Abstract
OBJECTIVE
An evidence-based synthesis of patient preferences for management of hyperglycemia is needed. Our objective was to systematically review patient preferences for noninsulin diabetes medications in adults with type 2 diabetes.
RESEARCH DESIGN AND METHODS
We searched the PubMed, Embase, CINAHL, and EconLit databases for articles published on or before 23 January 2013. We included English-language studies of adult patients with type 2 diabetes that assessed patient preferences for diabetes medication treatment. Titles, abstracts, and articles were reviewed by at least two independent reviewers. Study data and quality were abstracted with standard protocols.
RESULTS
Of 2,811 titles identified in our original search, 10 articles met inclusion criteria for the systematic review. Studies were conducted from 2007 to 2012 among diverse patient populations in the U.S., Sweden, Denmark, and the U.K. Methods used to assess patient preferences included discrete choice experiments (e.g., conjoint analysis), time tradeoff exercises, standard gamble, and patient surveys. Key attributes of diabetes medication associated with patient preferences included treatment benefits (e.g., glycemic control and weight loss/control), treatment burden (e.g., administration, frequency, and cost), and side effects (e.g., weight gain, gastrointestinal effects, and hypoglycemia).
CONCLUSIONS
Various clinical and quality of life–related factors influence patient preferences for noninsulin diabetes medications. Treatment efficacy with regard to glycemic control and weight loss/control and the risk of treatment-related hypoglycemia and gastrointestinal effects are reported to be important drivers of patient treatment selections. Future work is needed to identify practical methods for incorporating patient preferences into treatment decision making and patient-centered care.
Introduction
The importance of patient-centeredness in the care of patients with type 2 diabetes is now widely accepted (1), and an understanding of patient preferences relevant to their diabetes treatment is a necessary part of achieving patient-centered care in diabetes. The issue of treatment preferences is especially complex in type 2 diabetes because of the range of medication alternatives; medication-related benefits, harms, and burden; and the likelihood, uncertainty, and time horizons of these treatment-related outcomes.
Patient preferences measure a patient’s value for a specific outcome in relative (e.g., importance of weight loss vs. glycemic control) or absolute (e.g., importance of weight loss) terms (2). Additionally, patient preferences can be measured by how a patient chooses between treatment options or how a choice is influenced by the importance a patient places on a particular attribute of treatment. Preference measures differ from the better-studied patient-reported outcomes (PROs) such as health-related quality of life outcomes (HR-QoL) in that PROs provide information on a patient’s status at one point in time (e.g., HR-QoL after treatment with metformin) (2). Patient preferences seek to evaluate the relative importance of the attributes that contribute to the patient status at a future point in time. In other words, HR-QoL and other PROs (e.g., gastrointestinal side effects vs. improved glycemic control with metformin) can be considered as attributes of medication for which patients can express a preference.
Even though patient preferences are deemed important by major diabetes professional societies (1), little is actually known about patient preferences for the treatment of type 2 diabetes (2). Therefore, we conducted a systematic review to identify and analyze studies of patient preferences in patients with type 2 diabetes not on insulin.
Research Design and Methods
Study Design
We performed a systematic review of published studies that describe patient preferences for noninsulin diabetes medications (oral or injectable) in adults with type 2 diabetes. We also examined factors that could influence the risk of bias of study findings. A written study protocol was prepared in accordance with the PRISMA statement (3), and the review was registered with PROSPERO (systematic review record CRD42012002285).
Data Sources and Searches
We searched the PubMed, Embase, CINAHL, and EconLit databases for studies published on or before 23 January 2013. Methodology and content experts within our team developed comprehensive search strategies to identify relevant studies. Our search terms consisted of key words for diabetes medication treatment, as well as methods used to assess patient preferences (e.g., conjoint analysis, decision analysis, utilities, and stated preferences) about medication treatment. The detailed search strategies are included within Supplementary Table 1.
