Abstract
Bivalve molluscs are key components of the estuarine environments as contributors to the trophic chain, and as filter –feeders, for maintaining ecosystem integrity. Further, clams, oysters, and scallops are commercially exploited around the world both as traditional local shellfisheries, and as intensive or semi–intensive farming systems. During the past decades, populations of those species deemed of environmental or commercial interest have been subject to close monitoring given the realization that these can suffer significant decline, sometimes irreversible, due to overharvesting, environmental pollution, or disease. Protozoans of the genera Perkinsus, Haplosporidium, Marteilia, and Bonamia are currently recognized as major threats for natural and farmed bivalve populations. Since their identification, however, the variable publication rates of research studies addressing these parasitic diseases do not always appear to reflect their highly significant environmental and economic impact. Here we analyzed the peer– reviewed literature since the initial description of these parasites with the goal of identifying potential milestone discoveries or achievements that may have driven the intensity of the research in subsequent years, and significantly increased publication rates. Our analysis revealed that after initial description of the parasite as the etiological agent of a given disease, there is a time lag before a maximal number of yearly publications are reached. This has already taken place for most of them and has been followed by a decrease in publication rates over the last decade (20– to 30– year lifetime in the literature). Autocorrelation analyses, however, suggested that advances in parasite purification and culture methodologies positively drive publication rates, most likely because they usually lead to novel molecular tools and resources, promoting mechanistic studies. Understanding these trends should help researchers in prioritizing research efforts for these and other protozoan parasites, together with their development as model systems for further basic and translational research in parasitic diseases.
Introduction
Mollusc bivalves are key components of marine and estuarine environments because, as filter feeders, they play a critical role in maintaining water quality and ecosystem integrity. Marine bivalves are bottom dwellers attached to marine substrates for most of their life (e.g. oysters) or buried in the sand (e.g. clams, cockles); they can be displaced by heavy storms or moved as a result of human activities. The historical record indicates that bivalves were an abundant resource for the coastal inhabitants, although nowadays traditional local harvesting of the natural populations is being substituted worldwide by semi– intensive aquaculture initiatives. According to the Food and Agriculture Organization of the United Nations (http://www.fao.org/), the production of farmed clams, mussels, oysters, and scallops reached 14, 297, 010 metric tones with an estimated value of $13.7 bn (2010 data).
Protozoan parasites of the genera Perkinsus, Haplosporidium, Marteilia, and Bonamia severely affect a variety of mollusc species commercially harvested or farmed around the world. In particular, Perkinsus marinus, Perkinsus olseni, Marteilia refringens, Bonamia ostreae, and Bonamia exitiosa can infect abalones, clams, mussels, oyster, and scallops, and thus, are currently under surveillance by the World Organization for Animal Health (OIE; http://www.oie.int/; Aquatic Animal Health Code, Section 11: Diseases of Molluscs). Global warming and bivalve trading have been proposed as two of the main causes for expansion of parasitic diseases in molluscs [1]–[4]. As invertebrates lack adaptive immunity (although some immune memory has been proposed [5]), no vaccination approaches are feasible for bivalve molluscs, and intervention strategies are limited to management of the resource to enable commercialization of infected individuals prior to health decline or death, together with measures aimed at preventing the expansion of the parasite distribution range. Although selective breeding of individuals resistant to protozoan parasites has had variable success, it still remains as the most promising alternative [6]–[8]. Finally, the identification and use of anti– protozoan chemical agents has been hindered by the difficulties in treating marine molluscs due to both the inherent environmental toxicity of some of the compounds, and the farming practices in uncontained aquaculture settings [9]–[11]. Since their identification, however, the variable publication rates of research studies addressing the aforementioned parasitic diseases throughout the years do not always appear to reflect their highly significant environmental and economic impact. Previous quantitative analyses of trends in the literature have been used to investigate ecological hypothesis [12]. Here, we analyzed the peer– reviewed literature since the initial description of the aforementioned parasites, with the goal of identifying potential milestone discoveries or achievements that may have driven the intensity of the research in subsequent years, and significantly contributed to increase publication rates. Autocorrelation analyses suggested that advances in parasite purification and culture methodologies positively drive publication rates, most likely because they usually lead to novel molecular tools (e.g. transfection methodologies), resources (e.g. purification of proteins for drug profiling, crystallographic studies, antibody production), transcriptome and genome sequences, and ultimately, promote cellular and molecular mechanistic studies. These trends should help researchers and funding agencies in prioritizing research efforts for these and other protozoan parasites.
Materials and Methods
A search of the SCOPUS database (http://www.info.sciverse.com/scopus/), which contains over 20, 500 titles from 5,000 publishers worldwide and it goes back to 1823, was carried out following Ward and Lafferty [12], for peer– reviewed articles published since 1950 until 2013 with titles or abstracts (abstracts were available for most articles) containing specific protozoan parasite taxonomic or disease name strings (Table 1). The starting date chosen corresponded with the first record of the mass mortality attributed to the corresponding pathogen under analysis, and descriptive and taxonomy studies were included [12], whereas references to meeting proceedings were excluded. The data for comparing the numbers of published articles for each parasite was restricted to the period from 1980 to 2013, since prior to 1980 the number of papers on the protozoan parasites was negligible. References were imported to EndNote (Thompson-Reuters) and titles and abstracts were manually curated by eliminating duplicities; the search returned no false positives with the exception of Bonamia, which shares its name with plants of the Convolvulaceae (e.g. Bonamia spectabilis). Once in EndNote the references were manually searched for each parasite and sorted by year, and the number of publications was then used to build an Excel spreadsheet to generate the plots. Manual curation was used to assign the work to a particular geographic area/country; when the publication involved authors from several countries, the country that originated the sample took priority over where the work was performed. The generated time series of numbers of papers were analyzed for statistically significant trends, autocorrelation, and dominant periodicities. To standardize the lengths of the time series, the autocorrelation was performed on the period 1980–2013, a period during which all parasites analyzed were under investigation. The analysis was repeated using the 30 years after first record for each parasite. To determine dominant periodicities, a spectral analysis on the de– trended time series was carried out using a Fourier transform [13], and peaks were identified in the power spectra.
