Abstract
Study Objectives:
We aimed to validate the diagnostic accuracy and night-to-night variability of a simple 3-channel (type IV monitor) portable sleep monitor, ApneaLink (AL), in a population of morbidly obese subjects.
Design:
Cross-sectional validation and diagnostic accuracy study.
Setting:
Public tertiary care obesity center in Norway.
Participants:
A total of 105 (67 females) treatment seeking morbidly obese subjects were included, mean (SD) age 44.3 (11.4) years and BMI 43.6 (5.6) kg/m2.
Interventions:
The patients underwent two successive nights of recordings; the first night with the AL only, and the following night with both the reference instrument Embletta (E), a type III portable somnograph, and the AL.
Measurements and Results:
Main outcomes were diagnostic accuracy of AL as assessed by sensitivity, specificity and area under ROC curves, and level of agreement between AL and E. AL had high diagnostic accuracy at all levels of OSA, and the Bland-Altman plots showed good agreement between AL and E. The sensitivity and specificity of the instrument were 93% and 71% at the AHI cutoff 5 events/h, and 94% and 94% at the AHI cutoff 15, respectively. The night-to-night variability was low.
Conclusion:
Our results indicate that a simple 3-channel portable sleep monitor (ApneaLink) has a high diagnostic accuracy in diagnosing OSA in morbidly obese treatment seeking patients. Accordingly, this and similar instruments might help non-specialists to diagnose OSA in morbidly obese patients, and, importantly, help non-specialists to refer patients who need specific treatment to specialist without unnecessary delay.
Citation:
Fredheim JM, Røislien J, Hjelmesæth J. Validation of a portable monitor for the diagnosis of obstructive sleep apnea in morbidly obese patients. J Clin Sleep Med 2014;10(7):751-757.
Keywords: obstructive sleep apnea, validation, portable somnograph
Obstructive sleep apnea (OSA) is caused by the collapse of the upper airways during sleep and is the most prevalent sleep disordered breathing problem. OSA affects 2% to 26% of the general population depending on gender, age, body weight, and diagnostic criteria.1–3 In obese patients, narrowing of the upper airways and shortening of the trachea due to fat deposits increase upper airway collapsibility. Accordingly, the prevalence of OSA is particularly high in morbidly obese patients.4,5 Diabetes, hypertension, heart failure, cardiovascular disease, cerebrovascular disease, and traffic accidents are among the most frequent complications of OSA.6–11 Despite this high prevalence of comorbidities and severe complications many obese persons with OSA remain undiagnosed. Accordingly, the need for diagnostic sleep studies among morbidly obese subjects is particularly high.
The gold standard for diagnosing OSA is overnight polysomnography. This method is, however, time-consuming and expensive, and is only offered by a limited numbers of clinics. By contrast, several clinics use less complicated portable devices (e.g., Embletta) to diagnose OSA, and these have been shown to have a high diagnostic accuracy.12 However, even the use and interpretation of the Embletta system requires specialist assessment and may be too complicated to implement in primary care.
BRIEF SUMMARY
Current Knowledge/Study Rationale: The prevalence of obstructive sleep apnea in morbidly obese subjects is high, and overnight polysomnography is a resource demanding diagnostic method. This study validates a simple portable sleep monitor in a population of treatment seeking morbidly obese patients.
Study Impact: The results of this study might help non-specialists to diagnose obstructive sleep apnea in morbidly obese patients and refer patients for further sleep evaluation or treatment. Accordingly, a higher number of morbidly obese with obstructive sleep apnea might be diagnosed and treated appropriately.
As 60% to 90% of OSA patients have a body mass index (BMI) > 28 kg/m2, it is of importance to validate less complicated screening devices in obese subjects.13 The aim of this study was to validate the diagnostic accuracy of automated scoring in a simple 3-channel (type IV monitor) portable sleep monitor, ApneaLink (AL), against the manual scoring of the reference device, Embletta (E), in a population of morbidly obese subjects known to have a particularly high prevalence of OSA.5 We also wanted to analyze the night-to-night variability of OSA as measured by AL.
