Background: Mechanism by which Wnt expression was increased by carrageenan exposure was unknown.
Results: Sp1 activated Wnt9A transcription through changes in arylsulfatase B, chondroitin 4-sulfation, and galectin-3.
Conclusion: Decline in arylsulfatase B leads to transcriptional effects mediated by Sp1 and galectin-3.
Significance: Extracellular events can regulate transcription through changes in arylsulfatase B and chondroitin 4-sulfation.
Keywords: Animal Model, Cancer Biology, Chondroitin Sulfate, Galectin, Specificity Protein 1 (Sp1), Carrageenan, Wnt
Abstract
In cultured human colonic epithelial cells and mouse colonic tissue, exposure to the common food additive carrageenan leads to inflammation, activation of Wnt signaling, increased Wnt9A expression, and decline in the activity of the enzyme arylsulfatase B (ARSB; N-acetylgalactosamine-4-sulfatase). In this study, the novel transcriptional mechanism by which carrageenan and decline in ARSB increase Wnt9A expression in NCM460 and HT-29 human colonic epithelial cells and in mouse colon is presented. Increased expression of Wnt9A has been associated with multiple malignancies, including colon carcinoma, and with ectodermal and mesoendodermal morphogenesis. When ARSB activity was reduced by siRNA or by exposure to carrageenan (1 μg/ml for 24 h), degradation of chondroitin 4-sulfate (C4S) was inhibited, leading to accumulation of more highly sulfated C4S, which binds less galectin-3, a β-galactoside-binding protein. Nuclear galectin-3 increased and mediated increased binding of Sp1 to the Sp1 consensus sequence in the Wnt9A promoter, shown by oligonucleotide-binding assay and by chromatin immunoprecipitation assay. When galectin-3 was silenced, the increases in Sp1 binding to the Wnt9A promoter and in Wnt9A expression, which followed carrageenan or ARSB silencing, were inhibited. Mithramycin A, a specific inhibitor of Sp1 oligonucleotide binding, and Sp1 siRNA blocked the carrageenan- and ARSB siRNA-induced increases in Wnt9A expression. These studies reveal how carrageenan exposure can lead to transcriptional events in colonic epithelial cells through decline in arylsulfatase B activity, with subsequent impact on C4S, galectin-3, Sp1, and Wnt9A and can exert significant effects on Wnt-initiated signaling and related vital cell processes.
Introduction
The Wnt-signaling pathways are characterized as the canonical Wnt-signaling pathway, the non-canonical cell polarity pathway, and the non-canonical Wnt-calcium pathway. These signaling mechanisms are activated by an extracellular Wnt protein binding with a Frizzled cell receptor, with subsequent effects on the regulation of gene transcription, the organization of the cytoskeleton, and the mobilization of intracellular calcium. First identified in malignancies, the conserved Wnt pathways have been recognized as critically important in cell fate determinations in development and in tissue repair (1, 2).
In recent work, we have shown that the common food additive carrageenan, which is composed of sulfated or non-sulfated galactose residues, linked in alternating β-1,4 and α-1,3-galactosidic bonds, activated Wnt signaling in human colonic epithelial cells and in the mouse colon (3). This activation occurred through modulation of the nucleoredoxin-dishevelled interaction, by inhibition of thioredoxin reductase activity. The cytoplasmic β-catenin destruction complex, which blocks the nuclear translocation of β-catenin and thereby the subsequent transcriptional events that require nuclear β-catenin, was inhibited by the increased availability of dishevelled, which followed exposure to carrageenan. Increased availability of dishevelled resulted from less binding of dishevelled with nucleoredoxin when nucleoredoxin was oxidized due to impaired thioredoxin reductase activity. The increase in free dishevelled activated Wnt signaling due to effects on the cytoplasmic β-catenin destruction complex, which includes axin, casein kinase 1α, adenomatous polyposis coli, and glycogen synthase kinase-3 (4, 5).
Previous results also demonstrated that carrageenan exposure increased the expression of Wnt9A (also known as Wnt14) in the NCM460 human colonic epithelial cell line and in the C57BL/6J mouse colon (3, 6). The Wnt9 genes have been recognized as critically important in the ancestral patterning of ectodermal and mesoendodermal morphogenesis (7). These effects of carrageenan, on stimulation of both Wnt9A expression and Wnt/β-catenin signaling, demonstrate the profound influence of a common dietary component on a major pathway of colon carcinogenesis.
In this report, we have investigated the transcriptional mechanism by which carrageenan exposure leads to increase in Wnt9A expression in human colonic epithelial cells and in mouse colonic tissue. Increased expression of Wnt9A was previously recognized in human gastric and colonic carcinomas in association with high microsatellite instability (8) and in mammary, pancreatic, esophageal, cervical, and brain malignancies (9). Wnt9A promoter was identified as a bidirectional promoter (Wnt9A/CD558500), which was frequently hypermethylated, as shown in five of fifteen colonic carcinomas, of which two had a methylation density >40% (10).
The Wnt9A promoter has binding sites for the transcription factor Sp1 (specificity protein 1). Sp1 has zinc finger motifs by which it binds directly to DNA at the GC box element by the established consensus sequence 5′-GGGGCGGGG or (G/T)GGGCGG(G/A)(G/A)(C/T)-3′ (11). Multiple studies have confirmed that mutation of this consensus sequence leads to reduced promoter activation and expression of target genes (12–15). Sp1 was present in 74.8% of colon cancer tissues examined in one series, compared with presence in 42.2% of the normal colonic mucosa examined, and was overexpressed in colonic cancer stem cells (16). Sp1 has also been identified as a prognostic indicator in colorectal cancers (17, 18). Many established interactions of Sp1 with other proteins and enzymes involve post-translational modifications and include interactions with histone deacetylases (19). Interaction between Sp1 and galectin-3 was reported to enhance cyclin D1 promoter activity through Sp1 and cAMP-responsive element binding sites and to thereby contribute to enhanced proliferation in mammary epithelial cells (20).
In our previous work, we have reported a novel transcriptional mechanism, whereby decline in ARSB can lead to increased transcription of HIF-1α and of versican, due to reduced binding of galectin-3 with chondroitin 4-sulfate when chondroitin 4-sulfate (C4S)2 is more highly sulfated following decline in ARSB activity (21, 22). This finding is consistent with the reported decline in binding of galectin-3, -7, and -9 to more highly sulfated chondroitin, which was observed by frontal affinity chromatography (23). Coincident with reduced binding of galectin-3 to C4S, we have shown increases in nuclear galectin-3, which enabled transcriptional events mediated by AP-1, including increased expression of HIF-1α and versican, in human bronchial, colonic, and prostate cells (21, 22). Galectin-3 interacts with heterogeneous ribonucleoprotein and may contribute to malignant transformation in various epithelial tissues, including human colonic cells (21, 24–26).
Because carrageenan exposure reduces activity of arylsulfatase B (ARSB; N-acetylgalactosamine-4-sulfatase) (27), we have investigated the transcriptional mechanism whereby carrageenan leads to increased expression of Wnt9A in relation to decline in ARSB. The Wnt9A promoter has consensus oligonucleotide binding sites for Sp1 (28), and we investigated whether carrageenan exposure and ARSB silencing could lead to activation of the Wnt9A promoter and increased Wnt9A expression in colonic epithelium through increased oligonucleotide binding of Sp1, mediated by galectin-3.
Previous work identified reduction of ARSB in human colonic, prostatic, and mammary malignant cells and tissues, as well as in metastatic colonic cells, suggesting that the changes in chondroitin 4-sulfate that follow decline in ARSB can have profound effects on cell proliferation and vital cell processes (21, 29–33). Because carrageenan exposure reduces ARSB activity, we assessed the impact of the pathway involving arylsulfatase B, chondroitin-4-sulfate, galectin-3, and Sp1 on the transcriptional activation of Wnt9A, and present this pathway for the first time in this report.
EXPERIMENTAL PROCEDURES
Cell Lines and Mouse Models
NCM460 cells are from a nontransfected, human colonic epithelial cell line, originally derived from the normal colonic mucosa of a 68-year-old Hispanic male (34). NCM460 cells were grown in M3:10A medium (INCELL, San Antonio, TX) at 37 °C in a humidified 5% CO2 environment in multiwell culture plates and treated with 1 μg/ml of λ-carrageenan (Sigma-Aldrich) for 24 h. At the end of the treatment, cells were harvested, and spent media were collected from control and treated wells and stored at −80 °C until further analysis. The HT-29 (ATCC HTB-38) cell line, a human colonic adenocarcinoma cell line, was grown in DMEM with 10% FBS and treated similarly to the NCM460 cells.