Table 1. Search strings for protozoan parasites and bivalve groups.
| Search String | |
| Parasite | |
| Bonamia | Bonamia, Bonamiosis, Microcytos |
| Haplosporidium | Haplosporidium, MSX (Multi- nucleated Sphere X), Minchinia |
| Marteilia | Marteilia, Marteiloides, Aber Disease |
| Perkinsus | Dermo, Dermocystidium, Labyrinthomyxa, Perkinsosis, Pseudoperkinsus |
| Host * | |
| Oysters | Crassostrea, Ostrea, oysters |
| Clams | Clams, Tapes, Ruditapes, Venerupis |
*Abalones, mussels, cockles, and scallops, which can also be infected by some of these protozoan parasites, were not included in the host search for percent of literature reporting diseases.
Results
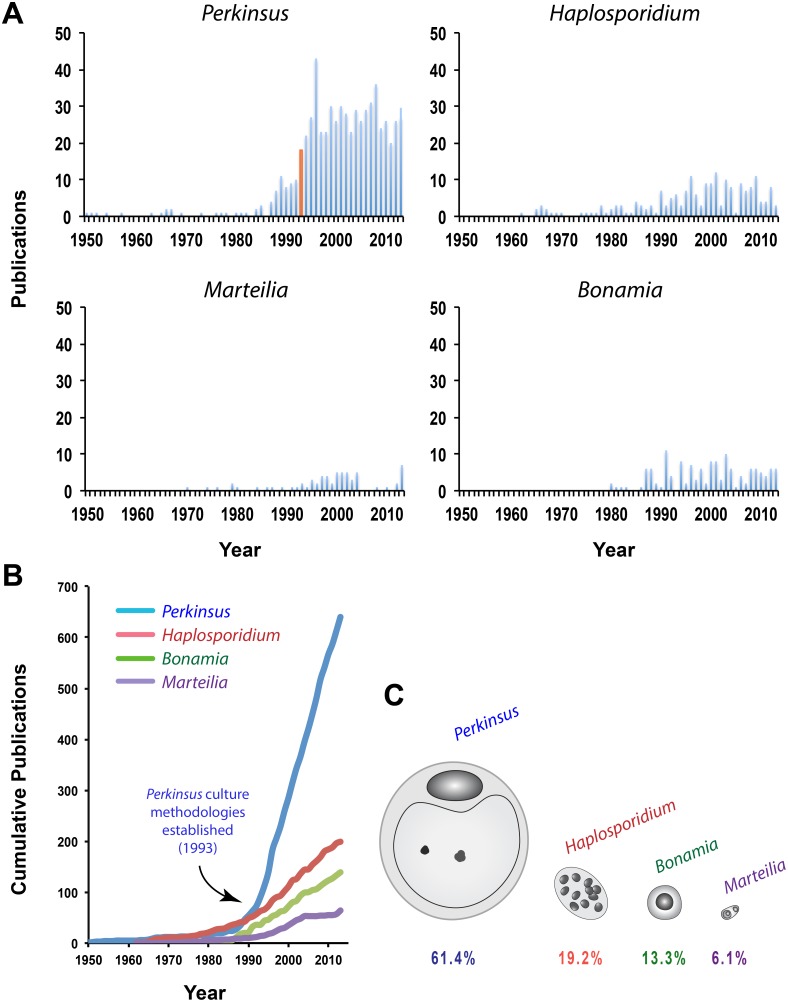
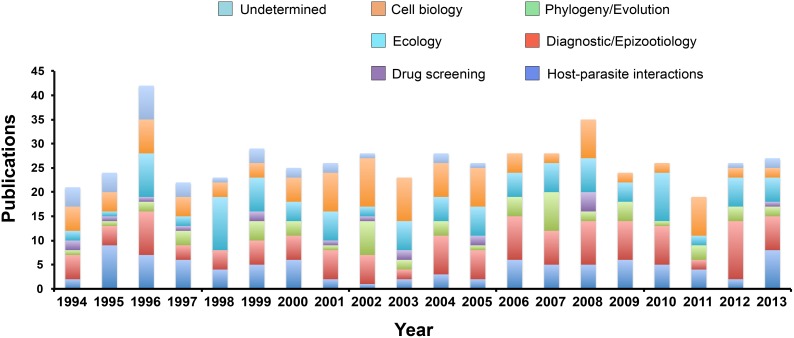
Perkinsus marinus was first described as associated with mass mortalities of eastern oysters in the Gulf coast during the 1950’s. Although the number of published papers was negligible during the following few years, during the late 1980’s and early 1990’s there was a sharp increase in publication rate, reaching more than 20 papers a year, a number that has remained relatively constant since then, and is significantly higher than those related to the other three parasite genera (Haplosporidium, Marteilia, and Bonamia) under study here (Figure 1A). For Haplosporidium, published articles appeared in the 1960’s, with most reports published in the 1990’s (though always below 10 papers/year) (Figure 1A). Marteilia refringens was first described in the 1970’s, with publications peaking in the 1990’s –early 2000’s, with a sharp decline over the last decade, and a spike in publications during 2013 (Figure 1A). The detection and description of Bonamia ostreae began in the early 1980’s and the profile of publication numbers, always lower than Perkinsus spp. and Haplosporidium spp., has been variable over the last three decades (Figure 1A). The number of spp. papers published on Perkinsus spp. has been about 5– to 13– fold over the other three protozoan parasites, and almost 3– fold over the three other species combined (Figure 1B). The autocorrelation analysis was carried out both on the four time series using data from 1980–2013, and using the first 30 years after the first record for each parasite. Using the 1980–2013 period, the Perkinsus spp. time series was significantly auto– correlated (p<0.05) out to a lag of nine years. Haplosporidium spp. and Bonamia spp. had no statistically significant autocorrelation, and Marteilia spp. had a significant two– year autocorrelation. All time series except Marteilia spp. had statistically significant increasing trends. For the de– trended data, Perkinsus spp. was significantly auto–correlated out to a lag of six years, and the other auto– correlations were unchanged. Using the 30 years following first record, results changed only for Perkinsus spp., which had no statistically significant auto– correlation or trend during this period (which predates the sharp increase in the 1990s), and for Marteilia spp., which was autocorrelated out to four years. In the spectral analysis, Haplosporidium spp. and Marteilia spp. had maximum peaks at a 20–30 year period and were mostly flat elsewhere, showing a strong 20–30 year mode underlying the signal. Bonamia spp. had a peak at 30 years as well, but also had comparable peaks at lower periods. Perkinsus spp. had a single maximum at 63 years, the full length of the time series, reflecting the dominance of the step change in the early 1990s. For the purpose of this study and based on the content, the papers were classified in six categories including cell biology, ecology, drug screening, phylogeny/evolution, diagnostic/epizootiology, and host– parasite interactions; papers that did not fall into these categories were assigned to the undetermined category (Figure 2).
Figure 1.
