Abstract
Aquaporin-2 (AQP2) is a water channel responsible for the final water reabsorption in renal collecting ducts. Alterations in AQP2 function induce nephrogenic diabetes insipidus (NDI), a condition characterized by severe polyuria and polydipsia. Three patients affected with severe NDI, who were compound heterozygous for the AQP2 mutations D150E and G196D, are presented here along with a mildly affected D150E homozygous patient from another family. Using Xenopus oocytes as an expression system, these two mutations (G196D and D150E) were compared with the wild-type protein (AQP2-wt) for functional activity (water flux analysis), protein maturation, and plasma membrane targeting. AQP2-wt induces a major increase in water permeability (Pf = 47.4 ± 12.2 × 10−4 cm/s) whereas D150E displays intermediate Pf values (Pf = 12.5 ± 3.0 × 10−4 cm/s) and G196D presents no specific water flux, similar to controls (Pf = 2.1 ± 0.8 × 10−4 cm/s and 2.2 ± 0.7 × 10−4 cm/s, respectively). Western blot and immunocytochemical evaluations show protein targeting that parallels activity levels with AQP2-wt adequately targeted to the plasma membrane, partial targeting for D150E, and complete sequestration of G196D within intracellular compartments. When coinjecting AQP2-wt with mutants, no (AQP2-wt + D150E) or partial (AQP2-wt + G196D) reduction of water flux were observed compared with AQP2-wt alone, whereas complete loss of function was found when both mutants were coinjected. These results essentially recapitulate the clinical profiles of the family members, showing a typical dominant negative effect when G196D is coinjected with either AQP2-wt or D150E but not between AQP2-wt and D150E mutant.
Keywords: functional expression, Xenopus oocyte, water channel
water reabsorption in the collecting duct of the kidney is performed by type 2 aquaporin (AQP2) located at the apical side of principal cells. This function is under the control of vasopressin that, through activation of its receptor (AVPR2), coordinates the amount of AQP2 present at the plasma membrane, thus controlling water reabsorption and ultimately defining the concentration of urine (1, 22, 23). The protein is assembled in the endoplasmic reticulum (ER) as a homotetramer and does not form heteromers with the other AQPs (AQP3 and 4) expressed in the same cell (26). Structurally, it is believed that each AQP2 subunit is independently functional (i.e., four independent water pores) and that, when expressed in oocytes, only one AQP2 monomer is N-glycosylated within the tetramer (11). Alterations in either AQP2 or AVPR2 function can lead to nephrogenic diabetes insipidus (NDI), a condition that presents with polyuria and polydipsia, among other symptoms (2, 6, 8).
To date, several NDI-inducing AQP2 mutations have been reported (http://www.medicine.mcgill.ca/nephros/aqp2.html). Although most of these were shown to be autosomal recessive traits (17, 20), a few were determined to be dominant (16, 17, 21). Generally, functional analysis of these mutations has shown impairment in protein synthesis that leads to the ER retention of misfolded products followed by eventual degradation (14). In some cases, when wild-type and mutated AQP2 proteins are expressed together, heteromeric combinations may occur that could induce retention of the wild-type protein in the ER with concomitant loss of function (dominant negative effect) (16). Finally, some AQP2 mutations were shown to be adequately targeted to the plasma membrane despite being nonfunctional (class IV mutations) (10).
Over the years, the Xenopus laevis oocyte has been used as a powerful expression system for the study of proteins such as channels and transporters, including aquaporins (20, 27). The oocyte can be used to evaluate many biochemical aspect of the protein including synthesis and regulation, as well as measuring protein activity. In the past, physiological evaluations in oocytes comparing wild-type to mutants forms of AQP2 have enabled thorough analysis of elements responsible for NDI (15, 20).
In the present study, two independent families bearing the D150E and G196D mutations were evaluated. In the first family three severely affected children are compound heterozygous (D150E; G196D) and in the second family a mildly affected child is homozygous for the D150E mutation. The D150E mutation was previously identified in a patient from Italy found to be severely affected and compound heterozygous (D150E; G215C) (12). Using the Xenopus oocyte system, we describe the characteristics of these mutations and have tentatively reproduced the different phenotypes of the family members through coexpression studies.
METHODS
Patients
The pedigree presented in Fig. 1 shows heterozygous individuals (parents and grandparents) for both D150E and G196D mutations and three male descendants presenting both mutations. Only individuals who are compound heterozygous for the two mutations display severe congenital NDI with polyuria, low urine osmolality (77 mmol/kgH2O), and lack of response to subcutaneous injection of 1-desamino-8d-arginine vasopressin (dDAVP). Evaluations of heterozygous individuals detected no abnormalities in 24-h urinary volumes or concentration abilities. DNA sequencing of the AVPR2 gene in these three male patients showed no mutations. DNA was obtained from patients and their family following acceptance by the Ethics Committee of Hôpital du Sacré-Cœur in Montreal.
Fig. 1.
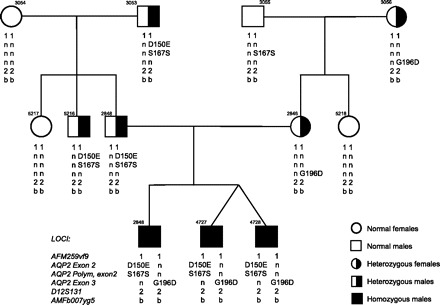
Pedigree of the first family with haplotype and mutational analysis. Squares and circles represent male and female subjects, respectively, with unaffected individuals (open symbols), carriers (half filled symbols), and affected individuals (solid symbol); n indicates normal allele. Haplotypes consist of markers that flank the AQP2 gene and that have been described previously (19). As expected, the alleles bearing the individual mutations are identical.
A boy from a second family, whose parents are second cousins and belong to a small ethnic group in Dagestan (Caucasus), was found to be homozygous for the D150E AQP2 mutation. He presented with polydipsia and polyuria in the first months of life but there was no reported episode of severe dehydration with hypernatremia. Upon referral at the age of 9 yr, his urine volume ranged from 6 to 10 l/day (7–12 ml·kg−1·h−1). Investigated twice with water deprivation tests, the urine osmolality increased from 160 to 614 mosmol/kgH2O and from 110 to 434 mosmol/kgH2O after 20 μg of intranasally administered dDAVP.
