Abstract
In this review, we examine the current status of Staphylococcus aureus vaccine development and the prospects for future vaccines. Examination of the clinical trials to date show that murine models have not predicted success in humans for active or passive immunization. A key factor in the failure to develop a vaccine to prevent S. aureus infections comes from our relatively limited knowledge of human protective immunity. More recent reports on the elements of the human immune response to staphylococci are analysed. In addition, there is some controversy concerning the role of antibodies for protecting humans, and these data are reviewed. From a review of the current state of understanding of staphylococcal immunity, a working model is proposed. Some new work has provided some initial candidate biomarker(s) to predict outcomes of invasive infections and to predict the efficacy of antibiotic therapy in humans. We conclude by looking to the future through the perspective of lessons gleaned from the clinical vaccine trials.
Introduction
Staphyloccus aureus is one of the most frequent causes of serious infections in humans. It strikes people of all ages, but it is most severe in the young, old, and patients with immunodeficienes such as neutropenia or HIV infection. Nevertheless, it can also strike people that are young and healthy with devasting effects [1]. Invasive disease is associated with a 20% and higher mortality [2-5] due to a combination of antibiotic resistance and multiple virulence factors (Table 1). Bacterial vaccines have been highly effective in reducing mortality from H. influenzae, S. pneumoniae, and N. meningiditis [6,7], but an effective staphyloccocal vaccine has not been made [8.9]. This is not due to a lack of clinical trials (see Table 2). To date, no human clinical trial has succeeded. The question is why do clinical trials fail when the vaccine candidates have performed well in animal models in general, and murine sepsis models in particular?
Table 1.
Analysis of publications concerning a potential association of hypogammaglobulinemia and increased incidence of S. aureus infections
| Immune deficiency in the paper | Higher incidence of S. aureus infections | Comment | Related to hypo- gammaglobu- linemia | Reference |
|---|---|---|---|---|
| Rheumatoid arthritis | Yes | Rheumatoid factor blocks effective opsonization [93] | No | Lee A [85] |
| Reduced T-cell activity | Yes | T-cell immunity is associated with S. aureus infections | No | Morrison [86] |
| Blocked receptors on PMNs | Yes | PMNs are needed for killing S. aureus | No | Foster [60] |
| Reduced Ig | No | Reports on S. albus and streptococci | Mickenberg [15] | |
| Reduced Ig | No | The number of S. aureus infections were lower than expected | Trakultivakorn [16] |
| Pagimaximab (Weisman) [91] | humanized mouse chimeric monoclonal antibody against lipoteichoic acid | Phase II | Randomized, double-blind, placebo-controlled dose ranging study for prevention of staphylococcal infection in patients with birth weight between 700- 1300 g (n=88) | Definite staphylococcal sepsis occurred in 0% (90mg/kg), 20% (60mg/kg), and 13% (placebo) (P=0.11). Findings not confirmed in Phase III trial |
| Active Immunization | ||||
| StaphVax (Shinefield) [58] | Bivalent vaccine of CP 5 & 8 conjugated individually to recombinant exoprotein A | Phase III | Randomized, double-blind, placebo controlled trial of StaphVax in prevention of S. aureus bacteremia in hemodialysis dependent adults (n=1804) | Efficacy in reduction of S. aureus bacteremia at 54 weeks non-significant (p=0.23); post-hoc efficacy estimate at 40 weeks: 57% (p=0.02) |
| V710 (Fowler) [2] | IsdB | Phase III | Randomized, double-blind, placebo controlled, event trial of efficacy of V710 to prevent major S..aureus infection in adults undergoing median sternotomy (n=8031) | Study stopped prematurely by data monitoring committee. No significant efficacy. Vaccine recipients who developed S. aureus infection were 5 times more likely to die than control recipients who developed S. aureus infection (23.0 vs 4.2 per 100 person-years |
Table 2.
Phase II and III Clinical Trials of Active and Passive Immunization for Staphylococcus aureus and Coagulase Negative Staphylococci.
| Compound | Product | Phase | Study design | Results |
|---|---|---|---|---|
| Passive immunization | ||||
| Treatment | ||||
| Aurexis Tefibazumab (Weems) [87] | Humanized monoclonal anti-clumping factor A antibodies | Phase II | Randomized, Double-blind, Placebo-controlled trial of standard treatment plus either Aurexis or placebo (n=63) | No differences in adverse events or rate of death, relapse, or complications |
| Altastaph (Rupp) [88] | Pooled human anti - capsular polysaccha- ride (CP) types 5 and 8 antibodies | Phase II | Randomized, Double-blind, Placebo-controlled trial of patients standard treatment plus Altastaph or placebo for S. aureus bacteremia in adults (n=40) | No significant mortality difference; Shorter length of stay in Altastaph vs placebo (9d vs. 14d; p=0.03) |
| Aurograb (not published in peer- reviewed journal) | Single-chain antibody variable fragment against ABC transporter component GrfA | Phase II | Unpublished by sponsor. | Addition of Aurograb to standard therapy for life- threatening staphylococcal infections failed to show efficacy |
| Prevention | ||||
| Altastaph (Benjamin) [89] | Phase II | Randomized, Double-blind, Placebo-controlled trial of Altastaph or placebo for prevention of nosocomial S. aureus infections in very low birth weight babies (n=206) | High levels of antibodies No difference in rate of invasive S. aureus infection | |
| Veronate(DeJonge) [90] | Pooled human IgG to ClfA (S. aureus) and SdrG (S. epidermidis) | Phase III | Double-blind, placebo-controlled trial of INH-21 vs placebo for prevention of staphylococcal late-onset sepsis in infants with birth weights 500 to 1250g (n=1983) | No difference in staphylococcal late-onset sepsis (5% INH-21 vs 6% placebo; p=0.34) |
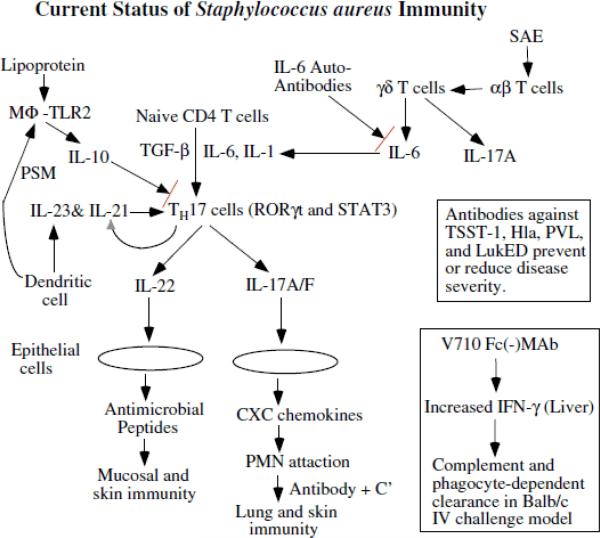
There may be several reasons behind the poor outcomes in the human trials. The most logical conclusion is that the protective immune response in humans is different from that of most animals, especially mice, and including non-human primates. While there is a good undertanding of the protective immunity in mice, there is limited understanding of the human immune response [8-11]. In addition, S. aureus is part of the normal flora of humans, which suggests that the organism has evolved to thwart most human immune responses [12-14]. Moreover, we also know that opsonic antibodies have been the basis for protection in mice and that the biomarker for efficacy of these antibodies has been activity in the neutrophil-based in vitro opsonophagocytic assay. However, while opsonophagocytic activity has proven to be a predictive biomarker for murine models of infection, this activity has not translated to protection of humans as all of the failed clinical trials used this biomarker [8-11]. Because we have no successful clinical trials of S. auerus vaccine based upon opsonphagocytic activity in vitro, we cannot define whether or not human serum already has sufficient opsonophagocytic activity, nor can we confirm that increasing the amount of activity will not promote protection. With this in mind, an alternative hypothetical model based upon the known immune responses in humans is shown in Figure 1. The evidence for this model will be discussed below and may serve as a starting point for defining the human immune response in more detail so as to provide a basis for identifying biomarkers for use in future clinical trials.
