Abstract
Background
Pulmonary abnormalities are found in both chronic heart failure (CHF) and pulmonary arterial hypertension (PAH). The differences of pulmonary function in chronic left heart failure and chronic right heart failure are not fully understood.
Material/Methods
We evaluated 120 patients with stable CHF (60 with chronic left heart failure and 60 with chronic right heart failure). All patients had pulmonary function testing, including pulmonary function testing at rest and incremental cardiopulmonary exercise testing (CPX).
Results
Patients with right heart failure had a significantly lower end-tidal partial pressure of CO2 (PetCO2), higher end-tidal partial pressure of O2 (PetO2) and minute ventilation/CO2 production (VE/VCO2) at rest. Patients with right heart failure had a lower peak PetCO2, and a higher peak dead space volume/tidal volume (VD/VT) ratio, peak PetO2, peak VE/VCO2, and VE/VCO2 slope during exercise. Patients with right heart failure had more changes in ΔPetCO2 and ΔVE/VCO2, from rest to exercise.
Conclusions
Patients with right heart failure had worse pulmonary function at rest and exercise, which was due to severe ventilation/perfusion (V/Q) mismatching, severe ventilation inefficiency, and gas exchange abnormality.
MeSH Keywords: Coronary Disease - rehabilitation, Heart Failure - prevention & control, Respiratory Function Tests - methods
Background
There are symptoms common to both chronic heart failure (CHF) and pulmonary arterial hypertension (PAH). The heart, lungs, kidneys, and muscles are affected in both diseases. Pulmonary abnormalities play an important role in the evaluation and prognosis of CHF and PAH. The differences of pulmonary function in chronic left heart failure and chronic right heart failure are not fully understood.
Cardiopulmonary exercise testing (CPX) is considered the criterion standard for studying cardiovascular, pulmonary, and metabolic adaptations to exercise in heart disease. Pulmonary abnormalities occur in both CHF and chronic right heart failure secondary to PAH [1–9]. Left heart failure with cardiac enlargement causes restrictive lung disease, interstitial edema, alveolar-capillary hydrostatic injury, and fatigue of respiratory muscles, which contributes to pulmonary abnormalities. PAH can cause ventilation/perfusion (V/Q) inequality secondary to pulmonary vascular bed damage. Hyperventilation may result from these pulmonary function changes.
We hypothesized that pulmonary function is different in patients with left heart failure and right heart failure secondary to PAH. This study may help understand relationships between pulmonary abnormalities and different types of heart failure.
Material and Methods
Patients
A single cardiologist evaluated 120 patients with clinically stable CHF, including 60 patients with chronic left heart failure and 60 patients with chronic right heart failure. Left heart failure was diagnosed using the American College of Cardiology Foundation (ACCF)/American Heart Association (AHA) guidelines for heart failure [10]. PAH was defined as WHO group1 PH according to the ACCF/AHA expert consensus document on pulmonary hypertension. We excluded patients with group 2, 3, 4, or 5, associated with congenital heart disease, portal hypertension, significant venous or capillary involvement, or persistent pulmonary hypertension of the newborn [11]. Right heart failure was defined as PAH and cardiac index (CI) <2.2 L/(min×m2) as measured by right heart catheterization. All patients had symptoms and/or signs of right heart failure. All patients had a forced expiratory volume in 1 s/forced vital capacity (FEV1/FVC) ratio >65% at rest. All testing was performed before treatment.
The study protocol adhered to the Declaration of Helsinki. Each patient provided written informed consent to participate in this study. The document was approved and recorded by the institutional Ethics Committee of Fuwai Hospital, China. The project approval number was 2012-401.
