Abstract
B-cell activating factor receptor (BAFF-R) is expressed on precursor B acute lymphoblastic leukemia ALL (pre-B ALL) cells but not on their pre-B normal counterparts. Thus, selective killing of ALL cells is possible by targeting this receptor. Here we have further examined therapeutic targeting of pre-B ALL based on the presence of the BAFF-R. Mouse pre-B ALL cells lacking BAFF-R function had comparable viability and proliferation to wild type cells but were more sensitive to drug treatment. Viability of human pre-B ALL cells was further reduced when antibodies to the BAFF-R were combined with other drugs, even in the presence of stromal protection. This indicates that inhibition of BAFF-R function reduces fitness of stressed pre-B ALL cells. We tested a novel humanized anti-BAFF-R monoclonal antibody optimalized for FcRγIII mediated, antibody-dependent cell killing by effector cells. Antibody binding to human ALL cells was inhibitable, in a dose-dependent manner, by recombinant human BAFF. There was no evidence for internalization of the antibodies. The antibodies significantly stimulated NK cell-mediated killing of different human patient-derived ALL cells. Moreover, incubation of such ALL cells with these antibodies stimulated phagocytosis by macrophages. When this was tested in an immunodeficient transplant model, mice that were treated with the antibody had a significantly decreased leukemia burden in bone marrow and spleen. In view of the restricted expression of the BAFF-R on normal cells and the multiple anti-pre-B ALL activities stimulated by this antibody, a further examination of its use for treatment of pre-B ALL is warranted.
Keywords: ADCC, antibody-mediated cellular cytotoxicity, ADCP, antibody-dependent cellular phagocytosis, BR3, BlyS, BAFF, Ph-positive, monoclonal antibodies, NK cells, macrophages
Introduction
Although the overall cure rate for adult precursor B-lineage acute lymphoblastic leukemia (pre-B ALL) has increased from 43% to 57% in the period between 1987 and 2007, a need remains for the development of more effective and less toxic treatments. The identification of targets for therapy that are expressed on a limited subset of cells, thus minimizing therapy side effects, is an important component of this. We were the first to report that precursor B-lineage acute lymphoblastic leukemia (pre-B ALL) cells aberrantly express the B cell activating factor receptor (BAFF-R) (1). This is the principal receptor for BAFF, a Tumor Necrosis Factor family member and a type II transmembrane protein found either in a membrane bound or soluble form on dendritic cells, stromal cells, macrophages and some T cells (2). The receptor for BAFF is expressed on normal immature and mature B cells, as well as on other malignant B-lineage cells including chronic lymphocytic leukemia, myeloma, hairy cell leukemia and B lymphoma (3-5). The BAFF—BAFF-R interaction is critical for survival of mature B cells, since profound loss of these cells is observed in both BAFF and BAFF-R null mutant mice. In normal mature B cells, the interaction between the ligand BAFF and the BAFF-R enhances B cell survival (6). Also, intracellular signal transduction cross-talk occurs between the B cell receptor (BCR) and the BAFF-R, and BCR stimulation induces BAFF-R mRNA expression in mature B cells (7, 8). Although the functional significance of the presence of the BAFF-R on pre-B ALL cells was unclear, the finding that normal pre-B cells lack BAFF-R expression (1, 9, 10) makes this receptor is an attractive target for ALL therapy. In one such approach, using a recombinant fusion protein between BAFF and the toxin Gelonin, we recently demonstrated that the presence of the BAFF-R can be used to selectively eradicate pre-B ALL cells (11).
Other potential therapeutic approaches showing promise include using monoclonal antibodies (mAbs) as immunotoxin conjugates or bispecific antibodies (5, 12-14). A different mechanism by which antibodies can be utilized to target cancer cells for eradication is through antibody-dependent cellular cytotoxicity (ADCC). This is mediated mainly by FcRγIII, a major triggering receptor on natural killer (NK) cells. Several therapeutic mAbs in use for cancer treatment mediate ADCC, including anti-CD20 rituximab (Rituxan®), anti-Her2 trastuzumab (Herceptin®), anti-TNF-α infliximab (Remicade®), and anti-RhD (15). ADCC-promoting antibodies that were developed for more mature B-cell cancers such as rituximab, alemtuzumab and epratuzumab are also being tested for treatment of B-cell precursor ALL (16, 17). Antibody coating of cells can also stimulate antibody-mediated phagocytosis (ADCP) by macrophage effector cells. Interestingly, two preclinical studies reported different outcomes using mAbs to stimulate effector-mediated eradication of precursor B-lineage ALL cells. Three MLL-positive ALL cell lines were resistant to NK-mediated ADCC in the presence of a CD19 antibody (13). The second study reported that Medi-551, a humanized anti-CD19 mAb, stimulates both ADCC by NK cells and phagocytosis by macrophages (18).
Although many antibodies generated against the BAFF-R inhibit BAFF-mediated B cell growth in vitro (19), to date, none of these were reported to be successful in therapeutical applications. In the current study, using a novel BAFF-R antibody, optimalized for ADCC, we demonstrate that this receptor is an extremely attractive target for effector-mediated eradication of pre-B ALL cells.
Materials and Methods
Reagents
Nilotinib and anti-BAFF-R B-1239 antibodies (unconjugated and Alexa-647 labeled) were from Novartis. These antibodies were selected from the Human Combinatorial Antibody Library (HuCAL GOLD{r}), using phage display technology (8). The B-1239 mAb was produced in a fucosyl-transferase deficient CHO cell line (BioWa Potelligent Technology, BioWa Inc., Princeton, NJ, USA) (20), with a humanized sequence to optimalize ADCC activity. B-1239 was selected based on its property of blocking the BAFF/BAFF-R interaction and signaling via BAFF. Anti-BAFF-R antibody clone 11c1, anti-human CD19 and CD10 antibodies were from BD Biosciences (San Jose, CA, USA). FITC-anti-human IgG was from Sigma Aldrich (CA, USA). Recombinant huBAFF and the function-blocking anti-BAFF-R antibodies were purchased from R&D Systems (MN, USA). Bcr N-20, BAFF-R and Gapdh antibodies for Western blotting were from Santa Cruz, eBiosciences and Millipore, respectively. Cell Mask Orange and Deep Red were from Life Technologies (Eugene, OR).
