Abstract
Background:
Elevated plasma levels of high-sensitivity C-reactive protein (hs-CRP) have been associated with increased adverse health outcomes. The aim of this study was to investigate the association of sleep duration with risk of elevated hs-CRP levels in Taiwanese adults.
Methods:
We examined the association between sleep duration and hs-CRP in 353 healthy adults recruited from the physical examination center at a regional hospital in southern Taiwan. Elevated hs-CRP was defined as a plasma level ≥ 0.20 mg/dL. Short sleep duration was defined as ≤ 5.5 h per day. Multiple logistic regression analysis was used to assess the association of short duration of sleep with elevated hs-CRP levels.
Results:
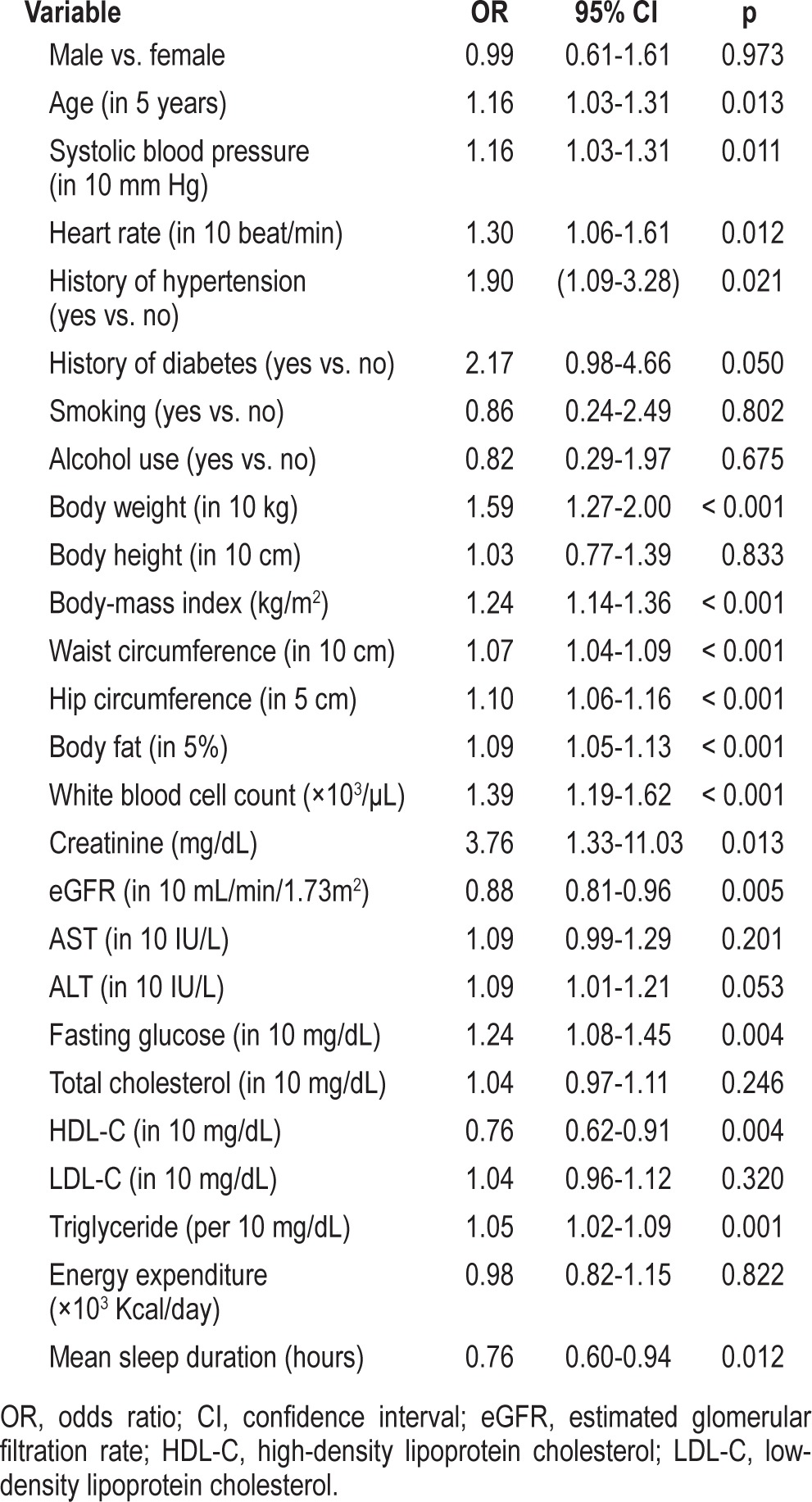
In this study, short duration of sleep (odds ratio [OR] = 2.20; 95% confidence interval [CI]: 1.11-4.30), aged 70 years or older (OR = 4.58; 95% CI: 1.70-12.66), menopause (OR = 2.81; 95% CI: 1.52-5.3), higher heart rate (OR = 1.38; 95% CI: 1.10-1.75), higher body mass index (OR = 1.20; 95% CI: 1.09-1.34), higher white blood cell count (OR = 1.38; 95% CI: 1.14-1.66), and higher uric acid level (OR = 1.31; 95% CI: 1.06-1.63) were significantly associated with an increased risk of elevated hs-CRP levels.
Conclusions:
In this study of healthy Taiwanese adults, short duration of sleep was significantly associated with elevated hs-CRP levels. Activation of pro-inflammatory pathways might represent a mechanism by which short sleep duration affects health.
Citation:
Chiang JK. Short duration of sleep is associated with elevated high-sensitivity C-reactive protein level in Taiwanese adults: a cross-sectional study. J Clin Sleep Med 2014;10(7):743-749.