Figure.
Abbreviations MΦ = macrophage; PSM = phenol soluble modulins; IL = interleukin; TGF-β = transforming growth factor-β; Th17 = T helper 17 cells; MAb = monoclonal antibody; V710 = IsdB antigen used in Merck Vaccine trail; C’ = complement; SAE = Staphylococcus aureus enterotoxin E; RORγτ = retinoid-related orphan receptor gamma; STAT3 = signal transducer and activator of transcription 3; Hla = α-toxin; TSST-1 = toxic shock syndrome toxin –1; Luk = leukocidin; PVL = Panton-Valentine Leukocidin (two component toxin made up of LukS-PV and LukF-PV); IFN-γ = interferon gamma
An analysis of publications concerning a potential association of hypogammaglobulinaemia and increased Staphylococcus aureus infections is provided (Table 1) by returning to the original reports. In one recent paper [94], references to articles on hypogammaglobulinemia were mentioned, attributing it to an increase in S. aureus infections [85,86], but the primary reports suggest otherwise. Another two papers [15,16] were cited in [17], but again, a link between hypogammaglobulinemia and S. aureus infections was not established.
Another two papers [15,16] were quoted by Anderson et al. [17] to support the concept that defects in humoral immunity resulted in more S. aureus infections. However, analysis of these two citations with acquired hypogammaglobulinemia showed that the patient sera were ineffective for S. albus (probably S. epidermidis), but the sera were fully opsonic in two of the three patients for S. aureus [15]. More importantly, none of the patients were noted to have S. aureus infections. Instead, patients developed what were in all likelihood pneumococcal infections, including recurrent pneumonia, sinusitis, and otitis. The second paper referenced by Anderson et al. was by Trakultivakorn et al. [16]. This study also failed to fully support the idea that low levels of gamma globulin was the causative factor for an increased incidence of S. aureus infections in hypogammaglobulinemic patients. Only one of the six patients had culture-confirmed S. aureus; however, the source was listed simply as being isolated from the “ear”, but details of the collection method were not given; hence, it easily could have been a skin contaminant. The paper by Trakultivakorn et al. also summarized data from some earlier papers about patients with X-linked aggamaglobulinemia. One of these summarized papers was by Lederman who found only two patients of 96 patients reported with S. aureus. Another 46 patients are reviewed in the Trakultivakron paper with no cases of S. aureus. Thus, of the total number of patients in the Trakultivakorn paper, only three S. aureus infections occurred in 148 children with invasive infections. When one considers that all of the patients included in the Trakultivakron paper were infected, this is very low incidence of S. aureus infections for children. Therefore, this paper actually supports the concept that gammaglobulin is not an important factor in preventing S. aureus infections. In summary, scant evidence supports the hypothesis that defects in opsonins contribute to increased numbers of S. aureus infections as has been previously note [8,9]. Finally, an analysis of antibody levels in humans showed no relationship between incidence of infection and antibody levels [11].
Are anti-idiotype Abs competing with natural Abs [18]? The investigators mixed human and murine antibodies directed against different types of carbohydrates found on the S. aureus surface, and these antibodies interfered with one another for opsonization. This is an interesting hypothesis, but the studies were performed in mice, and to date there are no clinical data to support this idea. Another question has been raised: Do anti-PNAG antibodies interfere with antibodies against capsular antigens, thereby causing vaacine failure [19]? Again, this is a novel question and some of the sera tested are from humans convalescing from bacteremia, but the data on interference are in vitro plus mouse model work, making this a long extrapolation to human disease.
In contrast, humans with defects in neutrophil and cell-mediated immunity do show increased infections with S. aureus [20,21]. Patients with Job's syndrome have a marked increase in S. aureus infections [22-24]. Patients with defects in cell-mediated immunity (CMI) also have a greater incidence of S. aureus infections. This includes patients receiving prednisone, having HIV infection, and defects in Th17/IL-17 [25,26]. Of interest, while the whole cell pertussis vaccine protected via antibodies, the subunit pertussis vaccine protects through Th17/IL-17 [27]. Further support for IL-17 in human infection comes from the observation that IL-6 auto-antibodies reduce IL-17 and increase incidence of S. aureus infections [28,29].
Animal model data support the idea that Th17/IL-17 are involved in S. aureus immunity. Transfer of Th17 cells, but not antibodies, protect mice from S. aureus infections [30,31]. IsdB protects via Th17 [32], and IL-17A is critical for ClfA-induced protection in mice [33]. Moreover, Th17 and IL-17 seem to be particularly important for skin and lung protection [23,24]. Of note, Th17 and IL-17 also drive antimicrobial peptide development in skin and mucosal cells [24], which may add to innate protection from S. aureus invading in the skin and respiratory tree. S. aureus antigen activation of dendritic cells releases IL-23 which drives Th17 activation [34]. In addition, TLR2 (MyD88/IRAK4) recognizes LTA and other lipoproteins and is required for S. aureus immunity [Chin 16; Fournier 29]. In contrast, circulating peptidoglycan increases IL-10 and attenuates Th1/Th17 cells [37,38]. Finally, Phenol Soluble Modulins (PSMs) reduce TNF, IL-12, and IL-6, but they increase IL-10 [39].
What does an antibody response to a S. aureus antigen tell us? It is a marker for an immune response, but it does not assure protection. A recent paper concerning antibody responses to S. aureus immunization overlooked this concept [40]. A good example robust antibody response failing to provide protection comes from work on the V710 vaccine (IsdB) [41,42]; whereas, the protective immune response occurs via Th17-mediated immunity [32]. Similar results were shown for other potential vaccine antigens aimed at S. aureus, where the antibodies produced against ClfA (collage binding protein) [33] and Als3p [30,43] were not protective, but Th17/IL-17 responses proved to be protective. It should be noted that some protection by antibodies did occur in the liver when V710 Fc(-) monoclonal antibody (MAb) were given to mice. These MAb stimulated IFN-γ that activates phagocytes for C’-mediated uptake/killing in murine models [44]. Of interest, Hla has been found to induce a Th17 response that contributes to lung damage in S. aureus pneumonia and that type I interferon provides an innate protective response by mitigating host cellular ATP loss [45]. Hence, neutralizing Hla with antibody reduces the direct damage to host from Hla and reducing the proinflammatory effects of Hla.
