Abstract
Background
The long-term durability and prognostic significance of improvement in renal function after mechanical circulatory support (MCS) has yet to be characterized in a large multicenter population. The primary goals of this analysis were to describe serial post-MCS changes in estimated glomerular filtration rate (eGFR) and determine their association with all-cause mortality.
Methods and Results
Adult patients enrolled in the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) with serial creatinine levels available (n=3363) were studied. Early post-MCS, eGFR improved substantially (median improvement, 48.9%; P<0.001) with 22.3% of the population improving their eGFR by ≥100% within the first few weeks. However, in the majority of patients, this improvement was transient, and by 1 year, eGFR was only 6.7% above the pre-MCS value (P<0.001). This pattern of early improvement followed by deterioration in eGFR was observed with both pulsatile and continuous-flow devices. Interestingly, poor survival was associated with both marked improvement (adjusted hazard ratio [HR], 1.64; 95% confidence interval [CI], 1.19–2.26; P=0.002) and worsening in eGFR (adjusted HR, 1.63; 95% CI, 1.15–2.13; P=0.004).
Conclusions
Post-MCS, early improvement in renal function is common but seems to be largely transient and not necessarily indicative of an improved prognosis. This pattern was observed with both pulsatile and continuous-flow devices. Additional research is necessary to better understand the mechanistic basis for these complex post-MCS changes in renal function and their associated survival disadvantage.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00119834.
Keywords: cardio-renal syndrome, heart failure, heart-assist devices, transplantation
Renal dysfunction (RD) is common in patients with heart failure (HF) and has emerged as one of the most important prognostic indicators.1,2 In patients requiring mechanical circulatory support (MCS), the prevalence of RD is particularly high, often negatively influencing patient selection for advanced therapies.3–5 Notably, many of the factors thought causal of HF-induced RD likely stem from the hemodynamic perturbations characteristic of severe HF, abnormalities which could improve after initiation of MCS. As a result, marked early improvement in renal function (IRF) post-MCS has now been described in several publications.4,6–12
Because the durability of MCS devices has improved, the cumulative effects of long-term support on noncardiac organ function have become an important area of interest.13 Notably, 2 small single-center studies in patients with continuous-flow devices have recently reported a signal for significant late deterioration in renal function.6,11 To date, the long-term durability of post-MCS IRF and the association with subsequent mortality has yet to be studied in a large multicenter population.
The Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) is a national registry for patients implanted with a Food and Drug Administration (FDA)-approved MCS device designed for long-term mechanical support.14 The primary goals of this study were to describe the early and late changes in renal function after MCS and to determine the potential clinical importance of these changes with respect to mortality in the large multicenter INTERMACS population.
Methods
INTERMACS Registry
INTERMACS is an audited registry of FDA-approved mechanical circulatory assist devices. Registry participation is mandatory for all Centers for Medicare and Medicaid Services–approved destination MCS implantation centers. The registry was created and maintained by the University of Alabama at Birmingham INTERMACS Data Coordinating Center since June 2005 and is supported by the National Heart, Lung, and Blood Institute, the FDA, and Centers for Medicare and Medicaid Services. Participating centers are required to obtain institutional review board approval before initiating data collection, and data are transmitted from sites using a Web-based system to a secure server provided by the United Network for Organ Sharing. Contributing centers to INTERMACS can be found on the INTERMACS web site (www.INTERMACS.org). The INTERMACS data were checked for completeness by the central collection facility. Values that fell outside of predetermined limits were validated with their site of origin; however, source documents are not routinely checked against the data submitted to INTERMACS. A Medical Events Committee reviewed primary cause of death as well as neurological dysfunction, infection, bleeding, and device malfunction.
Patient Population
Between June 23, 2006, until March 31, 2011, 4108 adult patients were prospectively enrolled into the INTERMACS database for primary implantation of a durable ventricular assist device. Approximately 10% of patients eligible for inclusion in INTERMACS are not enrolled most often secondary to either their refusal to consent or their inability to consent secondary to critical illness. Patients receiving a total artificial heart or right ventricular support only (n=152) and those with serum creatinine levels unavailable at baseline and 1 month (n=591) were excluded. Patients who received a left ventricular assist device and a right ventricular assist device in the same operating room visit were included (n=267).
Estimated glomerular filtration rate (eGFR) was calculated using the 4-variable Modified Diet and Renal Disease equation.15 As only age group is collected in INTERMACS, the median age of each group (ie, 50 for 40–59, etc) was used for calculation of eGFR. Renal function was evaluated preimplant (referred to as pre-MCS or baseline) and at 1 week, 1 month, 3 months, 6 months, and 1 year post-MCS. The minority of patients that were discharged from the hospital significantly before or after the 1-month post-MCS visit had both a discharge and a 1-month case report form available, and thus serum creatinine levels were available at both time points in some patients (n=1089). The primary outcome in the survival analyses was all-cause mortality, and censoring occurred at the time of cardiac transplantation, device explantation, or if the patient was alive with the device in place at the end of follow-up (March 31, 2011).