METHODS
Subjects
Consecutive treatment-seeking morbidly obese adults (BMI ≥ 40 kg/m2, or BMI ≥ 35 and < 40 kg/m2 with at least one obesity related comorbidity) were recruited between March 2009 and February 2011 from the Morbid Obesity Centre, a tertiary-care center in the southern part of Norway. The sleep studies were performed at the Otolaryngology Department's sleep center located in the same hospital as the Morbid Obesity Center. Informed consent was obtained from the study participants. The study protocol was approved by the regional ethics committee and all patients provided written informed consent. The exclusion criteria were immobile patients and inability to equip oneself with the recording devices.
Obstructive Sleep Apnea – Definition
OSA was defined as having ≥ 5 apneas or hypopneas, each event lasting ≥ 10 sec per hour during sleep.14 This was measured by the apnea-hypopnea index (AHI). OSA was categorized into severity levels of mild (AHI 5-14.9 events/h), moderate (15-29.9 events/h), and severe (≥ 30 events/h).
Design
Cross-sectional validation and diagnostic accuracy study. ApneaLink, a type IV portable somnograph with 3 channels, was validated against the reference instrument, the Embletta, a type II portable somnograph with 9 channels.12 In our study we utilized 5 of the available Embletta channels, so for practical purposes the Embletta functioned as a type III monitor. To validate AL and to test the night-to-night variability of AL, patients underwent 2 successive nights of recordings; the first night with the ApneaLink (AL1), and the following night with both the Embletta (E) and the ApneaLink (AL2). Both recordings were performed in the participants′ homes. Upon reception of the ApneaLink device, patients were also given written instructions of use, though no oral information was given at this stage. Prior to the second night of recording the E equipment setup was performed by a trained study nurse. The patients were all examined by the same otolaryngologist (the first author JMF) the day after the last sleep study. At this consultation the E recordings were scored (the scorer was blinded to the AL results), proper treatment decided and necessary information about the condition and different treatment options given, all in accordance with the recommendations of the American Academy of Sleep Medicine 2009 Adult Obstructive Sleep Apnea Task Force.15
Device Description
The ApneaLink is a 3-channel recorder and measures nasal airflow, pulse, and oxygen saturation. The recorder is worn around the chest and a nasal cannula and pulse oximetry is attached to it. The internal memory and battery allow approximately 10 h of recording. Accompanying software analyzes the recording and presents the data in a one-page report. The AHI is calculated based on total study time. It is possible to manually score the data, but the automatic report was the basis of our investigations. In our analyses we used the automatic scoring algorithm to explore whether a non-sleep specialist can trust the automatic report produced by the device. The default software settings were used. These defined an apnea as a reduction of nasal flow ≥ 80% of baseline lasting ≥ 10 seconds. Hypopneas were defined as a decrease in nasal flow by 50% to 80% of baseline for ≥ 10 sec plus oxygen desaturation ≥ 3%. The maximum possible duration of an apnea was 80 sec and the maximum possible duration of a hypopnea was 100 seconds. If apneas or hypopneas lasted longer than this, it was automatically interpreted by the software as an artifact.
The Embletta is a multi-channel recorder consisting of a nasal cannula, two piezoelectric belts, a finger pulse oximeter, and detection of body position. The nasal cannula monitors nasal airflow. The 2 piezoelectric belts are placed around the thorax and abdomen and monitor respiratory movement. All recordings from the Embletta were manually scored by the same person (JMF) to avoid inter-individual variations. Scoring rules were in accordance with the 2007 American Academy of Sleep Medicine manual for sleep scoring.14 An apnea was defined as a reduction of ≥ 90% of baseline nasal airflow lasting ≥ 10 seconds. Hypopneas were defined as a decrease in nasal flow of 50%-90% of baseline lasting ≥ 10 sec accompanied by an oxygen desaturation ≥ 3%.
