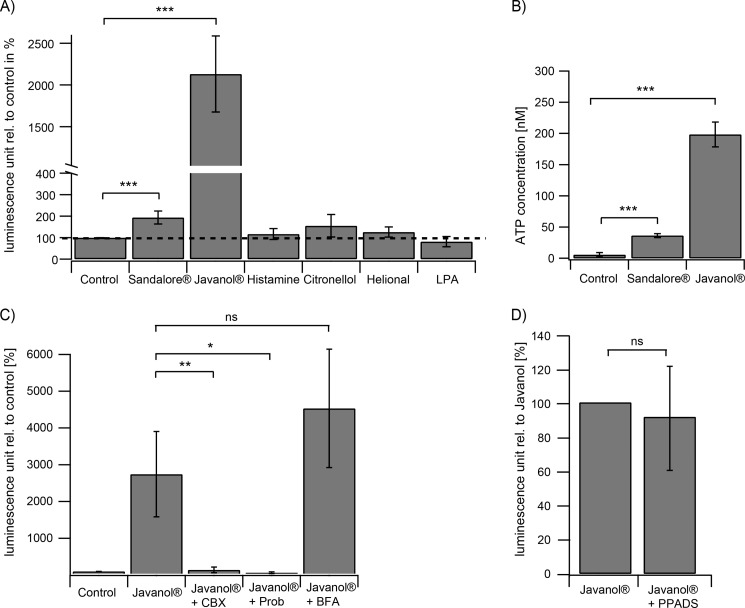
Keratinocytes are known to produce and secrete various substances and neurotransmitters that modulate adjacent cells in a paracrine and autocrine manner (27, 28, 39). Two substances, ATP and prostaglandin E2, are released from keratinocytes upon mechanical and thermal stimulation, as shown previously (27, 28, 39). To test whether ATP is also a mediator of chemical stimuli, ATP release assays were performed. For this purpose, HaCaT cells were cultured in 96-well plates and subjected to the luciferase-based ATP detection system. Luminescence measurements were performed subsequent to stimulation with the indicated substances (Fig. 4A). In the supernatant of stimulated HaCaT cells, the ATP concentration was significantly increased after a 1–2-min stimulation with the sandalwood derivatives (1 mm). Estimated from independent experiments, Sandalore® and Javanol® stimulation resulted in a release of ∼40 nm and 200 nm ATP into the culture medium, respectively. In contrast, no ATP increase was measured upon stimulation with other odorants, such as citronellol (1 mm) and helional (1 mm), and substances, such as LPA (200 nm). Interestingly, stimulation with histamine (100 μm), formerly shown to enhance trigeminal activity in co-cultures (Fig. 3, C and D), did not lead to ATP release. To exclude toxic effects of the sandalwood substances that might result in ATP leakage from dying cells, stimulated keratinocytes were analyzed with propidium iodide staining. Even long term incubation (24 h) of keratinocytes with 1 mm Javanol® and Sandalore® did not lead to cell damage (data not shown). Thus, the sandalwood-induced ATP release represents a physiological reaction of the skin cells.
FIGURE 4.
A, significantly increased ATP concentrations in the supernatant of sandalwood derivative-stimulated HaCaT cells (1 mm; n = 9). No ATP increase could be measured upon stimulation with histamine (100 μm; n = 15), citronellol (1 mm; n = 7), helional (1 mm; n = 8), and LPA (200 nm; n = 3). B, determination of the ATP concentration for Sandalore® (∼40 nm) and Javanol® (∼200 nm) from two independent experiments. C, quantitative analysis of the supernatant of Javanol®-stimulated HaCaT cells in the presence of carbenoxolon (CBX) (10 μm; n = 4), probenecid (Prob.; 10 μm; n = 3), and brefeldin A (BFA; 5 μg/ml; n = 4). Carbenoxolone and probenecid significantly inhibited the ATP release; CBX had no effect. D, the ATP receptor blocker PPADS had no effect on ATP release of HaCaT cells after stimulation with Javanol® (Javanol, 1 mm; PPADS, 200 μm; n = 4). PPADS application was initiated 90 s before and continued through the Javanol® stimulation (1 mm). *, p < 0.05; **, p < 0.01; **, p < 0.01; ns, not significant. Error bars, S.E.
The ATP Release by Keratinocytes Is Mediated by Pannexin 1 Hemichannels
Next, the mechanisms that lead to chemically induced ATP secretion from HaCaT keratinocytes were analyzed. A known mechanism of ATP release in keratinocytes is the release via connexins, pannexins, or exocytotic events (40, 41). For this purpose, the involvement of exocytotic events or gap junction-forming proteins was assayed by the use of specific inhibitors. The co-application of Javanol® and the connexin inhibitor carbenoxolone (42, 43) dramatically reduced the concentration of ATP released by HaCaT keratinocytes. Moreover, the co-application of the specific pannexin 1 inhibitor probenecid also blocked ATP release to the same extent, further implicating an involvement of pannexins and, more specifically, pannexin 1. Brefeldin A, a drug that blocks the movement of molecules from the endoplasmic reticulum to the trans-Golgi apparatus, had no inhibitory effect on the keratinocyte release of ATP (Fig. 4C).
