Abstract
The incidence of nontuberculous mycobacteria (NTM) has increased over the last decades. Elderly people are more susceptible to NTM and experience increased morbidities. NTM incidence is expected to rise due to an increasing elderly population at least up to 2050. Given the importance of NTM infection in the elderly, an increasing interest exists in studying NTM characteristics in aged population. In this review, we summarize the characteristics of NTM infection among elderly patients. We focus on epidemiology, clinical presentation, and treatment options of NTM in this age group. We highlight the differences in the diagnosis and treatment between rapid and slow growing mycobacterial infections. The current recommendation for treatment of NTM is discussed. We debate if in vitro susceptibility testing has a role in treatment of NTM. Drug-drug interaction between antibiotics used to treat NTM and other medications, particularly warfarin, is another important issue that we discuss. Finally, we review the prognosis of NTM disease in elderly patients.
Keywords: Nontuberculous mycobacterium, NTM, Elderly, Treatment
Case report
A 70 year-old woman was referred to our Bronchiectasis Clinic for chronic cough. Her past medical history was significant for 30 pack-year smoking and measles without pneumonia in childhood. She stated that for the last nine months she had a productive cough and chest pain. The cough had gradually progressed along with yellow, brown or blood tinged sputum. She first noticed hemoptysis 8 months prior and the last hemoptysis episode was a few weeks ago, but never expectorated gross blood. She received a course of levofloxacin with some temporary improvement in symptoms.
The chest pain was localized to midsternum and described as stabbing, burning, and heavy pressure feeling. It improved with sleep and worsened by singing, smoke exposure, coughing, eating oily food, sitting and standing. While she noted awakening due to coughing, she did not note a relation to position.
She had no dyspnea, orthopnea, paroxysmal nocturnal dyspnea, fever, chills, night sweats, weight loss as well as history of environmental or drug allergies asthma, tuberculosis (TB), diabetes, gastroesophageal reflux disease, seizure, HIV risks (including drugs, multiple sexual partners and transfusions) and exposure to TB patient, silica or significant asbestos. She worked in office jobs in the past.
Past medical history included measles, a throat abscess at age 12, tonsillectomy at age 13 and hemorrhagic gastritis 8 years ago. She had kidney stones that were removed. Family history was significant for emphysema and chronic bronchitis. She was taking low dose aspirin for Heart disease prevention and naproxen for joints pain.
Review of systems uncovered lightheadedness with palpitation, early satiety, and posterior neck pain.
On physical examination, the patient was a well-appearing, white woman in no distress. Her weight was 70.9 kg. Her vital signs included pulse 105/minute, blood pressure 122/78 mm Hg, and temperature 36.9C. Her oxygen saturation was 94% at room. The lungs had crackles at the bases but these improved with repeated breathing. She also had scant wheezing in the right anterior lower chest. The point of maximum impulse of the heart was not palpated. S1 and S2 were normal. She had a midsystolic click and I–II/VI systolic murmur. There was no gallop. The rest of physical examination was within normal range of limits.
The chest images showed many nodules, right middle lobe bronchiectasis, upper and lower lung circular opacities, suggesting bronchial and bronchiolar cuffing and lucencies that indicated cystic bronchiectasis. She also had mild scoliosis. (Images 1 and 2). Laboratory tests showed normal CBC and differentiation, total serum levels of immunoglobulins (Ig) E, A and G, blood chemistries and lipid profile. ECG was within normal limits.
Image 1.
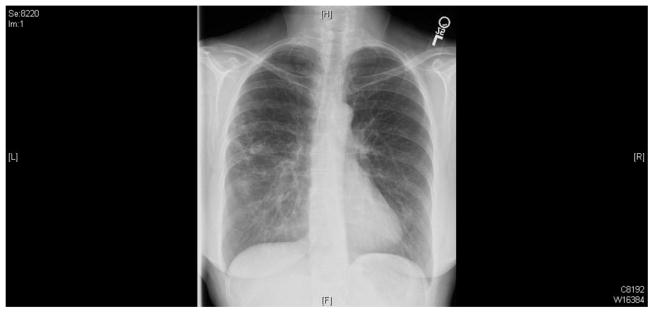
The chest X-ray shows diffuse interstitial fibrotic-type opacities throughout both lungs. There are ill-defined somewhat nodular appearing densities with a hint of cavitation in them on the right side, particularly in the apical segment of the lower lobe. Mild scoliosis is notable.
Image 3.