Mouse pre-B ALL WT and KO cells, human patient-derived and primary samples
BAFF-R-deficient and control C57Bl/6J mice were purchased from the Jackson Laboratories. Bcr/Abl-IRES-neo (p190), pHIT60 and pHIT123 (ecotropic envelope) plasmids were transfected to HEK 293FT cells using Lipofectamine 2000 (Life Technologies, NY, USA). Viral supernatant collected after 24 hrs was transferred to a 6 well plate coated with retronectin (Takara, CA, USA). After centrifuging the viral supernatant at 2000g for 90 min, pre-B cells were added onto the plates and again centrifuged at 600g for 30 min. Total bone marrow cells were cultured in the presence of 10 ng/ml recombinant mouse IL-7 for 12 days, after which cells were retrovirally transduced and IL-7 was withdrawn. We transformed bone marrows of three independent sets of WT and KO mice. US7 and US7R are Ph-negative ALLs; USFO2, BLQ1, BLQ11, P2 (Pt2, LAX2) and TXL2 are Ph-positive ALLs. BLQ1, BLQ11 and P2 contain a Bcr/Abl T315I mutation. ICN13 is a pro-B ALL with an MLL-AF4 fusion (1, 21, 22). TXL2R cells were derived from TXL2 and are able to proliferate in the presence of 500 nM nilotinib. Human ALL patient-derived cells have been passaged in NOD/SCID/IL2rg-/- (NSG) mice and were then grown on irradiated OP9 feeder layers. The AML was from a relapsed patient and grew directly in culture when provided with OP9 stromal support. Non-cultured primary human ALL samples were obtained after approval of the Committee on Clinical Investigations of Children’s Hospital Los Angeles. B-precursor ALLs (BM, PB) were gated to identify blasts and normal residual B-lymphocytes (mainly mature B cells) expressing high levels of CD45. Each group was independently assessed for BAFF-R expression and MFI.
Assay for receptor density and internalization
Cells were washed with autoMACs rinsing solution containing BSA and resuspended in human or mouse FcγR blocking reagent (Miltenyi Biotech, CA, USA). After a 15 min, cells were stained with 2.5 μg/ml Alexa-647 B-1329 or hIgG1 for 30 min, washed, then incubated with live/dead cell stain (Invitrogen). Cells were analyzed on a Fortessa (BD). Cell surface receptor numbers were quantified by running Quantum Simply Cellular anti-IgG Beads and APC-MESF beads (Bangs Laboratories) in parallel with the cell samples.
ImageSteam and confocal microscopy analysis
3×106 US7 cells were incubated for 4 hrs with antibodies, washed 1x with PBS, and then incubated for 5 min with plasma membrane stain. The cell surface was defined using 0.25 μg/ml Cell Mask Deep Red with 15 μl of internalizing probe (PE-BAFF antibodies, eBiosciences) or Cell Mask Orange with 0.6 μg/ml B-1239 Alexa 647. Acquisition was performed using an ImageStream Imaging Flow Cytometer (Amnis Corporation, Seattle, WA) using INSPIRE software. A 40x magnification was used for all samples. Data analysis was performed using the IDEAS v.6 software (Amnis Corporation). The Internalization score is defined as the ratio of signal intensity inside the cell to the intensity of the entire cell for a specific florescent signal, with higher internalization scores indicating higher internalization.
For confocal microscopy, cells were imaged on an LSM 710 confocal system mounted on an AxioObserver.Z1 microscope equipped with a 40x/1.2 C-APOCHROMAT water-immersion lens (Carl Zeiss Microimaging, Thornwood, NY). Fluorescence excitation was achieved with laser lines of 633 nm and emission ranges were 662-721 nm for Alexa Fluor 647. The confocal pinhole was set to 1 Airy unit. Brightfield images were acquired simultaneously with fluorescence. The confocal system was controlled by Zeiss ZEN 2010 software. Incubation times and antibody concentrations were as for ImageStream analysis.
In vitro antibody treatment
For assessment of BAFF-R antibody binding, ALL cells were incubated with different concentrations of the B-1239 BAFF-R antibody for 30 min at RT, washed with PBS and incubated with FITC conjugated anti-human IgG antibody and analyzed by FACS (Accuri Flow Cytometers Inc, MI, USA). Staining of these cells with FITC anti-human IgG alone served as control. For competition assays, US7 ALL cells were pre-incubated with recombinant human BAFF or PE-labeled α-BAFF-R antibody (BD Biosciences) for 2 hours, followed by incubation for 30 min with 5 μg/ml B-1239 α-BAFF-R antibody. In combination treatments, antibody and drug were added every alternate day. Non-adherent leukemia cells were collected from the stromal layer and viability of ALL cells was determined by manually counting Trypan blue-excluding lymphoblasts under a microscope.
ADCC assays
NK cells from normal human peripheral blood were isolated using a MACS negative separation column (Miltenyi Biotech, CA, USA). ALL cells (106) were labeled with calcein-AM (Lifescience Technologies, CA, USA) for 30 minutes at 37°C. Control human IgG Ab or B-1239 α-BAFF-R-Ab was added at 20,000, 10,000, 1000, 100 and 10 ng/ml to the ALL cells. After 1-2 hours of incubation, cells were washed with PBS-/- and counted. 10,000 cells per well were added to a 96-well plate. MACS-sorted human NK cells from PBMC were added at 50,000 / well (E: T ratio= 5:1). The optimal effector-to-target (E/T) ratio of 5:1 was determined in pilot experiments. After 4 hours of incubation, 100 μl of culture supernatant was transferred to a Black View Plate-96 well plate and arbitrary fluorescent units (AFU) were measured on a Tecan SPECTRAFLUOR (485 nm excitation/535 nm emission). The percentage of specific lysis from triplicate wells was determined using the following equation, in which “AFU mean spontaneous release” is calcein-AM release by target cells in the absence of antibody or NK cells and “AFU mean maximal release” is calcein-AM release by target cells upon lysis by detergent.
ADCP assays
Human mononuclear cells were isolated from normal PB by Ficoll centrifugation. Cells were plated on non-tissue culture-treated 10 cm dishes and activated with 100 nM PMA for 1hr. Floating cells were washed off and remaining adherent cells propagated for at least 6 days with fresh media (RPMI, 15% FBS, P/S, β-mercaptoethanol) added every other day. For measurement of ALL eradication by human macrophages, leukemia cells were first incubated for 30 minutes with 25 μg/ml B-1239. 5×105 washed ALL cells were then plated into wells containing 105 macrophages. Remaining Trypan blue excluding live cells were counted after 4 hours of incubation at 37°C. Human macrophages were also tested for ADCC activity using the calcein-AM release assay but no significant ADCC was measured.
Isolation of cells and phagocytosis was performed as described in detail in (23). In brief, Ficoll-purified normal blood mononuclear cells were incubated in a tissue culture dish for 45-90 minutes in monocyte adhesion medium (RPMI 1640 medium supplemented with 7.5% AB serum (Invitrogen), 1% L-glutamine and 1% penicillin/streptomycin), after which non-adherent cells were removed. After overnight incubation, cells were detached with PBS containing 5 mM EDTA. Detached cells were plated in 8-well chamber slides (LabTek) at 2×105 cells/well and allowed to differentiate for 7-10 days in macrophage culture medium (X-Vivo medium (Lonza, USA), 1% AB serum, 1% L-glutamine and 1% penicillin/streptomycin). For measurement of phagocytosis, US7 or TXL2 target cells were added to macrophage at a 1:1 ratio in the presence of 20 μg/ml B-1239 or human IgG as a control. After 2 h incubation, cells were fixed and stained with Diff Quick (Thermo Scientific) and percentage phagocytosis was determined by counting macrophages having engulfed one or more leukemia cells. At least 200 cells were counted for each experimental condition.
