Background: Translation of Tyr codons is highly prone to Phe misincorporation during amino acid limitation in CHO cells.
Results: CHO TyrRS is error-prone and readily aminoacylates tRNATyr with Phe.
Conclusion: Mammalian TyrRS has evolved to be significantly less accurate than its bacterial counterpart.
Significance: Different evolutionary constraints determine the accuracy of translation quality control in eukaryotes and bacteria.
Keywords: Aminoacyl tRNA Synthesis, Aminoacyl tRNA Synthetase, Protein Synthesis, Transfer RNA (tRNA), Translation
Abstract
Quality control operates at different steps in translation to limit errors to approximately one mistranslated codon per 10,000 codons during mRNA-directed protein synthesis. Recent studies have suggested that error rates may actually vary considerably during translation under different growth conditions. Here we examined the misincorporation of Phe at Tyr codons during synthesis of a recombinant antibody produced in tyrosine-limited Chinese hamster ovary (CHO) cells. Tyr to Phe replacements were previously found to occur throughout the antibody at a rate of up to 0.7% irrespective of the identity or context of the Tyr codon translated. Despite this comparatively high mistranslation rate, no significant change in cellular viability was observed. Monitoring of Phe and Tyr levels revealed that changes in error rates correlated with changes in amino acid pools, suggesting that mischarging of tRNATyr with noncognate Phe by tyrosyl-tRNA synthetase was responsible for mistranslation. Steady-state kinetic analyses of CHO cytoplasmic tyrosyl-tRNA synthetase revealed a 25-fold lower specificity for Tyr over Phe as compared with previously characterized bacterial enzymes, consistent with the observed increase in translation error rates during tyrosine limitation. Functional comparisons of mammalian and bacterial tyrosyl-tRNA synthetase revealed key differences at residues responsible for amino acid recognition, highlighting differences in evolutionary constraints for translation quality control.
Introduction
Translation accuracy is vital for the maintenance of cellular integrity. Accuracy in protein synthesis is dependent on a combination of sequential substrate recognition events, which include the synthesis of correct aminoacyl tRNAs (aa-tRNA)2 by aminoacyl tRNA synthetases (aaRSs), binding of elongation factor 1A (EF 1A) to the cognate aa-tRNA, and the selection of the correct aa-tRNA by the ribosome. All these steps have their own inherent error rate, which is thought to vary depending on various environmental conditions. The overall error rate of translation is generally believed to be 10−4 (1, 2), with the first step, synthesis of aa-tRNA, being the most error-prone. aa-tRNA synthesis is a two-step reaction: activation of an amino acid with ATP to form aminoacyl adenylate, followed by transfer of the aminoacyl moiety to the 3′ end of the tRNA (3). The error rate of this first step of translation is largely dependent on the specificity of the aaRS, that is selection of the correct amino acid and tRNA from the respective cellular pools of predominantly noncognate substrates. aaRSs select their cognate tRNAs by exploiting sequence-specific differences between various tRNAs during binding and aminoacylation, thereby resulting in a low error rate of 10−6 at this step (4, 5). In contrast, selection of the correct amino acid is often challenging due to the lack of sufficient discriminating functional groups in many amino acids and their analogs. To maintain a comparatively low error rate during translation, editing mechanisms have evolved to discriminate between substrates with close structural and chemical properties by hydrolyzing either the activated noncognate amino acid (pre-transfer editing) or mischarged tRNA (post-transfer editing) (6). The high specificity displayed by some aaRSs is also achieved by taking advantage of the unique structural and chemical properties of certain amino acids, leading to favorable binding affinities of cognate over noncognate substrates in the active site of the enzyme. For example, Phe and Tyr differ from each other by a single hydroxyl group, the specific recognition and binding of which allows bacterial tyrosyl-tRNA synthetase (TyrRS) to discriminate against noncognate Phe with a specificity of 105 (7).
Although quality control at different steps can limit errors to approximately one mistranslated codon per 10,000 during mRNA-directed protein synthesis (1, 2, 8), recent studies suggest that error rates vary considerably during translation. In Escherichia coli codon-specific differences in error rates of up to 18-fold were observed using a luciferase reporter assay (2). More dramatically, exposure of mammalian cells to a variety of stresses elevates tRNA mischarging to levels that could potentially lead to increases in the error rate of translation of 100-fold or more for some codons (9, 10). In a recent study, misincorporation rates of up to 0.2–3% for Phe at Tyr codons during protein synthesis were reported in mammalian cell culture under conditions of amino acid depletion (11). We now show that CHO TyrRS has a lower than expected specificity for Tyr over Phe, consistent with the previously observed error rates (11). We further examined how the active site of the eukaryotic enzyme evolved to confer lower specificity to the CHO TyrRS as compared with the bacterial enzyme. Residues lost from the eukaryotic enzyme but present in the bacterial counterpart were found to be important for substrate recognition and discrimination, illustrating how different evolutionary constraints have shaped the specificities of bacterial and higher eukaryotic TyrRS.
EXPERIMENTAL PROCEDURES
Cell Culture Experiment Setup and Analytics
CHO cells producing a recombinant monoclonal antibody were grown in chemically defined media. Tyrosine 2Na+·2H2O (SAFC Biosciences, Lenexa, KS) was used in the supplementation study. All media and stock solutions were filter-sterilized at 0.1 μm. Cells were grown in 500-ml vented shake flasks under 36 °C, 5% CO2, and 160 rpm. The inoculation density was 1 × 106 cells/ml, and the culture was grown for 16 days. Bolus feeds were added on days 5, 7, 9, 11, and 13 at 9% of current working volume. Tyrosine supplement was added on days 9, 11, and 13 targeting a 1 mm addition to the culture. Glucose (Life Technologies) was maintained in the range of 6–8 g/liter throughout production. Viable cell density and viability were measured using a Cedex automatic cell counter (Innovatis), and metabolites were measured using a NOVA BioProfile automated analyzer (NOVA Biomedical). Values of pH, pO2, and pCO2 were analyzed by the BioProfile pHox (NOVA Biomedical), and osmolality values were measured by the model 2020 osmometer (Advanced Instruments, Norwood, MA). Titer was measured by reverse-phase HPLC (Waters, Milford, MA) using a protein A column (Life Technologies). Free amino acids were measured by cation exchange HPLC (Agilent Technologies, Santa Clara, CA). HPLC-MS/MS analysis of amino acid substitutions in secreted recombinant antibodies was performed as described previously (12).
Cloning and Mutagenesis
The CHO TyrRS (EGW00102) gene, codon-optimized for expression in E. coli, was synthesized (GenScript) and subcloned under T7 promoter control into pET33b vector at NcoI and XhoI restriction sites. The resulting plasmid pET33b-TyrRS-His6 was used to transform E. coli BL21 (DE3) cells. CHO TyrRS mutations were constructed by PCR amplification and DpnI digestion using standard techniques. All cloning and mutagenesis were confirmed by sequencing, and the resulting plasmids were used to transform E. coli BL21 XJB (DE3).
