Abstract
Although the World Health Organization (WHO) categorizes spinal ependymomas into three histological grades, difference in surgical outcomes between WHO grades I and II tumors are unclear. For these benign tumors, prognosis may be best determined by factors other than tumor grade alone, such as extent of resection. To analyze the effects of the extent of resection on different grades of spinal ependymomas, we performed a comprehensive literature review to identify adult spinal ependymoma patients who received surgical resection with a clearly identifiable WHO grade. A total of 175 patients were identified. While grade III tumors carried the worst prognosis as expected (p < 0.001), grade I and II tumors did not differ significantly in outcomes following surgery. Overall, gross total resection (GTR, 68.7%, 114/166) provided significantly improved progression-free survival (PFS, p < 0.001) and overall survival (OS, p= 0.022) compared to the subtotal resection group. Surprisingly, the highest GTR rate was achieved for grade II tumors (78.8%, 78/99; p< 0.001) followed by grade I (58.9%, 33/56) and grade III tumors (27.3%, 3/11). Interestingly, PFS was significantly improved by GTR for grade II tumors (p < 0.001), but not for grade I (p = 0.705). Similar trends, although not statistically significant, were found for OS. Our results show that while GTR provides the best overall outcomes, GTR is most effective for classic grade II ependymomas, but not for grade I ependymomas. Despite having a lower WHO grade, myxopapillary ependymomas have a lower GTR rate, and benefit less from GTR.
Keywords: Ependymoma, Extent of resection, Recurrence, Spine, Tumor grade
1. Introduction
Ependymomas make up 3-6% of all central nervous system tumors1-3 and are thought to arise from radial glial cells that line the ventricles and spinal cord.4,5 Approximately 75% of these tumors occur in the spine,6 making them the most common glial tumors of the adult spine.2,3,7-11 While spinal ependymomas generally have a better prognosis compared to other intramedullary glial tumors,9,12 a significant portion of tumors can recur, leading to debilitating morbidities and mortality. While most ependymomas are considered histologically benign, recurrence rates can be as high as 50-70% without adjuvant therapy3,8,13 with a potential for widespread metastases.14-18 Thus, studies identifying preoperative features that can help predict outcomes are critical, not only for guiding treatment but in counseling patients before surgery.
One such factor shown to affect prognosis is the tumor grade.19-21 The World Health Organization (WHO) currently classifies ependymomas into three grades: grade I tumors include myxopapillary ependymomas and subependymomas; grade II includes classic ependymomas (consisting of cellular, papillary, clear cell, giant cell, and tanycytic subtypes); and grade III includes anaplastic ependymomas.22 While these grades help guide treatment, such as use of adjuvant radiotherapy,23-27 the prognostic value of this grading system is controversial.2 The prognostic value of distinguishing grade II and III tumors, for example, has been debated in regards to pediatric ependymomas.28 Moreover, the genetic heterogeneity of ependymomas of different subtypes, and even of the same histologic grade from spine and brain, make it difficult to establish tumor characteristics and prognosis based on WHO grade alone.29 Thus, better understanding of other prognostic factors, such as tumor location,2 extent of resection,7,30-34 length of clinical history,35 preoperative neurological status,36 presence of distant metastasis,37 adjuvant radiotherapy,23-27,38 and how these factors are related is needed to better determine the prognosis of spinal ependymoma patients.
The extent of resection with gross total resection (GTR) has been considered the most consistent variable in predicting good outcomes.1-3 Thus, the gold-standard therapy for spinal ependymomas remains en bloc GTR over piecemeal subtotal resection (STR).30-36 There are significant morbidities associated with surgery including limb weakness, sensory loss, dysesthesia, bowel and bladder dysfunction, wound infections, and cerebrospinal fluid leaks.39 These risks likely increase whenever aggressive resection is attempted. Given the critical role of the extent of resection in determining outcomes, we sought to analyze the association between the extent of resection and histological grade, specifically focusing on the two benign grades (WHO grade I and II), to determine whether aggressive surgical resection is more appropriate for certain tumors. We analyzed previously published patient data to determine how spinal ependymomas, stratified by WHO grade, are affected by extent of resection.
2. Methods
2.1 Article selection
A comprehensive systematic review of the English-language literature was carried out. An integrative analysis was performed, where individual patient data from studies was pooled and statistically analyzed. Articles were identified via a PubMed search using the key word “ependymoma”, and all manuscripts were individually reviewed to identify surgical spinal ependymoma patients where the WHO grade of tumor was clearly identifiable. Tumors reported as “benign” without specifying grade I versus grade II were omitted. Only patients 18 years of age and older were included. We identified 43 articles with total of 175 patients who met the criteria.15,18,25,36,40-78 Aggregated data sets, where individual patient data were grouped, were not included in this analysis.
2.3 Data Extraction
Data from case reports and institutional series were extracted with the following information: age, gender, WHO grade, extent of resection (GTR versus STR), adjuvant radiotherapy, recurrence or progression of disease, time to recurrence or progression of disease, mortality, time to mortality, tumor location (upper spinal: cervicomedullary, cervical, and cervicothoracic; lower spinal: thoracic, thoracolumbar, conus and cauda equina), and duration of follow-up. All mean values are presented with standard error of mean.