Study Selection
Two reviewers screened all titles independently. Titles were excluded if both reviewers determined that they did not meet inclusion criteria. Remaining articles and those with no titles proceeded to abstract review. Both reviewers independently screened abstracts of all remaining articles. Articles were excluded at this stage if both reviewers determined that they did not meet inclusion criteria. Disagreements about inclusion or exclusion based on abstract review were determined by consensus. Two investigators then reviewed the full text of all remaining articles and those with no abstracts. We included studies if they 1) included adult patients with type 2 diabetes and 2) assessed patient preferences related to medication treatment of type 2 diabetes. We defined a preference as a patient’s a priori selection or rating of one treatment alternative over another, given a choice of at least two treatment options (4). We excluded articles if they 1) were not written in English, 2) did not assess patient treatment preferences, 3) included only patients <18 years old, 4) contained no original data (e.g., review, commentary, editorial, or meeting abstract), 5) only assessed preferences for treating diabetes complications or comorbid medical conditions, 6) only included nonmedication diabetes treatment, 7) only assessed patient satisfaction with or adherence to treatment, or 8) only included insulin therapy. Consensus on study inclusion was reached by at least two reviewers.
Data Extraction and Quality Assessment
For each article that met our inclusion criteria, two reviewers extracted data, including information on study design, treatments compared, locations, sample size, participant characteristics, preference elicitation methods, funding sources, and treatment-related attributes associated with patient preferences. Reviewers resolved disagreements by discussion and adjudication with a third party (i.e., a health economist with expertise in assessing patient treatment preferences).
Two reviewers used a checklist adapted from previously published instruments evaluating general study quality (5,6) and the Purpose, Respondents, Explanation, Findings, Significance (PREFS) checklist (4) specifically developed based on guidelines for conjoint analysis, PROs, and randomized and nonrandomized trials to independently assess study reporting on factors that could influence the validity of findings, including study external validity (i.e., well-described inclusion and exclusion criteria) and factors influencing study internal validity (i.e., a well-defined study question that includes key PICOTS [population, intervention, comparator, outcomes, timing, and settings] components [6], a comprehensive description of treatment alternatives, appropriate measurement of patient preferences, appropriate analytical techniques for the given data, and prespecified analysis [Supplementary Table 2]).
Data Synthesis and Analysis
We decided a priori not to statistically combine results in a meta-analysis because we expected studies to be methodologically and clinically diverse. For instance, some studies ranked patient preferences using conjoint analysis or an alternative discrete choice experiment whereas others reported patient utilities for a particular treatment. Therefore, we qualitatively synthesized results for individual studies within summary evidence tables to help clarify the similarities and differences among studies that appear to address similar research questions across a variety of measures and patient populations.
Results
Search Results
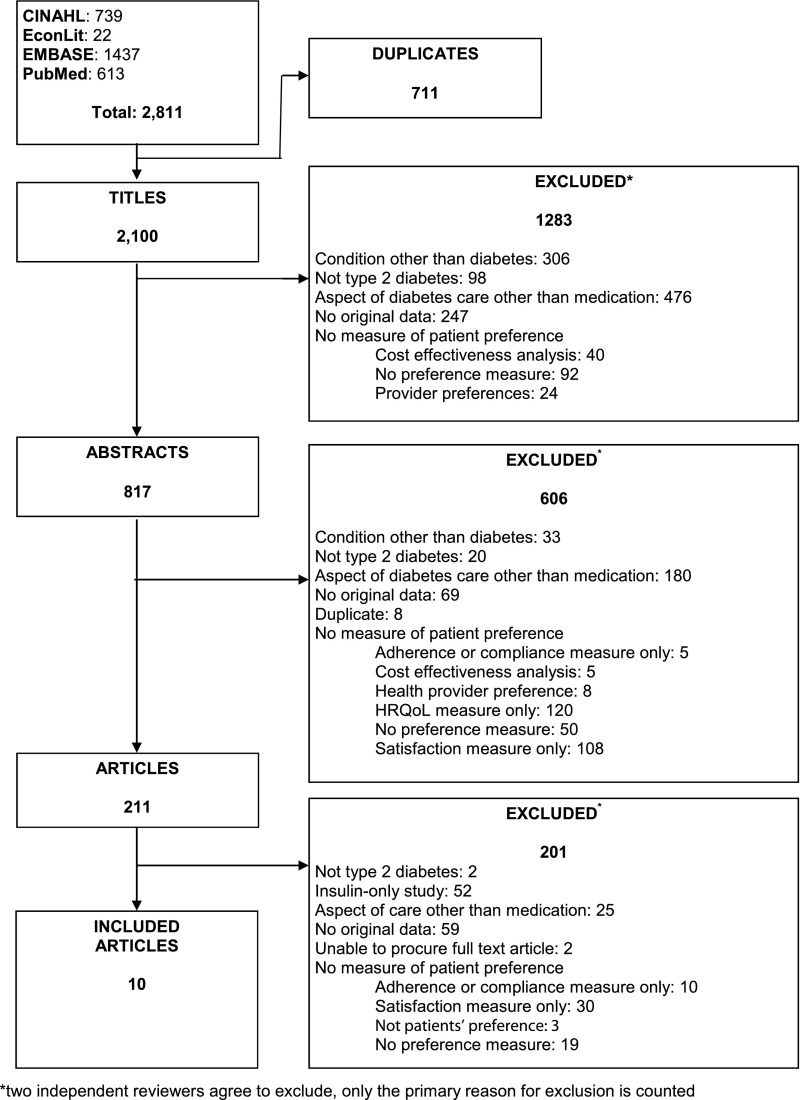
Of 2,811 total citations (613 in PubMed, 1,437 in Embase, 739 in CINAHL, and 22 in EconLit), 211 articles were eligible for full text review. We included 10 studies that met our inclusion criteria in the final review (7–16) (Fig. 1).