While we lack data for antibodies being determinative in preventing S. aureus infections, there are data suggesting that anti-toxin antibodies may reduce disease severity. Patients with antibodies against toxic shock toxin are less likely to develop toxic shock syndrome, but this has not been commercially developed because of the small number of cases limits the market [46]. Some clinical trials are underway employing anti-recombinant Hla and anti-recombinant LukS-PV [NCT01011335]. These trails are supported by the following animal model data: AT62-IgG (rabbit anti-Hla IgG) protected mice from pneumonia and intraperitoneal challenges [47]. A fusion of Hla and C. perfingens α-toxin (r-αCS) protected RBC's from lysis [48]. Anti-Hla antibodies reduce recurrent skin infections in children, but natural infection does not provoke durable immunity [49]. Similarly, anti-Hla antibodies protect rabbit and Jurkat T cells from lysis as does the ADAM10 (the mammalian receptor for Hla) inhibitor, R66 [50]. While antibodies to Hla and PVL are short-lived, anti-Hla antibody levels did correlate with protection against subsequent S. aureus infections in children [49]. Of note, cutaneous infections do not provide protective levels of antibody to Hla and PVL to prevent recurrences or invasive infection [49]. Leukotoxin ED binds CCR5 receptor on myeloid cells and T-lymphocytes. The HIV drug, maraviroc (CCR5 inhibitor), protects mice from S. aureus challenge and from LukED [51]. Finally, other potential toxoids may be suitable for vaccine based upon the observations that low anti-exotoxin antibody levels correlate more severe sepsis when patients are infected with enterotoxin-producing S. aureus strains [52], and higher anti-PVL antibody levels correlate with less severe pneumonia [53]. That enterotoxiods might need to be given as vaccines is suggested by the fact that S. aureus bacteremia in intravenous drug users failed to produce antibodies against enterotoxin superantigens [www.clinicaltrials.gov, NCT00548002], whereas non-eneterotoxin superantigens were common and increased following S. aureus bacteremia [54]. Thus, there is both human and animal data suggesting that anti-staphylococcal toxin antibodies may have efficacy in reducing disease severity.
Insights from Recent Clinical Studies of the Immune Response to S. aureus
In addition to the human clinical data on anti-toxin antibodies (above), several reports from clinical trials have revealed information about other aspects of the human immunity to S. aureus. Recently, Rose et al. provided a seminal paper on the human immune response during S. aureus bacteremia [55,56], which comfirms and extends our limited knowledge about the human immune response. Specifically, high IL-10 predicts high mortality; low IL-1β correlates with prolonged bacteremia. Previous studies had also suggested that a high IL-10/TNF-α ratio predicted higher mortality in febrile patients [57]. Of interest, circulating peptidoglycan increases IL-10 and attenuates Th1/Th17 cells, which may contribute to the mortality seen in high-level bacteremia [37,38]. Taken together, these data suggest that IL-10, TNF-α, and IL-1β levels may serve as biomarkers for disease severity, which could also serve as biomarkers for vaccine efficacy.
A Working Model of Immunity to S. aureus
Based upon the information provided above, a working model of S. auerus immunity can be developed (see Figure 1). Whenever possible, it based upon information known about human immunology and human resposnes to S. auerus infections. Th17 is placed at the center of the model as this organizes much of the current data. Activation of Th17 cells results in enhanced neutrophil activity as well as increased antimicrobial peptide activty on the mucosal and skin barriers. This scheme brings together the known increased incidence of S. aureus infections in patients with neutrophil and cell-mediated immune defects. The model also recognizes the role of IL-6 in activating Th17 cells and IL-10 in decreasing Th17 cell activity. Moreover, the immunomodulating effects of staphylococcal lipoproteins and phenol soluble modulins can be seen as stimulating the release of IL-10, which reduce Th17 cell activation. Moreover, the clinical findings that IL-6 autoantibodies increase the incidence of S. aureus infections may also be seen to reduce Th17 cell activation. Toxins such as SAE can activate αβ T cells, which then cause γδ T cells to release IL-6. Finally, a role for antibody neutralization of toxins, such as TSST-1, Hla, PVL, and LukED, is shown as distinct activity that is outside an opsonin role [52]. The fact that antibodies to IsdB did protect mice is also shown in a separate box because it protected via activation of IFN-γ to enhance clearance of organisms, but it is not known if such a mechanism is active in humans.
Conclusions Gleaned from Clinical Vaccine Trails
Both active and passive immunization has been attempted. For passive immunization, antibodies against SdrG, ClfA, LTA, types 5 and 8 capsules, and GrfA have been tested. Active immunization against IsdB, SEB, ClfA, types 5 and 8 capsules, LTA, and GrfA have been examined. All of the trials that have completed phases 1 and 2 have been based upon increasing opsonic antibody to surface antigens. There is a suspended phase 1 trial based upon anti-SEB (staphylococcal enteroxin B) trial from Bio-Therapeutics [NCT00974935] and the phase 1 immunogenicity trail by GlaxoSmithKline that includes PVL. There are some on-going clinical trials using the following antigens: rAls3p-N, fLukS-PV/rAT, Eap, GST-Can, His-Clf, SdrD, SdrE, IsdA, IsdB, and FhuD2. Most of the newer attempts are using multiple antigens.
As shown in Table 2, all of the completed phase 2 and phase 3 trials with both passive immunization and active immunization to date have failed to meet the pre-trial endpoints. With passive immunization, two strategies have been attempted: treatment of active staphylococcal infections as an adjunct in addition to standard treatment; and prevention of staphylococcal infections in at risk patient populations. In the setting of active S. aureus infection, three compounds have been evaluated. A Phase II trial to evaluate a monoclonal antibody against ClfA (tefibazumab/Aurexis; Inhibitex) for use as an adjunct to standard therapy for patients with S. aureus bacteremia failed to demonstrate any significant differences in a Composite Clinical End point of death, relapse, or development of a S. aureus-related complication not present at baseline. Hypersensitivity reactions developed in one in 30 patients. A Phase II trial of a polyclonal immune globulin of anti type 5 and anti type 8 antibodies (Altastaph; Nabi) also failed to show a significant difference in clinical outcomes among patients with complicated S. aureus bacteremia. Interestingly, length of stay among the Altastaph recipients was significantly shorter than placebo (9d vs. 14d; p = 0.03). Unfortunately, the sponsor has failed to publish the results of the trial involving Aurograb in a peer-reviewed format. Three compounds employing passive immunization have also been evaluated, all in neonates, for prevention of staphylococcal infections (both S. aureus and Staphylococcus epidemidis). Although pagimaximab (Biosynexus), humanized mouse chimeric monoclonal antibody against lipoteichoic acid, exhibited a promising trend in Phase II, these results were not substantiated in the registrational trial that followed.