2.4 Statistical analysis
Progression-free survival (PFS) and overall survival (OS) were analyzed by building Kaplan-Meier curves and differences assessed by log-rank test. The Cox proportional hazards model was fitted by backward stepwise model selection while accounting for confounding variables, including age, gender, tumor grade, tumor location, and adjuvant radiotherapy. Means of continuous variables were analyzed using analysis of variance, and categorical values were analyzed using the Pearson’s Chi-square test. Fisher’s exact test was used if the expected cell count in a contingency table was less than five. P values less than 0.05 were considered statistically significant. Analyses were performed using the Statistical Package for the Social Sciences 20 (SPSS, Chicago, Illinois, USA).
3. Results
3.1 Clinical characteristics
The literature search yielded 43 manuscripts with 175 patients who underwent surgical treatment for spinal ependymomas with clearly identifiable WHO grade.15,18,25,36,40-78 The mean age was 39.5 years with a range of 18 to 81. There was a significant difference in the mean age across tumor grades (p = 0.025) with the youngest age in the grade III (34.31 ± 3.85) and the oldest age in grade II (41.91 ± 1.33) groups (Table 1). Overall, there were more men (54.7%, 88/161) but gender was not associated with tumor grade (p = 0.866). The mean follow-up duration was 48.8 months (range, 0-240 months).
Table 1.
Patient demographic information was stratified by WHO grade. Notably, gross total resection (GTR) rate was lower for the grade I tumors compared to grade II tumors, while adjuvant radiotherapy was used more often for the grade I tumors compared to grade II tumors. Consistent with previous reports, myxopapillary tumors (grade I) were more prevalent in the lower spinal region.
| WHO Grade I | WHO Grade II | WHO Grade III | p | |
|---|---|---|---|---|
| N | 61 | 101 | 13 | |
| Age | 36.61 ± 1.89 | 41.91 ± 1.33 | 34.31 ± 3.85 | 0.025a |
| Gender: Male | 28/54 (52.5%) | 53/94 (56.4%) | 7/13 (53.8%) | 0.866b |
| GTR | 33/56 (58.9%) | 78/99 (78.8%) | 3/11 (27.3%) | < 0.001c |
|
Adjuvant
Radiotherapy |
27/56 (48.2%) | 11/99 (11.1%) | 5/11 (45.5%) | < 0.001c |
|
Tumor Location:
Lower Spinal |
52/61 (85.2%) | 44/101 (43.6%) | 8/13 (61.5%) | < 0.001c |
ANOVA (analysis of variance);
Chi-square test;
Fisher’s exact test
The overall GTR rate was 68.7% (114/166). There was a significant difference in the extent of resection by tumor grade (Table 1; p < 0.001). As expected, the GTR rate was lowest for grade III lesions (27.3%, 3/11). Unexpectedly, grade I tumors were found to have a lower GTR rate compared to grade II tumors (58.9%, 33/56 versus 78.8%, 78/99, respectively; p < 0.001). Moreover, adjuvant radiotherapy was used at a higher rate for grade I tumors (48.2%, 27/56) compared to grade II tumors (11.1%, 11/99; p < 0.001), likely due to the lower GTR rate in the grade I tumors. In fact, adjuvant therapy was used at a similar rate for patients with grade I and anaplastic tumors. We also analyzed tumor location for different histologic types and found that grade I myxopapillary tumors were mainly localized to the lower spine (85.2%, 52/61), consistent with previous reports.20,30,32
3.2 Gross total resection provides improved outcomes
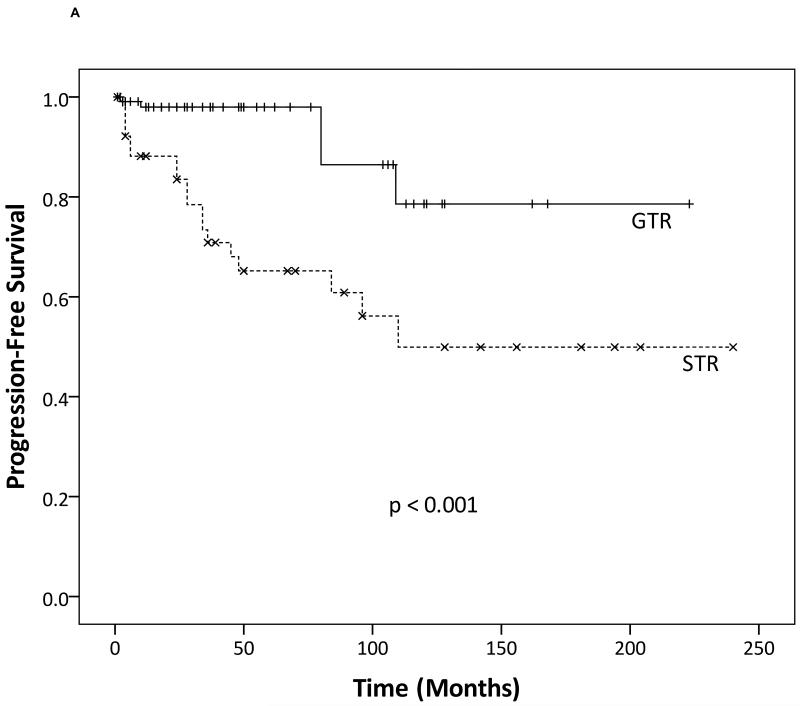
We first performed a Kaplan-Meier analysis to determine the effects of extent of resection on PFS and OS. Clearly, GTR provided better outcomes with respect to both PFS (Fig. 1a; p < 0.001) and OS (Fig. 1b; p = 0.022) compared to STR. These findings were confirmed by a multivariate Cox proportional hazards analysis, while controlling for other confounding variables, such as age, gender, tumor grade, tumor location, and adjuvant radiotherapy. Extent of resection (STR versus GTR) remained a significant factor for improved PFS with a hazard ratio of 7.35 (95% confidence interval 3.31-16.32; p < 0.01).