Variables/Measurements/Presentation
The apnea-hypopnea index (AHI) is the number of apneas or hypopneas per hour, and is used to measure the severity of OSA. The oxygen desaturation index (ODI) is a measure of how many desaturations patients have per hour of sleep. ODI is based on the peripheral oxygen saturation (pO2), which is continuously monitored throughout the night. All patients completed the Epworth Sleepiness Scale which measures daytime sleepiness on a scale from 0-24. Patients with scores > 11 were considered as having excessive daytime sleepiness.
Statistical Analysis
Bland-Altman plots with corresponding 95% limits of agreement (LoA) were used to assess agreement between the E and AL results and agreement between the two nights of AL recordings.16 The traditional Bland-Altman plot assumes that variance in the measurements is constant across the observed range. For data where the variance is proportional to the measured value, the limits of agreement will have to be correspondingly wider for higher measurement values than for lower.17 We applied the log transform to the data for calculation of LoA, with back transformation to original scale for interpretation, resulting in diagonal LoA lines. Sensitivity and specificity for diagnosing various categories of OSA (E as reference instrument) were calculated for different AHI values from the ApneaLink studies. Receiver operating characteristics curves (ROC curves) with corresponding area under the curve (AUC) were constructed to evaluate the diagnostic accuracy and to illustrate the true positive rate (sensitivity) against the false positive rate (1-specificity) at various AHI cutoff values (5, 15, and 30). Data were analyzed by the Statistical Package of the Social Science (SPSS) for Windows, version 17 (SPSS, Chicago, IL, USA) and R 3.0.1.18
RESULTS
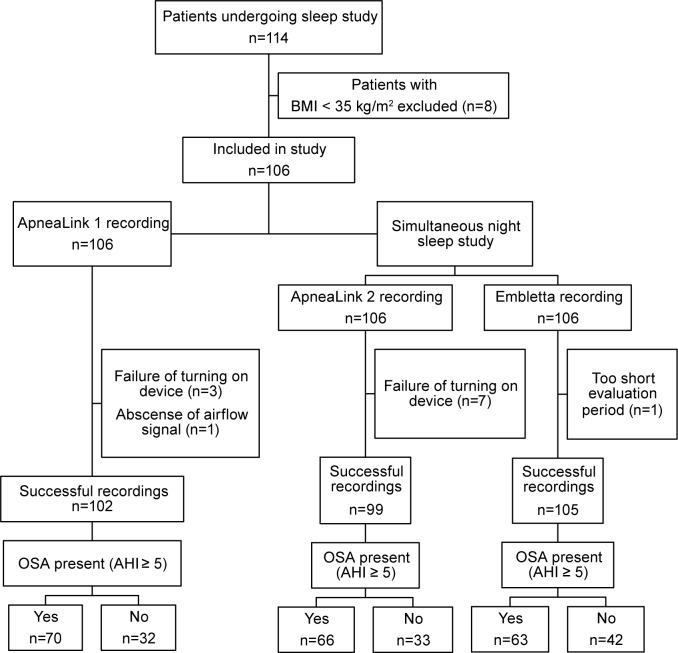
A total of 114 consecutive severely obese patients were eligible for inclusion. After exclusion of 8 patients with BMI < 35 kg/m2, 106 patients were included in the sleep study (Figure 1). Except for one patient with too short evaluation time (< 3 h), all study participants successfully underwent sleep studies with Embletta, leaving 105 subjects, 64% (n = 67) females, eligible for analysis. Four of the AL1 recordings and 7 of the AL2 recordings could not be interpreted because of insufficient data (failure to turn on the device properly (AL2/AL1 n = 7/3) or total absence of airflow signal (AL2/AL1 n = 0/1). All patients had at least one successful night of AL recording.
Figure 1. Flow chart of 114 eligible morbidly obese patients who underwent sleep studies with ApneaLink and Embletta.