Two registrational trials to date have evaluated vaccine candidates in active immunization strategies. Both trials have involved adult populations with high rates of S. aureus infection. While this strategy afforded important advantages in terms of sample size reduction due to the relatively high event rate of S. aureus infection, each patient population carried significant unavoidable complications to trial design related to their comorbid conditions, such as high rates of adverse events, relative immunocompromised state, etc. Nabi conducted a Phase III registrational trial of Staphvax, a bivalent vaccine of capsular protein 5 & 8, in 1804 hemodialysis patients with a primary fistula or synthetic graft vascular access. At the prespecified endpoint of 54 weeks following vaccination, the study failed to demonstrate protective efficacy in reduced rates of S. aureus bacteremia. In post hoc analyses, vaccination exhibited a statistically significant protective effect against S. aureus bacteremia as long as 40 weeks following vaccination (efficacy 57%; p = 0.02) [58]. Based upon this finding, Nabi conducted a second registrational trial, again in hemodialysis patients, in which the sample size was approximately doubled and the primary endpoint was moved to 6 months after vaccination (vs. 54 weeks with the Shinefield study). Inexplicably, this second StaphVax trial (never published in peer-reviewed literature) also failed to show efficacy. Merck tested V710, a vaccine targeting IsdB, in patients undergoing median sternotomy. The trial employed an event-driven design. After enrollment of ~ 8000 patients, the Data Monitoring Committee first held, then terminated, the trial based upon failure to demonstrate efficacy and a higher rate of multiorgan system failure–related deaths in the V710 recipients. Post hoc analysis revealed that vaccine recipients who developed S. aureus infection were 5 times more likely to die than control recipients who developed S. aureus infection (23.0 vs 4.2 per 100 person-years.
What have we learned? First, protection demonstrated in animal models, especially murine models has not translated into protection of humans. Second, staphylococcal anti-toxins have not been studied, yet anti-toxins approaches have worked very well in other gram-positive infections. Third, the use of multiple antigens has also not been studied, which may well be necessary given the very large number of virulence factors expressed by S. aureus. Fourth, prevention of S. aureus infection is a very high standard that may not be achievable in an organism that is part of the normal flora and that has learned to evade so many of the human host defenses. Aiming at reduced severity, and decreased cost/length of stay may be more achievable endpoints, although at least one pilot study to determine the effect of StaphVax on reduction of nasal carriage demonstrated no effect [59]. Fifth, V710 showed excess numbers of MRSA in vaccinated patients [2]. Some MRSA20CCmec types strains are more virulent [61-65]. However, while the mechanisms for this association are unknown, one could speculate that this may have played a role in the larger number of vaccinated patients showing multi-organ failure. In the end, the discovery that V710 recipients who developed S. aureus infection were five times more likely to die than controls that developed S. aureus infection suggests the potential for harm due to an “immune-priming” type process. While purely speculative, future studies will need to explicitly demonstrate the absence of such effects in pre-clinical and early clinical studies.
Future for a S. aureus Vaccine
We should not give up the hope of developing a staphylococcal vaccine. Carriers have more infections, but the infections are less severe [10,11], which strongly suggests that some form of immunity has developed during prolonged colonization. This provides another approach to vaccine development, specifically aiming at reducing severity of infections rather than prevention. All of the trials have aimed at prevention of infection, which is a very high standard for an organism that has evolved over milennia to survive with the human host. Possible directions for future follow.
Anti-toxoid vaccines have not been fully explored. Neutralizing antibodies have been shown to be important in protection against toxic shock syndrome [46], and there are data suggesting that anti-α-hemolysin (Hla) may reduce disease severity [49,50] and inhibitors of the Hla receptor also are protective [50]. Data surrounding anti-Panton-Valentine Leukocidin (PVL) toxins are more controversial, but they may have a role in preventing severe lung damage in post-influenza pneumonia [53]. Finally, naturally occuring antibodies against toxic shock syndrome toxin (TSST) are recognized to prevent TSS [66]. Other superantigens, e.g., enterotoxins, have also been recently evaluated as vaccine candidates [46]. Thus, the addition of anti-toxin neutralizing antibodies may prove valuable in a human vaccine to reduce disease severity, even though opsonic antibodies have not succeeded.
This brings us to another concept, i.e., multiple vaccines. Currently, multiple vaccines are being developed for E. coli, which is another pathogen that is part of the normal flora. Anti-fimbrial vaccines to prevent urinary tract infections or anti-Shiga toxoid to prevent hemolytic uremic syndrome or anti-adhesive antibodies for childhood diarrrhea [67-69]. Hence, another vaccine approach for S. aureus infections would be to design vaccines targeted to prevent foreign body infections, subcutaneous abscesses, endocarditis and hemodialysis infections, ICU pneumonia, etc. Naturally, more limited spectrum vaccines would be more costly.
While anti-toxin antibodies appear to be a good biomarker for the toxin-mediated S. aureus disease, the difficult questions arise because we still do not have a biomarker that predicts protection in humans. We do not know whether a particular surface antigen will provide protection in humans due to the lack of a biomarker. Of course, the lack of a biomarker(s) that predicts efficacy stems directly from our limited knowledge of the protective immune response in humans [8,9].
Numerous investigators have suggested that a multiple-antigen vaccine would be more effective [17,70-73], but a lack of biomarkers defining human protective immunity keep these proposals in the logical, but strictly hypothetical arena.
There are some data to suggest that improving non-antibody responses may be the key to a new and effective vaccine. Some data suggest that Th17 immune response is important. Thus, will Th17 adjuvants help? The duration of Th17 immunity in humans is unknown, but it is short in mice [74]. Can Th17 response be prolonged/enhanced by unique adjuvants that stimulate CD27/TRAFS? Phytol-based terpenoids, phytanol (PHIS-01) & phytanuyl Cl (PCl), have been shown to improve Th17/IL-17 responses [28,74,75]. Th17 immunity has been implicated in mucousal immunity against Clostridium difficile [76]; hence, one might speculate that it also might be valuable in decreasing mucosal colonization, which might preventing vaginal colonization and TSS or reduce nasal carriage [92]. Care must be exercised in developing a robust Th17 response as this arm of the immune system has been implicated in autoimmune diseases [77] and Th17 was induced by the IsdB (V710) vaccine [32], which was associated with an increase in multi-organ failure during S. aureus infections [2].
Other novel approaches include using a mutated protein A Ig binding domains as immunogen, which reduced virulence, improved immunogenicity of other Ag's, and enhanced opsonophagocytic activity in a murine model [78]. We know from the elegant work from the van Strijp lab that S. aureus produces a vast array of products that cripple the host immune system [13,14,79]. Thus, a possible approach that would complement many of the previous vaccine approaches would be to target SCINs, CHIPS, SSL's, etc. to potentiate PMN activity [79,80].
