Abstract
Bacterial lipopolysaccharide (LPS) in rodents is an established model for studying innate immune responses to gram-negative bacteria and mimicking symptoms of infections including reduced food intake associated with decreased circulating total ghrelin levels. The ghrelin-acylating enzyme, ghrelin-O-acyltransferase (GOAT) involved in the formation of acyl ghrelin (AG) was recently identified. We investigated changes in circulating AG, desacyl ghrelin (DG) and GOAT induced by intraperitoneal LPS (100μg/kg) and associated changes in food intake. Plasma AG and total ghrelin were assessed by radioimmunoassay, GOAT protein by Western blot and mRNA by RT-qPCR. DG was derived from total minus AG. Plasma AG and DG were decreased at 2h, 5h and 7h (p<0.01) post injection compared to vehicle and recovered at 24h. At 2h there was a significantly greater decrease of AG (-53%) than DG (-28%) resulting in a decreased AG/DG ratio (1:5, p <0.01), which thereafter returned to pre-injection values (1:3). This altered ratio was associated with a 38% decrease in plasma GOAT protein compared to vehicle (p <0.001), whereas gastric GOAT protein was slightly increased by 10% (p<0.05). GOAT mRNA expression was unchanged. Food intake was reduced by 58% measured during the 1.5-2h period post LPS injection. Decreased plasma AG and DG preceded the rise in rectal temperature and blood glucose that peaked at 7h. These data indicate that LPS induces a long-lasting reduction of AG and DG levels that may have a bearing with the decrease in food intake. The faster drop in AG than DG within 2h is associated with reduced circulating GOAT.
Keywords: acyl ghrelin, desacyl ghrelin, GOAT, LPS, RAPID method, rat
1. Introduction
Ghrelin was discovered in 1999 by Kojima and co-workers and identified as the endogenous ligand of the orphan growth hormone secretagogue receptor 1a (GHS-R1a) [17]. Ghrelin circulates in two major forms, acyl and desacyl ghrelin [11]. Acyl ghrelin has a unique octanoyl group on the serine in position three which is essential to activate the GHS-R1a [3]. Therefore, desacyl ghrelin that lacks this lipophilic group was initially considered a non-active molecular form. In recent years, growing evidence has indicated that desacyl ghrelin is a bioactive peptide exerting biological actions different from those of acyl ghrelin in a GHS-R1a independent manner [35]. Acyl ghrelin plays a major role as stimulator of food intake [35], whereas desacyl ghrelin may modulate this effect [13].
Initially, studies indicated that desacyl ghrelin accounts for >90% of the circulating ghrelin [11] with a ratio of acyl/desacyl ghrelin varying from 1:15 to 1:55 [11, 29]. We recently developed the new RAPID method for blood processing that prevents ex vivo peptide degradation and improves the recovery of endocrine gut peptides along with the detection of their correct molecular form [37]. For acyl ghrelin, the RAPID method reduced ex vivo conversion into the desacyl form by >80% [37] indicating the importance of optimizing blood sampling and processing to assess circulating levels of the molecular forms of the peptide.
The enzyme responsible for the acylation of ghrelin was unknown for almost one decade and has recently been identified in mice and humans as the fourth member of the superfamily of membrane-bound O-acyltransferases (MBOATs) and termed ghrelin-O-acyltransferase (GOAT) [9, 42]. GOAT is the only physiologically occurring ghrelin-acylating enzyme as shown by the complete absence of acyl ghrelin in GOAT knockout mice [9]. GOAT mRNA was shown to be highly co-expressed with ghrelin in X/A-like cells of the mouse gastric mucosa as assessed by in situ hybridization [32]. We recently demonstrated that in rats GOAT-immunoreactive cells were predominantly found in the lower third of glands and only half of the GOAT-positive cells co-expressed ghrelin whereas the other half co-labeled with histidine decarboxylase, a marker for enterochromaffin-like cells [36]. By contrast in mice, we found that nearly all gastric GOAT-immunopositive cells co-labeled with ghrelin indicating differential expression of GOAT in the mouse and rat gastric oxyntic mucosa [36]. Of interest was the detection of GOAT protein in the plasma and its regulation in relation with the metabolic status as shown by an increase after 24h fasting in rats and mice compared to ad libitum feeding [36]. These parallel changes suggest a regulatory function of GOAT in the circulation to influence the acylation of ghrelin [36, 37].
Injection of lipopolysaccharide (LPS), originating from cell walls of gram-negative bacteria such as Helicobacter pylori (H pylori), is a well characterized experimental model that mimics most of the clinical features of systemic infections related to acute phase responses such as increased body temperature and reduced appetite as well as gastric transit when LPS is injected at low doses in rodents [18, 19]. We previously reported that the LPS-induced reduction of food intake is associated with a sustained decrease of fasting plasma levels of total ghrelin in rats [2, 40]. Peripheral injection of ghrelin prevented the LPS-induced decrease in food intake, gastric emptying and gastrointestinal transit [7, 40] indicative of a potential role for altered ghrelin signaling in the decline of food ingestion and digestive processes. However, the impact of LPS on circulating acyl and desacyl ghrelin and whether those changes are associated with alterations of gastric and plasma GOAT protein expression have not been characterized.
In the present study, we investigated the 24-h time course of changes in plasma levels of acyl and desacyl ghrelin following intraperitoneal (ip) injection of LPS in conscious rats using the recently described RAPID method [37]. Rats were fasted to induce elevated circulating ghrelin levels and drive to eat as described before [40]. Changes in blood glucose and rectal temperature were monitored simultaneously as index of homeostatic changes induced by LPS. In addition the time course of the LPS-induced alterations of food intake response to a fast was monitored for 24 h in separate studies. Lastly, changes in gastric and plasma GOAT protein expression as well as gastric GOAT mRNA levels were assessed by Western blot and real-time reverse transcription polymerase chain reaction (RT-qPCR) respectively at 2 post LPS injection, when significant changes in the acyl/desacyl ghrelin ratio were observed.
2. Methods
2. 1. Animals
Adult male Sprague-Dawley rats (Harlan, San Diego, CA) weighing 280–320 g were housed 4/cage under controlled illumination (06:00-18:00 h) and temperature (21–23 °C). Animals had ad libitum access to standard rodent chow (Prolab RMH 2500; LabDiet, PMI Nutrition, Brentwood, MO) and tap water. Protocols were approved by the Animal Research Committees at the Veterans Affairs Greater Los Angeles Healthcare System (# 05-058-02). Experiments were started between 09:00 and 10:00 h.
2.2. Surgery
Intravenous (iv) cannulation was essentially performed as described before [40]. Briefly, rats were anesthetized with a mixture of ketamine (75 mg/kg ip; Fort Dodge Laboratories, Fort Dodge, IA) and xylazine (5 mg/kg ip; Mobay, Shawnee, KS). The right external jugular vein was cannulated using a sterile PE-50 tube filled with sterile saline, the catheter exteriorized between the scapulae via subcutaneous tunneling, secured to the skin, filled with heparin solution to maintain lumen patency (200 units/ml) and closed by wire obturator. Rats were single housed after surgery and allowed to recover for three days during which they were accustomed to the experimental procedures, namely handling for intraperitoneal (ip) injection, light hand-restraint for blood withdrawal and measurement of rectal temperature. Body weight was monitored before surgery and during the recovery period.