Keywords: sleep duration, C-reactive protein, high-sensitivity CRP, hs-CRP
C-reactive protein is secreted by the liver in response to acute inflammation and infection.1 The high-sensitivity C-reactive protein (hs-CRP) assay has low detection limits, and good performance for apparently healthy subjects.2 Elevated hs-CRP levels have been associated with inflammation, aging, cancer, adverse outcomes of angina pectoris, coronary artery disease, adverse outcomes of chronic obstructive pulmonary disease, stroke, and increased risk of mortality.3–10 In addition, elevated hs-CRP has been shown to be a stronger predictor of cardiovascular disease than low-density lipoprotein cholesterol.11 A recent study of more than 4,000 subjects followed for six years revealed that elevated hs-CRP was associated with type 2 diabetes.12 Another study with a follow-up of 11 years also demonstrated the same association of hs-CRP with diabetes.13 As expected, patients with lower glycosylated hemoglobin (A1C) levels were found to have significantly lower hs-CRP levels.14
In recent years it has been shown that sleep duration might affect health in a number of ways, such as its associations with insulin resistance, diabetes, hyperleptinemia, heart disease, and mortality.15–17 Specifically, shorter duration of sleep was associated with a greater risk of obesity, diabetes, hypertension, stroke, coronary heart disease, and all-cause mortality.18–21 Yet some people have short sleep duration due to their genetic make-up or any other reasons (e.g., taking a nap) so that they may not be sleep deprived. Copinschi showed that sleep deprivation can affect neuroendocrine regulation.22 Respiration with the assistance of continuous positive airway pressure can lower C-reactive protein levels in patients with sleep apnea.23 A cross-sectional study of self-reported sleep duration and biomarkers with 6,465 subjects showed that long sleep duration was associated with elevated C-reactive protein levels in males.24 Another study of subjects with short-term sleep deprivation reported elevated C-reactive protein levels.25 However, the association of long-term sleep duration with hs-CRP levels is still unclear. Therefore, the aim of this study was to explore the association of mean sleep duration with risk of elevated hs-CRP in healthy Taiwanese adults.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Higher plasma levels of C-reactive protein could increase cardiometabolic risk, but the association with duration of sleep is not clear. The aim of the current study was to investigate the association between short duration of sleep and elevated high-sensitivity C-reactive protein levels in Taiwanese adults.
Study Impact: The study demonstrated that short sleep duration was significantly associated with elevated high-sensitivity C-reactive protein levels, independent of adiposity.
METHODS
Study subjects were apparently healthy individuals recruited from the physical examination center at a regional hospital in southern Taiwan between August 2009 and June 2011. Individuals with cancers, end stage renal disease, and chronic infectious diseases were excluded.
Demographic information of the subjects including age, sex, body weight, body height, waist circumference, hip circumference, and blood pressure were assessed using a questionnaire administered face-to-face by a trained research assistant. Clinical data including white blood cell (WBC) count, creatinine, fasting glucose, total cholesterol, aspartate aminotransferase (AST), alanine aminotransferase (ALT), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides, and hs-CRP levels were also recorded. The methods used were described in previous studies.18,26 The coding of menopause was 1 = females with menopause, and 0 = otherwise (including males). The participants were asked for their average daily sleep durations, including nap times, for the previous 3 months. The average sleep durations during weekdays and weekends were recorded separately, and the mean duration of sleep per day was calculated as [(5/7 × duration of sleep per day during weekdays) + (2/7 × duration of sleep per day during weekends)].27
This study was approved by the Research Ethics Committee of the Buddhist Dalin Tzu Chi Hospital (Nos. B09801018-1 and B10101013). Written informed consent was obtained from all participants after full explanation of the study procedure.
Statistical Analysis
Means and proportions were calculated for males and females. Categorical variables were analyzed using the χ2 test, and continuous variables were compared using the t-test or Wilcoxon rank-sum test, as appropriate. Univariate logistic regression analysis was used to evaluate the association of elevated hs-CRP levels with the independent variables. Multiple logistic regression analysis was conducted with a stepwise procedure to examine the significant independent variables associated with elevated hs-CRP levels. In multivariate analysis, the stepwise variable selection procedure (with iterations between the forward and backward steps) was applied to obtain the best candidate final regression model. All the univariate significant and nonsignificant relevant covariates (listed in Table 2) and some of their interactions were put on the variable list to be selected. The significance levels for entry (SLE) and for stay (SLS) were set at 0.15 to be conservative. The best candidate final regression model was identified manually by dropping the covariates with p value > 0.05 one at a time until all regression coefficients were significantly different from 0. Any discrepancy between the results of the univariate analysis and multivariate analysis were likely due to the confounding effects of the uncontrolled covariates in univariate analysis. Generalized additive models (GAMs) were fitted to detect potential nonlinear effects of continuous covariates and identify an appropriate cutoff point for discretizing a continuous predictor such as sleep duration during the stepwise variable selection. Computationally, the vgam function (with the default values of smoothing parameters) of the VGAM package was used to fit GAMs for continuous, binary, and count responses in R.28,29 Analysis of areas under the curves (AUCs) for receiver-operating characteristic (ROC) curves was conducted. The estimated area under the receiver operating characteristic (ROC) curve (also called the c statistic), where 0 ≤ c ≤ 1, is a very popular goodness-of-fit (GOF) index for logistic regression models. In practice, c ≥ 0.7 suggests an acceptable level of discrimination for a fitted logistic regression model. The GOF of the logistic regression model was assessed by the Hosmer-Lemeshow test. Statistical analysis was performed using R (version 2.15.1). A two-sided p < 0.05 was considered to indicate statistical significance.
Table 2.