Number of papers in the literature (SCOPUS Database) reporting in the genera Perkinsus, Haplosporidium, Marteilia, and Bonamia; column in orange correspond to 1993, the year when the methodologies for culturing Perkinsus were published (A). Cumulative papers over the same period of time (B). Percent of the literature reporting each particular protozoan parasite (C).
Figure 2. Number of papers in the literature (SCOPUS Database) reporting in the genera Perkinsus, Haplosporidium, Marteilia, and Bonamia distributed into each of the six categories; papers that did not fall into those categories were assigned to the undetermined category.
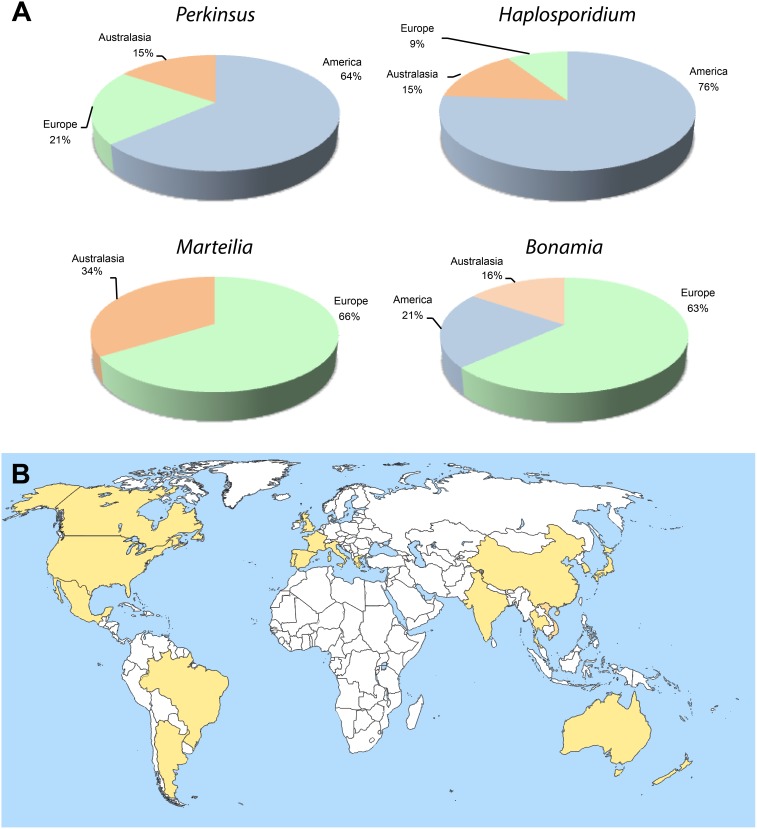
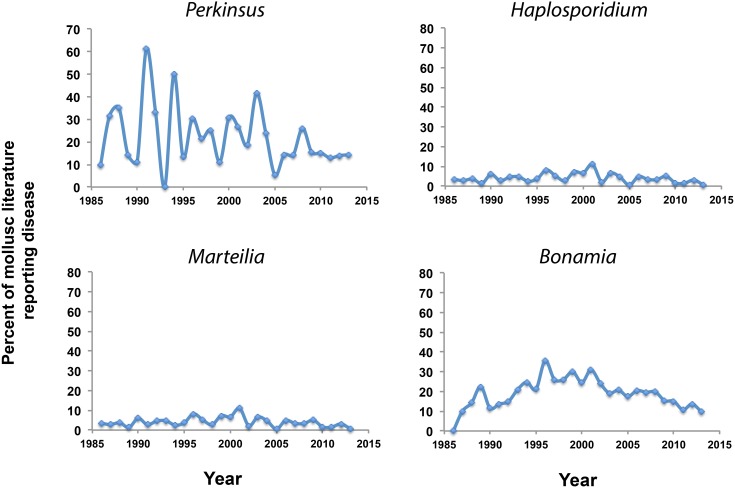
Although Perkinsus spp. have been detected in 20 countries, making it the protozoan parasite of molluscs with the widest range distribution reported to date (Figure 3B), most papers (64%) on Perkinsus spp. correspond to North America (Figure 3A). In the case of Haplosporidium spp., it is the North American continent where more papers have been published (76%), followed by Australasia (Figure 3B). Bonamia and Marteilia papers were mostly published with reference to European countries (64% and 65%, respectively) although in the case of Bonamia spp., the number of published papers is also noticeable from both North America and Australasia (Figure 3A). Overall, the number of papers published on any particular protozoan parasite in relation to the papers published on their host has been decreasing during the last decades (Figure 4).
Figure 3.
Distribution by continent of published papers in the literature reporting in the genera Perkinsus, Haplosporidium, Marteilia, and Bonamia (A). Countries where Perkinsus spp. have been reported by 2013 (B).
Figure 4. Percent of the literature (SCOPUS Database) in mollusc bivalves reporting disease by the protozoan parasites of the genera Perkinsus, Haplosporidium, Marteilia, and Bonamia.