Eight-week-old male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) were housed in the Institutional Animal Care and Use Committee (IACUC)-approved Veterinary Medicine Unit at the Jesse Brown Veterans Affairs Medical Center (Chicago, IL) with routine light-dark cycles. Heterozygous arylsulfatase B-deficient mice were obtained (strain 005598; The Jackson Laboratory) and bred (22, 35). Genotyping was performed to detect the mutation in ARSB (NM_009712.3), a G-to-T mutation at residue 94,560,057 (University of California, Santa Cruz database) in exon 2, leading to truncation and reduced activity. No changes in arylsulfatase A, galactose-6-sulfatase, or steroid sulfatase activity were detected in the ARSB-null mice. All procedures were performed in accordance with a protocol approved by the Animal Care Committee of the Jesse Brown Veterans Affairs Medical Center and the University of Illinois at Chicago. Mice were provided with standard mouse chow (65% carbohydrate, 20% protein, and 15% fat) and drank water ad libitum. In the carrageenan-exposed group of C57BL/6J mice, water was supplemented with undegraded carrageenan (10–100 μg/ml; Sigma) for 4 weeks. Weight and water consumption were measured routinely until the time of euthanasia, which was performed by carbon dioxide inhalation and cervical dislocation. Colonic tissue was immediately harvested and frozen at −80 °C until further experiments. For further studies, colon tissue was thawed, and the epithelium was scraped.
Wnt9A, ARSB, Galectin-3, and Sp1 Expression
To determine differences in mRNA expression following carrageenan exposure, ARSB silencing, and galectin-3 silencing, real-time polymerase chain reaction was performed (QRT-PCR; Realplex, Eppendorf, Hamburg, Germany). Total RNA was extracted and purified from cultured NCM460 cells and HT-29 cells and mouse colonic tissue by RNeasy Minikit (Qiagen, Valencia, CA). Equal amounts of purified RNAs from the control and treated cells were reverse-transcribed and amplified using Brilliant® SYBR Green QRT-PCR Master Mix (Stratagene). Human β-actin was used as an internal control. Cycle threshold (Ct) was determined for Wnt9A (NCBI gene ID 7483) and β-actin expression during the exponential phase of amplification. Primers were designed using Primer3 (36) and for Wnt9A were as follows: human, 5′-CAGCAGCAAGTTCGTCAAGC-3′ (forward) and 5′-TTGCCCACCTCATGGAAAG-3′ (reverse); and mouse, 5′-CATAAGCAAAAGGGTTGAGGAG-3′ (forward) and 5′-GGGCACAGGGTTACAAACAA-3′ (reverse). Primers for galectin-3 (LGALS3; NCBI gene ID 3958) were as follows: 5′-GGCCACTGATTGTGCCTTAT-3′ (forward) and 5′-AAGCGTGGGTTAAAGTGGAAG-3′ (reverse). Primers for ARSB (NCBI gene ID 411) expression were as follows: 5′-AGACTTTGGCAGGGGGTAAT-3′ (forward) and 5′-CAGCCAGTCAGAGATGTGGA-3′ (reverse). Fold changes in expression were determined from the differences between the Ct values using the formula: fold change = 2Δ3 with Δ3 = Δ1 − Δ2, where Δ1 represents Ctcontrol target gene − Ctcontrol actin and Δ2 indicates Cttreated target gene − Cttreated actin.
Quantification of ARSB protein in the NCM460 cells was performed by ARSB ELISA (MyBioSource, Inc., San Diego, CA) using the recommended methods. Galectin-3 protein was quantified by ELISA (R&D Systems, Minneapolis, MN) following the recommended procedures. Wnt9A protein was detected in the spent media of the NCM460 cells following 5× concentration of the media by Western blot with equal loading of protein (150 μg), as described previously (3). Sp1 protein was detected by Sp1 ELISA (MyBioSource, Inc.), using established procedures.
Measurements of ARSB Activity, Total Sulfated Glycosaminoglycans, and Chondroitin 4-Sulfate
ARSB was measured by a fluorometric assay, following a standard protocol using 20 μl of homogenate and 80 μl of assay buffer (0.05 m sodium acetate buffer, pH 5.6) with 100 μl of substrate (5 mm 4-methylumbelliferyl sulfate in assay buffer) in a black microplate, as reported previously (33). Total sulfated glycosaminoglycans, including C4S, chondroitin 6-sulfate, keratan sulfate, dermatan sulfate, heparan sulfate, and heparin, were measured in NCM460 cells, HT-29 cells, mouse colon following exposure to carrageenan, and colon of ARSB-null mice by sulfated glycosaminoglycan (GAG) assay (BlyscanTM, Biocolor, Ltd., Newtownabbey, Northern Ireland) as described previously (32). C4S was determined following immunoprecipitation of cell or tissue lysates with C4S antibody (4D1, Abnova, Littleton, CO or LY111 Amsbio, LLC, Lake Forest, CA).
ARSB, Galectin-3, and Sp1 Silencing by siRNA
Small interfering RNAs (siRNAs) used to silence human ARSB, human galectin-3, and Sp1 in the colonic epithelial cells were obtained commercially (Qiagen, Valencia, CA). siRNA sequences for ARSB (NM_000046) silencing were as follows: sense, 5′-GGGUAUGGUCUCUAGGCA-3′; antisense, 5′-UUGCCUAGAGACCAUACCC-3′. The sequence of the DNA template for human galectin-3 silencing (Hs_LGALS3_9) was 5′-ATGATGTTGCCTTCCACTTTA-3′ (22). For Sp1 siRNA, the DNA target sequence was 5′-TTGGGTAAGTGTGTTGTTTAA-3′. Cells at ∼60% confluence in 12-well culture plates were silenced by adding 0.6 μl of 20 μm siRNA (150 ng), mixed with 100 μl of serum-free medium and 12 μl of HiPerfect Transfection Reagent (Qiagen), as described previously. Confirmation of effectiveness of two of the siRNAs for ARSB silencing was determined by measurements of ARSB activity, protein content by ELISA (MyBioSource), and mRNA by QRT-PCR. Galectin-3 silencing was ascertained by galectin-3 QRT-PCR and by ELISA (ELISA kit (R&D Systems), which showed that galectin-3 declined ∼90% following treatment with three of the galectin-3 siRNAs. Similar effectiveness of Sp1 silencing was confirmed by ELISA (Active Motif, Carlsbad, CA).
Wnt9A Promoter Activity by Luciferase Reporter
Human Wnt9A promoter construct in a Renilla reniformis luciferase reporter gene (Ren SP) vector was used to determine differences in Wnt9A promoter activity following carrageenan, ARSB silencing, control silencing, galectin-3 silencing, and combined ARSB and galectin-3 silencing in the colonic epithelial cell lines (LightSwitch Assay, SwitchGear Genomics, Menlo Park, CA). The β-actin promoter (GoCloneTM) construct was used as a positive control, and a scrambled sequence (R01) was the negative control, both with Renilla luciferase reporters; these showed the effectiveness of transcription and the specificity of the reactions. Transfections were performed when cells were 70% confluent, following silencing for 24 h or carrageenan treatment (as described above), using FuGENE HD transfection reagent and proprietary LightSwitch assay reagent (SwitchGear). Luminescence was measured after incubation for 24 h and compared among the different cell preparations. Luminescence was read at 480 nm in a microplate reader (FLUOstar, BMG Labtech).
Oligonucleotide-based ELISA to Determine Nuclear Sp1
Oligonucleotide-binding assay (TransAM kit, Active Motif) was used to detect nuclear Sp1 in the NCM460 and HT-29 cells, following carrageenan, control silencing, ARSB silencing, galectin-3 silencing, combined ARSB and galectin-3 silencing, and in the untreated control cells, in the carrageenan-treated and control mouse tissue, and in the ARSB-null colonic tissue. Nuclear extracts were prepared using a nuclear extract preparation kit (Active Motif) and were added to the wells of a 96-well microtiter plate, precoated with the Sp1 consensus oligonucleotide sequence 5′-GGGGCGGGG-3′. Competition for Sp1 nuclear protein binding to the consensus oligonucleotide was performed using an Sp1-mutated oligonucleotide and an Sp1 WT consensus sequence oligonucleotide, prior to detection of bound Sp1 by Sp1 antibody. Sample values were normalized by total cell protein and expressed as percent of untreated control.
Inhibition of Sp1 Oligonucleotide Binding by Mithramycin A
Mithramycin A, a known inhibitor of Sp1 oligonucleotide binding (37, 38), was purchased (Sigma). Control, carrageenan-exposed, and ARSB-silenced NCM460 cells were treated with mithramycin A (250 nm for 24 h), and the impact on Wnt9A expression was determined by QRT-PCR, as above.