Figure 1.
Summary of literature search and article review process.
Study Characteristics
Eligible studies were conducted from 2007 to 2012. All studies were cross-sectional, and sample sizes ranged from 129 to 1,355 participants. Half of the studies were performed in the U.S. (9–11,15,16), and the remaining studies included participants from Sweden (12,13), Denmark (7), and the U.K. (8,11,14). Industry/pharmaceutical companies funded the majority of the studies (7,8,11–16) (Table 1).
Table 1.
Study characteristics
| Year | Author | Location | Design | Sample size | Patient population (age, sex, diabetes duration) | Method | Funding |
|---|---|---|---|---|---|---|---|
| 2012 | Jendle | Sweden | Cross-sectional | 461 | Age: N/R | Willingness to pay | Novo Nordisk A/S, Scandinavia |
| Male: N/R | |||||||
| Duration: N/R | |||||||
| 2011 | Bøgelund | Denmark | Cross-sectional | 270 | Age: 81% ≥50 years | Willingness to pay | Novo Nordisk A/S, Denmark |
| Sex: 66% male | |||||||
| Duration: 62% >5 years | |||||||
| 2011 | Boye | Scotland | Cross-sectional | 151 | Age (mean): 59.2 years | Standard gamble and visual analog scale | Eli Lilly and Company |
| Sex: 66.2% male | |||||||
| Mean age at diagnosis: 51.9 years | |||||||
| 2011 | Polonsky | U.S. | Cross-sectional | 1,355 | Age (mean): 58.02 years | Survey with Likert ratings scale | Amylin Pharmaceuticals; Eli Lilly and Company |
| Sex: 54.8% male | |||||||
| Mean duration: 10.09 years | |||||||
| 2010 | Jendle | Sweden | Cross-sectional | 461 | Age: 96.4% ≥50 years | Willingness to pay | Novo Nordisk, ESP Bioscience |
| Sex: 63.1% male | |||||||
| Duration: 62% >5 years | |||||||
| 2010 | Polster | U.S. | Cross-sectional | 382 | Age (mean): 52.7 years | Conjoint exercise and time tradeoff | Novo Nordisk A/S, Denmark |
| Sex: 48% male | |||||||
| Mean duration: 7.6 years | |||||||
| 2009 | Hauber | U.S.; U.K. | Cross-sectional | 407 | Age (mean): 57 years | Discrete choice experiment | Novartis Pharmaceuticals |
| Sex: 62% male | |||||||
| Duration: 81% = 1–10 years | |||||||
| 2008 | Brown | U.S. | Cross-sectional | 332 | Age: 100% ≥65 years | Time tradeoff | National Institutes of Health; Centers for Disease Control and Prevention; Chicago Center of Excellence in Health Promotion Economics |
| Sex: 42% male | |||||||
| Mean duration: 13 years (vulnerable group); 10 years (nonvulnerable group) | |||||||
| 2008 | Chin | U.S. | Cross-sectional | 473 | Age (mean): 73.7 years | Time tradeoff and visual analog scale | National Institutes of Health; Robert Wood Johnson Foundation |
| Sex: 37% male | |||||||
| Mean duration: 13.2 years | |||||||
| 2007 | Matza | Scotland; England | Cross-sectional | 129 | Age (mean): 55.9 years | Standard gamble | Eli Lilly and Company |
| Sex: 64.3% male | |||||||
| Mean age at diagnosis: 49.3 years |
Methodological Quality of Included Studies
All studies included a well-defined study question and conducted prespecified analyses. The majority described their inclusion and exclusion criteria well (70%), provided a comprehensive description of treatment alternatives (90%), used appropriate measures to assess patient preferences (90%), and conducted analyses that were appropriate for the study data (80%) (Supplementary Table 3).