Discussion
The protozoan parasites of the genera Perkinsus, Haplosporidium, Marteilia, and Bonamia have been described several decades ago but still affect significantly mollusc species that are environmentally and commercially relevant around the world, and are currently under surveillance by the OIE. Since no effective ant– parasitic treatments have been developed so far, the available intervention strategies are mostly limited to the improvement of management practices. Since their identification, however, the variable publication rates of research studies addressing these parasitic diseases do not always appear to reflect their highly significant environmental and economic impact. Here we analyzed the peer– reviewed literature since the initial description of these parasites with the goal of identifying potential milestone discoveries or achievements that may have driven the intensity of the research in subsequent years, and significantly increased publication rates. We did incorporate into this study protozoan parasites in the genus Haplosporidium [14], which, although they are not included in the OIE list of diseases, also have been associated to mass mortalities of oysters [15]. Although the herpes virus affecting Japanese oysters (Crassostrea gigas) has very recently become a problem especially for the production of spat [16], [17], this pathogen was not included in this study. This literature analysis initially confirmed that mass mortalities of bivalves were the main factor that prompted research aimed at the identification and description of the parasite as etiological agents. For example, although Perkinsus marinus (“Dermo” disease) was described upon mass mortalities of the eastern oyster, Crassostrea virginica, in Louisiana (USA) [18], [19], the analysis of archived material indicates that the parasite might have already been present in oysters from the same area circa 1920 [18]. In the late 1950’s, 90–95% of the oysters in Delaware Bay (USA) were killed by a disease named as MSX (Multinucleated Sphere X for “unknown”), the causative agent later identified as Minchinia nelsoni (currently Haplosporidium nelsoni) [15]. Marteilia refringens (“Aber” disease) was associated with mortalities of the European flat oyster, Ostrea edulis, in Aber Wrac’h, Brittany (France) in the 1970’s [20], [21]; similarly, Bonamia ostreae was also described after mass mortalities of O. edulis in Brittany (France) in 1979 [22]. There are several working hypotheses that could explain the onset of the epizooty, including the introduction of non– native host or parasite species, environmental conditions affecting the ability of the host to fight the parasites, and strains of variable virulence reaching the host (short generation time and large population size in the protozoan parasites provides the base for a high evolvability compared to the host). Testing this hypothesis, however, has proven to be not a trivial task since it would require rigorous examination of archived material that is not always available for the above– mentioned species. Thus we were interested in determining, once the etiological agent had been identified, how the studies on these diseases may have progressed over the last four to six decades. Remarkably, for the protozoan parasites analyzed here, there was a lag period between the description of the etiological agent and a noticeable increase in the number of published reports. Since their identification, the number of papers/year for all four parasites, have reached the maximum sometime during the past few years, and although the diseases they cause have not been resolved a significant decrease can be observed during the last decade. An exception is the noticeable rebound of published papers that has taken place for Marteilia in 2013; however, the next few years will reveal if this trend will continue. Interestingly, this overall decrease in the total of number of papers has been also accompanied by a decrease in the percent of the literature reporting the disease, reaching, in the last decade, the lowest ratios of papers on diseases compared to total papers on molluscs. This trend could be interpreted as a reduction of field monitoring programs, together with the acceptance that although the parasite is still a major concern and the management strategies available are judged sufficient [23]. The interested parties may put these to practice in the field without being reported in the peer– reviewed literature. Remarkably, and with the exception of Dermo and likely derived from the establishment of the culture methodologies, it appears that the numbers of papers published on these parasitic diseases follow 20– to 30– year modes. The local commercial importance of the shellfishery and the impact of the disease clearly play a significant role in the frequency of reports emerging from a particular area. Since the availability of research funds is a key factor that contributes to the publication rates, we attempted to identify those grants that specifically concern any aspects of the parasitic diseases addressed in this study. This search was limited to the National Science Foundation (NSF), because a searchable database for funded grant proposals is readily available (http://nsf.gov/awardsearch/). Since 1996, eighteen projects on Perkinsus biology (four of them include oyster resistance to both Perkinsus and Haplosporidium) have been funded by this agency, all of these addressing mechanistic or ecological aspects of Dermo disease. Nevertheless, it is also clear that programs supported by other funding agencies such as the state Sea Grant offices, and the Oyster Disease Research Program from the National Oceanic and Atmospheric Administration, have been and are still vital for supporting research on multiple aspects of these diseases in the USA, in both basic and translational studies. Studies on Bonamia spp. have been more prevalent in Europe where it depleted the flat oyster populations and still remains the main hindrance for the recovery of that shellfishery (http://oysterecover.eu/). Overall, the number of papers published on Perkinsus spp. greatly exceeds those published on the other three protozoan parasites combined and appears to have reached a plateau of paper/year far high than the other three parasite groups that are clearly beyond the plateau. This could be attributed not only to the widely recognized economic and environmental relevance of the eastern oyster in the coastal areas of USA and the significant detrimental impact of Dermo infections, but also to an increase in the number of Perkinsus spp. described worldwide [24]–[29], with first reports in Brazil, West coast of North America, China, Japan, and other regions in Asia [30]–[33]. The autocorrelation analysis suggested, however, that the major determinant in the increase of published papers was the establishment and optimization of the culture methodologies [34]–[37]. The strong autocorrelation in the Perkinsus time series implies that an increase in publications in one year corresponds to greater publications in subsequent years. The fact that this autocorrelation was absent in the time preceding the advances in culture methodologies for Perkinsus, as well as in the time series for the three other parasites, implies that these methodologies were the driving force in establishing momentum for the study of a parasite. Otherwise, the amount of research devoted to any particular parasite is effectively random from year to year, overlaid on a 20–30 year profile of rise and decline. Consequently, having unlimited amounts of the parasite infective stage has resulted in the development of diagnostic tools [38]–[42], essential to the description of new species [24]–[27], [29], more detailed epizootiology maps [2], [43], robust phylogenies [44], virulence studies [45]–[51], defense against the host responses [52]–[56], proteomics [57], and for the development of assays for the identification of drugs for intervention [9]–[11], [58]–[60]. Furthermore, it also accelerated the understanding of Perkinsus’ biology, including the parasite’s mechanisms for entry, survival, proliferation inside the host [53]–[56], [61]–[73], and factors controlling zoosporulation [74], as well as biotechnology and biomedicine applications [70], [75]. Moreover, the availability of the transcriptome [76], and the genome (http://www.ncbi.nlm.nih.gov/nuccore/126302507), together with recently developed transfection methodology [77], is likely to result in making Perkinsus a model organism to study protozoan parasitic diseases [78]. Over time, the most represented categories of papers included diagnostic/epizootiology, host – parasite interactions, and cell biology. The large numbers of diagnostic/epizootiology studies can be attributed to the increasing number of countries performing surveys as a result of current concerns about Dermo disease associated to the increase in worldwide trade of bivalve species. The increase in publications on host – parasite interactions and cell biology of the parasite suggests that more research groups are focusing on the mechanistic aspects of the parasitic disease. At a lesser scale, the development of methods for purification of Bonamia cells [79], also has favored several mechanistic studies [80]–[82]. Similarly, the identification of a probable intermediate host in the life cycle for Marteilia refringens [83], [84], appears to be responsible for the noticeable increase in publications during 2013.