Pretreatment of the Co-culture with the Global P2 Receptor Antagonist PPADS Inhibited the Indirect Activation of Trigeminal Neurons
To verify the participation of ATP as a mediator of the chemical communication events between human keratinocytes and mouse trigeminal neurons, calcium imaging experiments with co-cultures were performed in the presence of pyrodoxalphosphate-6-azophenyl-2′,4′-disulfonate (PPADS). PPADS is a previously described inhibitor of ATP receptors and affects a large number of P2X and P2Y receptors (reviewed by von Kügelgen (44)). Control experiments revealed that PPADS did not influence the odorant-mediated ATP release of HaCaT cells (Fig. 4D).
Application of PPADS for 90 s prior to and during the stimulation of co-cultures with Sandalore® and Javanol® inhibited trigeminal reactivity (Fig. 5A), whereas keratinocytes showed normal reactivity to the sandalwood substances. The quantification indicated the dramatic effect of PPADS co-application with either Javanol® or Sandalore® on trigeminal reactivity (Fig. 5B). In contrast, the histamine-induced cross-talk activity was not affected by co-application of PPADS in the co-culture approach (n = 82; Fig. 5, C and D).
FIGURE 5.
A, the ATP receptor blocker PPADS significantly inhibited the reactivity of trigeminal neurons in co-cultures. B, quantitative analysis of the responses of trigeminal neurons in co-cultures (TrigCoCu) and co-cultures in the presence of PPADS (TrigCoCu PPADS). PPADS lead to a decrease in the activity of trigeminal neurons upon Javanol® stimulation (Sandalore®: TrigCoCu n = 66 neurons in 23 co-cultures, TrigCoCu PPADS n = 88 in 17 co-cultures; Javanol®: TrigCoCu n = 25 neurons in 7 co-cultures, TrigCoCu PPADS n = 81 in 11 co-cultures). The ATP receptor blocker PPADS (200 μm) had no influence on reactivity (C) or calcium amplitudes (D) of trigeminal neurons in histamine (100 μm)-stimulated co-cultures (TrigCoCu, n = 29 neurons in 7 co-cultures) or histamine-stimulated co-cultures in the presence of PPADS (TrigCoCu PPADS, n = 89 neurons in 10 co-cultures). Amplitude levels were normalized to high potassium (K+; 45 mm). E, pannexin 1 inhibitor probenecid (10 μm) leads to a decrease in the activity of trigeminal neurons upon Javanol® stimulation (Javanol®: TrigCoCu n = 49 neurons in 5 co-cultures, TrigCoCu PPADS n = 47 in 6 co-cultures). F, quantification of Javanol®- and ATP-induced Ca2+ responses of HaCaT cells in co-cultures in the absence (n = 54 cells/72 cells) or presence of PPADS (n = 45 cells) or probenecid (n = 67 cells). Both inhibitors had no influence on reactivity of HaCaT cells in co-cultures. Shown are mean amplitudes of Ca2+ responses at repetitive applications of Javanol® (1–3; 1 mm) and ATP (100 μm, positive control). Amplitude levels were normalized to control measurements. G, quantification of high potassium (K+; 45 mm)-induced Ca2+ responses of trigeminal neurons in co-cultures in the absence (n = 43 neurons/48 neurons) or presence of PPADS (n = 43 neurons) or probenecid (n = 48 neurons). Amplitude levels were normalized to control measurements. Both inhibitors had no influence on reactivity of trigeminal neurons in co-cultures. In all measurements, PPADS (200 μm) or probenecid (10 μm) application was initiated 90 s before and continued through the Javanol® or K+ stimulation. *, p < 0.01; **, p < 0.01; ***, p < 0.001; ns, not significant; Fisher's exact test (B–E) or Student's t test (F and G).
To additionally prove that ATP serves as the mediating transmitter molecule released from skin cells after odor stimulation, we used the pannexin 1 inhibitor probenecid. In the presence of this blocker, a significant reduction of trigeminal activity was observed (Fig. 5E). Control experiments revealed that PPADS or probenecid did not influence keratinocyte or neuronal morphology or responsiveness to stimulation with 1 mm odorant or 100 μm ATP (positive control for HaCaT cells) and 100 μm high potassium (positive control for neurons) (Fig. 5, F and G).