Generation of AQP2 Mutant Constructs and cRNA
Both mutations were inserted by PCR reaction into the wild-type (wt) pT7TS-AQP2-wt plasmid. The primers used for site-specific mutagenesis were CCTCCACCGAAGAGCGCCGCG (forward) and CGCGGCGCTCTTCGGTGGAGG (reverse) for D150E and GTCGTCACTGACAAATTTGATC (forward) and CATCAAATTTGTCAGTGACGA (reverse) for G196D. A hemagglutinin (HA) epitope tag was added to AQP2 at the NH2-terminal position by subcloning full-length AQP2-wt into pT7TS-HA vector using the SpeI and BglII restriction sites. The pcDNA6-AQP2 vector used for expression in mIMCD-3 cells (a murine model of kidney inner medullary collecting duct) was generated by subcloning the full-length AQP2 from pT7TS-AQP2-wt using the BamH1 and Xba1 unique sites. Both mutations were also inserted in pcDNA6 using same strategy. All products were sequenced to validate mutations. Plasmids were linearized with SalI and capped mRNA was synthesized in vitro by using a T7 kit (Ambion, Austin, TX) according to the manufacturer's instructions.
Cell Culture Maintenance and Transfection
mIMCD-3 cells were routinely cultured in DMEM-F12 media supplemented with 10% FBS and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin) and maintained in a 95% air-5% CO2 atmosphere. For transfection with pcDNA6-AQP2 variants, cells were seeded on coverslips and transfection was performed by using Lipofectamine 2000 (Invitrogen) on 90% confluent monolayers (8 μg DNA/100-mm petri dish). After 16–24 h of incubation, coverslips were treated with forskolin (50 μM, 45 min) and fixed at −20°C for 20 min with formaldehyde (1%) diluted in methanol.
Oocyte Preparation, Injection, and Maintenance
All procedures involving animals (surgery and euthanasia) were made in accordance with Canadian guidelines and approved by the ethical committee of the Université de Montréal. Gravid Xenopus laevis (University of Alberta, Edmonton, Canada) were anesthetized using 2-aminobenzoic acid ethyl ester (1.3 g/l), and ovary nodes were surgically removed and then dissected by hand to collect mature oocytes (3). In brief, follicles were removed by collagenase treatment and oocytes were rinsed and kept at 18°C in Barth's solution [in mM: 88 NaCl, 3 KCl, 0.82 MgSO4, 0.4 CaCl2, 0.33 Ca(NO3)2 and 5 HEPES, pH 7.6] supplemented with horse serum (5%), sodium pyruvate (2.5 mM), and antibiotics (100 U/ml penicillin, 0.1 mg/ml streptomycin, 0.1 mg/ml kanamycin). Following a 24-h recovery period, oocytes were injected with HA-tagged or untagged wild-type (0.5 or 1 ng) and mutated forms (5 or 10 ng) of AQP2 cRNAs, as indicated in the figure legends, and further incubated for 48 to 72 h in Barth's solution at 18°C prior to experimentation.
Membrane Purification From Xenopus Oocytes
Total membranes and purified plasma membranes for Western blot detection of AQP2 were purified according to Leduc-Nadeau et al. (18). Briefly, for total membrane fractions, control and AQP2-injected oocytes were homogenized in PBS supplemented with protease inhibitor cocktail (Sigma Aldrich, St. Louis, MO) at a ratio of 1 oocyte per 10 μl of buffer. Oocyte homogenates were centrifuged at 4°C, first at 250 g for 10 min and then the supernatant was centrifuged again at 20,000 g for 20 min. The pellet was resuspended at 2 μl/oocyte in PBS.
For preparation of plasma membranes, 40 oocytes were treated with 0.35 U/ml subtilisin A (Sigma Aldrich; no. P5380) in modified basal salt solution (MBSS) (80 mM NaCl, 20 mM MES pH 6.0) then sequentially treated in MBSS with 1% Ludox CL colloidal silica (Sigma Aldrich; no. 420883) for 60 min at 4°C, rinsed three times and treated with 0.1% polyacrylic acid (Sigma Aldrich; molecular weight 30,000, no. 41.604-5) at 4°C for 60 min. Oocytes were rinsed and homogenized in 500 μl homogenization solution A (HbA; in mM: 5 MgCl2, 5 NaH2PO4, 1 EDTA, 80 sucrose, and 20 Tris, pH 7.4) via a 200-μl pipettor, adjusted to 1 ml with same solution, and then centrifuged two times at 16 g followed by two more centrifugations at 24 and 38 g. For each centrifugation, the top 950 μl was removed and replaced with fresh 950 μl HbA. The final pellet was diluted to 1 ml and centrifuged at 14,000 g for 20 min, and the pellet was resuspended in Laemmli buffer.
Western Blot
Western blots were performed as described earlier (18) on total membranes and purified plasma membranes from both control and AQP2-injected oocytes. Oocytes injected with wild-type (1 ng) or mutant AQP2 mRNA (10 ng) were incubated for 72 h prior to experimentation, and membrane equivalents of 1 oocyte (total membranes) or 40 oocytes (purified plasma membranes) were loaded on a 12% polyacrylamide gel, then transferred onto nitrocellulose membranes, blocked for nonspecific sites in TBS-T with 5% nonfat milk, and incubated overnight at 4°C with goat anti-AQP2 (N-20, at 1/100 dilution, Santa Cruz Biotechnology, Santa Cruz, CA) or horseradish peroxidase (HRP)-linked mouse anti-HA for targeting analysis (1/500 dilution, Roche, Laval, QC, Canada) in the same blocking solution. The nitrocellulose membranes were then rinsed, blocked again and incubated for 1 h with an appropriate secondary antibody (HRP-linked chicken anti-goat for anti-AQP2, Santa Cruz Biotechnology) at 1/25,000 dilution at room temperature in TBS-T with milk. Blots were revealed by enhanced chemiluminescence detection (Phototope-HRP, New England Biolabs, Pickering, ON, Canada). Molecular weights and densitometry analyses were determined by using software from alpha-imager 2000 (Alpha Innotech, San Leandro, CA).
Immunofluorescence
On oocytes.
Immunofluorescence detection on oocytes was performed according to previously published protocols for fixed oocytes (3). Control oocytes and oocytes injected with wild-type (1 ng) and mutant (10 ng) AQP2 mRNA were incubated for 72 h before the immunofluorescence assay. Oocytes were rinsed three times with Barth's solution and fixed for 15 min in ice-cold methanol solution containing 1% formaldehyde. Fixed oocytes were rinsed again three times and incubated overnight in a Barth's solution containing 30% sucrose. Oocytes were embedded in Tissue-tek embedding medium diluted 1/7 in water (Sakura Finetek), frozen, sliced (10 μm thickness) on a cryostat, and mounted on slides. Slices were blocked for 30 min at room temperature with a solution of 2% BSA in PBS to prevent nonspecific binding of antibody. Incubation with the anti-AQP2 antibody at a dilution of 1:20 in PBS + BSA was performed in a wet chamber at room temperature for 1 h, and slides were then rinsed three times in PBS. Incubation with a secondary antibody (Alexa Fluor 488-conjugated anti-goat IgG, 1:1,000 in blocking solution, Molecular Probes, Eugene, OR) was performed as for the first antibody and rinsed accordingly. Slides were mounted using an anti-quenching agent (Prolong antifade, Molecular Probes, Eugene OR) prior to observation.