Statistical Analysis
The primary focus of the analysis was (1) serial changes in eGFR post-MCS in the cohort and (2) the association of these changes with mortality. As such, the primary end points were (1) the magnitude and direction of serial post-MCS changes in eGFR and (2) the association between these changes and mortality. Given the large amount of missing data beyond 3 months post-MCS, much of which is likely missing not at random, the primary approach to analysis number 1 was descriptive. Plots were constructed based on initial device strategy (ie, destination versus bridge to transplant), patients that did or did not ultimately undergo cardiac transplantation, and device flow (pulsatile versus continuous). Plots were also constructed for only patients without missing data at the various time points to confirm that data missing not at random were not driving the results (data not shown). Values reported are mean±SD or median (interquartile range, IQR) for continuous variables, and percentile for categorical variables. Independent Student t test or the Wilcoxon rank-sum test was used to compare continuous variables. Pearson χ2 was used to evaluate associations between categorical variables. To examine renal function over time, mean eGFR at each interval was examined graphically for all patients, in patients stratified according to device strategy and device flow, in patients stratified according to baseline RD, and in patients with complete data through 1 year.
Cox models were used to evaluate the association between all-cause mortality and changes in eGFR. The primary analysis focused on the percent change in eGFR from baseline to 1-month post-MCS, and time zero of this analysis was 1-month postimplant. Because the percent change in eGFR variable included several extreme outliers, values beyond the largest and smallest 1% of the data were truncated at these percentiles. To capture nonlinearities in the relationship between change in eGFR and subsequent mortality, the predictor was modeled with a cubic spline using 3 degrees of freedom. This model gave substantially better Akaike Information Criteria than a simple linear model. For a more relevant clinical interpretation of this relationship, we divided eGFR into 5 quintiles of percent change in eGFR. Although interpretation is limited somewhat by missing data, these processes were repeated to examine the association between mortality and percent change in eGFR between 1 month and 3 months as an exploratory analysis. Time zero of this survival analysis was 3 months postimplant and only included patients who survived to this point (n=2416). Models were further adjusted for eGFR at various time intervals. Additional candidate covariates for multivariate modeling were selected by a combination of clinical judgment and precedence in the literature. Given the large number of events in this population, we used a low threshold to include any covariate with theoretical basis for impacting mortality or renal function, which included all baseline covariates presented in Table 1. Covariates ultimately entered into the models can be found in the text preceding the results of the model. Kaplan–Meier survival curves for all-cause mortality were constructed according to quintile of percent change in eGFR from baseline to 1 month post-MCS. These survival curves were also plotted excluding those patients with >200% IRF (top 5% of population) as a sensitivity analysis. Statistical analyses were performed using PASW Statistics version 18.0 (SPSS Inc, Chicago, IL) and R software version 2.14.2 (R Foundation for Statistical Computing, Vienna, Austria).
Table 1.
Baseline Characteristics
| Characteristic | Cohort (N=3363) |
|---|---|
| Demographics | |
| Age, y | 54.5±13.8 |
| White race | 69.6% |
| Male sex | 78.3% |
| Diabetes mellitus | 36.5% |
| Ischemic HF cause | 45.0% |
| NYHA class IV | 73.0% |
| COPD | 13.7% |
| INTERMACS profiles | |
| Profile 1 | 20.7% |
| Profile 2 | 42.4% |
| Profile 3 | 19.8% |
| Profile 4 | 11.0% |
| Profiles 5–7 | 6.1% |
| Device-related parameters | |
| LVAD alone | 92.1% |
| Continuous-flow device | 79.3% |
| Destination therapy | 17.7% |
| Medications | |
| β-Blockers | 51.2% |
| ACE inhibitors or ARBs | 38.4% |
| Loop diuretics | 78.1% |
| Inotropes | 80.8% |
| Renal function | |
| eGFR, mL/min per 1.73 m2 | 60.0±34.7 |
| Serum creatinine, mg/dL | 1.49±0.83 |
| eGFR ≥90 mL/min per 1.73 m2 | 11.7% |
| eGFR 60–89 mL/min per 1.73 m2 | 30.7% |
| eGFR 30–59 mL/min per 1.73 m2 | 46.6% |
| eGFR <30 mL/min per 1.73 m2 | 11.0% |
ACE indicates angiotensin converting enzyme; ARB, angiotensin receptor blocker; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; HF, heart failure; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; LVAD, left ventricular assist device; and NYHA, New York Heart Association.
Results
Baseline and Early Changes in Renal Function
Overall 3363 patients (81.9%) had data available to calculate pre-MCS and 1-month post-MCS eGFR. Baseline characteristics of this subset of the INTERMACS population are presented in Table 1. Preimplant RD was prevalent, with a mean pre-MCS eGFR of 60.9±34.7 mL/min per 1.73 m2, encompassing a broad spectrum of National Kidney Foundation chronic kidney disease stages (Table 1). Similar to previous reports, renal function improved substantially early post-MCS (Figure 1). The pattern of early rise in eGFR was consistent across subgroups of patients irrespective of initial device strategy (ie, destination versus bridge to transplant), patients that did or did not ultimately undergo cardiac transplantation, baseline INTERMACS Profile, and device flow (pulsatile versus continuous; Figure 1A–1C). Furthermore, this pattern of early changes in eGFR persisted among subgroups without missing data (data not shown). At 1 week, eGFR had improved to 79.6±42.6 mL/min per 1.73 m2 (P<0.001), and at 1 month, eGFR was 82.8±46.1 mL/min per 1.73 m2 (P<0.001). Notably, 61.3% of the population had an improvement of their eGFR by ≥20%, 39.3% improved by ≥50%, and 16.7% improved by ≥100% within 1 month post-MCS. significant early deterioration in renal function was less common during this time period, with a worsening in eGFR of ≥25% in 10.0%, and worsening of ≥50% in only 3.1% of patients.
Figure 1.