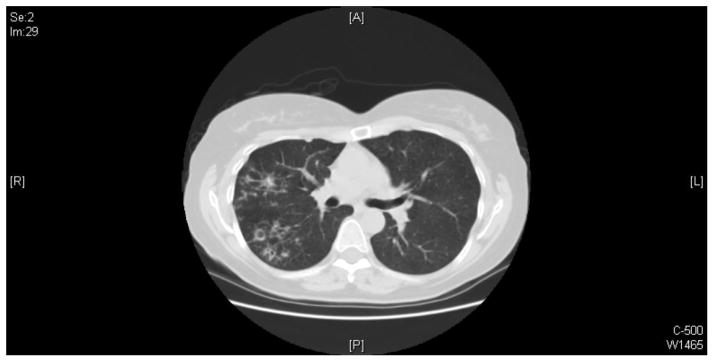
The representative image of chest CT scan shows that multiple small airways opacities are scattered in the right upper lobe, superior right lower lobe, and right middle lobe. Small areas of bronchiectasis are present in the right upper lobe. The largest area of cystic bronchiectasis/small cavity formation measures approximately 1 centimeter and it is present in the posterior right upper lobe.
Three sequential sputum specimens were sent for AFB smear, culture and drug susceptibility testing. Two days later, AFB smears reported positive and PCR test showed negative for M. tuberculous complex. From two sputum cultures Mycobacterium avium was isolated later. In vitro antibiotics susceptibility testing showed the M. avium isolate was sensitive to clarithromycin, ciprofloxacin, moxifloxacin, rifabutin, rifamycin, clofazimine and ethambutol, but resistant to cycloserine and amikacin, and intermediate to streptomycin and kanamycin.
Given clinical symptoms, chest x-ray and CT-scan results, and two positive sputum specimens for M. avium, pulmonary nontuberculous mycobacteria (NTM) was diagnosed and treatment was started with clarithromycin 500 milligram (mg) twice per day, rifampicin 600 mg once a day and ethambutol 1200 mg once a day. Her cough and chest pain were gradually improved. The sputum cultures for AFB were obtained monthly until sputum conversion. Her sputum cultures converted negative within 4 months after initiating antibiotics therapy. She was categorized as cured when completed 12 months treatment after sputum conversion. She was closely observed and no evidence of relapse was detected up to 2 years follow up.
Introduction
Nontuberculous mycobacteria have been recognized as human pathogens since the 1950, and to date, over 150 species of Mycobacterium have been identified [1–3]. Table 1 shows the most common NTM that cause infection in the elderly. They represent a diverse group of environmental organisms that can be isolated from water sources, soil, animals, and food [4, 5]. Human NTM infection is mainly acquired from environmental exposures [6, 7], although potential human-to-human transmission was recently suggested. [8] The NTM incidence has been increasing in the last decades. HIV was responsible for this increase from 1980s to 1990s. Afterward, the increase has mainly been in women without any of the classic risk factors. Although the exact cause is unclear, it may be a result of the improved methods of NTM detection, as well as growth of the elderly population. [9–12]
Table 1.
Most common NTM causing infections in elderly
| Slow-Growing Mycobacteria (SGM) | Rapid-Growing Mycobacteria (RGM) |
|---|---|
| M. avium complex | M. abscessus |
| M. Kansasii | M. chelonae |
| M. xenopi | M. fortuitum |
| M. simiae | M. marinum |
| M. malmoense | |
| M. szulgai |
Similar to the rest of the world, both the United States (US) and Europe are facing with an aging population and it is estimated that the number of people aged 65 and older in the US will increase to 88.5 million in 2050. It is almost twice the elderly population in 2010 (40.2 million). [13, 14]
Elderly people are more susceptible to NTM and most likely to need health and long term caring services. The average age for NTM infection is reported between 50 and 70 years old [15]. It was shown that age is an important prognostic factor for NTM disease. [16] Elderly HIV population is another concern [17, 18]. Given approximately 34 million people live with HIV infection in the world (2,300,000 of those in Europe) [19], HIV associated NTM will become to an important health concern in the coming years.
Despite the importance of NTM infection in the elderly, there is limited information on the characteristics of diseases caused by these pathogens. The aim of this study was to briefly review the epidemiology and clinical characteristics of NTM diseases among elderly patients.
Methods
A literature search was performed for articles published between 2000 and 2013 using the search terms ‘nontuberculous mycobacteria’ and ‘elderly’, ‘epidemiology’, ‘treatment’, ‘symptoms’, ‘prevention’ and ‘diagnosis’. PubMed, Cinahl, Embase and the Cochrane Library were reviewed. Titles of interest were further reviewed by abstract. Reference lists of relevant studies were hand-searched for additional studies.
Studies included in this review met the following criteria:
Study populations included patients with NTM.
Articles were full reports, case reports or reviews.
Articles were in English.
Articles were published in peer-reviewed journals.
1. What is the prevalence of NTM among elderly?
Although the incidence of pulmonary infection by NTM has been noted to be increasing, a formal epidemiological evaluation of this disease has been deficient until recently [20]. According to a laboratory assessment from 1993 to 1996 performed by the Centers for Disease Control and Prevention, the rate of positive NTM cultures was 7.5–8.2 cases per 100,000 persons. However, a recent survey showed a positive culture rate of 17.7 per 100,000 in non-HIV patients in the U.S. [21–23]. Moreover, the rate of pulmonary disease with Mycobacterium avium complex (MAC) has been reported to be 0.2 cases per 100,000 in Europe, while investigators in the United Kingdom estimated the incidence of NTM respiratory disease 2.0 per 100,000 [21, 24–26].
