Abstract
Background
Identifying reversible renal dysfunction (RD) in the setting of heart failure is challenging. The goal of this study was to evaluate whether elevated admission blood urea nitrogen/creatinine ratio (BUN/Cr) could identify decompensated heart failure patients likely to experience improvement in renal function (IRF) with treatment.
Methods and Results
Consecutive hospitalizations with a discharge diagnosis of heart failure were reviewed. IRF was defined as ≥20% increase and worsening renal function as ≥20% decrease in estimated glomerular filtration rate. IRF occurred in 31% of the 896 patients meeting eligibility criteria. Higher admission BUN/Cr was associated with inhospital IRF (odds ratio, 1.5 per 10 increase; 95% confidence interval [CI], 1.3–1.8; P<0.001), an association persisting after adjustment for baseline characteristics (odds ratio, 1.4; 95% CI, 1.1–1.8; P=0.004). However, higher admission BUN/Cr was also associated with post-discharge worsening renal function (odds ratio, 1.4; 95% CI, 1.1–1.8; P=0.011). Notably, in patients with an elevated admission BUN/Cr, the risk of death associated with RD (estimated glomerular filtration rate <45) was substantial (hazard ratio, 2.2; 95% CI, 1.6–3.1; P<0.001). However, in patients with a normal admission BUN/Cr, RD was not associated with increased mortality (hazard ratio, 1.2; 95% CI, 0.67–2.0; P=0.59; p interaction=0.03).
Conclusions
An elevated admission BUN/Cr identifies decompensated patients with heart failure likely to experience IRF with treatment, providing proof of concept that reversible RD may be a discernible entity. However, this improvement seems to be largely transient, and RD, in the setting of an elevated BUN/Cr, remains strongly associated with death. Further research is warranted to develop strategies for the optimal detection and treatment of these high-risk patients.
Keywords: cardiorenal syndrome, heart failure, mortality
Renal dysfunction (RD) is a common finding in heart failure (HF) and has emerged as one of the most potent prognostic indicators in these patients.1,2 However, multiple different mechanisms capable of initiating a reduction in glomerular filtration rate (GFR) exist in HF, and the mechanism underlying the reduction in GFR likely has important prognostic and therapeutic implications.3–6 Unfortunately, limited progress has been made with respect to differentiation of these potential mechanistic subtypes of RD.
The blood urea nitrogen/creatinine ratio (BUN/Cr) has been extensively used in clinical medicine for the differentiation of prerenal RD from intrinsic renal parenchymal disease.7 The discriminative ability of BUN/Cr is based on the intrarenal mechanisms governing tubular urea handling. In the setting of a prerenal stressor, such as dehydration, significant renal neurohormonal activation (ie, increases in vasopressin, renal sympathetic nerve activity, and the renin–angiotensin–aldosterone axis) causes a disproportionate reabsorption of urea compared with that of creatinine.8–11 Similarly, HF-induced RD has also traditionally been classified as a prerenal form of RD, and renal neurohormonal activation is hypothesized to represent a prominent mechanistic contributor to the genesis of this form of RD.12 As such, the same physiology that allows BUN/Cr to differentiate chronic intrinsic kidney disease from dehydration should also apply to differentiation of HF-induced RD. Notably, we have recently reported that BUN/Cr can differentiate clinically important subgroups of RD as evidenced by the finding that essentially all of the mortality risk attributable to RD is confined to patients with an elevated BUN/Cr.4
Given that most prerenal forms of RD are reversible if the appropriate treatment is instituted, it is plausible that some forms of HF-induced RD will also be reversible. The fact that improvement in renal function (IRF) seems to occur in up to 30% of acutely decompensated HF patients with their return to compensation supports this possibility.13,14 Given that an elevated BUN/Cr is often associated with reversible prerenal physiology, we hypothesized that an elevated admission BUN/ Cr would identify patients with reversible HF-induced RD that would improve with treatment of their decompensated HF. However, given that IRF is transient in the majority of patients, we also hypothesized that, despite this potential reversibility, RD, in the setting of an elevated BUN/Cr, would still be associated with worsened survival.13,14 The primary aim of this study was to determine whether baseline BUN/Cr could identify patients with reversible RD and to validate, in the same population, our previous observations that RD in the setting of an elevated BUN/Cr is associated with substantially worsened survival.
