Background: Chitinase inhibitors have potential applications as pesticides, fungicides, and antiasthmatics.
Results: Fully deacetylated chitooligosaccharides were determined to be GH18 chitinase inhibitors by thermodynamics and crystallography.
Conclusion: Crystal structures reveal a competitive inhibition mode.
Significance: This work first reports fully deacetylated chitooligosaccharides acting as chitinase inhibitors, perhaps providing a structural basis for developing eco-friendly inhibitors against chitinases.
Keywords: Chitinase, Crystal Structure, Enzyme Inhibitor, Glycoside Hydrolase, Thermodynamics, Deacetylated Chitooligosaccharides, Insect Molting
Abstract
Small molecule inhibitors against chitinases have potential applications as pesticides, fungicides, and antiasthmatics. Here, we report that a series of fully deacetylated chitooligosaccharides (GlcN)2–7 can act as inhibitors against the insect chitinase OfChtI, the human chitinase HsCht, and the bacterial chitinases SmChiA and SmChiB with IC50 values at micromolar to millimolar levels. The injection of mixed (GlcN)2–7 into the fifth instar larvae of the insect Ostrinia furnacalis resulted in 85% of the larvae being arrested at the larval stage and death after 10 days, also suggesting that (GlcN)2–7 might inhibit OfChtI in vivo. Crystal structures of the catalytic domain of OfChtI (OfChtI-CAD) complexed with (GlcN)5,6 were obtained at resolutions of 2.0 Å. These structures, together with mutagenesis and thermodynamic analysis, suggested that the inhibition was strongly related to the interaction between the −1 GlcN residue of the inhibitor and the catalytic Glu148 of the enzyme. Structure-based comparison showed that the fully deacetylated chitooligosaccharides mimic the substrate chitooligosaccharides by binding to the active cleft. This work first reports the inhibitory activity and proposed inhibitory mechanism of fully deacetylated chitooligosaccharides. Because the fully deacetylated chitooligosaccharides can be easily derived from chitin, one of the most abundant materials in nature, this work also provides a platform for developing eco-friendly inhibitors against chitinases.
Introduction
Glycoside hydrolase family 18 (GH18)3 chitinases (EC 3.2.1.14) catalyze the random hydrolysis of N-acetyl-β-d-glucosaminide (1→4)-β-linkages in chitin and chitodextrins, which play important roles in various life processes. For example, the well studied bacterium Serratia marcescens contains chitinases to degrade chitin for the purpose of nutrition (1–4). Insects secrete chitinases from epithelial cells to digest chitin during development (5, 6). Although chitin is absent in humans, there are two chitinases, chitotriosidase (HsCht) and acidic mammalian chitinase, that have been identified. HsCht is secreted by activated macrophages and most likely plays a role in the innate immune response to bacteria, fungi, and other pathogens (7, 8). Acidic mammalian chitinase is expressed in lung and stomach tissue, and an elevated level of acidic mammalian chitinase is often associated with asthma and allergic response (9, 10).
Small molecules against GH18 chitinases have been reported. Most of these inhibitors resemble the structure of oxazolinium ion intermediates or mimic carbohydrate-protein interactions. The pseudotrisaccharide allosamidin, which was isolated from the Streptomyces sp., is the most effective inhibitor, with Ki values at the nanomolar level (11–18). The cyclopentapeptides argifin and argadin, which were isolated from the cultured broths of fungal strains Gliocladium sp. FTD-0668 and Clonostachys sp. FO-7314, respectively (19–21), have IC50 values in the nanomolar to micromolar range (21–25). Other molecules, such as chitobiose and chitotriose thiazolines (26), xanthine derivatives (27), and CI-4 (28, 29), exhibit nanomolar to millimolar level inhibitory activities. Unfortunately, complex synthesis and/or limited availability of the starting materials limit the practical application of most of these potent molecules.
On the other hand, mixed randomly deacetylated chitooligosaccharides with different chain lengths have been reported to inhibit the activity of the bacterial chitinase B from S. marcescens (SmChiB) with IC50 values of 15–18 μm (30). This inhibition is easily explained based on the catalytic mechanism. Chitinases catalyze hydrolysis through a substrate-assisted mechanism in which the C2 N-acetyl group of the −1 sugar of a substrate is the nucleophile that attacks the C1 carbon (31). The GH18 chitinases contain a diagnostic DXDXE motif in which the glutamate is the catalytic acid/base. Binding of GlcNAc in the −1 subsite requires considerable distortion and is energetically unfavorable (32, 33). Therefore, if the −1 sugar is deacetylated, it may be energetically favorable to bind a GlcN in the −1 subsite (32). Thus, partially and fully deacetylated chitooligosaccharides (i.e. oligosaccharides only composed of β-(1–4)-linked d-glucosamine (GlcN, deacetylated unit)) can be inhibitors of GH18 chitinases. Partially deacetylated chitooligosaccharides can be recognized by chitinase as a substrate at a site with an acetylated GlcNAc. Therefore, the use of fully deacetylated inhibitors would prevent the degradation of the inhibitors by chitinase. In this study, we report the degree of polymerization-dependent inhibitory effects of fully deacetylated chitooligosaccharides with a degree of polymerization of 2–7. X-ray crystallographic analysis of the catalytic domain of the GH18 chitinase OfChtI (OfChtI-CAD) complexed with either (GlcN)5 or (GlcN)6 revealed that the crucial interaction is between the key catalytic residue and the −1 GlcN, which suggests that the inhibition is competitive. This work provides the first structural insight into the interaction of deacetylated chitooligosaccharide inhibitors with a chitinase.
EXPERIMENTAL PROCEDURES
Materials
(GlcN)2–(GlcN)7 were purchased from Qingdao BZ Oligo Biotech Co., Ltd. (Qingdao, China). The purities of the GlcN oligomers, determined by high performance liquid chromatography (HPLC), were 98.3, 98.0, 98.7, 97.3, 97.6, and 97.2% for dimer to heptamer, respectively. The GlcN oligomers were analyzed by mass spectrometry to ensure that no GlcNAc was present in the samples. The mixed (GlcN)2–7 was purchased from Dalian GlycoBio Co., Ltd. (Dalian, China). The components were determined by HPLC to contain 6.8% (GlcN)2, 21.2% (GlcN)3, 28.4% (GlcN)4, 21.5% (GlcN)5, 9.0% (GlcN)6, 3.1% (GlcN)7, and 10% minor components, including 0.6% GlcN, 2.5% (GlcN)8, 2.7% (GlcN)9, 2.0% (GlcN)10, 1.3% (GlcN)11, and 0.9% (GlcN)12.
Recombinant Expression and Purification
OfChtI (GenBankTM accession number DQ294305) from Ostrinia furnacalis was overexpressed and purified as described previously (34). Briefly, the coding sequence for residues Ala19–Leu554 of OfChtI was amplified using PCR, and a C-terminal His6 affinity tag was introduced. The DNA fragment was ligated into pPIC9 (Invitrogen), and the expression plasmid pPIC9-OfChtI was transformed into Pichia pastoris GS115 (Invitrogen) by electroporation. P. pastoris cells expressing OfChtI were grown in 200 ml of BMGY medium (2% peptone, 1% yeast extract, 1% glycerol, 1.34% yeast nitrogen, 0.2% biotin, and 100 mm potassium phosphate, pH 6.0) at 30 °C for 24 h, and then the cells were collected and resuspended in 1 liter of fresh BMMY medium (2% peptone, 1% yeast extract, 1% methanol, 1.34% yeast nitrogen, 0.2% biotin, and 100 mm potassium phosphate, pH 6.0). Methanol was added to a final concentration of 1% (v/v) at 24-h intervals as an inducer. After incubation for an additional 72 h, the supernatant was harvested via centrifugation. The supernatant was subjected to ammonium sulfate (70% saturation) precipitation at 4 °C. After the precipitate was dissolved, OfChtI was purified using metal chelating chromatography with a nickel-NTA-Sepharose high performance column (5 ml; GE Healthcare).