In the final analysis, work on the human immune response and biomarkers has come from Rose's lab [55,56]. Initial low levels of IL-1β[55,56] and high levels of TNF-α [81] were found in patients with S. aureus prolonged bacteremia (> 4 days) as compared to those that rapidly cleared their organisms. Of note, when the isolates from patients with prolonged bacteremia were tested in a whole blood killing assay, lower levels of IL-1β were produced in vitro when compared to rapidly cleared isolates [81]. In addition, elevated levels of the anti-inflammatory cytokine, IL10, were found in patients with higher levels of bacteremia and higher mortality [55,56]. These findings are similar to those reported for febrile patients wherein high IL-10 and low TNF-α correlated with increased mortality with multiple species of organisms [57]. Thus, these biomarkers predict mortality, clearance of bacteremia, and reflect the virulence of the particular isolate.
The importance of these seminal studies by Rose group should not be underestimated as they are predictive of outcome in human S. aureus bacteremia, and they are mimicked by cytokine responses in human neutrophils, thereby allowing in vitro study of future antigens. It should also be noted that these biomarkers also responded to successful antibiotic therapy, which also attests to their value, including their use in clinical trials where both vaccination and antibiotic therapy will inevitably be used together. These observations are included in the hypothetical model, which should provide testable hypotheses for confirming these biomarkers in clinical trials. Clearly, being able to factor in both the effectiveness of antimicrobial therapy and the virulence of the strain by using selected biomarkers will help to dissect out these host and organism variables from vaccine efficacy.
Finally, during our quest for a S. aureus vaccine, we should not overlook the immediately available solution of decolonization to prevent nosocomial S. aureus infections [49,83,84,95] in addition to excellent infection control practices. Mupirocin has been used, but resistance for disease reduction in surgical patients, but resistance is becoming a problem. Currently, a novel agent XF-73 that is extremely rapidly bactericidal is in phase 2 clinical trials for nasal decolonization (sponsored by NIH and NIAID) [Clinical Trials.gov NCT01592214].
Acknowledgements
This work was supported by the following grants from National Institutes of Health: 2R01-AI068804 and UM1-AI104681. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Transparency Declaration
VGF served as Chair of the V710 Scientific Advisory Committee (Merck), has received grant support from Cerexa, Pfizer, Advanced Liquid Logic and MedImmune, has grants under submission to Cubist, has been a paid consultant for Merck, Astellas, Affinium, Theravance, Cubist, Cerexa, Durata, Pfizer, NovaDigm, Novartis, Medicines Company, Biosynexus, MedImmune and Inimex, and has received honoraria from Merck, Astellas, Cubist, Pfizer, Theravance and Novartis. RAP has not declared any conflicts of interest.
Contributor Information
Vance G. Fowler, Jr., Division of Infectious Diseases Duke University Medical Center Durham, NC 27710.
Richard A. Proctor, University of Wisconsin School of Medicine and Public Health Madison, WI.
References
- 1.Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 2.Fowler VG, Allen KB, Moreira ED, et al. Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery: a randomized trial. JAMA. 2013;309:1368–1378. doi: 10.1001/jama.2013.3010. [DOI] [PubMed] [Google Scholar]
- 3.Koch K, Nørgaard M, Schønheyder HC, et al. Effect of socioeconomic status on mortality after bacteremia in working-age patients. A Danish population-based cohort study. PLoS One. 2013;8:e70082. doi: 10.1371/journal.pone.0070082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jung WJ, Kang YA, Park MS, et al. Prediction of methicillin-resistant Staphylococcus aureus in patients with non-nosocomial pneumonia. BMC Infect Dis. 2013;13:370. doi: 10.1186/1471-2334-13-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Su CH, Chang SC, Yan JJ, Tseng SH, Chien LJ, Fang CT. Excess mortality and long-term disability from healthcare-associated Staphylococcus aureus infections: a Population-Based Matched Cohort Study. PLoS One. 2013;8:e71055. doi: 10.1371/journal.pone.0071055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Payne AB, Link-Gelles R, Azonobi I, et al. Invasive pneumococcal disease among children with and without sickle cell disease in the United States, 1998–2009. Pediatr Infect Dis J. 2013;32:1308–1312. doi: 10.1097/INF.0b013e3182a11808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Snaebjarnard ottir K, Erlendsd ottir H, Reynisson IK, et al. Bacterial meningitis in children in Iceland, 1975–2010: a nationwide epidemiological study. Scand J Infect Dis. 2013;45:819–824. doi: 10.3109/00365548.2013.817680. [DOI] [PubMed] [Google Scholar]
- 8.Proctor RA. Is there a future for a Staphylococcus aureus vaccine? Vaccine. 2012;30:2921–2927. doi: 10.1016/j.vaccine.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Proctor RA. Challenges for a universal Staphylococcus aureus vaccine. Clin Infect Dis. 2012;54:1179–1186. doi: 10.1093/cid/cis033. [DOI] [PubMed] [Google Scholar]
- 10.Verkaik NJ, de Vogel CP, Boelens HA, et al. Anti-staphylococcal humoral immune response in persistent nasal carriers and noncarriers of Staphylococcus aureus. J Infect Dis. 2009;199:625–932. doi: 10.1086/596743. [DOI] [PubMed] [Google Scholar]
- 11.Verkaik NJ, van Wamel WJ, van Belkum A. Immunotherapeutic approaches against Staphylococcus aureus. Immunotherapy. 2011;3:1063–1073. doi: 10.2217/imt.11.84. [DOI] [PubMed] [Google Scholar]
- 12.van Belkum A, Melles DC, Nouwen J, et al. Co-evolutionary aspects of human colonisation and infection by Staphylococcus aureus. Infect Genet Evol. 2009;9:32–47. doi: 10.1016/j.meegid.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Bestebroer J, De Haas CJ, Van Strijp JA. How microorganisms avoid phagocyte attraction. FEMS Microbiol Rev. 2010;34:395–414. doi: 10.1111/j.1574-6976.2009.00202.x. [DOI] [PubMed] [Google Scholar]
- 14.Laarman A, Milder F, van Strijp J, Rooijakkers S. Complement inhibition by gram-positive pathogens: molecular mechanisms and therapeutic implications. J Mol Med (Berl) 2010;88:115–120. doi: 10.1007/s00109-009-0572-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mickenberg ID, Root RK, Wolff SM. Leukocytic function in hypogammaglobulinemia. J Clin Invest. 1970;49:1528–1538. doi: 10.1172/JCI106370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trakultivakorn M, Ochs HD. X-linked agammaglobulinemia in northern Thailand. Asian Pac J Allergy Immunol. 2006;24:57–63. [PubMed] [Google Scholar]