Univariate logistic regression analysis of factors associated with elevated hs-CRP levels
RESULTS
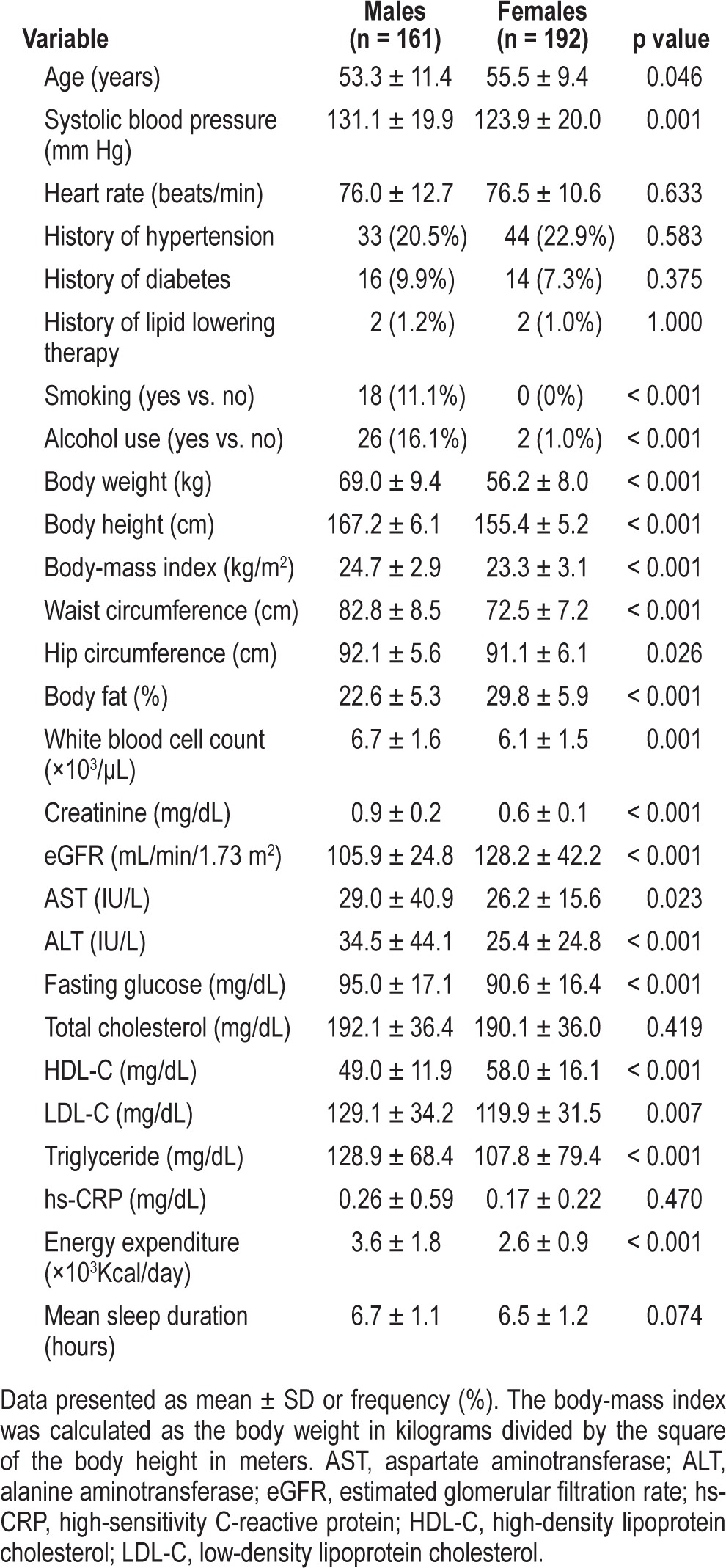
The characteristics of the 353 study participants are shown in Table 1. The mean of hs-CRP level was 0.21 mg/dL, the median 0.10 mg/dL, and the lower and upper quartiles 0.05 mg/dL and 0.20 mg/dL, respectively. The upper quartile value was considered the cutoff point for elevated hs-CRP levels in this study.30 The range of age was 25.2 to 84.6 years old. The minimum and maximum sleep durations were 4 and 11 hours, respectively.
Table 1.
Demographic, lifestyle, and clinical characteristics of participants (n = 353)
The results of the univariate analysis for elevated hs-CRP levels are shown in Table 2. Higher age, higher blood pressure, higher heart rate, history of hypertension, higher body weight, higher BMI, higher waist and hip circumference, higher body fat percent, higher WBC count, higher creatinine level, lower estimated glomerular filtration rate (eGFR), higher fasting glucose level, lower HDL-C level, higher triglycerides, and shorter mean sleep duration were associated with an increased risk of elevated hs-CRP levels. Table 3 shows the results of the multiple logistic and linear regression analyses of factors associated with hs-CRP levels, where the sleep duration (hours) was treated as a continuous predictor. Sleep duration was not a significant risk factor (p = 0.174) for hs-CRP levels using multiple linear regression after adjusting for the factors listed in Table 3. Longer sleep durations were associated with less probability of elevated hs-CRP levels (O.R. = 0.70, p = 0.006) after adjustments.
Table 3.