Mouse spleen was mechanically dispersed into a single cell suspension. After straining through a 70 μm strainer, cells were incubated overnight with L929-conditioned RPMI media with 10% FBS, P/S, β-mercaptoethanol on non-tissue culture treated dishes. Floating cells were removed by washing and cultures were expanded for around 10 days before use. Mouse bone marrow derived macrophages (BMDM) were obtained using standard procedures. To compare mouse BMDM and spleen-derived macrophages (SDM), human US7 were incubated with 25 μg/ml B-1239 for 1 hr in complete medium and then washed twice in PBS-/-. 5×105 washed ALL cells were plated into wells containing 105 macrophages for an E:T ratio of 1:5. Remaining ALL cells were identified after 30 minutes at 37°C using FACS and based on FSC/SSC. TXL2 and TXL2R cells were seeded at 1×106 cells into 24-well plates containing irradiated 105 OP9 cells and 105 spleen derived macrophages. After addition of 25 μg/ml B-1239, cells were incubated for 24 hrs. Remaining ALL cells were removed and live cells counted by FACS.
Human ALL transplant model
All animal experiments were carried out in concordance with Institutional IACUC and NIH guidelines. Human Ph positive TXL2 ALL cells were injected at 2×106 cells/animal into NOD.Cg-PrkdcscidIl2rgtm1Wjll/SzJ mice (NSG; Jackson Labs) mice. Cells were allowed to proliferate in vivo for 6 days before start of treatment. Transplanted mice (n = 5/group) were either injected i.p. with B-1239 α-BAFF-R antibody (10 mg/kg), orally administered 75 mg/kg of nilotinib, or treated with both antibody and nilotinib for consecutive 4 days. Control mice received peanut butter/oil and control 10 mg/kg human IgG. Mice were sacrificed 12 days after termination of treatment. Bone marrow and spleen were analyzed by FACS for the presence of human ALL cells using human CD19 and CD10 antibodies.
Statistical analysis
Student’s T-test was performed to assess statistical significance of the in vitro results. A p value <0.05 was considered significant. All experiments were done using triplicate wells for each experimental point.
Results and discussion
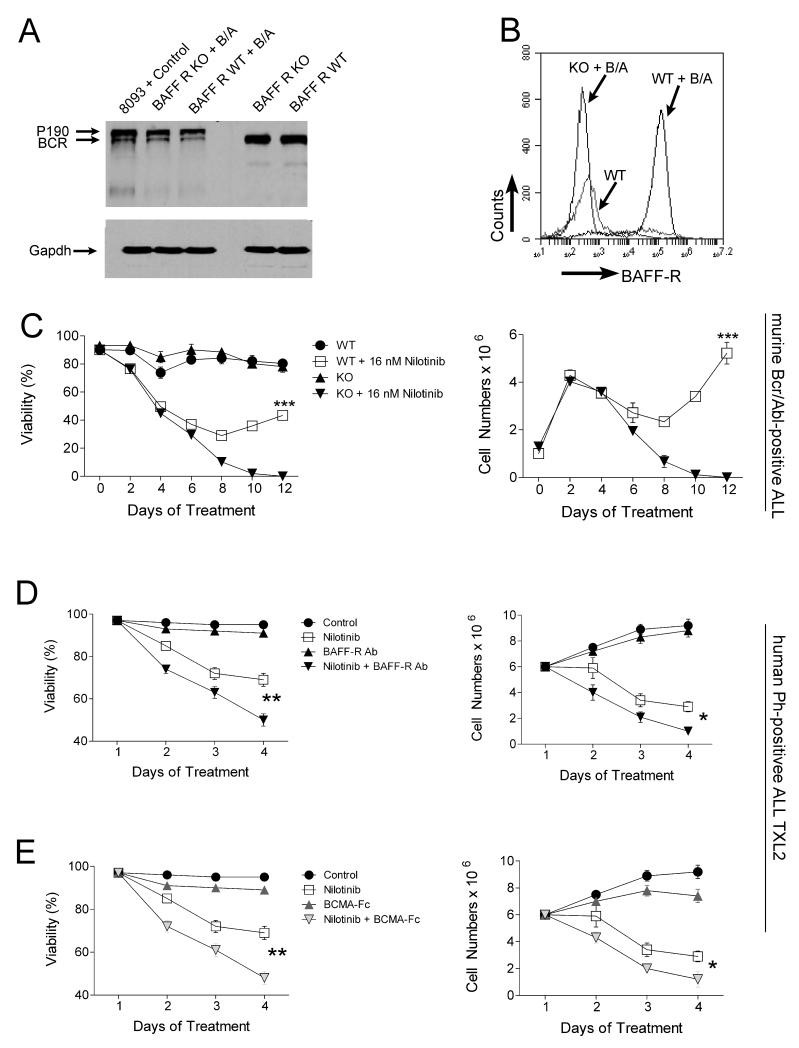
We previously reported that the addition of recombinant BAFF to ALL culture medium protects these cells from chemotherapy-induced apoptosis (1). To further investigate the survival advantage mediated by the BAFF-R to pre-B ALL cells, we generated pre-B ALL cells from bone marrows of wild type (WT) and baff-r null mutant (KO) mice with a retroviral vector carrying the BCR/ABL oncogene. Bcr/Abl expression was confirmed using Western blot analysis (Fig.1A). The proliferation and viability of WT and KO pre-B ALL cells was comparable (not shown). Interestingly, WT pre-B ALL cells expressed high levels of the BAFF-R (Fig.1B), confirming the aberrant appearance of this receptor on ALL cells, as reported by us and others in human ALL (1, 9, 10). We then treated both WT and KO deficient leukemic cells with 16 nM nilotinib for 12 days, in the absence of stroma. As shown in Figure 1C, WT leukemic cells developed resistance to nilotinib in 9-10 days, while BAFF-R deficient cells were eradicated. These results establish that the presence of the BAFF-R on pre-B ALL cells confers a survival advantage during drug treatment.
Figure 1.