2.3. Blood sampling and processing
Single housed rats were food deprived for 17h with free access to water. Starting at 09:00 vehicle (pyrogen-free saline, 300 μl) or LPS (Escherichia coli, serotype 055:B5; Sigma, St. Louis, MO, 100 μg/kg body weight in 300 μl saline) was injected ip. Repeated blood withdrawals (0.5 ml) were performed from the jugular vein cannula of conscious lightly hand-restrained rats directly before and at 2, 5, 7 and 24h post vehicle or LPS. During that time animals did not have food but free access to water.
Blood samples were processed according to the RAPID method as recently described in detail [37]. Briefly, immediately after withdrawal, blood was diluted 1:10 in ice-cold buffer (pH 3.6) containing 0.1 M ammonium acetate, 0.5 M NaCl, and enzyme inhibitors (diprotin A, E-64-d, antipain, leupeptin, chymostatin, 1 μg/ml; Peptides International, Louisville, KY), and centrifuged at 3000 rpm for 10 min at 4 °C. Sep-Pak C18 cartridges (360mg, 55-105μm, product-no. WAT051910, Waters Corporation, Milford, MA) were charged with 5 ml 100% acetonitrile and equilibrated with 10 ml 0.1% trifluoroacetate (TFA). The equilibrated cartridges were loaded with sample, rinsed with 3 ml 0.1% TFA and eluted with 2 ml 70% acetonitrile containing 0.1% TFA. Eluted samples were dried by vacuum centrifugation and powder stored at -80 °C until further processing. Samples were re-suspended in double distilled H2O according to the original volume of plasma. Total and acyl ghrelin levels were measured using specific radioimmunoassay kits (# GHRT-89HK and GHRA-88HK, Millipore, Billerica, MA). Acyl and total ghrelin levels will also comprise other forms of ghrelin such as des-Gln14-ghrelin which have been detected in low amounts in the stomach [12] since the antibodies used have been raised against the N- and C-terminus respectively (according to manufacturer's information). Desacyl ghrelin was calculated as the difference of total minus acyl ghrelin for each individual sample.
2.4. Gel electrophoresis and Western blot analysis of GOAT protein in plasma and gastric corpus
Overnight fasted rats (n=3/group) were injected ip (300 μl) with LPS (100 μg/kg body weight in saline) or vehicle (saline) and euthanized by decapitation at 2 h after injection. Gastric corpus and trunk blood were collected for assessment of GOAT protein expression. Crude protein fractions of gastric corpus mucosa and plasma were prepared as described before [36]. Briefly, trunk blood (1 ml) was collected in glass tubes, transferred to tubes containing aprotinin (0.6 Trypsin Inhibitory Unit; ICN Pharmaceuticals, Costa Mesa, CA), EDTA (7.5%, 10 μl/0.5 ml blood; Sigma, St. Louis, MO) and phenylmethylsulphonyl fluoride (PMSF, 1mM) using syringes rinsed with EDTA (7.5%) and immediately centrifuged at 4 °C for 10 min at 3000 g. The supernatants were collected and stored at -80 °C. Likewise, the stomachs were immediately opened and rinsed, the corpus mucosa scraped off and homogenized (Dounce, Wheaton, Millville, NY) on ice in ice-cold phosphate-buffered saline (PBS) containing one tablet of protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN) and PMSF (1mM). Crude protein was obtained by centrifugation of the homogenates in the Sorvall centrifuge at 12,000 g for 20 min at 4 °C to remove cell debris and nuclei. Protein concentrations for stomach mucosa and plasma were immediately determined using a BCA protein assay according to the manufacturer's protocol (Pierce Biotechnology, Rockford, IL).
Gel samples were prepared by mixing protein samples with gel sample buffer [4% SDS, 0.05% bromphenol blue (wt/vol), 20% glycerol, 1% mercaptoethanol (vol/vol) in 0.1 Tris buffer, pH 6.8]. The samples were boiled for 1 min before gel electrophoresis and equal amounts of protein (20 μg/lane) were loaded on a 4-12% SDS-polyacrylamide gel (SDS-PAGE, NuPage; Invitrogen, Carlsbad, CA) and run in 2-(N-morpholino)ethanesulfonic acid buffer. After SDS-PAGE, proteins were transferred by electrophoresis to nitrocellulose membranes (BioPlot-NC; Costar, Cambridge, MA) for 1h at 4 °C. Membranes were washed in distilled water and stained in Ponceau-S in 3% trichloroacetic acid solution, and an image was taken. Membranes were washed twice with Tween-Tris-buffered saline (TBS; 10 mM Tris, 150 mM NaCl, and 0.05% Tween, vol/vol) and incubated in Tween-TBS containing 5% (wt/vol) nonfat milk (Carnation, Nestlé, Glendale, CA). After 60 min, the membranes were incubated in anti-GOAT polyclonal antibody (GenScript Corporation, Piscataway, NJ) solution diluted 1:500 in Tween-TBS. This antibody was raised against the amino acids 273-286 of rat membrane-bound O-acyltransferase (MBOAT)4 protein and has been established previously to be suited for Western blot assessment of rat and mouse GOAT protein [36]. After 1h, membranes were washed five times with Tween-TBS and incubated with the secondary antibody solution (anti-rabbit IgG conjugated to alkaline phosphatase; Promega, Madison, WI) diluted 1:2000 in Tween-TBS. After 1h, membranes were washed three times before color development in alkaline phosphatase buffer [100 mM Tris, 100 mM NaCl, and 5 mM MgCl2 (pH 9.5)] containing 0.3% nitroblue tetrazolium solution (vol/vol) and 0.15% 5-bromo-4-chloro-3-indolyl-l-phosphate solution (vol/vol) according to the manufacturer's instructions for 5-10 min. The Western blot (four squares of 352 pixels each/lane) was analyzed using Scion Image 4.0.3 (Scion Corp., Frederick, MD). The same Western blot was stained again for β-actin (MW 45 kDa) using a polyclonal antibody (1:1000, Ab # 4967, Cell Signaling Technology Inc., Danvers, MA) following the protocol described above. Analysis was also performed with Scion Image 4.0.3 (Scion Corp.) and gastric GOAT protein expression was normalized to the housekeeping protein, β-actin.
2.5. RT-qPCR for GOAT mRNA expression in gastric corpus mucosa
Total RNA from the gastric corpus mucosa was isolated at 2h post LPS or vehicle injection in the same groups of rats described in section 2.4. RNA was denatured at 65 °C for 5 min and used to synthesize first-strand cDNA by reverse transcription with the ThermoScriptTM RT-PCR system (Invitrogen, CA). RT-qPCR for GOAT mRNA expression was performed using DNA Engine Opticon1 2 Detection System interfaced to the Opticon MONITORTM Analysis Software version 2.01 (MJ Research Inc., Waltham, MA) in a 20 μl reaction volume. The optimized reaction contained 10 μl of SYBR1 Premix Ex TaqTM (Perfect Real Time, Takara Mirus Bio Inc., Madison, WI), 1 μl each of oligonucleotide primers (10 mM), 1 μl of the cDNA synthesis reaction, and 7 μl of H2O. Selected primers for GOAT were GGACGACTCTCTCCTTCACG (forward, f) and TCACAGACCAGCACAGGAAG reverse (r) and for the housekeeping gene, hypoxanthine guanine ribotransferase (HPRT), CAGTCCCAGCGTCGTGATTA (f) and AGCAAGTCTTTCAGTCCTGTC (r) [6]. Each amplification was followed by a melting curve resulting in only one peak for each amplicon indicative of amplification of only one product. This was confirmed by agarose gel electrophoresis of the RT-PCR products. The cycle of threshold C(T) was determined as the fluorescent signal (binding of SYBR green to double-stranded cDNA) of 1 SD over background. All reactions were carried out in duplicate, and three separate amplifications for each primer pair were performed. Standard curves were constructed with four serial dilution points of control cDNA (combined cDNA from all samples, 100 ng–100 pg). Data presented were derived from starting quantity (SQ) values of each sample normalized to the housekeeping gene HPRT. The relative expression ratio of the target gene compared to the reference gene HPRT was calculated using the Pfaffl equation [28].