Fig. 1.
Kaplan-Meier analysis showing that gross total resection (GTR) provides (a) improved progression-free survival and (b) overall survival compared to subtotal resection (STR).
3.3 Anaplastic ependymomas portend poor prognosis
We then constructed Kaplan-Meier curves after stratifying patients by WHO grade (Fig. 2a, b). As expected, grade III tumors were associated with worse outcomes. Both PFS (grade I versus III and grade II versus III, p < 0.001) and OS (grade I versus III and grade II versus III, p < 0.001) were worse for grade III tumors compared to other WHO grades. However, there was no difference between grade I and grade II tumors for both PFS (p = 0.406) and OS (p = 0.499). These results suggest that while grade III tumors portend a poor prognosis, the distinction between myxopapillary grade I ependymomas and classic grade II tumors is, in terms of prognosis, small and non-significant.
Fig. 2.
Kaplan-Meier curves after stratifying patients by World Health Organization (WHO) tumor grade showing grade III ependymomas had the worst prognosis in terms of both (a) progression-free survival (PFS) and (b) overall survival (OS) compared to other WHO grades. Grade I and II tumors, however, did not differ significantly in (a) PFS and (b) OS.
3.4 Benefits of gross total resection are dependent on tumor grade
Given that outcomes were similar for the WHO grade I and II tumors, we wanted to investigate how different grades of tumors are affected by the extent of resection. We felt this analysis would be critically valuable as some authors have suggested that tumor grade alone is an insufficient indicator of prognosis,2,29 while extent of resection is considered the most consistent prognostic factor.1-3 Thus, we wanted to evaluate how tumor grade and extent of resection affect outcomes in combination.
Surprisingly, we found that GTR did not provide added benefits for the grade I tumors for either PFS (p = 0.705) or OS (p = 0.386) (Fig. 3a, b). There were four recurrences out of 33 patients in the GTR group, and six recurrences out of 23 patients in the STR group. The mortality rate was very low for grade I patients with only one death out of 51 patients.
Fig. 3.
Kaplan-Meier analysis of World Health Organization (WHO) grade I ependymomas showing that extent of resection did not significantly affect outcomes for (a) progression-free survival or (b) overall survival.
By contrast, PFS was significantly affected by the extent of resection in patients with grade II tumors (Fig. 4a; p = 0.001). Patients who received GTR had significantly longer PFS compared to those who received STR. There was only one recurrence out of 77 patients in the GTR group, while six out of 21 patients recurred in the STR group. Difference in OS, although present, did not reach a statistical significance (Fig. 4b; p = 0.213) with one death out of 78 patients in the GTR group and two deaths out of 22 patients in the STR group.
Fig. 4.
Kaplan-Meier analysis of World Health Organization (WHO) grade II ependymomas showing (a) progression-free survival was significantly improved by gross total resection (GTR) over subtotal resection (STR), (b) a trend towards higher mortality in the STR group compared to GTR group, but it is not statistically significant.
The difference in PFS (p = 0.162) and OS (p = 0.391) by the extent of resection also did not reach statistical significance for the anaplastic tumors (data not shown), although this is likely contributable to small sample size (GTR = 3, STR = 8) and the lack of events in the control (GTR) group: mainly, there were no recurrences or deaths in three patients who received GTR. By contrast, six recurred and five died out of eight patients with anaplastic tumors who received STR. Thus, patients in the STR group trended toward worse outcomes compared to those in the GTR group.
4. Discussion
Although surgery is accepted as the mainstay therapy for spinal ependymomas,1-3,30-36 the benefits of aggressive surgery must be weighed against the inherent risk of severe neurological deficits and other serious complications associated with surgery.39 An improved understanding of the factors affecting outcomes can help guide the surgeon’s decision on when aggressive surgical resection is appropriate, despite the possibility of serious complications. Thus, we analyzed how extent of resection affects outcomes of spinal ependymoma patients with different WHO grades using previously published data.
Our results show that GTR is significantly superior to STR with respect to PFS and OS (Fig. 1a, b) as previously reported.30-36 This result remained significant in our multivariate Cox regression analysis (hazard ratio 7.35, p < 0.01), even while adjusting for the effects of adjuvant radiotherapy. Overall GTR rate was quite high (68.7%), implying that optimal surgical outcomes can be achieved in at least two out of three patients with this disease. This was consistent with previously reported results.3,31,32,36,79 Unexpectedly, GTR rate was lower in grade I tumors (58.9%) compared to grade II tumors (78.8%, p < 0.001). There are likely many tumor features that affect the surgeon’s ability to achieve GTR, such as nerve root involvement, extradural versus intradural location, and involvement of the bony spine (i.e. sacrum). Since myxopapillary tumors occur more frequently in the lower spine (p < 0.001) (i.e. filum terminale, cauda equina, and sacrum),20,30 we hypothesize that the technical challenges associated with surgical resection in this anatomic region are likely responsible for the observed phenomenon. Furthermore, the infiltrative nature also likely affects the extent of resection, as demonstrated by the low GTR rate for anaplastic ependymomas (27.3%, p < 0.001).