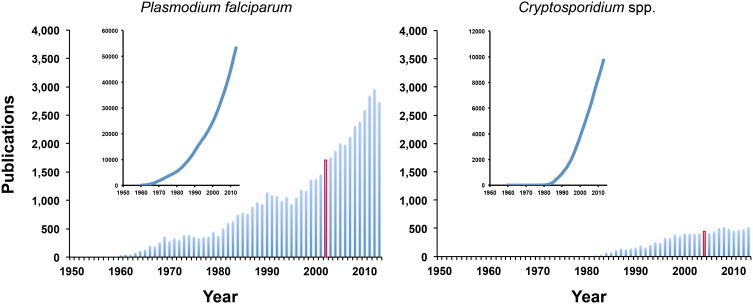
It would be expected that the sequencing of the genomes of protozoan parasites (as well as those of their hosts) would significantly increase not only the publication rates, but also the number of mechanistic studies. Surprisingly, the publication of the genomes of Plasmodium falciparum and Cryptosporidium spp. [85]–[87]) did not result in the expected increase in the total number of papers published per year (Figure 5); instead, both Plasmodium and Cryptosporidium species are highly autocorrelated out to >20 years, indicating that the amount of papers in any given year is strongly connected to the number of papers in previous years. Both time series have an underlying periodicity of 20–30 years, though for P. falciparum, the dominant period is the full length of the time series. This trend is perhaps the result of the continued high publication rates for these two particular parasite species during the past decade (in the range of 2,300 papers/year for Plasmodium and 474 papers/year for Cryptosporidium) and the fact that the full length sequences for numerous genes had been available much earlier than their genome drafts. It remains to be seen in the next few years if the availability of genome sequences for parasites from bivalve molluscs, obviously less popular than Plasmodium and Cryptosporidium, will significantly change the publication rates, particularly on mechanistic studies that may lead to the identification of therapeutic or ecological targets and the development of new intervention strategies against the disease to complement available strategies [23]. Finally, this is the first study that provides a side– by– side comparison of the publication record for the four main genera of protozoan parasites affecting molluscs of commercial interest. The trends identified here should help researchers in the field to fine– tune their research projects during these challenging times when available funding for research is limited, and to students interested in pursuing career in marine parasitology to make a more educated decision looking into their future careers. State and local agencies can also benefit from this study by prioritizing or compartmentalizing research efforts for these and other protozoan parasites, especially when the initial funding effort derived from a catastrophic event (such as mass mortalities) starts fading. Under these circumstances, the continuity of the research projects on a particular protozoan parasite depends on available resources and tools that are required to make it competitive with other model systems.
Figure 5. Number of papers published every year (1960–2013) and cumulative papers (inset figures) in the literature (SCOPUS Database) using Plasmodium and Cryptosporidium as search strings.
Columns in red correspond to the years when the genomes were published.
Acknowledgments
We thank Dr. Jarrod Scott (Bigelow Laboratory for Ocean Sciences) for the critical reading of the manuscript and Pamela Shephard (Bigelow Laboratory for Ocean Sciences) and Sofía Fernández Becerra for their help managing the scientific database.
Funding Statement
Institutional funds from Bigelow Laboratory for Ocean Sciences grants 1R21AI076797-01A2 from the National Institutes of Health, and IOS 1050518 and IOS 0958016 from the National Science Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ford SE, Chintala MM (2006) Northward expansion of a marine parasite: Testing the role of temperature adaptation. J Exp Mar Biol Ecol 339: 226–235. [Google Scholar]
- 2. Pecher WT, Alavi MR, Schott EJ, Fernández-Robledo JA, Roth L, et al. (2008) Assessment of the northern distribution range of selected Perkinsus species in eastern oysters (Crassostrea virginica) and hard clams (Mercenaria mercenaria) with the use of PCR-based detection assays. J Parasitol 94: 410–422. [DOI] [PubMed] [Google Scholar]
- 3.Elston RA, Farley CA, Kent ML (1996) Occurrence and significance of bonamiasis in European flat oysters Ostrea edulis in North America. Dis Aquat Organ: 49–54.
- 4. Grizel H, Mialhe E, Chagot D, Boulo V, Bachère E (1988) Bonamiasis: A model study of diseases in marine molluscs. Am Fisheries Soc Special Pub 18: 1–4. [Google Scholar]
- 5. Criscitiello MF, de Figueiredo P (2013) Fifty shades of immune defense. PLoS pathogens 9: e1003110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ragone Calvo LM, Harmon V, Burreson EM (1997) Selection of oysters for resistance to two protozoan parasites. J Shellfish Res 16: 327–328. [Google Scholar]
- 7. Morga B, Renault T, Faury N, Arzul I (2012) New insights in flat oyster Ostrea edulis resistance against the parasite Bonamia ostreae . Fish & shellfish immunology 32: 958–968. [DOI] [PubMed] [Google Scholar]
- 8. Culloty SC, Cronin MA, Mulcahy MF (2004) Potential resistance of a number of populations of the oyster Ostrea edulis to the parasite Bonamia ostreae . Aquaculture 237: 41–58. [Google Scholar]
- 9. Dungan CF, Hamilton RM (1995) Use of a tetrazolium-based cell proliferation assay to measure effects of in vitro conditions on Perkinsus marinus (Apicomplexa) proliferation. J Eukaryot Microbiol 42: 379–388. [Google Scholar]
- 10. Shridhar S, Hassan K, Sullivan DJ, Vasta GR, Fernández Robledo JA (2013) Quantitative assessment of the proliferation of the protozoan parasite Perkinsus marinus using a bioluminescence assay for ATP content. Int J Parasitol: Drug Drug Resist 3: 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Araujo NCP, Afonso R, Bringela A, Cancela ML, Cristiano MLS, et al. (2013) Peroxides with antiplasmodial activity inhibit proliferation of Perkinsus olseni, the causative agent of Perkinsosis in bivalves. Parasitol Int 62: 575–582. [DOI] [PubMed] [Google Scholar]
- 12. Ward JR, Lafferty KD (2004) The elusive baseline of marine disease: are diseases in ocean ecosystems increasing? PLoS biology 2: E120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chatfield C (1999) The analysis of time series: an introduction. 5th ed. CRC press.