2.6. Blood glucose
At the same time points when blood was collected for ghrelin measurement (before, 2, 5, 7 and 24h post vehicle or LPS injection), blood glucose levels were assessed by commercial test strips (One-Touch Ultra; LifeScan, Milpitas, CA).
2.7. Rectal temperature
During the same experiment, directly after blood collection rectal temperature was assessed before and 2, 5, 7 and 24h post vehicle or LPS injection in conscious animals using a thermometer lubricated with chlorhexidine gluconate (Surgilube, E. Fougera & Co., Atlanta Inc., NY), inserted 3 cm from the anus into the distal colon (Lumiscope Co., Inc., Piscataway, NJ) and left for 10 seconds in lightly hand restrained animals to obtain stable reading.
2.8. Food intake
In separate studies, rats were accustomed to single housing for 2, 8 and 24h as well as handled for ip injection during the week before the experiment. Then, they were single housed and food deprived overnight with free access to water. Fasted rats were injected ip with LPS (100 μg/kg body weight in 300 μl saline) or vehicle (300 μl saline) and pre-weighed standard rodent chow was made available at 1.5h post injection. Food intake was monitored at 2, 5, 7 and 24h post LPS or vehicle injection and expressed as g/300g body weight (bw).
2.9. Statistical analysis
Data are expressed as mean ± sem and were analyzed by ANOVA followed by all pair-wise multiple comparison procedures (Tukey post hoc test) or two-way ANOVA followed by Holm-Sidak method. The correlations between plasma levels of acyl and desacyl ghrelin and blood glucose or rectal temperature were determined by univariate linear regression. P < 0.05 was considered significant.
3. Results
3.1. LPS decreases acyl and desacyl ghrelin plasma levels
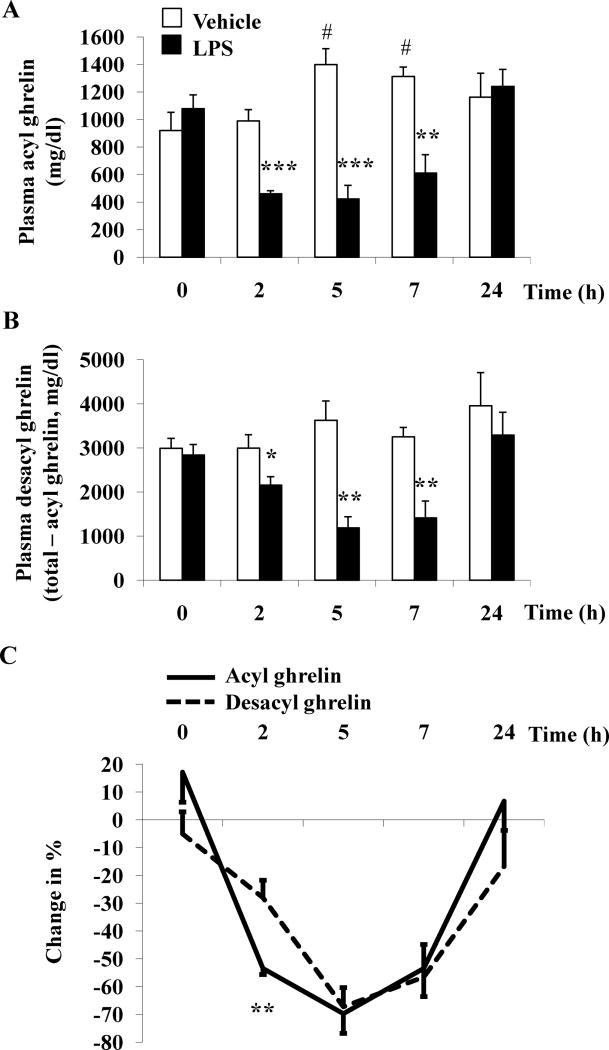
In rats fasted overnight, before injection of vehicle or LPS plasma levels of acyl ghrelin (921.0 ± 131.5 pg/ml and 1079.2 ± 99.9 pg/ml respectively, p > 0.05) and desacyl ghrelin (2991.0 ± 224.2 pg/ml and 2839.0 ± 237.9 pg/ml respectively, p > 0.05) were not significantly different. Intraperitoneal injection of LPS (100μg/kg) during the light phase significantly decreased acyl ghrelin levels from 2h (461.0 ± 22.3 vs. 990.2 ± 82.0 pg/ml, p < 0.001) to 7h (611.0 ± 133.7 vs. 1312.5 ± 68.2 pg/ml, p < 0.01) post injection compared to vehicle with a maximum response at 5h (423.4 ± 99.1 vs. 1399.3 ± 115.8 pg/ml, p < 0.001; Fig. 1A). Acyl ghrelin plasma levels were completely restored at 24h post injection compared to vehicle-treated animals (1241.2 ± 122.8 vs. 1162.5 ± 173.5 pg/ml, p > 0.05; Fig. 1A). In contrast to the LPS-treated group, in vehicle-treated animals, acyl ghrelin levels rose at 5h (1399.3 ± 115.8 pg/ml, p < 0.05) and 7h (1312.5 ± 68.2 pg/ml, p < 0.05) post injection compared to pre-injection values (921.0 ± 131.5 pg/ml, Fig. 1A). Two-way ANOVA showed a significant impact of treatment (F(1,37)=32.6, p < 0.001), time (F(4,37)=5.0, p < 0.01) and treatment × time (F(4,37)=10.0, p < 0.001).
Fig. 1.
LPS reduces plasma concentrations of acyl and desacyl ghrelin in conscious rats. LPS (100 μg/kg body weight) or vehicle was injected ip during the light phase in overnight fasted rats chronically implanted with a jugular catheter. Blood was withdrawn before and 2h, 5h, 7h and 24h post injection. Acyl ghrelin (A) and total ghrelin levels were assessed by radioimmunoassay. Desacyl ghrelin (B) levels were obtained by calculating the difference of total ghrelin minus acyl ghrelin. Each bar represents the mean ± sem of 5 rats/group. * p < 0.05, ** p < 0.01 and *** p < 0.001 vs. vehicle at the respective time point; # p < 0.05 vs. time point 0. Percentage of changes in plasma acyl and desacyl ghrelin levels induced by LPS (C). Data are expressed as % change (mean ± sem) in the LPS group compared to vehicle at the respective time point. ** p < 0.01 vs. % change of desacyl ghrelin.