Purification of CHO TyrRS and Variant Proteins
Protein was produced by growing the cells to an optical density at 600 nm (A600) of 0.6 at 37 °C, 250 rpm. Gene expression was induced with 0.5 mm isopropyl-β-d-thiogalactoside for 4 h. Cells were harvested; the pellet was resuspended in a buffer containing 25 mm Tris-HCl (pH 8.0), 300 mm NaCl, 10% glycerol, and 5 mm imidazole, and flash-frozen using liquid N2 before storage at −80 °C. Cell-free extract was produced by sonication of cells in buffer A (25 mm Tris-HCl, pH 8.0, 300 mm NaCl, 5 mm imidazole, and 10% glycerol) containing a protease inhibitor mixture tablet (Complete Mini, EDTA-free; Roche Applied Science) followed by centrifugation at 150,000 × g for 45 min. The resulting supernatant was loaded onto a pre-equilibrated 3-ml TALON® resin metal affinity column (Clontech) followed by washing, and the protein was eluted with buffer B (25 mm Tris-HCl, pH 8.0, 300 mm NaCl, 250 mm imidazole, and 5% glycerol). Fractions containing the protein of interest (judged by Coomassie Brilliant Blue staining after SDS-PAGE) were pooled and dialyzed twice against buffer C (25 mm Tris-HCl, pH 7.5, 0.1 mm EDTA, 10 Mm β-mercaptoethanol, and 5% glycerol) to remove any bound tyrosyl-adenylate from TyrRS. The enzyme was further dialyzed against two buffer changes of buffer D (50 mm Tris-HCl, pH 7.5, 140 mm KCl, 20 mm β-mercaptoethanol, 10 mm MgCl2, and 5% glycerol) and finally against buffer D with 50% glycerol and stored at −20 °C. Mini-TyrRS was produced as described for CHO TyrRS except that the region of pET33b-TyrRS-His6 encoding the endothelial monocyte-activating polypeptide (EMAP) II-like domain was removed.
Cloning and in Vitro Transcription of CHO tRNATyr
The gene for CHO tRNATyrGTA (CCTTCGATAGCTCAGTTGGTAGAGCGGAGGACTGTAGATCCTTAGGTCGCTGGTTCGATTCCGGCTCGAAGGACCA) was chosen from the various tRNATyr gene sequences predicted by tRNAscan-SE analysis of the available CHO genome. The tRNA gene was synthesized using synthetic DNA oligomers according to standard procedures (13). The 5′ nucleotide is a cytosine in CHO tRNATyr, which is a poor substrate for the T7 RNA polymerase, hence a hammerhead ribozyme was ligated between the T7 promoter and the tRNA sequence and cloned into pUC19 vector using BamHI and HindIII restriction sites to yield pUC19-T7 promoter-hammerhead ribozyme-CHO tRNATyr. This plasmid was digested with BstNI to generate 3′-CCA and used as a template for run-off transcription using T7 RNA polymerase. The tRNA transcript was purified on a denaturing 15% polyacrylamide gel and extracted by electrodialysis in 90 mm Tris borate/2 mm EDTA (pH 8.0). The tRNA was phenol- and chloroform-extracted, ethanol-precipitated, and resuspended in diethylpyrocarbonate-treated double-distilled dH2O.
Aminoacylation Assays
All aminoacylation reactions were performed at 37 °C in a reaction mixture containing 144 mm Tris-HCl, pH 7.78, 150 mm KCl, 10 mm MgCl2, 10 mm β-mercaptoethanol, 0.1 mg/ml BSA, 5 mm ATP, either CHO total tRNA or in vitro-transcribed CHO tRNATyr or E. coli tRNAPhe, l-[U-14C]tyrosine (482 mCi/mmol) or l-[U-14C]phenylalanine (487 mCi/mmol), and aaRSs at the indicated concentrations for specific experiments. CHO total tRNA containing native tRNATyr was prepared as described previously (14). The reaction was initiated with the addition of enzyme. Aliquots of reaction mixture were spotted on 3 MM filter paper presoaked in 5% TCA (w/v) at the required time intervals, washed in 5% TCA acid, and dried, and the level of radioactivity was determined by scintillation counting.
Steady-state Kinetics
Steady-state kinetic assays were carried out at 25 °C as described previously (15, 16). Reactions were carried out in buffer containing 144 mm Tris-HCl, pH 7.78, 150 mm KCl, 10 mm MgCl2, 10 mm β-mercaptoethanol, and 2 mm PPi. For ATP-PPi exchange assays to measure amino acid activation (17), concentrations of substrates were varied from 0.5 to 500 μm for Tyr and from 0.5 to 47 mm for Phe. Enzymes were added to a final concentration of 75 nm–5 μm. Kinetic parameters were calculated by fitting data to the Michaelis-Menten equation using nonlinear regression (KaleidaGraph, Synergy Software) and are presented as averages from three independent reactions with the corresponding standard errors.
RESULTS
Tyr Starvation Negatively Affects Cell Culture Performance
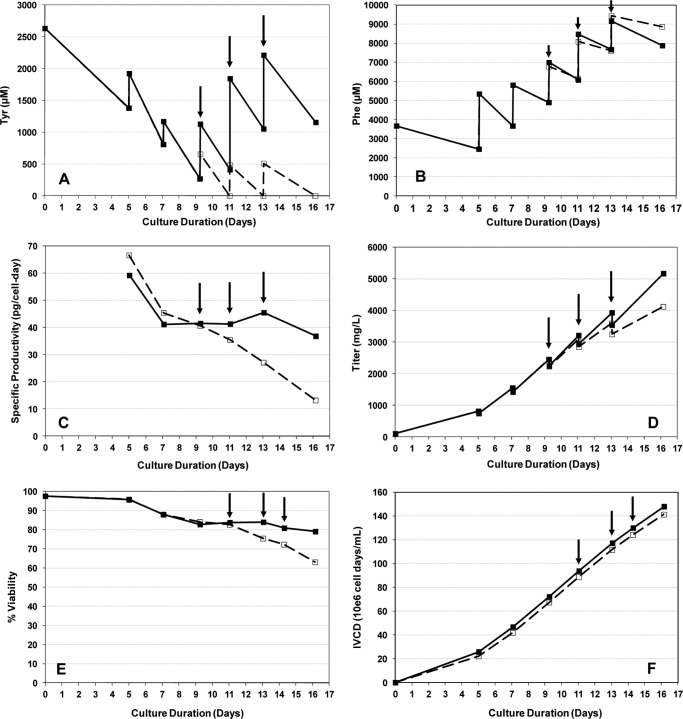
During growth of CHO cell cultures, Tyr starvation was observed in the latter half of fed-batch production with residual concentrations ranging from 0 to 500 μm, whereas Phe concentrations were maintained between 5 and 9 mm (Fig. 1, A and B). The routine decrease of Tyr concentration to zero during this period indicated that the intermittent feed was insufficient to support the culture's utilization of Tyr. A corresponding drop in recombinant protein-specific productivity, qp, on day 9 was observed despite the abundance of Phe in the culture, leading to a 3-fold reduction in qp by day 16 (Fig. 1C). The slope of the titer curve was also lowered as qp decreased and titer dropped by 1 g/liter at day 16 (Fig. 1D), and a drop in cell viability followed after day 11 (Fig. 1E). In the same experiment, Tyr supplementation was also tested in a separate culture with additions on days 9, 11, and 13. As a result, qp was maintained at a high level leading to the continual linear rise of the titer curve, whereas cell viability also significantly improved (Fig. 1, C–E). Cell growth was not impacted by Tyr starvation as shown by similar integrated viable cell density trends under the two conditions (Fig. 1F). Amino acid analysis showed that depletion of Tyr was prevented with the additional Tyr supplementation (Fig. 1A). Residual Phe concentrations remained similar in both conditions (Fig. 1B). Analyses of the secreted recombinant antibodies produced by these cultures showed multiple Phe misincorporation at a rate of ∼0.7% per Tyr codon during Tyr starvation. In the culture that was not depleted of Tyr, Phe misincorporation at Tyr codons was not observed (<0.01%).