On cells.
Immunofluorescence on cell monolayers was performed using confluent mIMCD-3 cells grown and transfected on coverslips as described earlier (3). AQP2 proteins were visualized by same protocol as for oocytes (shown green in figures). Prior to fixation, all coverslips were treated with forskolin (50 μM, 45 min; Ref. 5) to promote plasma membrane insertion and allow discrimination of mutant phenotypes. Protein disulfide isomerase (PDI) was used as an ER marker and visualized (red) by use of a specific antibody (mouse anti-PDI, 1/50 dilution, Santa Cruz Biotechnology) along with a corresponding secondary antibody (Alexa 568-labeled anti-mouse IgG, 1/1,000 dilution, Molecular Probes). Nuclei were stained by incubating slides in 4′,6-diamidino-2-phenylindole (DAPI) for 30 min (Sigma, 1 μg/ml in PBS). As was done for oocytes, slides were mounted by using an antiquenching agent (Prolong antifade, Molecular Probes) prior to visualization (×60) with an Olympus IX-81 microscope along with Image-Pro Plus v.5.0 software.
Volume Measurements
The functional activity of AQP2 was evaluated by optical measurements of oocytes challenged with hypotonic media. The procedure was described previously in studies describing SGLT1 and water transport (7, 28). The experimental chamber (volume of 0.07 ml) was specially designed to enable rapid solution changes (solution flux of 1.2 ml/min) to enable measurement of rapid changes in oocyte volume. The light source (2 mm × 5 mm) touches the surface of the solution to avoid distortion and reflection. The oocyte volume was calculated from its measured cross-section using an inverted microscope, a ×3 objective and a video camera (Dage MTI, model CCD72, Michigan City, IN). Images were sent to a PC (Intel 403E 486 30 MHz) through a video card (Image-1280, Matrox Electronic Systems) and our custom-made Windows-compatible software provided real-time values for the pixels comprising the oocyte image. This setup provides a sensitivity of 0.05% in the determination of the oocyte volume.
Volumes were evaluated for control, wild-type, and mutant AQP2-expressing oocytes after 24 and 48 h of incubation. Prior to volumetric analysis, oocytes were equilibrated for 15–30 min in a saline solution containing (in mM) 78 NaCl, 3 KCl, 0.82 MgCl2, 0.74 CaCl2, 5 HEPES pH 7.6, and 20 mannitol (final osmolality of 195 mosmol/kgH2O). Hypotonic shock of 20 mosmol/kgH2O was achieved by removal of mannitol (final osmolality of 175 mosmol/kgH2O). The osmolality of each solution was controlled within ± 1 mosmol/kgH2O using a vapor pressure osmometer (Advanced DigiMatic Osmometer, model 3D2, Advanced Instruments, Norwood, MA). For evaluation of cAMP-dependent inductions of activity, oocytes [noninjected (Ctl) or injected with either AQP2-wt (1 ng), AQP2-D150E (10 ng) or AQP2-G196D (10 ng) mRNA] were tested for water permeability before and after treatment with 10 μM forskolin (2 h) to perform paired analyses of Pf inductions. The data presented in Fig. 5 represent means ± SD of 14 to 39 individual determinations for each condition compiled in three to four experiments.
Fig. 5.
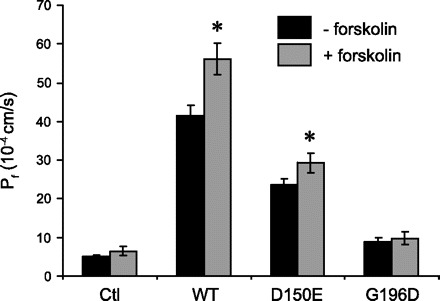
Effects of forskolin treatment on functionality of AQP2 variants. Oocytes were not injected (Ctl) or injected with either AQP2-wt (1 ng), AQP2-D150E (10 ng), or AQP2-G196D (10 ng) mRNAs and incubated for 3 days. Oocytes were tested for water permeability before and after treatment with 10 μM forskolin (2 h). Values are means ± SD of paired analyses; *significant induction in permeability by forskolin over nontreated oocytes (P < 0.05).
Data Analysis
Water permeabilities (Pf values) were calculated according to the following equation:
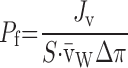 |
where Jv represents water flux (μl/s), S is the standard surface of an oocyte (0.4 cm2), v̄w is the specific volume of water (18 cm3/mol), and Δπ is the applied hypotonic shock (osmol/cm3). Water fluxes (Jv) were determined from regression of the linear portions of volume curves. Specific Pf values were calculated by subtracting Jv values before hypoosmotic shock to those determined after the shock. All Pf values represent measurements performed on five to eight oocytes, either noninjected (controls) or injected with various mRNAs. The values are listed as means ± SD for oocytes from a single donor. ANOVA analyses of data were performed where required with statistical difference (P < 0.05) indicated by an asterisk. All experiments were performed at least three times on oocytes from different donors and the figures present typical results.
RESULTS
Functional Evaluation of Wild-Type and Mutant AQP2
Figure 2A demonstrates a typical time curve of volume variations for control oocytes as well as oocytes injected with either wild-type (1 ng) or mutated forms of AQP2 mRNA (D150E and G196D, 10 ng). Following equilibration, the oocytes were submitted to a moderate hypoosmotic shock (−20 mosmol/kg), and the subsequent, initial linear segments of volume increase representing water flux were measured. Initial volumes of oocytes were set at a fixed 100% value to compare variations between conditions. As seen in the figure, volume increases were very rapid and the linear portions of curves were easily defined. The values for volume increase were used to determine the water permeabilities (Pf) that are presented in Fig. 2B. As shown in this figure, a small endogenous water permeability was observed in noninjected oocytes (Pf = 2.2 ± 0.7 × 10−4 cm/s). This value was not significantly different from that of AQP2-G196D-injected oocytes (Pf = 2.1 ± 0.8 × 10−4 cm/s), which was therefore considered to not contribute to water flux. On the other hand, AQP2-wt-injected oocytes demonstrated a net increase in water permeability (Pf = 47.4 ± 12.2 × 10−4 cm/s), as did AQP2-D150E-injected oocytes, although at a more modest level (Pf = 12.5 ± 3.0 × 10−4 cm/s).
Fig. 2.