Over the last 18 years, a study revealed that NTM isolates have increased in the Scottish Borders region and, interestingly, these cases have occurred predominantly among elderly [11]. In line with these results, a study performed in the US demonstrated that the prevalence of pulmonary NTM disease was highest in people aged over 50 years (15.5 cases per 100,000 persons) [27]. A report from Australia showed an increased number of NTM infection from 1999 to 2005 especially in elderly women [28]. Lai et al. showed the incidence of NTM increased in Taiwan from 2000 to 2008 [29]. (Table 2)
Table 2.
Major studies reporting the prevalence of NTM pulmonary disease
| Lead author | Year | Country | Prevalence* |
|---|---|---|---|
| Marras[4] | 1999–2000 | USA | 16.6 |
| Winthrop[27] | 2005–2006 | USA | 8.6 |
| Winthrop[60] | 2000–2008 | USA | 4.1 |
| Prevots[23] | 2004–2006 | USA | 5.5 |
| Moore[26] | 1995–2006 | England, Wales, and Northern Ireland | 2.9 |
| Lai[29] | 2000–2008 | Taiwan | 7.9 |
| Thomson[28] | 1999–2005 | Australia | 3.3 |
Nontuberculous mycobacteria infection prevalence rate per 100.000 population
Al-Houqani et al. demonstrated in a population-based study in Ontario, Canada that MAC lung disease increased substantially with age; from 1 in 100,000 in people <50 years old to 48 in100,000 in people over 79 years old [30].
MAC is a ubiquitous bacterium causing disease for human as well as animals in Europe, US and many regions of the world [31, 32]. It is a well-known pathogen for causing a pulmonary disease in elderly women known as Lady Windermere syndrome that presents with isolated middle lobe or lingular bronchiectasis [33]. Mycobacterium kansasii followed by M. fortuitum, M. scrofulaceum, M. chelonae and M. xenopi are reported as most common isolated pathogens after MAC [24, 31, 34]. Mycobacterium gordonae is frequently isolated from elderly patients with pulmonary symptoms and is considered as contamination [20]. However, it could be considered as a potential opportunistic pathogen in a patient with severe immunodeficiency and advance AIDS [35].
2. What are the risk factors for NTM in elderly?
NTM pulmonary infection typically occurs in two different groups of patients. The first group of patients usually are white middle-aged or elderly men who have classic mycobacterial risk factors such as smoking, alcohol abuse, structural lung diseases, and other comorbid conditions. The second group are mainly elderly nonsmoking women without any of these risk factors. [36, 37]. Patients with structural lung diseases such as chronic obstructive pulmonary disease (COPD), cystic fibrosis (CF), bronchiectasis, previous TB, pneumoconiosis and alveolar proteinosis are more at risk of NTM disease [38–40]. Considerably, structural lung disease has higher prevalence in elderly [41].
Genetic abnormalities in the cell immunity pathway like interleukin-12/interferon-γ synthesis, cystic fibrosis transmembrane conductance regulator (CFTR) mutations, human leukocyte antigen (HLA) alleles, polymorphisms of solute carrier 11A1, and the vitamin D receptor cause increased vulnerability to systemic NTM disease [42–45]. Impaired IFN-gamma pathway such as IFN-γ deficiency may also play a role for increasing susceptibility to NTM [46–48].
In HIV patients, disseminated NTM infection typically happens just after the CD4 T-lymphocyte cell counts drop lower than 50/μl, suggesting that T-cell activities or cytokines are necessary for mycobacterial resistance [49–51].
Lady Windermere syndrome is a typical example of NTM presentation in patients without any classical risk factors. It presents with right middle lobe and/or lingua involvement. It is proposed that this syndrome may be associated with a fastidious habit of voluntary cough suppression that causes secretions accumulation, which is ideal for growth of the organisms [33, 42]. Patient is commonly older age white female with no history of smoking and also without any previous lung diseases [52, 53]. Certain physical phenotypes seem to be more common among them, including a tall slender body habitus, scoliosis, pectus excavatum and mitral valve prolapsed [54–56]. It is suggested that leptin deficiency may play a role in susceptibility to NTM in thin elderly women [57]. This unique body morphology resemble those seen in inherited connective tissue disorders like Marfan’s syndrome [58]. Preceding reports assumed structural abnormalities of the chest might predispose elderly patients to MAC lung infections [55, 59]. Figure 1 shows the risk factors for NTM diseases in the elderly.
Figure 1.