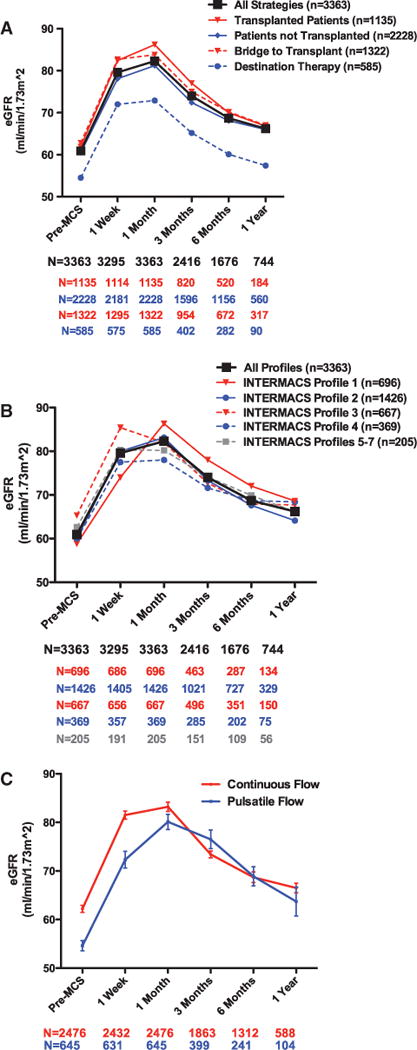
Mean eGFR over time grouped by device strategy, disease severity, and device flow. A, Mean eGFR over time by baseline device strategy or transplant status at end of follow-up. B, Mean eGFR over time by baseline INTERMACS Profile. C, Slope of the lines reflects the rate of change in eGFR over time. Sample sizes (n) refer to the number of patients in each group through 1 month, and sample sizes (N) refer to the number of patients with data available at each of the subsequent time points. eGFR indicates estimated glomerular filtration rate; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; and MCS, mechanical circularity support. Bridge to transplant defined as patients listed for transplantation or those deemed likely by the treating physician to be listed at the time of implantation.
Longer-Term Changes in Renal Function
Although post-MCS mean eGFR remained above the mean baseline value (P<0.0001 for all time points), after an initial small increase from 1 week to 1 month (P<0.0001), eGFR declined at all time points subsequent to 1 month (Figure 1). In those patients with data available at 1 year, the median improvement in eGFR was only 2.6 mL/min per 1.73 m2 (IQR, 10.1–17.2 mL/min per 1.73 m2) or 6.7% (IQR, 15%–35.8%) above the pre-MCS value. Similar to the early improvement, the pattern of late decline in eGFR was consistent across subgroups of patients based on initial device strategy, ultimate cardiac transplantation status, device flow, and among subgroups without missing data (Figure 1).
The prevalence of a ≥50% improvement in eGFR decreased over time from 39.3% at 1 month post-MCS to 27.6% at 3 months, 21.2% at 6 months, and 18.7% at 1 year. Similarly, late declines in eGFR ≥25% from 1 month post-MCS were also relatively common occurring in 28.0% at 3 months, 39.0% at 6 months, and 41.4% at 1 year. In the overall population, larger reductions in eGFR (≥50%) from the 1-month time point occurred in 3.9% by 3 months, 7.2% by 6 months, and 8.9% by 1 year. An important observation was that the late decline in eGFR was predominantly restricted to patients with early IRF (Figure 2). Ultimately, eGFR at both 6 months and 1 year was not different between patients that did or did not have a ≥50% IRF at 1 month (P≥0.23; Figure 2). However, in the group with IRF, despite a substantial decline subsequent to 1 month, eGFR remained meaningfully improved over the baseline value throughout the follow-up period (Figure 2). A significant pre-MCS to late post-MCS deterioration in eGFR was relatively uncommon with only 10.6% with ≥25% deterioration at 3 months, 14.8% at 6 months, and 15.7% at 1 year (in those patients surviving with the device in place free of transplant to the respective time points). Only 3.5% of patients experienced a ≥50% worsening from pre-MCS eGFR to 1 year.
Figure 2.
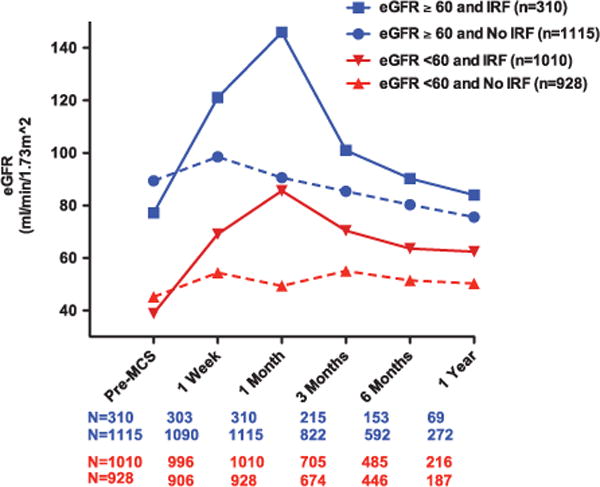
Mean eGFR over time in patients with and without pre-MCS renal dysfunction and post-MCS IRF. Mean eGFR according to presence or absence of baseline renal dysfunction further stratified by IRF at 1 mo post-MCS. Renal dysfunction defined as a pre-MCS eGFR <60 mL/min per 1.73 m2. IRF is defined as a ≥50% improvement in eGFR from pre-MCS to 1 mo post-MCS. Sample sizes (N) refer to the number of patients with data available at all time points. eGFR indicates estimated glomerular filtration rate; IRF, improvement in renal function; and MCS, mechanical circulatory support.
