Abstract
OBJECTIVE
To assess the efficacy of in-shoe orthoses that were designed based on shape and barefoot plantar pressure in reducing the incidence of submetatarsal head plantar ulcers in people with diabetes, peripheral neuropathy, and a history of similar prior ulceration.
RESEARCH DESIGN AND METHODS
Single-blinded multicenter randomized controlled trial with subjects randomized to wear shape- and pressure-based orthoses (experimental, n = 66) or standard-of-care A5513 orthoses (control, n = 64). Patients were followed for 15 months, until a study end point (forefoot plantar ulcer or nonulcerative plantar forefoot lesion) or to study termination. Proportional hazards regression was used for analysis.
RESULTS
There was a trend in the composite primary end point (both ulcers and nonulcerative lesions) across the full follow-up period (P = 0.13) in favor of the experimental orthoses. This trend was due to a marked difference in ulcer occurrence (P = 0.007) but no difference in the rate of nonulcerative lesions (P = 0.76). At 180 days, the ulcer prevention effect of the experimental orthoses was already significant (P = 0.003) when compared with control, and the benefit of the experimental orthoses with respect to the composite end point was also significant (P = 0.042). The hazard ratio was 3.4 (95% CI 1.3–8.7) for the occurrence of a submetatarsal head plantar ulcer in the control compared with experimental arm over the duration of the study.
CONCLUSIONS
We conclude that shape- and barefoot plantar pressure–based orthoses were more effective in reducing submetatarsal head plantar ulcer recurrence than current standard-of-care orthoses, but they did not significantly reduce nonulcerative lesions.
Introduction
It has long been recognized that a foot ulcer is a critical step in the causal pathway for most amputations in diabetes (1), and such ulcers result in significant morbidity and cost even when they do not lead to an amputation (2–4). After an initial foot ulcer, the risk of reulceration is extraordinarily high (5–7), in some studies >80% after 3 years (8). Approximately 40% (14–78%) of diabetic foot ulcers are located on the plantar surface related to metatarsal heads (MTHs) (9–13), where plantar pressure is generally highest (14). Thus, such lesions are the result of the repetitive forces of weight-bearing in patients whose gait is unmodified by sensory feedback (15). This implies that therapeutic footwear targeted to prevent plantar ulceration should maximally offload areas of high plantar pressure. Thus the variable results of studies exploring the ulcer prevention efficacy of diabetic footwear are likely to be at least partly due to the fact that efficacy of the footwear used, in terms of plantar offloading, is usually neither defined nor tested (16). To be eligible in the U.S. for Medicare coverage, custom in-shoe orthoses provided (the footwear component primarily responsible for plantar offloading) must be custom molded to the shape of the patient’s foot but, beyond that, the specifications are only generic, addressing the hardness and thickness of materials that are used (Healthcare Common Procedure Coding System [HCPCS] code A5513).
We have previously demonstrated that shape- and pressure-based orthoses provide superior MTH offloading compared with conventional A5513 diabetic orthoses (17). In this present Care For ULcer (CareFUL) Prevention trial, we tested the hypothesis that these same orthoses manufactured on the basis of both barefoot plantar pressure and foot shape are superior in preventing recurrence of plantar injury in neuropathic patients with a recently healed MTH-related plantar ulcer compared with standard-of-care orthoses manufactured on the basis of shape and clinical information alone.
Research Design and Methods
Study Setting
This study took place at 11 outpatient clinic sites specializing in diabetic foot care across the U.S. (two each in Pennsylvania, Illinois, California, and Arizona and one each in Ohio, Texas, and Colorado) and included academic medical centers, Veterans Affairs clinics, and private practice podiatry clinics (Supplementary Appendix 1). All site principal investigators (PIs) were clinicians with significant experience managing diabetes-related foot problems and had prior experience conducting clinical trials.
Study Participants
Most subjects were patients at the cooperating clinics and were recruited under institutional review board (IRB) approval. Local PIs considered consecutive patients with recently healed ulcers for inclusion in the study at each clinic site so that subjects who progressed to formal screening were already highly likely to meet the inclusion criteria, which are presented in full in Supplementary Appendix 2. In brief, inclusion criteria were as follows: men and women ≥18 years of age with diabetes and loss of protective sensation (inability to feel the 10-g monofilament at one or more sites [18]); at least one recently healed (>1 week but <4 months) plantar MTH-related foot ulcer; peak barefoot plantar pressure in the area of this previous ulcer (the “index ulcer”) >450 kPa; community ambulator; no current ulcer below the malleoli; partial foot amputation of no greater than two MTHs or rays per foot (no limit on toe amputations); no ankle–foot orthosis; no existing footwear intervention more complex than would be available through the study footwear and orthotic options (e.g., no rigid outsole, custom-molded shoes); and ability to comply with the protocol. All study questionnaires were available in English or Spanish. Peak barefoot plantar pressures were measured using a Novel emed D platform with a spatial resolution of four sensors per cm2 and a sampling frequency of 50 Hz using the average of five trials and a first walking step protocol (17).