While anaplastic tumors consistently demonstrated the worst prognosis when stratifying by histological grade, grade I tumors did not fare significantly better compared to grade II tumors, as might be expected (Fig. 2a, b). Interestingly, both PFS (p = 0.406) and OS (p = 0.499) were similar for grade I and grade II tumors. This was despite having lower GTR rate for the grade I tumors. However, more grade I tumors were treated with adjuvant radiotherapy (48.2%) compared to grade II tumors (11.1%); this would be expected to offset the lower rates of GTR among grade I tumors, but the difference in PFS and OS was still not significantly different compared to grade II lesions. Moreover, additional analysis after stratifying by tumor grade showed that GTR did not play a significant role in prolonging PFS for the grade I tumors (Fig. 3a), but GTR significantly improved PFS for the grade II tumors (Fig. 4a). The exact reasons for this finding is unclear, although differences in the genetics and molecular biology associated with different histological subtypes29 likely result in different tendencies for recurrence. Furthermore, GTR may be more difficult to obtain for grade I tumors than perceived by surgeons during surgery, as microtumors may be left behind on nerve roots, cauda equina, or filum terminale as the tumors are peeled away from these structures. It is also possible that myxopapillary grade I ependymomas are inherently more aggressive than grade II tumors, requiring adjuvant radiotherapy in order to achieve similar PFS and OS. Overall, our results indicate that WHO grade I spinal ependymomas do not fare better in outcomes compared to grade II tumors, suggesting that the current WHO grading system may need a further review, if it were to more accurately predict outcomes.
While we could not make firm conclusions about the role of the extent of resection for anaplastic ependymomas due to the small sample size, clear trends were seen for this histological grade. There were no recurrences or deaths in the three patients who received GTR. However, among the eight patients who underwent STR, six recurred and five died. We suspect that differences between the GTR and STR groups would reach a statistical significance with a larger population and that PFS and OS are both improved by GTR.
Because this is a retrospective analysis of pooled individual patient data from multiple studies, there are inherent limitations involved with this method. Individual patient data may not accurately reflect spinal ependymoma patients as a whole, since aggregated patient data (where individual patient data are grouped) was not used in this study. The extent of resection, which was determined by the surgeons or by postoperative imaging, may not be consistently reported across different studies due to surgeon or radiologist bias. Studies are also more likely to only report cases with good outcomes, and may be biased toward better outcomes than in reality. The differences in patient management at different institutions, such as the surgeon’s level of experience, whether adjuvant radiotherapy is used or not, follow-up protocol, and protocols involving treatment of recurrent tumors are not taken into account in this study and may affect results presented in this study.
5. Conclusion
The best outcomes for spinal ependymomas are achieved with GTR. More specifically, the classic grade II ependymomas may benefit most from aggressive resection. While myxopapillary grade I ependymomas did not have clear benefits from GTR, we hypothesize that this may be due to greater difficulty in achieving a “pure” GTR than previously perceived by gross appearance during surgery. While conclusions regarding anaplastic ependymomas are difficult to make, due to the small sample size and lack of events in the control GTR group, we hypothesize that aggressive resection likely benefits this tumor grade as well, given the trends found in our study. While GTR should be attempted whenever possible, further studies looking at the role of adjuvant radiotherapy for myxopapillary ependymomas, regardless of extent of resection are clearly warranted.
Acknowledgements
Dr. Oh received a National Research Service Award from the National Institutes of Health (F32NS073326-01). Dr. Parsa was partially funded by the Reza and Georgianna Khatib Endowed Chair in Skull Base Tumor Surgery.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest/Disclosures
The authors declare that they have no conflicts of interest in relation to this research and its publication.