Pulmonary function test at rest
Pulmonary function testing at rest was performed using a closed-circuit spirometer (COSMED, Italy) according to the American Thoracic Society (ATS) recommendations [12]. Dead space volume/tidal volume (VD/VT)=(PaCO2 – PECO2 mean)/PaCO2 – [VD (machine)/VT] where PaCO2 = arterial CO2 tension, PECO2 = the partial pressure of expired CO2. Minute ventilation/CO2 production (VE/VCO2) ratio was defined as VE/VCO2 = 863/[PaCO2 × (1-VD/VT)].
Cardiopulmonary exercise testing
Physician-supervised CPX was performed on a bicycle ergometer with a breath-by-breath system (COSMED, Italy) according to the ATS/American College of Chest Physicians (ACCP) Statement on CPX [13]. Exercise-induced right-to-left shunt (EIS) was performed according to criteria described by Sun [14].
Breathing reserve (BR) was defined as: BR = (MVV-peak VE)/MVV ×100% where MVV = maximal voluntary ventilation.
Change in CPX parameter from rest to peak exercise was defined as: Δ measure = (peak measure-rest measure)/rest measure ×100%.
Echocardiography
Two-dimensional echocardiography and Doppler ultrasound (Philips IE33, Netherlands) examinations were performed on the same day before CPX. Left ventricular ejection fraction (LVEF) was determined according to the recommendations of the European Association of Echocardiography [15].
Right heart catheterization
Right heart catheterization was performed 3 days after CPX. Pulmonary capillary wedge pressure (PCWP) and mean pulmonary artery pressure (mPAP) were determined with balloon flotation catheter (Edwards Lifesciences, USA). CI was determined by Frick method.
Statistical analyses
Data were analyzed using SPSS 13.0 (SPSS Inc; Chicago IL). Continuous variables are presented as mean ±SD and categorical variables as a percentage. The t test was used to compare continuous variables. The chi-square test was used to compare categorical variables. Multivariate linear regression was used to determine pulmonary function differences and the changes in CPX parameters between the 2 groups. To correct for demographic differences between the 2 groups of patients, variables that were either biologically plausible and/or significantly different between groups in univariate analysis were entered into the multivariate model. P<0.05 was considered statistically significant.
Results
Demographic data from the 2 patient groups are presented in Table 1. Patients with left heart failure were older, had a higher proportion of men and smokers, and had a higher BMI. These findings could affect pulmonary function.
Table 1.
Baseline characteristics.
| Characteristic | Left heart failure (n=60) | Right heart failure (n=60) | P value |
|---|---|---|---|
| Men, n | 53 | 10 | <0.001 |
| Age, years | 45.1±9.9 | 30.8±9.5 | <0.001 |
| BMI, kg/m2 | 24.12±3.94 | 22.13±2.95 | 0.002 |
| Smokers, n | 37 | 4 | <0.001 |
| NYHA, n | 0.131 | ||
| Class I/II | 18 | 27 | – |
| Class III/IV | 42 | 33 | – |
| LVEF,% | 27.92±8.99 | 64.65±6.31 | <0.001 |
| mPAP, mmHg | – | 54.35±16.91 | – |
| PCWP, mmHg | – | 9.20±3.83 | – |
| CI, L/min×m2 | – | 1.99±0.22 | – |
| ICM, n | 13 | – | – |
| NICM, n | 47 | – | – |
| IPAH, n | – | 44 | – |
| FPAH, n | 2 | ||
| APAH, n | 14 |
ICM – ischemic cardiomyopathy; NICM – non-ischemic cardiomyopathy; IPAH – idiopathic pulmonary arterial hypertension; FPAH – familial pulmonary arterial hypertension; APAH – associated with pulmonary arterial hypertension.
The anaerobic threshold (AT) was detectable in all patients. Among the patients with right heart failure, 21 showed EIS. Table 2 shows the results of univariate analysis of pulmonary function. Patients with right heart failure had lower oxygen uptake (VO2), VT, FEV1, FVC, MVV, and PetCO2, and higher end-tidal partial pressure of O2 (PetO2) and VE/VCO2 at rest. Right heart failure patients had lower peak VO2, peak VE, peak VT, and peak PetCO2, and had higher peak VD/VT, peak PetO2, peak VE/VCO2, and VE/VCO2 slope during exercise. Right heart failure patients had lower ΔVO2, ΔVE, ΔPetCO2, and ΔVE/VCO2, from rest to exercise.