- 14. Burreson EM, Ford SE (2004) A review of recent information on the Haplosporidia, with special reference to Haplosporidium nelsoni (MSX disease). Aquatic Liv Res 17: 499–517. [Google Scholar]
- 15. Ford SE, Haskin HH (1982) History and epizootiology of Haplosporidium nelsoni (MSX), an oyster pathogen in Delaware Bay, 1957–1980. J Invertebr Pathol 40: 118–141. [Google Scholar]
- 16. Clegg TA, Morrissey T, Geoghegan F, Martin SW, Lyons K, et al. (2014) Risk factors associated with increased mortality of farmed Pacific oysters in Ireland during 2011. Prev Vet Med 113: 257–267. [DOI] [PubMed] [Google Scholar]
- 17. Paul-Pont I, Dhand NK, Whittington RJ (2013) Spatial distribution of mortality in Pacific oysters Crassostrea gigas: Reflection on mechanisms of OsHV-1 transmission. Dis Aquatic Organ 105: 127–138. [DOI] [PubMed] [Google Scholar]
- 18. Andrews JD (1996) History of Perkinsus marinus, a pathogen of oysters in Chesapeake Bay 1950–1984. J Shellfish Res 15: 13–16. [Google Scholar]
- 19. Mackin JG, Owen HM, Collier A (1950) Preliminary note on the occurrence of a new protistan parasite, Dermocystidium marinum n. sp., in Crassostrea virginica (Gmelin). Science 111: 328–329. [DOI] [PubMed] [Google Scholar]
- 20. Grizel H, Comps M, Cousserans F, Bonami JR, Vago C (1974) Study of a parasite of the digestive gland observed during the current epizootic of the flat oyster. C R Acad Sci Hebd Séances Acad Sci D 279: 783–784. [PubMed] [Google Scholar]
- 21. Comps M (1970) Observations sur les causes d’une mortalite abnormale des huitres plates dans Ie Bassin de Marennes. Rev Trav Inst Pêch Marit 34: 317–326. [Google Scholar]
- 22. Pichot Y, Comps M, Tigé G, Grizel H, Rabouin MA (1979) Research on Bonamia ostreae gen. n., sp. n., a new parasite of the flat oyster Ostrea edulis L. Rev Trav Inst Pêch Marit 43: 131–140. [Google Scholar]
- 23.Fisher WS (1988) Disease Processes in Marine Bivalve Molluscs. Amer Fisheries Society (June 1988).
- 24. Coss CA, Robledo JAF, Ruiz GM, Vasta GR (2001) Description of perkinsus andrewsi n. sp. Isolated from the baltic clam (Macoma balthica) by characterization of the ribosomal RNA locus, and development of a species-specific PCR-based diagnostic assay. J Eukaryot Microbiol 48: 52–61. [DOI] [PubMed] [Google Scholar]
- 25. Coss CA, Robledo JAF, Vasta GR (2001) Fine structure of clonally propagated in vitro life stages of a Perkinsus sp. isolated from the baltic clam Macoma balthica . J Eukaryot Microbiol 48: 38–51. [DOI] [PubMed] [Google Scholar]
- 26. McLaughlin SM, Tall BD, Shaheen A, Elsayed EE, Faisal M (2000) Zoosporulation of a new Perkinsus species isolated from the gills of the softshell clam Mya arenaria . Parasite 7: 115–122. [DOI] [PubMed] [Google Scholar]
- 27. Moss JA, Xiao J, Dungan CF, Reece KS (2008) Description of Perkinsus beihaiensis n. sp., a new Perkinsus sp. parasite in oysters of Southern China. J Eukaryot Microbiol 55: 117–130. [DOI] [PubMed] [Google Scholar]
- 28. Casas SM, Grau A, Reece KS, Apakupakul K, Azevedo C, et al. (2004) Perkinsus mediterraneus n. sp., a protistan parasite of the European flat oyster Ostrea edulis from the Balearic Islands, Mediterranean Sea. Dis Aquat Organ 58: 231–244. [DOI] [PubMed] [Google Scholar]
- 29. Dungan CF, Reece KS (2006) In vitro propagation of two Perkinsus spp. parasites from Japanese Manila clams Venerupis philippinarum and description of Perkinsus honshuensis n. sp. J Eukaryot Microbiol 53: 316–326. [DOI] [PubMed] [Google Scholar]
- 30. da Silva PM, Vianna RT, Guertler C, Ferreira LP, Santana LN, et al. (2013) First report of the protozoan parasite Perkinsus marinus in South America, infecting mangrove oysters Crassostrea rhizophorae from the Paraíba River (NE, Brazil). J Invertebr Pathol 113: 96–103. [DOI] [PubMed] [Google Scholar]
- 31. Brandão RP, Boehs G, Sabry RC, Ceuta LO, Luz Mdos S, et al. (2013) Perkinsus sp. infecting oyster Crassostrea rhizophorae (Guilding, 1828) on the coast of Bahia, Brazil. J Invertebr Pathol 112: 138–141. [DOI] [PubMed] [Google Scholar]
- 32. Cáceres-Martínez J, Ortega MG, Vásquez-Yeomans R, García Tde J, Stokes NA, et al. (2012) Natural and cultured populations of the mangrove oyster Saccostrea palmula from Sinaloa, México, infected by Perkinsus marinus . J Invertebr Pathol 110: 321–325. [DOI] [PubMed] [Google Scholar]
- 33. Cáceres-Martínez J, Vásquez-Yeomans R, Padilla-Lardizabal G, del Río Portilla MA (2008) Perkinsus marinus in pleasure oyster Crassostrea corteziensis from Nayarit, Pacific coast of México. J Invertebr Pathol 99: 66–73. [DOI] [PubMed] [Google Scholar]
- 34. La Peyre JF, Faisal M, Burreson EM (1993) In vitro propagation of the protozoan Perkinsus marinus, a pathogen of the eastern oyster, Crassostrea virginica . J Eukaryot Microbiol 40: 304–310. [Google Scholar]
- 35. Kleinschuster SJ, Swink SL (1993) A simple method for the in vitro culture of Perkinsus marinus . The Nautilus 107: 76–78. [Google Scholar]
- 36. Gauthier JD, Vasta GR (1993) Continuous in vitro culture of the eastern oyster parasite Perkinsus marinus . J Invertebr Pathol 62: 321. [Google Scholar]
- 37. Gauthier JD, Vasta GR (1995) In vitro culture of the Eastern parasite Perkinsus marinus: optimization of the methodology. J Invertebr Pathol 66: 156–168. [Google Scholar]
- 38. Marsh AG, Gauthier JD, Vasta GR (1995) A semiquantitative PCR assay for assessing Perkinsus marinus infections in the eastern oyster, Crassostrea virginica . J Parasitol 81: 577–583. [PubMed] [Google Scholar]
- 39. Robledo JAF, Gauthier JD, Coss CA, Wright AC, Vasta GR (1998) Species-specificity and sensitivity of a PCR-based assay for Perkinsus marinus in the eastern oyster, Crassostrea virginica: A comparison with the fluid thioglycollate assay. J Parasitol 84: 1237–1244. [PubMed] [Google Scholar]