Methods Used to Assess Patient Preferences
Methods to assess patient preferences for noninsulin diabetes medications included the following: discrete choice experiments (7,11–13), time tradeoff methods (9,10), conjoint analysis exercises combined with time tradeoff (16), survey questions with Likert-type ratings scales (15), and standard gamble (8,14) (Tables 1 and 2).
Table 2.
Attributes examined when assessing patient preferences for noninsulin diabetes medications
| Attributes examined when assessing patient preferences |
||||||
|---|---|---|---|---|---|---|
| Year | Author | Relevant treatment comparisons | Methods of assessing preferences | Treatment benefits | Treatment burden | Side effects |
| 2012 | Jendle | Current medication vs. hypothetical drug (i.e., liraglutide, rosiglitazone, exenatide) | Willingness to pay | Weight loss/control | Administration frequency | Gastrointestinal effects |
| Glycemic control (HbA1c) | Blood glucose testing | Hypoglycemia events | ||||
| Systolic blood pressure | ||||||
| 2011 | Bøgelund | Oral antidiabetes drugs vs. injections | Willingness to pay (Danish kroner per month) | Glycemic control (HbA1c) | Mode of administration | Hypoglycemia events |
| Weight loss/control | Blood glucose testing | Transient nausea | ||||
| Blood pressure control | Payment per month | |||||
| Improved heart function | ||||||
| Possess driver’s license | ||||||
| 2011 | Boye | Hypothetical scenarios (i.e., oral medicine only vs. oral medicine plus daily injections) | Visual analog scale exercise and standard gamble vignette-based assessments | Dose frequency | Injection site reactions | |
| Dose flexibility (mealtimes) | ||||||
| 2011 | Polonsky | Oral medication vs. injectable medication | Patient-reported willingness to take medication given potential factors | Glycemic control | Treatment frequency | Hypoglycemia events |
| Weight loss/control | Costs | |||||
| 2010 | Jendle | Oral antidiabetes drugs vs. injections | Willingness to pay (Swedish krona/euro per month) | Glycemic control (HbA1c) | Mode of administration | Hypoglycemia events |
| Weight loss/control | Blood glucose testing | Transient nausea | ||||
| Blood pressure control | Payment per month | |||||
| Improved heart function | ||||||
| 2010 | Polster | Liraglutide vs. exenatide | Conjoint exercise and time tradeoff | Glycemic control (HbA1c) | Dosing schedule | Nausea |
| Hypoglycemia | ||||||
| 2009 | Hauber | Hypothetical oral medication profiles | Discrete choice experiment | Glycemic control (HbA1c) | Hypoglycemia events | |
| Water retention | ||||||
| Weight gain | ||||||
| Mild stomach upset | ||||||
| Heart attack risk | ||||||
| 2008 | Brown | Intensive vs. conventional glucose control; comprehensive diabetes care vs. comprehensive care with polypill | Time tradeoff questions | Method of delivery | Likelihood of side effects | |
| Laboratory testing | ||||||
| 2008 | Chin | Intensive therapy vs. conventional treatment | Time tradeoff and visual analog scale | Avoid complications | Treatment intensity | |
| Life expectancy | ||||||
| 2007 | Matza | Hypothetical type 2 diabetes–related health states | Standard gamble | Weight loss/control | Weight gain | |
| Nausea | ||||||
| Hypoglycemia | ||||||
Types of Treatment Comparisons Included When Assessing Patient Preferences
Studies compared a variety of noninsulin diabetes medications when assessing patient preferences for treatment. These comparisons included specific diabetes medications, such as liraglutide, rosiglitazone, and exenatide (12,16), as well as broader categories of medication types (e.g., oral drugs vs. injections) (7,13,15). Several studies included hypothetical medication profiles of treatment attributes (e.g., intensive vs. conventional treatment) to assess patient preferences (8–12,14) (Table 2).