Pre-B ALL cells are more vulnerable to therapeutic drug treatment when BAFF-R function is attenuated. A, Western blot analysis for Bcr/Abl on WT and BAFF-R-deficient (KO) pre-B cells before and after Bcr/Abl transduction using Bcr N-20 antibodies. Positive control, murine transgenic BCR/ABL 8093 ALL cells. B, FACS analysis of WT and BAFF-R deficient pre-B cells before and after Bcr/Abl transduction, using a BAFF-R antibody. C, Percent viability (left) and viable cell counts (right) of WT and BAFF-R-deficient (KO) Bcr/Abl transduced pre-B cells treated with 16 nM nilotinib for 12 days. ***p<0.001 for nilotinib-treated WT and KO ALL cells. 14 independent experiments; all experiments with triplicate samples. (D, E) Percentage viability (left) and viable cell counts (right) of human Ph-positive TXL2 ALL cells co-cultured with OP9 stroma and treated with (D), function-blocking BAFF-R antibody (5 μg/ml; R&D), nilotinib (300 nM) or a combination of both or (E), BCMA-Fc (10 μg/ml), nilotinib (300 nM) or a combination of both for 4 days. Control, DMSO vehicle. *p<0.05 and **p<0.01 nilotinib versus combination treatment with nilotinib.
To explore potential therapeutic applications, we inhibited the BAFF/BAFF-R interaction on human ALL cells using either a neutralizing antibody to the BAFF-R (R&D Systems) or a BCMAFc decoy receptor, combined with drug treatment. Antibody or BCMA-Fc monotreatment had little effect on cell viability (Fig. 1D, E left panels), or proliferation (not shown) but there was a reduction in the viability of ALL cells in the combination treatment group compared to the nilotinib monotreatment group (Fig. 1D, E).
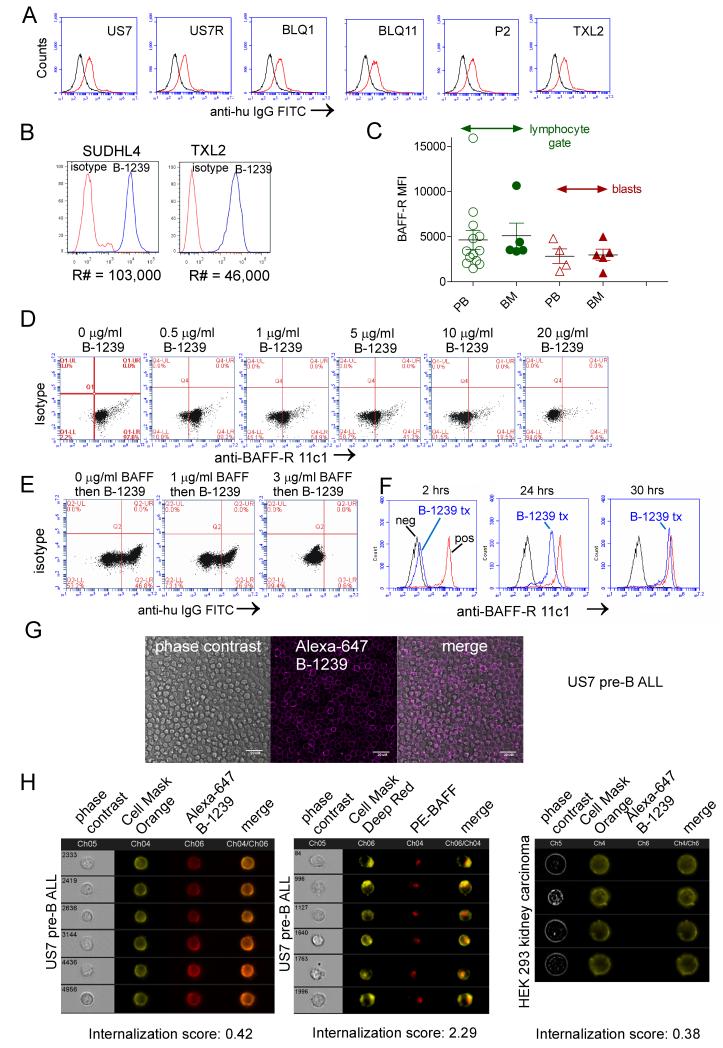
We next characterized B-1239, a human codon-optimized anti-BAFF-R mAb, for possible clinical application based on the presence of the BAFF-R on human pre-B ALL cells. Fig. 2A shows that the antibody bound to the BAFF-R on both Ph-positive and Ph-negative ALL cells, as detected indirectly with FITC-human IgG. We also compared relative copy numbers of the BAFF-R on a mature B-lineage lymphoma cell line to that on a pre-B ALL. As shown in Fig. 2B, BAFF-R copy numbers present on TXL2 pre-B ALL cells as measured by the B-1239 antibodies, were around 50% of that of a highly BAFF-R expressing diffuse large B-cell lymphoma cell line SUDHL4. Mean fluorescent intensity of BAFF-R expression was also high on normal residual mature lymphocytes present in ALL patient samples compared to blasts (average MFI normal BM/PB vs. ALL BM/PB, 4633/5097 vs. 2831/2981, respectively; see Fig.2C).
Figure 2.
Binding of humanized mAb B-1239 to the BAFF-R. A, Antibody binding of B-1239 (10 μg/ml, 2 hrs) α-BAFF-R antibody to the indicated patient-derived ALL cells detected using FITC anti-human IgG. Black histograms, control anti-human IgG; grey histograms, cells first incubated with B-1239, then stained with anti-human IgG antibody. B, Comparative analysis for BAFF-R copy number (R#) on the surface of SUDHL4 (diffuse large B-cell lymphoma) and Ph-positive ALL TXL2 as measured by B-1239. C, Comparison of mean fluorescent intensity (MFI) for BAFF-R expression on CD19+, BAFF-R+ residual normal B-lineage cells and CD19+, BAFF-R+ blasts for 4-9 different patient samples. Normal non-B lymphocytes did not express the BAFF-R. D, Binding of PE-labeled anti-BAFF-R antibody 11c1 clone (BD Biosciences) to human US7 ALL cells after pre-incubation with the indicated amounts of B-1239. E, Ability of recombinant BAFF to inhibit binding of 5 mg/ml B-1239 to the BAFF-R on US7 ALL cells as detected with FITC anti-human IgG antibody. F, Cell surface detection as measured by competition of PE-labeled anti-BAFF-R 11c1 antibodies with unlabeled B-1239 mAb on US7 pre-B ALL cells after addition of 30 μg/ml antibody on t =0. Control, unstained cells; blue, cells treated with B-1239. Red, no B-1239 added. G, Localization of Alexa-647-conjugated B-1239 antibodies using confocal microscopy. Bar, 20 μm. H, Representative Amnis ImageStream images showing specific cell surface attachment and lack of internalization of Alexa-627 B-1239 to US7 pre-B ALL cells, compared to positive control internalization of PE-anti-BAFF antibodies, and negative binding control for Alexa-647 B-1239 to HEK 293 cells which lack BAFF-R expression.
The B-1239 antibody inhibited binding of a different anti-BAFF-R antibody in a dose-dependent manner (Fig. 2D). Pre-incubation of ALL cells with human recombinant BAFF inhibited binding of the B-1239 antibody in a dose-dependent manner (Fig. 2E). These data suggest that the B-1239 antibody binds to epitopes on the BAFF-R that are part of the ligand-binding site.