Methods
Consecutive admissions from 2004 to 2009 to the cardiology and internal medicine services at the Hospital of the University of Pennsylvania with a primary discharge diagnosis of congestive HF were reviewed. Inclusion required an admission B-type natriuretic peptide level of >100 pg/mL within 24 hours of admission, a length of stay of 3 to 14 days, and availability of serum creatinine and BUN levels. There were 7 patients without admission serum BUN levels available who met all other inclusion criteria, accounting for the slightly lower number of patients in this cohort than the parent cohort from which it was derived.13 Patients on renal replacement therapy or those admitted to interventional cardiology services (to avoid confounding from contrast nephropathy) were excluded. In the event of multiple hospitalizations for a single patient, the first admission was retained. Post-discharge renal function was ascertained in the subset of patients with data available as previously described.13
Estimated GFR (eGFR) was calculated using the 4-variable modified diet and renal disease equation.15 Unless otherwise noted, IRF was defined as a ≥20% increase at any time during the hospitalization and post-discharge worsening renal function (WRF) as a ≥20% decrease in eGFR from the discharge to the outpatient value, consistent with previously published studies of IRF and WRF.3,5,6,13,14,16,17 All-cause mortality was determined via the Social Security death index.18 Loop diuretic doses were converted to furosemide equivalents with 1 mg bumetanide =20 mg torsemide =80 mg furosemide for oral diuretics, and 1 mg bumetanide =20 mg torsemide =40 mg furosemide for intravenous diuretics. The study was approved by the institutional review board of the Hospital of the University of Pennsylvania.
Statistical Analysis
The primary goal of this analysis was to evaluate the association between admission BUN/Cr and IRF during treatment of acute decompensated HF. For the purposes of the primary analysis and unless otherwise specified, BUN/Cr was treated as a continuous covariate. Values reported are mean±SD, median (25th–75th percentile), and percentage. The independent Student t test or the Wilcoxon rank-sum test was used to compare continuous variables. The Pearson χ2 was used to evaluate associations between categorical variables. Spearman correlation coefficients were used to examine statistical dependence between 2 variables. To facilitate interpretability of the descriptive statistics relating to BUN/Cr, this variable was dichotomized as ≥20 or <20 (in accordance with common clinical practice) for these analyses. Multivariable logistic regression analysis was performed to estimate the association between BUN/Cr and IRF after adjusting for potential confounders. Candidate covariates for the multivariable models were obtained by screening clinical characteristics for an association with IRF at P≤0.2.13 Using backward elimination, any covariate whose removal resulted in a change in the odds ratio (OR) for BUN/Cr >10% was retained in the final model. Additionally, any candidate variable associated with IRF with P<0.05 was retained in the model, regardless of its influence on the OR. Covariates that had a P>0.2, but a theoretical basis for potential confounding, were manually forced into and subsequently retained in the final model. Furthermore, covariates associated with mortality at P≤0.2 were also manually forced into (and retained in) the final model, to ensure the relationship between BUN/Cr and IRF was not driven by the greater disease severity in patients with either IRF or an elevated BUN/Cr. A total of 24 covariates were included in the first step of model building, and 17 variables were retained in the final model. ORs were reported for every 10 increase in BUN/Cr. Proportional hazards modeling was used to evaluate time-to-event associations with all-cause mortality. Candidate covariates entered in the model were those with univariate associations with all-cause mortality P≤0.2 and model building was done analogously to that described above for the logistic regression models. Hazard ratios (HRs) were also reported as per 10 increase in BUN/Cr. To examine the effect of BUN/Cr on the association between eGFR and mortality, Kaplan–Meier curves for death from any cause were plotted for the 4 combinations of groups between patients with and without an elevated BUN/Cr defined as a BUN/Cr in the highest compared with the lowest quartile (in accordance with previous studies) and those with and without significant RD (eGFR< 45 mL/min/1.73 m2).4 Statistical significance was determined with the log-rank test. Stratum-specific HRs and 95% confidence intervals (CIs) were derived from proportional hazards modeling of the individual strata, and the significance of the interactions was formally assessed in models incorporating terms for the main effect of renal function, the main effect of BUN/Cr, and the interaction between these variables. Statistical analyses were performed using Stata 12.0 (Statacorp, College Station, TX), and statistical significance was defined as a 2-sided P value <0.05, with the exception of tests of interaction where significance was defined as a P value <0.1.
Results
Overall, 896 patients met the inclusion criteria. Thirty-one percent of the population (n=278) experienced IRF during hospitalization with a mean improvement in eGFR in these patients of 43.7±27.2%. The remainder of the cohort experienced a mean improvement in eGFR from admission to the highest eGFR during hospitalization of only 5.3±6.7%. A detailed description of baseline characteristics, influence of treatment, and prognosis associated with IRF has previously been described.13
The mean baseline BUN/Cr in the cohort was 18.6±7.7 with a median value of 17 and an interquartile range of 13.3 to 22.2. Baseline BUN/Cr demonstrated very weak correlations with both admission serum creatinine (r=0.071; P=0.03) and eGFR (r=0.18; P<0.001). Baseline characteristics of patients with and without a BUN/Cr ≥20 are shown in Table 1. Notably, patients with an elevated BUN/ Cr were more likely to be white, to be older, and to have an ischemic cause for their HF. Markers of venous congestion were more prevalent in patients with an elevated BUN/Cr, including an elevated jugular venous pressure and presence of peripheral edema. Additionally, the elevated BUN/Cr group had multiple baseline indices consistent with greater HF disease severity, including lower baseline eGFR, serum sodium, hemoglobin, and systolic blood pressure, and a higher B-type natriuretic peptide.