FIGURE 1.
Effect of Tyr on CHO cells producing a recombinant monoclonal antibody. A, residual Tyr concentration. B, residual Phe concentration. C, specific productivity. D, titer. E, percentage of viability. F, integrated viable cell density (IVCD). Symbols: dashed line (□), cell culture without Tyr supplementation; solid line (■), cell culture with Tyr supplementation. Arrows indicate timing of tyrosine addition. Cells were grown in chemically defined media.
CHO TyrRS Has Low Specificity for Tyr over Phe
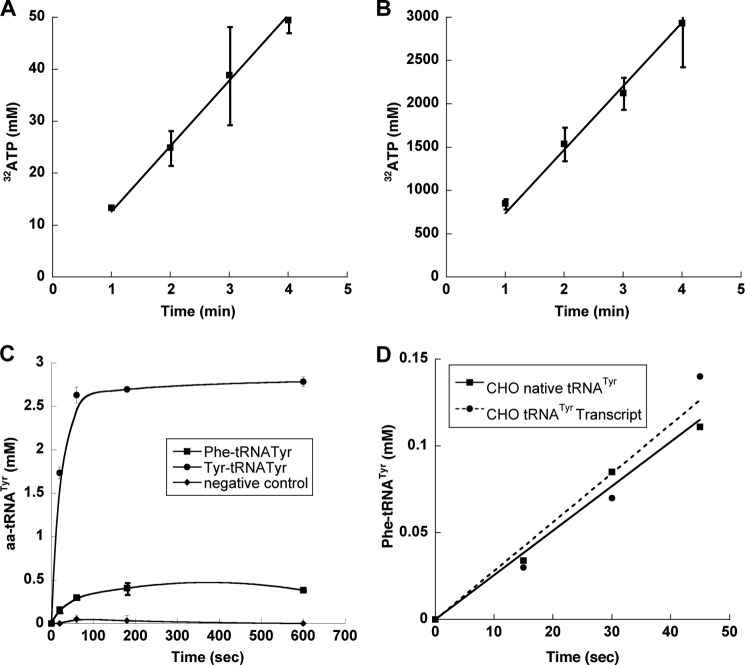
The observed changes in amino acid pools, as well as the absence of codon context or usage effects on misincorporation rates, suggest that mistranslation may result from errors during the aminoacylation of tRNATyr by TyrRS. To assess the specificity of amino acid activation by CHO cytoplasmic TyrRS, the corresponding gene sequence (EGW00102) was codon-optimized for protein production in E. coli. CHO TyrRS was found to misactivate Phe (Fig. 2A), and the specificity for Tyr over Phe was found to be 6100:1 (Table 1). The amino acid specificity of CHO TyrRS was almost 25-fold lower than that of the well characterized bacterial TyrRS from Geobacillus stearothermophilus (7), indicating a substantial reduction in the discrimination of near cognate amino acid in the eukaryotic enzyme.
FIGURE 2.
Activation and charging of Phe by TyrRS. A and B, ATP-PPi exchange assay of CHO TyrRS (250 nm) in the presence of 5 mm Phe (A) or Tyr (B) and CHO TyrRS (75 nm) in the presence of 100 μm Tyr (inset) at 37 °C. Error bars indicate ± S.D. C, aminoacylation of CHO tRNATyr (4 μm) by CHO TyrRS (100 nm) in the presence of 200 μm Phe (■) or 200 μm Tyr (●) and TyrRS (100 nm) in the presence of 100 μm Tyr without tRNA (♦) at 37 °C. D, aminoacylation reaction was carried out by CHO TyrRS (100 nm) in the presence of either 6 μm native tRNATyr (■) or 6 μm in vitro-transcribed tRNA (●) and 200 μm Phe at 37 °C.
TABLE 1.
Steady-state kinetic constants for ATP-[32P]PPi exchange for CHO cytosolic full length (FL) and mini-TyrRS
| Tyr |
Phea (kcat/Km) | Specificityb, Tyr/Phe | |||
|---|---|---|---|---|---|
| Km | kcat | kcat/Km | |||
| μm | s−1 | s−1/μm | s−1/μm | ||
| CHO FL TyrRS | 14.6 ± 4 | 12.5 ± 2 | 0.85 | 1.4 × 10−4 | 6100 |
| CHO mini-TyrRS | 16 ± 0.6 | 15 ± 3 | 0.93 | 1.15 × 10−4 | 8100 |
a kcat/Km was estimated using subsaturating Phe concentrations from the slope of the equation, V = kcat [E][S]/Km.
b Measured in kcat/Km.
Misacylation of tRNATyr by TyrRS
The comparatively low specificity of CHO TyrRS for Tyr over Phe during amino acid activation prompted us to investigate the ability of the enzyme to mischarge tRNATyr with Phe. Candidate tRNATyr genes encoded in the CHO genome were identified using the tRNAscan-SE software package (18). Two sequences, tRNATyr1 and tRNATyr2, were chosen from among the various candidates based on their similarity to known tRNATyr genes from related organisms. The two genes were used as templates for in vitro transcription, and both resulting tRNATyr variants were found to be equally efficient substrates for aminoacylation with tyrosine by TyrRS. CHO TyrRS had a Km for tRNATyr2 of 3 ± 1 μm and a kcat of 33 ± 5 s−1, both values within the range typically observed for in vitro-transcribed tRNAs with aaRSs, and this substrate was used for all further analyses. CHO TyrRS was able to attach Phe to both tRNATyr2 and CHO total tRNA containing native tRNATyr (Fig. 2, B and C), excluding the possibility that mischarging resulted from the lack of post-transcriptional modifications to the in vitro-transcribed substrate. Aminoacylation reactions were also performed using E. coli tRNAPhe with CHO TyrRS and CHO tRNATyr with E. coli PheRS to exclude the possibility that the mischarging observed was due to charging of CHO tRNATyr by an E. coli PheRS contaminant in the CHO TyrRS protein preparation (Fig. 3, A and B).
