Abstract
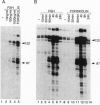
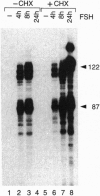
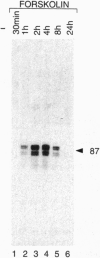
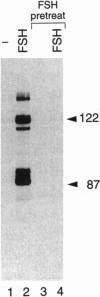
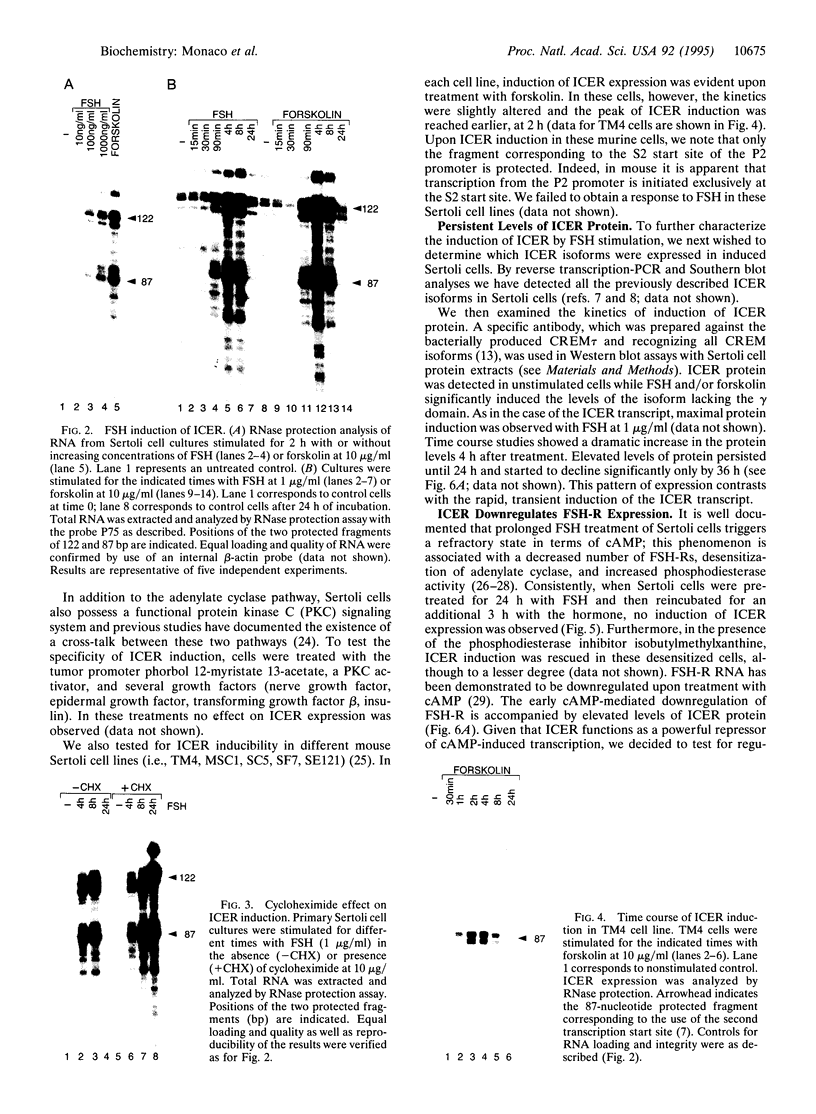
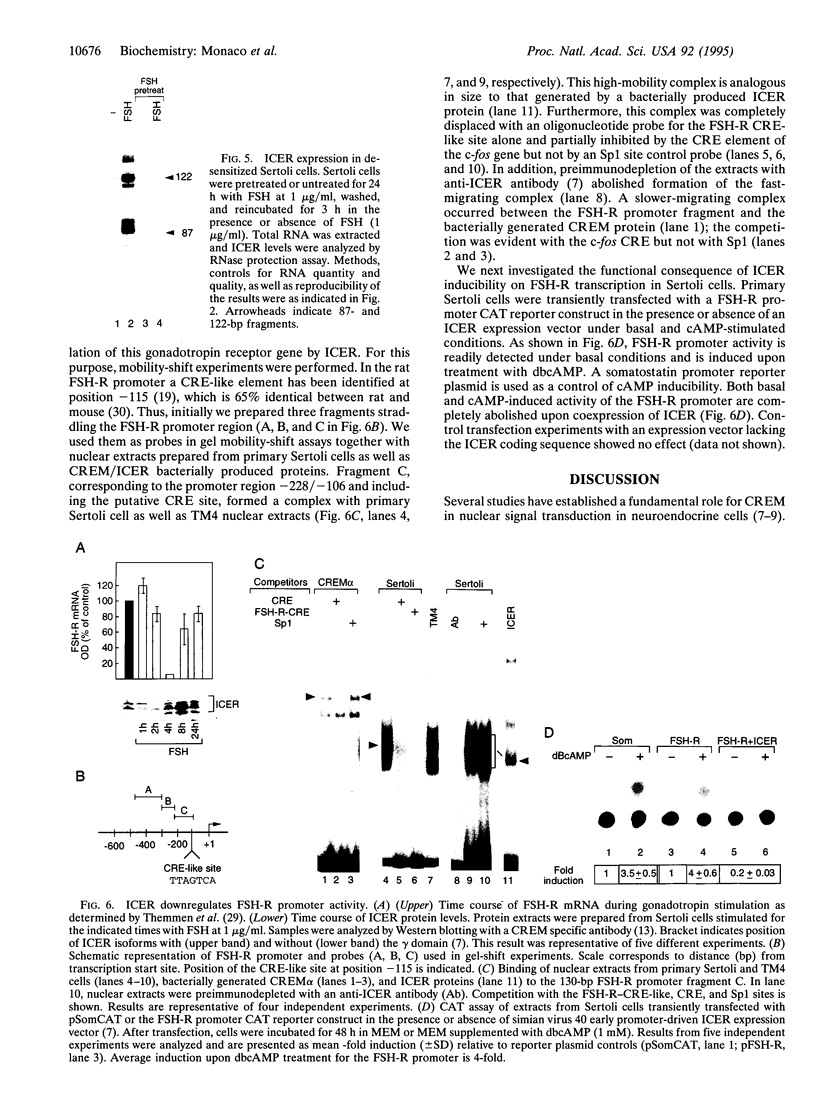
Transcription factor CREM (cAMP-responsive element modulator) plays a pivotal role in the nuclear response to cAMP in neuroendocrine cells. We have previously shown that follicle-stimulating hormone (FSH) directs CREM expression in male germ cells. The physiological importance of FSH in Sertoli cell function prompted us to analyze its effect on CREM expression in these cells. We observed a dramatic and specific increase in the CREM isoform ICER (inducible cAMP early repressor) expression, with a peak 4 h after FSH treatment of primary Sertoli cells. Interestingly, induced levels of ICER protein persist for a considerably longer time. Induction of the repressor ICER accompanies early down-regulation of the FSH receptor transcript, which leads to long-term desensitization. Here we show that ICER represses FSH receptor expression by binding to a CRE-like sequence in the regulatory region of the gene. Our results confirm the crucial role played by CREM in hormonal control and suggest its role in the long-term desensitization phenomenon of peptide membrane receptors.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews N. C., Faller D. V. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res. 1991 May 11;19(9):2499–2499. doi: 10.1093/nar/19.9.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli E., Montmayeur J. P., Foulkes N. S., Sassone-Corsi P. Signal transduction and gene control: the cAMP pathway. Crit Rev Oncog. 1992;3(4):321–338. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Conti M., Toscano M. V., Petrelli L., Geremia R., Stefanini M. Involvement of phosphodiesterase in the refractoriness of the Sertoli cell. Endocrinology. 1983 Nov;113(5):1845–1853. doi: 10.1210/endo-113-5-1845. [DOI] [PubMed] [Google Scholar]
- Delmas V., van der Hoorn F., Mellström B., Jégou B., Sassone-Corsi P. Induction of CREM activator proteins in spermatids: down-stream targets and implications for haploid germ cell differentiation. Mol Endocrinol. 1993 Nov;7(11):1502–1514. doi: 10.1210/mend.7.11.8114765. [DOI] [PubMed] [Google Scholar]