Study Design and Randomization
Eligible subjects were randomized in a 3:1:1:1 ratio (experimental orthosis:control orthosis 1:control orthosis 2:control orthosis 3; see below). Randomization was stratified by site and sex. Blocked randomization (block size 6) was used within strata. The randomization tables were developed by one of the investigators (D.T.M.) who was not directly involved in managing the trial. Randomization assignment was given by the coordinating center to each site for each subject after eligibility was confirmed. The study PI (J.S.U.), study statistician (D.T.M.), patients, and adjudicators were blinded as to the assignment of patients to treatment groups.
After randomization, visit two for dispensing of footwear occurred at 7.9 and 7.8 weeks in the experimental and control arms, respectively. During this time, the protocol recommended that subjects remain in their healing device or in a removable cast walker provided through the study. Subsequent visits were the same for all footwear conditions and were timed to facilitate frequent assessment, potential footwear modification, and subject education, particularly during the break-in period. The break-in process was formalized. Starting with dispensing of footwear (time 0), visits occurred at +1 week, +3 weeks, +6 weeks, and then every 3 months for another 15 months for a potential total follow-up time in footwear of 16.5 months. The 15-month follow-up time was selected based on experience from other published studies (19). Subjects were followed to primary end point, their volitional withdrawal, or termination of the study.
The study was registered at http://www.clinicaltrials.gov (NCT00803608). The overall study was reviewed and approved by the Essex IRB (121 Main Street, Lebanon, NJ 08833), and each study site was also reviewed and approved either by Essex or by a site-specific IRB.
Orthoses and Footwear
For all orthoses (control and experimental), foot shape was obtained using foam boxes, which were digitally scanned for manufacture of the experimental orthoses and sent to the manufacturer of the control insoles.
Manufacture of the experimental orthoses has been previously described (17). They are initially designed to be similar to a “shape only” insole and then modified using a computer-aided design process according to defined algorithms (17) based on the peak barefoot plantar pressure distribution contours. The orthoses are then milled from a block of ethylene vinyl acetate foam (Shore A 35 durometer) using a computer-aided manufacturing process. A laminated fabric-PPT (polyurethane foam) top cover is added, and the orthoses are hand-finished and shipped back to the clinical site for dispensing. For this study, the design algorithm focused on offloading the submetatarsal head region.
We chose to include standard orthoses from three different manufacturers in the control arm of the study in order to make the results more generalizable. However, the study was not powered for comparisons of the individual control orthoses. The three manufacturers were recognizable names in the field that routinely provide Medicare-reimbursed remotely manufactured diabetic in-shoe orthoses on the basis of foot shape and clinical information (HCPCS code A5513). The standard order form for each manufacturer was used, and the site PIs were encouraged to request additional modifications such as metatarsal pads, bars, reliefs, etc., as they would do in their clinical practice.
In all cases, subjects received three pairs of identical orthoses to be rotated while using the primary study footwear according to a written rotation protocol, changing the numbered orthoses in a set rotation every month. Subjects were offered one of two footwear models manufactured by p.w. Minor (Batavia, NY) regardless of study group assignment (Performance Walker and Leisure Time). The experimental insoles required more depth, and thus the DX2 specification was used for experimental subjects, whereas the Extra Depth specification was used for the control subjects. Subjects in all groups were also given a pair of Pedors Classic (Marietta, GA) shoes with 9.5-mm flat trilaminate orthoses and no customization for occasional steps at home. However, they were asked to use the primary footwear for the majority of their indoor and outdoor ambulation. Subjects could use protective overboots (e.g., Neos Overshoe) in inclement weather conditions.
The orthoses and shoes could be adjusted by the local site staff for fit at any point during the trial, but they could not be modified to alter the plantar offloading properties. Modifications such as trimming of orthoses, stretching of shoes, addition of tongue pads, and repairs were permitted, whereas addition of metatarsal pads and orthotic reliefs (after the initial prescription, which could include these modifications) or rocker outsoles were not permitted. In cases where the site PI or the subject was concerned about the safety of continued use of the study footwear and when permitted modifications could not alleviate such concerns, subjects discontinued use of study footwear. Any subject who discontinued use of the study footwear for any reason for >45 days was excluded from further footwear use and was deemed to have discontinued study footwear permanently but followed where possible to permit an intent-to-treat analysis. Shoes and/or orthoses could be replaced using prior data stored by the manufacturers if either were showing excessive wear in the opinion of the PI or of the subject.
Education and Motivation
Study coordinators discussed self-care behavior with all patients regardless of group assignment at each visit with a special focus on wearing the study shoes for all steps taken and on examining the feet daily to note and report problems. Study coordinators used worksheets to encourage adherence and also used nonthreatening open-ended questions in their conversations with study subjects. An educational brochure was given to patients as was a door hanger to remind subjects daily of what they should be doing (Supplementary Appendices 3 and 4).
End Point and Descriptive Measures
The primary end point for the study was either an ulcer or nonulcerative lesion involving the plantar surface and associated with an MTH. High-resolution standard view digital photographs of the feet and footwear were obtained at all study visits. The foot photographs were reviewed initially by one of the investigators blinded to group assignment, and a suspected ulcer or nonulcerative plantar lesion below the malleoli was referred to a panel of three blinded (as to group assignment) adjudicators, all experts in the field. Photographs were reviewed in the context of the full photographic series over time. Lesions were judged as absent/nonulcerative lesion/ulcer; involving/not involving the plantar surface (defined as involving the weight-bearing surface of the foot); and related to MTH/not related to MTH. Adjudicators did not confer with each other or with the study staff, and the majority opinion was taken as the final judgment. Ulcers were judged to be present if the integrity of both the epidermis and dermis was broken. A nonulcerative lesion (20) was defined for this study as hemorrhage into callus (all but one of such lesions adjudicated) or redness at a site of bony prominence persisting >20 min after removal of footwear and rest, based on repeat photographs.