- 17.Anderson AS, Miller AA, Donald RG, et al. Development of a multicomponent Staphylococcus aureus vaccine designed to counter multiple bacterial virulence factors. Hum Vaccin Immunother. 2012;8:1585–1594. doi: 10.4161/hv.21872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skurnik D, Kropec A, Roux D, Theilacker C, Huebner J, Pier GB. Natural antibodies in normal human serum inhibit Staphylococcus aureus capsular polysaccharide vaccine efficacy. Clin Infect Dis. 2012;55:1188–1197. doi: 10.1093/cid/cis624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pier GB. Will there ever be a universal Staphylococcus aureus vaccine? Hum Vaccin Immunother. 2013;9:1865–1876. doi: 10.4161/hv.25182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quilty S, Kwok G, Hajkowicz K, Currie B. High incidence of methicillin-resistant Staphylococcus aureus sepsis and death in patients with febrile neutropenia at Royal Darwin Hospital. Intern Med J. 2009;39:557–559. doi: 10.1111/j.1445-5994.2009.02003.x. [DOI] [PubMed] [Google Scholar]
- 21.White CJ, Gallin JI. Phagocyte defects. Clin Immunol Immunopathol. 1986;40:50–61. doi: 10.1016/0090-1229(86)90068-1. [DOI] [PubMed] [Google Scholar]
- 22.Donabedian H, Gallin JI. The hyperimmunoglobulin E recurrent-infection (Job's) syndrome. A review of the NIH experience and the literature. Medicine (Baltimore) 1983;62:195–208. doi: 10.1097/00005792-198307000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Ma CS, Chew GY, Simpson N, et al. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med. 2008;205:1551–1557. doi: 10.1084/jem.20080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minegishi Y, Saito M, Nagasawa M, et al. Molecular explanation for the contradiction between systemic Th17 defect and localized bacterial infection in hyper-IgE syndrome. J Exp Med. 2009;206:1291–1301. doi: 10.1084/jem.20082767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crum-Cianfione N, Weekes J, Bavarol M. Recurrent community-associated methicillin-resistant Staphylococcus aureus infections among HIV-infected persons: incidence and risk factors. AIDS Patient Care STDS. 2009;23:499–502. doi: 10.1089/apc.2008.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishigame H, Kakuta S, Nagai T, et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30:108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 27.Dunne A, Ross PJ, Pospisilova E, et al. Inflammasome activation by adenylate cyclase toxin directs Th17 responses and protection against Bordetella pertussis. J Immunol. 2010;185:1711–1719. doi: 10.4049/jimmunol.1000105. [DOI] [PubMed] [Google Scholar]
- 28.Mar odi L, Cypowyj S, T oth B, Chernyshova L, Puel A, Casanova JL. Molecular mechanisms of mucocutaneous immunity against Candida and Staphylococcus species. J Allergy Clin Immunol. 2012;130:1019–1027. doi: 10.1016/j.jaci.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puel A, Picard C, Lorrot M, et al. Recurrent staphylococcal cellulitis and subcutaneous abscesses in a child with autoantibodies against IL-6. J Immunol. 2008;180:647–654. doi: 10.4049/jimmunol.180.1.647. [DOI] [PubMed] [Google Scholar]
- 30.Spellberg B, Ibrahim AS, Yeaman MR, et al. The antifungal vaccine derived from the recombinant N terminus of Als3p protects mice against the bacterium Staphylococcus aureus. Infect Immun. 2008;76:4574–4580. doi: 10.1128/IAI.00700-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho JS, Pietras EM, Garcia NC, et al. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J Clin Invest. 2010;120:1762–1773. doi: 10.1172/JCI40891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joshi A, Pancari G, Cope L, et al. Immunization with Staphylococcus aureus iron regulated surface determinant B (IsdB) confers protection via Th17/IL17 pathway in a murine sepsis model. Hum Vaccin Immunother. 2012;8:336–346. doi: 10.4161/hv.18946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Narita K, Hu DL, Mori F, Wakabayashi K, Iwakura Y, Nakane A. Role of interleukin-17A in cell-mediated protection against Staphylococcus aureus infection in mice immunized with the fibrinogen-binding domain of clumping factor A. Infect Immun. 2010;78:4234–4242. doi: 10.1128/IAI.00447-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanke ML, Heim CE, Angle A, Sanderson SD, Kielian T. Targeting macrophage activation for the prevention and treatment of Staphylococcus aureus biofilm infections. J Immunol. 2013;190:2159–2168. doi: 10.4049/jimmunol.1202348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chin AC, Fournier B, Peatman EJ, Reaves TA, Lee WY, Parkos CA. CD47 and TLR-2 cross-talk regulates neutrophil transmigration. J Immunol. 2009;183:5957–5963. doi: 10.4049/jimmunol.0900789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fournier B. The function of TLR2 during staphylococcal diseases. Front Cell Infect Microbiol. 2012;2:167. doi: 10.3389/fcimb.2012.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chau TA, McCully ML, Brintnell W, et al. Toll-like receptor 2 ligands on the staphylococcal cell wall downregulate superantigen-induced T cell activation and prevent toxic shock syndrome. Nat Med. 2009;15:641–648. doi: 10.1038/nm.1965. [DOI] [PubMed] [Google Scholar]
- 38.Frodermann V, Chau TA, Sayedyahossein S, Toth JM, Heinrichs DE, Madrenas J. A modulatory interleukin-10 response to staphylococcal peptidoglycan prevents Th1/Th17 adaptive immunity to Staphylococcus aureus. J Infect Dis. 2011;204:253–262. doi: 10.1093/infdis/jir276. [DOI] [PubMed] [Google Scholar]
- 39.Schreiner J, Kretschmer D, Klenk J, et al. Staphylococcus aureus phenol-soluble modulin peptides modulate dendritic cell functions and increase in vitro priming of regulatory T cells. J Immunol. 2013;190:3417–3426. doi: 10.4049/jimmunol.1202563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bagnoli F, Bertholet S, Grandi G. Inferring reasons for the failure of Staphylococcus aureus vaccines in clinical trials. Front Cell Infect Microbiol. 2012;2:1–4. doi: 10.3389/fcimb.2012.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harro CD, Betts RF, Hartzel JS, et al. The immunogenicity and safety of different formulations of a novel Staphylococcus aureus vaccine (V710): results of two Phase I studies. Vaccine. 2012;30:1729–1736. doi: 10.1016/j.vaccine.2011.12.045. [DOI] [PubMed] [Google Scholar]