A total of 63 patients (60%) had OSA as diagnosed by the reference instrument (E). The prevalence of various categories of OSA and demographic characteristics according to gender are shown in Table 1. A total of 29 subjects had mild OSA, 12 subjects had moderate OSA, and 22 severe OSA. Men had a significantly higher prevalence and severity of OSA than women. No patients had Cheyne-Stokes respiration.
Table 1.
Demographic characteristics and apnea-hypopnea index values, based on the reference instrument (Embletta) of 105 morbidly obese subjects
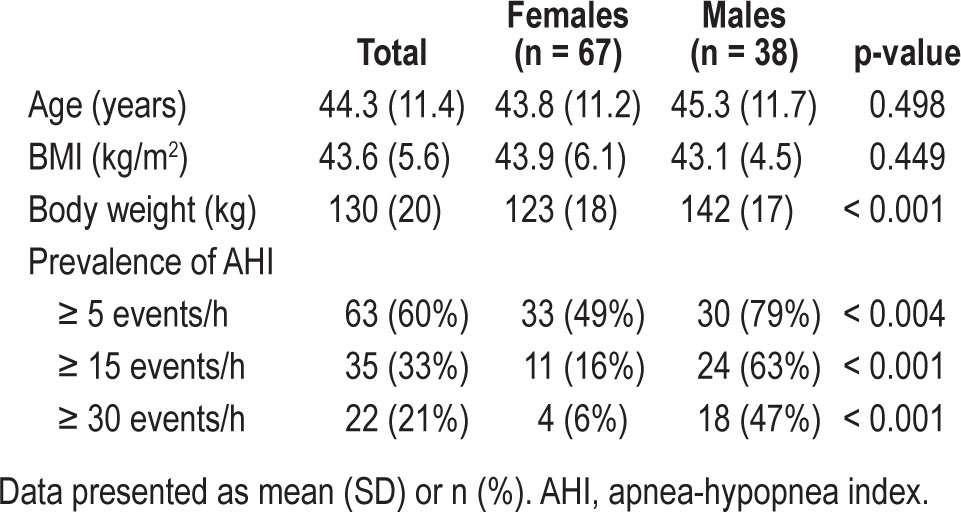
Table S1 (supplemental material) shows various sleep characteristics from the sleep studies (E, AL1, and AL2). The mean AHI, oxygen desaturation index (ODI), and peripheral oxygen saturation (pO2) did not differ significantly among the 3 measurements. Mean (SD) Epworth sleepiness score was 10.5 (4.8) with a range from 0 to 23.
Agreement between AL and E AHI Values
AHI values for AL2 and E and corresponding Bland-Altman plot with diagonal LoA are shown in Figure 2. The mean difference between the measurements was close to zero, indicating little systematic bias between the 2 pieces of apparatus. LoA were narrow for small AHI values, and overall the plot shows good agreement between the 2 methods. Figure 2C shows a zoomed in area of Figure 2B including AHI values especially important for the clinical diagnosis of OSA (cutoff level 5 events/h), illustrating that the AL had a tendency to slightly overestimate AHI at smaller values (Figure 2C).
Figure 2.
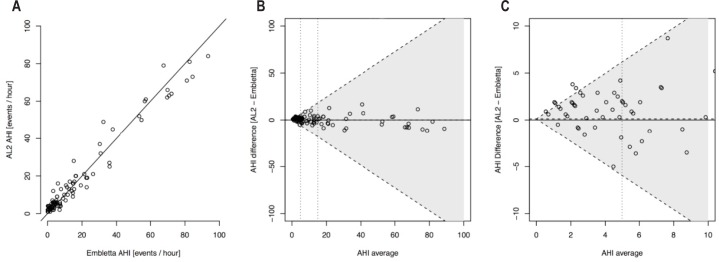
(A) Scatterplots of AHI values from ApneaLink (AL2) vs. Embletta. (B) Bland-Altman agreement plots for AHI measured by Embletta and ApneaLink (AL2) with diagonal 95% limits of agreement. The horizontal dotted line represents the mean AHI difference. (C) A zoomed-in area of Figure 2B showing Bland-Altman agreement plots for AHI measured by Embletta and ApneaLink (AL2) with diagonal 95% limits of agreement. The horizontal dotted line represents the mean AHI difference.