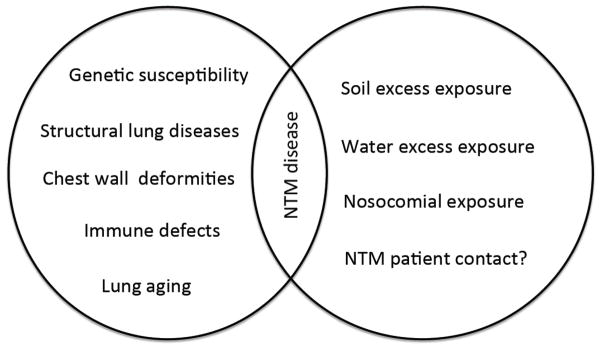
Illustration of risk factors for NTM disease. Immune defects include HIV/AIDS, immunosuppressant such as TNF-a inhibitors, chemotherapy agents and radiotherapy.
3. Is the NTM presentation different in the elderly in comparison to the adults?
Among the elderly, NTM can lead to both asymptomatic infection and symptomatic involvement. The most common clinical manifestation of NTM disease in this group of patients is lung disease, with MAC being the most frequent infection in both US and Europe [60]. Conversely to the older patients, children the clinical presentation of an NTM infection typically consists of a chronic unilateral cervical lymphadenopathy with spontaneous drainage and fistula [20, 61]. Nearly all patients have chronic or recurring cough. Other symptoms include sputum production, dyspnea, chest pain, hemoptysis, fatigue, fever and weight loss. Diagnosis is often hampered by symptoms caused by coexisting lung diseases [20, 62]. For instance, NTM diagnosis may be missed in a patient with non-CF bronchiectasis who chronically experiences all mentioned symptoms [63]. Physical examination is nonspecific as well and may reveal underlying pulmonary pathology, such as COPD and bronchiectasis. On auscultation, findings might consist of rhonchi, crackles and wheezes [2]. In HIV patients, NTM accompany by symptoms such as fever, night sweats and weight loss [51]. However, fever may be undetectable in some of elderly patients with pulmonary infection [64].
Clinically, pulmonary NTM can be categorized into either primary infection with no pre-existing lung disease or secondary to underlying lung diseases. Hypersensitivity pneumonitis (HP) and a nodular bronchiectatic pattern on CT scan (as seen in Lady Windermere syndrome) are the primary clinical presentations related to NTM infection [65, 66].
HP that is allergic reaction to MAC rather than an infection is generally related to aerosols of water including household water, pools, hot-tubs (hot-tub lung) and metalworking fluids [67, 68]. This is usually a disease of younger people [69, 70]. Older patients with Lady Windermere syndrome commonly present with the characteristic nodular-bronchiectatic changes and tree-in-bud appearance in high resolution computed tomography [69]. The nodular-bronchiectatic pattern has commonly been accompanied with MAC, although other species, including M. abscessus in the US and M. xenopi and M. malmoense in Europe, are also commonly related to this form of disease [71].
Studies show convincing evidence of NTM association with underlying lung diseases. It has been reported that NTM are associated with at least 10% of all adult patients with bronchiectasis [39]. The symptoms are nonspecific including chronic cough with or without sputum. Cavitation may occur in later stages of the disease. As we described earlier, NTM are commonly observed in the elderly women with scoliosis, pectus excavatum, and mitral valve prolapse without severe cardiopulmonary disease or significant smoking history [72]. A lean body habitus besides other musculoskeletal or soft tissue abnormalities and a high incidence of CFTR gene mutations have now been reported with this pattern of disease [73]. Progression can be very slow happening over months and years and may not occur at all [52].
4. How is NTM diagnosed in the elderly?
Methods for isolation/detection
NTM is a group of environmental pathogens that humans encounter on a daily basis. Isolation of NTM from one sputum sample in the setting of underlying lung disease such as bronchiectasis may represent ‘colonization’ of the respiratory tract rather than infection [70, 74, 75]. The American Thoracic Society (ATS) and the Infectious Diseases Society of America (IDSA) have established guidelines to aid physicians in making the diagnosis of NTM. These guidelines indicate that in order to diagnose pulmonary NTM disease, clinical, radiological, and microbiological evidence of the disease should be met [5]. Table 3 shows criteria for diagnosing NTM according to ATS/IDSA guidelines.
Table 3.
Summary of the American Thoracic Society diagnostic criteria for pulmonary nontuberculous mycobacterial infection
| General |
|
| Chest Images |
|
| Laboratory |
|
| Expert consultation is suggested when NTM isolates are either infrequently encountered or usually indicated environmental contamination |
| Patients who are believed to have NTM lung disease but not meet the diagnostic criteria should be followed until the diagnosis is established or excluded |
| Making the diagnosis of NTM lung disease does not demand the institution of therapy, which is a decision upon potential risks and benefits of therapy for every individual patient |
While useful in TB, neither IFN-γ release assay (IGRA) nor tuberculin skin test is useful to diagnose NTM. In fact, the main benefit of skin testing or IGRA is to rule out tuberculosis. Worth mentioning, M. kansasii, M. szulgai and M. marinum infections may cause false positive IGRA result due to cross reaction with ESAT-6 and CFP-10 antigens [76, 77].