- Dorrington J. H., Roller N. F., Fritz I. B. Effects of follicle-stimulating hormone on cultures of Sertoli cell preparations. Mol Cell Endocrinol. 1975 Jul;3(1):57–70. doi: 10.1016/0303-7207(75)90031-3. [DOI] [PubMed] [Google Scholar]
- Foulkes N. S., Borrelli E., Sassone-Corsi P. CREM gene: use of alternative DNA-binding domains generates multiple antagonists of cAMP-induced transcription. Cell. 1991 Feb 22;64(4):739–749. doi: 10.1016/0092-8674(91)90503-q. [DOI] [PubMed] [Google Scholar]
- Foulkes N. S., Mellström B., Benusiglio E., Sassone-Corsi P. Developmental switch of CREM function during spermatogenesis: from antagonist to activator. Nature. 1992 Jan 2;355(6355):80–84. doi: 10.1038/355080a0. [DOI] [PubMed] [Google Scholar]
- Foulkes N. S., Schlotter F., Pévet P., Sassone-Corsi P. Pituitary hormone FSH directs the CREM functional switch during spermatogenesis. Nature. 1993 Mar 18;362(6417):264–267. doi: 10.1038/362264a0. [DOI] [PubMed] [Google Scholar]
- Francis G. L., Brown T. J., Bercu B. B. Control of Sertoli cell response to FSH: regulation by homologous hormone exposure. Biol Reprod. 1981 Jun;24(5):955–961. doi: 10.1095/biolreprod24.5.955. [DOI] [PubMed] [Google Scholar]
- Ginty D. D., Kornhauser J. M., Thompson M. A., Bading H., Mayo K. E., Takahashi J. S., Greenberg M. E. Regulation of CREB phosphorylation in the suprachiasmatic nucleus by light and a circadian clock. Science. 1993 Apr 9;260(5105):238–241. doi: 10.1126/science.8097062. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Hermanowski A. L., Ziff E. B. Effect of protein synthesis inhibitors on growth factor activation of c-fos, c-myc, and actin gene transcription. Mol Cell Biol. 1986 Apr;6(4):1050–1057. doi: 10.1128/mcb.6.4.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromoll J., Dankbar B., Gudermann T. Characterization of the 5' flanking region of the human follicle-stimulating hormone receptor gene. Mol Cell Endocrinol. 1994 Jun;102(1-2):93–102. doi: 10.1016/0303-7207(94)90102-3. [DOI] [PubMed] [Google Scholar]