- 42.Zorman JK, Esser M, Raedler M, et al. Naturally occurring IgG antibody levels to the Staphylococcus aureus protein IsdB in humans. Hum Vaccin Immunother. 2013;9:1857–1864. doi: 10.4161/hv.25253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin L, Ibrahim AS, Xu X, et al. Th1–Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog. 2009;5:e1000703. doi: 10.1371/journal.ppat.1000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pancari G, Fan H, Smith S, et al. Characterization of the mechanism of protection mediated by CSD7, a monoclonal antibody to Staphylococcus aureus iron regulated surface determinant B (IsdB). Front Cell Infect Microbiol. 2012;2:36. doi: 10.3389/fcimb.2012.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frank KM, Zhou T, Moreno-Vinasco L, Hollett B, Garcia JGN, Bubeck Wardenburg J. Host response signature to Staphylococcus aureus a-hemolysin implicates pulmonary Th17 response. Infect Immun. 2012;80:3161–3169. doi: 10.1128/IAI.00191-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spaulding AR, Lin YC, Merriman JA, Brosnahan AJ, Peterson ML, Schlievert PM. Immunity to Staphylococcus aureus secreted proteins protects rabbits from serious illnesses. Vaccine. 2012;30:5099–5109. doi: 10.1016/j.vaccine.2012.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adhikari RP, Karauzum H, Sarwar J, et al. Novel structurally designed vaccine for S. aureus a-hemolysin: protection against bacteremia and pneumonia. PLoS One. 2012;7:e38567. doi: 10.1371/journal.pone.0038567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uppalapati SR, Kingston JJ, Murali HS, Batra HV. Generation and characterization of an inter-generic bivalent a domain fusion protein aCS from Clostridium perfringens and Staphylococcus aureus for concurrent diagnosis and therapeutic applications. J Appl Microbiol. 2012;113:448–458. doi: 10.1111/j.1365-2672.2012.05333.x. [DOI] [PubMed] [Google Scholar]
- 49.Fritz SA, Tiemann KM, Hogan PG, et al. A serologic correlate of protective immunity against community-onset Staphylococcus aureus infection. Clin Infect Dis. 2013;56:1554–1561. doi: 10.1093/cid/cit123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Foletti D, Strop P, Shaughnessy L, et al. Mechanism of action and in vivo efficacy of a human-derived antibody against Staphylococcus aureus a-hemolysin. J Mol Biol. 2013;425:1641–1654. doi: 10.1016/j.jmb.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 51.Alonzo F, 3rd, Benson MA, Chen J, Novick RP, Shopsin B, Torres VJ. Staphylococcus aureus leukocidin ED contributes to systemic infection by targeting neutrophils and promoting bacterial growth in vivo. Mol Microbiol. 2012;83:423–435. doi: 10.1111/j.1365-2958.2011.07942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adhikari RP, Ajao AO, Aman MJ, et al. Lower antibody levels to Staphylococcus aureus exotoxins are associated with sepsis in hospitalized adults with invasive S. aureus infections. J Infect Dis. 2012;206:915–923. doi: 10.1093/infdis/jis462. [DOI] [PubMed] [Google Scholar]
- 53.Rasigade JP, Sicot N, Laurent F, Lina G, Vandenesch F, Etienne J. A history of Panton–Valentine leukocidin (PVL)-associated infection protects against death in PVL-associated pneumonia. Vaccine. 2011;29:4185–4186. doi: 10.1016/j.vaccine.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 54.Grumann D, Ruotsalainen E, Kolata J, et al. Characterization of infecting strains and superantigen-neutralizing antibodies in Staphylococcus aureus bacteremia. Clin Vaccine Immunol. 2011;18:487–493. doi: 10.1128/CVI.00329-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rose WE, Eickhoff JC, Shukla SK, et al. Elevated serum interleukin-10 at time of hospital admission is predictive of mortality in patients with Staphylococcus aureus bacteremia. J Infect Dis. 2012;206:1604–1611. doi: 10.1093/infdis/jis552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rose W, Sakoulas G, Berti A, Nizet V, Shukla S. Biomarkers in Staphylococcus aureus bacteremia predicting bacteremia duration or patient mortalitys. 2013. ICAAC Abstract B-1432.
- 57.van Dissel JT, van Langevelde P, Westendorp RG, Kwappenberg K, Fr€olich M. Anti-inflammatory cytokine profile and mortality in febrile patients. Lancet. 1998;351:950–953. doi: 10.1016/S0140-6736(05)60606-X. [DOI] [PubMed] [Google Scholar]
- 58.Shinefield H, Black S, Fattom A, et al. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N Engl J Med. 2002;346:491–496. doi: 10.1056/NEJMoa011297. [DOI] [PubMed] [Google Scholar]
- 59.Climo MW, Yokoe DS, Warren DK, et al. Effect of daily chlorhexidine bathing on hospital-acquired infection. N Engl J Med. 2013;368:533–542. doi: 10.1056/NEJMoa1113849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Foster TJ. Immune evasion by staphylococci. Nat Rev Microbiol. 2005;8:948–958. doi: 10.1038/nrmicro1289. [DOI] [PubMed] [Google Scholar]
- 61.Chen SY, Liao CH, Wang JL, et al. Methicillin-resistant Staphylococcus aureus (MRSA) staphylococcal cassette chromosome mec genotype effects outcomes of patients with healthcare-associated MRSA bacteremia independently of vancomycin minimum inhibitory concentration. Clin Infect Dis. 2012;55:1329–1337. doi: 10.1093/cid/cis717. [DOI] [PubMed] [Google Scholar]
- 62.Ganga R, Riederer K, Sharma M, et al. Role of SCCmec type in outcome of Staphylococcus aureus bacteremia in a single medical center. J Clin Microbiol. 2009;47:590–595. doi: 10.1128/JCM.00397-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hota B, Lyles R, Rim J, et al. Predictors of clinical virulence in community-onset methicillin-resistant Staphylococcus aureus infections: the importance of USA300 and pneumonia. Clin Infect Dis. 2011;53:757–765. doi: 10.1093/cid/cir472. [DOI] [PubMed] [Google Scholar]
- 64.Lalani T, Federspiel JJ, Boucher HW, et al. Associations between the genotypes of Staphylococcus aureus bloodstream isolates and clinical characteristics and outcomes of bacteremic patients. J Clin Microbiol. 2008;46:2890–2896. doi: 10.1128/JCM.00905-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Popovich KJ, Weinstein RA, Hota B. Are community-associated methicillin-resistant Staphylococcus aureus (MRSA) strains replacing traditional nosocomial MRSA strains? Clin Infect Dis. 2008;46:787–794. doi: 10.1086/528716. [DOI] [PubMed] [Google Scholar]