FIGURE 3.
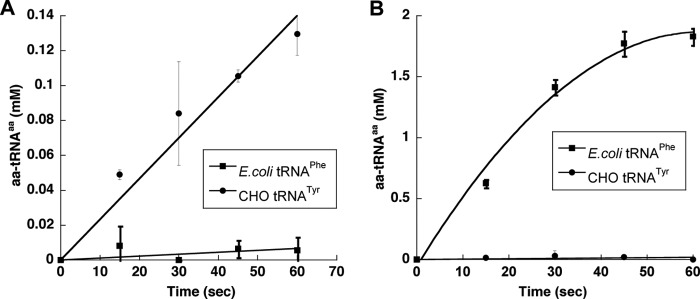
Mischarging of Phe by CHO TyrRS is not due to E. coli PheRS contamination. A and B, aminoacylation of 6 μm E. coli tRNAPhe (■) and 6 μm CHO tRNATyr (●) in the presence of 150 μm [14C] Phe CHO TyrRS (100 nm) (A) and E. coli PheRS (100 nm) (B) at 37 °C. Error bars indicate ± S.D.
The Endothelial Monocyte-activating Polypeptide II-like Domain of CHO TyrRS Does Not Compromise Amino Acid Specificity
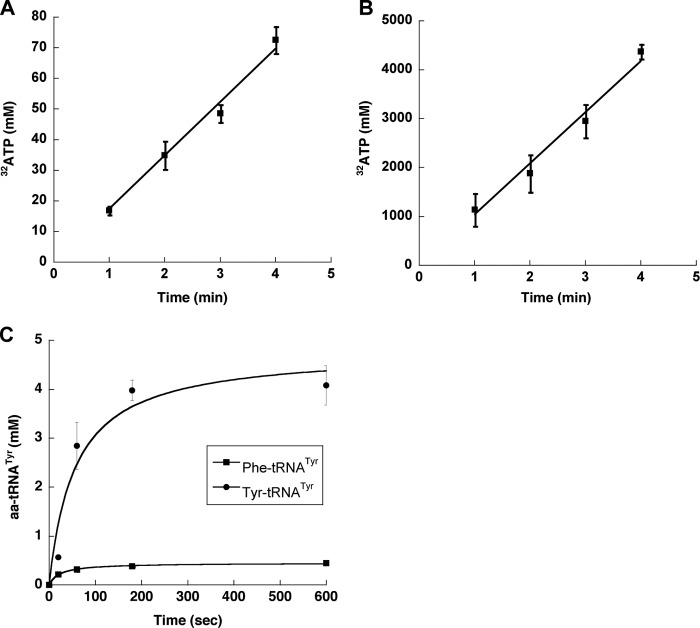
During apoptosis mammalian TyrRS is cleaved, releasing two fragments, an N-terminal mini-TyrRS and the EMAP II-like C-terminal domain, both of which are active cytokines (19). To investigate whether mini-TyrRS accumulation impacts mistranslation, the ability of the truncated enzyme to discriminate noncognate Phe was characterized. Mini-TyrRS activated and aminoacylated Phe and Tyr (Fig. 4, A–C), and the kinetic parameters for amino acid activation were found to be similar to those for full-length TyrRS (Table 1). These results indicate that the presence of the C-terminal EMAP II-domain has no effect on the recognition of cognate Tyr and discrimination against noncognate Phe, and so would not be expected to affect the level of mistranslation of Tyr codons.
FIGURE 4.
Activation and charging of Phe by mini-TyrRS. A and B, ATP-PPi exchange assay of CHO mini-TyrRS (250 nm) in the presence of 5 mm Phe (A) or Tyr (B) and CHO mini-TyrRS (75 nm) in the presence of 100 μm Tyr (inset) at 37 °C. Error bars indicate ± S.D. C, aminoacylation of CHO tRNATyr (4 μm) by CHO mini-TyrRS (100 nm) in the presence of 200 μm Phe (■) or 200 μm Tyr (●) at 37 °C. Error bars indicate ± S.D.
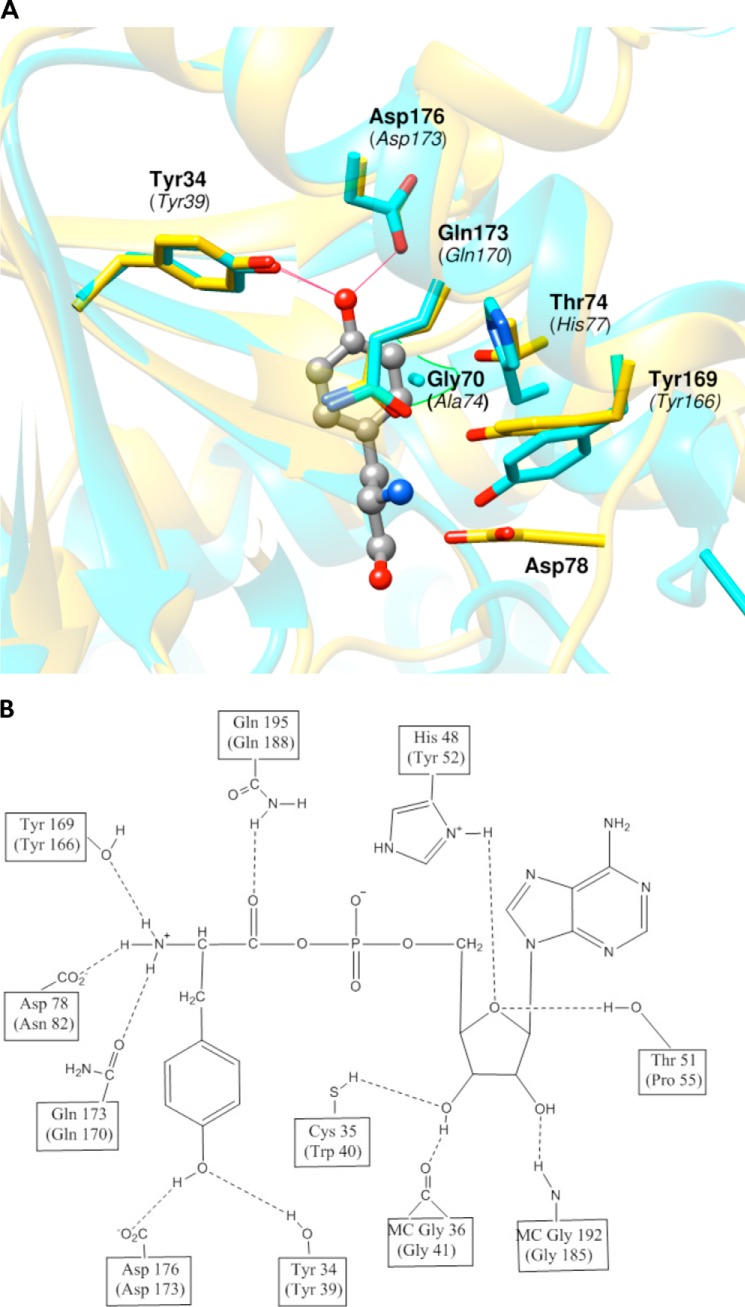
Comparison of Bacterial and CHO TyrRS Substrate Specificity Determinants
Comparison of TyrRS active site structures with Tyr bound would provide an ideal means to evaluate how the CHO enzyme evolved to have lower substrate specificity than its bacterial counterpart. Because the crystal structure of the CHO enzyme is not available, we compared the G. stearothermophilus (Protein Data Bank (PDB) 1tyd) and human (PDB 1q11) TyrRS active sites. The active site of G. stearothermophilus TyrRS has been studied in detail; the residues that make hydrogen-bonding interactions with the substrate tyrosine include Tyr-34, Asp-176, Tyr-169, Asp-78, and Gln-173. Comparison with the active sites of human and CHO TyrRS revealed that most of these residues are conserved except for Asp-78, the equivalent of which is an Asn residue in eukaryotes (Fig. 5). Mutation of CHO Asn-82 to Asp had minimal effect on the kcat and Km of Tyr and Phe, suggesting that the Asn residue is not important for substrate binding in the eukaryotic enzyme (Table 2). Most of the hydrophobic interactions in the CHO active site appear conserved except for Ala-74 and His-77, which are Gly and Thr, respectively, at the corresponding positions in the bacterial enzyme (Fig. 5). Replacement of Ala-74 with Gly increased the Km 7-fold and had no effect on kcat for Tyr. The kcat/Km for Phe decreased 5-fold. Mutation of His-77 to the smaller nonhydrophobic Thr increased the Km and decreased the kcat by 40-fold (Table 2). Substitutions that remove hydrophobic interactions typically lead to a loss of 1–2 kcal/mol of the interaction energy, consistent with the observed increase in the Km for Tyr. Several other positions not conserved between bacteria and eukaryotes are residues Cys-35, His-48, Thr-51, and Lys-233 in bacterial TyrRS, which interact with ATP during transition state formation (Fig. 5) (20). The