Attributes Associated With Patient Treatment Preferences
Treatment Benefits
Attributes related to treatment benefits included treatment efficacy in improving glycemic control, patient weight loss or control, blood pressure control, and heart function, as well as factors associated with enhanced quality of life, such as higher life expectancy, the avoidance of diabetes complications, and the ability to possess a driver’s license. Of these, glycemic control was the most frequently examined benefit (7,11,13,15,16), followed by weight loss/control (7,12,13,15).
Treatment Burden
Attributes related to treatment burden examined within studies included the method of delivery and mode of administration, dose frequency and flexibility, required blood glucose and laboratory testing, treatment-related costs, and treatment intensity. Of these, treatment/dose frequency and method of delivery/mode of administration were the most frequently examined (7,12,13,15,16).
Side Effects
Potential treatment-related side effects examined within studies included gastrointestinal effects (e.g., transient nausea and upset stomach), hypoglycemia, injection site reactions, weight gain, water retention, and increased risk of heart attacks. Hypoglycemia was the most frequently examined side effect (7,11–13,15,16), followed by gastrointestinal effects (7,11–13,16) (Table 2).
Relative Importance of Treatment Benefits Versus Treatment Burden and Side Effects When Assessing Patient Treatment Preferences
Seven of the included studies provided results (total of 54 direct comparisons) on the relative importance of treatment benefits and treatment burden and side effects in their assessment of patient preferences for noninsulin diabetes medications (7,8,11–13,15,16). The most common attribute comparisons were weight loss/control and glycemic control versus treatment-related burden and side effects (Table 3).
Table 3.
Relative importance of treatment benefits versus treatment burden and side effects when assessing patient preferences for noninsulin diabetes medications
| Treatment benefits |
||||||
|---|---|---|---|---|---|---|
| Weight loss/control | Glycemic control (glucose/HbA1c) | Blood pressure control | Improved heart function | Ability to have driver’s license | ||
| Treatment burden | Attribute ranked as most important (number of times ranked most important/total number of study comparisons) | |||||
| Administration or frequency | Weight loss/control (4/4) (7,12,13,15) | Glycemic control (4/4) (7,13,15,16) | Administration (1/1) (13) | Heart function (2/2) (7,13) | License (1/1) (7) | |
| Cost/payment | Weight loss/control (1/1) (15) | Glycemic control (1/1) (15) | — | — | — | |
| Glucose testing | Weight loss/control (2/2) (12,13) | Glycemic control (1/1) (13) | Blood pressure control (1/1) (13) | Heart function (1/1) (13) | — | |
| Side effects | Attribute ranked as most important (number of times ranked most important/total number of study comparisons) | |||||
| Gastrointestinal effects/nausea | Weight loss/control (3/3) (7,12,13) | Glycemic control (2/4) (11,16) | Gastrointestinal (1/1) (13) | Gastrointestinal (2/2) (7,13) | License (1/1) (7) | |
| Hypoglycemia | Weight loss/control (4/4) (7,12,13,15) | Glycemic control (5/5) (7,11,13,15,16) | Blood pressure control (1/1) (13) | Heart function (2/2) (7,13) | License (1/1) (7) | |
| Weight gain | Weight loss/control (2/3) (7,15) | Glycemic control (2/4) (7,11) | Weight gain (1/1) (13) | Heart function (1/2) (7) | License (1/1) (7) | |
In four studies (17 total comparisons), patients ranked weight loss/control as more important than treatment administration or frequency (4 of 4 comparisons), cost (1 of 1 comparison), glucose testing (2 of 2 comparisons), gastrointestinal effects (3 of 3 comparisons), hypoglycemia (4 of 4 comparisons), and potential weight gain (2 of 3 comparisons) (7,12,13,15). Similarly, in five studies (19 total comparisons) (7,11,13,15,16), glycemic control was ranked more important than treatment administration (4 of 4 comparisons), cost (1 of 1 comparison), glucose testing (1 of 1 comparison), gastrointestinal effects (2 of 4 comparisons), risk of hypoglycemia (5 of 5 comparisons), and potential weight gain (2 of 4 comparisons). Improved heart function was ranked as more important than treatment administration (2 of 2 comparisons), glucose testing (1 of 1 comparison), risk of hypoglycemia (2 of 2 comparisons), and potential weight gain (1 of 2 comparisons) in two studies (7,13). However, risk of gastrointestinal effects was ranked as more important than improved heart function (2 of 2 comparisons) in these two studies (7,13). Additional results on comparisons evaluated in a single study (7) are provided in Table 3.