Multiple logistic and linear regression analyses of factors associated with hs-CRP levels
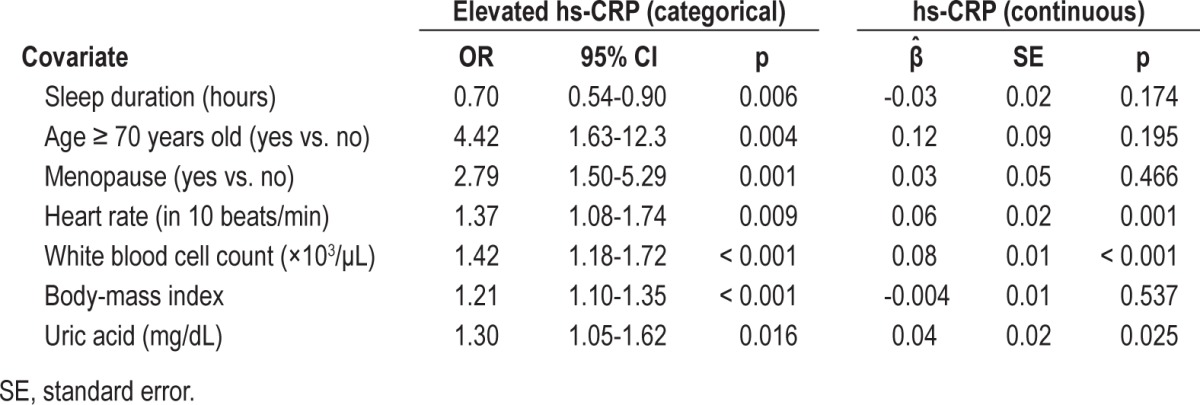
As shown in the GAM plot in Figure 1, the nonlinear effect of sleep duration on logit(P), where P = Pr(hs-CRP level was elevated), was not a U-shaped relationship after adjusting for the effects of the other covariates; and the cutoff point chosen to discretize it (5.5 h/day) was identified by locating the crosspoint between the smoothed curve and the horizontal line at logit(P) = 0. The interactive variables used in our regression analysis included (1) gender × age, gender × body fat percentage, gender × waist, gender × body weight, gender × energy expenditure; (2) menopause × age, menopause × body fat percentage, menopause × waist, menopause × body weight, menopause × energy expenditure; and (3) gender × short sleep duration. Yet none of them reached statistical significance in the fitted final regression model. The results of the multiple logistic regression analysis are shown in Table 4. Older age (≥ 75 years old), menopausal status, higher heart rate, higher WBC count, higher BMI, higher uric acid level, and short mean duration of sleep (≤ 5.5 h/day) were significantly associated with increased risk of an elevated hs-CRP level. The Nagelkerke R2 was 0.295, and the Hosmer-Lemeshow test was passed (p = 0.423) (Model 1 of Table 4). In our study, the effects of gender (p = 0.486)— even the interaction between gender and short sleep duration (p = 0.168)—were not significantly associated with elevated hs-CRP levels using the multiple logistic regression after adjustments.
Figure 1. Smoothing curve of sleep duration against probability of elevated hs-CRP levels adjusted for significant covariates listed in Model 1 of Table 3.
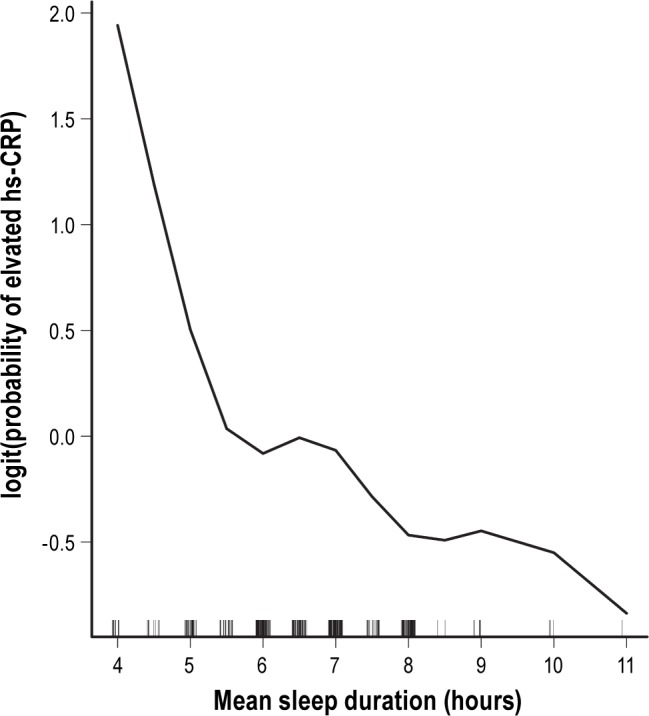
Table 4.
Multiple logistic regression analysis of factors associated with elevated hs-CRP levels
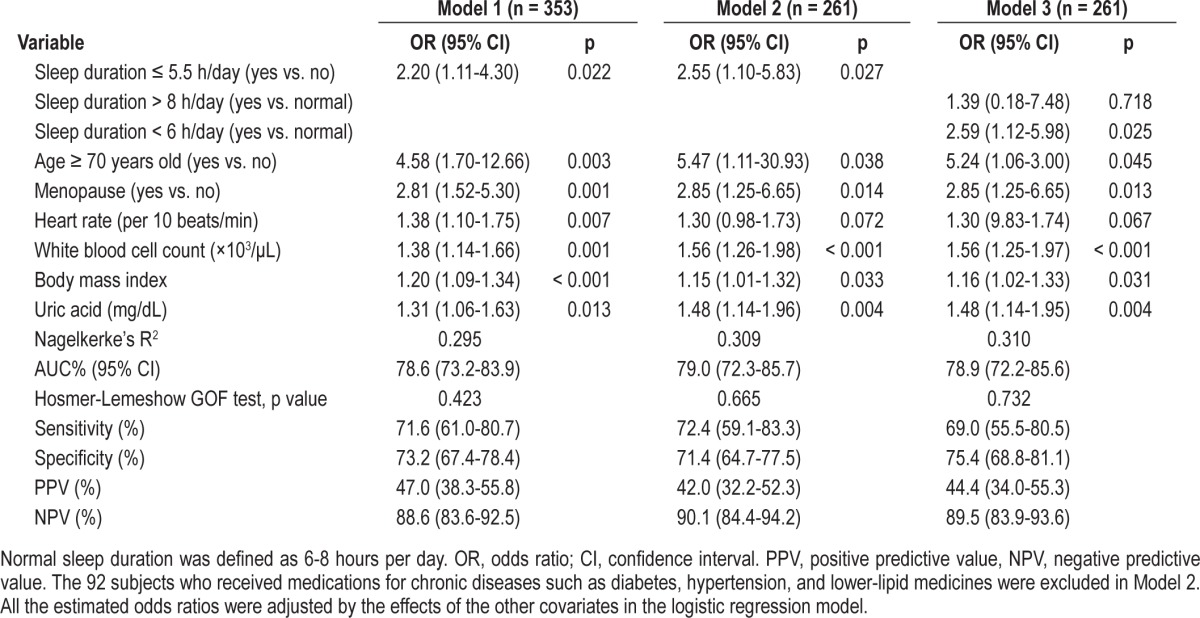
ROC curve analyses revealed that a cutoff value of 5.5 h/ day for mean duration of sleep yielded a sensitivity of 72% (95% confidence interval [CI]: 61%-81%), a specificity of 73% (95% CI: 67%-78%), a positive predictive value of 47% (95% CI: 38%-56%), a negative predictive value of 89% (95% CI: 84%-92%), and an AUC of 0.79 (95% CI: 0.73-0.84) for the prediction of an elevated hs-CRP level (Figure 2).