CHO TyrRS counterparts of these residues are Trp-40, Tyr-52, Pro-55, and Ser-225, all of which lack interactions with ATP (Fig. 5). Despite the absence of these interactions with ATP in the eukaryotic enzyme, the stabilities of the transition states for Tyr activation have been shown to be virtually identical for the human and G. stearothermophilus enzymes. Stabilization of the transition state in eukaryotic TyrRS is potentially achieved by interaction of a potassium ion, which has been shown to replace Lys-233, and Pro-55, which is believed to replace the Thr-51 interaction with ATP (21). To determine whether differences in ATP binding affect activation of cognate and noncognate substrates, the CHO residues Trp-40 and Tyr-52 were replaced with their bacterial equivalents, Cys and His, respectively, to generate single amino acid substitutions. The W40C mutation caused a 30-fold decrease of kcat for Tyr activation and a 30-fold increase of Km. The kcat/Km for Phe activation decreased 2000-fold, but no significant difference in the specificity for Tyr over Phe was observed, indicating that Trp-40 is not involved in amino acid discrimination. The Y52H mutation led to a 2-fold increase in Km and a 20-fold decrease in kcat. The kcat/Km for Phe decreased 280-fold, thereby increasing the specificity of the enzyme 6-fold. Replacement of Tyr-52 may disrupt a possible cation-π interaction between the aromatic ring of Tyr and the catalytically important potassium ion, consistent with the large decrease in kcat observed.
FIGURE 5.
Hydrogen-bonding and hydrophobic interaction between TyrRS and tyrosyl-adenylate. A, the image shows a comparison of the hydrogen-bonding interaction between TyrRS and substrate tyrosine. The image corresponds to a superposition of G. stearothermophilus and human TyrRS from PDB files 1tyd (chain E) and 1q11 (chain A). G. stearothermophilus TyrRS is in gold, human TyrRS is in cyan, and substrate tyrosine in the active site is in green. Amino acids making hydrogen-bonding and hydrophobic interactions are numbered with human TyrRS numbering in parentheses. B, hydrogen bonding between the tyrosyl adenylate and TyrRS in the active site of G. stearothermophilus with CHO TyrRS numbering in parentheses. MC, main chain.
TABLE 2.
Steady-state kinetic constants for ATP-[32P]PPi exchange for CHO cytosolic full length wild type and variant TyrRS
| TyrRS variant | Disrupted contact | Tyr |
Phea (kcat/Km) | Specificityb, Tyr/Phe | ||
|---|---|---|---|---|---|---|
| Km | kcat | kcat/Km | ||||
| μm | s−1 | s−1/μm | s−1/μm | kcat/Km | ||
| WT | 15 ± 4 | 13 ± 2 | 0.85 | 1.4 × 10−4 ± 3.5 × 10−5 | 6100 | |
| W40C | 510 ± 200 | 0.4 ± 0.14 | 7.9 × 10−4 | 7.3 × 10−8 ± 9 × 10−9 | 10,850 | |
| Y52H | 36 ± 4 | 0.7 ± 0.04 | 0.018 | 5 × 10−7 ± 1 × 10−7 | 36,000 | |
| A74G | Hydrophobic | 103 ± 45 | 12 ± 6 | 0.11 | 2.7 × 10−5 ± 1 × 10−5 | 4100 |
| H77T | Hydrophobic | 640 ± 51 | 0.3 ± 0.02 | 4.7 × 10−4 | 7.3 × 10−8 ± 1.4 × 10−9 | 6500 |
| N82D | Hydrogen bonding with substrate | 42 ± 16 | 5 ± 2 | 0.12 | 1.9 × 10−5 ± 1.5 × 10−6 | 6300 |
| G120N | 2° hydrogen bonding | 1070 ± 50 | 1.2 ± 0.2 | 1.2 × 10−3 | 5 × 10−7 ± 4 × 10−8 | 2400 |
| Y123W | 2° hydrogen bonding | 42 ± 2 | 7.3 ± 0.85 | 0.18 | 2.3 × 10−5 ± 6.2 × 10−6 | 7800 |
| D122N | 2° hydrogen bonding | 15 ± 2 | 9 ± 0.7 | 0.6 | 3 × 10−5 ± 1 × 10−5 | 20,000 |
| L125W | 2° hydrogen bonding | 12 ± 0.4 | 8.3 ± 1.2 | 0.72 | 1.3 × 10−4 ± 1.4 × 10−5 | 6000 |
a kcat/Km was estimated using subsaturating Phe concentrations from the slope of the equation, V = kcat [E][S]/Km.
b Measured in kcat/Km.