A fragment corresponding to human chitotriosidase (HsCht, GenBankTM accession number NM_003465) residues 22–466 (64–1398 bp) was ligated into the pPIC9 vector using the EcoRI restriction sites. The recombinant HsCht was overexpressed as a secreted protein from the P. pastoris GS115 and purified using metal chelating chromatography.
The genes encoding chitinase A and chitinase B from S. marcescens (SmChiA, GenBankTM accession number Z36294; SmChiB, GenBankTM accession number Z36295) were amplified from the S. marcescens genome, and a C-terminal His6 affinity tag was introduced. The DNA fragments were ligated into pET28a (Novagen, Madison, WI), and the expression plasmids pET28a–SmChiA and pET28a–SmChiB were transformed into Escherichia coli BL21 (DE3) (Novagen, Madison, WI). The cells were grown to an optical density (OD) of 1.8 at 600 nm before induction with 0.05 mm isopropyl 1-thio-β-d-galactopyranoside. After the addition of isopropyl 1-thio-β-d-galactopyranoside, the growth of cells was continued for 20 h at 16 °C. The target proteins were purified using metal chelating chromatography. The purity of these proteins was analyzed by SDS-PAGE and found to be >95% in all cases.
Site-directed Mutagenesis
The OfChtI E148Q, Y37A, N305A, F309A, and W372A mutants were constructed using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) according to the manufacturer's instructions. The mutated genes were sequenced to confirm that the desired mutations were present. The mutants were overexpressed and purified using the same procedure as for wild-type OfChtI.
Enzymatic Assay
Chitinase activities for OfChtI and its mutants, HsCht, SmChiA, and SmChiB, were determined using the chromogenic substrate 4-nitrophenyl β-d-N,N′-diacetylchitobioside (pNP-(GlcNAc)2; Sigma) in a standard assay. In a final volume of 60 μl, 50 nm enzyme was incubated with 0.1 mm substrate pNP-(GlcNAc)2 in 20 mm Tris-HCl, pH 7.0, at 30 °C. After the addition of 60 μl of 0.5 m sodium carbonate, the amount of 4-nitrophenylate released was determined by the absorbance at 405 nm. IC50 values of fully deacetylated chitooligosaccharides, (GlcN)2–7, were determined in the presence of different concentrations of inhibitor. For SmChiA and SmChiB, the Km values for pNP-(GlcNAc)2 were determined with increasing concentrations of the substrate from 0.02 to 0.5 mm, and the Ki values for (GlcN)5,6 were determined with concentrations of the substrate from 0.075 to 0.3 mm.
The IC50 value of (GlcN)5 against SmChiB was also determined with 4-methylumbelliferyl β-d-N,N′-diacetylchitobioside (MU-(GlcNAc)2; Sigma) as substrate. Standard reaction mixtures (60 μl) contained 2.5 nm SmChiB, 20 μm MU-(GlcNAc)2 in 20 mm Tris-HCl, pH 7.0, at 37 °C. The reaction was stopped with 60 μl of 1 m glycine/NaOH (pH 10.6). The released product, MU, was measured by a fluorescence microplate reader (Thermo Fisher Scientific, Waltham, MA) with excitation and emission wavelengths of 360 and 460 nm, respectively.
Isothermal Titration Calorimetry (ITC)
Isothermal titration calorimetry experiments were performed at 25 and 30 °C using a MicroCal iTC200 system (Microcal, Northampton, MA). For the experiments with (GlcN)2–7, OfChtI, at a concentration of 0.1–0.14 mm in 50 mm HEPES buffer, pH 8.0, was placed in the reaction cell with a volume of 202 μl, and (GlcN)2–7, at a concentration of 0.75–4.2 mm in 50 mm HEPES buffer, pH 8.0, was placed in the ITC syringe. Aliquots of 2–3 μl from the syringe were injected into the reaction cell at 120-s intervals with a stirring speed of 1000 rpm. The titrations were typically finished after 13 or 20 injections. The background was measured by injecting the inhibitors into the buffer.
The ITC data collected were processed by the MicroCal Origin version 7.0 software accompanying the ITC200 system. Before further analysis, all of the data were corrected for heat of dilution by subtracting the background. Using a nonlinear least squares algorithm in the Origin software, the data were analyzed using a single-site binding model. Using this binding model, the stoichiometry (n), the association constant (Ka), and the binding enthalpy change (ΔH) of the reaction were obtained. For (GlcN)2 and (GlcN)3 binding, a two-site binding model was used. The dissociation constant (Kd), the reaction free energy change (ΔG), and the binding entropy change (−TΔS) were calculated from the equation,
where R represents the gas constant (1.98 cal K−1 mol−1) and T is the absolute temperature in Kelvin (K).
Crystallization and Data Collection
The crystals of free OfChtI-CAD were obtained in 100 mm HEPES, pH 7.5, 25% (w/v) PEG 3350 by the vapor method as described previously (33). Crystals of the OfChtI-CAD·(GlcN)5 and OfChtI-CAD·(GlcN)6 complexes were obtained by transferring the crystals of free OfChtI-CAD to a solution consisting of 10 mm (GlcN)5 or (GlcN)6, 100 mm HEPES, pH 7.5, 25% (w/v) PEG 3350. These crystals were soaked for 20 h at 4 °C. Afterward, the crystals were soaked for 10 s in a reservoir solution containing 25% (v/v) glycerol as a cryoprotection reagent and subsequently flash-cooled in liquid nitrogen. X-ray diffraction data of the complexes were collected on the BL-17U at the Shanghai Synchrotron Radiation Facility in China. The diffraction data of the complexes were processed using the HKL-2000 package (35).
Structure Determination and Refinement
The structures of OfChtI-CAD complexed with (GlcN)5 and (GlcN)6 were solved by molecular replacement with Phaser (36) using the structure of free OfChtI-CAD as a model (PDB entry 3W4R). The PHENIX suite of programs (37) was used for structure refinement. Coot (38) was used for manually building and extending the molecular models. The stereochemical quality of the models was checked by PROCHECK (39). The coordinates of OfChtI-CAD with (GlcN)5 and OfChtI-CAD with (GlcN)6 were deposited in the Protein Data Bank as entries 3WQV and 3WQW, respectively. The structural figures were generated using PyMOL (40). The statistics for the diffraction data and the structure refinement are summarized in Table 1.
TABLE 1.
X-ray data collection and structure refinement statistics
| OfChtI-CAD·(GlcN)5 | OfChtI-CAD·(GlcN)6 | |
|---|---|---|
| Space group | P65 | P65 |
| Unit cell parameters | ||
| a = b (Å) | 94.222 | 94.219 |
| c (Å) | 122.43 | 122.366 |
| Wavelength (Å) | 0.97869 | 0.97930 |
| Temperature (K) | 100 | 100 |
| Resolution (Å) | 50–2.04 (2.08–2.04) | 50–2.0 (2.03–2.0) |
| Unique reflections | 39,003 | 41,613 |
| Observed reflections | 38,955 | 41,593 |
| Rmerge | 0.1 (0.37) | 0.11 (0.231) |
| Average multiplicity | 17.1 (17.1) | 13.9 (13.9) |
| 〈I/σ(I)〉 | 24.08 (11.76) | 18.67 (8.98) |
| Completeness (%) | 99 (100) | 100 (100) |
| R/Rfree | 0.1495/0.1676 | 0.1298/0.1572 |
| Protein atoms | 3086 | 3080 |
| Water molecules | 430 | 490 |
| Other atoms | 99 | 99 |
| Root mean square deviation from ideal | ||
| Bond lengths (Å) | 0.004 | 0.019 |
| Bond angles (degrees) | 0.87 | 1.66 |
| Wilson B factor (Å2) | 21.74 | 22.68 |
| Average B factor (Å2) | 33.40 | 24.8 |
| Protein atoms | 30.70 | 21.8 |
| Water molecules | 48.40 | 39.1 |
| Ramachandran plot (%) | ||
| Favored | 93.9 | 93.6 |
| Allowed | 6.1 | 6.4 |
| Outliers | 0 | 0 |
| PDB entry | 3WQV | 3WQW |
In Vivo Bioevaluation by Injection of Mixed (GlcN)2–7 into the Insect O. furnacalis
O. furnacalis larvae were reared using an artificial diet under a photo period of 16 h light and 8 h darkness and a relative humidity of 70–90% at 26–28 °C as described previously (41). In the experimental group, 100 μg of mixed (GlcN)2–7 that was dissolved in 2 μl of distilled water was injected into the penultimate abdominal segment of the fifth instar day 5 larvae using a microsyringe. In the control group, 2 μl of distilled water was injected instead. Each group contained 20 individual larvae with three independent replicates. After injection, all of the treated larvae were reared under identical conditions as described above. Mortality and developmental defects were recorded every day before eclosion.