Figure 2. Receiver operating characteristic curves for short duration of sleep (≤ 5.5 h/day) in predicting elevated hs-CRP levels.
The curve of the Model 3 was equal to that of the Model 2.
In addition, the findings persisted in a secondary analysis after excluding 92 subjects who received medications for hypertension, diabetes, and for decreasing lipid levels (14 diabetes, 61 hypertension, 13 diabetes and hypertension, 2 diabetes, hypertension and elevated lipids, 1 diabetes and elevated lipids, 1 hypertension and elevated lipids) (Model 2 of Table 4). Furthermore, sleep duration was discretized into 3 categories: < 6 h/day, 6-8 h/day (normal), and > 8 h/day. There was no significantly different risk of elevated hs-CRP levels between those with a sleep duration > 8 h and those with normal sleep duration (6-8 h), but those with a duration < 6 h had significantly higher risk of elevated hs-CRP levels than those with normal sleep duration (6-8 h) (p = 0.025) based on the multiple logistic regression analysis (Model 3 of Table 4). The results of Nagelkerke's R2 and AUCs for these 3 models are listed in Table 4. All 3 logistic regression models passed the Hosmer-Lemeshow GOF test.
DISCUSSION
The risk of elevated hs-CRP was 2.2 times greater in individuals with sleep duration of 5.5 hours or less per day compared with those with a longer duration after adjustments. The results of the current study have several important implications. First, this result implies that individuals with a short duration of sleep may be at increased risk of cardiovascular events, such as myocardial infarction.15 Second, physicians may take sleep duration into account when evaluating health risks. In fact, Kim et al. had done univariate analysis on the same issue and reported similar findings in their Table 1.31 To the best of our knowledge, we might be the first research team that has found such a nonlinear relationship and the corresponding cutoff point using the GAM method when we conducted logistic regression analysis. In particular, we adjusted the effects of the other covariates to uncover the true effect of sleep duration in the GAM analysis.
Kim et al. had shown that short sleep duration (< 5 h/ night) was significantly associated with an increased risk of elevated hs-CRP levels in univariate anaylysis.31 However, we conducted regression analysis to verify this finding by partialling out the contributions of multiple covariates and we applied the method of GAM to uncover nonlinear effects of continuous covariates in regression analysis. The unique contributions of the current work are as follows: (1) As shown in the GAM plot of Figure 1, the nonlinear relationship between sleep duration and logit(P), where P = Pr(hs-CRP level was elevated), were revealed and an appropriate cutoff point was identified using the modern GAM method. (2) In addition, the effects of the other covariates on elevated hs-CRP level were adjusted to uncover the true effect of sleep duration in the GAM analysis. Haevenaar-Blom et al. reported that subjects with less than 6 hours of sleep per night had a 15% higher risk of cardiovascular disease.32 Khera reported that CRP levels were influenced significantly by adiposity and were higher in females than in males.33 And Miller et al. showed that higher CRP levels were associated with shorter sleep duration in women but not in men.34 Nevertheless, Miller et al. explained that the observed elevation in hs-CRP in the short-sleeping women might reflect confounding factors not collected.34 We had seriously examined the gender effect by considering gender itself and its interactions with some other covariates (gender × age, gender × body fat percentage, gender × waist, gender × body weight, gender × energy expenditure, and gender × short sleep duration) in our regression analysis, but did not find any statistically significant gender effect after adjusting for the effects of the other predictors in the final regression model. Our speculation about this negative finding is that it may reflect the changing roles of women in workplace, performing jobs traditionally held by men. Dowd at al. reported that longer sleep duration (> 8 h) was associated with higher CRP levels.35 However, Dowd's study collected self-reported night time sleep duration, but not afternoon naps, and it is possible that total sleep duration was longer than reported. However, our study took naps into consideration, and unlike Dowd, we found longer sleep duration (> 8 h) was not a risk for elevated hs-CRP levels. However, McDade et al. found that higher CRP levels were not associated with sleep duration.36 The effects of sleep duration on health risks might thus be nonlinear and not always shown in studies which did not apply the appropriate statistical tools.