In contrast to previous studies, we included nonulcerative plantar lesions as part of the composite primary end point because we hypothesized that these lesions would also be prevented by the experimental footwear and because we felt it to be unethical to continue subjects in footwear that “allowed” such lesions to develop. Safety end points tracked in the study included skin injury below the malleoli not qualifying as primary end point as well as self-reported falls and fear of falling (21) and falls reported by sites as adverse events.
Information obtained at the screening visit included demographic details, information needed for inclusion/exclusion and for prescription of orthoses and footwear, as well as ankle–brachial index (ABI) (values absent due to noncompressible vessels were treated as missing data), standardized foot exam for deformity (in the analyses, the deformity index used was for the foot with the primary end point, or right foot when no end point occurred) (Supplementary Appendix 5), and baseline questionnaires assessing neuropathy-specific quality of life (22) (Supplementary Appendix 6), foot self-care (23) (Supplementary Appendix 7), and falls and fear of falls (21) (Supplementary Appendix 8). These three questionnaires were also administered at each follow-up visit, as was a questionnaire addressing subject satisfaction with footwear and footwear use (Supplementary Appendix 9).
Data Management and Statistical Analyses
Each site entered study data from paper source documents into a centralized data management system maintained and managed by the Department of Public Health Sciences, College of Medicine, Pennsylvania State University. Data were validated by the coordinating center against the primary source documents.
For the primary analyses, Kaplan-Meier survival curves were constructed for graphical displays of the time-to-event outcomes. Proportional hazards regression analyses were applied to test for statistical significance of the treatment effect. Separate analyses were performed for the composite outcome and for each of the two components, time to ulcer and time to nonulcerative lesion.
Secondary analyses using proportional hazard regression examined associations between primary end points and various patient characteristics. A linear mixed-effects model was used for analysis of patient-reported outcomes measured across time. All analyses were performed using the intent-to-treat paradigm with SAS version 9.2 (SAS Inc., Cary, NC).
The target sample size was 286 randomized patients, calculated to provide 80% statistical power for a two-sided, 0.05 significance level test, allowing for 15% withdrawals, to detect a 50% reduction in the rate of reulceration. We anticipated a 15-month ulcer recurrence rate of 30% in the control footwear group based on estimates from the literature (19) and 15% in the experimental footwear group based on a clinically meaningful risk reduction. Because recruitment was slower than anticipated, our funder (National Institute of Diabetes and Digestive and Kidney Diseases [NIDDK]) requested an unplanned interim analysis, and, based upon this assessment, we terminated recruitment and follow-up with a final sample size of 150 subjects randomized. The trial was ended because to achieve a statistically significant effect with respect to the primary composite end point (MTH-related plantar ulcer or nonulcerative lesion) would likely have required many more subjects and a significant effect in terms of ulcer prevention was already apparent.
Results
In all analyses, the effect of study site on outcomes was examined and there were no significant effects. Similarly, no differences were noted between the three control subgroups. Therefore in all subsequent analyses, the results are presented across the full trial comparing the experimental group and the control group as a whole.
Study Participants
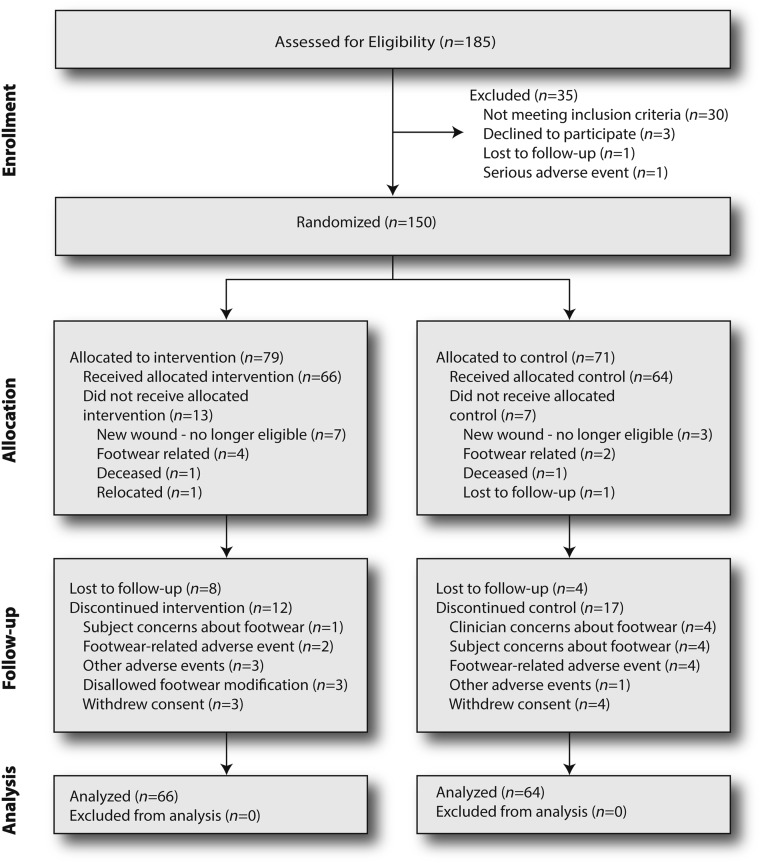
The flow of subjects through the various phases of the study is shown in the CONSORT diagram (24) in Fig. 1. Twenty patients did not receive the allocated intervention (13 experimental and 7 control). This reflects clinical reality where several weeks often elapse between footwear and orthoses being ordered and dispensed because of manufacturing and shipping times. Such was the case in the current study. During this time, events, including clinical events, can preclude footwear being dispensed at all. In the experimental arm, 7 of the 13 subjects developed new ulcers at various sites below the malleoli and therefore no longer met inclusion criteria; in the control arm this number was 3 of 7. In the experimental arm, footwear could not be made to fit or was otherwise deemed unsuitable in four patients; in the control arm, this number was two. There were no statistically significant differences in terms of the baseline characteristics (Table 1) between randomized subjects receiving footwear and not receiving footwear, except that those not receiving footwear reported higher scores for avoiding foot-damaging behaviors (0.96 vs. 0.90; P = 0.03).