Table 1.
Baseline Characteristics and Their Association With the Blood Urea Nitrogen/Creatinine Ratio
| Characteristics | Overall Cohort (n=896) | BUN/Cr ≥20
|
P | |
|---|---|---|---|---|
| No (n=571) | Yes (n=325) | |||
| Demographics | ||||
| Age, y | 62.8±15.8 | 59.6±16.0 | 68.5±13.8 | <0.001* |
| White | 33.9% | 24.3% | 50.8% | <0.001* |
| Male | 54.5% | 56.6% | 50.8% | 0.094 |
| Medical history | ||||
| Hypertension | 74.1% | 75.4% | 72.0% | 0.268 |
| Diabetes mellitus | 39.4% | 37.3% | 43.2% | 0.084 |
| Coronary artery disease | 42.8% | 38.7% | 50.5% | 0.001* |
| Ischemic cardiomyopathy | 24.6% | 21.7% | 29.5% | 0.009* |
| Ejection fraction ≥40% | 35.1% | 33.9% | 37.3% | 0.304 |
| Hyperlipidemia | 32.2% | 29.8% | 36.5% | 0.040* |
| Admission physical examination | ||||
| Heart rate, beats/min | 89.8±20.0 | 92.2±20.7 | 85.5±17.9 | <0.001* |
| Systolic blood pressure, mm Hg | 139.1±34.6 | 146.7±34.7 | 125.7±30.1 | 0.004* |
| Jugular venous distention | 35.5% | 32.6% | 40.3% | 0.025* |
| Moderate-to-severe edema | 15.4% | 13.3% | 19.2% | 0.020* |
| Medications | ||||
| β-Blocker | 67.0% | 63.1% | 73.9% | 0.001* |
| ACE inhibitor or ARB | 61.0% | 59.9% | 62.8% | 0.397 |
| Digoxin | 22.5% | 18.2% | 30.1% | <0.001* |
| Aldosterone antagonist | 15.1% | 11.6% | 21.1% | <0.001* |
| Loop diuretic dose, mg | 40 (0, 80) | 40 (0, 80) | 80 (20, 120) | <0.001* |
| Thiazide diuretics | 11.6% | 7.94% | 18.0% | <0.001* |
| Nitrates | 16.5% | 15.5% | 18.3% | 0.280 |
| Calcium channel blockers | 18.5% | 19.2% | 17.1% | 0.428 |
| Laboratory values (baseline) | ||||
| Serum sodium, mEq/L | 138.6±4.3 | 139.1±3.8 | 137.7±5.0 | <0.001* |
| Hemoglobin, g/dL | 12.2±2.1 | 12.3±2.1 | 12.0±2.0 | 0.032* |
| B-type natriuretic peptide, pg/mL | 1299 (659.0, 2368) | 1208 (637.2, 2849) | 1479 (738.0, 2848) | 0.004* |
| eGFR, mL/min per 1.73 m2 | 60.4±28.6 | 63.2±28.9 | 55.6±27.4 | <0.001* |
| Serum creatinine, mg/dL | 1.5±1.1 | 1.5±1.2 | 1.5±0.8 | 0.649 |
| Blood urea nitrogen, mg/dL | 28.3±20.2 | 21.2±12.9 | 40.8±24.3 | <0.001* |
ACE indicates angiotensin converting enzyme; ARB, angiotensin receptor blocker; BUN/Cr, blood urea nitrogen/creatinine ratio; and eGFR, estimated glomerular filtration rate.
Significant P value; () represents interquartile ranges.