RESULTS
Inhibitory Activities
To determine the inhibitory activities of GlcN oligomers against GH18 chitinases, the IC50 values of (GlcN)2–7 were evaluated by using pNP-(GlcNAc)2 as the substrate. Four GH18 chitinases from three different species were tested. They were the insect chitinase OfChtI, the human chitinase HsCht, and the bacterial chitinases SmChiA and SmChiB. The two bacterial enzymes, SmChiA and SmChiB, were selected due to their different hydrolysis directions; SmChiA degrades chitin from its reducing end, whereas SmChiB degrades chitin from the non-reducing end (42). As shown in Table 2, (GlcN)2–7 inhibited the enzymatic activities of the four chitinases with IC50 values ranging from 101 to 103 μm.
TABLE 2.
IC50 (μm) of (GlcN)2–7 toward four chitinases and three mutants of OfChtI at 30 °C (pH 7.0)
| IC50 |
||||||
|---|---|---|---|---|---|---|
| (GlcN)2 | (GlcN)3 | (GlcN)4 | (GlcN)5 | (GlcN)6 | (GlcN)7 | |
| μm | ||||||
| OfChtIa | ||||||
| Wild type | 2375.5 ± 433.6 | 776.0 ± 95.2 | 204.2 ± 17.3 | 16.5 ± 2.2 | 14.5 ± 1.8 | 11.4 ± 2.1 |
| N305A | 2117.1 ± 521.7 | 802.5 ± 107.2 | 225.7 ± 23.7 | 17.1 ± 2.9 | 15.0 ± 2.5 | 11.9 ± 1.7 |
| Y37A | 2402.2 ± 415.5 | 787.4 ± 95.2 | 375.6 ± 28.6 | 157.3 ± 11.8 | 151.5 ± 19.7 | 104.0 ± 13.2 |
| W372Ab | ||||||
| F309A | 6671.9 ± 496.0 | 2284.5 ± 242.9 | 778.1 ± 39.3 | 68.4 ± 4.7 | 58.0 ± 2.8 | 53.3 ± 3.7 |
| HsChta | 3422.5 ± 744.1 | 1610.0 ± 176.7 | 637.4 ± 115.6 | 163.1 ± 19.9 | 69.5 ± 10.1 | 37.8 ± 8.6 |
| SmChiAa,c | 4125.3 ± 720.9 | 1700.2 ± 205.3 | 659.7 ± 98.9 | 356.4 ± 47.1 | 169.7 ± 21.3 | 168.0 ± 28.4 |
| (320.7 ± 35.8)d | (145.5 ± 18.3)d | |||||
| SmChiBa,c,e | 2997.5 ± 352.6 | 560.3 ± 114.4 | 298.8 ± 36.7 | 116.4 ± 15.4 | 72.8 ± 8.6 | 65.7 ± 10.1 |
| (89.3 ± 4.2)d | (52.4 ± 5.8)d | |||||
a The IC50 values of (GlcN)2–7 toward four chitinases were determined with a substrate concentration of 0.1 mm.
b W372A showed no activity against pNP-(GlcNAc)2.
c The Km values for the substrate pNP-(GlcNAc)2 for SmChiA and SmChiB were determined by using Lineweaver-Burk plots. The Km value is 734.1 ± 33.8 μm for SmChiA and 320.2 ± 28.5 μm for SmChiB. The substrate concentrations used were 0.02–0.5 mm.
d The Ki values for (GlcN)5 and (GlcN)6 for SmChiA and SmChiB were determined by using Dixon plots (supplemental Fig. S2). The substrate concentrations used were 0.075–0.3 mm.
e The IC50 value of (GlcN)5 toward SmChiB with MU-(GlcNAc)2 as substrate is 127.6 ± 11.9 μm.
For OfChtI, (GlcN)2 was the weakest inhibitor. (GlcN)3–4 were moderate inhibitors, and (GlcN)5–7 were the most efficient inhibitors. By comparing the IC50 values of (GlcN)2, (GlcN)3, and (GlcN)4, we found that the addition of one GlcN residue was accompanied by a 3–4-fold decrease in IC50 values. However, if comparing the IC50 values of (GlcN)4 and (GlcN)5, the IC50 value was decreased by more than 12-fold with the addition of one GlcN residue.
For HsCht, a steady decrease in IC50 values by ∼2-fold was found to correspond to each increase in the polymerization degree of GlcN (from (GlcN)2 to (GlcN)7). Both (GlcN)2 and (GlcN)3 had IC50 values at the 103 μm level, which suggested that they were less active. The (GlcN)6 and (GlcN)7 were the most active inhibitors against HsCht. However, these two GlcN oligomers were 3–4 times less active against HsCht than toward OfChtI.
For SmChiA, (GlcN)2–7 were poor inhibitors, with the highest IC50 value at the 102 μm level. For SmChiB, (GlcN)2–7 acted in a similar manner as they did toward OfChtI. The obvious difference was that (GlcN)5, (GlcN)6, and (GlcN)7 exhibited 5–7-fold higher activity against OfChtI than against SmChiB. From these findings, we concluded that the fully deacetylated chitooligosaccharides (GlcN)2–7 are active molecules against chitinases, among which the insect chitinase OfChtI is the most susceptible enzyme.
In Vivo Bioevaluation
To test the in vivo efficacy of the fully deacetylated chitooligosaccharides, the mixed (GlcN)2–7 were injected into the larvae of the insect O. furnacalis at the larval-pupal transition stage, in which OfChtI plays an essential role. As shown in Fig. 1, 3 days after injection, 87% of water-injected larvae pupated, whereas only 15% of the mixed (GlcN)2–7-injected larvae pupated. Interestingly, 60% of the (GlcN)2–7-injected larvae died at the prepupal stage. Ten days after injection, all of the water-injected individuals completed adult eclosion, whereas 25% of the (GlcN)2–7-injected larvae died at the larval stage, and only 15% of the (GlcN)2–7-injected individuals completed adult eclosion.
FIGURE 1.