Table 2.
Univariate analysis of pulmonary function.
| Measure | Left heart failure | Right heart failure | P valure |
|---|---|---|---|
| Rest VO2, ml/min | 341.82±97.65 | 285.07±67.69 | <0.001 |
| Rest VE, L/min | 11.09±3.16 | 10.42±2.75 | 0.214 |
| Rest VT, L | 0.68±0.20 | 0.59±0.22 | 0.028 |
| Rest FEV1, L | 2.89±0.57 | 2.55±0.59 | 0.001 |
| Rest FVC, L | 3.69±0.69 | 3.23±0.74 | 0.001 |
| Rest FEV1/FVC, % | 79.28±5.41 | 79.25±7.70 | 0.984 |
| Rest MVV, L/min | 118.27±28.62 | 97.17±27.12 | <0.001 |
| Rest Rf, b/min | 17.02±4.69 | 18.82±5.44 | 0.054 |
| Rest VD/VT, % | 31.40±5.62 | 31.67±4.69 | 0.778 |
| Rest PetCO2, mmHg | 33.72±4.47 | 28.78±4.25 | <0.001 |
| Rest PetO2, mmHg | 110.68±7.48 | 115.80±6.65 | <0.001 |
| Rest VE/VCO2, L/min/L/min | 37.96±6.50 | 43.45±6.79 | <0.001 |
| Peak VO2, ml/min | 1092.85±309.73 | 756.83±271.30 | <0.001 |
| Peak VE, L/min | 43.62±11.79 | 36.91±14.71 | 0.007 |
| Peak BR, % | 61.80±9.77 | 63.83±12.19 | 0.315 |
| Peak VT, L | 1.47±0.41 | 1.19±0.36 | <0.001 |
| Peak Rf, b/min | 30.30±6.01 | 31.24±8.04 | 0.468 |
| Peak VD/VT, % | 27.18±4.02 | 28.83±4.04 | 0.027 |
| Peak PetCO2, mmHg | 33.42±6.04 | 24.32±5.30 | <0.001 |
| Peak PetO2, mmHg | 117.17±6.10 | 124.43±5.61 | <0.001 |
| Peak VE/VCO2, L/min/L/min, | 36.13±7.97 | 48.50±11.95 | <0.001 |
| VE/VCO2 slope | 32.74±7.52 | 45.70±16.18 | 0.001 |
| EIS, n | – | 21 | |
| ΔVO2, % | 236.40±108.37 | 171.28±82.76 | <0.001 |
| ΔVE, % | 323.56±168.47 | 262.41±118.19 | 0.023 |
| ΔVT, % | 126.49±59.07 | 114.34±68.37 | 0.300 |
| ΔRf, % | 88.70±58.52 | 72.89±45.66 | 0.054 |
| ΔVD/VT, % | −11.71±15.45 | −7.72±14.21 | 0.300 |
| ΔPetCO2, % | −0.61±14.16 | −15.61±12.91 | <0.001 |
| ΔPetO2,% | 6.18±7.06 | 7.65±5.12 | 0.196 |
| ΔVE/VCO2, % | 4.55±16.28 | −11.46±18.68 | <0.001 |
Rf – respiratory frequency.
Table 3 shows the result of multivariate regression analysis of pulmonary function. Patients with right heart failure had lower PetCO2, and higher PetO2 and VE/VCO2 at rest. Right heart failure patients had a lower peak PetCO2, and higher peak VD/VT, peak PetO2, peak VE/VCO2, and VE/VCO2 slope during exercise. Right heart failure patients had lower ΔPetCO2 and ΔVE/VCO2, from rest to exercise.