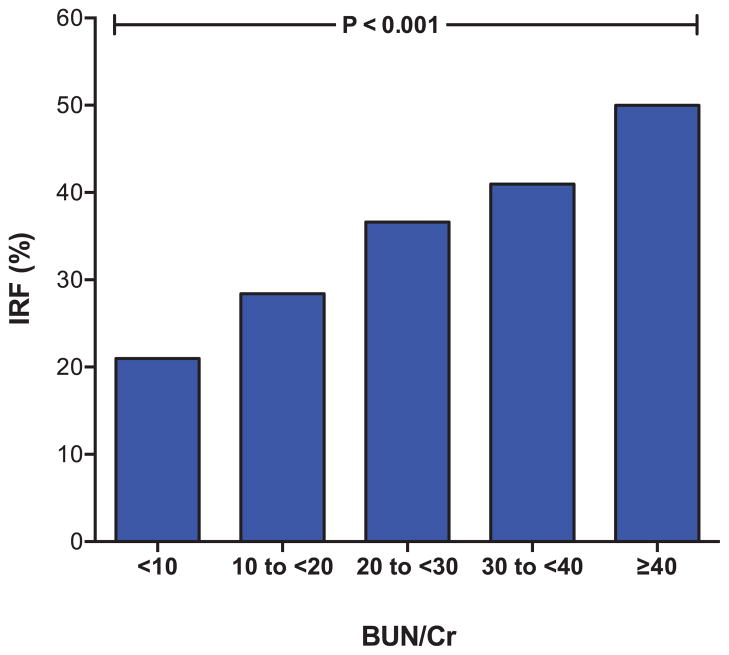
The admission BUN/Cr was significantly associated with IRF (OR, 1.5 per 10 increase in BUN/Cr; 95% CI, 1.3–1.8; P<0.001) (Figure 1). The association between BUN/Cr and IRF was linear across values of BUN/Cr as evaluated graphically with a lowess smoother.19 Patients with higher BUN/Cr also had a significantly greater likelihood of continuing to meet criteria for IRF at the time of discharge (OR, 1.5; 95% CI, 1.2–1.8; P<0.001). The BUN/Cr remained associated with IRF after adjustment for baseline eGFR (OR, 1.4; 95% CI, 1.2–1.7; P<0.001) and baseline factors associated with IRF (age, race, hypertension, diabetes mellitus, ischemic HF pathogenesis, jugular venous distention, ejection fraction, systolic blood pressure, loop diuretic dose, angiotensin converting enzyme or angiotensin receptor blocker use, β-blocker use, spironolactone use, thiazide use, nitrate use, B-type natriuretic peptide level, serum sodium, and serum hemoglobin) (OR, 1.4; 95% CI, 1.1–1.8; P=0.004). Results were similar when comparing BUN/Cr ≥20 with BUN/Cr <20, the top BUN/Cr quartile versus the remainder of population, the top BUN/Cr tertile versus the remainder of population, and BUN/Cr above and below the median for both the adjusted and unadjusted analyses (Table 2). Admission BUN demonstrated a significant univariate association with IRF (OR, 1.02; 95% CI, 1.01–1.02; P<0.001). When both admission BUN and BUN/ Cr were examined together in a regression model adjusted for admission eGFR, only BUN/Cr retained a significant relationship with IRF (BUN/Cr OR, 1.4; 95% CI, 1.09–1.82; P=0.01; BUN OR, 1.0; 95% CI, 0.99–1.01; P=0.93).
Figure 1.
Incidence of improvement in renal function during hospitalization with a progressively higher baseline blood urea nitrogen/creatinine ratio (BUN/Cr). IRF indicates improvement in renal function. IRF defined as a ≥20% improvement in glomerular filtration rate. Test for trend P<0.001.
Table 2.
Unadjusted and Adjusted Associations of Blood Urea Nitrogen/Creatinine Ratio With IRF by Varied Definitions of BUN/Cr
| BUN/Cr Definition | Unadjusted
|
Adjusted
|
||
|---|---|---|---|---|
| OR (95% CI) | P * | OR (95% CI) | P * | |
| BUN/Cr as a continuous parameter† | 1.51 (1.26–1.81) | <0.001 | 1.43 (1.12–1.83) | 0.004 |
| BUN/Cr ≥20† | 1.86 (1.39–2.49) | <0.001 | 1.66 (1.15–2.41) | 0.007 |
| BUN/Cr dichotomized as top quartile vs remainder of population | 1.72 (1.25–2.37) | <0.001 | 1.54 (1.02–2.32) | 0.038 |
| BUN/Cr dichotomized as top tertile vs remainder of population | 1.86 (1.40–2.50) | <0.001 | 1.73 (1.19–2.50) | 0.004 |
| BUN/Cr dichotomized as above vs below median | 1.70 (1.27–2.26) | <0.001 | 1.47 (1.01–2.14) | 0.044 |
BUN/Cr indicates blood urea nitrogen/creatinine ratio; CI, confidence interval; and OR, odds ratio.
Significant P value;
OR reported for BUN/Cr as a continuous covariate are for every 10 increase in BUN/Cr. Adjusted analyses included adjustment for age, race, hypertension, diabetes mellitus, ischemic heart failure pathogenesis, jugular venous distention, ejection fraction, systolic blood pressure, loop diuretic dose, angiotensin converting enzyme inhibitor or angiotensin receptor blocker use, β-blocker use, spironolactone use, thiazide use, nitrate use, brain natriuretic peptide level, serum sodium, and serum hemoglobin.
In the overall population, BUN/Cr increased on average 16.6±40.2% from admission to discharge (P<0.001). Interestingly, there was not a significant difference in the degree of increase in BUN/Cr between patients that met criteria for IRF at discharge compared with those that did not (14.7±39.5% versus 17.0±40.3% increase; P=0.50). This lack of difference seemed to be predominantly driven by the fact that patients with IRF had a relatively larger improvement in serum creatinine (25.0% improvement) compared with their improvement in BUN (13.8% improvement), ultimately leading to a net worsening in the ratio.