Similarly, desacyl ghrelin plasma levels calculated as the difference of total ghrelin minus acyl ghrelin significantly declined following LPS treatment from 2h (2156.0 ± 188.7 vs. 2997.6 ± 299.8 pg/ml, p < 0.05) to 7h (1411.2 ± 383.3 vs. 3253.3 ± 208.6 pg/ml, p < 0.01) post injection compared to vehicle-treated rats with a nadir response at 5h post injection (1186.0 ± 250.0 vs. 3625.1 ± 439.1 pg/ml, p < 0.01; Fig. 1B). As observed for acyl ghrelin, desacyl ghrelin levels recovered at 24h post injection compared to vehicle (3291.8 ± 514.1 vs. 3957.5 ± 750.9 pg/ml, p > 0.05; Fig. 1B). Time course studies of desacyl ghrelin plasma levels after ip injection of vehicle showed that there were no significant changes throughout the 24h experimental period (p > 0.05; Fig. 1B). Two-way ANOVA indicated a significant influence of treatment (F(1,37)=24.5, p < 0.001), time (F(4,37)=3.8, p < 0.05) and treatment × time (F(4,37)=3.0, p < 0.05).
Comparison of the percentage of suppression showed a 53% decrease of acyl ghrelin compared to a 28% reduction of desacyl ghrelin levels at 2h post LPS injection (p < 0.01; Fig. 1C). This resulted in a significantly decreased acyl/desacyl ghrelin ratio at 2h post LPS injection (p < 0.01) whereas no significant changes in ratio were observed at later time points (Table 1).
Table 1. Acyl/desacyl ghrelin plasma ratio after intraperitoneal injection of LPS.
| Treatmenta | Acyl/desacyl ghrelin plasma ratio | ||||
|---|---|---|---|---|---|
| 0h | 2h | 5h | 7h | 24h | |
| Vehicle | 1:3 | 1:3 | 1:3 | 1:3 | 1:4 |
| LPS | 1:3 | 1:5* | 1:3 | 1:2 | 1:3 |
Overnight fasted rats were injected ip with LPS (100μg/kg body weight) or vehicle (pyrogen-free saline) and acyl and total ghrelin levels were assessed before, and at 2, 5, 7 and 24h post injection. Desacyl ghrelin levels were obtained by calculating the difference between total and acyl ghrelin. The acyl/desacyl ghrelin ratio was calculated from the mean values of 5 rats/group; p < 0.05 vs. vehicle.
3.2. LPS decreases plasma GOAT protein concentration whereas gastric GOAT proteinexpression is increased
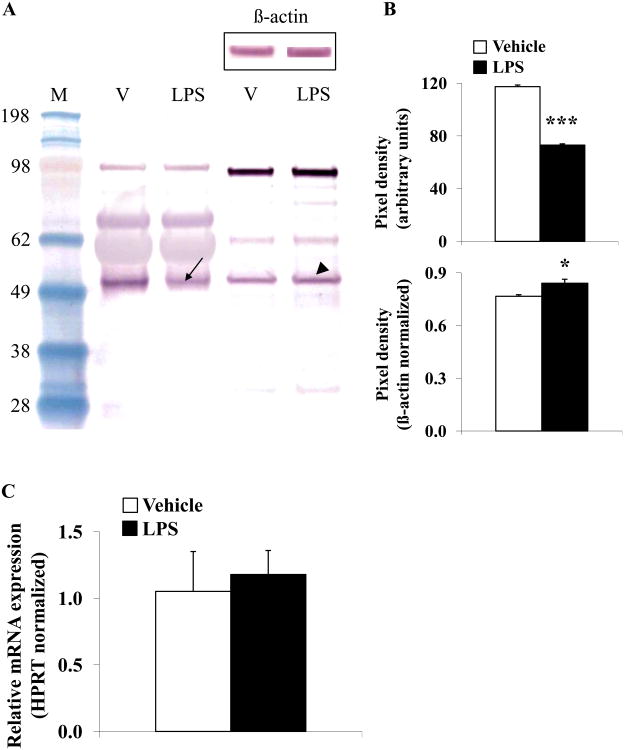
Since we observed a more rapid decrease of acyl ghrelin compared to desacyl ghrelin at 2h post LPS as reflected in a significantly altered acyl/desacyl ghrelin ratio, we investigated the concentration of the ghrelin-acylating enzyme, GOAT at that time. Plasma GOAT protein levels were significantly decreased by 38% at 2h post LPS injection compared to vehicle treated rats (p < 0.001; Fig. 2A-B), whereas in the gastric corpus mucosa GOAT protein expression levels increased slightly by 10% (p < 0.05; Fig. 2A-B). No alteration of gastric GOAT mRNA expression levels was detected at 2h post LPS injection compared to vehicle (p > 0.05; Fig. 2C).
Fig. 2.
LPS decreases plasma GOAT concentration, whereas protein content in the gastric corpus is slightly increased and mRNA expression unchanged in rats. Overnight fasted rats were injected ip with LPS (100μg/kg body weight) or vehicle (pyrogen-free saline) and trunk blood and stomach were collected at 2h post injection. Equal amounts of protein were loaded and plasma and gastric corpus mucosa GOAT concentrations were assessed using Western blot followed by semi-quantitative analysis. Lane 1 contains the molecular weight standards, lane 2 plasma proteins after vehicle injection, lane 3 plasma proteins after LPS, lane 4 gastric corpus mucosa proteins after vehicle and lane 5 gastric corpus mucosa proteins after LPS (A). The blot shows two dominant bands at ~50 and ~100 kDa. The 50 kDa band represents monomeric GOAT, whereas the 100 kDa band likely represents an SDS-stable dimer. Injection of LPS reduced the 50 kDa band (arrow) compared to vehicle demonstrating reduced plasma concentration of GOAT, whereas GOAT in the gastric corpus mucosa was increased (arrowhead, A). Re-staining of the Western blot with β-actin demonstrates equal gastric corpus mucosal protein concentration (A, insert). Quantification of GOAT plasma and stomach protein expression is shown in (B). Gastric GOAT mRNA expression did not change at 2h post LPS injection compared to vehicle treated rats (C). * p < 0.05 and *** p < 0.001 vs. vehicle; LPS, lipopolysaccharide; M, standard molecular weight marker; V, vehicle.