Pre-MCS RD and Post-MCS Changes in Renal Function
Patients with moderate to severe pre-MCS RD (eGFR <60 mL/min per 1.73 m2; 57.6% of the population) were also likely to experience significant early IRF by 1 month (P<0.0001) followed by a progressive decline in eGFR through 1 year postimplant (Figure 2; Figure I in the Data Supplement). In both patients with or without pre-MCS RD, the majority of the late decline in eGFR was derived from the group that experienced early IRF (Figure 2). Despite the late decline in eGFR, patients with pre-MCS RD had a 1-year mean eGFR that remained significantly above their pre-MCS baseline (Figure 2; Figure I in the Data Supplement; P<0.001), whereas those without RD sustained a relative decrement in mean eGFR compared with pre-MCS (P<0.0001; Figure 2; Figure I in the Data Supplement). Although limited by smaller numbers of patients with both normal (eGFR >90 mL/min per 1.73 m2) and severely reduced baseline renal function (eGFR <30 mL/min per 1.73 m2), further examination of renal function over time stratified by pre-MCS chronic kidney disease stage generally revealed similar patterns of early improvement followed by progressive decline (Figure 3; Figure I in the Data Supplement). However, patients with normal pre-MCS renal function (eGFR ≥90 mL/min per 1.73 m2) were more likely to experience worsening renal function (WRF) 1 month post-MCS (P<0.001; Figure 3) and less likely to experience marked IRF (P<0.001; Figure 3). Patients with severe RD (eGFR <30 mL/min per 1.73 m2) who received MCS were more likely to experience IRF at 1 month post-MCS and at 3 months post-MCS (P<0.001 for both; Figure 3).
Figure 3.
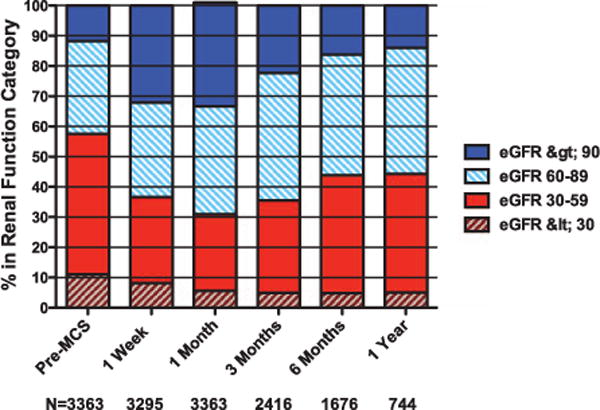
Proportion of patients across stages of renal function over time. Sample sizes (N) refer to the number of patients with data available at each time point. eGFR indicates estimated glomerular filtration rate (mL/min per 1.73 m2); and MCS, mechanical circulatory support.
Changes in Renal Function and Survival
Out of 3363 subjects surviving to 1 month after implantation, 562 died (16.7%) during a median follow-up of 7.0 (IQR, 3.4–12.7) months. The association between pre-MCS to 1 month post-MCS change in renal function and risk for mortality was roughly U-shaped with a nadir in the region of a small improvement in eGFR and higher risk associated with the extremes of both improvement and worsening in eGFR. Consistent with this nonlinear association, quintiles of pre-MCS to 1-month post-MCS change in eGFR were strongly associated with mortality (P<0.00001; Table 2). Notably, both the top quintile (>88% improvement) and the bottom quintile (<0% improvement, ie, any worsening) were both strongly and similarly associated with mortality, with 178 deaths in the bottom quintile and 137 deaths in the top quintile of improvement, respectively (Table 2; Figure 4). Interestingly, patients in quintile 2 (<22% improvement) or quintile 4 (47%–88% improvement) tended to have inferior survival compared with patients in quintile 3 (22%–47% improvement) who had the best outcome with only 67 deaths during the study period (Table 2; Figure 4). The increased mortality associated with large IRF (>88%) persisted despite exclusion of those patients with >200% improvement (top ≈5% of population; P<0.001; data not shown). Furthermore, the association between early post-MCS changes in renal function and mortality was only minimally affected by adjusting for patient and device characteristics (age, race, sex, history of diabetes mellitus, history of pulmonary disease, ischemic disease, New York Heart Association class, baseline medication use, device strategy [ie, destination or bridge to transplant], device type, device implant year, device flow, INTERMACS Profile, and need for hemodialysis) or by adjusting for pre-MCS or 1-month post-MCS eGFR (ie, time zero of the survival analysis; Table 2). Additionally, the magnitude of the mortality disadvantage associated with the various quintiles of percent change in eGFR did not differ when baseline renal function was dichotomized into >60 or <60 mL/min/1.73 m2 (P interaction=0.77) or based on baseline stage of chronic kidney disease (P interaction=0.48). Moreover, there was no significant interaction between baseline eGFR as a continuous parameter and quintiles of percent change in eGFR (P interaction=0.27). In a sensitivity analysis using quintiles of absolute change in eGFR, again, the top quintile (≥45 mL/min per 1.73 m2 improvement; adjusted hazards ratio [HR], 1.49; 95% confidence interval [CI], 1.06–2.09; P=0.021) and the bottom quintile (any worsening; adjusted HR, 1.35; 95% CI, 1.01–1.80; P=0.040) were similarly associated with reduced survival.
Table 2.