Acid-fast microscopy (Insensitive)
This method is the simplest, cheapest and fastest way of identifying patients with mycobacterial infections [78]. The specificity of AFB staining for detecting acid-fast bacilli is high, however, the overall sensitivity of the microscopy test is only 22–65% [10, 79]. Currently, there are different methods of acid-fast stains that Kinyoun and Ziehl-Neelsen stains are the most commonly used [80].
Fluorescence in situ hybridization (FISH) (Insensitive)
The acid-fast stain is unable to differentiate between M. tuberculosis (MTB) complex organism and NTM. However, a molecular biology approach using FISH could be helpful [81, 82]. This method is limited by poor function to identify the presence of the relatively common isolated species including M. flavescens, M. fortuitum, and M. xenopi [81].
Mycobacterial culture (Gold standard for isolation)
Acid-fast microscopy is the first important method to diagnose mycobacterial infections, but it should be followed by culture for confirmation of presence of NTM and species identification. In addition, culture is required to perform drug susceptibility testing and genotyping [10, 83]. Many species of NTM need a couple of weeks to grow on solid media and are called slow growing mycobacteria (SGM). Instead, the rapidly growing Mycobacteria (RGM) grow in a short time, approximately a week [84]. Therefore, knowing the growth time of isolate may help the clinician to narrow the differential diagnosis of the possible pathogen [20]. For example, MAC as the most common NTM disease is known as a SGM. To increase probability of NTM isolation, it is recommended to incubate both liquid and solid media at 35 and 30°C [85].
Methods for identification
Nucleic acid hybridization methods
The AccuProbe (Gen-Probe Inc., San Diego, CA) nucleic acid hybridization kits allows for rapid identification of the MTB complex, the M. avium complex, M. kansasii and M. gordonae. This assay offers rapid identification within 2 hours as soon as sufficient colonies are achieved following growth in culture [86]. It was shown that the test has very high sensitivity and specificity (more than 95%) to differentiate mycobacteria species [87].
High-performance liquid chromatography (HPLC)
HPLC for mycolic acids of the cell wall has been approved to be a rapid (less than 2 hours) and inexpensive method to recognize a wide range of mycobacteria species either from sputum or culture [88]. However, the assay needs highly trained staff due to the visual interpretation of the chromatographic patterns, high-cost instruments, expertise in instrument maintenance and standardized growth conditions including the need for a large biomass when using Ultraviolet-HPLC [89]. This test is not available in diagnostic laboratories.
Polymerase chain reaction (PCR) and Restriction fragment length polymorphism (RFLP)
This rapid tool usually is performed on AFB isolates that grow either in liquid or on solid media. Since it is amplified by PCR, the assay involves less biomass than either the AccuProbe or HPLC [90]. Due to the interspecies genetic variability, there is a risk of misidentification with this test [10]. Consequently, to conquer this problem, two new diagnostic algorithms are established [91, 92].
5. How to treat NTM in elderly patients?
Antibiotic therapy for NTM disease involves multiple medications. Consequently, the risk of drug toxicities is relatively high, especially in elderly subjects [93]. For instance, elderly patients with MAC disease commonly complain of gastrointestinal symptoms from the long-standing use of clarithromycin and azithromycin [94]. Therefore, a few elemental questions should be addressed for decision-making on NTM treatment. Does the patient have any clinical symptoms? Will the patient benefit from multidrug therapy? Can the patient be examined regularly to assess the evidence of disease establishment and progression? Will therapy be more unbearable than the disease, particularly for elderly patients with trivial symptoms?
A decision to treat NTM is mainly based on clinical and radiological characteristics, underlying diseases and NTM species. Subsequently, antibiotic therapy is planned by the species identified, the pattern and the extent of lung involvement and possibly drug susceptibility testing. It was shown that not offering a treatment to the patient who met NTM diagnosis, or poor therapeutic response for any reason including inappropriate antibiotic therapy may conclude extensive pleural thickening, atelectasis, advance bronchiectasis and cavitation [95–97].
The treatment of pulmonary NTM disease, as outlined in the latest ATS/IDSA guideline, includes seven essential concepts [20]. The first six instructions are generalizations linking to treatment of infection caused by slowly growing NTM. The seventh concept gives a perspective about differences in management of infections caused by rapidly growing NTM. (Table 4) In addition, it has been shown that if pulmonary NTM disease has caused localized lung involvement, a combined surgical/medical management may worth considering [2].
Table 4.
Seven concepts on treatment of pulmonary NTM diseases
| NTM classification | Therapy |
|---|---|
| Slowly growing NTM (SGM) |
|
| Rapidly growing NTM(RGM) |
|
There is increasing evidence that following the results of in vitro antimicrobial susceptibility tests may increase NTM clinical response [98–100]. The authors believe antibiotic susceptibility testing has a significant role in the selection of the most effective therapy for NTM. The disagreement reported between in vitro susceptibility and clinical response in some circumstances may drive from unstandardized laboratory methods [101]. Clinical and Laboratory Standards Institute (CLSI) recently established recommendations for drug susceptibility testing for NTM with standardized methods and antimicrobial breakpoints [102]. However, limited data are available about drug susceptibility guided NTM therapy.