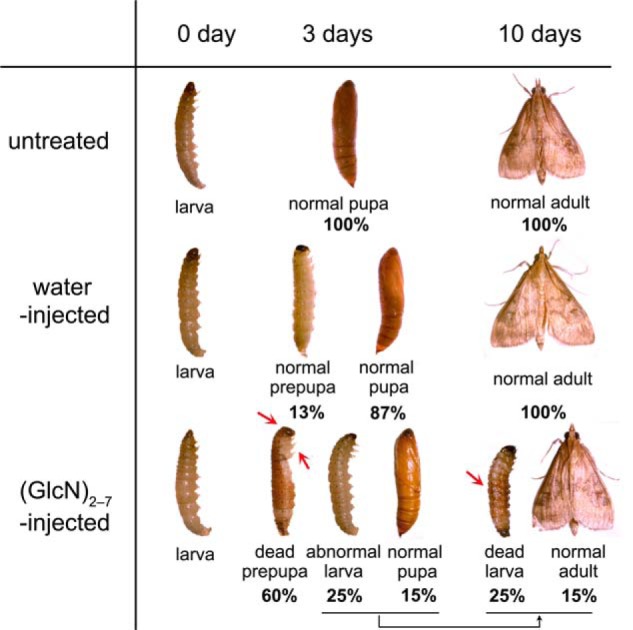
Phenotypes observed after the injection of mixed (GlcN)2–7 into the fifth instar day 5 of the insect O. furnacalis. Three days after injection with mixed (GlcN)2–7, 60% of the larvae died at the prepupal stage, with the head capsule and the thoracic legs attached to the body (red arrow). Ten days after injection with mixed (GlcN)2–7, 25% of the larvae died with their body shrunk seriously (red arrow). No malformation was found in water-injected control groups.
As shown in Fig. 1, 25% of the (GlcN)2–7-injected larvae died with their body shrunk seriously. The deaths of the 60% of the (GlcN)2–7-injected larvae that molted to prepupal stage appeared to be caused by a failure to shed their old cuticle. For the group that died at the prepupal stage, both the head capsule and the thoracic legs were attached to the body. As observed in Fig. 1, the new pupal cuticle had formed under the old cuticle and tanned underneath. Thus, these larvae died by being trapped in their old cuticles.
Thermodynamics
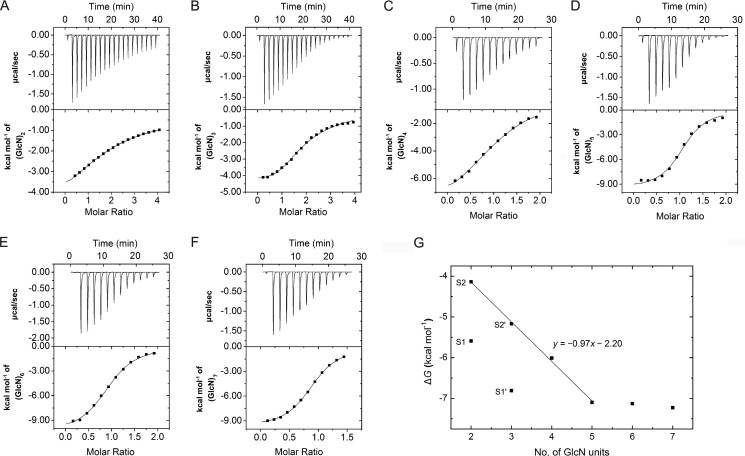
To investigate the binding of (GlcN)2–7 to OfChtI, the thermodynamics of the binding of these inhibitors to OfChtI was determined by using ITC. The binding was assessed in 50 mm HEPES, pH 8.0, at 25 °C. Thermograms and titration curves for (GlcN)2–7 are shown in Fig. 2. The derived thermodynamic parameters (n, Kd, ΔG, ΔH, and −TΔS) are summarized in Table 3.
FIGURE 2.
A–F, thermograms and binding isotherms with theoretical fits for the binding of (GlcN)2–7 to OfChtI: (GlcN)2 (A), (GlcN)3 (B), (GlcN)4 (C), (GlcN)5 (D), (GlcN)6 (E), and (GlcN)7 (F). G, free energy changes for (GlcN)2–7 binding to OfChtI relative to the number of GlcN units in the saccharide chain. (GlcN)2 and (GlcN)3 bind to OfChtI at two sites. For (GlcN)2, S1 is the strong binding site with a low Kd value, and S2 is the weak binding site with a high Kd value. For (GlcN)3, S1′ is the strong binding site with a low Kd value, and S2′ is the weak binding site with a high Kd value.
TABLE 3.
Thermodynamic parameters of (GlcN)2–7 binding to OfChtI derived from ITC at 25 °C (pH 8.0)
| Ligand | n | Kd | ΔG | ΔH | −TΔS |
|---|---|---|---|---|---|
| μm | kcal mol−1 | kcal mol−1 | kcal mol−1 | ||
| (GlcN)2 | 1.95 ± 0.13 | Kd1, 79.4 ± 8.7; | ΔG1, −5.59; | ΔH1, −3.96 ± 0.13; | −TΔS1, −1.63; |
| Kd2, 917.2 ± 113.5 | ΔG2, −4.14 | ΔH2, −13.51 ± 0.86 | −TΔS2, 9.38 | ||
| (GlcN)3 | 1.96 ± 0.10 | Kd1, 10.2 ± 1.6; | ΔG1, −6.81; | ΔH1, −4.23 ± 0.17; | −TΔS1, −2.58; |
| Kd2, 161.1 ± 23.7 | ΔG2, −5.17 | ΔH2, −7.76 ± 0.31 | −TΔS2, 2.59 | ||
| (GlcN)4 | 1.06 ± 0.02 | 39.2 ± 3.2 | −6.01 | −9.08 ± 0.10 | 3.07 |
| (GlcN)5 | 1.05 ± 0.01 | 6.2 ± 0.3 | −7.10 | −9.52 ± 0.24 | 2.41 |
| (GlcN)6 | 1.05 ± 0.02 | 5.9 ± 0.3 | −7.13 | −9.66 ± 0.09 | 2.54 |
| (GlcN)7 | 0.98 ± 0.05 | 5.0 ± 0.4 | −7.23 | −10.34 ± 0.42 | 3.11 |
As shown in Fig. 2, A and B, the titration curves for (GlcN)2–3 fit very well into a two-site binding model with a stoichiometry number of 1.95–1.96. For convenience, we named the site corresponding to Kd1 the “strong binding site” and the site corresponding to Kd2 the “weak binding site.” (GlcN)2 and (GlcN)3 bound to OfChtI with greater than 1 order of magnitude affinity difference (for (GlcN)2, Kd1 = 79.4 μm and Kd2 = 917.2 μm; for (GlcN)3, Kd1 = 10.2 μm and Kd2 = 161.1 μm) in the two binding sites. (GlcN)3 bound to OfChtI with ∼8-fold and 5-fold higher affinities than (GlcN)2 at the strong and weak binding sites, respectively. As shown in Table 3, the enthalpy values for (GlcN)2–3 at the four sites were negative, which suggested that both strong and weak binding types were exothermic. The two binding processes were enthalpically driven (|ΔH| > |−TΔS|). The entropy values (−TΔS1) for (GlcN)2–3 were greater than zero, which suggested that entropy partly contributed to the strong binding sites of (GlcN)2–3.
The titration curves of (GlcN)4–7 (Fig. 2, C–F) fit the single-site binding model, which suggested the presence of only one binding site. This result, together with the presence of two binding sites for (GlcN)2–3, suggested that the long active cleft of OfChtI provides an additional binding site for GlcN oligomers with a low degree of polymerization (n = 2, 3). Based on the Kd values of 917.2, 161.1, 39.2, and 6.2 μm for (GlcN)2 at the weak site, (GlcN)3 at the weak site, (GlcN)4, and (GlcN)5, respectively, an increase in the polymerization degree of GlcN by one unit results in a gain in affinity of 4–6-fold. Interestingly, (GlcN)4 exhibited 4-fold weaker binding than (GlcN)3 in the strong binding site. The binding affinities of (GlcN)5, (GlcN)6, and (GlcN)7 to OfChtI were equivalent, which was in agreement with the inhibitory activity data. The enthalpy values were in the range of −9.08 to −10.34 kcal/mol, and entropy values (−TΔS) were positive, and based on these results, we concluded that the binding of all of the inhibitors examined is exothermic and enthalpically driven. These data suggested that inhibitor binding is accompanied by hydrogen bonding and/or electrostatic-electrostatic interaction.