- Hall S. H., Joseph D. R., French F. S., Conti M. Follicle-stimulating hormone induces transient expression of the protooncogene c-fos in primary Sertoli cell cultures. Mol Endocrinol. 1988 Jan;2(1):55–61. doi: 10.1210/mend-2-1-55. [DOI] [PubMed] [Google Scholar]
- Hamil K. G., Conti M., Shimasaki S., Hall S. H. Follicle-stimulating hormone regulation of AP-1: inhibition of c-jun and stimulation of jun-B gene transcription in the rat Sertoli cell. Mol Cell Endocrinol. 1994 Mar;99(2):269–277. doi: 10.1016/0303-7207(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Hamil K. G., Hall S. H. Cloning of rat Sertoli cell follicle-stimulating hormone primary response complementary deoxyribonucleic acid: regulation of TSC-22 gene expression. Endocrinology. 1994 Mar;134(3):1205–1212. doi: 10.1210/endo.134.3.8161377. [DOI] [PubMed] [Google Scholar]
- Heckert L. L., Daley I. J., Griswold M. D. Structural organization of the follicle-stimulating hormone receptor gene. Mol Endocrinol. 1992 Jan;6(1):70–80. doi: 10.1210/mend.6.1.1738373. [DOI] [PubMed] [Google Scholar]
- Hofmann M. C., Narisawa S., Hess R. A., Millán J. L. Immortalization of germ cells and somatic testicular cells using the SV40 large T antigen. Exp Cell Res. 1992 Aug;201(2):417–435. doi: 10.1016/0014-4827(92)90291-f. [DOI] [PubMed] [Google Scholar]
- Klein-Hitpass L., Ryffel G. U., Heitlinger E., Cato A. C. A 13 bp palindrome is a functional estrogen responsive element and interacts specifically with estrogen receptor. Nucleic Acids Res. 1988 Jan 25;16(2):647–663. doi: 10.1093/nar/16.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalli E., Sassone-Corsi P. Signal transduction and gene regulation: the nuclear response to cAMP. J Biol Chem. 1994 Jul 1;269(26):17359–17362. [PubMed] [Google Scholar]
- Lalli E., Sassone-Corsi P. Thyroid-stimulating hormone (TSH)-directed induction of the CREM gene in the thyroid gland participates in the long-term desensitization of the TSH receptor. Proc Natl Acad Sci U S A. 1995 Oct 10;92(21):9633–9637. doi: 10.1073/pnas.92.21.9633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means A. R., Dedman J. R., Tash J. S., Tindall D. J., van Sickle M., Welsh M. J. Regulation of the testis sertoli cell by follicle stimulating hormone. Annu Rev Physiol. 1980;42:59–70. doi: 10.1146/annurev.ph.42.030180.000423. [DOI] [PubMed] [Google Scholar]