Hydrogen-bonding interactions between bacterial TyrRS residues Asp-176 and Tyr-34 and the substrate Tyr hydroxyl group help confer amino acid specificity to the enzyme (7, 22). These residues also hydrogen-bond other residues surrounding the inner core of residues that interact directly with substrate; for example Trp-126 and Asn-123 are both hydrogen-bonded to Asp-176 (Fig. 6). Trp-126 and Asn-123 are not conserved across the three domains of life, the equivalent residues in eukaryotes being Tyr and Gly, respectively (Fig. 6). The similarity between bacterial and CHO TyrRS is around 16%, and a number of possible alignments were used, together with available crystal structures, to guide further mutagenesis. Comparing the G. stearothermophilus TyrRS and human TyrRS crystal structure indicated that CHO Gly-120 is present at the position corresponding to Asn-123 and lacks hydrogen-bonding interactions with any residue, which could potentially interact with the OH group of Tyr (Fig. 6). Replacing Gly-120 with Asn resulted in a 70-fold increase in Km, an 11-fold decrease in kcat for Tyr, and a 280-fold decreased kcat/Km for Phe. Replacement of Tyr-123 (equivalent to bacterial Trp-126) with Trp had minimal effect on the kcat and Km of Tyr and Phe activation. When additional alignment-directed mutations were made (Fig. 7), a slight increase in the specificity for the cognate amino acid Tyr was observed. Replacement of Leu-125 with the bacterial equivalent Trp had no effect on the kcat/Km for either Tyr or Phe; however, changing Asp-122 to Asn led to 40-fold increased kcat/Km for Phe but had no effect on the Km and kcat of Tyr activation, leading to 3-fold increases in the specificity of the CHO enzyme. These changes indicate that Asp-122 is involved in the discrimination of cognate over noncognate amino acid.
FIGURE 6.
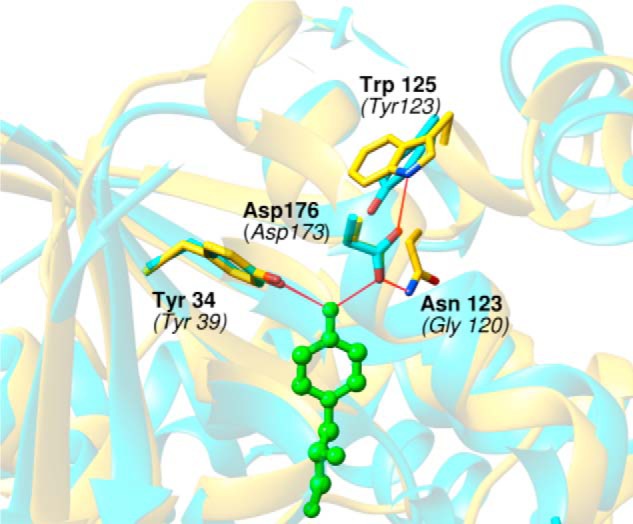
Hydrogen-bonding network of G. stearothermophilus TyrRS Asp-176 (CHO Asp-173). The image shows the specificity site of TyrRS formed by two residues that interact directly with the tyrosine hydroxyl (substrate) by hydrogen bonding: Asp-176 and Tyr-34 with CHO numbering in parentheses. The image corresponds to a superposition of G. stearothermophilus and human TyrRS from PDB files 1tyd (chain E) and 1q11 (chain A). G. stearothermophilus TyrRS is in gold, human TyrRS is in cyan, and substrate tyrosine in the active site is in green. Amino acids making hydrogen-bonding interactions are numbered with human/CHO TyrRS numbering in parentheses.
FIGURE 7.
Alignment of amino acid sequences for TyrRS. The alignment shown is based on the ClustalW alignment of TyrRS amino acid sequences from G. stearothermophilus, E. coli, Thermococcus kodakarensis, Homo sapiens, S. cerevisiae (cytoplasmic), and CHO. Shaded regions indicate highly conserved amino acids.
DISCUSSION
Amino Acid Imbalance Increases Translation Error Rates
aaRSs maintain translational fidelity either by accurate binding of the correct amino acid or by proofreading and editing of noncognate products. Previous studies have shown that errors in translation can occur randomly at a rate of about 4 × 10−4 to 5 × 10−5 per codon or 0.005–0.04% per site under normal growth conditions (23, 24). Various studies have suggested that error rates in protein synthesis can increase under a variety of conditions such as oxidative stress, (9, 10, 25), changes in codon bias (26, 27), genetic heterogeneity (28), heterologous overproduction (29), and amino acid starvation (24). To determine the rate and extent of mistranslation under different conditions, accurate detection and quantification of amino acid misincorporation are critical (30, 31). Aside from the technical challenges, measuring mistranslation is further complicated by the expectation that misincorporation could lead to protein misfolding and subsequent degradation by the cellular protein quality control machinery. This potential problem can be partly addressed by measuring misincorporation rates in a secreted recombinant antibody, which revealed Phe substitutions at Tyr codons at an overall error rate of ∼0.7% during Tyr starvation. The higher than expected level of Tyr to Phe misincorporation was effectively suppressed by supplementing the medium with Tyr, indicating that the increase in error rate was due to an imbalance in cellular amino acid pools. Amino acid imbalances are encountered by mammalian cells under various conditions such as, for example, bacterial infections that trigger acute intracellular amino acid starvation due to host membrane damage (32) or cancer tumor microenvironments that become nutritionally deprived due to rapid cell proliferation. Our findings suggest that under such growth conditions, protein synthesis error rates may also rise significantly, but whether this would be expected to have beneficial or detrimental effects on the cell is unclear and may depend on the level of mistranslation in a particular system (31).
Misincorporation of Phe at Tyr Codons Is due to Mischarging of tRNATyr
It has previously been proposed that aaRSs should exhibit a selectivity of at least 3000-fold for cognate over noncognate amino acids to maintain error rates below 10−4 during translation (33). Selectivity is the product of the specificity of the aaRS and the ratio of cognate versus noncognate amino acid (34). The specificity of bacterial TyrRS for Tyr over Phe is 10−5 (7), and the Phe: Tyr ratio in a dividing bacterial cell is typically around 1.9:1 (35). These two factors combined give a rate of misincorporation comparable with other amino acid pairs and show that, in the absence of an editing mechanism, bacterial TyrRS can discriminate between tyrosine and phenylalanine by simple preferential binding and activation. In the case of CHO TyrRS, steady-state kinetic analysis revealed a lower specificity of 6100 for Tyr over Phe as compared with the value of 150,000 reported for bacterial TyrRS. Under Tyr starvation the relative concentration of Phe to Tyr was as high as 18:1, thereby decreasing the selectivity for cognate over noncognate amino acid to around 350:1. Hence, although the selectivity of CHO TyrRS is above the threshold of 3000:1 under normal growth conditions, it is significantly lower during Tyr limitation and results in mistranslation. This increased susceptibility to translation errors observed in higher eukaryotes as compared with bacteria suggests that protein synthesis quality control has evolved with different constraints in each kingdom. On a practical level, our data also illustrate the utility of determining optimal cognate to noncognate amino acid concentrations in the medium to overcome poor aaRS specificity and mitigate tRNA mischarging. This approach of understanding specificity requirements may be applicable to reduce other amino acid misincorporation caused by tRNA mischarging such as Ser → Asn in CHO-produced antibodies (24).