To analyze the binding free energy changes of these GlcN oligomers, the free energies of (GlcN)2–7 binding were plotted against the number of sugar residues (Fig. 2G). Interestingly, the binding energies of (GlcN)2 and (GlcN)3 at the weak sites, (GlcN)4, and (GlcN)5 correlated well to the function, y = −0.97x − 2.20 (where y represents free energy, and x is the number of GlcN residues). Every increase in x (from (GlcN)2 to (GlcN)5) resulted in an average free energy gain of approximately −1.0 kcal mol−1. The free energies of (GlcN)6 and (GlcN)7 binding were equal to the free energy of (GlcN)5 and thus did not fit the model. This result suggested that (GlcN)6 and (GlcN)7 occupied the same number of binding subsites as (GlcN)5.
To investigate the role of the catalytic acid Glu148 in inhibitor binding, the mutant E148Q was constructed. The binding of (GlcN)5 to wild-type OfChtI and the mutant E148Q was determined by ITC at 30 °C because of the low heat of (GlcN)5 binding to E148Q at 25 °C. The mutant E148Q bound (GlcN)5 with 19-fold lower affinity than wild-type OfChtI (for OfChtI, Kd = 3.9 μm; for E148Q, Kd = 73.0 μm), as listed in Table 4. The binding of (GlcN)5 to E148Q was entropically driven with an enthalpic contribution (−TΔS = −3.02 kcal mol−1, ΔH = −2.71 kcal mol−1), whereas the binding of (GlcN)5 to wild-type OfChtI was strictly enthalpically driven (ΔH = −11.63 kcal mol−1, −TΔS = 4.15 kcal mol−1). The reduced binding capacity does not impair ΔG (−1.75 kcal mol−1) very much. However, a dramatic reduction of enthalpic change (ΔΔH = −8.92 kcal mol−1) was observed, which accompanied with a dramatic reduction of entropic change (Δ−TΔS = −7.17 kcal mol−1). This result confirms that a tight interaction is formed between the inhibitors and the catalytic residue Glu148.
TABLE 4.
Thermodynamic parameters of (GlcN)5 binding to OfChtI and the mutant E148Q derived from ITC at 30 °C (pH 8.0)
| Protein | Ligand | n | Kd | ΔG | ΔH | −TΔS |
|---|---|---|---|---|---|---|
| μm | kcal mol−1 | kcal mol−1 | kcal mol−1 | |||
| OfChtI | (GlcN)5 | 1.02 ± 0.03 | 3.9 ± 0.5 | −7.48 | −11.63 ± 0.34 | 4.15 |
| E148Q | (GlcN)5 | 0.91 ± 0.04 | 73.0 ± 13.2 | −5.73 | −2.71 ± 0.27 | −3.02 |
Crystallography
To further investigate the inhibitory mechanism of the GlcN oligomers, crystals of OfChtI-CAD were obtained by soaking with the inhibitors (GlcN)5 and (GlcN)6. A nomenclature for sugar-binding subsites was proposed by Davies et al. (43), where subsite +n represents the reducing end, subsite −n is the non-reducing end, and the cleavage point is between the −1 and the +1 subsites.
Both of the structures of OfChtI-CAD in complex with (GlcN)5 and (GlcN)6 were solved at a resolution of 2.0 Å. The sixth GlcN residue was surprisingly missing in the OfChtI-CAD·(GlcN)6 complex. The electron density at the +1 subsite could be easily identified as a HEPES molecule, so the missing sixth GlcN residue was not possibly located at the +1 subsite. It is worth noting that some blurry electron density close to the −5 sugar was observed. We thus deduce that the sixth GlcN residue should be the −6 sugar.
The overall structures of OfChtI-CAD·(GlcN)5 and OfChtI-CAD·(GlcN)6 are very similar to the structure of the wild-type enzyme, and all matching atoms had a root mean square deviation of 0.19 Å. Both of the ligands bind to the active cleft (Fig. 3) and could be superposed (Fig. 4A). Most of the interactions are between the −1 sugar and the enzyme. As shown in Fig. 3, the pyranose ring of the −1 sugar interacts with the side chain of residue Trp372 via a stacking interaction. The O1, O3, and O6 atoms of the −1 sugar form hydrogen bonds with residues Tyr217, Trp107, and Asp218/Arg274, respectively. The C2 amino group of the −1 sugar is 2.7 Å from the side chain carboxyl oxygen atoms of the catalytic residues Asp146 and Glu148. Compared with the wild-type OfChtI-CAD structure, the side chain of the catalytic Asp146 in the OfChtI-CAD·(GlcN)5 structure rotates 91° and faces toward the catalytic Glu148. Additionally, weaker interactions between the −2 to −5 sugars and the enzyme are observed. The O6 atom of the −2 sugar interacts with the amide group of the main chain of residue Ala108 through a hydrogen bond. The pyranose ring of the −3 sugar stacks with the aromatic side chain of Trp34 via hydrophobic interactions. The glycosidic bond that joins the −3 sugar and the −4 sugar is twisted, which causes the plane of the −4 sugar to lie perpendicular to the planes of the −3, −2, and −1 sugars. The −5 sugar adopts the same orientation as the −4 sugar and is positioned at the end of the substrate-binding cleft. The distance is 3.3 Å between the 3-OH of the −5 GlcN residue and the acetyl oxygen in the GlcNAc conjugated to the glycosylated residue Asn305. This is the distance most likely to form a hydrogen bond.
FIGURE 3.
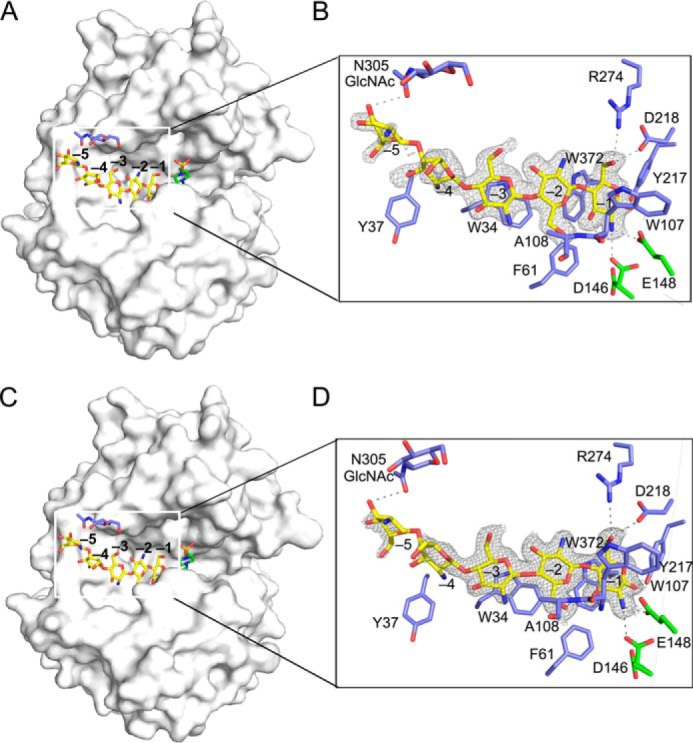
Overall structures of OfChtI-CAD complexed with (GlcN)5 and (GlcN)6. A and C, surface representations of OfChtI-CAD complexed with (GlcN)5 (A) and (GlcN)6 (C). The ligands (GlcN)5 and (GlcN)6, the Asn305-linked N-GlcNAc, and the ligand HEPES are shown as sticks with yellow, blue, and green carbon atoms, respectively. The numbers indicate the subsite to which the GlcN residue is bound. B and D, details of the active cleft with the interactions between OfChtI-CAD and (GlcN)5 (B) or (GlcN)6 (D). The ligand is represented as a stick with yellow carbon atoms, and the 2Fo − Fc electron density map around the ligand is contoured at the 2.0σ level. The catalytic residues and the amino acids that interact with the ligand are labeled and are shown as sticks with green and blue carbon atoms, respectively. The numbers indicate the subsite to which the GlcN residue is bound. Hydrogen bonds between the enzyme and the ligand are drawn as dashed lines.