3.3. LPS causes a late onset rise in rectal temperature and blood glucose
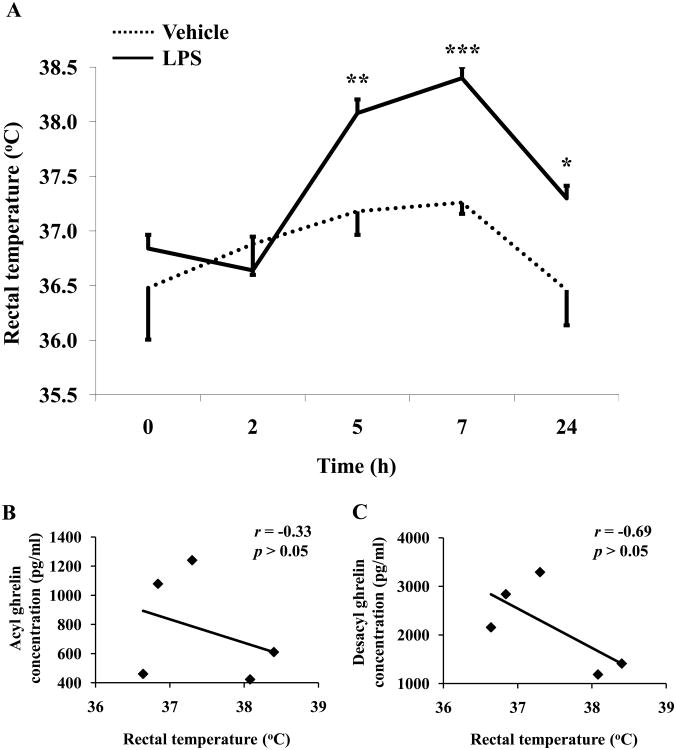
We simultaneously assessed relevant functional and homeostatic changes during our experiments. Injection of LPS (100 μg/kg, ip) during the light phase significantly elevated rectal temperature from 5h (38.1 ± 0.1 vs. 37.2 ± 0.2 °C, p < 0.01) to 24h (37.3 ± 0.1 vs. 36.5 ± 0.3 °C, p < 0.05) post injection compared to vehicle-treated (saline, ip) animals (Fig. 3A). The peak response was observed at 7h after LPS injection (38.4 ± 0.1 vs. vehicle 37.3 ± 0.1 °C, p < 0.001; Fig. 3A). This elevated temperature occurred later than the maximum suppressive effect on circulating acyl and desacyl ghrelin. No correlation was observed between temperature and acyl ghrelin (Fig. 3B) or desacyl ghrelin (Fig. 3C) at any time point (p > 0.05). No significant changes in rectal temperature were observed after ip injection of vehicle throughout the 24h experimental period (p > 0.05; Fig. 3A). Two-way ANOVA showed a significant impact of treatment (F(1,40)=14.6, p < 0.001) and time (F(4,40)=9.4, p < 0.001).
Fig. 3.
LPS increases rectal temperature in rats. LPS (100μg/kg body weight) or vehicle was injected ip during the light phase in overnight fasted rats and rectal temperature measured in conscious lightly hand-restrained rats before and 2h, 5h, 7h and 24h post injection (A). Each line represents the mean ± sem of 5 rats/group. * p < 0.05, ** p < 0.01 and *** p < 0.001 vs. vehicle. Correlations between plasma acyl (B) or desacyl ghrelin (C) and rectal temperature from 0 - 24h post LPS injection. Values for r and p are indicated in each graph.
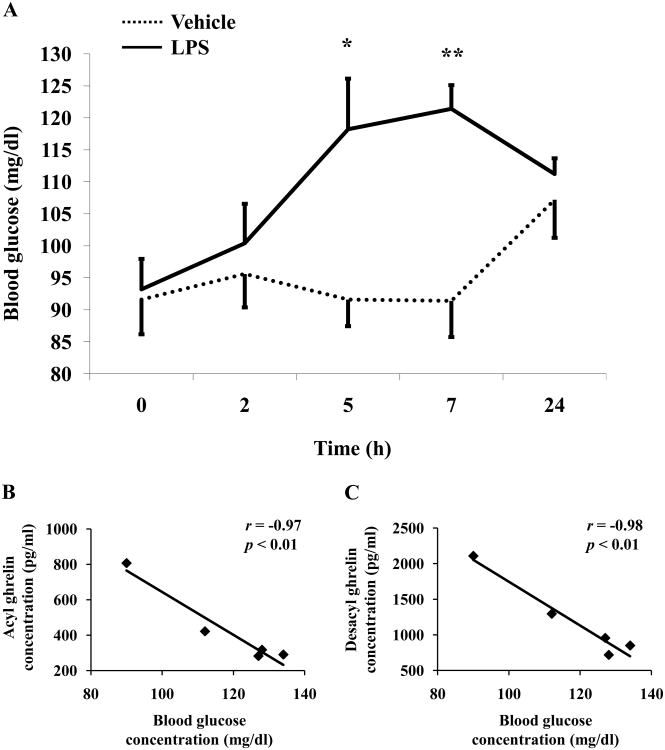
LPS significantly increased blood glucose levels in conscious rats compared to vehicle from 5h (118.2 ± 7.9 vs. 91.6 ± 4.2 mg/dl, p < 0.05) to 7h (121.4 ± 3.7 vs. 91.4 ± 5.6 mg/dl, p < 0.01; Fig. 4A) after LPS treatment. Blood glucose levels normalized at 24h post injection compared to vehicle (111.2 ± 2.5 vs. 107.2 ± 5.9 mg/dl, p > 0.05; Fig. 4A). Time course studies of blood glucose levels after ip vehicle did not show any significant changes throughout the 24h experimental period (p > 0.05; Fig. 4A). Two-way ANOVA indicated a significant influence of treatment (F(1,40)=15.8, p < 0.001), time (F(4,40)=3.3, p < 0.05) and treatment × time (F(4,40)=3.3, p < 0.05). Acyl ghrelin and blood glucose were negatively correlated at 5h post LPS injection (r = -0.97, p < 0.01; Fig. 4B), whereas at earlier or later time points no correlation was observed (p > 0.05). Similarly, desacyl ghrelin levels were negatively correlated with blood glucose at 5h (r = -0.98, p < 0.01; Fig. 4C) and 7h (r = -0.95, p < 0.05) post LPS injection.
Fig. 4.
LPS increases blood glucose levels in rats. Overnight fasted rats were injected ip with LPS (100μg/kg body weight) or vehicle (saline) and blood was withdrawn at the time points indicated at the x-axis for measurement of acyl and total ghrelin levels. At the same time, blood glucose levels were assessed (A). Each line represents the mean ± sem of 5 rats/group. * p < 0.05 and ** p < 0.01 vs. vehicle. Correlations between plasma acyl (B) or desacyl ghrelin (C) and blood glucose from 5h post LPS injection. Values for r and p are indicated in each graph.
3.4. LPS decreases the food intake response to fasting
In overnight fasted rats injected ip 1.5h before re-feeding started, LPS significantly reduced the food intake monitored during the 1.5-2h, 5-7h and 7-24h periods post injection compared to vehicle (p < 0.05; Table 2).
Table 2. LPS decreases food intake in rats.
| Treatmenta | Food intake/period (g/300g body weight) | |||
|---|---|---|---|---|
| 1.5-2h | 2-5h | 5-7h | 7-24h | |
| Vehicle | 4.8 ± 0.3 | 2.5 ± 0.7 | 4.6 ± 0.4 | 20.9 ± 0.9 |
| LPS | 2.0 ± 0.6*** | 1.8 ± 0.4 | 3.1 ± 0.5* | 16.8 ± 0.9** |
Overnight fasted rats were injected ip with LPS (100μg/kg body weight) or vehicle (saline) and re-fed at 1.5h post injection. Food intake was monitored at 2, 5, 7 and 24h post injection and expressed as food intake/period (g/300g bw). Each line represents the mean ± sem of 7-8 rats/group.
p < 0.05,
p < 0.01 and
p < 0.001 vs. vehicle.