Divergence in Amino Acid Discrimination between Bacterial and Eukaryotic TyrRS
Bacterial TyrRS displays high specificity toward its cognate amino acid Tyr as compared with the noncognate substrate Phe (7). Our results demonstrate that higher eukaryotic TyrRSs have evolved to have a lower specificity for Tyr over Phe than their bacterial counterparts (Table 1). Although the active site residues in G. stearothermophilus and human tyrosyl-tRNA synthetases are largely conserved, several key differences exist between the two enzymes. Asp-78 mediates one important hydrogen-bonding interaction missing from the eukaryotic enzyme in the bacterial system, which is located in a loop region between helix α4 and helix α5. This loop is located at the entrance to the tyrosine-binding site and undergoes a substantial conformational change upon binding of the substrate. This loop region of the bacterial enzyme is also more hydrophilic than its eukaryotic counterpart. In the eukaryotic enzyme, the loop provides a hydrophobic lid over the tyrosine-binding pocket and the conformational change is thought to play a role in sequestering the activated amino acid from water during the catalytic reaction (36). The active site of the eukaryotic TyrRS also has two hydrophobic interactions, via His-77 and Ala-74, which are missing in the bacterial enzyme. His-77 was found to be important for substrate binding (Table 2), illustrating how the eukaryotic enzyme has evolved in a way that may improve binding of the more hydrophobic phenylalanine in the active site of CHO TyrRS. In addition to differences in direct enzyme-substrate interactions, changes in the hydrogen-bonding network are also evident in the eukaryotic TyrRS. Secondary interaction between the catalytically important Asp-173 and residue Asp-122 in the eukaryotic enzyme was found to contribute to discrimination between the cognate versus the noncognate substrate. This residue is located on helix η6 and the loop connecting helix η2 and α6 and could influence specificity by, for example, inducing a conformational change in the loop itself or by altering the protein backbone conformation as shown recently for aspartate aminotransferase (37). Mutation of Tyr-52, which is located in the ATP-binding region, removes a potential interaction with the catalytically important potassium ion, which must be replaced, by an interaction somewhere else in the protein. This mutation decreases Phe activation more than Tyr, thereby generating a TyrRS variant with 6-fold higher specificity for the cognate amino acid. The future deployment of aaRS variants with improved amino acid specificity, as described here, may help to significantly reduce elevated amino acid misincorporation during heterologous protein over production.
Aminoacyl-tRNA synthetases have drawn interest as potential targets for the development of new antibiotics. For example, a series of related competitive inhibitors that bind 40,000-fold more tightly to Staphylococcus aureus than to Saccharomyces cerevisiae TyrRS has been identified (38, 39). The selectivity of these inhibitors is consistent with the differences described here between the active sites of the bacterial and eukaryotic enzymes, and supports the utility of TyrRS as a target for antimicrobial therapeutics.
Acknowledgments
We thank Dr. Todd Lowe (University of California, Santa Cruz) for tRNA gene annotation and Dr. Assaf Katz for critical reading of the manuscript.
This work was supported by National Science Foundation (NSF) Grant MCB-1052344 (to M. I.) an NSF-Grant Opportunities for Academic Liaison with Industry (NSF-GOALI) fellowship (to M. R) and NIH training grant fellowships (GM008512 and GM086252 to A.M.).
- aa-tRNA
- aminoacyl tRNA(s)
- aaRS
- aminoacyl tRNA synthetase
- TyrRS
- tyrosyl-tRNA synthetase
- PheRS
- phenylalanyl-tRNA synthetase
- EMAP
- endothelial monocyte-activating polypeptide.
REFERENCES
- 1. Loftfield R. B., Vanderjagt D. (1972) The frequency of errors in protein biosynthesis. Biochem. J. 128, 1353–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kramer E. B., Farabaugh P. J. (2007) The frequency of translational misreading errors in E. coli is largely determined by tRNA competition. RNA 13, 87–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ibba M., Soll D. (2000) Aminoacyl-tRNA synthesis. Annu. Rev. Biochem. 69, 617–650 [DOI] [PubMed] [Google Scholar]
- 4. Giegé R., Sissler M., Florentz C. (1998) Universal rules and idiosyncratic features in tRNA identity. Nucleic Acids Res. 26, 5017–5035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guth E. C., Francklyn C. S. (2007) Kinetic discrimination of tRNA identity by the conserved motif 2 loop of a class II aminoacyl-tRNA synthetase. Mol. Cell 25, 531–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yadavalli S. S., Ibba M. (2012) Quality control in aminoacyl-tRNA synthesis its role in translational fidelity. Adv. Protein Chem. Struct. Biol. 86, 1–43 [DOI] [PubMed] [Google Scholar]
- 7. Fersht A. R., Shi J. P., Knill-Jones J., Lowe D. M., Wilkinson A. J., Blow D. M., Brick P., Carter P., Waye M. M., Winter G. (1985) Hydrogen bonding and biological specificity analysed by protein engineering. Nature 314, 235–238 [DOI] [PubMed] [Google Scholar]
- 8. Kurland C. G. (1992) Translational accuracy and the fitness of bacteria. Annu. Rev. Genet. 26, 29–50 [DOI] [PubMed] [Google Scholar]
- 9. Netzer N., Goodenbour J. M., David A., Dittmar K. A., Jones R. B., Schneider J. R., Boone D., Eves E. M., Rosner M. R., Gibbs J. S., Embry A., Dolan B., Das S., Hickman H. D., Berglund P., Bennink J. R., Yewdell J. W., Pan T. (2009) Innate immune and chemically triggered oxidative stress modifies translational fidelity. Nature 462, 522–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jones T. E., Alexander R. W., Pan T. (2011) Misacylation of specific nonmethionyl tRNAs by a bacterial methionyl-tRNA synthetase. Proc. Natl. Acad. Sci. U.S.A. 108, 6933–6938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Feeney L., Carvalhal V., Yu X. C., Chan B., Michels D. A., Wang Y. J., Shen A., Ressl J., Dusel B., Laird M. W. (2013) Eliminating tyrosine sequence variants in CHO cell lines producing recombinant monoclonal antibodies. Biotechnol. Bioeng. 110, 1087–1097 [DOI] [PubMed] [Google Scholar]