FIGURE 4.
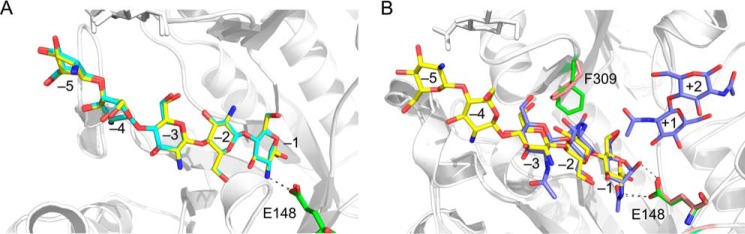
A, the superposition of the ligands (GlcN)5 and (GlcN)6 in the active cleft of OfChtI-CAD. (GlcN)5 and (GlcN)6 are shown as sticks with yellow and cyan carbon atoms, respectively. The catalytic residue Glu148 in two complex structures is labeled and shown as sticks with green carbon atoms. The numbers indicate the subsite to which the GlcN residue is bound. Hydrogen bonds between Glu148 and the ligands are drawn as a dashed line. B, superposition of the inhibitor (GlcN)5 and the substrate (GlcNAc)2/3 (PDB entry 3WL1) in the substrate-binding cleft of OfChtI-CAD. (GlcN)5 and (GlcNAc)2/3 are shown as sticks with yellow and blue carbon atoms, respectively. The catalytic Glu148 and Phe309 in the structures in complex with (GlcN)5 and (GlcNAc)2/3 are labeled and shown as sticks with green and pink carbon atoms, respectively. The numbers indicate the subsite to which the GlcN and GlcNAc residue is bound. Hydrogen bonds between Glu148 and the ligands are drawn as dashed lines.
Deduction of the Substrate-binding Subsite −5
The crystal structure of OfChtI-CAD complexed with either (GlcN)5 or (GlcN)6 shows that a hydrogen bond is probably formed between the 3-OH of the −5 GlcN residue and the acetyl oxygen of the glycosylated GlcNAc conjugated to Asn305. Thus, we proposed that this interaction may account for the lower IC50 value of (GlcN)5 compared with (GlcN)2–4. To verify this hypothesis, Asn305 was mutated to Ala305, and the activities of oligosaccharides (GlcN)2–7 against the mutant N305A were tested individually. Surprisingly, the mutant N305A was as sensitive as the wild type (Table 2). This result indicated that an interaction was unlikely to take place between the −5 GlcN residue and the glycosylated GlcNAc.
To determine the reason for the 12-fold higher activity of (GlcN)5 compared with (GlcN)4, the structure of a known GH18 chitinase, SmChiA, was analyzed (44). The structure of SmChiA in complex with (GlcNAc)8 (PDB entry 1EHN) indicates that SmChiA possesses a substrate binding subsite for the −5 sugar of this substrate. According to the structure-based comparison, OfChtI-CAD is thought to possess a −5 subsite composed of the residue Tyr37. Mutation of Tyr37 to Ala37 was performed, and inhibitory activities of the oligosaccharides (GlcN)2–7 were tested individually. As shown in Table 2, (GlcN)2 and (GlcN)3 exhibited similar effects against both Y37A and the wild-type OfChtI. However, (GlcN)4 and (GlcN)5–7 showed 2- and 10-fold weaker inhibitory activity against Y37A than the wild-type enzyme, respectively. Although this was not observed in the crystal structures, we believe that these results suggest that the −5 GlcN is most likely to interact with the aromatic side chain of Tyr37 under native conditions.
The mutations along with the inhibitory activity data perhaps suggested the presence of the subsite −5 of OfChtI-CAD. However, further work is needed to verify this assumption.
DISCUSSION
Here we report the inhibitory activities of fully deacetylated chitooligosaccharides. The crystal structures of OfChtI-CAD complexed with the oligomers (GlcN)5 or (GlcN)6 provide structural insights into the inhibitory mechanism.
Inhibitory Mechanism
The crystal structures reveal that the complexed ligand, either (GlcN)5 or (GlcN)6, is located in the binding cleft of OfChtI-CAD at the start of the subsite −1 and interacts with residues that form the cleft wall. A structural comparison of the inhibitor·enzyme complex, OfChtI-CAD·(GlcN)5, with the previously reported substrate·enzyme complex (OfChtI-CAD·(GlcNAc)2/3) (PDB entry 3WL1) reveals several differences in the binding modes (Fig. 4B).
First, the interactions between the catalytic Glu148 residue and the −1 sugar are different (Fig. 4B). In the substrate·enzyme complex, the distance is 2.5 Å between the catalytic oxygen of the side chain of Glu148 and the C1-OH of the −1 GlcNAc, which suggests that a strong interaction is formed for proton transfer during catalysis. This interaction can be formed if the −1 GlcNAc of the substrate adopts a “boat” conformation. Additionally, the other oxygen of the side chain of Glu148 interacts with the C2 acetamido group of the −1 GlcNAc within a distance of 3.0 Å. In the inhibitor·enzyme complex, the catalytic oxygen of Glu148 cannot interact with the C1-OH of the −1 GlcN of the inhibitor because the −1 GlcN adopts a “chair” conformation. The only interaction is between the carbonyl oxygen of the side chain of Glu148 and the C2 amino group of the −1 GlcN within a distance of 2.5–2.7 Å. This distance is shorter than the distance between Glu148 and the C2 acetamido group of the −1 GlcNAc, suggesting that a stronger interaction occurs. The binding of the inhibitor (GlcN)5 to the mutant E148Q exhibited 19-fold lower affinity if compared with the wild type. This result confirms that the catalytic residue Glu148 strongly interacts with the inhibitors. Also, the result agrees with the assumption made by Eijsink and co-workers (32) that binding of GlcN in the −1 subsite could be energetically less unfavorable than binding of a GlcNAc.
Second, the comparison indicates that the steric presence of the acetyl group of the −2 GlcNAc has led the Phe309 to rotate about 90°, resulting in a farther distance of 3.6 Å between the Phe309 and the acetamido group. However, in the enzyme·inhibitor complex, the absence of the N-acetyl group lets the Phe309 remain still if compared with the unliganded enzyme (PDB code 3W4R). Instead, there is a water-mediated interaction between the residue Arg274 and the amino group of the −2 GlcN. It is worth noting that the mutation of the Phe309 to Ala309 results in only 15% enzymatic activity loss but 4-fold inhibitory activity decrease, perhaps meaning that Phe309 is more crucial for the −2 GlcN binding to OfChtI. Taken together, we presume that the lack of an acetyl group at the −2 sugar would facilitate a water molecule to come and to form a water-mediated interaction between the enzyme (Arg274) and the −2 GlcN. Because the Phe309 in OfChtI is absent in SmChiB, we notice that the effect of an acetyl group at the −2 sugar in OfChtI is quite different from that in SmChiB.
Third, based on the complex structures, both the acetamido group of −3 GlcNAc (in the complex OfChtI·substrate) and the amino group of −3 GlcN (in the complex OfChtI·inhibitor) do not interact directly with the enzyme. However, in both complex structures, a water-mediated interaction is observed. We thus assume that the presence or absence of an acetyl group of the −3 sugar would not harm the binding of a ligand to the enzyme. From the above findings, we conclude that the −1 and −2 subsites are crucial for (GlcN)5, 6 inhibiting the activity of OfChtI.