Chromatin Immunoprecipitation Assay for Sp1 and Galectin-3
Binding of Sp1 and galectin-3 to the Wnt9A promoter was assessed by chromatin immunoprecipitation (ChIP) assay (22). For ChIP, IgG control, control silenced, ARSB-silenced, carrageenan-treated, carrageenan-exposed, and galectin-3 silenced, and ARSB- and galectin-3-silenced colonic epithelial cells were fixed with 1% formaldehyde for 10 min at room temperature. This was followed by shearing of chromatin by sonication (ChIP Assay, Active Motif, Carlsbad, CA), and the sheared DNA was incubated with rabbit polyclonal anti-galectin-3 (sc-20157, Santa Cruz Biotechnology, Santa Cruz, CA) and anti-Sp-1 (Active Motif) antibodies for 1 h, as well as with IgG control. Protein-DNA complexes were precipitated by protein A/G-coupled magnetic beads, and the DNA was purified from the immunoprecipitated complexes by reversal of cross-linking, followed by proteinase K treatment. Then, real-time RT-PCR was performed using Brilliant SYBR Green QRT-PCR master mix (Stratagene, La Jolla, CA) and Mx3000 (Stratagene) to amplify the Wnt9A promoter. The sequences for the Wnt9A promoter (5′-TTCGGGTAGGTGCCTTCG-3′ (left) and 5′-AGAGAGGCGGGTGGACC-3′ (right)) encompassed a putative Sp1-binding site (5′-GGGGCGGGG-3′) in the promoter (28). Band intensity was compared among the carrageenan-treated, ARSB-silenced, ARSB-silenced, and galectin-3 silenced, carrageenan- and galectin-3 silenced, control-silenced, control cell, and IgG control samples on a 1.5% agarose gel.
Statistics
The significance of the differences between samples was determined by one-way analysis of variance with Tukey-Kramer post tests for multiple comparisons using InStat software (GraphPad Software, San Diego, CA) unless stated otherwise. Unpaired two-tailed t tests were used to compare determinations between two groups. At least three independent biological samples with technical replicates of each measurement were analyzed. p values ≤ 0.05 were considered significant. In the figures, a single asterisk represents p ≤ 0.05; double asterisks represent p ≤ 0.01; and triple asterisks represent p ≤ 0.001.
RESULTS
Impact of Carrageenan on Total Sulfated Glycosaminoglycans, Chondroitin 4-Sulfate, and Galectin-3 in Colonic Epithelium
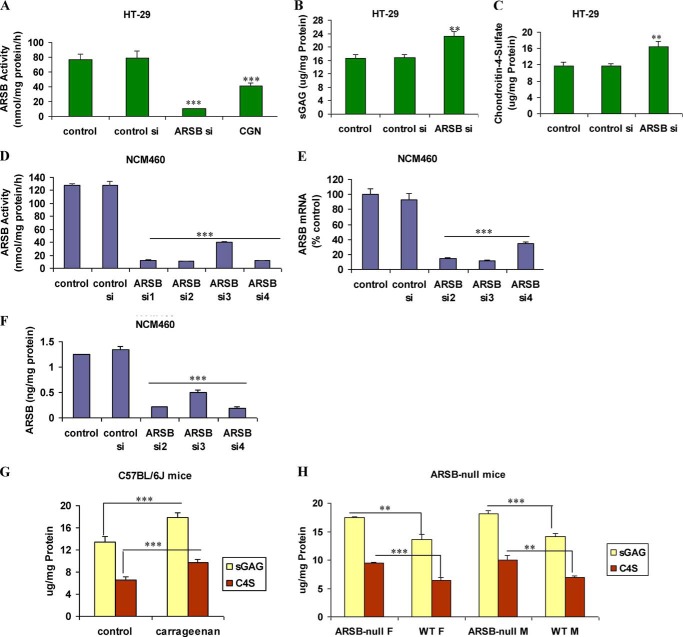
Following exposure to λ-carrageenan, the ARSB activity in the NCM460 cells decreased markedly, from 126.5 ± 1.1 nmol/mg protein/h to 75.2 ± 4.6 nmol/mg protein/h, as reported previously (23). Correspondingly, in the NCM460 cells, the total sulfated GAGs increased from 14.6 ± 0.4 μg/mg protein to 20.4 ± 1.2 μg/mg protein and the C4S increased from 7.4 ± 0.3 μg/mg protein to 11.4 ± 0.4 μg/mg protein, as reported previously (27). In the malignant HT-29 cell line, exposure to carrageenan reduced the ARSB activity from 77.0 ± 7.4 to 41.2 ± 3.7 nmol/mg protein/h. ARSB siRNA reduced the activity further, to 10.5 ± 0.4 nmol/mg protein/h (p < 0.001) (Fig. 1A), with corresponding increases in total sulfated GAGs (from 16.6 ± 1.2 to 23.1 ± 1.5 μg/mg protein) (p < 0.01) (Fig. 1B) and in C4S from 11.8 ± 0.5 μg/mg protein to 16.5 ± 1.2 μg/mg protein (p < 0.01) (Fig. 1C). Effectiveness of ARSB silencing by siRNA was shown by ARSB activity assay (Fig. 1D), ARSB ELISA (Fig. 1E), and ARSB mRNA by QRT-PCR (Fig. 1F).
FIGURE 1.
Impact of carrageenan on sulfated glycosaminoglycans and chondroitin 4-sulfate in HT-29 cells and in mouse models. A, ARSB activity was significantly inhibited by both ARSB siRNA and carrageenan exposure in the HT-29 cells (p < 0.001). B, consistent with the decline in ARSB, the total sulfated GAGs increased significantly following ARSB silencing (p < 0.01). C, the majority of the increase in the sulfated GAGs was attributable to the increase in C4S following ARSB silencing (p < 0.01). D, ARSB siRNA markedly reduced the ARSB activity in the NCM460 cells. E, ARSB protein was significantly reduced by ARSB siRNA in the NCM460 cells. F, QRT-PCR for ARSB demonstrated marked decline in ARSB mRNA expression following ARSB siRNA treatment. G, in the C57BL/6J mice exposed to carrageenan in their water supply, sulfated GAGs and C4S were significantly increased over the unexposed controls (p < 0.0001, unpaired t test, two-tailed). H, in both female and male ARSB-deficient mice, the total sulfated GAGs (p = 0.002 (female), p = 0.0012 (male), unpaired t test, two-tailed), and the C4S (p = 0.0009 (female), p = 0.0026 (male), unpaired t test, two-tailed) were significantly increased over the values in the control mice. sGAG, sulfated glycosaminoglycan; CGN, carrageenan; WT, wild-type; F, female; M, male; si, siRNA.
In the C57BL/6J mice exposed to κ-λ carrageenan (10–100 μg/ml for 4 weeks), total sulfated GAGs increased from 13.4 ± 1.0 μg/mg protein to 17.8 ± 0.9 μg/mg protein. This increase was largely accounted for by the increase in C4S from 6.5 ± 0.6 μg/mg protein in the unexposed control to 9.7 ± 0.6 μg/mg protein following carrageenan (p < 0.0001, unpaired t test, two-tailed) (Fig. 1G), as the ARSB declined from a baseline value of 116.9 ± 10.6 nmol/mg protein/h to 79.6 ± 4.8 nmol/mg protein/h. In male ARSB-deficient mice, the total sulfated GAG content in the colonic epithelium was 18.1 ± 0.6 μg/mg protein, with 10.1 ± 0.8 μg/mg protein of C4S accounting for most of the difference from the value in the wild-type mice (Fig. 1H). Similarly, in female ARSB-deficient mice, the total sulfated glycosaminoglycans and C4S content were significantly higher than in control, age-matched mice.