Association of Quintiles of Percent Change in eGFR Over Time and All-Cause Mortality
| Quintile of eGFR Change | HR, 95% CI | P Value | HR, 95% CI | P Value | HR, 95% CI | P Value | HR, 95% CI | P Value |
|---|---|---|---|---|---|---|---|---|
| Change in eGFR from pre-MCS to 1 mo post-MCS | Unadjusted | Adjusted for pre-MCS eGFR | Adjusted for 1 mo eGFR | Adjusted for patient characteristics*,† | ||||
| 1 (<0%, any worsening) | 1.96, 1.48–2.60 | <0.0001 | 2.22, 1.68–2.95 | <0.0001 | 1.72, 1.29–2.29 | 0.0002 | 1.63, 1.15–2.13 | 0.004 |
| 2 (0%–22% improvement) | 1.31, 0.94–1.84 | 0.12 | 1.36, 0.97–1.90 | 0.08 | 1.25, 0.89–1.76 | 0.19 | 1.25, 0.87–1.78 | 0.22 |
| 3 (22%–47% improvement) | Reference group | … | … | … | … | … | … | … |
| 4 (47%–88% improvement) | 1.45, 1.07–1.96 | 0.02 | 1.36, 1.01–1.84 | 0.049 | 1.52, 1.12–2.06 | 0.007 | 1.40, 1.02–1.93 | 0.04 |
| 5 (>88% improvement) | 2.02, 1.51–2.70 | <0.0001 | 1.61, 1.19–2.19 | 0.003 | 2.20, 1.64–2.96 | <0.0001 | 1.64, 1.19–2.26 | 0.002 |
| Change in eGFR from 1 mo to 3 months post MCS (n=2416) | Unadjusted | Adjusted for 1 mo eGFR | Adjusted for 3 mo eGFR | Adjusted for patient characteristics*,‡ | ||||
| 1 (>30% worsening) | 2.07, 1.43–3.00 | 0.0001 | 2.34, 1.62–3.40 | <0.0001 | 1.87, 1.28–2.71 | 0.001 | 1.74 1.17–2.58 | 0.006 |
| 2 (16%–30% worsening) | 1.51, 1.02–2.22 | 0.04 | 1.59, 1.08–2.35 | 0.02 | 1.47, 1.00–2.17 | 0.05 | 1.36, 0.90–2.04 | 0.14 |
| 3 (0%–16% worsening) | Reference group | … | … | … | … | … | … | … |
| 4 (0.1%–17% improvement) | 1.28, 0.87–1.87 | 0.21 | 1.21, 0.82–1.77 | 0.33 | 1.34, 0.91–1.96 | 0.14 | 1.10, 0.72–1.65 | 0.66 |
| 5 (>17% improvement) | 1.80, 1.24–2.61 | 0.002 | 1.42, 0.97–2.10 | 0.07 | 1.93, 1.32–2.80 | 0.0006 | 1.54, 1.03–2.30 | 0.03 |
CI indicates confidence interval; eGFR, estimated glomerular filtration rate; HR, hazards ratio; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; and MCS, mechanical circulatory support.
Adjusted for age, sex, race, diabetes mellitus, chronic lung disease, ischemic cause, New York Heart Association class, device type, device strategy (destination vs bridge to transplant), INTERMACS Profile, year of MCS implant, baseline β-blocker use, baseline angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use, baseline loop diuretic use, baseline inotrope use, and dialysis requirement.
Adjusted for 1-mo eGFR.
Adjusted for 3-mo eGFR.
Figure 4.
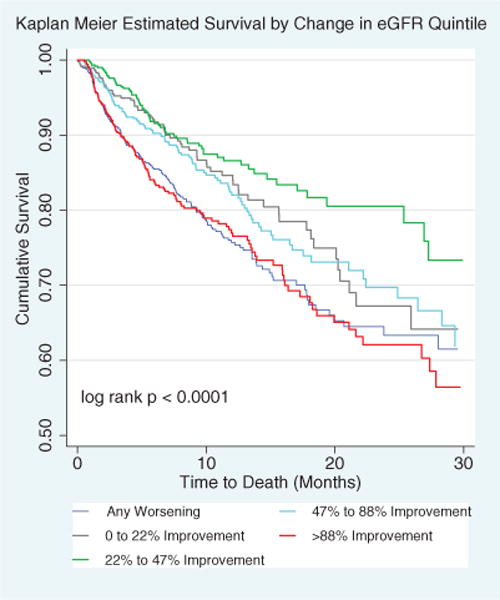
Relationship between early post-MCS changes in renal function and risk of death. Kaplan–Meier survival curves according to percent change in eGFR quintile. Percent change in eGFR is from pre-MCS to 1 mo post-MCS. eGFR indicates estimated glomerular filtration rate; and MCS, mechanical circulatory support.
In a subset of patients with data on baseline right ventricular function available (n=1544), both the top quintile (>88% improvement; HR, 2.25; 95% CI, 1.44–3.51; P<0.001) and the bottom quintile (any worsening; HR, 1.89; 95% CI, 1.22–2.93; P=0.004) in eGFR were similarly associated with increased mortality. Adjustment for the presence of baseline moderate to severe right ventricular dysfunction had minimal impact on these associations, with large IRF (>88% IRF; HR, 2.20; P<0.001) and any WRF (HR, 1.90; P=0.004) still conferring a substantially increased mortality risk.