To address how long the B-1239 antibody remains on the cell surface, we performed a time course to determine when loss of inhibition of binding of the other anti-BAFF-R antibody happens. Fig. 2F shows that after 30 hours inhibition is lost. To determine if loss of B-1239 antibody is due to internalization, we imaged cells incubated with Alexa-647-labeled B-1239 using confocal microscopy (Fig. 2G) and ImageStream (Fig. 2H). Both analysis yielded similar results with no detection of internalization of the antibody.
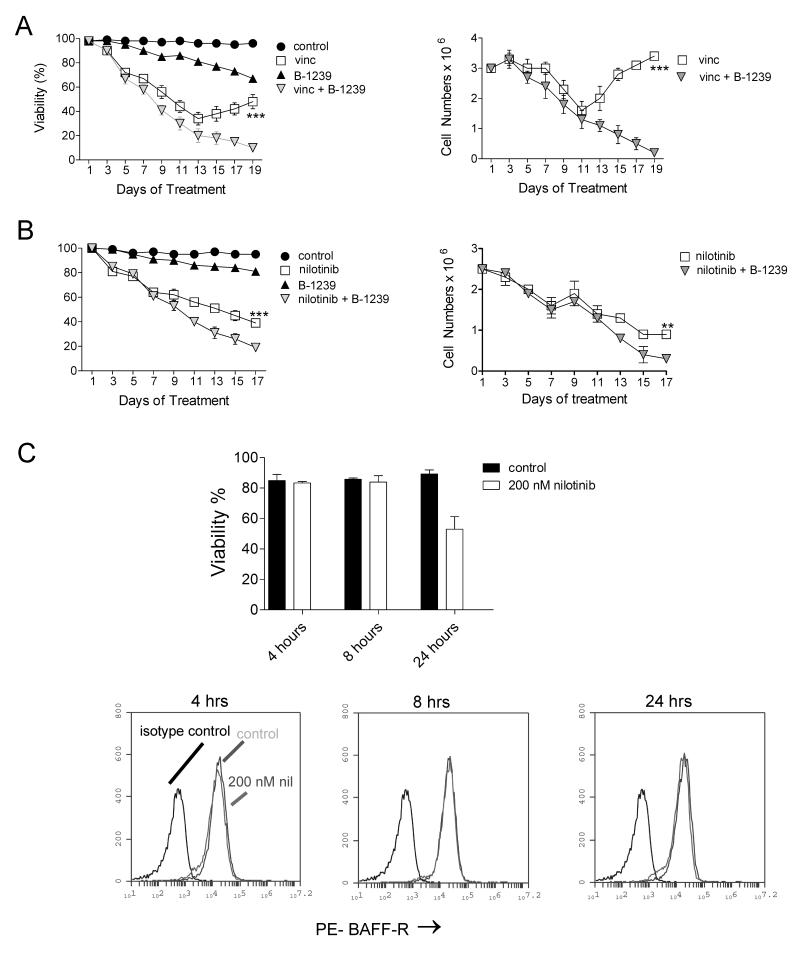
Since B-1239 competes with BAFF for BAFF-R binding, we tested if blocking of the BAFF-R with it would enhance chemotherapy sensitivity in these cells. As shown in Fig. 3A and B, this antibody alone also had little effect on cell viability or proliferation. However, there was a reduction in the viability of ALL cells in the combination treatment group, especially in combination with vincristine (Fig. 3B). To address the possibility that nilotinib as tyrosine kinase inhibitor could affect BAFF-R expression we compared its expression on cells treated for varying periods of time with nilotinib. However, no changes in expression were seen (Fig. 3C).
Figure 3.
Combination treatment of pre-B ALL cells with chemotherapy and B-1239 antibodies. A, Viability (left) and cell counts (right) of US7 ALL cells treated with 20 μg/ml B-1239 (daily), 2.5 nM vincristine (on alternate days) or both over a period of 19 days. B, Viability (left) and cell counts (right) of TXL2 ALL cells treated with 20 μg/ml B-1239 (daily), 300 nM nilotinib (fresh on alternate days) or both over a period of 17 days. Control, 20 μg/ml human IgG. ***p<0.001 and **p<0.01 for vincristine or nilotinib monotreatment compared to combination treatment with B-1239. One of two experiments on triplicate samples. Antibody was added freshly every day and drugs every alternate day. C, Evaluation of effect of nilotinib treatment on BAFF-R cell surface expression on TXL2 cells. Control, cells treated with DMSO.
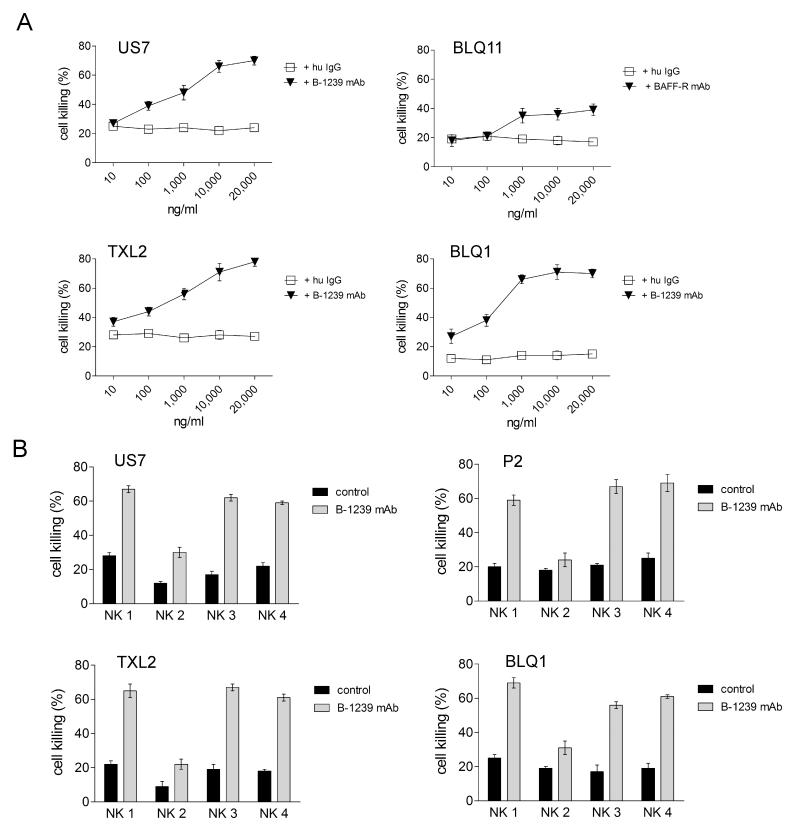
The cell surface expression of the BAFF-R on ALL cells can be used as a therapeutic target independent of its function: binding of monoclonal antibodies can stimulate cell killing by effector cells that express the FcγR (24). Since the B-1239 antibody was designed to promote antibody-dependent cellular cytotoxicity (ADCC) we tested this using NK cells. After comparing different ratios of effector (NK) to target (ALL) cells (not shown) we chose a ratio of 5:1 for ADCC experiments. As shown in Fig. 4A, compared to control human IgG, B-1239 had a dose-dependent specific effect on stimulating NK-cell mediated cytotoxicity. We also tested the ADCC-promoting activity of the antibody on NK cells isolated from different normal donors. Fig. 4B shows that depending on the donor NK cells, 20-70% of the ALL cells were killed within four hours of incubation. Both Ph-positive (BLQ1, BLQ11, P2, TXL2) and negative (US7) ALL cells were killed by NK cells. The results shown here validate this novel BAFF-R antibody as a very promising tool for therapy of pre-B ALL.