Expression of Wnt9A Is Increased after Carrageenan or ARSB siRNA and Increases Are Inhibited by Galectin-3 Silencing
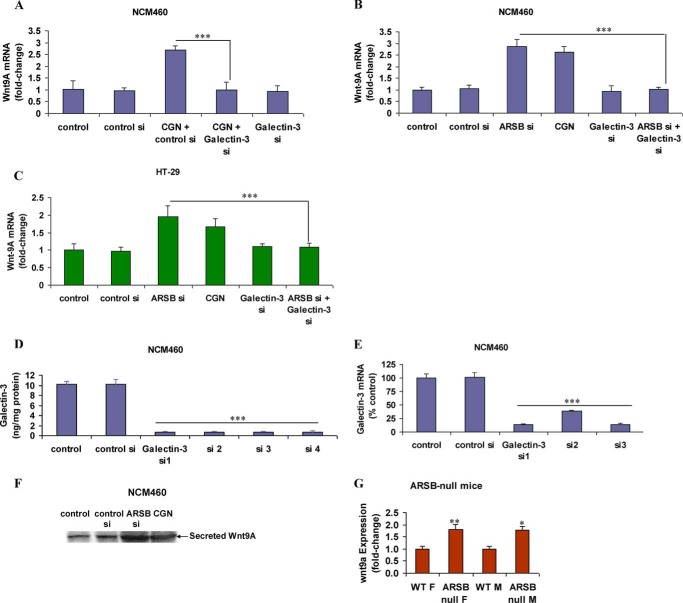
Previously, experiments demonstrated that carrageenan exposure reduced ARSB activity (27) and that decline in ARSB led to transcriptional events that were attributable to the reduced association of galectin-3 with the more highly sulfated C4S present when ARSB activity was less (21, 22). Wnt9A expression increased to 2.8 times the baseline in NCM460 cells and to 2.4 times the baseline in C57BL/6J mice following exposure to carrageenan, and exposure to the reactive oxygen species scavenger Tempol inhibited the increase in Wnt9A in the NCM460 cells (3). When galectin-3 was silenced by siRNA, the carrageenan-induced increase in Wnt9A expression was inhibited in the NCM460 cells (Fig. 2A). ARSB silencing increased the Wnt9A mRNA expression to 2.0 ± 0.3 times the baseline, and carrageenan exposure increased Wnt9A mRNA to 1.7 ± 0.2 times the baseline. The combination of ARSB silencing and galectin-3 silencing in NCM460 (Fig. 2B) and HT-29 (Fig. 2C) cells inhibited the increases in Wnt9A expression. Effectiveness of galectin-3 silencing was demonstrated by ELISA (Fig. 2D) and by QRT-PCR (Fig. 2E). Increases of secreted Wnt9A in the spent media of the NCM460 cells followed ARSB silencing and carrageenan exposure, as shown by Western blot (Fig. 2F). In the colonic epithelium of the male and female ARSB-null mice, wnt9A expression was significantly increased, compared with expression in wild-type control mice (Fig. 2G).
FIGURE 2.
Increase in Wnt9A expression following ARSB silencing and carrageenan with inhibition by galectin-3 silencing. A, following carrageenan expression, the Wnt9A mRNA expression was significantly increased in the NCM460 cells and was inhibited by galectin-3 silencing (p < 0.001). B and C, in the NCM460 and HT-29 cells, galectin-3 silencing inhibited the ARSB siRNA-induced increase in Wnt9A expression (p < 0.001). ARSB silencing had a greater impact on Wnt9A expression than carrageenan, consistent with their effects on ARSB activity and expression. D, galectin-3 ELISA demonstrated the effectiveness of galectin-3 silencing by siRNA in the NCM460 cells. E, QRT-PCR for galectin-3 following silencing indicated marked decline in mRNA expression in the NCM460 cells. F, Western blot demonstrated increase in secreted Wnt9A following carrageenan exposure and ARSB silencing in the NCM460 cells. G, Wnt9A expression was increased in the female and male ARSB-deficient mice, compared with ARSB female and male wild-type mice (p = 0.009 and p = 0.03, respectively, unpaired t test, two-tailed). ***, p < 0.001, **, p < 0.01, and *, p < 0.05. ARSB, arylsulfatase B; CGN, carrageenan; si, siRNA; WT, wild-type; F, female; M, male.
Decline in Galectin-3 Binding to C4S and Increase in Nuclear Galectin-3 Following Carrageenan or ARSB Silencing
In association with the decline in ARSB activity by siRNA or by carrageenan exposure, the galectin-3 that co-immunoprecipitated with C4S declined. From baseline of 9.2 ± 0.3 ng/mg protein, the galectin-3 bound to C4S declined to 3.8 ± 0.4 ng/mg protein in the NCM460 cells following carrageenan (Fig. 3A). In the HT-29 cells, the galectin-3 that co-immunoprecipitated with C4S declined from 7.8 ± 0.5 ng/mg protein in the control to 2.1 ± 0.1 ng/mg protein following ARSB silencing and to 3.0 ± 0.2 ng/mg protein following carrageenan exposure (Fig. 3B). These declines occurred in association with more than 2-fold increases in the nuclear galectin-3 in the NCM460 cells (Fig. 3C) and in the HT-29 cells (Fig. 3D). Exposure to carrageenan produced less of an increase in the nuclear galectin-3 than ARSB siRNA, but the increase was still significant (p < 0.001). In the colonic epithelium of the carrageenan-exposed C57BL/6J mice, the nuclear galectin-3 was significantly greater than in the unexposed controls (12.6 ± 0.8 versus 6.7 ± 0.2 ng/mg protein) (Fig. 3E). Similarly, in both the male and female ARSB-deficient mice, the nuclear galectin-3 was more than 2-fold times the level in the wild-type control mice (Fig. 3F).
FIGURE 3.
ARSB decline and corresponding increase in C4S mediate decline in galectin-3 that co-immunoprecipitates with C4S and increase in nuclear galectin-3. A and B, after carrageenan exposure or ARSB silencing, the galectin-3 that co-immunoprecipitated with C4S declined significantly in the NCM460 cells and in the HT-29 cells (p < 0.001). C and D, following carrageenan exposure or ARSB silencing, the nuclear galectin-3 was significantly increased in the NCM460 and HT-29 cells (p < 0.001) compared with controls. In the HT-29 cells, the increase following ARSB silencing by siRNA was significantly greater (p < 0.01) than the increase following carrageenan. E, following carrageenan ingestion, the colonic nuclear galectin-3 increased significantly (p < 0.001) over the value in the control C57BL/6J mice. F, in the ARSB-deficient mice (male and female), the nuclear galectin-3 was significantly higher than in the wild-type mice (p < 0.001). CGN, carrageenan; WT, wild-type; F, female; M, male. ***, p < 0.001.
Carrageenan Exposure and ARSB Silencing Increase Wnt9A Promoter Activity
Wnt9A promoter activity in the NCM460 and HT-29 cells was determined by Renilla luciferase assay using a promoter construct, as detailed in the Methods. When NCM460 cells were exposed to carrageenan, the luciferase activity increased to more than 2.0 times the control level. Following ARSB silencing, the luciferase activity increased to ∼2.6 times the control silencing level (Fig. 4A). Luciferase activity increased to 1.7 times baseline following carrageenan and to 2.1 times control silencing level in the malignant HT-29 cells (Fig. 4B). No significant changes occurred in the vector control. Galectin-3 silencing reduced the Wnt9A promoter activity to the baseline level, demonstrating dependence on galectin-3 for the Wnt9A promoter activation.
FIGURE 4.
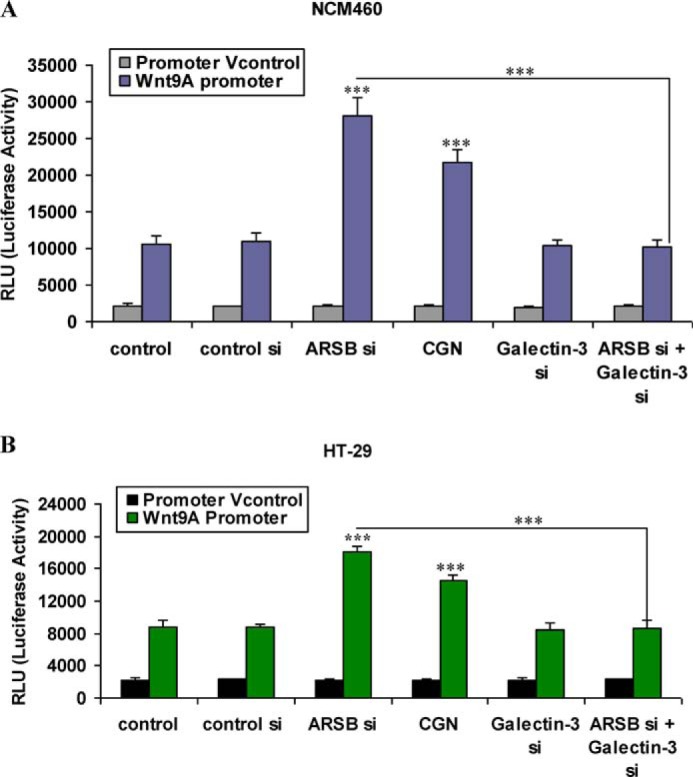
Carrageenan exposure or ARSB silencing increase Wnt9A promoter activity, and galectin-3 silencing inhibits the increase in Wnt9A promoter activity. A and B, Renilla luciferase assay showed significant increase in activation following carrageenan exposure or ARSB silencing. These increases were inhibited when galectin-3 was silenced in the NCM460 and HT-29 cells (p < 0.001). The increase following carrageenan was significantly less than from ARSB siRNA. CGN, carrageenan; RLU, relative luciferase unit; si, siRNA; Vcontrol, vector control.