Late Changes in eGFR and Survival
A total of 2416 (71.8% of the initial cohort) survived to the 3-month time point (ie, alive, free of transplant, without missing eGFR data) and were available for exploratory analysis of the change in eGFR from 1 month post-MCS to 3 months. In this subset of patients, 430 deaths were observed. Once again, there was a nonlinear relationship between percent change in eGFR and mortality (Table 2). Similar to the early changes in renal function, extreme improvement (80 deaths observed) and extreme worsening in renal function (86 deaths observed) were associated with increased mortality as compared with 42 deaths in the middle quintile (0%–16% worsening). Unlike the early post-MCS changes, worsening in renal function seemed to predominate this association as substantial improvement was rare (Table 2). These associations remained significant after adjustment for either 1-month eGFR (baseline eGFR of this analysis; P<0.0001) or 3-month eGFR (ie, time zero of this survival analysis; P=0.003) and persisted with adjustment for patient and device characteristics (Table 2). Importantly, the significant survival disadvantage associated with significant worsening in eGFR from 1 to 3 months (both with ≥25% and ≥50% WRF) was similar regardless if a patient had experienced an early ≥50% IRF (P interaction >0.44 for both).
Discussion
The principal findings of this study are that: (1) post-MCS, most patients experience a substantial early improvement in kidney function; (2) much of this early improvement is sustained only for a few weeks to months; and (3) large early and late changes in renal function, regardless whether worsening or improvement, are associated with worsened survival. Overall, these results reveal that dynamic changes in renal function post-MCS are common and given the strong association with mortality, potentially of clinical importance.
MCS results in significant resolution of the hemodynamic perturbations thought to be ultimately responsible for HF-induced RD. As such, it is not unexpected that significant IRF early after MCS has been described by several authors. Because much of the hemodynamic improvement post-MCS is presumed to be sustained, one might expect that the IRF would be as well, but recent single-center studies of continuous-flow populations have raised the possibility of significant long-term decline in eGFR during continued support.6,11,12 The current analysis of the large multicenter INTERMACS registry confirms that a decline in eGFR is commonly observed in the large majority of patients. However, an important incremental finding from this study is the observation that the long-term deterioration in renal function seems predominantly confined to patients who experienced significant early improvement. Although this group has a substantial decline from their peak eGFR and ultimately ends up with an eGFR similar to patients that did not experience early IRF, the long-term eGFR in these patients remains meaningfully higher than the baseline value. There are important potential mechanistic implications in the above observations. For example, if the sole driver of the late deterioration were a direct adverse effect of the device on the kidney, unless early improvement confers or identifies a specific vulnerability, a late decline would be expected to occur in all patients and not be primarily restricted to those with marked early improvement. Furthermore, the observation that a late deterioration in renal function is associated with worsened survival makes it unlikely that change in body composition (leading to nonrenal increases in serum creatinine) is the sole mechanism responsible for the late worsening, given that increases in skeletal muscle mass would be expected to be associated with neutral or improved survival.
The mortality disadvantage associated with early post-MCS WRF is not surprising considering the large body of epidemiological evidence linking postcardiac surgery acute kidney injury with worsened outcomes.16–18 Interestingly, a significant worsening in eGFR occurring late after MCS implantation was also associated with worsened survival, despite the fact that eGFR remained above the pre-MCS level. The finding that the risk persisted despite adjustment for either the pre- or postworsening eGFR suggests that the risk is not simply a reflection of the eGFR ultimately achieved but factors related to the change itself.
Remarkably, large improvements in kidney function were actually associated with reduced survival of a similar magnitude to WRF. This seemingly counterintuitive finding may stem from the fact that there was no true control group (eg, patients with the potential for IRF who did not undergo MCS), and hence the comparison was to those patients receiving MCS that had relatively stable post-MCS renal function. A requisite for treatment-induced recovery of renal function is to have a reversible form of RD at baseline, with the most likely cause being HF-induced RD. As a result, it is not surprising that comparison of patients with substantial improvement to patients with relatively stable renal function (indicative of a lesser degree of pre-MCS HF-induced RD and thus lower pre-MCS disease severity) revealed greater mortality in patients with larger degrees of IRF.
Another notable observation from this data is that people with the most severe RD at baseline tended to have the most durable IRF. An important caveat in the interpretation of the above observation is that many of the patients with severe RD who undergo MCS are a highly selected group that was thought to have reversible RD at the time of MCS evaluation. As such, caution must be exercised before concluding that unselected HF patients (ie, patients being considered for MCS) with severe RD would have renal outcomes similar to the few patients in INTERMACS who were ultimately selected to receive MCS.
Lastly, there are several biologically plausible mechanisms by which chronic exposure to nonpulsatile flow might directly cause a deterioration of renal function, including periarteritis, hyperplasia of renal arterial smooth muscle cells, and activation of the renin–angiotensin–aldosterone system.19–22 Although multiple limitations in this data set preclude formal comparison of changes in renal function between device flow types, qualitatively, a substantial early improvement followed by late decline in renal function was observed with both pulsatile and continuous-flow devices. As such, it would seem unlikely that the late decline in renal function can be entirely attributed to direct adverse effects of continuous flow. Furthermore, the superior survival in patients with continuous-flow devices highlights that many factors beyond renal function ultimately contribute to patient outcomes.