Figure 1.
CONSORT diagram.
Table 1.
Baseline demographic and other characteristics of subjects who received footwear
| Characteristic | Experimental group | Control group |
|---|---|---|
| Number of subjects | 66 | 64 |
| Number of male subjects | 50 (75.8%) | 52 (81.3%) |
| Age in years* (range) | 60.5 ± 10.1 (33–83) | 58.5 ± 10.7 (35–88) |
| BMI (kg/m2)* | 32.3 ± 7.1 | 31.4 ± 5.5 |
| Smoking | 6 (9.1%) | 12 (18.8%) |
| Ethnicity | ||
| Hispanic or Latino | 21 (31.8%) | 20 (30.2%) |
| Race | ||
| White† | 55 (83.3%) | 51 (79.7%) |
| African American† | 10 (15.2%) | 11 (17.2%) |
| Other† | 1 (1.5%) | 2 (3.1%) |
| Socioeconomic | ||
| At least high school graduate | 49 (74.2%) | 52 (82.3%) |
| Graduated college | 12 (18.2%) | 18 (28.1%) |
| Not living alone | 50 (75.8%) | 47 (73.4%) |
| ABI*‡ | 1.05 ± 0.16 | 1.13 ± 0.18 |
| Index ulcer location§ | ||
| MTH 1 | 36 (54.5%) | 29 (45.3%) |
| MTH 2 | 13 (19.7%) | 10 (15.6%) |
| MTH 3 | 2 (3.0%) | 11 (17.2%) |
| MTH 4 | 5 (7.6%) | 8 (12.5%) |
| MTH 5 | 13 (19.7%) | 11 (17.2%) |
| Barefoot peak plantar pressure at prior index ulcer site (kPa)* | 946 ± 266 | 967 ± 233 |
| Barefoot peak plantar pressure at any site in either foot (kPa)* | 1,109 ± 173 | 1,085 ± 191 |
| Prior minor amputation|| | 21 (31.8%) | 24 (37.5%) |
| Foot deformity index*¶ | 28.4 ± 14.6 | 28.9 ± 17.3 |
| NeuroQOL*# | ||
| Physical symptoms | 1.56 ± 0.85 | 1.38 ± 0.87 |
| Psychological symptoms | 1.57 ± 1.13 | 1.64 ± 1.16 |
| Foot self-care*,** | ||
| Preventive | 0.66 ± 0.21 | 0.68 ± 0.20 |
| Avoid damaging behaviors | 0.88 ± 0.14 | 0.92 ± 0.12 |
| Falls | ||
| Number recalling a fall last year | 28 (42.4%) | 34 (53.1%) |
| Number who worry about falling (%)* | 47.1 ± 31.2 | 45.8 ± 33.9 |
NeuroQOL, neuropathy-specific quality of life. *Mean ± SD.
†Some selected more than one race.
‡Average of all legs.
§Some had more than one index ulcer.
||See exclusion criteria.
¶Scale 0–100.
#Scale 0–4, 0 = no symptoms.
**Scale 0–1, 1 = best behavior.
At baseline, the mean ABI was higher in the control group (P = 0.02), and subjects entering the control group showed a trend toward higher scores on avoiding foot-damaging behaviors (P = 0.07); both these biases would favor better outcomes in the control group. The sample as a whole was typical of diabetic neuropathic patients being predominantly male, in late middle age, and obese. The group was ethnically and racially diverse.
Footwear
In the control arm, metatarsal pads or bars were provided in 59% of cases and metatarsal reliefs (removal of material under an MTH) in 33%, based on the opinion/wishes of the prescribing clinician. By design, all experimental orthoses had patient-specific metatarsal bars and reliefs (17). Nine experimental and five control subjects received replacement/supplemental footwear in an average of 277 days after the original footwear was dispensed (237 days experimental and 351 days control).