Baseline BUN/Cr and Post-Discharge Renal Function
The incidence of post-discharge WRF (data available n=452, 50.4% of the population) was 39.2%. Importantly, there was no significant difference between baseline BUN/Cr (18.3±7.4 versus 18.9±7.9; P=0.2) or IRF (30.4% versus 31.5%; P=0.7) in those patients with and without post-discharge data available. Patients with higher baseline BUN/Cr were more likely to experience post-discharge WRF (OR, 1.4 per 10 increase; 95% CI, 1.1–1.8; P=0.011). Notably, this association was unchanged after adjustment for admission eGFR (OR, 1.4 per 10 increase; 95% CI, 1.1–1.8; P=0.016), discharge eGFR (OR, 1.4 per 10 increase; 95% CI, 1.1–1.9; P=0.006), or the admission to discharge change in eGFR (OR, 1.4 per 10 increase in BUN/Cr; 95% CI, 1.1–1.8; P=0.020). Notably, there was not a significant difference in the strength of association between BUN/Cr and post-discharge WRF between patients that did or did not experience in-hospital IRF (p interaction =0.96). This relationship was not detectable for discharge BUN/Cr (OR, 1.1 per 10 increase; 95% CI, 0.9–1.6; P=0.28), a finding unchanged by adjustment for admission eGFR (P=0.33), discharge eGFR (P=0.22), or the change in eGFR (P=0.30).
Baseline BUN/Cr, RD, and Mortality
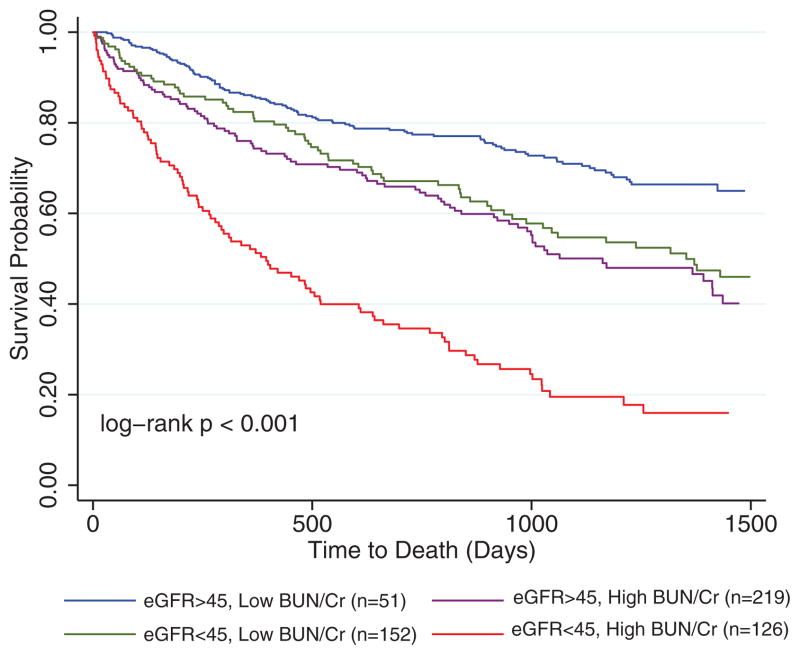
Forty-four percent of the population died during a median follow-up of 2.6 years. Baseline BUN/Cr was significantly associated with increased mortality in this population (HR, 1.8 per 10 increase; 95% CI, 1.6–2.0; P<0.001), an association that persisted when adjusted for baseline eGFR (Table 3). Adjusting for baseline characteristics, chronic medical conditions, medication use, and admission laboratory data did not eliminate the independent association of increasing BUN/Cr with mortality (Table 3). Admission eGFR was also significantly associated with mortality (HR, 1.1 per 10 mL/min per 1.73 m2 decrease in eGFR; 95% CI, 1.1–1.2; P<0.001), an association which persisted after adjustment for baseline BUN/Cr (HR, 1.1 per 10 mL/min per 1.73 m2 decrease in eGFR; 95% CI, 1.1–1.2; P<0.001) and baseline characteristics (HR, 1.1 per 10 mL/min per 1.73 m2 decrease in eGFR; 95% CI, 1.0–1.1; P=0.017). Consistent with our previously published findings in other populations, there was significant effect modification by BUN/Cr on the association between eGFR and mortality (p interaction for continuous variables =0.04).4 Notably, in patients with a BUN/Cr in the top quartile, the risk of death associated with admission eGFR remained significant (HR, 1.2 per 10 mL/min per 1.73 m2 decrease in eGFR; 95% CI, 1.1–1.3; P<0.001). However, in patients with a BUN/Cr in the bottom quartile, eGFR was no longer associated with death (HR, 1.0 per 10 mL/min per 1.73 m2 decrease in eGFR; 95% CI, 0.97–1.1; P=0.25; p interaction=0.029). Consistent with our previously published findings, this effect modification was strengthened by adjustment for baseline characteristics, including age, sex, race, hypertension, coronary artery disease, B-type natriuretic peptide, serum sodium, systolic blood pressure, heart rate, loop diuretic dose, and angiotensin converting enzyme inhibitor or angiotensin receptor blocker use (p interaction for continuous variables=0.016). Similar results were noted when eGFR was dichotomized to patients with or without moderate-to-severe RD (eGFR ≤45 mL/min per 1.73 m2), where the risk associated with RD was substantial in those with a BUN/Cr in the top quartile (HR, 2.2; 95% CI, 1.6–3.1; P<0.001) and undetectable in those with a BUN/Cr in the bottom quartile (HR, 1.2; 95% CI, 0.67–2.0; P=0.59; p interaction =0.03) (Figure 2). Although the risk of death associated with RD also differed between those with an admission BUN in the top versus bottom quartile (p interaction=0.08), when both the interaction between admission BUN and RD as well as the interaction between admission BUN/Cr and RD were examined in the same model, only the interaction between BUN/Cr and RD remained significantly associated with mortality (p interaction BUN/Cr×RD=0.03; p interaction BUN×RD=0.26).