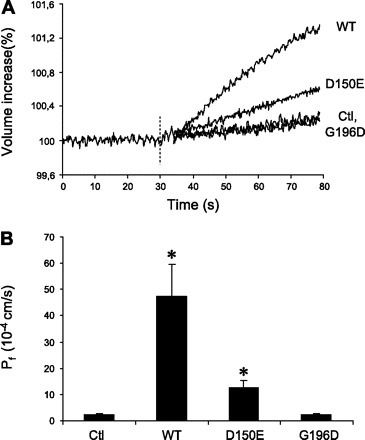
Determination of water permeabilities (Pf) of wild-type (WT) AQP2 and mutants expressed in Xenopus oocytes. Oocytes were injected with either AQP2-wt (1 ng), AQP2-D150E (10 ng), or AQP2-G196D (10 ng) mRNAs and incubated for 2 days prior to assay. Determination of water permeabilities was performed by evaluation of volume increase in oocytes as induced by a 20 mosmol/kgH2O hypotonic shock (see methods). A: time course of oocyte volume presented as percent increase from isotonic condition. Dashed line indicates moment of switch to hypotonic shock treatment. The reading precision allows for determination limit of less than 0.05% of cell volume. B: histogram presenting water permeabilities for the 3 different AQP2 variants along with control (Ctl). Data are Pf values (10−4 cm/s) ± SD and represents means of 5 oocytes for each condition. *Significant difference from control oocytes (P < 0.05). Results shown are typical of 3 experiments performed on different oocytes.
Biochemical Evaluation of Wild-Type and Mutant AQP2
To correlate the functional analysis of the different AQP2s with expression and localization patterns, biochemical evaluation of the proteins was performed using both Western blots (Fig. 3) and immunofluorescence (Fig. 4). Western blot analyses were performed on total membranes as well as on purified plasma membrane fractions. In total membrane fractions, a 29-kDa band was observed for all AQP2 forms tested. Wild-type protein (A) presents a broader band at 39–45 kDa representing the mature, glycosylated form of AQP2 which is not observed with the two mutants (lanes B and C) that only exhibit the core-glycosylated forms, seen as 31-kDa bands. In purified plasma membrane fractions, the intensity of the 29-kDa bands correlates to the activity levels shown in Fig. 2 with high staining for AQP2-wt, moderate staining for AQP2-D150E, and no staining for AQP2-G196D. It should be noted that, even in overexposed blots, the 31-kDa band found in the total membrane fractions could not be seen in the purified plasma membrane fractions. Immunofluorescence was performed on control and AQP2-injected oocytes to confirm the results found in Western blots. As shown in Fig. 4, only the AQP2-wt-injected oocyte (Fig. 4B) showed net labeling at the plasma membrane. Oocytes injected with AQP2-G196D showed only intracellular staining (Fig. 4D) whereas oocytes injected with AQP2-D150E displayed intracellular staining with faint but distinct plasma membrane labeling (Fig. 4C). As expected, control oocytes (Fig. 4A) showed no specific staining whatsoever. AQP2-expressing oocytes were also treated with forskolin to see whether the cAMP-dependent increase in functionality normally found with AQP2-wt could also be seen with either D150E or G196D. As shown in Fig. 5, incubating oocytes for 2 h with 10 μM forskolin stimulated the activity of both AQP2-wt (40 ± 11%, P < 0.05) and D150E (30 ± 14%, P < 0.05) but not G196D.
Fig. 3.
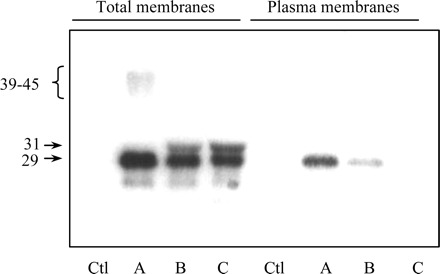
Western blot analysis of oocytes expressing AQP2-wt or AQP2 mutants. Oocytes were injected with either AQP2-wt (1 ng), AQP2-D150E (10 ng), or AQP2-G196D (10 ng) mRNAs and incubated for 3 days. Oocytes were treated for the purification of plasma membranes or total membranes as described in methods. Lanes represents control (Ctl), AQP2-wt (A), AQP2-D150E (B), or AQP2-G196D (C). One oocyte per sample was loaded for total membranes and plasma membrane lanes employed purified fractions from 40 oocytes. SDS-PAGE was performed on a 12% gel and the primary antibody was used at 1/100 dilution.
Fig. 4.
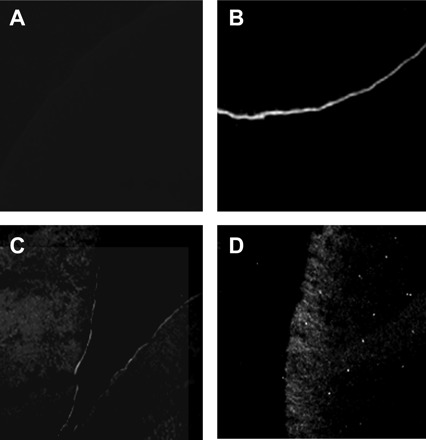
Immunofluorescence of AQP2 expressed in oocytes. Oocytes were not injected (A) or injected with either AQP2-wt (B, 1 ng), AQP2-D150E (C, 10 ng) or AQP2-G196D (D, 10 ng) mRNAs and incubated for 3 days prior to assay. Oocytes were immunostained and visualized with antibodies to AQP2 as described inmethods.
Wild-type and mutated forms of AQP2 were also transiently expressed in mIMCD-3 cells to visualize the cellular distribution for each AQP2 species and also to evaluate the possible mistargeting of both mutants, similar to what is found in oocytes. Again, all samples were treated with forskolin (50 μM, 45 min) prior to fixation to promote plasma membrane insertion. As seen in Fig. 6, immunofluorescence analyses demonstrated that both AQP2-wt (top) and D150E (middle) were adequately targeted to the plasma membrane (straight arrows) whereas G196D (bottom) was completely retained within intracellular stores compatible with ER (PDI staining, in red) as seen by colocalization staining (orange, dotted arrows).
Fig. 6.
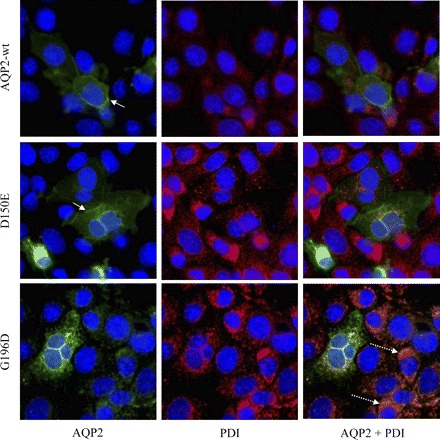
Immunofluorescence analysis of AQP2 in transfected IMCD3 cells. IMCD3 cells were transfected with pcDNA6-AQP2-wt (top), -D150E (middle), or -G196D (bottom), incubated for 16–24 h, and treated with forskolin (50 μM, 45 min) prior to fixation. Protein disulfide isomerase (PDI) was used as an endoplasmic reticulum (ER) marker (red), and 4′,6-diamidino-2-phenylindole (DAPI) was used as a nuclear stain (blue). Plasma membrane staining are found for both AQP2-wt and D150E (solid arrows) but not for G196D (dotted arrows). ER colocalization with AQP2 products is seen in merged images (orange). Magnification ×60.