ATS/IDSA guidelines suggest to perform macrolide susceptibility test routinely for all MAC isolates. These guidelines also recommend M. Kansasii isolates should be tested for rifamycins. Routine drug susceptibility test for RGM should be requested as well. M. abscessus, M. chelonae and M. fortuitum should be tested for amikacin, imipenem, doxycycline, fluoroquinolones, trimethoprim-sulfamethoxazole, cefepim, clarithromycin, linezolid, tigacyclin and tobramycin [20]. We suggest susceptibility test for all patients who remain culture-positive after 6 months of antibiotic therapy.
Ideally, sputum culture should be requested monthly during antibiotic therapy until sputum converting to negative in two consequence months. At that point, sputum culture should be monitored every 3 months until the end of the treatment. If any new pulmonary symptom occurs, an extra sputum culture should be requested.
There are little data regarding genotyping of NTM. It should be considered that NTM genotyping may be helpful in a patient with recurrent presence of positive culture during treatment. To meet the cure definition, combined antibiotic therapy should be continued for 12 months after sputum conversion.
6. What are the risks of drug interactions induced by NTM medications in the elderly?
Drug-drug interaction is an important issue in elderly. Macrolides, rifamycins and fluoroquinolones are vastly used for NTM treatment particularly SGM. They usually cause interaction with metabolisms of other drugs via interacting with cytochrome P-450 (CYP) [103]. CYP isoenzymes are a group of enzymes located in the endoplasmic reticulum of hepatocytes [104]. Macrolides inhibit oxidation of several drugs via CYP. Some of those include, but are not limited to, alprazolam, midazolam, clozapine, carbamazepine, simvastatin, lovastatin, warfarin and cyclosporine [103]. A patient who takes warfarin and macrolide concomitantly is in risk for increasing international normalized ratio (INR). Therefore, monitoring INR carefully is advised [105]. The risk of over anticoagulation in patients prescribed azithromycin on a warfarin regimen is controversial. Recent studies showed the addition of azithromycin to a stable warfarin regimen resulted in a significant change in the INR and warfarin dosage alteration [106]. Macrolides, particularly clarithromycin, may have interaction with direct thrombin inhibitors and factor Xa inhibitors, new oral anticoagulants. Clarithromycin increases anticoagulant effect of rivaroxaban and dabigatran [107]. Therefore, caution is needed in patient concurrently receiving clarithromycin with this group of drugs.
Rifamycin group drugs (rifampin, rifabutin and rifapentine) are strong inducer of CYP enzyme. They may lower efficacy of several drugs if use concomitantly. However, rifapentine causes less drug interaction. Concomitant administration of warfarin may decrease its serum level and cause INR goes to under therapeutic level. The drug interaction with rifampin has been recently reviewed [108].
Quinolones are another group of inhibitors of CYP enzyme. They inhibit metabolism of nonsteroidal antiinflamatory drugs and theophylline. It should be considered that quinolones absorption is inhibited with concomitant use of aluminum and magnesium antacids, calcium supplements and dairy products [109]. Medication list of any NTM patient should be reviewed before starting antibiotics. Consulting with a clinical pharmacist is well worth in patients on multiple medications.
7. What is the prognosis of NTM in elderly?
NTM-related mortality is increasing. There is a strong association between age and NTM mortality [110, 111]. The mortality rate from limited NTM infections (lung, skin and so on) is low, but mortality rate due to disseminated NTM infection is reported to be about 30% to 40% [16, 112]. Indeed, the mortality was found to be higher in patients older than 65 years. Besides male gender and high levels of comorbidities, advanced age was assumed to be a strong predictor of five-year mortality [16]. Additionally, according to the British Thoracic Society studies, M. xenopi was reported with the highest mortality rate [113]. The impact of pulmonary NTM on pulmonary remodeling (fibrosis, pulmonary hypertension and airway diseases) is unclear and should be investigated.
Conclusions
The incidence of NTM infections is growing, probably due to a combination of factors including advancing age. They are being recognized with improvements in laboratory methodology, liquid culture techniques and advance molecular methods. Because of the ubiquitous nature of NTM as environmental pathogens, it is vital to differentiate between clinical disease and colonization. There is a considerable variation in treatment management that should be deliberated before starting the treatment. While the US and European populations are aging and NTM diseases are rising in elderly population with a high mortality rate, we would hope to see an increasing focus on research in NTM infection, and multicenter trials. Creation of regional referral institutions can improve management of this challenging group of diseases.
Learning points.
The Epidemiology of NTM infection has been changing in the last decades. The incidence of NTM is increasing and the increase has mainly been in elderly women without any of the classic risk factors.