Figure 4.
B-1239 promotes NK-cell mediated ADCC activity against human pre-B ALL cells. A, Percent lysis of ALL cells pre-incubated with different concentrations of B-1239, after 4 hours of incubation with NK cells. All graphs were generated using an effector : target ratio of 5 : 1 and NK cells from one donor. B, ADCC mediated by NK cells from different normal donors (NK 1- NK 4) using an effector : target ratio = 5 : 1 in all graphs and 10 μg/ml B-1239. Bars, mean ±SEM of triplicate samples.
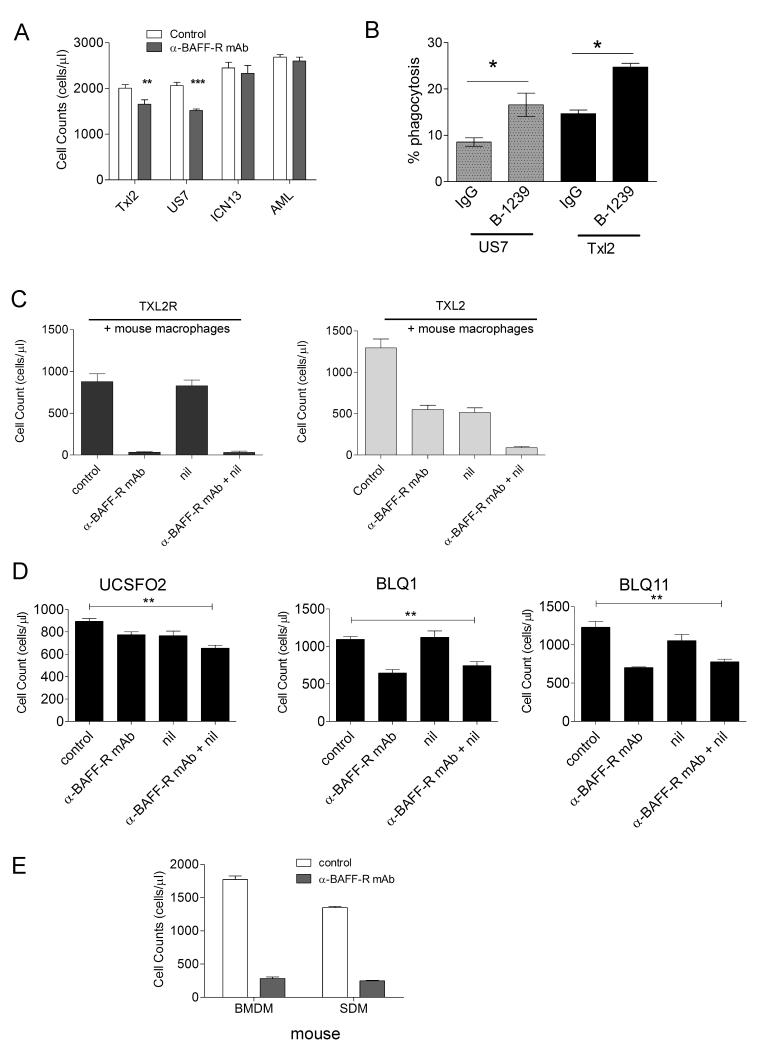
Some monoclonal antibodies promote antibody-mediated phagocytosis (ADCP) and macrophages can be a major effector cell type that mediates cell killing through antibodies. We therefore tested human macrophage-mediated cell eradication against TXL2 and US7, representative of Ph-positive and negative ALLs. As negative controls, we included ICN13, a pro-B ALL and an AML. Based on FACS, neither expresses the BAFF-R (not shown). As shown in Fig. 5A, cell counts of ICN13 and AML incubated with macrophages with and without B-1239 were identical, indicating that in the absence of the BAFF-R these antibodies do not stimulate phagocytosis. However, B-1239 clearly stimulated eradication of ALL cells expressing the BAFF-R, demonstrating the specificity of this effect. We also directly measured phagocytosis and found specific stimulation by B-1239 (Fig. 5B).
Figure 5.
B-1239 stimulates ADCP by human and mouse macrophages. A, Cell counts of TXL2, US7, ICN13 or AML cells preincubated or not (control) with 25 μg/ml B-1239 followed by a 4-hour co-incubation with human macrophages. **p<0.01, ***p<0.001 compared to control. B, Percentage phagocytosis by human macrophages of US7 or TXL2 cells in an E:T ratio of 1:1 after 2 hrs in the presence of 20 μg/ml B-1239 or 20 μg/ml control human IgG. One of two experiments with independent macrophage isolates. C, Live cell counts of TXL2 and TXL2R in the presence of mouse SDM without further treatment (control) or with 100 nM nilotinib, 25 μg/ml B-1239, or both for 24 hrs. D, Live cell counts of the indicated Ph-positive pre-B ALLs treated as in (C). **p<0.01, for combination treatment compared to control. E, Live cell counts of US7 cells incubated with mouse BMDM or SDM in the presence or absence of 25 μg/ml B-1239.
As reviewed by Galluzzi et al (25), some targeted drugs may also indirectly decrease tumor cell survival in vivo through mechanisms involving immune cell function. There is little information if inhibitors such as nilotinib will affect functioning of non-malignant immune cells. Therefore, we evaluated B-1239-stimulated eradication of TXL2 cells by mouse SDM in the presence of nilotinib. As control, we included TXL2R, which is not responsive to nilotinib. Fig. 5C shows that nilotinib treatment did not affect TXL2R cell numbers when there were macrophages present (compare control to nilotinib monotreatment). As expected, B-1239 stimulated phagocytosis of TXL-2R, and macrophage ADCP function was not affected by the presence of nilotinib. In contrast and as expected, nilotinib significantly reduced TXL2 cell numbers within 24 hours. B-1239 treatment had a similar effect, and a combined treatment with nilotinib and B-1239 was additive. These results further suggest that nilotinib acts as a chemosensitizer for phagocytosis. Additional experiments with three other Ph-positive pre-B ALLs from relapsed patients, which are nilotinib-insensitive, confirmed that nilotinib acts as a sensitizer, since it had no additive effect combined with the anti-BAFF-R antibody on these pre-B ALL cells (Fig. 5D).