(GlcN)5 against SmChiB
The effect of (GlcN)5 against SmChiB is interesting. Previous studies by Cederkvist et al. (30, 45) suggested that the partially deacetylated chitooligosaccharides, perhaps mainly the DADAA (D for GlcN and A for GlcNAc), exhibited a very good inhibitory effect with an IC50 value of 16 μm by using the (GlcNAc)3 analog MU-(GlcNAc)2 as substrate. However, in our case, the fully deacetylated chitooligosaccharide, (GlcN)5 (i.e. DDDDD according to their nomenclature), exhibited a 8-fold lower inhibitory activity against SmChiB. Based on the structural information of the SmChiB·(GlcNAc)5 complex (46), Cederkvist et al. concluded that the −2 subsite strongly preferred an acetylated sugar (30). Our result provided a supportive datum to their conclusion.
Structural Differences between Chitinases
It is very surprising that the inhibitory effects of (GlcN)2–7 are so different between the chitinases. A structural comparison was then performed. As shown in supplemental Fig. S1, OfChtI possesses an open and the longest hydrophobic cleft, in the middle of which are the +1 and −1 subsites responsible for cleavage occurring. Besides the catalytic site, seven aromatic residues are lined up along the cleft. Among them, four residues, including Trp223, Phe194, Tyr243, and Trp241, follow the +1 subsite, and three residues, including Phe61, Trp34, and Tyr37, follow the −1 subsite. For both SmChiA and HsCht, there are two aromatic residues (Phe396 and Tyr418 in SmChiA and Trp218 and Tyr190 in HsCht) located by the right side of the +1 subsite. However, if compared with the SmChiA, the HsCht possesses a wider-ended cleft for binding the non-reducing end of substrates. Unlike OfChtI, SmChiA, and HsCht, which possess open clefts, SmChiB has a blocked cleft with two aromatic residues, Phe12 and Phe51, positioned around the −1 subsite. Taken together, we assume that the obvious differences in the architecture of substrate-binding clefts might account for the different effects of (GlcN)2–7 between the chitinases.
In summary, we report the polymerization degree-dependent activities of fully deacetylated chitooligosaccharides against chitinases. The crystal structures of the insect chitinase OfChtI complexed with (GlcN)5,6 identify a structural explanation for the inhibitory mechanism.
Supplementary Material
Acknowledgments
We thank Professor Yuguang Du (Dalian Institute of Chemical Physics, Chinese Academy of Science) for kindly providing the fully deacetylated chitooligosaccharides. We thank all of the staff at beamline BL17U of the Shanghai Synchrotron Radiation Facility (China).
This work was supported by National High Technology Research and Development Program of China Grant 2011AA10A204; National Key Project for Basic Research Grant 2010CB126100; National Natural Science Foundation of China Grant 61202252; National Key Technology R&D Program Grant 2011BAE06B05; and Specialized Research Fund for the Doctoral Program of Higher Education of China Grant 20120041120052.

This article contains supplemental Figs. S1 and S2.
The atomic coordinates and structure factors (codes 3WQV and 3WQW) have been deposited in the Protein Data Bank (http://wwpdb.org/).
- GH18
- glycoside hydrolase family 18
- pNP
- 4-nitrophenolate
- ITC
- isothermal titration calorimetry
- MU
- methylumbelliferyl
- PDB
- Protein Data Bank.
REFERENCES
- 1. Fuchs R. L., McPherson S. A., Drahos D. J. (1986) Cloning of a Serratia marcescens gene encoding chitinase. Appl. Environ. Microbiol. 51, 504–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jones J. D., Grady K. L., Suslow T. V., Bedbrook J. R. (1986) Isolation and characterization of genes encoding two chitinase enzymes from Serratia marcescens. EMBO J. 5, 467–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Suzuki K., Taiyoji M., Sugawara N., Nikaidou N., Henrissat B., Watanabe T. (1999) The third chitinase gene (chiC) of Serratia marcescens 2170 and the relationship of its product to other bacterial chitinases. Biochem. J. 343, 587–596 [PMC free article] [PubMed] [Google Scholar]
- 4. Vaaje-Kolstad G., Horn S. J., Sørlie M., Eijsink V. G. (2013) The chitinolytic machinery of Serratia marcescens: a model system for enzymatic degradation of recalcitrant polysaccharides. FEBS J. 280, 3028–3049 [DOI] [PubMed] [Google Scholar]
- 5. Genta F. A., Blanes L., Cristofoletti P. T., do Lago C. L., Terra W. R., Ferreira C. (2006) Purification, characterization and molecular cloning of the major chitinase from Tenebrio molitor larval midgut. Insect Biochem. Mol. Biol. 36, 789–800 [DOI] [PubMed] [Google Scholar]
- 6. Fukamizo T., Kramer K. J. (1985) Mechanism of chitin hydrolysis by the binary chitinase system in insect moulting fluid. Insect Biochem. 15, 141–145 [Google Scholar]
- 7. Barone R., Simporé J., Malaguarnera L., Pignatelli S., Musumeci S. (2003) Plasma chitotriosidase activity in acute Plasmodium falciparum malaria. Clin. Chim. Acta 331, 79–85 [DOI] [PubMed] [Google Scholar]
- 8. Labadaridis I., Dimitriou E., Theodorakis M., Kafalidis G., Velegraki A., Michelakakis H. (2005) Chitotriosidase in neonates with fungal and bacterial infections. Arch. Dis. Child Fetal Neonatal Ed. 90, F531–F532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Donnelly L. E., Barnes P. J. (2004) Acidic mammalian chitinase: a potential target for asthma therapy. Trends Pharmacol. Sci. 25, 509–511 [DOI] [PubMed] [Google Scholar]
- 10. Kawada M., Hachiya Y., Arihiro A., Mizoguchi E. (2007) Role of mammalian chitinases in inflammatory conditions. Keio J. Med. 56, 21–27 [DOI] [PubMed] [Google Scholar]
- 11. Andersen O. A., Dixon M. J., Eggleston I. M., van Aalten D. M. (2005) Natural product family 18 chitinase inhibitors. Nat. Prod. Rep. 22, 563–579 [DOI] [PubMed] [Google Scholar]
- 12. Sakuda S., Isogai A., Matsumoto S., Suzuki A. (1987) Search for microbial insect growth regulators. II. Allosamidin, a novel insect chitinase inhibitor. J. Antibiot. 40, 296–300 [DOI] [PubMed] [Google Scholar]
- 13. Rao F. V., Houston D. R., Boot R. G., Aerts J. M., Sakuda S., van Aalten D. M. (2003) Crystal structures of allosamidin derivatives in complex with human macrophage chitinase. J. Biol. Chem. 278, 20110–20116 [DOI] [PubMed] [Google Scholar]
- 14. Bortone K., Monzingo A. F., Ernst S., Robertus J. D. (2002) The structure of an allosamidin complex with the Coccidioides immitis chitinase defines a role for a second acid residue in substrate-assisted mechanism. J. Mol. Biol. 320, 293–302 [DOI] [PubMed] [Google Scholar]
- 15. Papanikolau Y., Tavlas G., Vorgias C. E., Petratos K. (2003) De novo purification scheme and crystallization conditions yield high-resolution structures of chitinase A and its complex with the inhibitor allosamidin. Acta Crystallogr. D Biol. Crystallogr. 59, 400–403 [DOI] [PubMed] [Google Scholar]
- 16. Vaaje-Kolstad G., Houston D. R., Rao F. V., Peter M. G., Synstad B., van Aalten D. M., Eijsink V. G. (2004) Structure of the D142N mutant of the family 18 chitinase ChiB from Serratia marcescens and its complex with allosamidin. Biochim. Biophys. Acta 1696, 103–111 [DOI] [PubMed] [Google Scholar]