Transcription Factor Sp1 Binding to Wnt9A Promoter Increased Following Carrageenan and ARSB Silencing
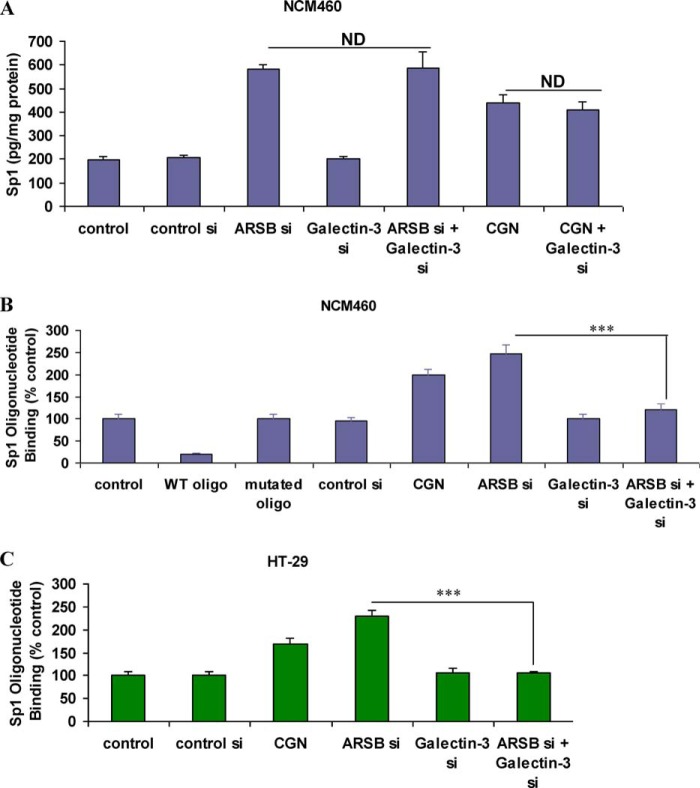
Because the Wnt9A promoter has consensus sequences for Sp1, measurements of nuclear Sp1 were performed in NCM460 cells following treatment with carrageenan or ARSB silencing. Nuclear Sp1 increased from 195 ± 19 pg/mg protein in control to 440 ± 32 pg/mg protein following carrageenan and to 582 ± 17 pg/mg protein following ARSB silencing (Fig. 5A). These increases in the nuclear Sp1 were not inhibited by galectin-3 silencing. Sp1 binding with the Wnt9A promoter consensus oligonucleotide increased to 2.0 ± 0.1 times the baseline in the NCM460 cells following carrageenan and to 2.5 ± 0.2 times the baseline following ARSB silencing (Fig. 5B). Competition by addition of exogenous consensus oligonucleotide completely inhibited the Sp1 binding, and competition by a mutated consensus oligonucleotide did not impact upon the Sp1 binding, as detected by Sp1 antibody. In the malignant HT-29 cell line, the Sp1 binding to the consensus oligonucleotide in the Wnt9A promoter increased to 1.7 ± 0.1 times the baseline following carrageenan and to 2.3 ± 0.1 times the baseline following ARSB silencing (Fig. 5C). Galectin-3 silencing inhibited the increases in Sp1 binding to the Wnt9A promoter in the colonic epithelial cell lines.
FIGURE 5.
Nuclear Sp1 and Sp1 oligonucleotide binding increased following carrageenan and ARSB silencing. A, in the NCM460 cells, nuclear Sp1 was significantly increased following exposure to carrageenan or ARSB silencing by siRNA (p < 0.001). Galectin-3 silencing did not inhibit the increase in the nuclear Sp1. ARSB silencing produced greater increase in nuclear Sp1 than carrageenan exposure (p < 0.01; 2.98 times control versus 2.26 times control). B, in NCM460 cells, oligonucleotide binding assay demonstrated an increase in Sp1 binding to the Sp1 oligonucleotide consensus sequence following ARSB silencing (p < 0.001). This increase was inhibited when galectin-3 was silenced. Addition of exogenous Sp1 wild-type consensus oligonucleotide completely inhibited the nuclear Sp1 binding as detected by Sp1 antibody. Baseline binding was unaffected by addition of mutated Sp1 consensus oligonucleotide. C, In HT-29 cells, oligonucleotide binding assay indicated an increase in Sp1 binding to the Sp1 oligonucleotide consensus sequence following ARSB silencing (p < 0.001) and inhibition when galectin-3 was silenced. CGN, carrageenan; oligo, oligonucleotide; si, siRNA; WT, wild-type; ND, no difference.
Chromatin Immunoprecipitation Assay Confirms Binding of Sp1 to Wnt9A Promoter
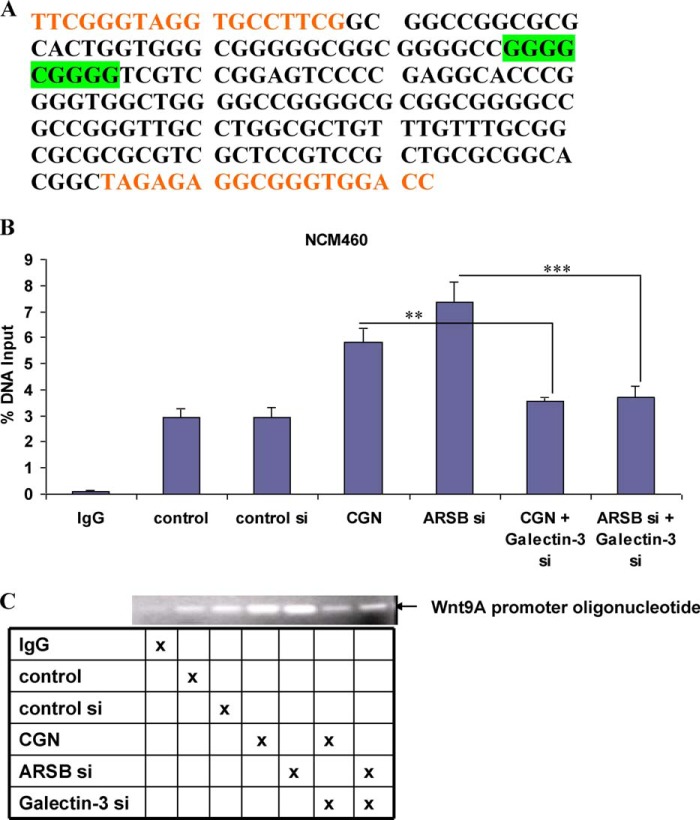
The consensus sequence for Sp1 and the primers used to amplify the promoter sequence in the colonic epithelial cells are shown (Fig. 6A). Chromatin immunoprecipitation assay confirmed that the binding of Sp1 to the Wnt9A promoter oligonucleotide was increased following exposure to carrageenan or to ARSB siRNA (Fig. 6B). Agarose gel demonstrated increased intensity of bands following ARSB siRNA or carrageenan exposure. These increases in band intensity were inhibited when galectin-3 was silenced (Fig. 6C).
FIGURE 6.
Chromatin immunoprecipitation demonstrates increase in Sp1 binding to the Wnt9A promoter following carrageenan and ARSB silencing and inhibition of the increases when galectin-3 is silenced. A, consensus oligonucleotide sequence for Sp1 in the Wnt9A promoter is highlighted in green. The primers used to amplify this region are in orange type. B, ChIP assay showed significant increases in the measured RT-PCR product following carrageenan or ARSB silencing (p < 0.001). These increases were inhibited when galectin-3 was silenced in the NCM460 cells. C, the agarose gel confirms the increases as measured above by RT-PCR in the Sp1 binding to the Wnt9A promoter oligonucleotide with the targeted Sp1 binding site (as indicated in A) following ARSB silencing or carrageenan exposure, and the inhibition of these increases by galectin-3 silencing. CGN, carrageenan; si, siRNA.
Sp1 siRNA and Mithramycin A Inhibit the Effect of Carrageenan and of ARSB Silencing on Wnt9A Expression
Sp1 siRNAs were shown to effectively silence Sp1 by ELISA (Fig. 7A). When HT29 and NCM460 cells were treated with Sp1 siRNA, Wnt9A expression was inhibited (p < 0.001) (Fig. 7B). The combination of Sp1 silencing and ARSB silencing suppressed the expression to the level of the control silencing.
FIGURE 7.