The Bland-Altman plots in Figure 3 show good agreement between AHI values for AL1 and AL2 and a mean difference between methods close to zero.
Figure 3.
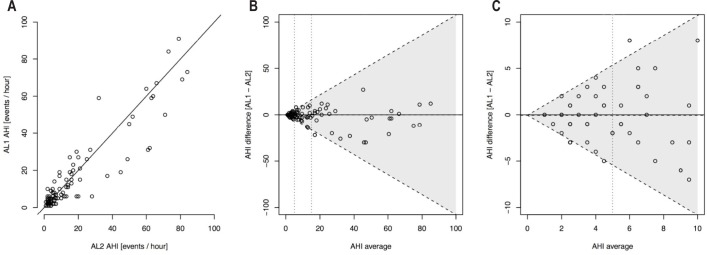
(A) Scatterplots of AHI values from the two ApneaLink recordings; AL1 vs. AL2. (B) Bland-Altman agreement plot for AHI measured by two consecutive nights of ApneaLink (AL1 and AL2) with diagonal 95% limits of agreement. The horizontal dotted line represents the mean AHI difference. (C) A zoomed-in area of Figure 3B showing Bland-Altman agreement plot for AHI measured by two consecutive nights of ApneaLink (AL1 and AL2) with diagonal 95% limits of agreement. The horizontal dotted line represents the mean AHI difference.
Diagnostic Accuracy of AL
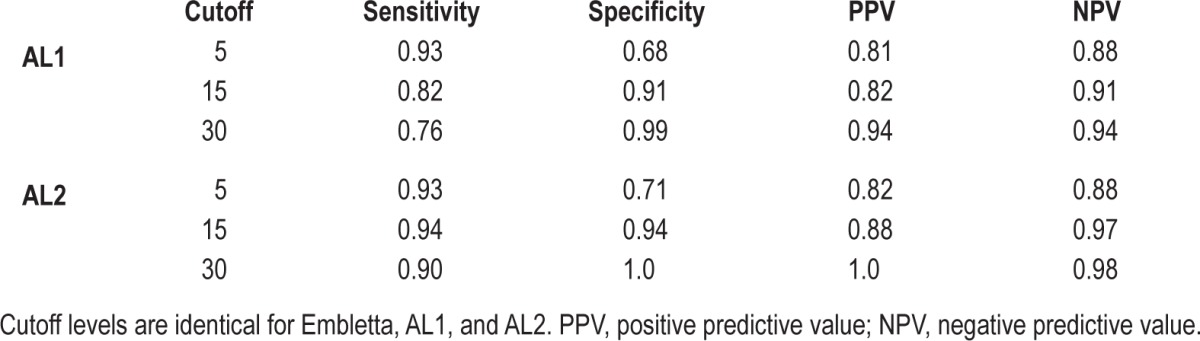
Sensitivity, specificity, positive predictive value, and negative predictive value from AL1 and AL2 at various cutoff values of AHI are presented in Table 2. The AHI cutoff values used in Table 2 were equal for E, AL1, and AL2. The AL2 recordings misclassified 12 non-OSA patients (E-AHI < 5) to have OSA, all of which were categorized as having mild OSA. Further, 4 OSA patients (E-AHI ≥ 5) were misclassified as non-OSA. All patients with severe OSA (AHI ≥ 30) were identified by the AL.
Table 2.
Diagnostic accuracy of both ApneaLink recordings at various AHI cutoff levels.
Figures 4A-4C demonstrate that the diagnostic accuracy of AL was high at all levels of OSA (mild, moderate, severe, all AUC > 0.94). The respective sensitivity and specificity values are presented in Table S2 (supplemental material).
Figure 4.