Table 3.
Multivariate regression analysis of pulmonary function.
| Measure | β | P value |
|---|---|---|
| Rest VO2, ml/min | −0.052 | 0.700 |
| Rest VT, L | 0.058 | 0.687 |
| Rest FEV1, L | −0.139 | 0.304 |
| Rest FVC, L | −0.004 | 0.977 |
| Rest MVV, L/min | −0.049 | 0.705 |
| Rest Rf, b/min | 0.064 | 0.664 |
| Rest PetCO2, mmHg | −0.435 | 0.001 |
| Rest PetO2, mmHg | 0.329 | 0.023 |
| Rest VE/VCO2, L/min/L/min | 0.320 | 0.020 |
| Peak VO2, ml/min | −0.174 | 0.118 |
| Peak VE, L/min | 0.213 | 0.102 |
| Peak VT, L | 0.179 | 0.149 |
| Peak VD/VT,% | 0.376 | 0.010 |
| Peak PetCO2, mmHg | −0.704 | <0.001 |
| Peak PetO2, mmHg | 0.655 | <0.001 |
| Peak VE/VCO2, L/min/L/min, | 0.614 | <0.001 |
| VE/VCO2 slope | 0.506 | 0.001 |
| ΔVO2, % | −0.195 | 0.171 |
| ΔVE, % | 0.003 | 0.984 |
| ΔRf, % | −0.081 | 0.591 |
| ΔPetCO2, % | −0.657 | <0.001 |
| ΔVE/VCO2, % | −0.622 | <0.001 |
Left heart failure = 0, right heart failure = 1, female = 0, male = 1, non-smoker = 0, smoker = 1.
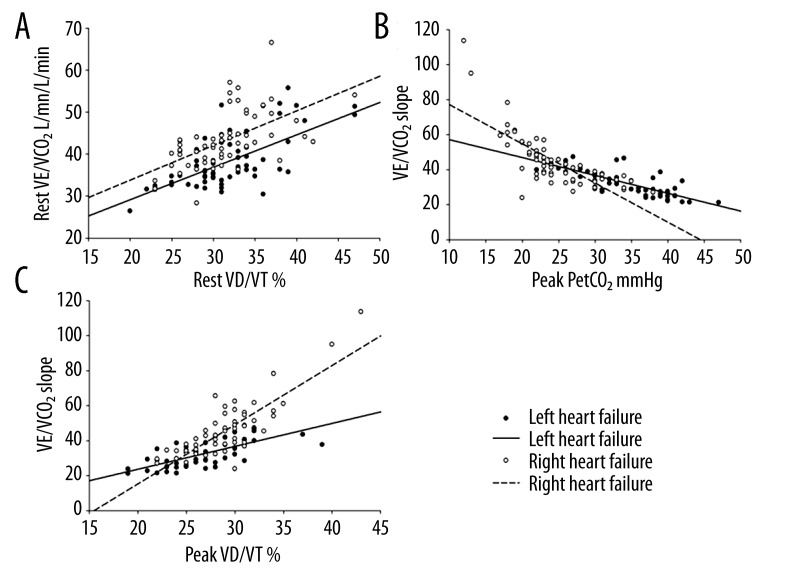
Figure 1A shows the result of VD/VT ratio versus VE/VCO2 at rest. The curves show patients with right heart failure had higher VE/VCO2 and lower PetCO2 at any given VD/VT ratio at rest. Figure 1B shows the result of peak PetCO2 versus VE/VCO2 slope. The abrupt curve of right heart failure was suggestive of EIS in the right heart failure patients during exercise. Most right heart failure patients had higher VE/VCO2 slope and peak VD/VT at any given peak PetCO2. Figure 1C shows the result of peak VD/VT ratio versus VE/VCO2 slope. The curves show that most right heart failure patients had a lower PetCO2 and higher VD/VT ratio at any given.
Figure 1.