Study Limitations
Although INTERMACS represents high-quality registry data, limitations inherent to the retrospective analyses of such data apply, and causality is impossible to demonstrate. Because of the inclusion of only patients with FDA-approved devices, device selection was driven primarily by approval status at the time of implant, leading to the prevalence of pulsatile flow devices clustered in the early portion of the registry and exclusion of a relatively large population of patients with investigational devices. Furthermore, confounding by indication among approved devices (ie, anticipation of the potential requirement for biventricular support) likely occurred. Patients with significant RD deemed irreversible are often not referred for MCS, limiting generalizability, particularly in the subgroup of patients with baseline RD. Additionally, the probability for informative censoring exists because significant IRF may contribute to the decision to list a patient for cardiac transplantation. As such, the survival analyses should be interpreted as hypothesis-generating only. To maintain confidentiality, protected health information was largely not collected and, therefore, the registry cannot confirm death or cardiac transplantation status, possibly underestimating the frequency of these events. Moreover, information on age was limited to age ranges spanning ≈20 years, potentially leading to both over-and underestimates of static eGFR; however, because all estimates of GFR in an individual patient used the same numeric age, relative differences in GFR over time are likely less biased. Still, INTERMACS was not specifically designed to examine serial changes in renal function. Physicians were not blinded to the renal function data and likely altered treatment based on this information. Furthermore, the interval between renal function data points is relatively long in the context of how quickly changes in renal function can occur and thus may not encapsulate the fluctuations in renal function that may have occurred between them. Given the limited data available, we were also unable to adjust for the potential effects of postoperative right ventricular dysfunction or ultrafiltration on these fluctuations. Furthermore, at 3 months post-MCS, there is a significantly different level of medical supervision, therapies, and adverse events such as gastrointestinal bleeding/infection compared with 1 month when many patients are still in the hospital or recently discharged. These differences may have played a role in the observed changes in renal function. Additionally, competing pathophysiologic events may occur such that a patient could have both some resolution of HF-induced RD and perioperative acute tubular necrosis (which has been reported to occur in a substantial proportion of cardiac surgery patients), leading to an unpredictable net effect on eGFR.17,23 Lastly, using creatinine-based estimates of GFR in a population known to have large fluctuations in body composition, such as post-MCS patients, is prone to produce biased results.
Conclusions
In a contemporary multicenter population, substantial early improvement in eGFR after MCS is common but seems to be largely transient. Large post-MCS changes in renal function, both improvement and worsening, identify patients at high risk of death. Further research is necessary to better understand these changes in eGFR, their associated mortality disadvantage, and if the adverse renal and clinical outcomes can be modified by changes in medical or device strategies.
Supplementary Material
CLINICAL PERSPECTIVE.
Because the durability of mechanical circulatory support (MCS) devices has improved, the cumulative effects of long-term support on noncardiac organ function have become an important area of interest. significant early improvement in renal function (IRF) post-MCS has now been described in several series, which is reassuring given the theoretical concerns and experimental data regarding possible detrimental effects of nonpulsatile flow on the kidney. However, several small studies have recently reported a significant late deterioration in renal function after MCS. In the present study, we leveraged the strengths of the multicenter Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) registry to provide a detailed and objective description of the changes in renal function and their associated clinical implications, longitudinally after MCS placement. We found that post-MCS, renal function improved substantially, but in the majority of patients, this improvement seems to be largely transient. Importantly, patients with continuous-flow and pulsatile devices qualitatively experienced similar changes in renal function over time. Surprisingly, both marked improvement and worsening in renal function were associated with increased mortality. Although the trajectory of renal function post-MCS was somewhat more favorable in patients with severe baseline renal dysfunction, there remained a significant hazard associated with IRF even in these patients. Part of this seemingly paradoxical association between IRF and worsened survival may be driven by the high rate of recurrence of baseline renal dysfunction with prolonged support. Additional investigation to better understand the mechanisms and potential treatment strategies for these high-risk changes in renal function is imperative.
Acknowledgments
We would like to thank Dr Frank Pagani (Ann Arbor, Michigan) and Dr David Naftel (Birmingham, Alabama) for their review and revisions of the article.
Sources of Funding
This project has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health (NIH), Department of Health and Human Services, under Contract No. HHSN268201100025C, and with NIH grant numbers 5T32HL007891, 5T32HL007843-15, 1K23HL114868-02, and K24DK090203.
Footnotes
The Data Supplement is available at http://circheartfailure.ahajournals.org/lookup/suppl/doi:10.1161/CIRCHEARTFAILURE.113.000507/-/DC1.
Disclosures
None.