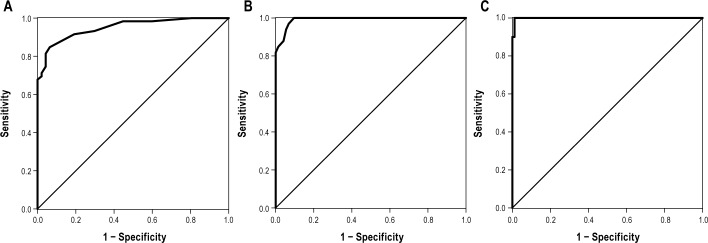
(A) ROC curve with Embletta AHI cutoff value of 5 events/h for AL2 with a corresponding area under the curve (AUC) of 0.950. (B) ROC curve with Embletta AHI cutoff value of 15 events/h for AL2 with a corresponding area under the curve (AUC) of 0.992. (C) ROC curve with Embletta AHI cutoff value of 30 events/h for AL2 with a corresponding area under the curve (AUC) of 0.999.
DISCUSSION
This study demonstrates that a simple 3 channel (type IV monitor) portable sleep monitor (AL) may be used for the screening and diagnosis of OSA in treatment seeking morbidly obese patients. Using the somnograph E as reference, the AL had a high diagnostic accuracy in the screening of patients with mild, moderate, or severe OSA. The night-to-night variability between the AL recordings was negligible (close to zero).
The high sensitivity (93%) of the AL to diagnose OSA (AHI ≥ 5 events/h), and the relatively high predictive value of a positive test (81%) may have important clinical implications. First, in patients diagnosed with mild sleep apnea, this diagnosis may motivate patients to weight loss or prevent further weight gain, and thereby prevent deterioration of, or reduce the severity of sleep apnea. Second, given the particularly high diagnostic accuracy in identifying patients with moderate or severe sleep apnea (AHI ≥ 15 events/h), patients in need of treatment with continuous positive airway pressure (CPAP) might be diagnosed by their primary care physician and referred directly to a specialist to initiate CPAP treatment without unnecessary delay.
Our finding of a high diagnostic sensitivity of the AL to identify subjects with OSA is in accordance with previous validation studies in normal weight to overweight populations and in adolescents.19–21 Importantly, we report a somewhat higher specificity than previous studies, and the high predictive value of a positive test might be useful for the primary care physicians in terms of better tailoring future treatment of severely obese patients diagnosed with OSA.
The high sensitivity of the AL to diagnose patients with moderate to severe OSA is in accordance with most previous studies from obese and non-obese populations.19,22,23 The specificity regarding the diagnosis of moderate or severe OSA was, however, higher in our study than those reported by others, and the predictive value of a positive test was higher than or equal to others. In addition, our validation study of AL with three channels (airflow, oximetry, and pulse) showed better sensitivity, specificity, and positive predictive value than previous studies in moderately obese subjects of AL with only one channel.24,25
While a specificity of 71% in the diagnosis of OSA might be considered low, it probably has few negative clinical implications. Most false positive recordings with the AL (AHI ≥ 5) showed AHI values only marginally higher than the E, and no patients without OSA were diagnosed as having moderate or severe OSA. This is in accordance with the Bland-Altman plot in Figure 2, where AL shows a slight tendency of overestimating AHI compared to E at lower values, but not for higher AHI values. Nevertheless, in cases where the diagnosis was in doubt, it is still recommended that the measurement be repeated or a more sophisticated method utilized (e.g., a type II monitor). Importantly, to initiate CPAP treatment of those diagnosed with mild OSA, the patient should have symptoms of OSA, in addition to an AHI ≥ 5 events/hour.15
According to guidelines for the treatment of adult sleep apnea, all patients with AHI ≥ 15 events/h should be offered treatment with CPAP or other treatment (e.g. mandibular advancement device, surgery, or lifestyle modification) if CPAP is declined or otherwise not suitable.15 Our results indicate that it might be safe and appropriate to initiate OSA treatment in treatment seeking morbidly obese patients if the AL shows an AHI ≥ 15. Importantly, patients with obesity hypoventilation syndrome, Cheyne-Stokes respiration, or central apneas should be referred to a specialist. If the patient has a negative test, but clinical symptoms, the test might be repeated.