(A) VD/VT ratio versus VE/VCO2 at rest. The curves showed that patients with right heart failure had a higher VE/VCO2 at any given VD/VT ratio at rest. (B) Peak PetCO2 versus VE/VCO2 slope. The abrupt curve of right heart failure was suggestive of EIS in the right heart failure patients during exercise. (C) Peak VD/VT ratio versus VE/VCO2 slope. The curves showed that most right heart failure patients had a lower PetCO2 at any given VD/VT ratio.
Discussion
Patients with right heart failure had lower PetCO2, and higher PetO2 and VE/VCO2 at rest. Patients with right heart failure showed a higher peak VD/VT, peak PetO2, peak VE/VCO2, and VE/VCO2 slope, and a lower PetCO2 during exercise. Patients with right heart failure had more changes in ΔPetCO2 and ΔVE/VCO2 from rest to exercise. These results show that patients with right heart failure had worse pulmonary function at rest and exercise.
Pulmonary function at rest
Pulmonary function changes compatible with restrictive lung disease are observed in most patients with severe CHF [16,17], and findings compatible with airway obstruction are common in patients with right heart failure caused by idiopathic pulmonary arterial hypertension (IPAH) [18]. We did not observed any different ventilatory measures at rest in the 2 groups.
PetCO2 reflects ventricular function [19]. Right heart failure patients had a lower PetCO2 (Table 3, Figure 1A). Figure 1A shows that right heart failure patients had a lower PetCO2 at any given VD/VT. These results demonstrate that patients with right heart failure had more ventilation at given CO2 discharge. Considering there was no difference in respiratory frequency or VE, the lower PetCO2 was due to hyperventilation in alveolus with well-perfusion and hypoperfusion of well ventilated alveolus in patients with right heart failure [20,21]. The lower PetCO2 could explain the higher VE/VCO2 in patients with right heart failure. The higher VE/VCO2 showed that right heart failure patients had lower ventilation efficiency. The higher PetO2 was a secondary effect of lower PetCO2.
The VD/VT ratio reflects the V/Q mismatching [20]. There was no difference in VD/VT ratio between the 2 groups. This result means that there was no difference in V/Q mismatching at rest in the 2 groups.
Pulmonary function during exercise
PetCO2 was lower in both CHF and PAH, reflecting the ventilation impairment [3,8,20–25]. We found that right heart failure patients had a lower PetCO2 during exercise (Table 3). The reason for the lower peak PetCO2 during exercise in patients with right heart failure was similar to the reason for lower PetCO2 at rest. Sun et al. [14] found that patients with PAH had an EIS resulting in abrupt decreased PetCO2, had increased PetO2, and increased VE/VCO2 ratio during exercise. The abrupt curve of right heart failure demonstrated that patients with right heart failure had EIS (Figure 1B). EIS also contributed to the lower peak PetCO2.
We found that patients with right heart failure had significantly higher peak VD/VT ratio (Table 3). Figure 1C shows that most patients with right heart failure had a lower peak PetCO2 and a higher VE/VCO2 slope. These results demonstrate that patients with right heart failure had severe V/Q mismatching at peak exercise. This means that primary pulmonary vesicular damage played a greater role in V/Q mismatching during exercise than did pulmonary congestion. Moreover, the EIS was involved in the higher VD/VT in patients with right heart failure.
VE/VCO2 and VE/VCO2 slope are important to reflect disease severity and prognosis in CHF and PAH, and reflect V/Q mismatching in PAH [21,25]. We found that peak VE/VCO2 and VE/VCO2 slope were higher in patients with right heart failure. These results demonstrate that patients with right heart failure had more severe ventilation inefficiency and gas exchange abnormality compared to patients with left heart failure. These results were due to lower peak PetCO2 and higher peak VD/VT in right heart failure patients.