Because of the ubiquitous nature of NTM as environmental pathogens, it is vital to differentiate between clinical disease and colonization.
Acid-fast microscopy is the first important method to diagnose mycobacterial infections, but it should be followed by culture for confirmation of presence of NTM and species identification.
A decision to treat NTM is mainly based on clinical and radiological characteristics, underlying diseases and NTM species.
Antibiotic therapy for NTM disease involves multiple medications. The treatment of pulmonary NTM disease, as outlined in the latest ATS/IDSA guideline, concludes seven essential concepts.
Drug-drug interaction is an important issue in elderly. Consulting with a clinical pharmacist is well worth in patients on multiple medications.
Highlights.
Elderly women are the most susceptible group to NTM.
Lung disease is the most common clinical manifestation of NTM in the elderly.
Acid-fast microscopy and culture are the most important methods to diagnose NTM.
Clinical data and NTM species should be evaluated before starting treatment.
Drug-drug interaction is an important issue in the elderly.
Acknowledgments
Authors would like to thank Dr. Travis Yamanaka for his editorial help.
Abbreviations
- TB
Tuberculosis
- Ig
Immunoglobulins
- NTM
Nontuberculous mycobacteria
- US
United States
- MAC
Mycobacterium avium complex
- COPD
Chronic obstructive pulmonary disease
- CF
Cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- HLA
human leukocyte antigen
- HP
Hypersensitivity pneumonitis
- ATS
American Thoracic Society
- IDSA
Infectious Diseases Society of America
- IGRA
IFN-γ release assay
- FISH
Fluorescence in situ hybridization
- MTB
M. tuberculosis
- SGM
Slow growing mycobacteria
- RGM
Rapidly growing Mycobacteria
- HPLC
High-performance liquid chromatography
- PCR
Polymerase chain reaction
- RFLP
Restriction fragment length polymorphism
- CLSI
Clinical and Laboratory Standards Institute
- CYP
Cytochrome P-450
- INR
International normalized ratio
Footnotes
Conflict of interests
The authors have no conflicts of interests to declare related to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Masson AM, Prissick FH. Cervical lymphadenitis in children caused by chromogenic Mycobacteria. Can Med Assoc J. 1956;75(10):798–803. [PMC free article] [PubMed] [Google Scholar]
- 2.Cook JL. Nontuberculous mycobacteria: opportunistic environmental pathogens for predisposed hosts. Br Med Bull. 2010;96:45–59. doi: 10.1093/bmb/ldq035. [DOI] [PubMed] [Google Scholar]
- 3.Dai J, Chen Y, Lauzardo M. Web-accessible database of hsp65 sequences from Mycobacterium reference strains. J Clin Microbiol. 2011;49(6):2296–2303. doi: 10.1128/JCM.02602-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan K, Wang J, Marras TK. Nontuberculous mycobacterial sensitization in the United States: national trends over three decades. Am J Respir Crit Care Med. 2007;176(3):306–313. doi: 10.1164/rccm.200702-201OC. [DOI] [PubMed] [Google Scholar]
- 5.Lai CC, Tan CK, Lin SH, Liu WL, Liao CH, Huang YT, Hsueh PR. Clinical significance of nontuberculous mycobacteria isolates in elderly Taiwanese patients. Eur J Clin Microbiol Infect Dis. 2011;30(6):779–783. doi: 10.1007/s10096-011-1155-8. [DOI] [PubMed] [Google Scholar]
- 6.von Reyn CF, Arbeit RD, Horsburgh CR, Ristola MA, Waddell RD, Tvaroha SM, Samore M, Hirschhorn LR, Lumio J, Lein AD, Grove MR, Tosteson AN. Sources of disseminated Mycobacterium avium infection in AIDS. J Infect. 2002;44(3):166–170. doi: 10.1053/jinf.2001.0950. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka E, Kimoto T, Matsumoto H, Tsuyuguchi K, Suzuki K, Nagai S, Shimadzu M, Ishibatake H, Murayama T, Amitani R. Familial pulmonary Mycobacterium avium complex disease. Am J Respir Crit Care Med. 2000;161(5):1643–1647. doi: 10.1164/ajrccm.161.5.9907144. [DOI] [PubMed] [Google Scholar]
- 8.Bryant JM, Grogono DM, Greaves D, Foweraker J, Roddick I, Inns T, Reacher M, Haworth CS, Curran MD, Harris SR, Peacock SJ, Parkhill J, Floto RA. Whole-genome sequencing to identify transmission of Mycobacterium abscessus between patients with cystic fibrosis: a retrospective cohort study. Lancet. 2013;381(9877):1551–1560. doi: 10.1016/S0140-6736(13)60632-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al Houqani M, Jamieson F, Chedore P, Mehta M, May K, Marras TK. Isolation prevalence of pulmonary nontuberculous mycobacteria in Ontario in 2007. Can Respir J. 2011;18(1):19–24. doi: 10.1155/2011/865831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Somoskovi A, Mester J, Hale YM, Parsons LM, Salfinger M. Laboratory diagnosis of nontuberculous mycobacteria. Clin Chest Med. 2002;23(3):585–597. doi: 10.1016/s0272-5231(02)00018-7. [DOI] [PubMed] [Google Scholar]