Primary and Secondary End Points
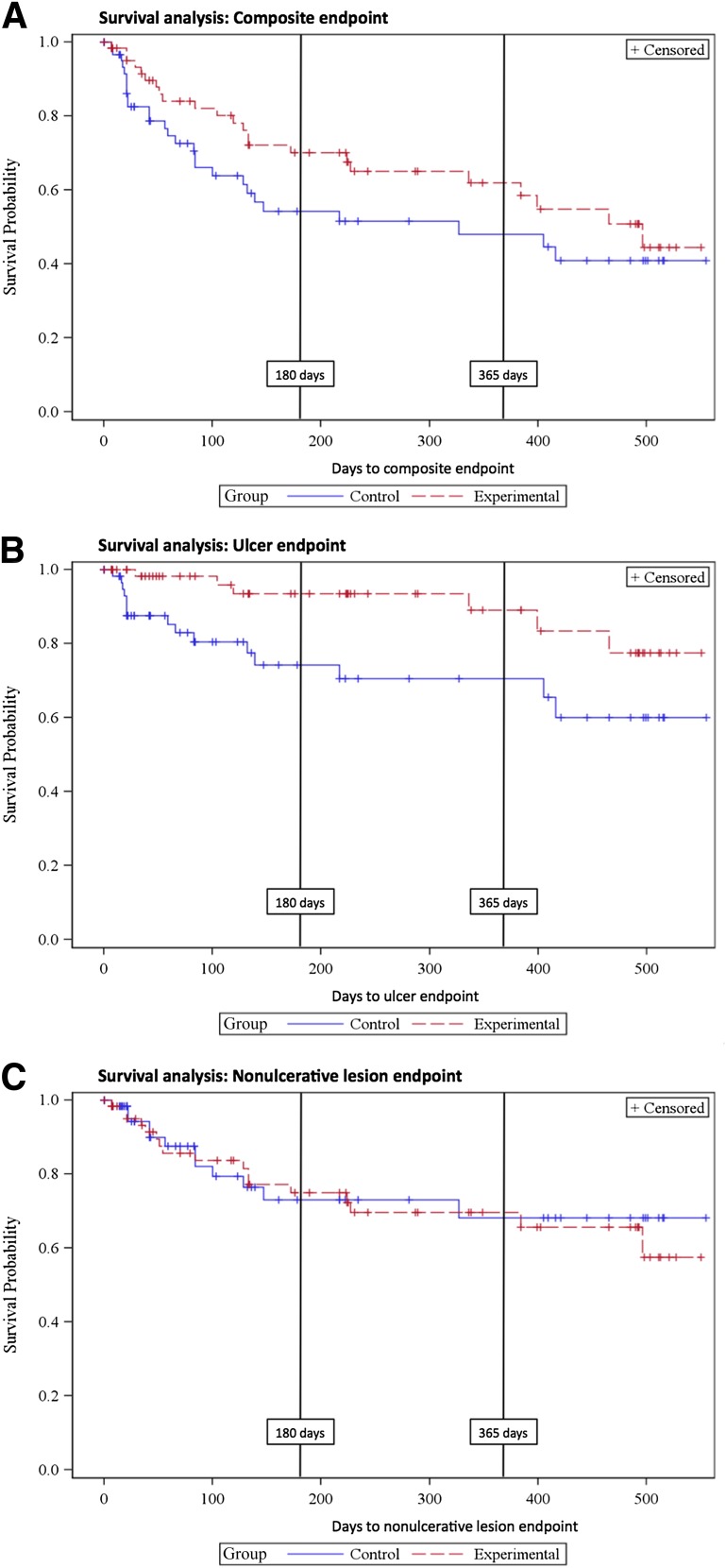
As shown in Fig. 2A, there is a clear trend toward a difference in the composite primary end point (ulcers or nonulcerative lesions) across the full follow-up period (P = 0.13). This trend is due to a marked difference in ulcer occurrence (P = 0.007, greater in control) (Fig. 2B), whereas there is no difference in the rate of nonulcerative lesions (P = 0.76) (Fig. 2C). Examination of the Kaplan-Meier curves suggests that most of the benefit accrues over the first few months, after which the event rates appear to be parallel. Thus at 180 days, the ulcer prevention effect of the experimental condition is already significant (P = 0.003) when compared with the control condition; at this point, the benefit of the experimental condition with respect to the composite end point is also significant (P = 0.042).
Figure 2.
A: Combined end point Kaplan-Meier curve. B: Ulcer end point Kaplan-Meier curve. C: Nonulcerative lesion end point Kaplan-Meier curve.
The P values at 1 year, the point at which footwear is usually replaced, were P = 0.073 and P = 0.0041 for the composite and ulcer end points, respectively. Across the full study, a primary end point occurred in 37.9% (25 of 66) of subjects in the experimental arm (ulcers in 9.1%; 6 of 66) and in 45.3% (29 of 64) of subjects in the control group (ulcers in 25.0%; 16 of 64). The hazard ratio was 3.4 (95% CI 1.3–8.7) for the occurrence of an ulcer in the control footwear compared with the experimental condition over the duration of the study.
None of the baseline characteristics except barefoot plantar pressure predicted outcomes, composite or component, in either arm. Higher end point foot-specific (or right if no end point) forefoot peak plantar barefoot pressure was associated with higher risk of end point lesions. Thus, the peak barefoot plantar pressure was on average 1,131, 1,042, and 984 kPa in feet that developed ulcers, nonulcerative lesions, and no end point, respectively (P = 0.04). In each case, the barefoot plantar pressures trended higher in the experimental compared with control groups (1,170 vs. 1,116 kPa for ulcers and 1,071 vs. 999 kPa for nonulcerative lesions; P = 0.3).