- 40. Robledo JA, Coss CA, Vasta GR (2000) Characterization of the ribosomal RNA locus of Perkinsus atlanticus and development of a polymerase chain reaction-based diagnostic assay. J Parasitol 86: 972–978. [DOI] [PubMed] [Google Scholar]
- 41. Penna MS, Khan M, French RA (2001) Development of a multiplex PCR for the detection of Haplosporidium nelsoni, Haplosporidium costale and Perkinsus marinus in the eastern oyster (Crassostrea virginica, Gmelin, 1971). Mol Cell Prob 15: 385–390. [DOI] [PubMed] [Google Scholar]
- 42. Elandalloussi LM, Leite RM, Afonso R, Nunes PA, Robledo JAF, et al. (2004) Development of a PCR-ELISA assay for diagnosis of Perkinsus marinus and Perkinsus atlanticus infections in bivalve molluscs. Mol Cell Prob 18: 89–96. [DOI] [PubMed] [Google Scholar]
- 43. Reece KS, Dungan CF, Burreson EM (2008) Molecular epizootiology of Perkinsus marinus and P. chesapeaki infections among wild oysters and clams in Chesapeake Bay, USA. Dis Aquat Organ 82: 237–248. [DOI] [PubMed] [Google Scholar]
- 44. Bachvaroff TR, Handy SM, Place AR, Delwiche CF (2011) Alveolate phylogeny inferred using concatenated ribosomal proteins. J Eukaryot Microbiol 58: 223–233. [DOI] [PubMed] [Google Scholar]
- 45. Bushek D, Allen SK Jr (1996) Host-parasite interactions among broadly distributed populations of the eastern oyster Crassostrea virginica and the protozoan Perkinsus marinus . Mar Ecol Prog Ser 139: 127–141. [Google Scholar]
- 46. Bushek D, Ford SE, Chintala MM (2002) Comparison of in vitro-cultured and wild-type Perkinsus marinus. III. Fecal elimination and its role in transmission. Dis Aquat Organ 51: 217–225. [DOI] [PubMed] [Google Scholar]
- 47. Chintala MM, Bushek D, Ford SE (2002) Comparison of in vitro-cultured and wild-type Perkinsus marinus. II. Dosing methods and host response. Dis Aquat Organ 51: 203–216. [DOI] [PubMed] [Google Scholar]
- 48. Ford SE, Chintala MM, Bushek D (2002) Comparison of in vitro-cultured and wild-type Perkinsus marinus. I. Pathogen virulence. Dis Aquat Organ 51: 187–201. [DOI] [PubMed] [Google Scholar]
- 49. Casas SM, Reece KS, Li Y, Moss JA, Villalba A, et al. (2008) Continuous culture of Perkinsus mediterraneus, a parasite of the European flat oyster Ostrea edulis, and characterization of its morphology, propagation, and extracellular proteins in vitro . J Eukaryot Microbiol 55: 34–43. [DOI] [PubMed] [Google Scholar]
- 50. Garreis KA, La Peyre JF, Faisal M (1996) The effects of Perkinsus marinus extracellular products and purified proteases on oyster defence parameters in vitro . Fish Shellfish Immun 6: 581–597. [Google Scholar]
- 51. La Peyre JF, Schafhauser DY, Rizkalla EH, Faisal M (1995) Production of serine proteases by the oyster pathogen Perkinsus marinus (Apicomplexa) in vitro . J Eukaryot Microbiol 42: 544–551. [Google Scholar]
- 52. Ahmed H, Schott EJ, Gauthier JD, Vasta GR (2003) Superoxide dismutases from the oyster parasite Perkinsus marinus: Purification, biochemical characterization, and development of a plate microassay for activity. Anal Biochem 318: 132–141. [DOI] [PubMed] [Google Scholar]
- 53. Schott EJ, Pecher WT, Okafor F, Vasta GR (2003) The protistan parasite Perkinsus marinus is resistant to selected reactive oxygen species. Exp Parasitol 105: 232–240. [DOI] [PubMed] [Google Scholar]
- 54. Schott EJ, Vasta GR (2003) The PmSOD1 gene of the protistan parasite Perkinsus marinus complements the sod2Δ mutant of Saccharomyces cerevisiae, and directs an iron superoxide dismutase to mitochondria. Mol Bioch Parasitol 126: 81–92. [DOI] [PubMed] [Google Scholar]
- 55. Asojo OA, Schott EJ, Vasta GR, Silva AM (2006) Structures of PmSOD1 and PmSOD2, two superoxide dismutases from the protozoan parasite Perkinsus marinus . Acta Cryst F: Struct Biol Cryst Commun 62: 1072–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fernández-Robledo JA, Schott EJ, Vasta GR (2008) Perkinsus marinus superoxide dismutase 2 (PmSOD2) localizes to single-membrane subcellular compartments. Biochem Biophys Res Commun 375: 215–219. [DOI] [PubMed] [Google Scholar]
- 57. Fernández-Boo S, Chicano-Gálvez E, Alhama J, Barea JL, Villalba A, et al. (2014) Comparison of protein expression profiles between three Perkinsus spp., protozoan parasites of molluscs, through 2D electrophoresis and mass spectrometry. J Invertebr Pathol 118: 47–58. [DOI] [PubMed] [Google Scholar]
- 58. Elandalloussi LM, Leite RB, Rodrigues PM, Afonso R, Nunes PA, et al. (2005) Effect of antiprotozoal drugs on the proliferation of the bivalve parasite Perkinsus olseni . Aquaculture 243: 9–17. [Google Scholar]
- 59. Lund ED, Soudant P, Chu F-LE, Harvey E, Bolton S, et al. (2005) Effects of triclosan on growth, viability and fatty acid synthesis of the oyster protozoan parasite Perkinsus marinus . Dis Aquat Organ 67: 217–224. [DOI] [PubMed] [Google Scholar]
- 60. Stelter K, El-Sayed NM, Seeber F (2007) The expression of a plant-type ferredoxin redox system provides molecular evidence for a plastid in the early dinoflagellate Perkinsus marinus . Protist 158: 119–130. [DOI] [PubMed] [Google Scholar]
- 61. Soudant P, E. Chu FL, Volety A (2013) Host-parasite interactions: Marine bivalve molluscs and protozoan parasites, Perkinsus species. J Invertebr Pathol 114: 196–216. [DOI] [PubMed] [Google Scholar]
- 62. Allam B, Carden WE, Ward JE, Ralph G, Winnicki S, et al. (2013) Early host-pathogen interactions in marine bivalves: Evidence that the alveolate parasite Perkinsus marinus infects through the oyster mantle during rejection of pseudofeces. J Invertebr Pathol 113: 26–34. [DOI] [PubMed] [Google Scholar]