Patch Clamp Characterization of Responding Trigeminal Neurons in the Co-culture
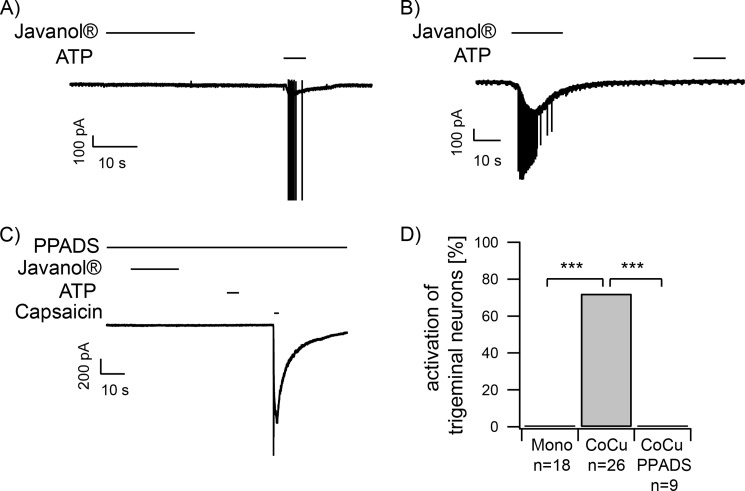
To further investigate the chemically induced communication events between skin cells and trigeminal neurons, patch clamp experiments were performed. This method allows for a rough differentiation between ATP-responsive neurons by comparing the signal amplitudes and enables the identification of the subgroup of ATP receptors potentially involved in the communication with keratinocytes. Recordings were conducted using the whole-cell mode with a holding potential of −60 mV. Javanol®, the most potent odorant enhancer of trigeminal activity in co-cultures, was applied. Again, application of this substance (1 mm) had no effect on monocultured trigeminal neurons (Fig. 6A). In the co-culture, trigeminal neurons located in close proximity to keratinocytes were preferentially examined. Upon keratinocyte stimulation with the given substance, activating inward currents were detected in ∼73% of the co-cultured neurons (Fig. 6, B and D). Interestingly, trigeminal neurons without any visible contact with keratinocytes (cell-cell contact or neurites) did not show activating currents after Javanol® stimulation (n = 4; data not shown). Moreover, ∼50% of the neurons reacted with a burst of action potentials. However, the onset of the inward sodium current that occurred subsequent to stimulation differed strongly. Inward currents in trigeminal neurons induced by Javanol stimulation of co-cultures desensitized within 10 s. With few exceptions, all responding neurons reacted only once. Subsequent ATP stimulation did not induce a current, probably indicating the desensitization of ATP-activated currents. However, the subsequent application of the TrpV1 agonist capsaicin (3.3 μm), used to perform a rough classification of the responding neurons, did induce inward currents signals in all Javanol®-reactive neurons in the co-culture.
FIGURE 6.
A and B, patch clamp recordings of trigeminal neurons in monoculture (A) and in co-culture with HaCaT keratinocytes (B). A, application of Javanol® (1 mm) did not induce currents in monocultured trigeminal neurons. ATP (20 μm) was used as a viability control. In co-cultures, Javanol® stimulation led to an inward current (B), which was not observed in the presence of 100 μm PPADS (C). D, quantification of the results presented in A–C. Javanol®-induced activation in percent for monocultured trigeminal neurons (Mono; n = 18 in 9 monocultures), co-cultured trigeminal neurons (CoCu; n = 26 neurons in 9 co-cultures), and co-cultured trigeminal neurons in the presence of PPADS (CoCu PPADS; n = 9 neurons in 3 co-cultures). ***, p < 0.001; Fisher's exact test.
According to our previous findings in calcium imaging, trigeminal reactivity was abolished in the presence of 100 μm PPADS (Fig. 5C). The inhibitory effect of PPADS was complete, as indicated by a lack of inward currents upon external ATP stimulation at the end of the experiment. Quantification of the PPADS effect revealed a complete reduction of the Javanol® effect (Fig. 6D). This indicates that the blocker was sufficient to completely inhibit Javanol®-induced communication events between HaCaT keratinocytes and trigeminal neurons in the co-culture.
DISCUSSION
Our data show that keratinocytes communicate with trigeminal neurons in response to odorant stimulation via ATP signaling. Keratinocytes release ATP upon chemical stimulation, leading to the subsequent activation of co-cultured trigeminal neurons, which fail to react to such stimuli in monoculture. These findings strongly support the hypothesis that trigeminal neurons receive environmental signals indirectly by transmission from adjacent cells in their peripheral innervation area. Previous studies have shown that mechanical and thermal stimulation of skin cells also leads to the release of ATP, which subsequently activates trigeminal neurons (27, 45). This result is strongly indicative for similar processing mechanisms and a general skin-nerve communication.
Almost all odorants induce trigeminal sensations; however, even at high concentrations, monocultured trigeminal neurons failed to react to several odorants. Thus, the complex receptor expression pattern of trigeminal neurons does not explain all chemosensory features associated with the trigeminal nerve. Keratinocytes respond with calcium transients to stimulation with substances from various chemical groups with the ability to elicit clear trigeminal excitation and thus, in some cases, are generally thought to be trigeminal activators (46). Because synthetic sandalwood odorants are often added to perfumes or creams and thus frequently contact skin cells, it is interesting that keratinocytes are able to detect and convert such stimuli through intrinsic pathways. The stimulation of keratinocytes with synthetic sandalwood derivatives, such as Sandalore® and Javanol® led to a robust increase in intracellular calcium in all tested primary keratinocyte cultures from various donors but never in primary trigeminal neuron cultures. We thus used Sandalore® and Javanol® to investigate possible communication events between keratinocytes and trigeminal neurons in a co-culture approach. The subsequent activation of co-cultured trigeminal neurons in response to prior keratinocyte stimulation by communication processes shows that sandalwood derivatives induced communication processes from keratinocytes to trigeminal neurons. Thus, we have demonstrated for the first time that, for several chemical cues, skin cells and not trigeminal neurons function as primary chemodetectors and, further, that the subsequent neuron excitation is caused indirectly by ATP release from the keratinocytes. Moreover, because trigeminal neurons in monoculture fail to react to such stimuli, our findings demonstrate an essential role of skin cells in trigeminal chemodetection.