Conclusions
In this systematic review, we identified weight loss/control and glycemic control as key attributes of diabetes treatment that drive patient preferences when these factors were compared with treatment burden and side effects. Gastrointestinal effects were ranked as more important than hypoglycemia by patients within the included studies. Evidence on patient preferences related to other treatment-related attributes of risk and burden was sparse.
For the clinician, our review provides the best available information on the relative importance to patients of factors that likely influence their treatment decision making related to noninsulin medications for type 2 diabetes. Type 2 diabetes is a particularly preference-sensitive condition because of the multitude of available treatments, which have varied benefits, risks, and burdens. In this study, we identified weight loss/control, glycemic control, and gastrointestinal side effects as particularly important to patients when considering treatment side effects/burden. Of note, patient BMI was also reported to be significantly associated with patient preferences about weight loss/control (e.g., patients with a BMI >30 kg/m2 reported a higher willingness to pay for weight reduction than patients with a BMI <30 kg/m2) (7,13). Although these findings may not apply to individual patients, the consistency of results should raise awareness of the importance of these factors in the initiation and evaluation of medication outcomes. For example, perceived ineffectiveness of a medication on the part of a clinician could actually be related to nonadherence because of side effects that could be avoided with a different medication; a priori understanding of patient preferences could prevent these types of treatment obstacles. An important consideration in interpreting our results is that they apply to noninsulin diabetes medications generally; in fact, most studies did not focus on a specific medication, and only one study compared patient preferences relative to two specific medications (liraglutide and exenatide) (16); the others either did not specify medications (e.g., oral hypoglycemic vs. injectables) (7,13,15) or used hypothetical scenarios (8–12,14). Clinically, we expect that preferences for treatment-related attributes are independent of the specific treatment (e.g., a patient’s preference for gastrointestinal side effects relative to hypoglycemia should be the same regardless of which medication is causing these side effects), and therefore, understanding general preferences regarding benefits and risks can be considered with specific medication attribute profiles to facilitate the overall medication decision. Thus, the included studies of both real and hypothetical medication profiles provide relevant information on preferences for treatment and move us closer to patient-centered care in diabetes.
A major strength of this review is the conduct of a carefully designed literature search of several databases and the use of detailed methods consistent with PRISMA guidelines (3) throughout the study. We also used a detailed quality assessment instrument to evaluate factors influencing the risk of bias of study findings and anticipate that this instrument could be used in the future evaluation of preference studies. Included studies were conducted among both men and women with variation in age; were diverse in setting; and used a variety of methods to assess preferences, including discrete choice experiments, time tradeoff methods, standard gamble, and survey questionnaires. A majority of studies included a well-defined study question, described their inclusion and exclusion criteria well, and provided a comprehensive description of treatment alternatives.
As with all systematic reviews, the strength of our conclusions is dependent on the quality of available studies, and limitations of our study deserve consideration. We found that all of the studies meeting our inclusion criteria reported outcomes at a single time point, with many studies incorporating hypothetical scenarios or medication profiles. Thus, we were unable to assess the extent to which reported patient preferences align with actual treatment decision making when patients are faced with these decisions in real-world settings, and we could not evaluate patient preferences regarding treatment benefits or side effects over time. Also, studies rarely performed subgroup analyses of patient preferences and did not always account for important clinical (e.g., comorbidities) and demographic characteristics (e.g., race/ethnicity) that could potentially influence patient treatment preferences. Although all of the studies that performed statistical analyses conducted analyses that were appropriate for study data, there were several studies that performed little or no statistical analyses. Our quality assessment tool did not explicitly penalize studies for not performing statistical analyses, and thus this important limitation of assessing study internal validity should be noted. Although this literature provided evidence on patient preferences using a wide variety of methods, differences between preference assessment methods may have influenced the relative values patients placed on treatment-related attributes. Few studies tested whether using multiple preference assessment methods, such as both conjoint analysis and time tradeoff methods, would result in different patient choices. Studies also varied in their presentation of preference choices to patients. For example, some studies described “gastrointestinal effects” as transient nausea whereas other studies described it as upset stomach. Thus, caution is required when comparing the relative importance of treatment-related attributes across studies. We did exclude non–English language articles, but the included studies were comprised of differing patient populations representing five countries. Therefore, we anticipate that the exclusion of any non–English language articles would not significantly change our observed findings. Finally, the majority of studies were funded by pharmaceutical and device manufacturing companies, which may introduce the risk of publication bias.