Change in CPX parameters from rest to peak exercise
We found that patients with right heart failure had higher absolute values of ΔPetCO2 and ΔVE/VCO2 from rest to peak exercise (Table 2 and 3). These results show that right heart failure patients had larger changes of pulmonary function from rest to exercise. The larger change of ΔPetCO2 demonstrates that patients with right heart failure had more severe hyperventilation from rest to exercise. This should be attributed to more complex ventilation drive in PAH, including exercise-induced hypoxia and EIS [20].
We found that patients with right heart failure had worse ventilation efficiency, severe V/Q mismatching, and gas exchange abnormality, even without differences in peak VO2.
This study had some limitations. It was a single-center study and demographic differences were detected between the 2 groups. The etiology of heart failure in the 2 groups resulted in differences of sex and age. Because there was no control group, we could not confirm a normal breathing pattern at rest. We could not obtain diffusion function measures from all patients. This influenced the evaluation of pulmonary function. We could not perform invasive hemodynamic testing in all patients, which influenced our evaluation of left heart failure. We merged the 4 disease classes into 2 groups because there were not enough patients with class IV NYHA right heart failure.
Conclusions
Patients with right heart failure had worse pulmonary function at rest and during exercise. The differences in pulmonary function at rest were due to different breathing patterns and worse gas exchange in patients with right heart failure. The differences during exercise were due to severe V/Q mismatching, EIS, alveolar ventilation disorder, and oxygenation dysfunction secondary to pulmonary vascular damage in patients with right heart failure.
Acknowledgments
We thank Xiu-Ping, MA for excellent work in data collection.
Footnotes
Source of support: Zhi-Hong Liu received financial support from the National Key Technology R&D Program, China (Project Number: 2011BAI11B15)
References
- 1.Wright RS, Levine MS, Bellamy PE, et al. Ventilatory and diffusion abnormalities in potential heart transplant recipients. Chest. 1990;98(4):816–20. doi: 10.1378/chest.98.4.816. [DOI] [PubMed] [Google Scholar]
- 2.Agostoni P, Cattadori G, Guazzi M, et al. Cardiomegaly as a possible cause of lung dysfunction in patients with heart failure. Am Heart J. 2000;140:e24. doi: 10.1067/mhj.2000.110282. [DOI] [PubMed] [Google Scholar]
- 3.Wasserman K, Zhang YY, Gitt A, et al. Lung function and exercise gas exchange in chronic heart failure. Circulation. 1997;96(7):2221–27. doi: 10.1161/01.cir.96.7.2221. [DOI] [PubMed] [Google Scholar]
- 4.Papazachou O, Anastasiou-Nana M, Sakellariou D, et al. Pulmonary function at peak exercise in patients with chronic heart failure. Int J Cardiol. 2007;118(1):28–35. doi: 10.1016/j.ijcard.2006.04.091. [DOI] [PubMed] [Google Scholar]
- 5.De Feo S, Franceschini L, Brighetti G, et al. Ischemic etiology of heart failure identifies patients with more severely impaired exercise capacity. Ischemic etiology of heart failure identifies patients with more severely impaired exercise capacity. Int J Cardiol. 2005;104(3):292–97. doi: 10.1016/j.ijcard.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 6.Ferrazza AM, Martolini D, Valli G, Palange P. Cardiopulmonary exercise testing in the functional and prognostic evaluation of patients with pulmonary diseases. Respiration. 2009;77:3–17. doi: 10.1159/000186694. [DOI] [PubMed] [Google Scholar]
- 7.D’Alonzo GE, Gianotti LA, Pohil RL, et al. Comparison of progressive exercise performance of normal subjects and patients with primary pulmonary hypertension. Chest. 1987;92(1):57–62. doi: 10.1378/chest.92.1.57. [DOI] [PubMed] [Google Scholar]