Functional Analysis in Coexpression Studies
To determine possible interactions between wild-type and mutant AQP2 proteins, oocytes coinjected with both wild-type and mutant forms of AQP2 were tested for their functionality. To that end, preliminary experiments using volume measurements (AQP2-wt, D150E) and Western blots (AQP2-wt, D150E, and G196D) indicated a linear relationship between the expression levels observed and the amount of mRNA injected in the case of AQP2-wt (up to 1.5 ng), D150E (up to 15 ng), and G196D (up to 20 ng). Figure 7 presents the water permeabilities of oocytes injected with one or with a combination of two AQP2 mRNAs. As already shown in Fig. 2, background water permeability in noninjected oocytes was minimal (Pf = 3.5 ± 1.1 × 10−4 cm/s) and was not significantly increased in AQP2-G196D-injected oocytes, either with 5 (Pf = 4.2 ± 1.1 × 10−4 cm/s) or 10 ng (Pf = 4.1 ± 1.0 × 10−4 cm/s) of mRNA. Injecting 5 ng of AQP2-D150E mRNA produced a modest increase in Pf with respect to noninjected oocytes (Pf = 7.3 ± 2.1 × 10−4 cm/s), and a further increase was observed with 10-ng injections (Pf = 13.8 ± 5.1 × 10−4 cm/s). In three different batches of oocytes, the Pf stimulation, with respect to noninjected oocytes, was roughly proportional to the amount of mRNA injected. In the case of AQP2-wt, only 0.5 ng of mRNA was injected to promote oligomeric interactions between wild-type and mutant proteins. The addition of 10 ng of AQP2-D150E mRNA to 0.5 ng AQP2-wt mRNA did not significantly alter the water permeability of the oocyte (Pf = 27.8 ± 7.5 × 10−4 cm/s for AQP2-wt alone, and 28.4 ± 7.1 × 10−4 cm/s for AQP2-wt + AQP2-D150E). On the other hand, coexpressing AQP2-wt along with AQP2-G196D dramatically reduced the water permeability of the oocyte compared with AQP2-wt alone (Pf = 12.0 ± 4.8 × 10−4 cm/s, P < 0.05). Interestingly, injecting 5 ng of AQP2-G196D mRNA also produced a significant inhibitory effect compared with the Pf obtained with oocytes injected with 5 ng of AQP2-D150E cRNA (Pf = 4.4 ± 1.1 × 10−4 cm/s). Similar coexpression results were observed for three different oocyte batches.
Fig. 7.
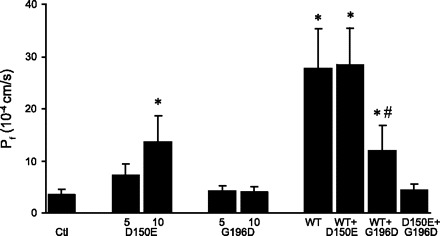
Evaluation of water permeabilities in oocytes expressing pure forms or coexpressing wild-type with mutant forms of AQP2. Oocytes were injected with either pure AQP2-wt (0.5 ng), AQP2-D150E (5 and 10 ng), or AQP2-G196D (5 and 10 ng) mRNAs or with a mixture of mRNAs: AQP2-wt + AQP2-D150E (0.5 + 10 ng), AQP2-wt + AQP2-G196D (0.5 + 10 ng), or AQP2-D150E + AQP2-G196D (5 ng each). Oocytes were incubated for 2 days prior to assay. Values are presented as Pf values (10−4 cm/s) ± SD and are the means of 5 oocytes. Symbols indicate significant difference (P < 0.05) from control (*) or pure AQP2-wt-injected (#) oocytes. Experiment is typical of 3 assays using different donor animals.
Western blots were also used to evaluate the impact of coexpression on expression level and plasma membrane distribution of AQP2-wt. Using an HA-tagged version of AQP2-wt, we demonstrated that the coexpression conditions used herein did not significantly alter the level of expression of HA-AQP2-wt, as detected through evaluation of the HA tag in total membrane samples (Fig. 8A). On the other hand, using purified plasma membranes from the same experiment, the presence of G196D greatly impeded the normal plasma membrane targeting of HA-AQP2-wt whereas D150E was without effect (Fig. 8B). These results are in agreement with the functional assays presented in Fig. 7.
Fig. 8.
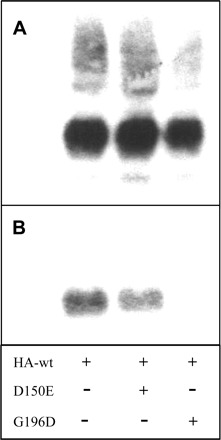
Effects of coexpression conditions on plasma membrane targeting of AQP2-wt. Oocytes were injected either with pure HA-AQP2-wt (0.5 ng) or with the same mRNA plus untagged AQP2-D150E (10 ng) or AQP2-G196D (10 ng) and incubated for 2 days prior to assay. Oocytes were treated by preparation of total membrane or purified plasma membrane fractions as described in methods. An equivalent of 1 oocyte per condition was loaded for total membranes (A) and plasma membrane lanes employed purified fractions from 40 oocytes (B); blots were probed using anti-HA antibody to specifically visualize AQP2-wt.
DISCUSSION
In the past, Xenopus oocytes have proven to be very useful for investigating AQP2 expression, both for the wild-type and for the mutated forms of the protein responsible for NDI. With this expression system, the fundamentals of AQP2 physiology were determined in both its biochemical and functional aspects. Up to now, case reports of AQP2-related NDI usually presented affected homozygotes (patients) along with nonaffected heterozygotes (parents). In this report, we had access both to affected patients compound heterozygous for the D150E and G196D mutations and also to patients homozygous for the “mild” D150E mutation. Family members bearing only one mutation (heterozygotes) did not demonstrate the typical traits of NDI such as high urinary volumes or inability to concentrate urine. The oocyte expressing system was used here primarily to evaluate the functionality and biochemistry of each mutation, and also to determine their possible interrelations through oligomeric structures within mutants or along with the wild-type form of AQP2. Ultimately, we attempted to correlate the actual family phenotypes to the heteromeric conditions of AQP2.