There were no changes in the reported quality of life, fear of falling, and satisfaction with footwear over the duration of the study. All subjects reported better average foot self-care habits after being instructed (P < 0.01 baseline vs. follow-up). No differences between study arms were apparent for these four measures over the follow-up period, and there were no statistically significant associations between any of these measures and outcomes. Most end point lesions occurred in the same forefoot as the index ulcer (49 of 54 for the composite end point, 19 of 22 for ulcers). There were no significant differences between groups in the occurrence of adverse events other than primary end points (Supplementary Appendix 10).
Conclusions
The findings of the current study indicate that patient-specific orthoses manufactured on the basis of foot shape and barefoot plantar pressure are superior to orthoses manufactured only on the basis of foot shape and clinical insight. Specifically, the hazard ratio was 3.4 (95% CI 1.3–8.7) for the occurrence of an ulcer in the control footwear compared with the experimental condition over the duration of the study. This observation extends the previous finding that such orthoses are superior in offloading the forefoot (17).
There was also a clear trend toward the experimental condition preventing the composite end point of skin ulcer and nonulcerative lesion (P = 0.04, P = 0.07, and P = 0.13 at the three analysis time points). However, it is also apparent that this trend was due to the ulcer-prevention effect of the experimental orthoses. The hypothesis that the experimental condition was different from the control condition regarding the frequency of nonulcerative lesions was therefore rejected.
One possible explanation for the lack of difference in the occurrence of nonulcerative lesions between the two groups is that some anatomical locations that would develop nonulcerative lesions in control footwear may escape damage altogether in the experimental footwear. Furthermore, some locations that would ulcerate in the control condition may result in nonulcerative lesions in experimental footwear. This hypothesis is supported by the observed trend differences in plantar pressure, where end points tended to occur in feet with higher forefoot barefoot pressures in the experimental group than in the control. Also in this study, as in others (15), occurrence of end point lesions was associated with higher forefoot barefoot plantar pressure regardless of study arm.
Nonulcerative skin lesions, particularly hemorrhage into callus, which is the most common lesion, are an established risk factor for ulceration if left untreated (5). However, treatment to prevent a full ulcer is usual and might include debridement of callus, a further modification to or change of footwear or a change in patient behavior to more consistently comply with footwear use, use of a pedometer to manage activity, etc.
The high rate of ulcer occurrence during the first weeks of use of the control insoles is consistent with the clinical observation that this is a particularly high-risk time. It also indicates the relative inadequacy of the control orthoses for ulcer prevention. The overall ulcer rates in this study were similar to those seen in other intervention studies with high-risk patients (19,20).
Because subjects in the experimental arm received double-extra depth rather than just extra depth shoes, it is plausible that this difference in the shoes somehow contributed to the positive findings in the study. Similarly, since footwear use was not measured, it is plausible that differences in footwear use between experimental and control subjects contributed to the observed effects. However, we think it likely that this effect would favor the control condition since the double-extra depth shoes were larger and possibly less acceptable to the subjects.
Most of the benefit apparent from the experimental treatment had already been achieved after ∼6 months of wear. After that, the survival curves are more or less parallel, and it therefore appears that footwear-related lesions occur relatively early after footwear is dispensed. It is possible that lesions occurring after the initial few months have less to do with footwear design but rather may be a consequence of variable activity patterns, noncompliance with footwear, etc. This finding is in contrast to some other studies, where the curves representing the effect of nonfootwear interventions continue to diverge throughout follow-up (19). Based on our data, it may be sufficient to conduct future footwear intervention trials for 6 months, or perhaps a maximum of 1 year when shoes and orthoses are customarily replaced.
We intentionally did not compare efficacy of the pressure- and shape-based orthoses to efficacy of custom orthoses manufactured by hand by a skilled orthotist or pedorthist. Examination of the efficacy of remotely produced pressure-based orthoses in comparison with such handcrafted orthoses would be of interest, since the manufacturing cost of the latter is much higher.
Taken together with the recent studies from the Netherlands (20,25), our data offer some clarification of the previously uncertain (16,25) foundation for the effectiveness of diabetic footwear. To increase the probability of ulcer prevention, therapeutic footwear must demonstrably reduce plantar pressure at defined high-risk locations and the patient must wear their footwear consistently.
The present findings are limited to ulcers under the MTHs since this was the scope of the current design algorithm and the site of prior ulcers in this sample. Future studies should examine other regions of the foot where similar relationships between plantar pressure reduction and risk reduction may be expected (16,20). In addition, a future study quantitatively examining the degradation in pressure relief of various orthoses as a function of time would inform guidelines for replacement/reimbursement. Although a definitive statement will require a formal cost-benefit analysis, the huge costs associated with foot ulcers and subsequent amputation in diabetic patients (2–4) (estimated to be $10.7 billion in 2001) (4) suggest that clinical use of the orthoses tested in this study would result in significant cost savings to the health care system.
In summary, this study demonstrates that orthoses individually designed and manufactured on the basis of both measured barefoot plantar pressure and foot shape prevent plantar ulceration at MTHs in high-risk patients better than current Medicare-approved orthoses. Data from barefoot plantar pressure measurement should be used as described by Owings et al. (17) in the design of orthoses for high-risk patients with diabetes and neuropathy.