Major sites of ALL cell infiltration and proliferation include the bone marrow and the spleen. We therefore compared the ability of mouse bone marrow-derived macrophages (BMDM) and spleen-derived macrophages (SDM) to phagocytose human ALL cells. Fig. 5E shows that macrophages from both locations had significant ADCP activity in the presence of B-1239.
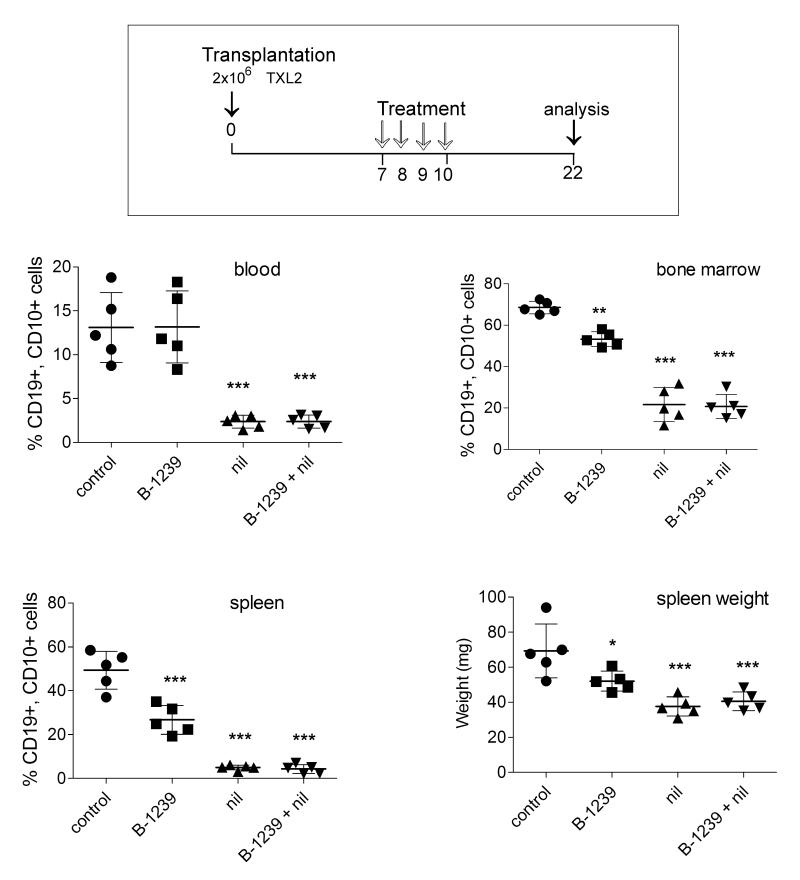
To evaluate the ability of B-1239 to stimulate pre-B ALL killing in vivo we transplanted NSG mice with TXL2 cells and allowed the cells to proliferate for 6 days to form an appreciable tumor burden (Fig. 6). Next, either 10 mg/kg B-1239 or human IgG was administered as four daily treatments. We also included treatment groups with 75 mg/kg nilotinib or a combination of nilotinib and B-1239, and all mice were sacrificed to evaluate leukemia cell burden. As shown in Fig. 6, FACS analysis showed that 12 days after the last treatment, leukemia cell numbers in the circulation of all treated groups were comparable. However, a significant inhibition of ALL cell growth in the bone marrow and spleens of mice treated with BAFF-R antibody compared to control groups was noted. The spleen weight of BAFF-R antibody-treated mice was also significantly reduced compared to controls (Fig. 6). Nilotinib monotreatment significantly reduced the ALL cell burden in spleen and bone marrow. Although no added effect of combination treatment was seen, a second in vivo experiment in which mice had a lower overall ALL burden suggested a small additive benefit (not shown).
Figure 6.
Treatment of mice with B-1239. Top, Schematic of in vivo treatment with 10 mg/kg B-1239, control 10 mg/kg human IgG or combined with 75 mg/kg nilotinib. Bottom, Percentage of human CD19+ CD10+ ALL cells as detected by FACS in blood, bone marrow and spleen of NSG leukemic mice and spleen weights of control and treated mice on d22. *p<0.05, **p<0.01, ***p<0.001; all comparisons with control. Nil versus nil+ B-1239, n.s. One of two independently performed experiments.
We previously reported common expression of the BAFF-R in patient-derived B-precursor ALL samples (11), in agreement with Maia et al who detected expression using RT/PCR (9). Because thirteen other primary CD19+, CD10bright, sIgM- B-precursor ALL samples were BAFF R-positive (manuscript in preparation) this receptor may be expressed in a major percentage of ALL samples. We conclude that B-1239, which interferes with binding of BAFF to the BAFF-R, is a promising antibody-based treatment that should be further evaluated because it may reduce the fitness of pre-B ALL cells when combined with other drugs. Importantly, it has very significant other activities including ADCP in vitro and in vivo, and promotes NK cell-mediated killing of ALL cells. Together, these activities are likely to be extremely useful to eradicate pre-B ALL cells.
Acknowledgements
Yong-mi Kim is acknowledged for the primary AML sample. We thank Anne George for help with the ImageStream, Jennifer Johnson for receptor copy number analysis and Esteban Fernandez of the Cellular Imaging Core at CHLA for expert help with the confocal microscopy.
Grant Support Supported by PHS NIH CA090321, CA172040, Alex’s Lemonade Stand Foundation, the V-Foundation and Novartis.
Grant support: NIH PHS CA090321, CA172040, ALSF, the V-foundation and Novartis (N. Heisterkamp).
Disclosure of potential conflicts of interest: Studies using the B-1239 α-BAFF-R antibody were supported by a grant from Novartis.
Abbreviations
- ADCC
antibody-dependent cellular cytotoxicity
- ADCP
antibody-mediated phagocytosis
- BAFF-R
B-cell activating factor receptor
- BCR
B cell receptor
- BMDM
bone marrow-derived macrophages
- E : T
effector-to-target
- MFI
mean fluorescent intensity
- NK
natural killer
- NSG
NOD.Cg-PrkdcscidIl2rgtm1Wjll/SzJ
- pre-B ALL
precursor B acute lymphoblastic leukemia ALL
- SDM
spleen-derived macrophages
- WT
wild type
Footnotes
Authors Contributions Conception and design: R. Parameswaran, M. Lim, H. Abdel-Azim, M. McLaughlin, N. Heisterkamp
Development of methodology: H. Gram
Acquisition of data: R. Parameswaran, M. Lim, F. Fei, H. Abdel-Azim, A. Arutyunyan, I. Schiffer, H. Huet, H. Gram
Analysis and interpretation of data: R. Parameswaran, M. Lim, F. Fei, H. Abdel-Azim, I. Schiffer, J. Groffen, N. Heisterkamp.