To date, the evidence base for high-quality comparative effectiveness research on how attributes of treatment efficacy, treatment burden, and potential side effects drive patient preferences for management of type 2 diabetes has been relatively lean. To our knowledge, we provide the first systematic review of patient preferences for noninsulin diabetes medication among adults with type 2 diabetes. Our review provides a comprehensive synthesis of available evidence regarding attributes of diabetes medication that are important to patient-centered decision making in the noninsulin treatment of type 2 diabetes.
One important consideration addressed by our methodology should be explored in future studies. Since there is a lack of a widely accepted definition of patient preferences and how they should be measured, we adopted a working theoretical definition of preferences, which most closely resembles the economics conceptualization of preferences, to guide our study inclusion criteria (4). On this basis, it is possible that we excluded articles that may contain useful preference information because they used definitions that did not meet our inclusion criteria. However, the uniform application of selection criteria is the bedrock of a well-conducted systematic review, and we believe that our definition of preferences was sensitive and specific, enabling us to identify the relevant articles. Guidelines on the definition of preferences are needed, and we believe that our study can provide a starting point for these.
Finally, preferences for treatment-related attributes must be combined with the probability of treatment-related outcomes to make a patient-centered decision. These types of analyses are outside the scope of the current study, but future studies should apply proven decision analysis methods, such as the Analytic Hierarchy Process (17). We describe the use of this method for medication decision making in type 2 diabetes in a separate article (18).
In summary, our systematic review of the evidence on patient preferences relevant to noninsulin type 2 diabetes medications reveals that weight loss/control and glycemic control may drive patient preferences when compared with treatment-related burden and side effects. Of the studied medication side effects, risk of gastrointestinal effects was an important attribute associated with treatment preferences. Clinicians can consider the patient preferences identified in this study in the care of their patients, and researchers should build upon this by developing evidence-based guidelines for the future conduct and evaluation of preference studies. Preference elicitation provides a necessary stepping stone in the path to individualized care and patient-centered decision making in type 2 diabetes as recommended by major professional organizations (1,19). Ultimately, we must develop and implement clinical decision support tools that incorporate preferences in order to truly provide patient-centered care in the treatment of type 2 diabetes.
Article Information
Acknowledgments. The authors sincerely thank Sonal Singh, Johns Hopkins University School of Medicine, Baltimore, MD, and Jodi Segal, Johns Hopkins University School of Medicine, Baltimore, MD, for their contributions to the conception and planning of this study.
Funding. This study was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (Grant 5T32-HL-007180 to T.S.P.) and from the U.S. Food and Drug Administration, Department of Health and Human Services (Contract HHSF2232010000072C to S.J., E.L., J.F.P.B., and N.M.).
The funders had no role in the design and conduct of the study, interpretation of the data, or preparation of the manuscript.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. T.S.P. developed the criteria for identifying preference studies and rating their quality, conducted the systematic review, and led the writing of the manuscript. S.J. and E.L. developed the criteria for identifying preference studies and rating their quality and conducted the systematic review. J.F.P.B. developed the criteria for identifying preference studies and rating their quality. N.M. developed the criteria for identifying preference studies and rating their quality, conducted the systematic review, provided clinical insights, and led the writing of the manuscript. All authors contributed to the writing of the manuscript and approved the final version.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-2527/-/DC1.