Functionality Studies
The setup used to analyze water fluxes (7) is highly sensitive since it can detect volume changes as small as 0.05%. This enables the use of relatively small hypotonic shocks (−20 mosmol/kgH2O) in functional evaluation of AQP2, which minimizes possible stress and regulatory mechanisms that could be associated with more dramatic osmolarity changes. In this study, AQP2-related Pf values were found to be similar to those reported earlier (4, 20) with the expected linear relation between the injected AQP2-wt mRNA and the AQP2 activity, for mRNA amounts up to 1.5 ng (data not shown). With efficient functional tests, amounts of mRNA for AQP2-wt could be kept in the low linear range (0.5 and 1 ng), which is relevant in coexpression studies (13).
When AQP2-G196D was expressed in oocytes, no specific water permeability increase could be observed (Fig. 2), regardless of the amount of mRNA injected into the oocyte (up to 46 ng, data not shown). When performing immunofluorescence studies, as shown in Fig. 4D, there is also no evidence of any targeting to the plasma membrane. This result is consistent with Western blot analysis (Fig. 3), in which no trace is found for this mutant when probing purified plasma membranes. Since this protein was synthesized in sufficient amounts to be detected, as shown in the same blot (total membranes, lane C), it seems apparent that AQP2-G196D lacked appropriate processing, which prevented its normal targeting to the plasma membrane. This finding was substantiated by immunofluorescent analysis in mIMCD-3 cells (Fig. 6) where, even after forskolin treatment to promote plasma membrane insertion, AQP2-G196D was still retained in an intracellular store compatible with the ER, as seen by colocalization with PDI (orange staining, dotted arrows). In such systems, leakage from the normal cellular location is often found when heterologous proteins are overexpressed. This is found with high-expressing cells in the same micrograph, where colocalization with ER is seen as punctate yellow and orange staining along noncolocalized areas (green in merged image) representing leakage from the ER. Still, plasma membrane labeling remained absent under such conditions. mIMCD-3 cells were chosen for this investigation as this cell line adequately synthesizes, processes and targets AQP2 when expressed heterologously (25). The profile found for AQP2-G196D is consistent with a class II mutant, which is the case for most AQP2 mutations studied so far (20). Also, with AQP2-G196D sequestered inside the cell, we do not yet know whether the protein is actually functional or not; active AQP2 mutants trapped in intracellular stores have been reported in the past (4). In Western blots performed on total membranes (Fig. 3), the protein generated two bands of low molecular weight. The lowest represents the unglycosylated form (29 kDa) whereas the band above (31 kDa) represents a high-mannose form of the protein. This latter band is routinely found in Western blots of AQP2 mutants expressed in oocytes and is indicative of retention within the ER (20). As mentioned earlier, when expressed in oocytes, only one subunit in the tetrameric AQP2 structure is believed to be glycosylated, and its processing through the ER is found to be quite fast. For this reason, high-mannose species of AQP2-wt are almost never detected on Western blots (24). Instead, the normal signature for AQP2-wt in Western blots is a single band of low molecular weight (29 kDa, in this case) with occasionally a high-molecular-weight band spanning around 39–45 kDa, representing a mature complex-glycosylated form of AQP2 (16).
The expression of AQP2-D150E in oocytes is somewhat similar to that of AQP2-G196D. Yet low APQ2 activity levels are found in functional assays on this mutant, which was corroborated by all analyses performed. In functional assays, AQP2-D150E generated about one-third of the water permeability elicited by AQP2-wt (Fig. 2). When considering that 10 times more mRNA was injected for the mutant (10 ng) compared with AQP2-wt (1 ng), the overall efficiency of AQP2-D150E can thus be estimated at about 1/30 of AQP2-wt. The activity level also correlated with the amount of cRNA injected (5 and 10 ng injected, Fig. 7). In accordance with this weak activity, immunofluorescent labeling of AQP2-D150E in oocytes (Fig. 4C) showed faint but distinct labeling at the plasma membrane as well as major intracellular staining. Furthermore, the discrete band seen in Western blots of plasma membranes purified from AQP2-D150E expressing oocytes (lane B) confirmed that at least low amounts of the protein were adequately targeted to the plasma membrane (Fig. 3). Still, by comparing the proportion of the protein detected in the homogenate to that found in the plasma membrane fraction, it must be concluded that almost all of the AQP2-D150E protein was retained within intracellular stores. This phenotype, determined for pure D150E expression, correlates well with that observed in the homozygous patient described in the second family, in which mild NDI was observed (clinical data not shown here). These findings are in contradiction with those of Iolascon et al. (12) who concluded that there was a complete absence of activity for this mutation through immunological detection using D150E-transfected cells. The actual physiological activity was not measured. Our water permeability assays clearly demonstrated that D150E displays partial functionality (Figs. 2 and 6), which correlates with its partial plasma membrane targeting (Figs. 3–5), and, most importantly, with the fact that the affected homozygous individual is responsive to dDAVP treatment. This sensitivity to dDAVP is mimicked here by the use of forskolin. As shown in Fig. 5, the functionality of AQP2-D150E was induced in oocytes to the same degree as was AQP2-wt (30 ± 14 and 40 ± 11%, respectively). Furthermore, when expressed in IMCD cells, plasma membrane labeling of AQP2-D150E was again observed similarly to AQP2-wt (Fig. 6, top and middle). This result is in contradiction to that found in M-1 cells where AQP2-D150E does not reach the plasma membrane even after forskolin treatment (12). It is possible that the different cell type used may explain this discrepancy. On the other hand, forskolin could not exert any significant functionality on AQP2-G196D expressed in oocytes, nor could it promote plasma membrane insertion in IMCD cells, which is also compatible with its phenotype. Since partial expression was found with AQP2-D150E, recovery assays using chemical chaperones may be envisaged here (16).
Coexpression Studies
Coexpression studies were undertaken to see whether the actual phenotypes described for individual family members could be explained using the oocyte expression system and possibly reproduced. For these experiments, the amount of each cRNA injected may be crucial since ratios between the different forms of AQP2 may (or may not) induce heteromeric structures (13). Whereas mutant forms of AQP2 are easily sequestered as high-molecular-weight structures (not shown here), we have determined through quantification of individual bands in Western blots (Fig. 3) that only about one-tenth of the protein is present as the low-molecular-weight structure (29 kDa, similar to that of AQP2-wt), a form believed to be more susceptible to association into an oligomeric structure. This condition is the one shown in Fig. 3 where equivalent amounts of the 29-kDa proteins were found in total membranes. To promote heteromeric structures between wild-type and mutant proteins, we imposed a twofold ratio of unbound protein for mutant (29 kDa) over the wild-type form of AQP2, which was achieved with a fixed mRNA ratio of 20 to 1 for AQP2 mutants over AQP2-wt, or 10 to 0.5 ng as seen in Fig. 7.