References
- 1.Chamberlain MC. Ependymomas. Curr Neurol Neurosci Rep. 2003 May;3(3):193–199. doi: 10.1007/s11910-003-0078-x. [DOI] [PubMed] [Google Scholar]
- 2.Gilbert MR, Ruda R, Soffietti R. Ependymomas in adults. Curr Neurol Neurosci Rep. 2010 May;10(3):240–247. doi: 10.1007/s11910-010-0109-3. [DOI] [PubMed] [Google Scholar]
- 3.Ruda R, Gilbert M, Soffietti R. Ependymomas of the adult: molecular biology and treatment. Curr Opin Neurol. 2008 Dec;21(6):754–761. doi: 10.1097/WCO.0b013e328317efe8. [DOI] [PubMed] [Google Scholar]
- 4.Poppleton H, Gilbertson RJ. Stem cells of ependymoma. Br J Cancer. 2007 Jan 15;96(1):6–10. doi: 10.1038/sj.bjc.6603519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor MD, Poppleton H, Fuller C, et al. Radial glia cells are candidate stem cells of ependymoma. Cancer Cell. 2005 Oct;8(4):323–335. doi: 10.1016/j.ccr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz TH, Kim S, Glick RS, et al. Supratentorial ependymomas in adult patients. Neurosurgery. 1999 Apr;44(4):721–731. doi: 10.1097/00006123-199904000-00018. [DOI] [PubMed] [Google Scholar]
- 7.Abdelwahab M, Etuk B, Palermo J, et al. Spinal cord gliomas: A multi-institutional retrospective analysis. International Journal of Radiation OncologyBiologyPhysics. 2006;64(4):1060–1071. doi: 10.1016/j.ijrobp.2005.09.038. [DOI] [PubMed] [Google Scholar]
- 8.Cooper PR. Outcome after operative treatment of intramedullary spinal cord tumors in adults: intermediate and long-term results in 51 patients. Neurosurgery. 1989 Dec;25(6):855–859. doi: 10.1097/00006123-198912000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Helseth A, Mork SJ. Primary intraspinal neoplasms in Norway, 1955 to 1986. A population-based survey of 467 patients. J Neurosurg. 1989 Dec;71(6):842–845. doi: 10.3171/jns.1989.71.6.0842. [DOI] [PubMed] [Google Scholar]
- 10.Parsa AT, Lee J, Parney IF, Weinstein P, McCormick PC, Ames C. Spinal cord and intradural-extraparenchymal spinal tumors: current best care practices and strategies. J Neurooncol. 2004 Aug-Sep;69(1-3):291–318. doi: 10.1023/b:neon.0000041889.71136.62. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz TH, McCormick PC. Intramedullary ependymomas: clinical presentation, surgical treatment strategies and prognosis. J Neurooncol. 2000 May;47(3):211–218. doi: 10.1023/a:1006414405305. [DOI] [PubMed] [Google Scholar]
- 12.Tseng JH, Tseng MY. Survival analysis of 459 adult patients with primary spinal cancer in England and Wales: a population-based study. Surg Neurol. 2007 Jan;67(1):53–58. doi: 10.1016/j.surneu.2006.04.011. discussion 58. [DOI] [PubMed] [Google Scholar]
- 13.Guidetti B, Mercuri S, Vagnozzi R. Long-term results of the surgical treatment of 129 intramedullary spinal gliomas. J Neurosurg. 1981 Mar;54(3):323–330. doi: 10.3171/jns.1981.54.3.0323. [DOI] [PubMed] [Google Scholar]
- 14.Cameron MM. Surgical management of multiple neuraxial ependymomas. Case report. Eur Neurol. 1976;14(5):365–369. doi: 10.1159/000114760. [DOI] [PubMed] [Google Scholar]
- 15.de Divitiis E, Spaziante R, Stella L. Giant intramedullary ependymoma. A case report. Neurochirurgia (Stuttg) 1978 Mar;21(2):69–72. doi: 10.1055/s-0028-1090325. [DOI] [PubMed] [Google Scholar]
- 16.Mork SJ, Risberg G, Krogness K. Case report. Anaplastic ependymoma of the spinal cord. Neuropathol Appl Neurobiol. 1980 Jul-Aug;6(4):307–311. doi: 10.1111/j.1365-2990.1980.tb00215.x. [DOI] [PubMed] [Google Scholar]
- 17.Newton HB, Henson J, Walker RW. Extraneural metastases in ependymoma. J Neurooncol. 1992 Oct;14(2):135–142. doi: 10.1007/BF00177617. [DOI] [PubMed] [Google Scholar]
- 18.Schuurmans M, Vanneste JAL, Verstegen MJT, Furth WR. Spinal extramedullary anaplastic ependymoma with spinal and intracranial metastases. Journal of Neuro-Oncology. 2006;79(1):57–59. doi: 10.1007/s11060-005-9114-9. [DOI] [PubMed] [Google Scholar]
- 19.Waldron JN, Laperriere NJ, Jaakkimainen L, et al. Spinal cord ependymomas: a retrospective analysis of 59 cases. Int J Radiat Oncol Biol Phys. 1993 Sep 30;27(2):223–229. doi: 10.1016/0360-3016(93)90231-j. [DOI] [PubMed] [Google Scholar]