Article Information
Acknowledgments. The contribution of Joseph L. Loomis, DIApedia LLC, to this project over the past 10 years is very much appreciated. The contribution of all participants in the study is gratefully acknowledged and greatly appreciated. The study would not have been possible without the hard work of the site investigators and study coordinators, which is also greatly appreciated. They were as follows: Blair Medical, Altoona, PA: Harold L. Penny (PI), Julia Benton (Research Manager), Heidi Sprouse (Research Coordinator); Carl T. Hayden VA Medical Research Foundation, Phoenix, AZ: Robert G. Frykberg (PI), Shannon Fears (Research Coordinator); Center For Clinical Research, San Francisco, CA: Alexander M. Reyzelman (PI), Gayana Sarkisova (Research Coordinator); Cleveland Clinic Foundation, Cleveland, OH: Georgeanne G. Botek (PI), Tammy Owings (Research Coordinator); Complete Family Foot Care, McAllen, TX: Joseph M. Caporusso (PI), Joseph F. Bender, Bryan J. Prukop (Sub-I), Tracy Rodriguez, Brenda Hernandez (Research Coordinators); Diabetic Foot and Wound Center, Denver, CO: Eric D. Jaakola (PI), Jeffrey L. Jensen (PI), Brian Gillin (CPed), Patricia Nelson (Research Coordinator); Edward Hines Jr. VA Hospital, Hines, IL: Rodney M. Stuck (PI), Coleen Napolitano, Nicholas Vogelsang (Sub-I), Anne Garabedian, Ana Zuluaga (Research Coordinators); Innovative Medical Technologies, Inc., Los Angeles, CA: Gabriel J. Halperin (PI), Jack Morgan (Sub-I), Francis Morfin (Research Coordinator); Southern Arizona Limb Salvage Alliance, Tucson, AZ: David G. Armstrong (PI), Maricela Cervantez, Marcy Watchman (Research Coordinators); Temple University School of Podiatric Medicine, Philadelphia, PA: James B. McGuire (PI), Jinsup Song (Sub-I), Dana Tango (Research Coordinator); Weil Foot and Ankle Institute, Des Plaines, IL: Lowell S. Weil Sr. (PI), Jeffrey Baker, George Enriquez, Erin Klein (Sub-I), Robert DeLeon, Jessica Knight, Usman Akram (Research Assistants), Iwona Putowska, Francesca Rivera (Research Coordinators).
A critical contribution to this project was made by the blinded photograph adjudicators: Michael J. Mueller (Washington University School of Medicine, St. Louis, MO), Neil M. Scheffler (Baltimore Podiatry Group, Baltimore, MD), Stephanie C.S. Wu (Rosalind Franklin University, Chicago, IL). The authors thank James Wrobel, DPM, MS (University of Michigan, Ann Arbor, MI), for providing data and safety oversight for the duration of the study and to members of the ad hoc data and safety monitoring board assembled to review the interim data analysis: Benjamin Wilfond, MD (Professor, Department of Pediatrics, University of Washington [Chair]), Ann Melvin, MD, MPH (Associate Professor of Pediatrics, Division of Infectious Disease, University of Washington), Patrick Heagerty, PhD (Professor, Biostatistics, University of Washington), and Susan Ellenberg, PhD (Professor, Biostatistics, University of Pennsylvania). The patient education materials were developed with the help of the late Richard Rubin, PhD (The Johns Hopkins University School of Medicine, Baltimore, MD), whose input is hereby acknowledged with gratitude. Last, the authors thank the staff at DIApedia LLC, Alesia Dumas, Heidi McClelland, Angie Morrison, and Linda Zedalis, who were responsible for overall trial coordination.
Funding. This study was supported by Grant 2R44-DK-059074 from the National Institutes of Health (NIDDK) to DIApedia LLC.
Duality of Interest. J.S.U., T.H., and P.R.C. all have equity in DIApedia LLC, which designed and manufactured the experimental orthoses. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. All authors participated in the design of the trial. J.S.U. served as the PI for the trial overall and wrote the manuscript. T.H. managed clinical site interactions and edited the manuscript. D.T.M. conducted the statistical analysis and edited the manuscript. P.R.C. wrote the manuscript. J.S.U. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT00803608, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-2956/-/DC1.