- 8.Arena R, Lavie CJ, Milani RV, et al. Cardiopulmonary exercise testing in patients with pulmonary arterial hypertension: an evidence-based review. J Heart Lung Transplant. 2010;29(2):159–73. doi: 10.1016/j.healun.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Reybrouck T, Mertens L, Schulze-Neick I, et al. Ventilatory inefficiency for carbon dioxide during exercise in patients with pulmonary hypertension. Clin Physiol. 1998;18:337–44. doi: 10.1046/j.1365-2281.1998.00109.x. [DOI] [PubMed] [Google Scholar]
- 10.Jessup M, Abraham WT, Casey DE, et al. 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119(14):1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 11.McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation. 2009;119:2250–94. doi: 10.1161/CIRCULATIONAHA.109.192230. [DOI] [PubMed] [Google Scholar]
- 12.American Thoracic Society. Standardization of Spirometry, 1994 Update. Am J Respir Crit Care Med. 1995;152:1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 13.American Thoracic Society; American College of Chest Physicians. ATS/ACCP Statement on cardiopulmonary exercise testing. Am J RespirCrit Care Med. 2003;167(2):211–77. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 14.Sun XG, Hansen JE, Oudiz RJ, Wasserman K. Gas exchange detection of exercise-induced right-to-left shunt in patients with primary pulmonary hypertension. Circulation. 2002;105(1):54–60. doi: 10.1161/hc0102.101509. [DOI] [PubMed] [Google Scholar]
- 15.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Agostoni P, Bussotti M, Cattadori G, et al. Gas diffusion and alveolar-capillary unit in chronic heart failure. Eur Heart J. 2006;27:2538–43. doi: 10.1093/eurheartj/ehl302. [DOI] [PubMed] [Google Scholar]
- 17.Olson TP, Beck KC, Johnson BD. Pulmonary function changes associated with cardiomegaly in chronic heart failure. J Card Fail. 2007;13:100–7. doi: 10.1016/j.cardfail.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jing ZC, Xu XQ, Badesch DB, et al. Pulmonary function testing in patients with pulmonary arterial hypertension. Respir Med. 2009;103(8):1136–42. doi: 10.1016/j.rmed.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Yano T, Yunoki T, Matsuura R, Arimitsu T. Relationship between hyperventilation and excessive CO2 output during recovery from repeated cycling sprints. Physiol Res. 2009;58:529–35. doi: 10.33549/physiolres.931565. [DOI] [PubMed] [Google Scholar]
- 20.Wasserman K, Hansen J, Sue D, et al. Priniciples of Exercise Testing and Interpretation. Lippincott Williams & Wilkins; 2004. pp. 80–145. [Google Scholar]
- 21.Ting H, Sun XG, Chuang ML, et al. A noninvasive assessment of pulmonary perfusion abnormality in patients with primary pulmonary hypertension. Chest. 2001;119:824–32. doi: 10.1378/chest.119.3.824. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto A, Itoh H, Eto Y, et al. End-tidal CO2 pressure decreases during exercise in cardiac patients: association with severity of heart failure and cardiac output reserve. J Am Coll Cardiol. 2000;36:242–49. doi: 10.1016/s0735-1097(00)00702-6. [DOI] [PubMed] [Google Scholar]
- 23.Myers J, Gujja P, Neelagaru S, et al. End-tidal CO2 pressure and cardiac performance during exercise in heart failure. Med Sci Sports Exerc. 2009;41:19–25. doi: 10.1249/MSS.0b013e318184c945. [DOI] [PubMed] [Google Scholar]
- 24.Dimopoulos S, Anastasiou-Nana M, Katsaros F, et al. Impairment of autonomic nervous system activity in patients with pulmonary arterial hypertension: a case control study. J Card Fail. 2009;15(10):882–89. doi: 10.1016/j.cardfail.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Guazzi M, Adams V, Conraads V, et al. EACPR/AHA Scientific Statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation. 2012;126:2261–74. doi: 10.1161/CIR.0b013e31826fb946. [DOI] [PMC free article] [PubMed] [Google Scholar]