In the co-culture approach, the majority of subsequently activated neurons reacted only once. However, the latencies between calcium signal induction in skin cells and trigeminal neurons varied and did not appear to be linked to the distance between reacting cells. This result probably indicates that neurites, invisible due to the high culture confluence, proceed among the keratinocyte layers and come into direct contact with reacting keratinocytes at a distance. In this context, we observed that trigeminal neurons in direct contact with keratinocytes or neurons connected with keratinocytes via their neurites were able to communicate. This also explains why not all neurons of the co-culture communicated with keratinocytes. Former studies describe the axonal outgrowth of sensory neurons supported by co-culturing with keratinocytes (47, 48). However, no junctions, such as synaptic contacts, have been identified between the co-cultured keratinocytes and sensory fibers under these conditions. In the intact skin, ultrastructural analysis revealed that keratinocytes and fibers of dorsal root ganglion neurons come in close contact through membrane-membrane apposition (49). Antibody staining of respective pre- and postsynaptic markers failed, supporting the lack of synaptic contacts between keratinocytes and trigeminal neurons in the co-culture approach described here.
Supernatant analysis identified ATP as the transmitting substance of chemical signals, and this result was verified by blocking the communication events by the ATP receptor blocker PPADS and the pannexin 1 inhibitor probenecid. ATP appears to be a common transmitter of external stimuli. Numerous mechanisms for the release of ATP from keratinocytes have been discussed to date: ATP-binding cassettes, exocytosis, connexins, and pannexins (40, 41, 50, 51). However, the successful inhibition of Javanol®-induced communication using the selective inhibitor probenecid (42) implicates pannexin 1. This was further emphasized by the described lack of synaptic structures between keratinocytes and trigeminal neurons in the co-culture system. However, the mechanism of how calcium ions activate pannexins has not yet been fully elucidated (52).
ATP release from keratinocytes has already been described as a consequence of mechanical and thermal stimulation and subsequently induces processing of mechanical information through the activation of P2Y2 or P2X2 receptors on trigeminal neurons (27, 45, 53). An odorant-induced ATP release from olfactory receptor neurons was recently observed by Spehr et al. (13). Because these different types of stimuli induce ATP release from skin cells, the stimulus quality has to be transported in another manner, probably by the co-release of additional transmitters. The activation of P2 receptors is an important tool for regulating processes in many tissues, such as skin blood flow and wound healing (54, 55). P2 receptors comprise two subgroups, the ionotropic P2X receptors and the metabotropic P2Y receptors (56). Subtypes from both groups show tissue-dependent expression (57–60). A previous investigation in our laboratory investigated ATP-mediated currents in trigeminal neurons (13). A comparison of the kinetics of the inward currents induced by the sandalwood derivatives measured by patch clamp analysis with our previous data suggests an involvement of P2X2 receptors in the communication processes described here. In contrast to P2X3, P2X2 shows inward currents that weakly desensitize during ATP application. Because these receptors are located on the somata and neuron processes, the stimulation may occur via neurites that are located in close proximity to the HaCaT cells (13). 20% of the trigeminal neurons express P2X3, 36% express P2X2/3, and 40% express P2X2 receptors (13). In contrast to weakly desensitizing homomultimeric P2X2 receptors, P2X3-containing receptors quickly desensitize. Therefore, communication events based on P2X3 receptors probably cannot be detected in neurons of our co-culture system. Therefore, the rate of communication is probably even higher, as suggested by our measurements.
In the skin, nucleotide signaling is involved in proliferation and differentiation and thus essentially contributes to the regulation of the epidermal barrier. Primary keratinocytes, HaCaT cells (61), and trigeminal neurons express functional P2 receptors of either subgroup. As described previously (13, 62), trigeminal neurons express a series of purinergic receptors. PPADS was used to antagonize P2X and P2Y receptors with effects on numerous receptor subtypes (reviewed by von Kügelgen (44)). In patch clamp analysis, PPADS reduced trigeminal activity after Javanol® stimulation to control levels. We showed that the amount of ATP released from keratinocytes after Sandalore® or Javanol® stimulation was sufficient to activate the purinergic receptors of the co-cultured trigeminal neurons. These results indicate that extracellular communication by ATP plays a major role in intercellular signaling.