Writing, review and/or revision of manuscript: R. Parameswaran, M. Lim, H. Abdel-Azim, N. Heisterkamp
References
- 1.Parameswaran R, Muschen M, Kim YM, Groffen J, Heisterkamp N. A functional receptor for B-cell-activating factor is expressed on human acute lymphoblastic leukemias. Cancer Res. 2010;70:4346–56. doi: 10.1158/0008-5472.CAN-10-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mackay F, Schneider P, Rennert P, Browning J. BAFF AND APRIL: a tutorial on B cell survival. Annu Rev Immunol. 2003;21:231–64. doi: 10.1146/annurev.immunol.21.120601.141152. [DOI] [PubMed] [Google Scholar]
- 3.Lyu MA, Cheung LH, Hittelman WN, Marks JW, Aguiar RC, Rosenblum MG. The rGel/BLyS fusion toxin specifically targets malignant B cells expressing the BLyS receptors BAFF-R, TACI, and BCMA. Mol Cancer Ther. 2007;6:460–70. doi: 10.1158/1535-7163.MCT-06-0254. [DOI] [PubMed] [Google Scholar]
- 4.Kern C, Cornuel JF, Billard C, Tang R, Rouillard D, Stenou V, et al. Involvement of BAFF and APRIL in the resistance to apoptosis of B-CLL through an autocrine pathway. Blood. 2004;103:679–88. doi: 10.1182/blood-2003-02-0540. [DOI] [PubMed] [Google Scholar]
- 5.Asselin BL, Gaynon P, Whitlock JA. Recent advances in acute lymphoblastic leukemia in children and adolescents: an expert panel discussion. Curr Opin Oncol. 2013;25(Suppl 3):S1–13. doi: 10.1097/CCO.0000000000000017. quiz S4-6. [DOI] [PubMed] [Google Scholar]
- 6.Mackay F, Browning JL. BAFF: a fundamental survival factor for B cells. Nat Rev Immunol. 2002;2:465–75. doi: 10.1038/nri844. [DOI] [PubMed] [Google Scholar]
- 7.Khan WN. B cell receptor and BAFF receptor signaling regulation of B cell homeostasis. J Immunol. 2009;183:3561–7. doi: 10.4049/jimmunol.0800933. [DOI] [PubMed] [Google Scholar]
- 8.Smith SH, Cancro MP. Cutting edge: B cell receptor signals regulate BLyS receptor levels in mature B cells and their immediate progenitors. J Immunol. 2003;170:5820–3. doi: 10.4049/jimmunol.170.12.5820. [DOI] [PubMed] [Google Scholar]
- 9.Maia S, Pelletier M, Ding J, Hsu YM, Sallan SE, Rao SP, et al. Aberrant expression of functional BAFF-system receptors by malignant B-cell precursors impacts leukemia cell survival. PLoS One. 2011;6:e20787. doi: 10.1371/journal.pone.0020787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Onda K, Iijima K, Katagiri YU, Okita H, Saito M, Shimizu T, et al. Differential effects of BAFF on B cell precursor acute lymphoblastic leukemia and Burkitt lymphoma. Int J Hematol. 2010;91:808–19. doi: 10.1007/s12185-010-0567-z. [DOI] [PubMed] [Google Scholar]
- 11.Parameswaran R, Yu M, Lyu MA, Lim M, Rosenblum MG, Groffen J, et al. Treatment of acute lymphoblastic leukemia with an rGel/BLyS fusion toxin. Leukemia. 2012;26:1786–96. doi: 10.1038/leu.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathisen MS, Kantarjian HM, Jabbour EJ. Emerging drugs for acute lymphocytic leukemia. Expert Opin Emerg Drugs. 2014;19:37–50. doi: 10.1517/14728214.2014.872629. [DOI] [PubMed] [Google Scholar]
- 13.Rytting M, Triche L, Thomas D, O’Brien S, Kantarjian H. Initial experience with CMC-544 (inotuzumab ozogamicin) in pediatric patients with relapsed B-cell acute lymphoblastic leukemia. Pediatr Blood Cancer. 2014;61:369–72. doi: 10.1002/pbc.24721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah NN, Dave H, Wayne AS. Immunotherapy for pediatric leukemia. Front Oncol. 2013;3:166. doi: 10.3389/fonc.2013.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Natsume A, Niwa R, Satoh M. Improving effector functions of antibodies for cancer treatment: Enhancing ADCC and CDC. Drug Des Devel Ther. 2009;3:7–16. [PMC free article] [PubMed] [Google Scholar]
- 16.Hoelzer D. Targeted therapy with monoclonal antibodies in acute lymphoblastic leukemia. Curr Opin Oncol. 2013;25:701–6. doi: 10.1097/CCO.0000000000000009. [DOI] [PubMed] [Google Scholar]
- 17.Daver N, O’Brien S. Novel therapeutic strategies in adult acute lymphoblastic leukemia--a focus on emerging monoclonal antibodies. Curr Hematol Malig Rep. 2013;8:123–31. doi: 10.1007/s11899-013-0160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matlawska-Wasowska K, Ward E, Stevens S, Wang Y, Herbst R, Winter SS, et al. Macrophage and NK-mediated killing of precursor-B acute lymphoblastic leukemia cells targeted with a-fucosylated anti-CD19 humanized antibodies. Leukemia. 2013;27:1263–74. doi: 10.1038/leu.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee CV, Hymowitz SG, Wallweber HJ, Gordon NC, Billeci KL, Tsai SP, et al. Synthetic anti-BR3 antibodies that mimic BAFF binding and target both human and murine B cells. Blood. 2006;108:3103–11. doi: 10.1182/blood-2006-03-011031. [DOI] [PubMed] [Google Scholar]
- 20.Beck A, Reichert JM. Marketing approval of mogamulizumab: a triumph for glyco-engineering. MAbs. 2012;4:419–25. doi: 10.4161/mabs.20996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fei F, Stoddart S, Muschen M, Kim YM, Groffen J, Heisterkamp N. Development of resistance to dasatinib in Bcr/Abl-positive acute lymphoblastic leukemia. Leukemia. 2010;24:813–20. doi: 10.1038/leu.2009.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duy C, Hurtz C, Shojaee S, Cerchietti L, Geng H, Swaminathan S, et al. BCL6 enables Ph+ acute lymphoblastic leukaemia cells to survive BCR-ABL1 kinase inhibition. Nature. 2011;473:384–8. doi: 10.1038/nature09883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davies JQ, Gordon S. Isolation and culture of human macrophages. Methods Mol Biol. 2005;290:105–16. doi: 10.1385/1-59259-838-2:105. [DOI] [PubMed] [Google Scholar]
- 24.Pillay V, Gan HK, Scott AM. Antibodies in oncology. N Biotechnol. 2011;28:518–29. doi: 10.1016/j.nbt.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 25.Galluzzi L, Senovilla L, Zitvogel L, Kroemer G. The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov. 2012;11:215–33. doi: 10.1038/nrd3626. [DOI] [PubMed] [Google Scholar]