4. Discussion
We previously reported that LPS injected ip at a low dose (100 μg/kg) induces a long lasting decrease in total circulating ghrelin levels following ip injection in fasted rats [2, 40]. In the present study, we extend these observations by characterizing the inhibitory response of the two major circulating forms, acyl ghrelin and desacyl ghrelin, the latter being derived from total and acyl ghrelin values as well as changes in the ghrelin-acylating enzyme, GOAT using a similar ip dose of LPS as in our previous studies [2, 40]. We found that LPS decreased both acyl and desacyl ghrelin plasma levels with a nadir decrease of -70% and -67% respectively at 5h which started to recover at 7h post injection in fasted rats. The levels of both circulating forms were fully recovered at 24h post injection. The non-selective cyclooxygenase 1/2 inhibitors, indomethacin or ketorolac, blocked the early reduction of total ghrelin levels at 3h while not preventing the later nadir of total ghrelin decrease occurring at 5h post injection [24, 40]. Moreover, this early LPS-induced decrease of total ghrelin was inhibited by an interleukin-1 receptor antagonist in fasted rats [40] and was not observed in mice lacking the type 1 interleukin-1 receptor [24]. Subsequent studies established in fasted rats, that ghrelin mRNA-containing cells express the prostacyclin receptor (PGI2), whereas the type 1 interleukin-1β receptor is localized in other than X/A-like cells of the gastric oxyntic mucosa [24]. In line with these morphological findings, injection of PGI2 also decreased circulating total ghrelin levels, whereas prostaglandin E2 or carbocyclic thromboxane A2 did not [24]. Taken together, these data suggest an involvement of interleukin-1β-stimulated release of prostacyclin which acts directly on PGI2 receptor-expressing ghrelin-producing X/A-like cells of the gastric oxyntic mucosa to reduce circulating levels of total ghrelin occurring during the first 3 h post injection of LPS. The Toll-like receptor 4 (TLR4) is known to transduce LPS signaling and has been recently reported to be expressed in human enterochromaffin cells and the LPS-induced serotonin release was inhibited by the TLR4 antagonist, E. coli K12 LPS in vitro [16]. Whether LPS can, in addition, act directly on ghrelin-producing X/A-like cells through TLR4 to influence acyl ghrelin production and/or release warrants further investigation. The LPS-induced suppression of acyl or total ghrelin levels is not restricted to elevated fasting peptide levels and occurs also in non-fasted rats [10]. However, 10- and 100-fold higher LPS doses were required in these studies while 100 μg/kg had no effect [10]. This may be related to the different serotypes of LPS used (026:B6) [10] known to have a lower potency than 055:B5 [41] used in the present and previous studies [2, 24, 40].
Similar to the 53% reduction of plasma acyl ghrelin at 2 h post LPS injection, the food intake response to a fast was reduced by 58% as monitored during the 1.5-2h period post LPS injection indicative of an association between the reduction of circulating acyl ghrelin and the drive to eat at this time point. Previous studies showed that the anorexia induced by LPS at 100μg/kg body weight ip is sustained, with a reduction of cumulative food intake for up to 24-h in fasted rats [23]. Likewise, in the present study food intake remained lower compared to vehicle at the 5-7h and 7-24h periods post LPS injection with a significant 33% and 20% decrease respectively. However a direct correlation between the magnitude of changes in food intake in fed rats and acyl ghrelin plasma levels in fasted rats during these same time periods cannot be performed since feeding versus fasting conditions modify the secretion of acyl ghrelin [4]. Nonetheless, the sustained reduction of plasma acyl ghrelin observed in fasted rats may impact on the feeding behavior.
In the present study, LPS induced a 53% reduction of plasma acyl ghrelin levels at 2 h post injection, whereas desacyl ghrelin was reduced by 28%. This lower reduction of desacyl ghrelin compared to acyl ghrelin results in a temporary alteration of the acyl/desacyl ghrelin ratio of 1/5 at 2h instead of 1/3 or 1/2 at other time points or after injection of vehicle. In contrast, under similar conditions of blood processing, we previously observed that different metabolic conditions associated with 24-h fasting or ad libitum feeding alter acyl and desacyl ghrelin levels without changing their ratio [37]. GOAT is a key enzyme that catalyzes the n-octanoyl modification of desacyl ghrelin resulting in acyl ghrelin [30]. Therefore, in addition to acyl and desacyl ghrelin, we investigated changes of GOAT protein levels in the gastric oxyntic mucosa and plasma at the 2 h time point associated with the altered acyl/desacyl ghrelin ratio. There was a 38% reduction in plasma GOAT protein concentration following injection of LPS, whereas gastric corpus GOAT concentration slightly increased by 10% and gastric GOAT mRNA levels were not modified as monitored by RT-qPCR. These data point towards an inhibition of GOAT protein release from the stomach rather than changes in GOAT mRNA expression resulting in slightly increased gastric GOAT protein and significantly reduced circulating GOAT protein. It has been established that the highest gene expression of GOAT is found in the stomach [42] and our previous immunohistochemical analysis of gastric mucosal X/A-like cells indicated that GOAT immunoreactivity is located in vesicles [36] suggesting that circulating GOAT may be derived from gastric release. The latter is likely to contribute to the more rapid decrease of plasma acyl ghrelin induced by LPS. However, it cannot be ruled out that other endocrine organs expressing GOAT such as the anterior pituitary gland [36] and adrenal gland [31] which are also the target of the LPS action, could contribute to the decrease in circulating GOAT as well. Conversely, we previously found that 24-h fasting in rats induced a 20% increase in plasma levels of GOAT protein [36] associated with that of acyl ghrelin [37]. Collectively, these data may point towards a regulatory role of circulating GOAT protein in the formation of the acyl form of ghrelin. Time course studies indicated that in vehicle-treated animals food-deprived for 41 h in total, desacyl ghrelin levels do not further increase suggesting that production and release of desacyl ghrelin are already maximally stimulated by the initial 17 h of fasting. This is in agreement with a previous study indicating that total ghrelin levels do not increase further when compared at 24h and 48h fasting [33]. However, circulating acyl ghrelin levels rose further at 5h and 7h post vehicle injection corresponding to PM samplings while values were similar in the AM samplings at 0 or 2 and 24 h time points. We previously found an up-regulation of circulating GOAT protein expression after a 24h fast [36] that may contribute to the rise occurring at the 5 h and 7 h post vehicle injection and matching 22-24 h fasting. Whether these variations in the vehicle group are also indicative of a diurnal rhythm of circulating or gastric GOAT that impacts on acyl ghrelin formation under these conditions warrants further investigations.
Consistent with established actions of LPS on thermoregulation and glucoregulation [38, 39], rectal temperature and blood glucose levels rose at 5h post injection with a peak response at 7h and subsequent decrease at 24h. Neither rectal temperature nor blood glucose reached baseline values at 24h post injection in fasted rats. Therefore, the onset and duration of hyperglycemia and hyperthermia were different from those of circulating acyl and desacyl ghrelin levels. A negative correlation between ghrelin and insulin has been previously well established [15, 22], whereas changes in ghrelin and glucose are not always correlated [40]. In LPS-treated rats, circulating glucose levels correlated with acyl ghrelin only at 5h and with desacyl ghrelin levels at 5 and 7h post LPS injection. We previously reported that the hyperglycemic response to LPS results in a rise in plasma insulin levels which were negatively correlated with total ghrelin levels [40]. Another study showed that arginine- or glucagon-induced hyperglycemia did not affect circulating ghrelin levels [5] pointing towards a regulatory role of insulin rather than glucose itself. Likewise, LPS at 100 μg/kg or chronic infusion-induced changes in body temperature do not temporarily correlate with the reduction in food intake in rats [20, 27]. We also did not find a correlation between rectal temperature and plasma acyl or desacyl ghrelin levels at any time point. Taken together, it is unlikely that the LPS-induced changes in blood glucose and rectal temperature influence the observed changes in acyl and desacyl ghrelin levels particularly at the early time point of 2h due to the delayed increase and different time course of normalization.