- 20.Mork SJ, Loken AC. Ependymoma: a follow-up study of 101 cases. Cancer. 1977 Aug;40(2):907–915. doi: 10.1002/1097-0142(197708)40:2<907::aid-cncr2820400247>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 21.Armstrong TS, Vera-Bolanos E, Bekele BN, Aldape K, Gilbert MR. Adult ependymal tumors: prognosis and the M. D. Anderson Cancer Center experience. Neuro-Oncology. 2010;12(8):862–870. doi: 10.1093/neuonc/noq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007 Aug;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akyurek S, Chang EL, Yu TK, et al. Spinal myxopapillary ependymoma outcomes in patients treated with surgery and radiotherapy at M.D. Anderson Cancer Center. J Neurooncol. 2006 Nov;80(2):177–183. doi: 10.1007/s11060-006-9169-2. [DOI] [PubMed] [Google Scholar]
- 24.Gomez DR. High failure rate in spinal ependymomas with long-term follow-up. Neuro-Oncology. 2005;7(3):254–259. doi: 10.1215/S1152851704001231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin YH, Huang CI, Wong TT, et al. Treatment of spinal cord ependymomas by surgery with or without postoperative radiotherapy. J Neurooncol. 2005 Jan;71(2):205–210. doi: 10.1007/s11060-004-1386-y. [DOI] [PubMed] [Google Scholar]
- 26.Wahab SH, Simpson JR, Michalski JM, Mansur DB. Long term outcome with post-operative radiation therapy for spinal canal ependymoma. Journal of Neuro-Oncology. 2007;83(1):85–89. doi: 10.1007/s11060-006-9310-2. [DOI] [PubMed] [Google Scholar]
- 27.Whitaker SJ, Bessell EM, Ashley SE, Bloom HJ, Bell BA, Brada M. Postoperative radiotherapy in the management of spinal cord ependymoma. J Neurosurg. 1991 May;74(5):720–728. doi: 10.3171/jns.1991.74.5.0720. [DOI] [PubMed] [Google Scholar]
- 28.Godfraind C. Classification and controversies in pathology of ependymomas. Childs Nerv Syst. 2009 Oct;25(10):1185–1193. doi: 10.1007/s00381-008-0804-4. [DOI] [PubMed] [Google Scholar]
- 29.Mack SC, Taylor MD. The genetic and epigenetic basis of ependymoma. Childs Nerv Syst. 2009 Oct;25(10):1195–1201. doi: 10.1007/s00381-009-0928-1. [DOI] [PubMed] [Google Scholar]
- 30.Sonneland PR, Scheithauer BW, Onofrio BM. Myxopapillary ependymoma. A clinicopathologic and immunocytochemical study of 77 cases. Cancer. 1985 Aug 15;56(4):883–893. doi: 10.1002/1097-0142(19850815)56:4<883::aid-cncr2820560431>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 31.Asazuma T, Toyama Y, Suzuki N, Fujimura Y, Hirabayshi K. Ependymomas of the spinal cord and cauda equina: An analysis of 26 cases and a review of the literature. Spinal Cord. 1999 Nov;37(11):753–759. doi: 10.1038/sj.sc.3100902. [DOI] [PubMed] [Google Scholar]
- 32.McCormick PC, Torres R, Post KD, Stein BM. Intramedullary ependymoma of the spinal cord. J Neurosurg. 1990 Apr;72(4):523–532. doi: 10.3171/jns.1990.72.4.0523. [DOI] [PubMed] [Google Scholar]
- 33.Celli P, Cervoni L, Cantore G. Ependymoma of the filum terminale: treatment and prognostic factors in a series of 28 cases. Acta Neurochir (Wien) 1993;124(2-4):99–103. doi: 10.1007/BF01401130. [DOI] [PubMed] [Google Scholar]
- 34.Fischer G, Mansuy L. Total removal of intramedullary ependymomas: follow-up study of 16 cases. Surg Neurol. 1980 Oct;14(4):243–249. [PubMed] [Google Scholar]
- 35.Cervoni L, Celli P, Fortuna A, Cantore G. Recurrence of spinal ependymoma. Risk factors and long-term survival. Spine (Phila Pa 1976) 1994 Dec 15;19(24):2838–2841. doi: 10.1097/00007632-199412150-00019. [DOI] [PubMed] [Google Scholar]
- 36.Hanbali F, Fourney DR, Marmor E, et al. Spinal cord ependymoma: radical surgical resection and outcome. Neurosurgery. 2002 Nov;51(5):1162–1172. doi: 10.1097/00006123-200211000-00010. discussion 1172-1164. [DOI] [PubMed] [Google Scholar]
- 37.Marks JE, Adler SJ. A comparative study of ependymomas by site of origin. Int J Radiat Oncol Biol Phys. 1982 Jan;8(1):37–43. doi: 10.1016/0360-3016(82)90382-0. [DOI] [PubMed] [Google Scholar]
- 38.Peschel RE, Kapp DS, Cardinale F, Manuelidis EE. Ependymomas of the spinal cord. Int J Radiat Oncol Biol Phys. 1983 Jul;9(7):1093–1096. doi: 10.1016/0360-3016(83)90402-9. [DOI] [PubMed] [Google Scholar]
- 39.Nagasawa DT, Smith ZA, Cremer N, Fong C, Lu DC, Yang I. Complications associated with the treatment for spinal ependymomas. Neurosurg Focus. 2011 Oct;31(4):E13. doi: 10.3171/2011.7.FOCUS11158. [DOI] [PubMed] [Google Scholar]
- 40.Akutsu H, Shibata Y, Okazaki M, Hyodo A, Matsumura A. Intramedullary clear cell ependymoma in the cervical spinal cord: case report. Neurosurgery. 2000 Dec;47(6):1434–1437. discussion 1437-1438. [PubMed] [Google Scholar]