- 17. Cederkvist F. H., Saua S. F., Karlsen V., Sakuda S., Eijsink V. G., Sørlie M. (2007) Thermodynamic analysis of allosamidin binding to a family 18 chitinase. Biochemistry 46, 12347–12354 [DOI] [PubMed] [Google Scholar]
- 18. Sakuda S., Isogai A., Matsumoto S., Suzuki A., Koseki K. (1986) The structure of allosamidin, a novel insect chitinase inhibitor, produced by Streptomyces sp. Tetrahedron Lett. 27, 2475–2478 [Google Scholar]
- 19. Arai N., Shiomi K., Iwai Y., Omura S. (2000) Argifin, a new chitinase inhibitor, produced by Gliocladium sp. FTD-0668. II. Isolation, physico-chemical properties, and structure elucidation. J. Antibiot. 53, 609–614 [DOI] [PubMed] [Google Scholar]
- 20. Omura S., Arai N., Yamaguchi Y., Masuma R., Iwai Y., Namikoshi M., Turberg A., Kölbl H., Shiomi K. (2000) Argifin, a new chitinase inhibitor, produced by Gliocladium sp. FTD-0668. I. Taxonomy, fermentation, and biological activities. J. Antibiot. 53, 603–608 [DOI] [PubMed] [Google Scholar]
- 21. Arai N., Shiomi K., Yamaguchi Y., Masuma R., Iwai Y., Turberg A., Kölbl H., Omura S. (2000) Argadin, a new chitinase inhibitor, produced by Clonostachys sp. FO-7314. Chem. Pharm. Bull. 48, 1442–1446 [DOI] [PubMed] [Google Scholar]
- 22. Rao F. V., Houston D. R., Boot R. G., Aerts J. M., Hodkinson M., Adams D. J., Shiomi K., Omura S., van Aalten D. M. (2005) Specificity and affinity of natural product cyclopentapeptide inhibitors against A. fumigatus, human, and bacterial chitinases. Chem. Biol. 12, 65–76 [DOI] [PubMed] [Google Scholar]
- 23. Dixon M. J., Andersen O. A., van Aalten D. M., Eggleston I. M. (2005) An efficient synthesis of argifin: a natural product chitinase inhibitor with chemotherapeutic potential. Bioorg. Med. Chem. Lett. 15, 4717–4721 [DOI] [PubMed] [Google Scholar]
- 24. Andersen O. A., Nathubhai A., Dixon M. J., Eggleston I. M., van Aalten D. M. (2008) Structure-based dissection of the natural product cyclopentapeptide chitinase inhibitor argifin. Chem. Biol. 15, 295–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rush C. L., Schüttelkopf A. W., Hurtado-Guerrero R., Blair D. E., Ibrahim A. F., Desvergnes S., Eggleston I. M., van Aalten D. M. (2010) Natural product-guided discovery of a fungal chitinase inhibitor. Chem. Biol. 17, 1275–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Macdonald J. M., Tarling C. A., Taylor E. J., Dennis R. J., Myers D. S., Knapp S., Davies G. J., Withers S. G. (2010) Chitinase inhibition by chitobiose and chitotriose thiazolines. Angew. Chem. Int. Ed. Engl. 49, 2599–2602 [DOI] [PubMed] [Google Scholar]
- 27. Schüttelkopf A. W., Andersen O. A., Rao F. V., Allwood M., Lloyd C., Eggleston I. M., van Aalten D. M. (2006) Screening-based discovery and structural dissection of a novel family 18 chitinase inhibitor. J. Biol. Chem. 281, 27278–27285 [DOI] [PubMed] [Google Scholar]
- 28. Houston D. R., Eggleston I., Synstad B., Eijsink V. G., van Aalten D. M. (2002) The cyclic dipeptide CI-4 [cyclo-(l-Arg-d-Pro)] inhibits family 18 chitinases by structural mimicry of a reaction intermediate. Biochem. J. 368, 23–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Houston D. R., Synstad B., Eijsink V. G., Stark M. J., Eggleston I. M., van Aalten D. M. (2004) Structure-based exploration of cyclic dipeptide chitinase inhibitors. J. Med. Chem. 47, 5713–5720 [DOI] [PubMed] [Google Scholar]
- 30. Cederkvist F. H., Parmer M. P., Vårum K. M., Eijsink V. G. H., Sørlie M. (2008) Inhibition of a family 18 chitinase by chitooligosaccharides. Carbohydr. Polym. 74, 41–49 [Google Scholar]
- 31. Tews I., Terwisscha van Scheltinga A. C., Perrakis A., Wilson K. S., Dijkstra B. W. (1997) Substrate-assisted catalysis unifies two families of chitinolytic enzymes. J. Am. Chem. Soc. 119, 7954–7959 [Google Scholar]
- 32. Aam B. B., Heggset E. B., Norberg A. L., Sørlie M., Vårum K. M., Eijsink V. G. (2010) Production of chitooligosaccharides and their potential applications in medicine. Mar. Drugs 8, 1482–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen L., Liu T., Zhou Y., Chen Q., Shen X., Yang Q. (2014) Structural characteristics of an insect group I chitinase, an enzyme indispensable to moulting. Acta Crystallogr. D Biol. Crystallogr. 70, 932–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu Q., Liu T., Yang Q. (2013) Cloning, expression and biocharacterization of OfCht5, the chitinase from the insect Ostrinia furnacalis. Insect Sci. 20, 147–157 [DOI] [PubMed] [Google Scholar]
- 35. Otwinowski Z., Minor W. (1997) Processing of x-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 [DOI] [PubMed] [Google Scholar]
- 36. McCoy A. J., Grosse-Kunstleve R. W., Adams P. D., Winn M. D., Storoni L. C., Read R. J. (2007) Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Adams P. D., Afonine P. V., Bunkóczi G., Chen V. B., Davis I. W., Echols N., Headd J. J., Hung L. W., Kapral G. J., Grosse-Kunstleve R. W., McCoy A. J., Moriarty N. W., Oeffner R., Read R. J., Richardson D. C., Richardson J. S., Terwilliger T. C., Zwart P. H. (2010) PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Emsley P., Cowtan K. (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 39. Laskowski R. A., MacArthur M. W., Moss D. S., Thornton J. M. (1993) PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 26, 283–291 [Google Scholar]
- 40. DeLano W. L. (2012) The PyMOL Molecular Graphics System, version 1.5.0.1, Schroedinger, LLC, New York [Google Scholar]
- 41. Qu M., Liu T., Yang J., Yang Q. (2011) The gene, expression pattern and subcellular localization of chitin synthase B from the insect Ostrinia furnacalis. Biochem. Biophys. Res. Commun. 404, 302–307 [DOI] [PubMed] [Google Scholar]
- 42. Hult E. L., Katouno F., Uchiyama T., Watanabe T., Sugiyama J. (2005) Molecular directionality in crystalline β-chitin: hydrolysis by chitinases A and B from Serratia marcescens 2170. Biochem. J. 388, 851–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Davies G. J., Wilson K. S., Henrissat B. (1997) Nomenclature for sugar-binding subsites in glycosyl hydrolases. Biochem. J. 321, 557–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Papanikolau Y., Prag G., Tavlas G., Vorgias C. E., Oppenheim A. B., Petratos K. (2001) High resolution structural analyses of mutant chitinase A complexes with substrates provide new insight into the mechanism of catalysis. Biochemistry 40, 11338–11343 [DOI] [PubMed] [Google Scholar]
- 45. Cederkvist F. H., Zamfir A. D., Bahrke S., Eijsink V. G., Sørlie M., Peter-Katalinić J., Peter M. G. (2006) Identification of a high-affinity-binding oligosaccharide by (+) nanoelectrospray quadrupole time-of-flight tandem mass spectrometry of a noncovalent enzyme-ligand complex. Angew. Chem. Int. Ed. Engl. 45, 2429–2434 [DOI] [PubMed] [Google Scholar]
- 46. van Aalten D. M., Komander D., Synstad B., Gåseidnes S., Peter M. G., Eijsink V. G. (2001) Structural insights into the catalytic mechanism of a family 18 exo-chitinase. Proc. Natl. Acad. Sci. U.S.A. 98, 8979–8984 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.