References
- 1.Inzucchi SE, Bergenstal RM, Buse JB, et al. American Diabetes Association (ADA) European Association for the Study of Diabetes (EASD) Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012;35:1364–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bridges JFPOE, Johnson FR, Hauber AB. Patient preference methods - a patient centered evaluation paradigm [Internet], 2007. Available from http://www.ispor.org/news/articles/Dec07/PPM.asp Accessed 6 September 2013
- 3.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joy SM, Little E, Maruthur NM, Purnell TS, Bridges JF. Patient preferences for the treatment of type 2 diabetes: a scoping review. Pharmacoeconomics 2013;31:877–892 [DOI] [PubMed] [Google Scholar]
- 5.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52:377–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agency for Healthcare Research and Quality. Methods Guide for Effectiveness and Comparative Effectiveness Reviews Rockville, MD, AHRQ, 2012. (AHRQ publ. no. 10(12)-EHC063-EF) [PubMed]
- 7.Bøgelund M, Vilsbøll T, Faber J, Henriksen JE, Gjesing RP, Lammert M. Patient preferences for diabetes management among people with type 2 diabetes in Denmark - a discrete choice experiment. Curr Med Res Opin 2011;27:2175–2183 [DOI] [PubMed] [Google Scholar]
- 8.Boye KS, Matza LS, Walter KN, Van Brunt K, Palsgrove AC, Tynan A. Utilities and disutilities for attributes of injectable treatments for type 2 diabetes. Eur J Health Econ 2011;12:219–230 [DOI] [PubMed] [Google Scholar]
- 9.Brown SE, Meltzer DO, Chin MH, Huang ES. Perceptions of quality-of-life effects of treatments for diabetes mellitus in vulnerable and nonvulnerable older patients. J Am Geriatr Soc 2008;56:1183–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chin MH, Drum ML, Jin L, Shook ME, Huang ES, Meltzer DO. Variation in treatment preferences and care goals among older patients with diabetes and their physicians. Med Care 2008;46:275–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hauber AB, Mohamed AF, Johnson FR, Falvey H. Treatment preferences and medication adherence of people with type 2 diabetes using oral glucose-lowering agents. Diabet Med 2009;26:416–424. [DOI] [PubMed]
- 12.Jendle J, Torffvit O, Ridderstråle M, Ericsson A, Nilsen B, Bøgelund M. Willingness to pay for diabetes drug therapy in type 2 diabetes patients: based on LEAD clinical programme results. J Media Econ 2012;15(Suppl. 2):1–5 [DOI] [PubMed] [Google Scholar]
- 13.Jendle J, Torffvit O, Ridderstråle M, Lammert M, Ericsson A, Bøgelund M. Willingness to pay for health improvements associated with anti-diabetes treatments for people with type 2 diabetes. Curr Med Res Opin 2010;26:917–923 [DOI] [PubMed] [Google Scholar]
- 14.Matza LS, Boye KS, Yurgin N, et al. Utilities and disutilities for type 2 diabetes treatment-related attributes. Qual Life Res 2007;16:1251–1265 [DOI] [PubMed]
- 15.Polonsky WH, Fisher L, Hessler D, Bruhn D, Best JH. Patient perspectives on once-weekly medications for diabetes. Diabetes Obes Metab 2011;13:144–149 [DOI] [PubMed] [Google Scholar]
- 16.Polster M, Zanutto E, McDonald S, Conner C, Hammer M. A comparison of preferences for two GLP-1 products–liraglutide and exenatide–for the treatment of type 2 diabetes. J Med Econ 2010;13:655–661 [DOI] [PubMed] [Google Scholar]
- 17.Dolan JG, Isselhardt BJ, Jr., Cappuccio JD. The analytic hierarchy process in medical decision making: a tutorial. Med Decis Making 1989;9:40–50 [DOI] [PubMed]
- 18.Maruthur NM, Joy S, Dolan J, Segal JB, Shihab HM, Singh S. Systematic assessment of benefits and risks: study protocol for a multi-criteria decision analysis using the Analytic Hierarchy Process for comparative effectiveness research. F1000Res 2013;2:160 [DOI] [PMC free article] [PubMed]
- 19.Qaseem A, Snow V, Owens DK, Shekelle P, Clinical Guidelines Committee of the American College of Physicians The development of clinical practice guidelines and guidance statements of the American College of Physicians: summary of methods. Ann Intern Med 2010;153:194–199 [DOI] [PubMed] [Google Scholar]