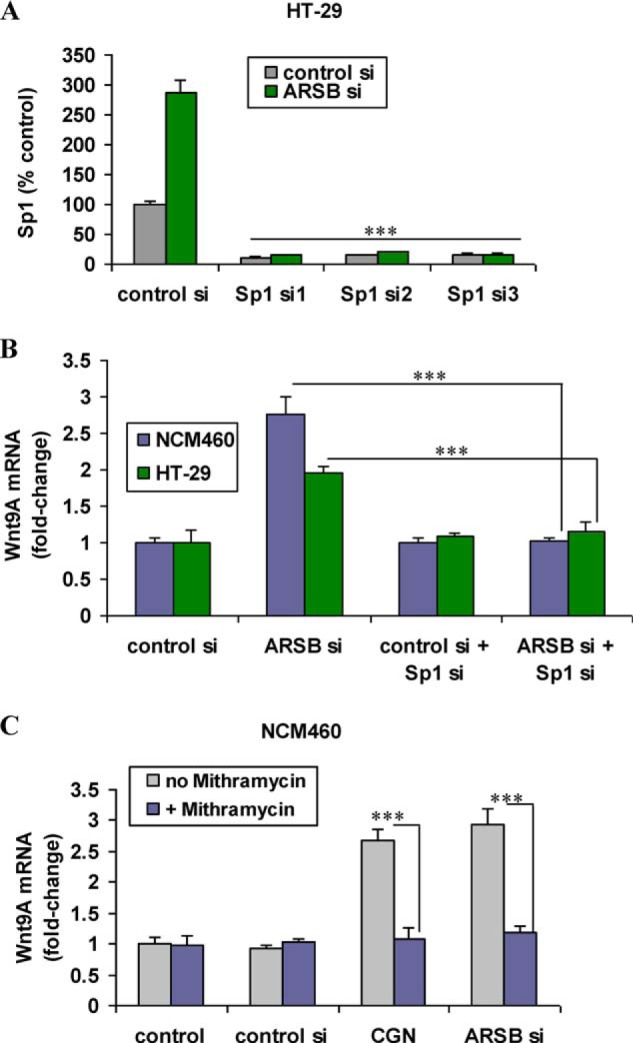
Sp1 siRNA and mithramycin A inhibit the effect of carrageenan and of ARSB silencing on Wnt9A expression in the colonic epithelial cells. A, Sp1 siRNA effectively inhibited expression of Sp1 in HT-29 cells. B, Sp1 siRNA suppressed mRNA expression of Wnt9A in the HT-29 and NCM460 cells. The mRNA expression of Wnt9A was similar in the control and with the combination of ARSB silencing and Sp1 silencing. C, when NCM460 cells were exposed to mithramycin A, a specific inhibitor of Sp1 oligonucleotide binding, the increases in Wnt9A expression that followed carrageenan exposure or ARSB silencing were inhibited (p < 0.001). CGN, carrageenan; si, siRNA.
Mithramycin A, recognized as an inhibitor of Sp1 oligonucleotide binding (37, 38), also inhibited the increases in Wnt9A expression that followed carrageenan and ARSB silencing in the NCM460 cells (Fig. 7C). These findings are a further demonstration of the involvement of Sp1 in the transcriptional mechanism by which carrageenan exposure and ARSB silencing lead to increased expression of Wnt9A.
DISCUSSION
The study findings have demonstrated that exposure to the common food additive carrageenan increases the mRNA expression of Wnt9A in human colonic epithelial cells and in C57BL/6J mice. This transcriptional effect is mediated through decline in activity of the enzyme ARSB, leading to increase in chondroitin-4-sulfation and reduced binding of galectin-3 to the more highly sulfated C4S. In association with the diminished binding to C4S, nuclear galectin-3 increased and mediated an increase in Sp1 binding to the Wnt9A promoter, thereby activating Wnt9A expression. Wnt9A has been associated with several malignancies, and activation of this signaling pathway may contribute to malignant transformation in the colonic epithelium.
Carrageenan exposure has been implicated in the development of colonic neoplasms for decades (39, 40). In hundreds of animal experiments, exposure to carrageenan was associated with polyps, adenomas, and adenocarcinomas. Carrageenan was also noted to act as a promoter of colonic malignancies, when given in combination with azoxymethane, nitrosomethylurea, and dimethylhydrazine (41–43). These and other reports caused low molecular weight carrageenan to be identified as a class IIB agent by the International Agency for Research on Cancer (40). In rats, a lifetime exposure level of 2310 mg/kg body weight of degraded carrageenan was implicated in development of large intestinal malignancies in half of the exposed animals (TD50) by the Carcinogenic Potency Database (44). This corresponds to a lifetime exposure of ∼138 g in a 60-kg person. Daily intake of carrageenan in humans is estimated to be ∼250 mg (45), of which ∼10% is anticipated to be degraded (39). Hence, the TD50 consumption of degraded carrageenan would be ingested in ∼5.5 years with regular dietary consumption of carrageenan, consistent with the gradual emergence of colorectal cancer over the lifespan.
The basis of the carcinogenicity of carrageenan was considered to be similar to the development of neoplasia that occurs with ulcerative colitis, with chronic inflammation and increased mucosal turnover (46). However, the current study findings demonstrate that a transcriptional mechanism leading to increased expression of Wnt9A and attributable to carrageenan-induced decline in ARSB activity, contributes to the carcinogenic potential of carrageenan in human colonic epithelium. Previously, decline in ARSB was demonstrated in malignant colonic tissues and in metastatic colonic epithelial cells (30, 31).
The reactive oxygen species inhibitor Tempol only partially inhibits the carrageenan-induced increases in NF-κB and IL-8 because carrageenan-initiated inflammation proceeds through both a reactive oxygen species-mediated pathway and an innate immune-mediated signaling pathway requiring TLR4 and BCL10 (47, 48). In contrast, Tempol completely blocked the increase in Wnt9A expression and the carrageenan-induced increase in Wnt signaling in NCM460 cells (3), as well as increases in total sulfated GAGs and C4S. These findings indicate that the effect of carrageenan on Wnt9A expression is independent of the carrageenan-stimulated innate immune pathway of inflammation.
The precise intermediates involved in the inhibition of ARSB by carrageenan have not been elucidated yet. The sulfate groups of carrageenan might mimic the 4-sulfate groups at the non-reducing end of C4S or dermatan sulfate, which are the targets of ARSB enzymatic activity. The unusual α-d-Gal(1→3)d-Gal bond of carrageenan may constrain the proper release of the sulfate group and the subsequent renewal of ARSB (27, 49). By inhibition of ARSB activity, carrageenan exposure induces changes in the ARSB-C4S-galectin-3 signaling axis, leading to increase in Sp1 binding to the Wnt9A promoter and increased Wnt9A expression. Previously, we have shown that carrageenan exposure activates the Wnt signaling pathway (3), and the combination of increase in Wnt9A production and increase in activation of Wnt signaling is expected to profoundly impact upon vital Wnt-mediated cell processes and affect proliferation and development of colonic tissue in critical ways. The impact of carrageenan exposure might be cumulative over time, based on the ongoing consumption of carrageenan-containing foods in the human diet.
In this report, the impact of decline in ARSB on Sp1 in colonic epithelium has been shown using specific inhibitors of Sp1, including Sp1 siRNA and mithramycin A. Studies to address possible contributions of other SP family members are of interest because Sp transcription factor paralogs, including Sp3 and Sp4, have co-evolved with Sp1, and competition for binding at GC box consensus sites can lead to complex regulatory interactions, particularly involving Sp1 and Sp3 (50–52). The potential interactions between multiple transcriptionally active SP binding sites indicate that intricate, complex regulatory mechanisms are involved (53–56). Distinctive properties and effects of Sp4 have also been recognized (57). Recently, specific transcriptional effects of neuronal Sp4 on the regulation of Na+/K+-ATPase and of glutaminergic receptors were reported (58, 59). Future studies will clarify whether or not the interactions of ARSB, C4S, and galectin-3 also influence effects of Sp3 or Sp4 on Wnt9A transcription, or on transcription of other Wnt family members, in colonic epithelium and in other tissues. Sp1 has also been associated with activation of WNT5A, both in human embryonic tissues and in airway smooth muscle cells following stimulation by TGF-β (60, 61).
In addition to impact on Sp1-mediated transcriptional events, the carrageenan exposure is anticipated to also affect other ARSB-C4S-galectin-3-mediated transcriptional processes, including AP-1 transcriptional effects. Other work has shown that this pathway affects expression of HIF-1α and versican through AP-1 (21, 22). Galectin-3 was reported to affect cyclin D1 through Sp1 in mammary epithelial cells (20), suggesting potential impact of ARSB on cell cycle regulation. Coordinated transcriptional effects mediated by AP-1 and Sp1 may profoundly impact the cell fate of colonic epithelial cells in vivo. Recognizing that activation of Wnt expression and Wnt signaling can disturb fundamental biological processes and that the human colonic epithelium is exposed to significant quantities of carrageenan daily, additional initiatives to reduce dietary intake of carrageenan should be undertaken.
Footnotes
- C4S
- chondroitin 4-sulfate
- ARSB
- arylsulfatase B
- QRT-PCR
- quantitative RT-PCR
- GAG
- glycosaminoglycan.