This study has a number of limitations. The AL provides nasal airflow, oxygen saturation, and pulse information, but unlike the gold standard PSG, data related to sleep stages and positioning are not recorded by AL. Accordingly, it is not possible to define exact sleep time. We have not analyzed the prevalence of central apneas in this study. In addition, the generalizability of our results is limited by our assessment of treatment-seeking white morbidly obese patients only.
Our study is strengthened by the inclusion of morbidly obese subjects in which AL previously has not been validated. In addition, recordings from both devices were performed in the patients′ homes and not in a foreign environment like a sleep lab.
Advantages of AL and other portable monitors over full PSG include cost-effectiveness and the fact that more patients might be diagnosed early, leading to substantial economical savings through the prevention of morbidity, accidents, and absenteeism/presenteeism.26 Compared to more elaborate equipment, the AL is practical to use, easy to interpret, and its size and simplicity helps retain a more natural sleep and facilitates usage by non-specialists.
In contrast to the 2007 report from the portable monitoring task force, which suggests a minimum of four channels on portable monitors,27 our findings suggest that the three-channel AL may be sufficient to diagnose OSA in morbidly obese patients. Based on our results, we argue that morbidly obese patients with an AL-recorded AHI ≥ 15 events/h may be referred directly to a specialist for CPAP treatment.
CONCLUSIONS
The results of the present study indicate that a simple three-channel (type IV monitor) portable sleep monitor (Apnea-Link) may help non-specialists to screen and diagnose OSA in morbidly obese patients.
DISCLOSURE STATEMENT
Dr. Fredheim has received an unrestricted grant from ResMed Norway. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the patients for their participation and the nurses Marit Skiaker and Astrid Hillestad for their effort in the investigation.
SUPPLEMENTAL MATERIAL
Sleep characteristics of morbidly obese subjects
Sensitivity and specificity at different ApneaLink 2 AHI values for Embletta AHI cutoff values of 5, 15, and 30 events/hour
REFERENCES
- 1.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 2.Kryger MH. Diagnosis and management of sleep apnea syndrome. Clin Cornerstone. 2000;2:39–47. doi: 10.1016/s1098-3597(00)90039-5. [DOI] [PubMed] [Google Scholar]
- 3.Hrubos-Strom H, Randby A, Namtvedt SK, et al. A Norwegian population-based study on the risk and prevalence of obstructive sleep apnea. The Akershus Sleep Apnea Project (ASAP) J Sleep Res. 2011;20:162–70. doi: 10.1111/j.1365-2869.2010.00861.x. [DOI] [PubMed] [Google Scholar]
- 4.Isono S. Obesity and obstructive sleep apnoea: mechanisms for increased collapsibility of the passive pharyngeal airway. Respirology. 2012;17:32–42. doi: 10.1111/j.1440-1843.2011.02093.x. [DOI] [PubMed] [Google Scholar]
- 5.Fredheim JM, Rollheim J, Omland T, et al. Type 2 diabetes and pre-diabetes are associated with obstructive sleep apnea in extremely obese subjects: a cross-sectional study. Cardiovasc Diabetol. 2011;10:84. doi: 10.1186/1475-2840-10-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165:670–6. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 7.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 8.Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med. 1999;160:1101–6. doi: 10.1164/ajrccm.160.4.9903020. [DOI] [PubMed] [Google Scholar]
- 9.Hamilton GS, Solin P, Naughton MT. Obstructive sleep apnoea and cardiovascular disease. Intern Med J. 2004;34:420–6. doi: 10.1111/j.1445-5994.2004.00596.x. [DOI] [PubMed] [Google Scholar]