- 41.Arnautovic K, Arnautovic A. Extramedullary intradural spinal tumors: a review of modern diagnostic and treatment options and a report of a series. Bosn J Basic Med Sci. 2009 Oct;9(Suppl 1):40–45. doi: 10.17305/bjbms.2009.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baldwin H, Hadley MN, Pittman H, Spetzler RF, Drayer BP. Gadolinium-DTPA enhancement of a recurrent intramedullary ependymoma: a case report. Surg Neurol. 1989 Mar;31(3):220–223. doi: 10.1016/0090-3019(89)90121-3. [DOI] [PubMed] [Google Scholar]
- 43.Barbagallo GMV, Caltabiano R, Parisi G, Albanese V, Lanzafame S. Giant cell ependymoma of the cervical spinal cord: case report and review of the literature. European Spine Journal. 2008;18(S2):186–190. doi: 10.1007/s00586-008-0789-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boccardo M, Telera S, Vitali A. Tanycytic ependymoma of the spinal cord. Case report and review of the literature. Neurochirurgie. 2003 Dec;49(6):605–610. [PubMed] [Google Scholar]
- 45.Choudry UH, Moran SL, Karacor Z. Functional Reconstruction of the Pelvic Ring With Simultaneous Bilateral Free Fibular Flaps Following Total Sacral Resection. Annals of Plastic Surgery. 2006;57(6):673–676. doi: 10.1097/01.sap.0000237058.57395.1d. [DOI] [PubMed] [Google Scholar]
- 46.Clover LL, Hazuka MB, Kinzie JJ. Spinal cord ependymomas treated with surgery and radiation therapy. A review of 11 cases. Am J Clin Oncol. 1993 Aug;16(4):350–353. doi: 10.1097/00000421-199308000-00016. [DOI] [PubMed] [Google Scholar]
- 47.Di Marco A, Griso C, Pradella R, Campostrini F, Garusi GF. Postoperative management of primary spinal cord ependymomas. Acta Oncol. 1988;27(4):371–375. doi: 10.3109/02841868809093557. [DOI] [PubMed] [Google Scholar]
- 48.Fakhrai N, Neophytou P, Dieckmann K, et al. Recurrent spinal ependymoma showing partial remission under Imatimib. Acta Neurochirurgica. 2004;146(11):1255–1258. doi: 10.1007/s00701-004-0374-5. [DOI] [PubMed] [Google Scholar]
- 49.Fourney DR, Siadati A, Bruner JM, Gokaslan ZL, Rhines LD. Giant cell ependymoma of the spinal cord. Case report and review of the literature. J Neurosurg. 2004 Jan;100(1 Suppl Spine):75–79. doi: 10.3171/spi.2004.100.1.0075. [DOI] [PubMed] [Google Scholar]
- 50.Graca J, Gultasli N, D’Haene N, Brotchi J, Salmon I, Baleriaux D. Cystic extramedullary ependymoma. AJNR Am J Neuroradiol. 2006 Apr;27(4):818–821. [PMC free article] [PubMed] [Google Scholar]
- 51.Heuer GG, Stiefel MF, Bailey RL, Schuster JM. Acute paraparesis from hemorrhagic spinal ependymoma: diagnostic dilemma and surgical management. Journal of Neurosurgery: Spine. 2007;7(6):652–655. doi: 10.3171/SPI-07/12/652. [DOI] [PubMed] [Google Scholar]
- 52.Higgins GS, Smith C, Summers DM, Statham PXF, Erridge SC. Myxopapillary ependymoma with intracranial metastases. British Journal of Neurosurgery. 2005;19(4):356–358. doi: 10.1080/02688690500305373. [DOI] [PubMed] [Google Scholar]
- 53.Ito T, Ozaki Y, Nakagawara J, Nakamura H, Tanaka S, Nagashima K. A case of cervicomedullary junction tanycytic ependymoma associated with marked cyst formation. Brain Tumor Pathology. 2005;22(1):29–33. doi: 10.1007/s10014-005-0174-5. [DOI] [PubMed] [Google Scholar]
- 54.Joaquim AF, Santos MJ, Tedeschi H. Surgical management of intramedullary spinal ependymomas. Arq Neuropsiquiatr. 2009 Jun;67(2A):284–289. doi: 10.1590/s0004-282x2009000200021. [DOI] [PubMed] [Google Scholar]
- 55.Kaner T, Sasani M, Oktenoglu T, Solmaz B, Sarloglu AC, Ozer AF. Clinical analysis of 21 cases of spinal cord ependymoma: positive clinical results of gross total resection. J Korean Neurosurg Soc. 2010 Feb;47(2):102–106. doi: 10.3340/jkns.2010.47.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Katoh S, Ikata T, Inoue A, Takahashi M. Intradural extramedullary ependymoma. A case report. Spine (Phila Pa 1976) 1995 Sep 15;20(18):2036–2038. doi: 10.1097/00007632-199509150-00017. [DOI] [PubMed] [Google Scholar]
- 57.Kawano N, Yagishita S, Oka H, et al. Spinal tanycytic ependymomas. Acta Neuropathol. 2001 Jan;101(1):43–48. doi: 10.1007/s004010000265. [DOI] [PubMed] [Google Scholar]
- 58.Kim NR, Chung DH, Lee SK, Ha SY. Intramedullary clear cell ependymoma in the thoracic spinal cord: a case with its crush smear and ultrastructural findings. J Korean Med Sci. 2007 Sep;22(Suppl):S149–153. doi: 10.3346/jkms.2007.22.S.S149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kocak Z, Garipagaoglu M, Adli M, Uzal MC, Kurtman C. Spinal cord ependymomas in adults: analysis of 15 cases. J Exp Clin Cancer Res. 2004 Jun;23(2):201–206. [PubMed] [Google Scholar]