CRP is a sensitive and stable marker of systemic inflammation, with a long half-life and no circadian variability.37 Vascular and extravascular agents of inflammation increase serum proinflammatory cytokines, which then stimulate interleukin-6 to induce hepatic production of CRP. CRP plays a direct role in local inflammation and endothelial dysfunction.38 Sleep deprivation has been shown to elevate pro-inflammatory cytokine levels including CRP. Chronic elevation in CRP levels is associated with an increased risk of adverse health outcomes, such as diabetes and heart disease.27
Other significant risk factors of this study were consistent with those in previous reports. As expected, advanced age and menopausal status were associated with elevated CRP levels.39,40 Iannuzzi et al. found that higher hs-CRP levels were associated with obstructive sleep apnea, and the effects were mediated by being overweight and obese.41 Similarly, decreasing body mass index by lifestyle interventions such as exercise and dietary restrictions was shown to decrease hs-CRP levels in a randomized study of 439 postmenopausal women.42 In a study of acute infection, CRP levels were positively correlated with heart rate and WBC count.43,44 An increased number of leukocytes in the peripheral circulation has also been shown to be associated with 36 and 64 hours of sleep deprivation.45 Other evidence has revealed that WBC count may be associated with accelerated atherosclerosis.44 A study of 3,518 subjects without clinical cardiovascular disease revealed that uric acid was positively associated with hs-CRP.46
In a systematic review, Prasad reported that cyclooxygenase inhibitors, platelet aggregation inhibitors, lipid-lowering agents, β-adrenoreceptor antagonists, vitamin E, some angiotensin converting enzyme inhibitors, some angiotensin receptor blockers, and some antidiabetic agents could decrease CRP levels.47 In our secondary analysis, we excluded patients receiving medications for diabetes, hypertension, and dyslipidemia, and similar results were obtained, i.e., short sleep duration was still a significant predictor of elevated hs-CRP levels. Based on the variables obtained from our multiple logistic regression modeling, the probability of an elevated hs-CRP level can be estimated according to the formula shown in the appendix.
Several limitations of our study deserve mention. First, habitual sleep duration was ascertained by self-report, and thus subject to recall error. Lauderdale et al. showed that subjectively reported sleep duration averaged 0.8 hours longer than measured sleep duration. They found that persons sleeping 5 and 7 hours might over report, on average, 1.3 and 0.3 hours per night more, respectively.48 On the other hand, it is much more useful to rely on subjectively reported sleep duration in clinical practice. Second, the proportion of Buddhists among the subjects was higher than in many other hospitals in Taiwan. Nevertheless, we did not find any significant association between religion and hs-CRP levels. Third, the potential confounding variables included sleep quality,24 fatty liver,31 and ulcers.49 However, the area under the ROC curve was around 0.79 (see Table 4), indicating that the logistic regression models, which included a number of important covariates, were able to fit the observed data well.
CONCLUSIONS
A significant association was observed between short duration of sleep and risk of elevated hs-CRP levels in healthy Taiwanese adults, and the association remained after adjustments for confounding variables. Since insufficient sleep is a very common problem in modern societies, its association with a greater risk of elevated hs-CRP levels, and in turn, potentially adverse impacts on metabolic and endocrine processes, has important clinical implications. Future studies will be needed to clarify the role of sleep duration as a modifiable risk factor of the effects of hs-CRP levels on health.
DISCLOSURE STATEMENT
This was not an industry supported study. The work was performed at the Buddhist Dalin Tzu Chi Hospital. Dr. Chiang received research grants from Buddhist Dalin Tzu Chi Hospital (DTCRD 101(2)-I-04). Dr. Chiang has indicated no financial conflicts of interest.
ABBREVIATIONS
- A1C
glycosylated hemoglobin
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- AUC
area under the curve
- CI
confidence interval
- eGFR
estimated glomerular filtration rate
- GAM
generalized additive model
- GOF
goodness-of-fit
- HDL-C
high-density lipoprotein cholesterol
- hs-CRP
high-sensitivity C-reactive protein
- LDL-C
low-density lipoprotein cholesterol
- OR
odds ratio
- ROC curve
receiver-operating characteristic curve
APPENDIX
Programming code in OpenOfficeCalc, Microsoft Excel, and R environment for calculating the probability of elevated high-sensitivity C-reactive protein (> 0.20 mg/dL) based on our multiple logistic regression model.