In addition, non-odorant substances, such as histamine, induced strong repetitive calcium signals in keratinocytes that were also transmitted to trigeminal neurons. Histamine plays a central role in allergic responses of external sensitizers in the body. In addition, histamine is a transmitter molecule in inflammatory processes and induces burning sensations (63–67), leading to itching or pain (68, 69). Histamine release occurs from vesicles in mast cells or basophils in IgE-mediated allergic reactions (70). This leads to the activation of keratinocytes, which in turn release a transmitter to excite free nerve endings in the epidermis. However, histamine stimulation did not result in ATP release from keratinocytes, and the histamine-released transmitting molecule is still unknown. Nevertheless, in our co-culture experiments, signal transmission to trigeminal neurons was observed. This result demonstrates that, depending on the type of the chemical stimulus, different signal mechanisms exist for how skin cells transmit signals to adjacent neurons. The occurrence of different transduction mechanisms would enable the skin to encode information regarding stimulus identity.
In summary, we have shown for the first time that chemical stimulation of keratinocytes and HaCaT cells, here induced by the sandalwood derivative Sandalore® or Javanol®, leads to an increase in intracellular calcium concentrations. In addition, it induces a release of ATP mediated by pannexin 1. Secreted ATP, in turn, activates ATP receptors, probably P2X2, in co-cultured trigeminal neurons and, thus, mediates information processing between the skin and the nervous system upon chemical stimulation. The described mechanism emphasizes a cutaneous contribution to trigeminal function.
Acknowledgments
We thank Harry Bartels and Franziska Mössler for excellent technical assistance and Dennis Roggenkamp for suggestions regarding the manuscript.
Footnotes
- TG
- trigeminal ganglia
- Trp
- transient receptor potential
- LPA
- lysophosphatidic acid
- PPADS
- pyridoxalphosphate-6-azophenyl-2′,4′-disulfonate
- TrigMono
- trigeminal neurons in monocultures
- TrigCoCu
- trigeminal neurons in co-cultures.
REFERENCES
- 1. Tucker D. (1971) Nonolfactory Responses from the Nasal Cavity: Jacobson's Organ and the Trigeminal System (Beidler L. M., ed), pp. 164–169, Springer, Berlin [Google Scholar]
- 2. Luzardo-Baptista M. (1973) Intraepithelial nerve fibers in the human oral mucosa: an electron microscopic study. Oral. Surg Oral. Med. Oral. Pathol. 35, 372–376 [DOI] [PubMed] [Google Scholar]
- 3. Zahm D. S., Munger B. L. (1985) The innervation of the primate fungiform papilla: development, distribution and changes following selective ablation. Brain Res. 356, 147–186 [DOI] [PubMed] [Google Scholar]
- 4. Finger T. E., Böttger B. (1990) Transcellular labeling of taste bud cells by carbocyanine dye (DiI) applied to peripheral nerves in the barbels of the catfish, Ictalurus punctatus. J. Comp. Neurol. 302, 884–892 [DOI] [PubMed] [Google Scholar]
- 5. Silver W. L., Farley L. G., Finger T. E. (1991) The effects of neonatal capsaicin administration on trigeminal nerve chemoreceptors in the rat nasal cavity. Brain Res. 561, 212–216 [DOI] [PubMed] [Google Scholar]
- 6. Royster M., Driscoll P., Kelly P. A., Freemark M. (1995) The prolactin receptor in the fetal rat: cellular localization of messenger ribonucleic acid, immunoreactive protein, and ligand-binding activity and induction of expression in late gestation. Endocrinology 136, 3892–3900 [DOI] [PubMed] [Google Scholar]
- 7. Caterina M. J., Schumacher M. A., Tominaga M., Rosen T. A., Levine J. D., Julius D. (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824 [DOI] [PubMed] [Google Scholar]
- 8. Bonaventure P., Voorn P., Luyten W. H., Leysen J. E. (1998) 5HT1B and 5HT1D receptor mRNA differential co-localization with peptide mRNA in the guinea pig trigeminal ganglion. NeuroReport 9, 641–645 [DOI] [PubMed] [Google Scholar]
- 9. Liu L., Chang G. Q., Jiao Y. Q., Simon S. A. (1998) Neuronal nicotinic acetylcholine receptors in rat trigeminal ganglia. Brain Res. 809, 238–245 [DOI] [PubMed] [Google Scholar]
- 10. Zhu J. J., Uhlrich D. J. (1998) Cellular mechanisms underlying two muscarinic receptor-mediated depolarizing responses in relay cells of the rat lateral geniculate nucleus. Neuroscience 87, 767–781 [DOI] [PubMed] [Google Scholar]
- 11. Alimohammadi H., Silver W. L. (2000) Evidence for nicotinic acetylcholine receptors on nasal trigeminal nerve endings of the rat. Chem. Senses 25, 61–66 [DOI] [PubMed] [Google Scholar]