- 60.Langford LA, Barre GM. Tanycytic ependymoma. Ultrastruct Pathol. 1997 Mar-Apr;21(2):135–142. doi: 10.3109/01913129709021312. [DOI] [PubMed] [Google Scholar]
- 61.Lonjon M, Goh KY, Epstein FJ. Intramedullary spinal cord ependymomas in children: treatment, results and follow-up. Pediatr Neurosurg. 1998 Oct;29(4):178–183. doi: 10.1159/000028718. [DOI] [PubMed] [Google Scholar]
- 62.Miller CA, Torack RM. Secretory ependymoma of the filum terminale. Acta Neuropathol. 1970;15(3):240–250. doi: 10.1007/BF00686770. [DOI] [PubMed] [Google Scholar]
- 63.Miralbell R, Louis DN, O’Keeffe D, Rosenberg AE, Suit HD. Metastatic ependymoma of the sacrum. Cancer. 1990 May 15;65(10):2353–2355. doi: 10.1002/1097-0142(19900515)65:10<2353::aid-cncr2820651032>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 64.Ohata K, Takami T, Gotou T, et al. Surgical outcome of intramedullary spinal cord ependymoma. Acta Neurochir (Wien) 1999;141(4):341–346. doi: 10.1007/s007010050309. discussion 346-347. [DOI] [PubMed] [Google Scholar]
- 65.Okawara S. Ruptured spinal ependymoma simulating bacterial meningitis. Arch Neurol. 1983 Jan;40(1):54–55. doi: 10.1001/archneur.1983.04050010074023. [DOI] [PubMed] [Google Scholar]
- 66.Parekh HC, Sharma RR, Bertolis G, Davis CH, Prabhu S. Primary dorsal exophytic ependymoma. Br J Neurosurg. 1993;7(2):201–203. doi: 10.3109/02688699309103480. [DOI] [PubMed] [Google Scholar]
- 67.Plans G, Brell M, Cabiol J, Villà S, Torres A, Acebes JJ. Intracranial retrograde dissemination in filum terminale myxopapillary ependymomas. Acta Neurochirurgica. 2006;148(3):343–346. doi: 10.1007/s00701-005-0693-1. [DOI] [PubMed] [Google Scholar]
- 68.Plotkin SR, O’Donnell CC, Curry WT, Bove CM, MacCollin M, Nunes FP. Spinal ependymomas in neurofibromatosis Type 2: a retrospective analysis of 55 patients. J Neurosurg Spine. 2011 Apr;14(4):543–547. doi: 10.3171/2010.11.SPINE10350. [DOI] [PubMed] [Google Scholar]
- 69.Sato K, Kubota T, Ishida M, Handa Y. Spinal tanycytic ependymoma with hematomyelia--case report. Neurol Med Chir (Tokyo) 2005 Mar;45(3):168–171. doi: 10.2176/nmc.45.168. [DOI] [PubMed] [Google Scholar]
- 70.Scott M. Infiltrating ependymomas of the cauda equina. Treatment by conservative surgery plus radiotherapy. J Neurosurg. 1974 Oct;41(4):446–448. doi: 10.3171/jns.1974.41.4.0446. [DOI] [PubMed] [Google Scholar]
- 71.Seigel RS, Williams AG, Mettler FA, Jr., Wicks JD. Intraspinal, extradural ependymoma. J Comput Assist Tomogr. 1982 Feb;6(1):189–192. doi: 10.1097/00004728-198202000-00037. [DOI] [PubMed] [Google Scholar]
- 72.Shamji MF, Benoit BG, Perry A, Jansen GH. Giant Cell Ependymoma of the Thoracic Spine. Neurosurgery. 2009;64(3):E566–E567. doi: 10.1227/01.NEU.0000338428.01654.A4. [DOI] [PubMed] [Google Scholar]
- 73.Shaw EG, Evans RG, Scheithauer BW, Ilstrup DM, Earle JD. Radiotherapeutic management of adult intraspinal ependymomas. Int J Radiat Oncol Biol Phys. 1986 Mar;12(3):323–327. doi: 10.1016/0360-3016(86)90345-7. [DOI] [PubMed] [Google Scholar]
- 74.Shintaku M, Nagata N, Itoh H. Tanycytic ependymoma of the spinal cord with anaplastic cytological features. Brain Tumor Pathology. 2009;26(1):7–10. doi: 10.1007/s10014-008-0239-3. [DOI] [PubMed] [Google Scholar]
- 75.Smith JA, Northcroft GB. Lumbar ependymoma. Br J Clin Pract. 1974 Jun;28(6):220–222. [PubMed] [Google Scholar]
- 76.Tait MJ, Chelvarajah R, Garvan N, Bavetta S. Spontaneous hemorrhage of a spinal ependymoma: a rare cause of acute cauda equina syndrome: a case report. Spine (Phila Pa 1976) 2004 Nov 1;29(21):E502–505. doi: 10.1097/01.brs.0000143663.27275.7f. [DOI] [PubMed] [Google Scholar]
- 77.Tzekov C, Naydenov E, Kalev O. Ependymoma of the cauda equina starting with communicating hydrocephalus: a case report. Pediatr Neurosurg. 2007;43(5):399–402. doi: 10.1159/000106390. [DOI] [PubMed] [Google Scholar]
- 78.Yucesoy K, Ozer E, Koyuncuoglu M. Parenchymal brain metastasis of a spinal myxopapillary ependymoma after extradural manipulation. Acta Neurochir (Wien) 2001 Oct;143(10):1071–1072. doi: 10.1007/s007010170014. [DOI] [PubMed] [Google Scholar]
- 79.Volpp PB, Han K, Kagan AR, Tome M. Outcomes in Treatment for Intradural Spinal Cord Ependymomas. International Journal of Radiation Oncology*Biology*Physics. 2007;69(4):1199–1204. doi: 10.1016/j.ijrobp.2007.04.058. [DOI] [PubMed] [Google Scholar]