REFERENCES
- 1. Clevers H. (2006) Wnt/β-catenin signaling in development and disease. Cell 127, 469–480 [DOI] [PubMed] [Google Scholar]
- 2. Reya T., Clevers H. (2005) Wnt signaling in stem cells and cancer. Nature 434, 843–850 [DOI] [PubMed] [Google Scholar]
- 3. Bhattacharyya S., Feferman L., Borthakur S., Tobacman J. K. (2014) Common food additive carrageenan stimulates Wnt/β-catenin signaling in colonic epithelium by inhibition of nucleoredoxin reduction. Nutr. Cancer 66, 117–127 [DOI] [PubMed] [Google Scholar]
- 4. Funato Y., Miki H. (2010) Redox regulation of Wnt signaling via nucleoredoxin. Free Radic. Res. 44, 379–388 [DOI] [PubMed] [Google Scholar]
- 5. Schwarz-Romond T., Fiedler M., Shibata N., Butler P. J., Kikuchi A., Higuchi Y, Bienz M. (2007) The DIX domain of Dishevelled confers Wnt signaling by dynamic polymerization. Nat. Struct. Mol. Biol. 14, 484–492 [DOI] [PubMed] [Google Scholar]
- 6. Bhattacharyya S., Borthakur A., Dudeja P. K., Tobacman J. K. (2007) Carrageenan reduces bone morphogenetic protein-4 (BMP4) and activates Wnt/β-catenin pathway in normal human colonic epithelial cells. Dig. Dis. Sci. 52, 2766–2774 [DOI] [PubMed] [Google Scholar]
- 7. Cox A. A., Jezewski P. A., Fang P. K., Payne-Ferreira T. L. (2010) Zebrafish Wnt9a, 9b paralog comparisons suggest ancestral roles for Wnt9 in neural, oral-pharyngeal ectoderm and mesoendoderm. Gene Expr. Patterns 10, 251–258 [DOI] [PubMed] [Google Scholar]
- 8. An C. H., Kim S. S., Kang M. R., Kim Y. R., Kim H. S., Yoo N. J., Lee S. H. (2010) Frameshift mutations of ATBF1, WNT9A, CYLD, and PARK2 in gastric and colorectal carcinomas with high microsatellite instability. Pathology 42, 583–585 [DOI] [PubMed] [Google Scholar]
- 9. Kirikoshi H., Sekihara H., Katoh M. (2001) Expression of WNT14 and WNT14B mRNAs in human cancer, up-regulation of WNT14 by IFN γ and up-regulation of WNT14B by β-estradiol. Int. J. Oncol. 19, 1221–1225 [DOI] [PubMed] [Google Scholar]
- 10. Shu J., Jelinek J., Chang H., Shen L., Qin T., Chung W., Oki Y., Issa J. P. (2006) Silencing of bidirectional promoters by DNA methylation in tumorigenesis. Cancer Res. 66, 5077–5084 [DOI] [PubMed] [Google Scholar]
- 11. Olender T., Safran M., Edgar R., Stelzer G., Nativ N., Rosen N., Shtrichman R., Mazor Y., West M. D., Keydar I., Rappaport N., Belinky F., Warshawsky D., Lancet D. (2013) An overview of synergistic data tools for biological scrutiny. Isr. J. Chem. 53, 185–198 [Google Scholar]
- 12. Zhu G. H., Lenzi M., Schwartz E. L. (2002) The Sp1 transcriptional factor contributes to the tumor necrosis factor-induced expression of the angiogenic factor thymidine phosphorylase in human colon carcinoma cells. Oncogene 21, 8477–8485 [DOI] [PubMed] [Google Scholar]
- 13. Kovarik A., Lu P. J., Peat N., Morris J., Taylor-Papadimitriou J. (1996) Regulation of the activity of the epithelium-specific MUC1 promoter. J. Biol. Chem. 271, 18140–18147 [DOI] [PubMed] [Google Scholar]
- 14. Keith W. N., Vulliamy T., Zhao J., Ar C., Erzik C., Bilsland A., Ulku B., Marrone A., Mason P. J., Bessler M., Serakinci N., Dokal I. (2004) A mutation in a functional Sp1 binding site of the telomerase RNA gene (hTERC) promoter in a patient with Paroxysmal Nocturmal Haemoglobinuria. BMC Blood Disord. 4, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Onishi T., Yamakawa K., Franco O. E., Kawamura J., Watanabe M., Shiraishi T., Kitazawa S. (2001) Mitogen-activated protein kinase pathway is involved in α6 integrin gene expression in androgen-independent prostate cancer cells: role of proximal Sp1 consensus sequence. Biochim. Biophys. Acta 1538, 218–227 [DOI] [PubMed] [Google Scholar]
- 16. Zhao Y., Zhang W., Guo Z., Ma F., Wu Y., Bai Y., Gong W., Chen Y., Cheng T., Zhi F., Zhang Y., Wang J., Jiang B. (2013) Inhibition of the transcription factor Sp1 suppresses colon cancer stem cell growth and induces apoptosis in vitro and in nude mice. Oncol. Rep. 30, 1782–1792 [DOI] [PubMed] [Google Scholar]
- 17. Wang F., Ma Y. L., Zhang P., Shen T. Y., Shi C. Z., Yang Y. Z., Moyer M. P., Zhang H. Z., Chen H. Q., Liang Y., Qin H. L. (2013) Sp1 mediates the link between methylation of the tumour suppressor miR-149 and outcome in colorectal cancer. J. Pathol. 229, 12–24 [DOI] [PubMed] [Google Scholar]
- 18. Maurer G. D., Leupold J. H., Schewe D. M., Biller T., Kates R. E., Hornung H. M., Lau-Werner U., Post S., Allgayer H. (2007) Analysis of specific transcriptional regulators as early predictors of independent relevance in resected colorectal cancer. Clin. Cancer Res. 13, 1123–1132 [DOI] [PubMed] [Google Scholar]
- 19. Chang W. C., Hung J. J. (2012) Functional role of post-translational modifications of Sp1 in tumorigenesis. J. Biomed. Sci. 19, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lin H. M., Pestell R. G., Raz A., Kim H. R. (2002) Galectin-3 enhances cyclin D1 promoter activity through SP1 and a cAMP-responsive element in human breast epithelial cells. Oncogene 21, 8001–8010 [DOI] [PubMed] [Google Scholar]
- 21. Bhattacharyya S., Tobacman J. K. (2012) Hypoxia reduces arylsulfatase B activity and silencing arylsulfatase B replicates and mediates the effects of hypoxia. PLoS One 7, e33250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bhattacharyya S., Feferman L., Tobacman J. K. (2013) Arylsulfatase B regulates versican expression by galectin-3 and AP-1 mediated transcriptional effects. Oncogene 10.1038/onc.2013.483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Iwaki J., Minamisawa T., Tateno H., Kominami J., Suzuki K., Nishi N., Nakamura T., Hirabayashi J. (2008) Desulfated galactosaminoglycans are potential ligands for galectins: evidence from frontal affinity chromatography. Biochem. Biophys. Res. Commun. 373, 206–212 [DOI] [PubMed] [Google Scholar]
- 24. Yoo B. C., Hong S. H., Ku J. L., Kim Y. H., Shin Y. K., Jang S. G., Kim I. J., Jeong S. Y., Park J. G. (2009) Galectin-3 stabilizes heterogeneous nuclear ribonucleoprotein Q to maintain proliferation of human colon cancer cells. Cell Mol. Life Sci. 66, 350–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang L., Inohara H., Pienta K. J., Raz A. (1995) Galectin-3 is a nuclear matrix protein which binds RNA. Biochem. Biophys. Res. Commun. 217, 292–303 [DOI] [PubMed] [Google Scholar]
- 26. Song S., Byrd J. C., Mazurek N., Liu K., Koo J. S., Bresalier R. S. (2005) Galectin-3 modulates MUC2 mucin expression in human colon cancer cells at the level of transcription via AP-1 activation. Gastroenterology 129, 1581–1591 [DOI] [PubMed] [Google Scholar]
- 27. Yang B., Bhattacharyya S., Linhardt R., Tobacman J. (2012) Exposure to common food additive carrageenan leads to reduced sulfatase activity and increase in sulfated glycosaminoglycans in human epithelial cells. Biochimie 94, 1309–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heinemeyer T., Wingender E., Reuter I., Hermjakob H., Kel A. E., Kel O. V., Ignatieva E. V., Ananko E. A., Podkolodnaya O. A., Kolpakov F. A., Podkolodny N. L., Kolchanov N. A. (1998) Databases on transcriptional regulation: TRANSFAC, TRRD, and COMPEL. Nucleic Acids Res. 26, 362–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Feferman L., Bhattacharyya S., Deaton R., Gann P., Guzman G., Kajdacsy-Balla A., Tobacman J. K. (2013) Arylsulfatase B (N-acetylgalactosamine-4-sulfatase): potential role as a biomarker in prostate cancer. Prostate Cancer Prostatic Dis. 16, 277–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Prabhu S. V., Bhattacharyya S., Guzman-Hartman G., Macias V., Kajdacsy-Balla A., Tobacman J. K. (2011) Extra-lysosomal localization of arylsulfatase B in human colonic epithelium. J. Histochem. Cytochem. 59, 328–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bhattacharyya S., Tobacman J. K. (2009) Arylsulfatase B regulates colonic epithelial cell migration by effects on MMP9 expression and RhoA activation. Clin. Exp. Metastasis 26, 535–545 [DOI] [PubMed] [Google Scholar]