- 12. Lang P. M., Burgstahler R., Sippel W., Irnich D., Schlotter-Weigel B., Grafe P. (2003) Characterization of neuronal nicotinic acetylcholine receptors in the membrane of unmyelinated human C-fiber axons by in vitro studies. J. Neurophysiol. 90, 3295–3303 [DOI] [PubMed] [Google Scholar]
- 13. Spehr J., Spehr M., Hatt H., Wetzel C. H. (2004) Subunit-specific P2X-receptor expression defines chemosensory properties of trigeminal neurons. Eur. J. Neurosci. 19, 2497–2510 [DOI] [PubMed] [Google Scholar]
- 14. McKemy D. D., Neuhausser W. M., Julius D. (2002) Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416, 52–58 [DOI] [PubMed] [Google Scholar]
- 15. Gu Y., Huang L.-Y. (1994) Modulation of glycine affinity for NMDA receptors by extracellular Ca2+ in trigeminal neurons. J. Neurosci. 14, 4561–4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Durkin M. M., Gunwaldsen C. A., Borowsky B., Jones K. A., Branchek T. A. (1999) An in situ hybridization study of the distribution of the GABA(B2) protein mRNA in the rat CNS. Brain Res. Mol. Brain Res. 71, 185–200 [DOI] [PubMed] [Google Scholar]
- 17. Silver W. (1992) Neural and pharmacological basis for nasal irritation. Ann. N.Y. Acad. Sci. 641, 152–163 [DOI] [PubMed] [Google Scholar]
- 18. Finger T. E., Böttger B., Hansen A., Anderson K. T., Alimohammadi H., Silver W. L. (2003) Solitary chemoreceptor cells in the nasal cavity serve as sentinels of respiration. Proc. Natl. Acad. Sci. U.S.A. 100, 8981–8986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cometto-Muñiz J. E., Cain W. S., Abraham M. H. (2004) Chemosensory additivity in trigeminal chemoreception as reflected by detection of mixtures. Exp. Brain Res. 158, 196–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lumpkin E. A., Caterina M. J. (2007) Mechanisms of sensory transduction in the skin. Nature 445, 858–865 [DOI] [PubMed] [Google Scholar]
- 21. Lin W., Ogura T., Margolskee R. F., Finger T. E., Restrepo D. (2008) TRPM5-expressing solitary chemosensory cells respond to odorous irritants. J. Neurophysiol. 99, 1451–1460 [DOI] [PubMed] [Google Scholar]
- 22. Doty R. L., Brugger W. E., Jurs P. C., Orndorff M. A., Snyder P. J., Lowry L. D. (1978) Intranasal trigeminal stimulation from odorous volatiles: psychometric responses from anosmic and normal humans. Physiol. Behav. 20, 175–185 [DOI] [PubMed] [Google Scholar]
- 23. Mason J. R., Silver W. L. (1983) Trigeminally mediated odor aversions in starlings. Brain Res. 269, 196–199 [DOI] [PubMed] [Google Scholar]
- 24. Denda M., Fuziwara S., Inoue K., Denda S., Akamatsu H., Tomitaka A., Matsunaga K. (2001) Immunoreactivity of VR1 on epidermal keratinocyte of human skin. Biochem. Biophys. Res. Commun. 285, 1250–1252 [DOI] [PubMed] [Google Scholar]
- 25. Inoue K., Koizumi S., Fuziwara S., Denda S., Inoue K., Denda M. (2002) Functional vanilloid receptors in cultured normal human epidermal keratinocytes. Biochem. Biophys. Res. Commun. 291, 124–129 [DOI] [PubMed] [Google Scholar]
- 26. Denda M., Denda S. (2007) Air-exposed keratinocytes exhibited intracellular calcium oscillation. Skin Res. Technol. 13, 195–201 [DOI] [PubMed] [Google Scholar]
- 27. Koizumi S., Fujishita K., Inoue K., Shigemoto-Mogami Y., Tsuda M., Inoue K. (2004) Ca2+ waves in keratinocytes are transmitted to sensory neurons: the involvement of extracellular ATP and P2Y2 receptor activation. Biochem. J. 380, 329–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tsutsumi M., Inoue K., Denda S., Ikeyama K., Goto M., Denda M. (2009) Mechanical-stimulation-evoked calcium waves in proliferating and differentiated human keratinocytes. Cell Tissue Res. 338, 99–106 [DOI] [PubMed] [Google Scholar]
- 29. Kalmes M., Neumeyer A., Rio P., Hanenberg H., Fritsche E., Blömeke B. (2006) Impact of the arylhydrocarbon receptor on eugenol- and isoeugenol-induced cell cycle arrest in human immortalized keratinocytes (HaCaT). Biol. Chem. 387, 1201–1207 [DOI] [PubMed] [Google Scholar]
- 30. Fulbright R. K., Skudlarski P., Lacadie C. M., Warrenburg S., Bowers A. A., Gore J. C., Wexler B. E. (1998) Functional MR imaging of regional brain responses to pleasant and unpleasant odors. Am. J. Neuroradiol. 19, 1721–1726 [PMC free article] [PubMed] [Google Scholar]
- 31. Boukamp P., Petrussevska R. T., Breitkreutz D., Hornung J., Markham A., Fusenig N. E. (1988) Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 106, 761–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jacob T. J., Wang L., Jaffer S., McPhee S. (2006) Changes in the odor quality of androstadienone during exposure-induced sensitization. Chem. Senses 31, 3–8 [DOI] [PubMed] [Google Scholar]