-
In OpenOfficeCalc or Microsoft Excel:
Key in the values for sleep duration (≤ 5.5 hours/day = 1, > 5.5 hours/day = 0) in the A1 cell, age (≥ 70 years old, yes = 1, no = 0) in the A2 cell, menopause (yes = 1, no = 0) in the A3 cell, heart rate (per min) in the A4 cell, white blood cell count (per 103/μL) in the A5 cell, body-mass index in the A6 cell, and uric acid levels (mg/dL) in the A7 cell. Key in the following formula in any empty cell on the same spreadsheet to obtain the estimated probability of elevated high-sensitivity C-reactive protein (> 0.20 mg/dL):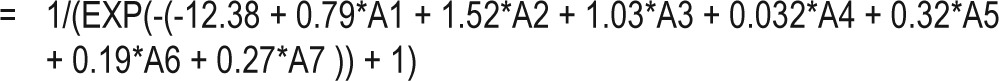
-
In an R environment:
To calculate the probability of hyperleptinemia, substitute the values for the variables X1 to X2 in the following regression equation and execute in the R console:
REFERENCES
- 1.Braga F, Panteghini M. Biologic variability of C-reactive protein: is the available information reliable? Clin Chim Acta. 2012;413:1179–83. doi: 10.1016/j.cca.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Roberts WL. CDC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: laboratory tests available to assess inflammation--performance and standardization: a background paper. Circulation. 2004;110:e572–6. doi: 10.1161/01.CIR.0000148986.52696.07. [DOI] [PubMed] [Google Scholar]
- 3.Lichtenstern C, Brenner T, Bardenheuer HJ, Weigand MA. Predictors of survival in sepsis: what is the best inflammatory marker to measure? Curr Opin Infect Dis. 2012;25:328–36. doi: 10.1097/QCO.0b013e3283522038. [DOI] [PubMed] [Google Scholar]
- 4.Colak E, Majkic-Singh N, Zoric L, Radosavljevic A, Kosanovic-Jakovic N. The role of CRP and inflammation in the pathogenesis of age-related macular degeneration. Biochem Med (Zagreb) 2012;22:39–48. doi: 10.11613/bm.2012.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allin KH, Nordestgaard BG. Elevated C-reactive protein in the diagnosis, prognosis, and cause of cancer. Crit Rev Clin Lab Sci. 2011;48:155–70. doi: 10.3109/10408363.2011.599831. [DOI] [PubMed] [Google Scholar]
- 6.Koukkunen H, Penttila K, Kemppainen A, et al. C-reactive protein, fibrinogen, interleukin-6 and tumour necrosis factor-alpha in the prognostic classification of unstable angina pectoris. Ann Med. 2001;33:37–47. doi: 10.3109/07853890109002058. [DOI] [PubMed] [Google Scholar]
- 7.Harada K, Amano T, Uetani T, et al. Association of inflammatory markers with the morphology and extent of coronary plaque as evaluated by 64-slice multidetector computed tomography in patients with stable coronary artery disease. Int J Cardiovasc Imaging. 2013;29:1149–58. doi: 10.1007/s10554-013-0181-2. [DOI] [PubMed] [Google Scholar]
- 8.Tofan F, Rahimi-Rad MH, Rasmi Y, Rahimirad S. High sensitive C-reactive protein for prediction of adverse outcome in acute exacerbation of chronic obstructive pulmonary disease. Pneumologia. 2012;61:160–2. [PubMed] [Google Scholar]
- 9.Di Napoli M, Elkind MS, Godoy DA, Singh P, Papa F, Popa-Wagner A. Role of C-reactive protein in cerebrovascular disease: a critical review. Expert Rev Cardiovasc Ther. 2011;9:1565–84. doi: 10.1586/erc.11.159. [DOI] [PubMed] [Google Scholar]
- 10.Oluleye OW, Folsom AR, Nambi V, Lutsey PL, Ballantyne CM. ARIC Study Investigators: Troponin T, B-type natriuretic peptide, C-reactive protein, and cause-specific mortality. Ann Epidemiol. 2013;23:66–73. doi: 10.1016/j.annepidem.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ridker PM. High-sensitivity C-reactive protein and cardiovascular risk: rationale for screening and primary prevention. Am J Cardiol. 2003;92:17K–22K. doi: 10.1016/s0002-9149(03)00774-4. [DOI] [PubMed] [Google Scholar]
- 12.Wang C, Yatsuya H, Tamakoshi K, et al. Positive association between high sensitivity C-reactive protein and incidence of type-2 diabetes mellitus in Japanese workers: 6-year follow-up. Diabetes Metab Res Rev. 2013;29:398–405. doi: 10.1002/dmrr.2406. [DOI] [PubMed] [Google Scholar]
- 13.Rubio-Martin E, Soriguer F, Gutierrez-Repiso C, et al. C-reactive protein and incidence of type 2 diabetes in the Pizarra study. Eur J Clin Invest. 2013;43:159–67. doi: 10.1111/eci.12027. [DOI] [PubMed] [Google Scholar]
- 14.Schnell O, Amann-Zalan I, Jelsovsky Z, et al. Changes in A1C levels are significantly associated with changes in levels of the cardiovascular risk biomarker hs-CRP: results from SteP Study. Diabetes Care. 2013;36:2084–9. doi: 10.2337/dc12-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koo M, Lai NS, Chiang JK. Short duration of sleep is associated with hyperleptinemia in Taiwanese adults. J Clin Sleep Med. 2013;15:1049–55. doi: 10.5664/jcsm.3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel SR, Ayas NT, Malhotra MR, et al. A prospective study of sleep duration and mortality risk in women. Sleep. 2004;27:440–4. doi: 10.1093/sleep/27.3.440. [DOI] [PubMed] [Google Scholar]
- 17.Chang JK, Koo M, Kao VY, Chiang JK. Association of sleep duration and insulin resistance in Taiwanese vegetarians. BMC Public Health. 2012;12:666. doi: 10.1186/1471-2458-12-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knutson KL, Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Ann N Y Acad Sci. 2008;1129:287–304. doi: 10.1196/annals.1417.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faraut B, Touchette E, Gamble H, et al. Short sleep duration and increased risk of hypertension: a primary care medicine investigation. J Hypertens. 2012;30:1354–63. doi: 10.1097/HJH.0b013e32835465e5. [DOI] [PubMed] [Google Scholar]
- 20.Cappuccio FP, Cooper D, D'Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32:1484–92. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- 21.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2011;33:585–92. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Copinschi G. Metabolic and endocrine effects of sleep deprivation. Essent Psychopharmacol. 2005;6:341–7. [PubMed] [Google Scholar]
- 23.Friedman M, Samuelson CG, Hamilton C, et al. Effect of continuous positive airway pressure on C-reactive protein levels in sleep apnea: a meta-analysis. Otolaryngol Head Neck Surg. 2012;147:423–33. doi: 10.1177/0194599812451840. [DOI] [PubMed] [Google Scholar]