- 63. Feng C, Ghosh A, Amin MN, Giomarelli B, Shridhar S, et al. (2013) The galectin CvGal1 from the eastern oyster (Crassostrea virginica) binds to blood group a oligosaccharides on the hemocyte surface. J Biol Chem 288: 24394–24409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Earnhart CG, Kaattari SL (2005) Potential novel epitopes in the extracellular products of oyster homogenate-supplemented Perkinsus marinus cells are not detected by subtractive immunization. J Parasitol 91: 689–691. [DOI] [PubMed] [Google Scholar]
- 65. McLaughlin SM, Elsayed EE, Faisal M (2000) Analysis of extracellular proteins of two Perkinsus spp. isolated from the softshell clam Mya arenaria in vitro . Comp Bioch Physiol - B Bioch Mol Biol 126: 587–598. [DOI] [PubMed] [Google Scholar]
- 66. La Peyre JF, Xue QG, Itoh N, Li Y, Cooper RK (2010) Serine protease inhibitor cvSI-1 potential role in the eastern oyster host defense against the protozoan parasite Perkinsus marinus . Devel Comp Immunol 34: 84–92. [DOI] [PubMed] [Google Scholar]
- 67. Tasumi S, Vasta GR (2007) A galectin of unique domain organization from hemocytes of the eastern oyster (Crassostrea virginica) is a receptor for the protistan parasite Perkinsus marinus . J Immunol 179: 3086–3098. [DOI] [PubMed] [Google Scholar]
- 68. Lin Z, Fernández-Robledo JA, Cellier MFM, Vasta GR (2011) The natural resistance-associated macrophage protein from the protozoan parasite Perkinsus marinus mediates iron uptake. Biochemistry 50: 6340–6355. [DOI] [PubMed] [Google Scholar]
- 69. Robledo JAF, Courville P, Cellier MFM, Vasta GR (2004) Gene organization and expression of the divalent cation transporter Nramp in the protistan parasite Perkinsus marinus . J Parasitol 90: 1004–1014. [DOI] [PubMed] [Google Scholar]
- 70. Fernández-Robledo JA, Vasta GR (2010) Production of recombinant proteins from protozoan parasites. Trends Parasitol 26: 244–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Chu FL, Lund E, Soudant P, Harvey E (2002) De novo arachidonic acid synthesis in Perkinsus marinus, a protozoan parasite of the eastern oyster Crassostrea virginica . Mol Bioch Parasitol 119: 179–190. [DOI] [PubMed] [Google Scholar]
- 72. La Peyre JF, Yarnall HA, Faisal M (1996) Contribution of Perkinsus marinus extracellular products in the infection of eastern oysters (Crassostrea virginica). J Invertebr Pathol 68: 312–313. [DOI] [PubMed] [Google Scholar]
- 73. Pales Espinosa E, Winnicki S, Allam B (2013) Early host-pathogen interactions in a marine bivalve: Crassostrea virginica pallial mucus modulates Perkinsus marinus growth and virulence. Dis Aquat Organ 104: 237–247. [DOI] [PubMed] [Google Scholar]
- 74. Casas SM, La Peyre JF (2013) Identifying factors inducing trophozoite differentiation into hypnospores in Perkinsus species. Europ J Protistol 49: 201–209. [DOI] [PubMed] [Google Scholar]
- 75. Wijayalath W, Majji S, Kleschenko Y, Pow-Sang L, Brumeanu TD, et al. (2014) Humanized HLA-DR4 mice fed with the protozoan pathogen of oysters Perkinsus marinus (Dermo) do not develop noticeable pathology but elicit systemic immunity. PLoS One 9: e87435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Joseph SJ, Fernández-Robledo JA, Gardner MJ, El-Sayed NM, Kuo CH, et al. (2010) The Alveolate Perkinsus marinus: Biological insights from EST gene discovery. BMC Genomics 11: 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Fernández-Robledo JA, Lin Z, Vasta GR (2008) Transfection of the protozoan parasite Perkinsus marinus . Mol Bioch Parasitol 157: 44–53. [DOI] [PubMed] [Google Scholar]
- 78. Fernández Robledo JA, Caler E, Matsuzaki M, Keeling PJ, Shanmugam D, et al. (2011) The search for the missing link: A relic plastid in Perkinsus? Int J Parsitol 41: 1217–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mialhe E, Bachere E, Chagot D, Grizel H (1988) Isolation and purification of the protozoan Bonamia ostreae (Pichot, et al. 1980), a parasite affecting the flat oyster Ostrea edulis L. Aquaculture 71: 293–299. [Google Scholar]
- 80. Morga B, Arzul I, Chollet B, Renault T (2009) Infection with the protozoan parasite Bonamia ostreae modifies in vitro haemocyte activities of flat oyster Ostrea edulis . Fish Shellfish Immunol 26: 836–842. [DOI] [PubMed] [Google Scholar]
- 81. Morga B, Renault T, Faury N, Chollet B, Arzul I (2011) Cellular and molecular responses of haemocytes from Ostrea edulis during in vitro infection by the parasite Bonamia ostreae . Inter J Parasitol 41: 755–764. [DOI] [PubMed] [Google Scholar]
- 82. Hervio D, Bachère E, Boulo V, Cochennec N, Vuillemin V, et al. (1995) Establishment of an experimental infection protocol for the flat oyster, Ostrea edulis, with the intrahaemocytic protozoan parasite, Bonamia ostreae: Application in the selection of parasite-resistant oysters. Aquaculture 132: 183–194. [Google Scholar]
- 83. Audemard C, Sajus MC, Barnaud A, Sautour B, Sauriau PG, et al. (2004) Infection dynamics of Marteilia refringens in flat oyster Ostrea edulis and copepod Paracartia grani in a claire pond of Marennes-Oleron Bay. Dis Aquat Organ 61: 103–111. [DOI] [PubMed] [Google Scholar]
- 84. Audemard C, Le Roux F, Barnaud A, Collins C, Sautour B, et al. (2002) Needle in a haystack: Involvement of the copepod Paracartia grani in the life-cycle of the oyster pathogen Marteilia refringens . Parasitology 124: 315–323. [DOI] [PubMed] [Google Scholar]
- 85. Gardner MJ, Hall N, Fung E, White O, Berriman M, et al. (2002) Genome sequence of the human malaria parasite Plasmodium falciparum . Nature 419: 498–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Xu P, Widmer G, Wang Y, Ozaki LS, Alves JM, et al. (2004) The genome of Cryptosporidium hominis . Nature 431: 1107–1112. [DOI] [PubMed] [Google Scholar]
- 87. Abrahamsen MS, Templeton TJ, Enomoto S, Abrahante JE, Zhu G, et al. (2004) Complete genome sequence of the Apicomplexan, Cryptosporidium parvum . Science 304: 441–445. [DOI] [PubMed] [Google Scholar]