- 33. Story G. M., Peier A. M., Reeve A. J., Eid S. R., Mosbacher J., Hricik T. R., Earley T. J., Hergarden A. C., Andersson D. A., Hwang S. W., McIntyre P., Jegla T., Bevan S., Patapoutian A. (2003) ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112, 819–829 [DOI] [PubMed] [Google Scholar]
- 34. Macpherson L. J., Hwang S. W., Miyamoto T., Dubin A. E., Patapoutian A., Story G. M. (2006) More than cool: promiscuous relationships of menthol and other sensory compounds. Mol. Cell. Neurosci. 32, 335–343 [DOI] [PubMed] [Google Scholar]
- 35. Stotz S. C., Vriens J., Martyn D., Clardy J., Clapham D. E. (2008) Citral sensing by TRANSient receptor potential channels in dorsal root ganglion neurons. PLoS One 3, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ohkawara S., Tanaka-Kagawa T., Furukawa Y., Nishimura T., Jinno H. (2010) Activation of the human transient receptor potential vanilloid subtype 1 by essential oils. Biol. Pharm. Bull. 33, 1434–1437 [DOI] [PubMed] [Google Scholar]
- 37. Giustizieri M. L., Albanesi C., Fluhr J., Gisondi P., Norgauer J., Girolomoni G. (2004) H1 histamine receptor mediates inflammatory responses in human keratinocytes. J. Allergy Clin. Immunol. 114, 1176–1182 [DOI] [PubMed] [Google Scholar]
- 38. Lichte K., Rossi R., Danneberg K., ter Braak M., Kürschner U., Jakobs K. H., Kleuser B., Meyer zu Heringdorf D. (2008) Lysophospholipid receptor-mediated calcium signaling in human keratinocytes. J. Invest. Dermatol. 128, 1487–1498 [DOI] [PubMed] [Google Scholar]
- 39. Huang S. M., Lee H., Chung M.-K., Park U., Yu Y. Y., Bradshaw H. B., Coulombe P. A., Walker J. M., Caterina M. J. (2008) Overexpressed transient receptor potential vanilloid 3 ion channels in skin keratinocytes modulate pain sensitivity via prostaglandin E2. J. Neurosci. 28, 13727–13737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stout C. E., Costantin J. L., Naus C. C., Charles A. C. (2002) Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J. Biol. Chem. 277, 10482–10488 [DOI] [PubMed] [Google Scholar]
- 41. Coco S., Calegari F., Pravettoni E., Pozzi D., Taverna E., Rosa P., Matteoli M., Verderio C. (2003) Storage and release of ATP from astrocytes in culture. J. Biol. Chem. 278, 1354–1362 [DOI] [PubMed] [Google Scholar]
- 42. Silverman W., Locovei S., Dahl G. (2008) Probenecid, a gout remedy, inhibits pannexin 1 channels. Am. J. Physiol. Cell Physiol. 295, C761–C767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ma W., Hui H., Pelegrin P., Surprenant A. (2009) Pharmacological characterization of pannexin-1 currents expressed in mammalian cells. J. Pharmacol. Exp. Ther. 328, 409–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. von Kügelgen I. (2008) Pharmacology of mammalian P2X- and P2Y-receptors. BIOTREND Review 3/9-2008, BIOTREND Chemicals AG, Wangen, Switzerland [Google Scholar]
- 45. Mandadi S., Sokabe T., Shibasaki K., Katanosaka K., Mizuno A., Moqrich A., Patapoutian A., Fukumi-Tominaga T., Mizumura K., Tominaga M. (2009) TRPV3 in keratinocytes transmits temperature information to sensory neurons via ATP. Pflugers Arch. 458, 1093–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Inoue T., Bryant B. P. (2005) Multiple types of sensory neurons respond to irritating volatile organic compounds (VOCs): calcium fluorimetry of trigeminal ganglion neurons. Pain 117, 193–203 [DOI] [PubMed] [Google Scholar]
- 47. Ulmann L., Rodeau J.-L., Danoux L., Contet-Audonneau J.-L., Pauly G., Schlichter R. (2007) Trophic effects of keratinocytes on the axonal development of sensory neurons in a coculture model. Eur. J. Neurosci. 26, 113–125 [DOI] [PubMed] [Google Scholar]
- 48. Roggenkamp D., Falkner S., Stäb F., Petersen M., Schmelz M., Neufang G. (2012) Atopic keratinocytes induce increased neurite outgrowth in a coculture model of porcine dorsal root ganglia neurons and human skin cells. J. Invest. Dermatol. 132, 1892–1900 [DOI] [PubMed] [Google Scholar]
- 49. Hilliges M., Wang L., Johansson O. (1995) Ultrastructural evidence for nerve fibers within all vital layers of the human epidermis. J. Invest. Dermatol. 104, 134–137 [DOI] [PubMed] [Google Scholar]
- 50. Darby M., Kuzmiski J. B., Panenka W., Feighan D., MacVicar B. A. (2003) ATP released from astrocytes during swelling activates chloride channels. J. Neurophysiol. 89, 1870–1877 [DOI] [PubMed] [Google Scholar]