- 24.Jackowska M, Kumari M, Steptoe A. Sleep and biomarkers in the English Longitudinal Study of Ageing: Associations with C-reactive protein, fibrinogen, dehydroepiandrosterone sulfate and hemoglobin. Psychoneuroendocrinology. 2013;38:1484–93. doi: 10.1016/j.psyneuen.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 25.Meier-Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–83. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 26.Chiang JK, Koo M. Lipid accumulation product: a simple and accurate index for predicting metabolic syndrome in Taiwanese people aged 50 and over. BMC Cardiovasc Disord. 2012;12:78. doi: 10.1186/1471-2261-12-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel SR, Zhu X, Storfer-Isser A, et al. Sleep duration and biomarkers of inflammation. Sleep. 2009;32:200–4. doi: 10.1093/sleep/32.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yee TW. VGAM: Vector generalized linear and additive models. R package. 2013. version 0.9-2 (URL: http://CRAN.R-project.org/package=VGAM)
- 29.Yee TW, Wild CJ. Vector generalized additive models. J R Stat Soc Series B Stat Methodol. 1996;58:481–93. [Google Scholar]
- 30.Low AF, Seow SC, Yeoh KG, Lim YT, Tan HC, Yeo TC. High-sensitivity C-reactive protein is predictive of medium-term cardiac outcome in high-risk Asian patients presenting with chest pain syndrome without myocardial infarction. Ann Acad Med Singapore. 2004;33:407–12. [PubMed] [Google Scholar]
- 31.Kim CW, Yun KE, Jung HS, et al. Sleep duration and quality in relation to non-alcoholic fatty liver disease in middle-aged workers and their spouses. J Hepatol. 2013;59:351–7. doi: 10.1016/j.jhep.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 32.Hoevenaar-Blom MP, Spijkerman AMW, Kromhout D, van den Berg JF, Monique Verschuren WM. Sleep duration and sleep quality in relation to 12-year cardiovascular disease incidence: the MORGEN study. Sleep. 2011;34:1487–92. doi: 10.5665/sleep.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khera A, Vega GL, Das SR, et al. Sex differences in the relationship between C-reactive protein and body fat. J Clin Endocrinol Metab. 2009;94:3251–8. doi: 10.1210/jc.2008-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller M, Kandala NB, Kivimaki M, et al. Gender differences in the cross-sectional relationships between sleep duration and markers of inflammation: Whitehall II study. Sleep. 2009;32:857–64. [PMC free article] [PubMed] [Google Scholar]
- 35.Dowd JB, Goldman N, Weinstein M. Sleep duration, sleep quality, and biomarkers of inflammation in a Taiwanese population. Ann Epidemiol. 2011;21:799–806. doi: 10.1016/j.annepidem.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDade TW, Hawkley LC, Cacioppo JT. Psychosocial and behavioral predictors of inflammation in middle-aged and older adults: the Chicago health, aging and social relations study. Psychosom Med. 2006;68:376–81. doi: 10.1097/01.psy.0000221371.43607.64. [DOI] [PubMed] [Google Scholar]
- 37.Verma S, Szmitko PE, Yeh ETH. C-reactive protein: structure affects function. Circulation. 2004;109:1914–7. doi: 10.1161/01.CIR.0000127085.32999.64. [DOI] [PubMed] [Google Scholar]
- 38.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–54. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 39.Bruunsgaard H, Skinhoj P, Pedersen AN, Schroll M, Pedersen BK. Ageing, tumour necrosis factor-alpha (TNF-alpha) and atherosclerosis. Clin Exp Immunol. 2000;121:255–60. doi: 10.1046/j.1365-2249.2000.01281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woodard GA, Mehta VG, Mackey RH, et al. C-reactive protein is associated with aortic stiffness in a cohort of African American and white women transitioning through menopause. Menopause. 2011;18:1291–7. doi: 10.1097/gme.0b013e31821f81c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iannuzzi A, Licenziati MR, De Michele F, et al. C-reactive protein and carotid intima-media thickness in children with sleep disordered breathing. J Clin Sleep Med. 2013;9:493–8. doi: 10.5664/jcsm.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Imayama I, Ulrich CM, Alfano CM, et al. Effects of a caloric restriction weight loss diet and exercise on inflammatory biomarkers in overweight/ obese postmenopausal women: a randomized controlled trial. Cancer Res. 2012;72:2314–26. doi: 10.1158/0008-5472.CAN-11-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khoueiry Z, Roubille C, Nagot N, et al. Could heart rate play a role in pericardial inflammation? Med Hypotheses. 2012;79:512–5. doi: 10.1016/j.mehy.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 44.Albert MA, Ridker PM. Inflammatory biomarkers in African Americans: a potential link to accelerated atherosclerosis. Rev Cardiovasc Med. 2004;5(Suppl):S22–7. [PubMed] [Google Scholar]
- 45.Dinges DF, Douglas SD, Zaugg L, et al. Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. J Clin Invest. 1994;93:1930–9. doi: 10.1172/JCI117184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keenan T, Blaha MJ, Nasir K, et al. Relation of uric acid to serum levels of high-sensitivity C-reactive protein, triglycerides, and high-density lipoprotein cholesterol and to hepatic steatosis. Am J Cardiol. 2012;110:1787–92. doi: 10.1016/j.amjcard.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prasad K. C-reactive protein (CRP)-lowering agents. Cardiovasc Drug Rev. 2006;24:33–50. doi: 10.1111/j.1527-3466.2006.00033.x. [DOI] [PubMed] [Google Scholar]
- 48.Lauderdale DS, Knutson KL, Rathouz PJ, Yan LL, Hulley SB, Liu K. Sleep duration: how well do self-reports reflect objective measure? The CARDIA sleep study. Epidemiology. 2008;19:838–45. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jafarzadeh A, Hassanshahi GH, Nemati M. Serum levels of high-sensitivity C-reactive protein (hs-CRP) in Helicobacter pylori-infected peptic ulcer patients and its association with bacterial CagA virulence factor. Dig Dis Sci. 2009;54:2612–6. doi: 10.1007/s10620-008-0686-z. [DOI] [PubMed] [Google Scholar]