As already mentioned, AQP2-D150E displays a weak activity level that is proportional to the amount of mRNA injected (5 and 10 ng). On the other hand, AQP2-G196D does not show any activity at all, even at 10 ng mRNA. When AQP2-wt (0.5 ng) was coinjected with AQP2-D150E (10 ng), no modification of the activity level was found. It thus seems that this mutant does not interact, at least functionally, with AQP2-wt. Since 10 ng of AQP2-D150E was known to confer some activity (40% of AQP2-wt for the experiments depicted in Fig. 7), an increase of the Pf value would have been expected. Although care was taken to remain in linear conditions for protein expression, it is possible that overexpression of D150E might partially hinder the normal expression of AQP-wt or, on the other hand, that the expression of AQP2-wt inhibited or superseded whatever small proportion of AQP2-D150E that would normally be targeted to the plasma membrane. Still, in accordance with clinical data reported in heterozygous parents bearing the AQP2-D150E mutation, no NDI symptoms are found in these individuals. When AQP2-wt was coinjected with AQP2-G196D, a 50% loss of activity was found. This mutant form of AQP2 thus displays a typical dominant negative effect over AQP2-wt, a common condition found among COOH-terminal AQP2 mutants (9). This behavior is explained through heteromeric relations between AQP2-G196D and the wild-type protein, which are retained in cell compartments. As already mentioned, corresponding heterozygous parents with one AQP2-G196D allele were not identified with any specific NDI symptoms. This discrepancy between the actual phenotype reported in wt/G196D individuals and functionality assays determined in oocytes may originate from this particular experimental condition, which would favor G196D retention action on the wild-type protein, a condition that is not encountered in heterozygotes. It is also possible that these individuals are in fact mildly affected with NDI, although not adequately detected. Also, as mentioned above, overexpressing a mutant form of AQP2 may affect the normal expression level of the wild-type protein when expressed in lower amounts. Iolascon et al. (12) did not report functional data for the D150E and G215C mutations identified in a male patient who was compound heterozygote and presented severe NDI with hypernatremia, a urine osmolality of 80 mosmol/kgH2O, and no response to dDAVP. In their report, no signs or symptoms of NDI were reported in heterozygous parents. By contrast, the patient homozygous for the D150E reported here has a clinical phenotype recapitulating the functional studies observed following D150E expression in Xenopus oocytes. In our studies, coinjecting AQP2-D150E with AQP2-G196D (5 ng each) eliminated all AQP2-linked activity with a Pf value similar to control levels. Once again, AQP2-G196D may interfere with AQP2-D150E through heteromeric structures retained in ER, thus eliminating whatever small function it might otherwise have generated. This condition refers to the patient with compound mutations, the only one who presents acute NDI symptoms.
D150 is a conserved residue located in the second intracellular loop whereas G196, also conserved, is situated in the last extracellular loop (Fig. 9). Based on what is already known about the pathophysiology of NDI, it was expected that both of these mutations would be transmitted as recessive traits and that they would not be able to functionally interfere with APQ2-wt. In fact, the literature indicates that, up to now, only COOH-terminal mutations have the ability to oligomerize with the wild-type counterpart of AQP2 and are thus inherited as dominant traits (dominant negative behavior). Our results presented herein indicate that, whereas D150E typically behaves as a recessive trait, G196D, has the ability to impede AQP2-wt functionality, similar to what is found with dominant traits. These conclusions about the capacity to functionally interfere with AQP2-wt are also demonstrated in Fig. 8, where we show that AQP2-G196D can efficiently impede plasma membrane targeting of the wild-type protein whereas AQP2-D150E was without effect. In such a case, G196D would be the most NH2-terminal mutation reported so far to display such behavior. The fact that the G196D mutation is inherited as a recessive trait while displaying behavior typical of dominant trait in oocytes (dominant negative effect over AQP2-wt) can be explained by the weak translation efficacy of this mutation; in heterozygotes, the low level of AQP2-G196D protein forbids any notable effect on the normal expression and plasma membrane targeting of the wild-type counterpart.
Fig. 9.
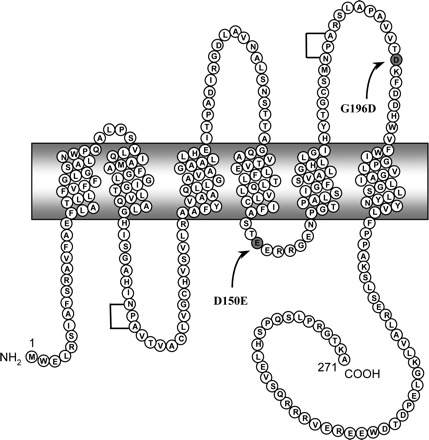
Schematic representation of AQP2 protein. Depiction of the AQP2 protein showing both intracellular D150E and extracellular G196D mutations. Brackets indicate conserved NPA motifs.
To our knowledge, this is the first functional evidence of a compound mutation combining both partial (D150E) and complete (G196D) NDI causing mutations. From a clinical point of view it is possible that homozygous patients for the AQP2-D150E mutation are poorly recognized since the symptoms and signs are mild to moderate and since some urinary osmolality increase is observed after dehydration or dDAVP. The exact interactions between D150E and other AQP2 mutants will have to be assessed with further experiments.
Conclusion
We have presented two new mutations for AQP2 that were analyzed by using Xenopus oocytes as expression system. The AQP2-D150E mutation is expressed at the plasma membrane in small proportions whereas AQP2-G196D is completely sequestered within intracellular stores. Still, only AQP2-G196D presents a dominant negative effect when coinjected with AQP2-wt or AQP2-D150E. The basic characteristics determined herein are in agreement with the phenotypes reported for family members. These new mutants may be useful in future studies concerning quaternary structure of AQP2 (using AQP2-G196D), protein processing, or rescue assays of misfolded protein (using AQP2-D150E).
GRANTS
This work was supported by the Canada Research Chair in the Genetics of Renal Disease to D. G. Bichet and by le Fonds de la Recherche en Santé du Québec (FRSQ) general funding program to the GÉPROM.
Acknowledgments
We thank Dr. Michael J. Coady and Yolaine Dodier for technical assistance with molecular biology.