The majority of pre-clinical reports using LPS injection in rats [2, 40] as well as clinical reports investigating patients with H pylori infection [8, 14, 26, 34] focus on total ghrelin levels. Previous studies suggested that acyl and desacyl ghrelin are equally regulated [1, 25] and therefore, total ghrelin levels were considered a good surrogate marker not only for desacyl but also acylated ghrelin. The present demonstration of a more pronounced decrease of acyl ghrelin at 2h post injection of low dose LPS resulting in a temporarily changed acyl/desacyl ghrelin ratio indicates that an immunological stressor can differentially modulate the two major forms of circulating ghrelin and points towards the relevance of measuring both forms of ghrelin. The observed sustained reduction of acyl ghrelin levels may be involved in the decrease in food intake and delay in gastric transit as observed in rats under these conditions of LPS treatment [2, 40] (present study). This may have a bearing with similar clinical manifestations of infections with gram-negative bacteria such as H pylori, a condition where patients present lower plasma ghrelin levels [8, 34] associated with a decreased density of ghrelin-immunoreactive cells [21]. In addition, the present findings provide new insight in the regulation of circulating compared to gastric GOAT under conditions of immune challenge induced by a low dose of LPS. The rapid decrease in plasma GOAT levels and slightly increased gastric GOAT protein levels at 2 h post injection when acyl/desacyl ghrelin ratio is reduced suggests inhibition of gastric GOAT release and an important role of circulating GOAT in the formation of acyl ghrelin which is still to be further characterized.
Research highlights.
Plasma acyl ghrelin (AG) decreases more than desacyl ghrelin (DG) at 2h post LPS
Both forms equally decrease at 5 and 7h and return to pre-injection levels at 24h
At 2h post LPS, plasma GOAT protein decreases while gastric GOAT protein increases
This may account for lower plasma acyl ghrelin levels
Decline in AG and DG is not secondary to increased temperature and blood glucose
Acknowledgments
This work was supported by Department of Veterans Affairs Merit Awards (N.W.G.L. and Y.T.) and a VA Research Career Scientist Award (Y.T.), NIHDK 33061 (Y.T.), Center grant DK-41301 (Animal Core, Y.T. and Peptidomic, Radioimmunoassay and Proteomic Core, J.R.R., Jr.) and German Research Foundation grants STE 1765/1-1 (A.S.) and GO 1718/1-1 (M.G.). We are grateful to Mrs. Honghui Liang for her excellent technical support and we thank Ms. Eugenia Hu for reviewing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ariyasu H, Takaya K, Hosoda H, Iwakura H, Ebihara K, Mori K, et al. Delayed short-term secretory regulation of ghrelin in obese animals: evidenced by a specific RIA for the active form of ghrelin. Endocrinology. 2002;143:3341–50. doi: 10.1210/en.2002-220225. [DOI] [PubMed] [Google Scholar]
- 2.Basa NR, Wang L, Arteaga JR, Heber D, Livingston EH, Taché Y. Bacterial lipopolysaccharide shifts fasted plasma ghrelin to postprandial levels in rats. Neurosci Lett. 2003;343:25–8. doi: 10.1016/s0304-3940(03)00312-4. [DOI] [PubMed] [Google Scholar]
- 3.Bednarek MA, Feighner SD, Pong SS, McKee KK, Hreniuk DL, Silva MV, et al. Structure-function studies on the new growth hormone-releasing peptide, ghrelin: minimal sequence of ghrelin necessary for activation of growth hormone secretagogue receptor 1a. J Med Chem. 2000;43:4370–6. doi: 10.1021/jm0001727. [DOI] [PubMed] [Google Scholar]
- 4.Bodosi B, Gardi J, Hajdu I, Szentirmai E, Obal F, Jr, Krueger JM. Rhythms of ghrelin, leptin, and sleep in rats: effects of the normal diurnal cycle, restricted feeding, and sleep deprivation. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1071–9. doi: 10.1152/ajpregu.00294.2004. [DOI] [PubMed] [Google Scholar]
- 5.Broglio F, Gottero C, Prodam F, Destefanis S, Gauna C, Me E, et al. Ghrelin secretion is inhibited by glucose load and insulin-induced hypoglycaemia but unaffected by glucagon and arginine in humans. Clin Endocrinol (Oxf) 2004;61:503–9. doi: 10.1111/j.1365-2265.2004.02121.x. [DOI] [PubMed] [Google Scholar]
- 6.Caminos JE, Bravo SB, Gonzalez CR, Garces MF, Cepeda LA, Gonzalez AC, et al. Food-intake-regulating-neuropeptides are expressed and regulated through pregnancy and following food restriction in rat placenta. Reprod Biol Endocrinol. 2008;6:14. doi: 10.1186/1477-7827-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen YT, Tsai SH, Sheu SY, Tsai LH. Ghrelin improves LPS-induced gastrointestinal motility disturbances: roles of NO and prostaglandin E2. Shock. 2010;33:205–12. doi: 10.1097/SHK.0b013e3181ae841b. [DOI] [PubMed] [Google Scholar]
- 8.Chuang CH, Sheu BS, Yang HB, Lee SC, Kao AW, Cheng HC, et al. Gender difference of circulating ghrelin and leptin concentrations in chronic Helicobacter pylori infection. Helicobacter. 2009;14:54–60. doi: 10.1111/j.1523-5378.2009.00653.x. [DOI] [PubMed] [Google Scholar]
- 9.Gutierrez JA, Solenberg PJ, Perkins DR, Willency JA, Knierman MD, Jin Z, et al. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci U S A. 2008;105:6320–5. doi: 10.1073/pnas.0800708105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hataya Y, Akamizu T, Hosoda H, Kanamoto N, Moriyama K, Kangawa K, et al. Alterations of plasma ghrelin levels in rats with lipopolysaccharide-induced wasting syndrome and effects of ghrelin treatment on the syndrome. Endocrinology. 2003;144:5365–71. doi: 10.1210/en.2003-0427. [DOI] [PubMed] [Google Scholar]
- 11.Hosoda H, Kojima M, Matsuo H, Kangawa K. Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem Biophys Res Commun. 2000;279:909–13. doi: 10.1006/bbrc.2000.4039. [DOI] [PubMed] [Google Scholar]
- 12.Hosoda H, Kojima M, Matsuo H, Kangawa K. Purification and characterization of rat des-Gln14-Ghrelin, a second endogenous ligand for the growth hormone secretagogue receptor. J Biol Chem. 2000;275:21995–2000. doi: 10.1074/jbc.M002784200. [DOI] [PubMed] [Google Scholar]
- 13.Inhoff T, Mönnikes H, Noetzel S, Stengel A, Goebel M, Dinh QT, et al. Desacyl ghrelin inhibits the orexigenic effect of peripherally injected ghrelin in rats. Peptides. 2008;29:2159–68. doi: 10.1016/j.peptides.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jang EJ, Park SW, Park JS, Park SJ, Hahm KB, Paik SY, et al. The influence of the eradication of Helicobacter pylori on gastric ghrelin, appetite, and body mass index in patients with peptic ulcer disease. J Gastroenterol Hepatol. 2008;23(Suppl 2):S278–85. doi: 10.1111/j.1440-1746.2008.05415.x. [DOI] [PubMed] [Google Scholar]