- Mellström B., Naranjo J. R., Foulkes N. S., Lafarga M., Sassone-Corsi P. Transcriptional response to cAMP in brain: specific distribution and induction of CREM antagonists. Neuron. 1993 Apr;10(4):655–665. doi: 10.1016/0896-6273(93)90167-p. [DOI] [PubMed] [Google Scholar]
- Meyer T. E., Habener J. F. Cyclic adenosine 3',5'-monophosphate response element binding protein (CREB) and related transcription-activating deoxyribonucleic acid-binding proteins. Endocr Rev. 1993 Jun;14(3):269–290. doi: 10.1210/edrv-14-3-269. [DOI] [PubMed] [Google Scholar]
- Molina C. A., Foulkes N. S., Lalli E., Sassone-Corsi P. Inducibility and negative autoregulation of CREM: an alternative promoter directs the expression of ICER, an early response repressor. Cell. 1993 Dec 3;75(5):875–886. doi: 10.1016/0092-8674(93)90532-u. [DOI] [PubMed] [Google Scholar]
- Monaco L., Conti M. Inhibition by phorbol esters and other tumor promoters of the response of the Sertoli cell to FSH: evidence for dual site of action. Mol Cell Endocrinol. 1987 Feb;49(2-3):227–236. doi: 10.1016/0303-7207(87)90217-6. [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy P. J. FSH receptor autoregulation and cyclic AMP production in the immature rat testis. Biol Reprod. 1980 Nov;23(4):810–814. doi: 10.1095/biolreprod23.4.810. [DOI] [PubMed] [Google Scholar]
- Skinner M. K. Cell-cell interactions in the testis. Endocr Rev. 1991 Feb;12(1):45–77. doi: 10.1210/edrv-12-1-45. [DOI] [PubMed] [Google Scholar]
- Stehle J. H., Foulkes N. S., Molina C. A., Simonneaux V., Pévet P., Sassone-Corsi P. Adrenergic signals direct rhythmic expression of transcriptional repressor CREM in the pineal gland. Nature. 1993 Sep 23;365(6444):314–320. doi: 10.1038/365314a0. [DOI] [PubMed] [Google Scholar]
- Takahashi J. S. Circadian rhythms. ICER is nicer at night (sir!). Curr Biol. 1994 Feb 1;4(2):165–168. doi: 10.1016/s0960-9822(94)00040-0. [DOI] [PubMed] [Google Scholar]
- de Groot R. P., den Hertog J., Vandenheede J. R., Goris J., Sassone-Corsi P. Multiple and cooperative phosphorylation events regulate the CREM activator function. EMBO J. 1993 Oct;12(10):3903–3911. doi: 10.1002/j.1460-2075.1993.tb06068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]