REFERENCES
- 1.Agre P, Nielsen S. The aquaporin family of water channels in kidney. Nephrologie 17: 409–415, 1996 [PubMed] [Google Scholar]
- 2.Bichet DG. Nephrogenic diabetes insipidus. Am J Med 105: 431–442, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Bissonnette P, Noel J, Coady MJ, Lapointe JY. Functional expression of tagged human Na+-glucose cotransporter in Xenopus laevis oocytes. J Physiol 520: 359–371, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buck TM, Eledge J, Skach WR. Evidence for stabilization of aquaporin-2 folding mutants by N-linked glycosylation in endoplasmic reticulum. Am J Physiol Cell Physiol 287: C1292–C1299, 2004 [DOI] [PubMed] [Google Scholar]
- 5.De Mattia F, Savelkoul PJ, Kamsteeg EJ, Konings IB, van der Sluijs P, Mallmann R, Oksche A, Deen PM. Lack of arginine vasopressin-induced phosphorylation of aquaporin-2 mutant AQP2–R254L explains dominant nephrogenic diabetes insipidus. J Am Soc Nephrol 16: 2872–2880, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Deen PM, Marr N, Kamsteeg EJ, van Balkom BW. Nephrogenic diabetes insipidus. Curr Opin Nephrol Hypertens 9: 591–595, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Duquette PP, Bissonnette P, Lapointe JY. Local osmotic gradients drive the water flux associated with Na+/glucose cotransport. Proc Natl Acad Sci USA 98: 3796–3801, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frokiaer J, Marples D, Knepper MA, Nielsen S. Pathophysiology of aquaporin-2 in water balance disorders. Am J Med Sci 316: 291–299, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Fujiwara TM, Bichet DG. Molecular biology of hereditary diabetes insipidus. J Am Soc Nephrol 16: 2836–2846, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Goji K, Kuwahara M, Gu Y, Matsuo M, Marumo F, Sasaki S. Novel mutations in aquaporin-2 gene in female siblings with nephrogenic diabetes insipidus: evidence of disrupted water channel function. J Clin Endocrinol Metab 83: 3205–3209, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Hendriks G, Koudijs M, van Balkom BW, Oorschot V, Klumperman J, Deen PM, van der Sluijs P. Glycosylation is important for cell surface expression of the water channel aquaporin-2 but is not essential for tetramerization in the endoplasmic reticulum. J Biol Chem 279: 2975–2983, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Iolascon A, Aglio V, Tamma G, D'Apolito M, Addabbo F, Procino G, Simonetti MC, Montini G, Gesualdo L, Debler EW, Svelto M, Valenti G. Characterization of two novel missense mutations in the AQP2 gene causing nephrogenic diabetes insipidus. Nephron Physiol 105: p33–P41, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Kamsteeg EJ, Deen PM. Importance of aquaporin-2 expression levels in genotype -phenotype studies in nephrogenic diabetes insipidus. Am J Physiol Renal Physiol 279: F778–F784, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Kamsteeg EJ, Deen PM, van Os CH. Defective processing and trafficking of water channels in nephrogenic diabetes insipidus. Exp Nephrol 8: 326–331, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Kamsteeg EJ, Heijnen I, van Os CH, Deen PM. The subcellular localization of an aquaporin-2 tetramer depends on the stoichiometry of phosphorylated and nonphosphorylated monomers. J Cell Biol 151: 919–930, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamsteeg EJ, Wormhoudt TA, Rijss JP, van Os CH, Deen PM. An impaired routing of wild-type aquaporin-2 after tetramerization with an aquaporin-2 mutant explains dominant nephrogenic diabetes insipidus. EMBO J 18: 2394–2400, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuwahara M, Iwai K, Ooeda T, Igarashi T, Ogawa E, Katsushima Y, Shinbo I, Uchida S, Terada Y, Arthus MF, Lonergan M, Fujiwara TM, Bichet DG, Marumo F, Sasaki S. Three families with autosomal dominant nephrogenic diabetes insipidus caused by aquaporin-2 mutations in the C-terminus. Am J Hum Genet 69: 738–748, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leduc-Nadeau A, Lahjouji K, Bissonnette P, Lapointe JY, Bichet DG. Elaboration of a novel technique for the purification of plasma membranes from Xenopus laevis oocytes. Am J Physiol Cell Physiol 292: C1132–C1136, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Lin SH, Bichet DG, Sasaki S, Kuwahara M, Arthus MF, Lonergan M, Lin YF. Two novel aquaporin-2 mutations responsible for congenital nephrogenic diabetes insipidus in Chinese families. J Clin Endocrinol Metab 87: 2694–2700, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Marr N, Bichet DG, Hoefs S, Savelkoul PJ, Konings IB, De Mattia F, Graat MP, Arthus MF, Lonergan M, Fujiwara TM, Knoers NV, Landau D, Balfe WJ, Oksche A, Rosenthal W, Muller D, Van Os CH, Deen PM. Cell-biologic and functional analyses of five new Aquaporin-2 missense mutations that cause recessive nephrogenic diabetes insipidus. J Am Soc Nephrol 13: 2267–2277, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Mulders SM, Bichet DG, Rijss JP, Kamsteeg EJ, Arthus MF, Lonergan M, Fujiwara M, Morgan K, Leijendekker R, van der Sluijs P, van Os CH, Deen PM. An aquaporin-2 water channel mutant which causes autosomal dominant nephrogenic diabetes insipidus is retained in the Golgi complex. J Clin Invest 102: 57–66, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nielsen S, Frokiaer J, Marples D, Kwon TH, Agre P, Knepper MA. Aquaporins in the kidney: from molecules to medicine. Physiol Rev 82: 205–244, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Nielsen S, Kwon TH, Christensen BM, Promeneur D, Frokiaer J, Marples D. Physiology and pathophysiology of renal aquaporins. J Am Soc Nephrol 10: 647–663, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Tamarappoo BK, Verkman AS. Defective aquaporin-2 trafficking in nephrogenic diabetes insipidus and correction by chemical chaperones. J Clin Invest 101: 2257–2267, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Umenishi F, Narikiyo T, Schrier RW. Effect on stability, degradation, expression, and targeting of aquaporin-2 water channel by hyperosmolality in renal epithelial cells. Biochem Biophys Res Commun 338: 1593–1599, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Van Balkom BW, van Raak M, Breton S, Pastor-Soler N, Bouley R, van der Sluijs P, Brown D, Deen PM. Hypertonicity is involved in redirecting the aquaporin-2 water channel into the basolateral, instead of the apical, plasma membrane of renal epithelial cells. J Biol Chem 278: 1101–1107, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Wagner CA, Friedrich B, Setiawan I, Lang F, Broer S. The use of Xenopus laevis oocytes for the functional characterization of heterologously expressed membrane proteins. Cell Physiol Biochem 10: 1–12, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Zeuthen T, Meinild AK, Klaerke DA, Loo DD, Wright EM, Belhage B, Litman T. Water transport by the Na+/glucose cotransporter under isotonic conditions. Biol Cell 89: 307–312, 1997 [DOI] [PubMed] [Google Scholar]