- 15.Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Oikawa S. Effects of insulin, leptin, and glucagon on ghrelin secretion from isolated perfused rat stomach. Regul Pept. 2004;119:77–81. doi: 10.1016/j.regpep.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Kidd M, Gustafsson BI, Drozdov I, Modlin IM. IL1beta- and LPS-induced serotonin secretion is increased in EC cells derived from Crohn's disease. Neurogastroenterol Motil. 2009;21:439–50. doi: 10.1111/j.1365-2982.2008.01210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–60. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 18.Langhans W. Anorexia of infection: current prospects. Nutrition. 2000;16:996–1005. doi: 10.1016/s0899-9007(00)00421-4. [DOI] [PubMed] [Google Scholar]
- 19.Langhans W. Bacterial products and the control of ingestive behavior: clinical implications. Nutrition. 1996;12:303–15. doi: 10.1016/s0899-9007(96)80052-9. [DOI] [PubMed] [Google Scholar]
- 20.Larson SJ, Collins SM, Weingarten HP. Dissociation of temperature changes and anorexia after experimental colitis and LPS administration in rats. Am J Physiol. 1996;271:R967–72. doi: 10.1152/ajpregu.1996.271.4.R967. [DOI] [PubMed] [Google Scholar]
- 21.Liew PL, Lee WJ, Lee YC, Chen WY. Gastric ghrelin expression associated with Helicobacter pylori infection and chronic gastritis in obese patients. Obes Surg. 2006;16:612–9. doi: 10.1381/096089206776945002. [DOI] [PubMed] [Google Scholar]
- 22.Lippl F, Kircher F, Erdmann J, Allescher HD, Schusdziarra V. Effect of GIP, GLP-1, insulin and gastrin on ghrelin release in the isolated rat stomach. Regul Pept. 2004;119:93–8. doi: 10.1016/j.regpep.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Lugarini F, Hrupka BJ, Schwartz GJ, Plata-Salaman CR, Langhans W. A role for cyclooxygenase-2 in lipopolysaccharide-induced anorexia in rats. Am J Physiol Regul Integr Comp Physiol. 2002;283:R862–8. doi: 10.1152/ajpregu.00200.2002. [DOI] [PubMed] [Google Scholar]
- 24.Madison LD, Scarlett JM, Levasseur P, Zhu X, Newcomb K, Batra A, et al. Prostacyclin signaling regulates circulating ghrelin during acute inflammation. J Endocrinol. 2008;196:263–73. doi: 10.1677/JOE-07-0478. [DOI] [PubMed] [Google Scholar]
- 25.Murakami N, Hayashida T, Kuroiwa T, Nakahara K, Ida T, Mondal MS, et al. Role for central ghrelin in food intake and secretion profile of stomach ghrelin in rats. J Endocrinol. 2002;174:283–8. doi: 10.1677/joe.0.1740283. [DOI] [PubMed] [Google Scholar]
- 26.Nwokolo CU, Freshwater DA, O'Hare P, Randeva HS. Plasma ghrelin following cure of Helicobacter pylori. Gut. 2003;52:637–40. doi: 10.1136/gut.52.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Reilly B, Vander AJ, Kluger MJ. Effects of chronic infusion of lipopolysaccharide on food intake and body temperature of the rat. Physiol Behav. 1988;42:287–91. doi: 10.1016/0031-9384(88)90084-4. [DOI] [PubMed] [Google Scholar]
- 28.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raff H. Total and active ghrelin in developing rats during hypoxia. Endocrine. 2003;21:159–61. doi: 10.1385/ENDO:21:2:159. [DOI] [PubMed] [Google Scholar]
- 30.Romero A, Kirchner H, Heppner K, Pfluger P, Tschoep M, Nogueiras R. GOAT - the master switch for the ghrelin system? Eur J Endocrinol. 2010 doi: 10.1530/EJE-10-0099. in press. [DOI] [PubMed] [Google Scholar]
- 31.Rucinski M, Ziolkowska A, Tyczewska M, Malendowicz LK. Expression of prepro-ghrelin and related receptor genes in the rat adrenal gland and evidences that ghrelin exerts a potent stimulating effect on corticosterone secretion by cultured rat adrenocortical cells. Peptides. 2009;30:1448–55. doi: 10.1016/j.peptides.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 32.Sakata I, Yang J, Lee CE, Osborne-Lawrence S, Rovinsky SA, Elmquist JK, et al. Colocalization of ghrelin O-acyltransferase and ghrelin in gastric mucosal cells. Am J Physiol Endocrinol Metab. 2009;297:E134–41. doi: 10.1152/ajpendo.90859.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato T, Fukue Y, Teranishi H, Yoshida Y, Kojima M. Molecular forms of hypothalamic ghrelin and its regulation by fasting and 2-deoxy-d-glucose administration. Endocrinology. 2005;146:2510–6. doi: 10.1210/en.2005-0174. [DOI] [PubMed] [Google Scholar]
- 34.Shiotani A, Miyanishi T, Uedo N, Iishi H. Helicobacter pylori infection is associated with reduced circulating ghrelin levels independent of body mass index. Helicobacter. 2005;10:373–8. doi: 10.1111/j.1523-5378.2005.00343.x. [DOI] [PubMed] [Google Scholar]
- 35.Stengel A, Goebel M, Wang L, Taché Y. Ghrelin, des-acyl ghrelin and nesfatin-1 in gastric X/A-like cells: role as regulators of food intake and body weight. Peptides. 2010;31:357–69. doi: 10.1016/j.peptides.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stengel A, Goebel M, Wang L, Taché Y, Sachs G, Lambrecht NW. Differential distribution of ghrelin-O-acyltransferase (GOAT) immunoreactive cells in the mouse and rat gastric oxyntic mucosa. Biochem Biophys Res Commun. 2010;392:67–71. doi: 10.1016/j.bbrc.2009.12.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stengel A, Keire D, Goebel M, Evilevitch L, Wiggins B, Taché Y, et al. The RAPID method for blood processing yields new insight in plasma concentrations and molecular forms of circulating gut peptides. Endocrinology. 2009;150:5113–8. doi: 10.1210/en.2009-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsushima H, Mori M. Involvement of protein kinase C and tyrosine kinase in lipopolysaccharide-induced anorexia. Pharmacol Biochem Behav. 2001;69:17–22. doi: 10.1016/s0091-3057(01)00500-7. [DOI] [PubMed] [Google Scholar]
- 39.Virkamaki A, Yki-Jarvinen H. Role of prostaglandins in mediating alterations in glucose metabolism during acute endotoxemia in the rat. Endocrinology. 1995;136:1701–6. doi: 10.1210/endo.136.4.7895681. [DOI] [PubMed] [Google Scholar]
- 40.Wang L, Basa NR, Shaikh A, Luckey A, Heber D, St-Pierre DH, et al. LPS inhibits fasted plasma ghrelin levels in rats: role of IL-1 and PGs and functional implications. Am J Physiol Gastrointest Liver Physiol. 2006;291:G611–20. doi: 10.1152/ajpgi.00533.2005. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe K, Jaffe EA. Comparison of the potency of various serotypes of E. coli lipopolysaccharides in stimulating PGI2 production and suppressing ACE activity in cultured human umbilical vein endothelial cells. Prostaglandins Leukot Essent Fatty Acids. 1993;49:955–8. doi: 10.1016/0952-3278(93)90181-u. [DOI] [PubMed] [Google Scholar]
- 42.Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132:387–96. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]