References
- 1.Bock JS, Gottlieb SS. Cardiorenal syndrome: new perspectives. Circulation. 2010;121:2592–2600. doi: 10.1161/CIRCULATIONAHA.109.886473. [DOI] [PubMed] [Google Scholar]
- 2.Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52:1527–1539. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 3.Khot UN, Mishra M, Yamani MH, Smedira NG, Paganini E, Yeager M, Buda T, McCarthy PM, Young JB, Starling RC. Severe renal dysfunction complicating cardiogenic shock is not a contraindication to mechanical support as a bridge to cardiac transplantation. J Am Coll Cardiol. 2003;41:381–385. doi: 10.1016/s0735-1097(02)02823-1. [DOI] [PubMed] [Google Scholar]
- 4.Sandner SE, Zimpfer D, Zrunek P, Dunkler D, Schima H, Rajek A, Grimm M, Wolner E, Wieselthaler GM. Renal function after implantation of continuous versus pulsatile flow left ventricular assist devices. J Heart Lung Transplant. 2008;27:469–473. doi: 10.1016/j.healun.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Lund LH, Matthews J, Aaronson K. Patient selection for left ventricular assist devices. Eur J Heart Fail. 2010;12:434–443. doi: 10.1093/eurjhf/hfq006. [DOI] [PubMed] [Google Scholar]
- 6.Sandner SE, Zimpfer D, Zrunek P, Rajek A, Schima H, Dunkler D, Grimm M, Wolner E, Wieselthaler GM. Renal function and outcome after continuous flow left ventricular assist device implantation. Ann Thorac Surg. 2009;87:1072–1078. doi: 10.1016/j.athoracsur.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 7.Kamdar F, Boyle A, Liao K, Colvin-adams M, Joyce L, John R. Effects of centrifugal, axial, and pulsatile left ventricular assist device support on end-organ function in heart failure patients. J Heart Lung Transplant. 2009;28:352–359. doi: 10.1016/j.healun.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Butler J, Geisberg C, Howser R, Portner PM, Rogers JG, Deng MC, Pierson RN., 3rd Relationship between renal function and left ventricular assist device use. Ann Thorac Surg. 2006;81:1745–1751. doi: 10.1016/j.athoracsur.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 9.Demirozu ZT, Etheridge WB, Radovancevic R, Frazier OH. Results of HeartMate II left ventricular assist device implantation on renal function in patients requiring post-implant renal replacement therapy. J Heart Lung Transplant. 2011;30:182–187. doi: 10.1016/j.healun.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 10.Russell SD, Rogers JG, Milano CA, Dyke DB, Pagani FD, Aranda JM, Klodell CT, Jr, Boyle AJ, John R, Chen L, Massey HT, Farrar DJ, Conte JV, HeartMate II Clinical Investigators Renal and hepatic function improve in advanced heart failure patients during continuous-flow support with the HeartMate II left ventricular assist device. Circulation. 2009;120:2352–2357. doi: 10.1161/CIRCULATIONAHA.108.814863. [DOI] [PubMed] [Google Scholar]
- 11.Hasin T, Topilsky Y, Schirger JA, Li Z, Zhao Y, Boilson BA, Clavell AL, Rodeheffer RJ, Frantz RP, Edwards BS, Pereira NL, Joyce L, Daly R, Park SJ, Kushwaha SS. Changes in renal function after implantation of continuous-flow left ventricular assist devices. J Am Coll Cardiol. 2012;59:26–36. doi: 10.1016/j.jacc.2011.09.038. [DOI] [PubMed] [Google Scholar]
- 12.Singh M, Shullo M, Kormos RL, Lockard K, Zomak R, Simon MA, Bermudez C, Bhama J, McNamara D, Toyoda Y, Teuteberg JJ. Impact of renal function before mechanical circulatory support on posttransplant renal outcomes. Ann Thorac Surg. 2011;91:1348–1354. doi: 10.1016/j.athoracsur.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 13.Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, Sun B, Tatooles AJ, Delgado RM, III, Long JW, Wozniak TC, Ghumman W, Farrar DJ, Frazier OH, HeartMate II Investigators Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 14.Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, Miller MA, Baldwin JT, Young JB. The Fourth INTERMACS Annual Report: 4,000 implants and counting. J Heart Lung Transplant. 2012;31:117–126. doi: 10.1016/j.healun.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F, Chronic Kidney Disease Epidemiology Collaboration Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 16.Alba AC, Rao V, Ivanov J, Ross HJ, Delgado DH. Predictors of acute renal dysfunction after ventricular assist device placement. J Card Fail. 2009;15:874–881. doi: 10.1016/j.cardfail.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 17.Hobson CE, Yavas S, Segal MS, Schold JD, Tribble CG, Layon AJ, Bihorac A. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009;119:2444–2453. doi: 10.1161/CIRCULATIONAHA.108.800011. [DOI] [PubMed] [Google Scholar]
- 18.Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53:961–973. doi: 10.1053/j.ajkd.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Welp H, Rukosujew A, Tjan TD, Hoffmeier A, Kösek V, Scheld HH, Drees G. Effect of pulsatile and non-pulsatile left ventricular assist devices on the renin-angiotensin system in patients with end-stage heart failure. Thorac Cardiovasc Surg. 2010;58(suppl 2):S185–S188. doi: 10.1055/s-0029-1240709. [DOI] [PubMed] [Google Scholar]
- 20.Kihara Sai, Litwak KN, Nichols L, Litwak P, Kameneva MV, Wu Z, Kormos RL, Griffith BP. Smooth muscle cell hypertrophy of renal cortex arteries with chronic continuous flow left ventricular assist. The Ann Thorac Surg. 2003;75:178–183. doi: 10.1016/s0003-4975(02)04087-0. discussion 183. [DOI] [PubMed] [Google Scholar]
- 21.Ootaki C, Yamashita M, Ootaki Y, Kamohara K, Weber S, Klatte RS, Smith WA, Massiello AL, Emancipator SN, Golding LA. Reduced pulsatility induces periarteritis in kidney: role of the local renin–angiotensin system. J Thorac Cardiovasc Surg. 2008;136:150–158. doi: 10.1016/j.jtcvs.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saito S, Westaby S, Piggot D, Dudnikov S, Robson D, Catarino PA, Clelland C, Nojiri C. End-organ function during chronic nonpulsatile circulation. Ann Thorac Surg. 2002;74:1080–1085. doi: 10.1016/s0003-4975(02)03846-8. [DOI] [PubMed] [Google Scholar]
- 23.Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol. 2006;1:19–32. doi: 10.2215/CJN.00240605. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