Table 3.
Association Between BUN/Cr and All-Cause Mortality
| Association | HR (95% CI) | P |
|---|---|---|
| Unadjusted | 1.8 (1.6–2.0) | <0.001 |
| Adjusted for admission eGFR | 1.7 (1.5–1.9) | <0.001 |
| Adjusted for baseline characteristics* | 1.3 (1.1–1.5) | 0.001 |
BUN/Cr was analyzed as a continuous parameter and HR are per 10 increase in BUN/Cr. BUN/Cr indicates blood urea nitrogen/creatinine ratio; CI, confidence interval; eGFR, estimated glomerular filtration rate; and HR, hazard ratio.
Adjusted for age, race, hypertension, diabetes mellitus, coronary artery disease, preserved ejection fraction, systolic blood pressure, heart rate, loop diuretic dose, angiotensin converting enzyme inhibitor or angiotensin receptor blockers, β-blockers, digoxin, thiazide and spironolactone use, serum sodium, hemoglobin, B-type natriuretic peptide level, and admission eGFR.
Figure 2.
Kaplan–Meier survival curves grouped by blood urea nitrogen/creatinine ratio (BUN/Cr) and renal dysfunction. eGFR indicates estimated glomerular filtration rate. BUN/Cr dichotomized as the top vs bottom quartile.
Discussion
The primary finding of this study is the strong association between an elevated admission BUN/Cr and significant improvement in kidney function during the treatment of acute decompensated HF. Even after adjustment for comorbidities known to impact renal function, as well as medications that influence GFR, an elevated BUN/Cr at admission continued to be strongly associated with IRF. However, the IRF observed after standard decompensated HF treatment was frequently transient, and RD in the setting of an elevated BUN/ Cr remained strongly associated with reduced survival. These findings provide proof of concept that not only may prospective identification of potentially reversible forms of RD be possible, but also that this form of RD seems to represent, perhaps, the most prognostically important cardiorenal phenotype in HF.
Urea plays a fundamental and direct role in fluid and sodium homeostasis, processes tightly regulated by neurohormonal systems.8,9,20,21 As a result, during times of fluid and sodium avidity, such as intravascular volume depletion or HF, the rate of urea excretion is reduced out of proportion to the reduction in GFR, ultimately leading to an elevated BUN/ Cr.10,22 However, with intrinsic renal parenchymal disease, the primary defect leading to RD is irreversible nephron loss rather than neurohormonal activation. As a result, the rate of urea clearance is reduced in parallel to GFR, resulting in a normal BUN/Cr. This neurohormonally mediated disassociation between urea reabsorption and glomerular filtration forms the basis for the widespread clinical application of BUN/Cr for the differentiation of prerenal RD from intrinsic renal parenchymal disease. Given the key role for neurohormones in the pathogenesis of both HF and reversible prerenal forms of RD, this physiology may represent the common thread linking the finding of reversibility and the increased risk for death.
We have previously reported that the majority of patients who experience IRF during the treatment of decompensated HF actually have post-discharge recurrence of the RD.13,14 Similarly, in the current analysis, we found that an elevated admission BUN/Cr was also associated with an increased incidence of post-discharge WRF, independent of the discharge eGFR or changes in eGFR during hospitalization. These observations allow some speculation as to how BUN/ Cr could identify a form of RD that is potentially reversible and also associated with significantly increased mortality. Because currently available HF treatments are not capable of increasing renal function to supranormal levels, for improvement in kidney function to be possible, reversible RD must be present at baseline (with the most likely pathogenesis being RD induced by severe HF). Given that patients experiencing IRF were likely sicker at baseline and the improvement in disease severity is largely transient, it is understandable how an elevated BUN/Cr could potentially be associated with reversible RD but also worsened survival. However, we have also previously reported that in the few patients who maintain IRF long term, there may actually be improved survival associated with the IRF.13 Although again speculative, this observation raises the possibility that strategies aimed at inducing and maintaining IRF could potentially lead to improved outcomes. Markers, such as BUN/Cr, may allow the prospective identification of patients with the potential for IRF, facilitating interventional trials that can actually prove or disprove causality for these highly complex associations.