- 12. Zhang Z., Shah B., Bondarenko P. V. (2013) G/U and certain wobble position mismatches as possible main causes of amino acid misincorporations. Biochemistry 52, 8165–8176 [DOI] [PubMed] [Google Scholar]
- 13. Sampson J. R., Uhlenbeck O. C. (1988) Biochemical and physical characterization of an unmodified yeast phenylalanine transfer RNA transcribed in vitro. Proc. Natl. Acad. Sci. U.S.A. 85, 1033–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Polycarpo C., Ambrogelly A., Ruan B., Tumbula-Hansen D., Ataide S. F., Ishitani R., Yokoyama S., Nureki O., Ibba M., Söll D. (2003) Activation of the pyrrolysine suppressor tRNA requires formation of a ternary complex with class I and class II lysyl-tRNA synthetases. Mol. Cell 12, 287–294 [DOI] [PubMed] [Google Scholar]
- 15. Roy H., Ling J., Irnov M., Ibba M. (2004) Post-transfer editing in vitro and in vivo by the β subunit of phenylalanyl-tRNA synthetase. EMBO J. 23, 4639–4648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roy H., Ling J., Alfonzo J., Ibba M. (2005) Loss of editing activity during the evolution of mitochondrial phenylalanyl-tRNA synthetase. J. Biol. Chem. 280, 38186–38192 [DOI] [PubMed] [Google Scholar]
- 17. Francklyn C. S., First E. A., Perona J. J., Hou Y. M. (2008) Methods for kinetic and thermodynamic analysis of aminoacyl-tRNA synthetases. Methods 44, 100–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lowe T. M., Eddy S. R. (1997) tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 25, 955–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wakasugi K., Schimmel P. (1999) Two distinct cytokines released from a human aminoacyl-tRNA synthetase. Science 284, 147–151 [DOI] [PubMed] [Google Scholar]
- 20. Kleeman T. A., Wei D., Simpson K. L., First E. A. (1997) Human tyrosyl-tRNA synthetase shares amino acid sequence homology with a putative cytokine. J. Biol. Chem. 272, 14420–14425 [DOI] [PubMed] [Google Scholar]
- 21. Austin J., First E. A. (2002) Potassium functionally replaces the second lysine of the KMSKS signature sequence in human tyrosyl-tRNA synthetase. J. Biol. Chem. 277, 20243–20248 [DOI] [PubMed] [Google Scholar]
- 22. Brick P., Blow D. M. (1987) Crystal structure of a deletion mutant of a tyrosyl-tRNA synthetase complexed with tyrosine. J. Mol. Biol. 194, 287–297 [DOI] [PubMed] [Google Scholar]
- 23. Parker J. (1989) Errors and alternatives in reading the universal genetic code. Microbiol. Rev. 53, 273–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wen D., Vecchi M. M., Gu S., Su L., Dolnikova J., Huang Y. M., Foley S. F., Garber E., Pederson N., Meier W. (2009) Discovery and investigation of misincorporation of serine at asparagine positions in recombinant proteins expressed in Chinese hamster ovary cells. J. Biol. Chem. 284, 32686–32694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ling J., Söll D. (2010) Severe oxidative stress induces protein mistranslation through impairment of an aminoacyl-tRNA synthetase editing site. Proc. Natl. Acad. Sci. U.S.A. 107, 4028–4033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Calderone T. L., Stevens R. D., Oas T. G. (1996) High-level misincorporation of lysine for arginine at AGA codons in a fusion protein expressed in Escherichia coli. J. Mol. Biol. 262, 407–412 [DOI] [PubMed] [Google Scholar]
- 27. Yu X. C., Borisov O. V., Alvarez M., Michels D. A., Wang Y. J., Ling V. (2009) Identification of codon-specific serine to asparagine mistranslation in recombinant monoclonal antibodies by high-resolution mass spectrometry. Anal. Chem. 81, 9282–9290 [DOI] [PubMed] [Google Scholar]
- 28. Harris R. J., Murnane A. A., Utter S. L., Wagner K. L., Cox E. T., Polastri G. D., Helder J. C., Sliwkowski M. B. (1993) Assessing genetic heterogeneity in production cell lines: detection by peptide mapping of a low level Tyr to Gln sequence variant in a recombinant antibody. Biotechnology 11, 1293–1297 [DOI] [PubMed] [Google Scholar]
- 29. Kurland C., Gallant J. (1996) Errors of heterologous protein expression. Curr. Opin. Biotechnol. 7, 489–493 [DOI] [PubMed] [Google Scholar]
- 30. Drummond D. A., Wilke C. O. (2009) The evolutionary consequences of erroneous protein synthesis. Nat. Rev. Genet. 10, 715–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reynolds N. M., Lazazzera B. A., Ibba M. (2010) Cellular mechanisms that control mistranslation. Nat. Rev. Microbiol. 8, 849–856 [DOI] [PubMed] [Google Scholar]
- 32. Tattoli I., Sorbara M. T., Vuckovic D., Ling A., Soares F., Carneiro L. A., Yang C., Emili A., Philpott D. J., Girardin S. E. (2012) Amino acid starvation induced by invasive bacterial pathogens triggers an innate host defense program. Cell Host Microbe 11, 563–575 [DOI] [PubMed] [Google Scholar]
- 33. Fersht A. R. (1981) Enzymic editing mechanisms and the genetic code. Proc. R Soc. Lond. B Biol. Sci. 212, 351–379 [DOI] [PubMed] [Google Scholar]
- 34. Ling J., Reynolds N., Ibba M. (2009) Aminoacyl-tRNA synthesis and translational quality control. Annu. Rev. Microbiol. 63, 61–78 [DOI] [PubMed] [Google Scholar]
- 35. Reynolds N. M., Ling J., Roy H., Banerjee R., Repasky S. E., Hamel P., Ibba M. (2010) Cell-specific differences in the requirements for translation quality control. Proc. Natl. Acad. Sci. U.S.A. 107, 4063–4068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang Y., Wang L., Schultz P. G., Wilson I. A. (2005) Crystal structures of apo wild-type M. jannaschii tyrosyl-tRNA synthetase (TyrRS) and an engineered TyrRS specific for O-methyl-l-tyrosine. Protein Sci. 14, 1340–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Oue S., Okamoto A., Yano T., Kagamiyama H. (1999) Redesigning the substrate specificity of an enzyme by cumulative effects of the mutations of non-active site residues. J. Biol. Chem. 274, 2344–2349 [DOI] [PubMed] [Google Scholar]
- 38. Berge J. M., Broom N. J., Houge-Frydrych C. S., Jarvest R. L., Mensah L., McNair D. J., O'Hanlon P. J., Pope A. J., Rittenhouse S. (2000) Synthesis and activity of analogues of SB-219383: novel potent inhibitors of bacterial tyrosyl tRNA synthetase. J. Antibiot. 53, 1282–1292 [DOI] [PubMed] [Google Scholar]
- 39. Jarvest R. L., Berge J. M., Brown P., Hamprecht D. W., McNair D. J., Mensah L., O'Hanlon P. J., Pope A. J. (2001) Potent synthetic inhibitors of tyrosyl tRNA synthetase derived from C-pyranosyl analogues of SB-219383. Bioorg. Med. Chem. Lett. 11, 715–718 [DOI] [PubMed] [Google Scholar]