- 10.Bassetti C, Aldrich MS. Sleep apnea in acute cerebrovascular diseases: final report on 128 patients. Sleep. 1999;22:217–23. doi: 10.1093/sleep/22.2.217. [DOI] [PubMed] [Google Scholar]
- 11.Smolensky MH, Di ML, Ohayon MM, Philip P. Sleep disorders, medical conditions, and road accident risk. Accid Anal Prev. 2011;43:533–48. doi: 10.1016/j.aap.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Ng SS, Chan TO, To KW, et al. Validation of Embletta portable diagnostic system for identifying patients with suspected obstructive sleep apnoea syndrome (OSAS) Respirology. 2010;15:336–42. doi: 10.1111/j.1440-1843.2009.01697.x. [DOI] [PubMed] [Google Scholar]
- 13.Nerfeldt P, Nilsson BY, Mayor L, Udden J, Friberg D. A two-year weight reduction program in obese sleep apnea patients. J Clin Sleep Med. 2010;6:479–86. [PMC free article] [PubMed] [Google Scholar]
- 14.Iber C, Ancoli-Israel S, Chesson A, Quan SF. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. [Google Scholar]
- 15.Epstein LJ, Kristo D, Strollo PJ, Jr., et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–76. [PMC free article] [PubMed] [Google Scholar]
- 16.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- 17.Bland JM, Altman DG. Measurement error proportional to the mean. BMJ. 1996;313:106. doi: 10.1136/bmj.313.7049.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.R Core Team. R: A Language and Environment for Statistical Computing. 2013.
- 19.Ragette R, Wang Y, Weinreich G, Teschler H. Diagnostic performance of single airflow channel recording (ApneaLink) in home diagnosis of sleep apnea. Sleep Breath. 2010;14:109–14. doi: 10.1007/s11325-009-0290-2. [DOI] [PubMed] [Google Scholar]
- 20.Ng SS, Chan TO, To KW, et al. Validation of a portable recording device (ApneaLink) for identifying patients with suspected obstructive sleep apnoea syndrome. Intern Med J. 2009;39:757–62. doi: 10.1111/j.1445-5994.2008.01827.x. [DOI] [PubMed] [Google Scholar]
- 21.Lesser DJ, Haddad GG, Bush RA, Pian MS. The utility of a portable recording device for screening of obstructive sleep apnea in obese adolescents. J Clin Sleep Med. 2012;8:271–7. doi: 10.5664/jcsm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nigro CA, Dibur E, Aimaretti S, Gonzalez S, Rhodius E. Comparison of the automatic analysis versus the manual scoring from ApneaLink device for the diagnosis of obstructive sleep apnoea syndrome. Sleep Breath. 2011;15:679–86. doi: 10.1007/s11325-010-0421-9. [DOI] [PubMed] [Google Scholar]
- 23.Gantner D, Ge JY, Li LH, et al. Diagnostic accuracy of a questionnaire and simple home monitoring device in detecting obstructive sleep apnoea in a Chinese population at high cardiovascular risk. Respirology. 2010;15:952–60. doi: 10.1111/j.1440-1843.2010.01797.x. [DOI] [PubMed] [Google Scholar]
- 24.Oktay B, Rice TB, Atwood CW, Jr, et al. Evaluation of a single-channel portable monitor for the diagnosis of obstructive sleep apnea. J Clin Sleep Med. 2011;7:384–90. doi: 10.5664/JCSM.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erman MK, Stewart D, Einhorn D, Gordon N, Casal E. Validation of the ApneaLink for the screening of sleep apnea: a novel and simple single-channel recording device. J Clin Sleep Med. 2007;3:387–92. [PMC free article] [PubMed] [Google Scholar]
- 26.Swanson LM, Arnedt JT, Rosekind MR, Belenky G, Balkin TJ, Drake C. Sleep disorders and work performance: findings from the 2008 National Sleep Foundation Sleep in America poll. J Sleep Res. 2011;20:487–94. doi: 10.1111/j.1365-2869.2010.00890.x. [DOI] [PubMed] [Google Scholar]
- 27.Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3:737–47. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sleep characteristics of morbidly obese subjects
Sensitivity and specificity at different ApneaLink 2 AHI values for Embletta AHI cutoff values of 5, 15, and 30 events/hour