- 51. Braunstein G. M., Roman R. M., Clancy J. P., Kudlow B. A., Taylor A. L., Shylonsky V. G., Jovov B., Peter K., Jilling T., Ismailov I. I., Benos D. J., Schwiebert L. M., Fitz J. G., Schwiebert E. M. (2001) Cystic fibrosis transmembrane conductance regulator facilitates ATP release by stimulating a separate ATP release channel for autocrine control of cell volume regulation. J. Biol. Chem. 276, 6621–6630 [DOI] [PubMed] [Google Scholar]
- 52. Braet K., Aspeslagh S., Vandamme W., Willecke K., Martin P. E., Evans W. H., Leybaert L. (2003) Pharmacological sensitivity of ATP release triggered by photoliberation of inositol-1,4,5-trisphosphate and zero extracellular calcium in brain endothelial cells. J. Cell. Physiol. 197, 205–213 [DOI] [PubMed] [Google Scholar]
- 53. Mizumoto N., Mummert M. E., Shalhevet D., Takashima A. (2003) Keratinocyte ATP release assay for testing skin-irritating potentials of structurally diverse chemicals. J. Invest. Dermatol. 121, 1066–1072 [DOI] [PubMed] [Google Scholar]
- 54. Gendaszewska-Darmach E., Kucharska M. (2011) Nucleotide receptors as targets in the pharmacological enhancement of dermal wound healing. Purinergic Signal. 7, 193–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Koganezawa T., Ishikawa T., Fujita Y., Yamashita T., Tajima T., Honda M., Nakayama K. (2006) Local regulation of skin blood flow during cooling involving presynaptic P2 purinoceptors in rats. Br. J. Pharmacol. 148, 579–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Abbracchio M. P., Burnstock G., Boeynaems J. M., Barnard E. A., Boyer J. L., Kennedy C., Knight G. E., Fumagalli M., Gachet C., Jacobson K. A., Weisman G. A. (2006) International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol. Rev. 58, 281–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pillai S., Bikle D. D. (1992) Adenosine triphosphate stimulates phosphoinositide metabolism, mobilizes intracellular calcium, and inhibits terminal differentiation of human epidermal keratinocytes. J. Clin. Invest. 90, 42–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Reimer W. J., Dixon S. J. (1992) Extracellular nucleotides elevate [Ca2+]i in rat osteoblastic cells by interaction with two receptor subtypes. Am. J. Physiol. 263, C1040–C1048 [DOI] [PubMed] [Google Scholar]
- 59. Ralevic V., Burnstock G. (1998) Receptors for purines and pyrimidines. Pharmacol. Rev. 50, 413–492 [PubMed] [Google Scholar]
- 60. Dixon C. J., Bowler W. B., Littlewood-Evans A., Dillon J. P., Bilbe G., Sharpe G. R., Gallagher J. A. (1999) Regulation of epidermal homeostasis through P2Y2 receptors. Br. J. Pharmacol. 127, 1680–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Burrell H. E., Bowler W. B., Gallagher J. A., Sharpe G. R. (2003) Human keratinocytes express multiple P2Y-receptors: evidence for functional P2Y1, P2Y2, and P2Y4 receptors. J. Invest. Dermatol. 120, 440–447 [DOI] [PubMed] [Google Scholar]
- 62. Damann N., Rothermel M., Klupp B. G., Mettenleiter T. C., Hatt H., Wetzel C. H. (2006) Chemosensory properties of murine nasal and cutaneous trigeminal neurons identified by viral tracing. BMC Neurosci. 7, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dekanski J. (1945) The effect of cutaneous burns on histamine in mice. J. Physiol. 104, 151–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Weltman J. K. (2000) Update on histamine as a mediator of inflammation. Allergy Asthma Proc. 21, 125–128 [DOI] [PubMed] [Google Scholar]
- 65. MacGlashan D. (2003) Histamine: a mediator of inflammation. J. Allergy Clin. Immunol. 112, S53–S59 [DOI] [PubMed] [Google Scholar]
- 66. Bell J. K., McQueen D. S., Rees J. L. (2004) Involvement of histamine H4 and H1 receptors in scratching induced by histamine receptor agonists in Balb C mice. Br. J. Pharmacol. 142, 374–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rossbach K., Wendorff S., Sander K., Stark H., Gutzmer R., Werfel T., Kietzmann M., Bäumer W. (2009) Histamine H4 receptor antagonism reduces hapten-induced scratching behaviour but not inflammation. Exp. Dermatol. 18, 57–63 [DOI] [PubMed] [Google Scholar]
- 68. Mobarakeh J. I., Sakurada S., Katsuyama S., Kutsuwa M., Kuramasu A., Lin Z. Y., Watanabe T., Hashimoto Y., Watanabe T., Yanai K. (2000) Role of histamine H1 receptor in pain perception: a study of the receptor gene knockout mice. Eur. J. Pharmacol. 391, 81–89 [DOI] [PubMed] [Google Scholar]
- 69. Shim W.-S., Oh U. (2008) Histamine-induced itch and its relationship with pain. Mol. Pain 4, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Church M. K., Hiroi J. (1987) Inhibition of IgE-dependent histamine release from human dispersed lung mast cells by anti-allergic drugs and salbutamol. Br. J. Pharmacol. 90, 421–429 [DOI] [PMC free article] [PubMed] [Google Scholar]