- 32. Bhattacharyya S., Kotlo K., Shukla S., Danziger R. S., Tobacman J. K. (2008) Distinct effects of N-acetylgalactosamine-4-sulfatase and galactose-6-sulfatase expression on chondroitin sulfate. J. Biol. Chem. 283, 9523–9530 [DOI] [PubMed] [Google Scholar]
- 33. Bhattacharyya S., Tobacman J. K. (2007) Steroid sulfatase, arylsulfatases A and B, galactose 6-sulfatase, and iduronate sulfatase in mammary cells and effects of sulfated and non-sulfated estrogens on sulfatase activity. J. Steroid Biochem. Mol. Biol. 103, 20–34 [DOI] [PubMed] [Google Scholar]
- 34. Moyer M. P., Manzano L. A., Merriman R. L., Stauffer J. S., Tanzer L. R. (1996) NCM460, a normal human colon mucosal epithelial cell line. In Vitro Cell Dev. Biol. Anim. 32, 315–317 [DOI] [PubMed] [Google Scholar]
- 35. Curtain M. M., Donahue L. R. (2009) A mutation in the Arsb gene: a mouse model that resembles Maroteaux-Lamy syndrome. Mouse Genome Informatics, The Jackson Laboratory, J:149960 [Google Scholar]
- 36. Rozen S., Skaletsky H. (2000) Primer3 on WWW for general users and for biologist programmers. Methods Mol. Biol. 132, 365–386 [DOI] [PubMed] [Google Scholar]
- 37. Sleiman S. F., Langley B. C., Basso M., Berlin J., Xia L., Payappilly J. B., Kharel M. K., Guo H., Marsh J. L., Thompson L. M., Mahishi L., Ahuja P., MacLellan W. R., Geschwind D. H., Coppola G., Rohr J., Ratan R. R. (2011) Mithramycin is a gene-selective Sp1 inhibitor that identifies a biological intersection between cancer and neurodegeneration. J. Neurosci. 31, 6858–6870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Blume S. W., Snyder R. C., Ray R., Thomas S., Koller C. A., Miller D. M. (1991) Mithramycin inhibits SP1 binding and selectively inhibits transcriptional activity of the dihydrofolate reductase gene in vitro and in vivo. J. Clin. Invest. 88, 1613–1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tobacman J. K. (2001) Review of harmful gastrointestinal effects of carrageenan in animal experiments. Environ. Health Perspect. 109, 983–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. IARC Working Group on the Evaluation of the Carcinogenic Risk of Chemicals to Humans (1983) Carrageenan. IARC Monogr. Eval. Carcinog. Risk Chem. Hum. 31, 79–94 [PubMed] [Google Scholar]
- 41. Watanabe K., Reddy B. S., Wong C. Q., Weisburger J. H. (1978) Effect of dietary undegraded carrageenan on colon carcinogenesis in F344 rats treated with azoxymethane or methylnitrosourea. Cancer Res. 38, 4427–4430 [PubMed] [Google Scholar]
- 42. Kawaura A., Shibata M., Togei K., Otsuka H. (1982) Effect of dietary degraded carrageenan on intestinal carcinogenesis in rats treated with 1,2-dimethylhydrazine dihydrochloride. Tokushima J. Exp. Med. 29, 125–129 [PubMed] [Google Scholar]
- 43. Arakawa S., Okumura M., Yamada S., Ito M., Tejima S. (1986) Enhancing effect of carrageenan on the induction of rat colonic tumors by 1,2-dimethylhydrazine and its relation to β-glucuronidase activities in feces and other tissues. J. Nutr. Sci. Vitaminol. 32, 481–485 [DOI] [PubMed] [Google Scholar]
- 44. Gold L. S., Slone T. H., Manley N. B., Garfinkel G. A., Rohrbach L., Ames B. N. (1997) Carcinogenic Potency Database in The Handbook of Carcinogenic Potency and Genotoxicity Databases (Gold L. S., Zeiger E., eds) pp. 116–117, CRC Press, Inc., New York [Google Scholar]
- 45. Encyclopædia Britannica Macropedia (2010) Algae. Volume 13, p. 236, Encyclopædia Britannica, Inc., Chicago [Google Scholar]
- 46. Serafini E. P., Kirk A. P., Chambers T. J. (1981) Rate and pattern of epithelial cell proliferation in ulcerative colitis. Gut 22, 648–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bhattacharyya S., Dudeja P. K., Tobacman J. K. (2008) Carrageenan-induced NFκB activation depends on distinct pathways mediated by reactive oxygen species and Hsp27 or by Bcl10. Biochim. Biophys. Acta 1780, 973–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bhattacharyya S., Gill R., Chen M. L., Zhang F., Linhardt R. J., Dudeja P. K., Tobacman J. K. (2008) Toll-like receptor 4 mediates induction of Bcl10-NFκB-IL-8 inflammatory pathway by carrageenan in human intestinal epithelial cells. J. Biol. Chem. 283, 10550–10558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bhattacharyya S., Liu H., Zhang Z., Jam M., Dudeja P. K., Michel G., Linhardt R. J., Tobacman J. K. (2010) Carrageenan-induced innate immune response is modified by enzymes that hydrolyze distinct galactosidic bonds. J. Nutr. Biochem. 21, 906–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yokoyama K. D., Pollock D. D. (2012) SP Transcription factor paralogs and DNA-binding sites coevolve and adaptively converge in mammals and birds. Genome Biol. Evol. 4, 1102–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hagen G., Müller S., Beato M., Suske G. (1994) Sp1-mediated transcriptional activation is repressed by Sp3. EMBO J. 13, 3843–3851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Majello B., De Luca P., Suske G., Lania L. (1995) Differential transcriptional regulation of c-myc promoter through the same DNA binding sites targeted by Sp1-like proteins. Oncogene 10, 1841–1848 [PubMed] [Google Scholar]
- 53. Yu B., Datta P. K., Bagchi S. (2003) Stability of the Sp3-DNA complex is promoter-specific: Sp3 efficiently competes with Sp1 for binding to promoters containing multiple Sp-sites. Nucleic Acids Res. 31, 5368–5376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pagliuca A., Gallo P., Lania L. (2000) differential role for Sp1/Sp3 transcription factors in the regulation of the promoter activity of multiple cyclin-dependent kinase inhibitor genes. J. Cell Biochem. 76, 360–367 [DOI] [PubMed] [Google Scholar]
- 55. Encarnacao P. C., Ramirez V. P., Zhang C., Aneskievich B. J. (2013) Sp sites contribute to basal and inducible expression of the human TNIP1 (TNFα-inducible protein 3-interaction protein 1) promoter. Biochem. J. 452, 519–529 [DOI] [PubMed] [Google Scholar]
- 56. Li L., Davie J. R. (2010) the role of Sp1 and Sp3 in normal and cancer cell biology. Ann. Anat. 192, 275–283 [DOI] [PubMed] [Google Scholar]
- 57. Hagen G., Dennig J., Preiss A., Beato M., Suske G. (1995) Functional analyses of the transcription factor Sp4 reveal properties distinct from Sp1 and Sp3. J. Biol. Chem. 270, 24989–24994 [DOI] [PubMed] [Google Scholar]
- 58. Johar K., Priya A., Wong-Riley M. T. (2014) Regulation of Na+/K+-ATPase by neuron-specific transcription factor Sp4: implication in the tight coupling of energy production, neuronal activity and energy consumption in neurons. Eur. J. Neurosci. 39, 566–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Priya A., Johar K., Nair B., Wong-Riley M. T. (2014) Specificity protein 4 (Sp4) regulates the transcription of AMPA receptor subunit GluA2 (Gria2). Biochim. Biophys. Acta 1843, 1196–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Danielson K. G., Pillarisetti J., Cohen I. R., Sholehvar B., Huebner K., Ng L. J., Nicholls J. M., Cheah K. S., Iozzo R. V. (1995) Characterization of the complete genomic structure of the human WNT-5A gene, functional analysis of its promoter, chromosomal mapping, and expression in early human embryogenesis. J. Biol. Chem. 270, 31225–31234 [DOI] [PubMed] [Google Scholar]
- 61. Kumawat K., Menzen M. H., Slegtenhorst R. M., Halayko A. J., Schmidt M., Gosens R. (2014) TGF-β-activated kinase 1 (TAK1) signaling regulates TGF-β-induced WNT-5A expression in airway smooth muscle cells via Sp1 and β-catenin. PLoS One 9, e94801. [DOI] [PMC free article] [PubMed] [Google Scholar]