Despite the above promising proof-of-concept findings, BUN/Cr is a less than ideal measure of renal urea handling and is influenced by non-renal factors, such as diet and protein catabolism.23 Furthermore, creatinine-based estimates of GFR also have significant limitations secondary to factors, such as the dependence of serum creatinine on muscle mass and tubular secretion.24 Recently, several novel renal biomarkers, such as neutrophil gelatinase-associated lipocalin (NGAL), N-acetyl-β-D-glucosaminidase (NAG), and kidney injury molecule 1 (KIM-1), have demonstrated high specificity in the detection of acute kidney injury.25 Furthermore, at present, the widely available filtration marker cystatin C offers the advantage of limited influence from lean body mass and tubular secretion. It is reasonable to hypothesize that given the strong signals demonstratable using a crude metric, such as BUN/Cr, the aforementioned renal biomarkers may provide superior discriminative ability.
Limitations
There are several limitations that must be considered when interpreting these results. First, given the retrospective study design, causality is impossible to demonstrate and residual confounding cannot be excluded. Physicians were not blinded to measures of renal function and, thus, may have altered treatment decisions in response to these data. Furthermore, given that proven methodology to detect and optimally treat reversible RD in this population was unavailable to the treating physicians, it is highly likely that some patients with reversible RD may have been refractory to the treatment they received (or possibly received treatment that led to no improvement or worsening in renal function) and, thus, did not experience IRF. This possibility may have led to a substantial underestimation of the magnitude of the association between BUN/Cr and reversible RD. Additionally, non-neurohormonal factors, such as diet and protein catabolism, that influence urea reabsorption may have introduced potential uncontrolled confounding. Given the slow equilibration time and nonrenal factors that influence serum creatinine, assessment of IRF based on creatinine-based eGFR may also have introduced bias. The analysis of post-discharge renal function has a large degree of missing data, which are likely missing not at random and, thus, may have significant bias inherent to the results. As a result of the above limitations, our findings should be considered hypothesis-generating and serve primarily to initiate further investigation.
Conclusions
In the setting of decompensated HF, an elevated BUN/Cr identifies patients likely to experience IRF, providing proof of concept that reversible RD may be a discernible entity. However, the improved kidney function observed following standard decompensated HF treatment seems to be largely transient and, perhaps as a result, RD in the setting of an elevated BUN/Cr remains strongly associated with worsened survival. Further research to develop methodology for the optimal detection and treatment of these high-risk patients is warranted with the goal of facilitating sustained improvements in renal function and potentially clinical outcomes.
CLINICAL PERSPECTIVE.
Renal dysfunction (RD) has emerged as one of the most potent risk factors for death in patients with heart failure (HF). In some patients, RD is a direct result of HF and potentially reversible. In others, the RD is primarily because of irreversible renal parenchymal disease, such as that caused by diabetes mellitus or hypertension. To date, methodology has not been identified that can differentiate reversible HF-induced RD from intrinsic RD. In this study, we investigated whether an elevated admission blood urea nitrogen/creatinine ratio, a marker often used to distinguish prerenal physiology from chronic kidney disease, could identify patients with reversible HF-induced RD. We found that decompensated HF patients with an elevated admission blood urea nitrogen/creatinine ratio had a significantly greater incidence of improvement in renal function with the return to compensation. Despite the improvement in these patients, recurrence of RD was common after discharge, and the greatest survival disadvantage clustered in patients with RD and an elevated admission blood urea nitrogen/creatinine ratio. These findings provide proof of concept that prospective identification of potentially reversible HF-induced RD is possible. Further research is warranted to develop strategies for the optimal detection and treatment of these high-risk patients.
Acknowledgments
Sources of Funding
The study was supported by National Institutes of Health grant numbers 5T32HL007891, 5T32HL007843-15, and 1K23HL11486-01.
Footnotes
Disclosures
None.