- 66.Quan L, Morita R, Kawakami S. Toxic shock syndrome toxin-1 (TSST-1) antibody levels in Japanese children. Burns. 2010;36:716–721. doi: 10.1016/j.burns.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 67.Ahmed T, Bhuiyan TR, Zaman K, Sinclair D, Qadri F. Vaccines for preventing enterotoxigenic Escherichia coli (ETEC) diarrhoea. Cochrane Database Syst Rev. 2013;7:CD009029. doi: 10.1002/14651858.CD009029.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brumbaugh AR, Mobley HL. Preventing urinary tract infection: progress toward an effective Escherichia coli vaccine. Expert Rev Vaccines. 2012;11:663–676. doi: 10.1586/erv.12.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thomas DE, Elliott EJ. Interventions for preventing diarrhea-associated hemolytic uremic syndrome: systematic review. BMC Public Health. 2013;13:799. doi: 10.1186/1471-2458-13-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Daum RS, Spellberg B. Progress toward a Staphylococcus aureus vaccine. Clin Infect Dis. 2012;54:560–567. doi: 10.1093/cid/cir828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.DeDent A, Kim HK, Missiakas D, Schneewind O. Exploring Staphylococcus aureus pathways to disease for vaccine development. Semin Immunopathol. 2012;34:317–333. doi: 10.1007/s00281-011-0299-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Projan SJ, Nesin M, Dunman PM. Staphylococcal vaccines and immunotherapy: to dream the impossible dream? Curr Opin Pharmacol. 2006;6:473–479. doi: 10.1016/j.coph.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 73.Schaffer AC, Lee JC. Staphylococcal vaccines and immunotherapies. Infect Dis Clin North Am. 2009;23:153–171. doi: 10.1016/j.idc.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 74.Pepper M, Linehan JL, Pag an AJ, et al. Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nat Immunol. 2010;11:83–89. doi: 10.1038/ni.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roy Chowdhury R, Fitch RW, Ghosh SK. Efficacy of phytol-derived diterpenoid immunoadjuvants over alum in shaping the murine host's immune response to Staphylococcus aureus. Vaccine. 2013;31:1178–1186. doi: 10.1016/j.vaccine.2012.12.069. [DOI] [PubMed] [Google Scholar]
- 76.Buonomo EL, Madan R, Pramoonjago P, Li L, Okusa MD, Petri WA., Jr Role of interleukin 23 signaling in Clostridium difficile colitis. J Infect Dis. 2013;208:917–920. doi: 10.1093/infdis/jit277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marwaha AK, Leung NJ, McMurchy AN, Levings MK. Th17 cells in autoimmunity and immunodeficiency: protective or pathogenic? Front Immunol. 2012;3:1–8. doi: 10.3389/fimmu.2012.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim HK, Cheng AG, Kim HY, Missiakas DM, Schneewind O. Nontoxigenic protein A vaccine for methicillin-resistant Staphylococcus aureus infections in mice. J Exp Med. 2010;207:1863–1870. doi: 10.1084/jem.20092514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Haas PJ, de Haas CJ, Kleibeuker W, et al. N-terminal residues of the chemotaxis inhibitory protein of Staphylococcus aureus are essential for blocking formylated peptide receptor but not C5a receptor. J Immunol. 2004;173:5704–5711. doi: 10.4049/jimmunol.173.9.5704. [DOI] [PubMed] [Google Scholar]
- 80.McCarthy AJ, Lindsay JA. Genetic variation in Staphylococcus aureus surface and immune evasion genes is lineage associated: implications for vaccine design and host–pathogen interactions. BMC Microbiol. 2010;10:173. doi: 10.1186/1471-2180-10-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burke SL, Shukla SK, Haney MS, Rose WE. Differential whole blood killing and IL-1b response in Staphylococcus aureus isolates from patients with persistent and rapidly cleared bacteremia. [ICAAC Abstract B-490, 2013]
- 82. ClinicalTrials.gov identifier: NCT01592214: A Two-Part Phase I Study to Establish and Compare the Safety and Local Tolerability of Two Nasal Formulations of XF-73 for Decolonization of Staphylococcus aureus: A Previously Investigated 0.5 mg/g Viscosified Gel Formulation Versus a Modified Formulation. [DOI] [PMC free article] [PubMed]
- 83.Huang SS, Septimus E, Kleinman K, et al. AHRQ DECIDE Network and Healthcare-Associated Infections Program. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med. 2013;368:2255–2265. doi: 10.1056/NEJMoa1207290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bode LG, Kluytmans JA, Wertheim HF, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med. 2010;362:9–17. doi: 10.1056/NEJMoa0808939. [DOI] [PubMed] [Google Scholar]
- 85.Lee A, Levinson AI, Schumacher HR., Jr Hypogammaglobulinemia and rheumatic disease. Semin Arthritis Rheum. 1993;22:252–264. doi: 10.1016/0049-0172(93)80073-o. [DOI] [PubMed] [Google Scholar]
- 86.Morrison VA. The infectious complications of chronic lymphocytic leukemia. Semin Oncol. 1998;25:98–106. [PubMed] [Google Scholar]
- 87.Weems JJ, Jr, Steinberg J, Filler S, et al. Veronate human polyclonal IgG with high anti-Staphylococcus aureus and anti-polyclonal antibodies tested in neonates. Antimicrob Agents Chemother. 2006;50:2751–2755. doi: 10.1128/AAC.00096-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rupp ME, Holley HP, Jr, Lutz J, et al. Phase II randomized, multicenter, double-blind, placebo-controlled trial of a polyclonal anti-Staphylococcus aureus capsular polysaccharide immune globulin in treatment of Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2007;51:4249–4254. doi: 10.1128/AAC.00570-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Benjamin DK, Schelonka R, White R, et al. A blinded, randomized, multicenter study of an intravenous Staphylococcus aureus immune globulin. J Perinatol. 2006;26:290–295. doi: 10.1038/sj.jp.7211496. [DOI] [PubMed] [Google Scholar]
- 90.DeJonge M, Burchfield D, Bloom B, et al. Clinical trial of safety and efficacy of INH-A21 for the prevention of nosocomial staphylococcal bloodstream infection in premature infants. J Pediatr. 2007;151:260–265. doi: 10.1016/j.jpeds.2007.04.060. [DOI] [PubMed] [Google Scholar]
- 91.Weisman LE, Thackray HM, Steinhorn RH, et al. A randomized study of a monoclonal antibody (pagibaximab) to prevent staphylococcal sepsis. Pediatrics. 2011;128:271–279. doi: 10.1542/peds.2010-3081. [DOI] [PubMed] [Google Scholar]
- 92.Creech CB, 2nd, Johnson BG, Alsentzer AR, Hohenboken M, Edwards KM, Talbot TR., 3rd Vaccination as infection control: a pilot study to determine the impact of Staphylococcus aureus vaccination on nasal carriage. Vaccine. 2009;28:256–260. doi: 10.1016/j.vaccine.2009.09.088. [DOI] [PubMed] [Google Scholar]
- 93.Gupta RC, Laforce FM, Mills DM. Polymorphonuclear leukocyte inclusions and impaired bacterial killing in patients with Felty's syndrome. J Lab Clin Med. 1976;88:183–193. [PubMed] [Google Scholar]
- 94.Holtfreter S, Kolata J, Br€oker BM. Towards the immune proteome of Staphylococcus aureus—the anti-S. aureus antibody response. Int J Med Microbiol. 2010;300:176–192. doi: 10.1016/j.ijmm.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 95.von Eiff C, Becker K, Machka K, Stammer H, Peters G. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N Engl J Med. 2001;344:11–16. doi: 10.1056/NEJM200101043440102. [DOI] [PubMed] [Google Scholar]