References
- 1.Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation. Basis for prevention. Diabetes Care 1990;13:513–521 [DOI] [PubMed] [Google Scholar]
- 2.Driver VR, Yao M. Discussion. The economics of limb salvage in diabetes. Plast Reconstr Surg 2011;127(Suppl. 1):296S–297S [DOI] [PubMed] [Google Scholar]
- 3.Driver VR, Fabbi M, Lavery LA, Gibbons G. The costs of diabetic foot: the economic case for the limb salvage team. J Vasc Surg 2010;52(Suppl.):17S–22S [DOI] [PubMed] [Google Scholar]
- 4.Gordois A, Scuffham P, Shearer A, Oglesby A, Tobian JA. The health care costs of diabetic peripheral neuropathy in the US. Diabetes Care 2003;26:1790–1795 [DOI] [PubMed] [Google Scholar]
- 5.Dubský M, Jirkovská A, Bem R, et al. Risk factors for recurrence of diabetic foot ulcers: prospective follow-up analysis in the Eurodiale subgroup. Int Wound J 2013;10:555–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kloos C, Hagen F, Lindloh C, et al. Cognitive function is not associated with recurrent foot ulcers in patients with diabetes and neuropathy. Diabetes Care 2009;32:894–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maciejewski ML, Reiber GE, Smith DG, Wallace C, Hayes S, Boyko EJ. Effectiveness of diabetic therapeutic footwear in preventing reulceration. Diabetes Care 2004;27:1774–1782 [DOI] [PubMed] [Google Scholar]
- 8.Edmonds ME, Blundell MP, Morris ME, Thomas EM, Cotton LT, Watkins PJ. Improved survival of the diabetic foot: the role of a specialized foot clinic. Q J Med 1986;60:763–771 [PubMed] [Google Scholar]
- 9.Cowley MS, Boyko EJ, Shofer JB, Ahroni JH, Ledoux WR. Foot ulcer risk and location in relation to prospective clinical assessment of foot shape and mobility among persons with diabetes. Diabetes Res Clin Pract 2008;82:226–232 [DOI] [PubMed] [Google Scholar]
- 10.Arts ML, Waaijman R, de Haart M, Keukenkamp R, Nollet F, Bus SA. Offloading effect of therapeutic footwear in patients with diabetic neuropathy at high risk for plantar foot ulceration. Diabet Med 2012;29:1534–1541 [DOI] [PubMed] [Google Scholar]
- 11.Altindas M, Kilic A, Cinar C, Bingol UA, Ozturk G. The epidemiology of foot wounds in patients with diabetes: a description of 600 consecutive patients in Turkey. J Foot Ankle Surg 2011;50:146–152 [DOI] [PubMed] [Google Scholar]
- 12.Strbová L, Krahulec B, Waczulíková I, Gaspar L, Ambrózy E. [Characteristics of foot ulcers in diabetic patients]. Vnitr Lek 2009;55:918–924 [in Slovak] [PubMed] [Google Scholar]
- 13.Prompers L, Huijberts M, Apelqvist J, et al. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia 2007;50:18–25 [DOI] [PubMed] [Google Scholar]
- 14.Pham H, Armstrong DG, Harvey C, Harkless LB, Giurini JM, Veves A. Screening techniques to identify people at high risk for diabetic foot ulceration: a prospective multicenter trial. Diabetes Care 2000;23:606–611 [DOI] [PubMed] [Google Scholar]
- 15.Veves A, Murray HJ, Young MJ, Boulton AJ. The risk of foot ulceration in diabetic patients with high foot pressure: a prospective study. Diabetologia 1992;35:660–663 [DOI] [PubMed] [Google Scholar]
- 16.Bus SA, Valk GD, van Deursen RW, et al. The effectiveness of footwear and offloading interventions to prevent and heal foot ulcers and reduce plantar pressure in diabetes: a systematic review. Diabetes Metab Res Rev 2008;24(Suppl. 1):S162–S180 [DOI] [PubMed] [Google Scholar]
- 17.Owings TM, Woerner JL, Frampton JD, Cavanagh PR, Botek G. Custom therapeutic insoles based on both foot shape and plantar pressure measurement provide enhanced pressure relief. Diabetes Care 2008;31:839–844 [DOI] [PubMed] [Google Scholar]
- 18.Boulton AJ, Armstrong DG, Albert SF, et al. American Diabetes Association. American Association of Clinical Endocrinologists Comprehensive foot examination and risk assessment: a report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care 2008;31:1679–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lavery LA, Higgins KR, Lanctot DR, et al. Preventing diabetic foot ulcer recurrence in high-risk patients: use of temperature monitoring as a self-assessment tool. Diabetes Care 2007;30:14–20 [DOI] [PubMed] [Google Scholar]
- 20.Bus SA, Waaijman R, Arts ML, et al. Effect of custom-made footwear on foot ulcer recurrence in diabetes: a multicenter randomized controlled trial. Diabetes Care 2013;36:4109–4116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cavanagh PR, Derr JA, Ulbrecht JS, Maser RE, Orchard TJ. Problems with gait and posture in neuropathic patients with insulin-dependent diabetes mellitus. Diabet Med 1992;9:469–474 [DOI] [PubMed] [Google Scholar]
- 22.Vileikyte L, Peyrot M, Bundy C, et al. The development and validation of a neuropathy- and foot ulcer-specific quality of life instrument. Diabetes Care 2003;26:2549–2555 [DOI] [PubMed] [Google Scholar]
- 23.Vileikyte L, Gonzalez JS, Leventhal H, et al. Patient interpretation of neuropathy (PIN) questionnaire: an instrument for assessment of cognitive and emotional factors associated with foot self-care. Diabetes Care 2006;29:2617–2624 [DOI] [PubMed] [Google Scholar]
- 24.Moher D, Hopewell S, Schulz KF, et al. CONSORT CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg 2012;10:28–55 [DOI] [PubMed] [Google Scholar]
- 25.Waaijman R, Keukenkamp R, de Haart M, Polomski WP, Nollet F, Bus SA. Adherence to wearing prescription custom-made footwear in patients with diabetes at high risk for plantar foot ulceration. Diabetes Care 2013;36:1613–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]