References
- 1.Bock JS, Gottlieb SS. Cardiorenal syndrome: new perspectives. Circulation. 2010;121:2592–2600. doi: 10.1161/CIRCULATIONAHA.109.886473. [DOI] [PubMed] [Google Scholar]
- 2.Rastogi A, Fonarow GC. The cardiorenal connection in heart failure. Curr Cardiol Rep. 2008;10:190–197. doi: 10.1007/s11886-008-0033-1. [DOI] [PubMed] [Google Scholar]
- 3.Testani JM, Coca SG, McCauley BD, Shannon RP, Kimmel SE. Impact of changes in blood pressure during the treatment of acute decompensated heart failure on renal and clinical outcomes. Eur J Heart Fail. 2011;13:877–884. doi: 10.1093/eurjhf/hfr070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Testani JM, Coca SG, Shannon RP, Kimmel SE, Cappola TP. Influence of renal dysfunction phenotype on mortality in the setting of cardiac dysfunction: analysis of three randomized controlled trials. Eur J Heart Fail. 2011;13:1224–1230. doi: 10.1093/eurjhf/hfr123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Testani JM, Kimmel SE, Dries DL, Coca SG. Prognostic importance of early worsening renal function after initiation of angiotensin-converting enzyme inhibitor therapy in patients with cardiac dysfunction. Circ Heart Fail. 2011;4:685–691. doi: 10.1161/CIRCHEARTFAILURE.111.963256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Testani JM, Chen J, McCauley BD, Kimmel SE, Shannon RP. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation. 2010;122:265–272. doi: 10.1161/CIRCULATIONAHA.109.933275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cecil RL, Goldman L, Ausiello DA. Cecil Medicine. Philadelphia, PA: Saunders Elsevier; 2008. [Google Scholar]
- 8.Fenton RA. Essential role of vasopressin-regulated urea transport processes in the mammalian kidney. Pflugers Arch. 2009;458:169–177. doi: 10.1007/s00424-008-0612-4. [DOI] [PubMed] [Google Scholar]
- 9.Cowley AW., Jr Role of the renal medulla in volume and arterial pressure regulation. Am J Physiol. 1997;273(1 pt 2):R1–15. doi: 10.1152/ajpregu.1997.273.1.R1. [DOI] [PubMed] [Google Scholar]
- 10.Lindenfeld J, Schrier RW. Blood urea nitrogen a marker for adverse effects of loop diuretics? J Am Coll Cardiol. 2011;58:383–385. doi: 10.1016/j.jacc.2011.01.054. [DOI] [PubMed] [Google Scholar]
- 11.Kazory A. Emergence of blood urea nitrogen as a biomarker of neurohormonal activation in heart failure. Am J Cardiol. 2010;106:694–700. doi: 10.1016/j.amjcard.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 12.Braunwald E, Bonow RO. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. Philadelphia, PA: Saunders; 2012. [Google Scholar]
- 13.Testani JM, McCauley BD, Chen J, Coca SG, Cappola TP, Kimmel SE. Clinical characteristics and outcomes of patients with improvement in renal function during the treatment of decompensated heart failure. J Card Fail. 2011;17:993–1000. doi: 10.1016/j.cardfail.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Testani JM, McCauley BD, Kimmel SE, Shannon RP. Characteristics of patients with improvement or worsening in renal function during treatment of acute decompensated heart failure. Am J Cardiol. 2010;106:1763–1769. doi: 10.1016/j.amjcard.2010.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 16.Testani JM, Cappola TP, McCauley BD, Chen J, Shen J, Shannon RP, Kimmel SE. Impact of worsening renal function during the treatment of decompensated heart failure on changes in renal function during subsequent hospitalization. Am Heart J. 2011;161:944–949. doi: 10.1016/j.ahj.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Testani JM, McCauley BD, Chen J, Shumski M, Shannon RP. Worsening renal function defined as an absolute increase in serum creatinine is a biased metric for the study of cardiorenal interactions. Cardiology. 2010;116:206–212. doi: 10.1159/000316038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quinn J, Kramer N, McDermott D. Validation of the Social Security Death Index (SSDI): an important readily-available outcomes database for researchers. West J Emerg Med. 2008;9:6–8. [PMC free article] [PubMed] [Google Scholar]
- 19.Royston P, Sauerbrei W. Building multivariable regression models with continuous covariates in clinical epidemiology–with an emphasis on fractional polynomials. Methods Inf Med. 2005;44:561–571. [PubMed] [Google Scholar]
- 20.Sands JM, Layton HE. The physiology of urinary concentration: an update. Semin Nephrol. 2009;29:178–195. doi: 10.1016/j.semnephrol.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang B, Bankir L. Urea and urine concentrating ability: new insights from studies in mice. Am J Physiol Renal Physiol. 2005;288:F881–F896. doi: 10.1152/ajprenal.00367.2004. [DOI] [PubMed] [Google Scholar]
- 22.Schrier RW. Blood urea nitrogen and serum creatinine: not married in heart failure. Circ Heart Fail. 2008;1:2–5. doi: 10.1161/CIRCHEARTFAILURE.108.770834. [DOI] [PubMed] [Google Scholar]
- 23.Maroni BJ, Steinman TI, Mitch WE. A method for estimating nitrogen intake of patients with chronic renal failure. Kidney Int. 1985;27:58–65. doi: 10.1038/ki.1985.10. [DOI] [PubMed] [Google Scholar]
- 24.Brenner BM, Rector FC. Brenner and Rector’s the Kidney. Philadelphia, PA: Saunders Elsevier; 2008. [Google Scholar]
- 25.Coca SG, Parikh CR. Urinary biomarkers for acute kidney injury: perspectives on translation. Clin J Am Soc Nephrol. 2008;3:481–490. doi: 10.2215/CJN.03520807. [DOI] [PMC free article] [PubMed] [Google Scholar]