- 11.McCallum AD, Watkin SW, Faccenda JF. Non-tuberculous mycobacterial infections in the Scottish Borders: identification, management and treatment outcomes--a retrospective review. The journal of the Royal College of Physicians of Edinburgh. 2011;41(4):294–303. doi: 10.4997/JRCPE.2011.403. [DOI] [PubMed] [Google Scholar]
- 12.Cox JN, Brenner ER, Bryan CS. Changing patterns of mycobacterial disease at a teaching community hospital. Infect Control Hosp Epidemiol. 1994;15(8):513–515. doi: 10.1086/646968. [DOI] [PubMed] [Google Scholar]
- 13.Rynning E. The ageing populations of Europe--implications for health systems and patients’ rights. Eur J Health Law. 2008;15(3):297–306. doi: 10.1163/157180908x338241. [DOI] [PubMed] [Google Scholar]
- 14.Wiener JM, Tilly J. Population ageing in the United States of America: implications for public programmes. Int J Epidemiol. 2002;31(4):776–781. doi: 10.1093/ije/31.4.776. [DOI] [PubMed] [Google Scholar]
- 15.Simons S, van Ingen J, Hsueh PR, Van Hung N, Dekhuijzen PN, Boeree MJ, van Soolingen D. Nontuberculous mycobacteria in respiratory tract infections, eastern Asia. Emerg Infect Dis. 2011;17(3):343–349. doi: 10.3201/eid1703100604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andrejak C, Thomsen VO, Johansen IS, Riis A, Benfield TL, Duhaut P, Sorensen HT, Lescure FX, Thomsen RW. Nontuberculous pulmonary mycobacteriosis in Denmark: incidence and prognostic factors. Am J Respir Crit Care Med. 2010;181(5):514–521. doi: 10.1164/rccm.200905-0778OC. [DOI] [PubMed] [Google Scholar]
- 17.Murcia-Aranguren MI, Gomez-Marin JE, Alvarado FS, Bustillo JG, de Mendivelson E, Gomez B, Leon CI, Triana WA, Vargas EA, Rodriguez E. Frequency of tuberculous and non-tuberculous mycobacteria in HIV infected patients from Bogota, Colombia. BMC Infect Dis. 2001;1:21. doi: 10.1186/1471-2334-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakagawa F, Lodwick RK, Smith CJ, Smith R, Cambiano V, Lundgren JD, Delpech V, Phillips AN. Projected life expectancy of people with HIV according to timing of diagnosis. AIDS. 2012;26(3):335–343. doi: 10.1097/QAD.0b013e32834dcec9. [DOI] [PubMed] [Google Scholar]
- 19.Data on the size of the HIV/AIDS epidemic: Data by WHO region by WHO region. 2013 avaialble at: http://apps.who.int/gho/data/node.main.619?lang=en.
- 20.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ, Jr, Winthrop K Subcommittee ATSMD, American Thoracic S, Infectious Disease Society of A. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 21.Bodle EE, Cunningham JA, Della-Latta P, Schluger NW, Saiman L. Epidemiology of nontuberculous mycobacteria in patients without HIV infection, New York City. Emerg Infect Dis. 2008;14(3):390–396. doi: 10.3201/eid1403.061143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butler WCJ, Shutt K. Nontuberculous mycobacteria reported to the Public Health Laboratory Information System by state public health laboratories, United States, 1993–1996. Centers for Disease Control and Prevention. 1999 [Google Scholar]
- 23.Prevots DR, Shaw PA, Strickland D, Jackson LA, Raebel MA, Blosky MA, Montes de Oca R, Shea YR, Seitz AE, Holland SM, Olivier KN. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med. 2010;182(7):970–976. doi: 10.1164/rccm.201002-0310OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henry MT, Inamdar L, O’Riordain D, Schweiger M, Watson JP. Nontuberculous mycobacteria in non-HIV patients: epidemiology, treatment and response. Eur Respir J. 2004;23(5):741–746. doi: 10.1183/09031936.04.00114004. [DOI] [PubMed] [Google Scholar]
- 25.Maugein J, Dailloux M, Carbonnelle B, Vincent V, Grosset J. Sentinel-site surveillance of Mycobacterium avium complex pulmonary disease. Eur Respir J. 2005;26(6):1092–1096. doi: 10.1183/09031936.05.00148604. [DOI] [PubMed] [Google Scholar]
- 26.Moore JE, Kruijshaar ME, Ormerod LP, Drobniewski F, Abubakar I. Increasing reports of non-tuberculous mycobacteria in England, Wales and Northern Ireland, 1995–2006. BMC public health. 2010;10:612. doi: 10.1186/1471-2458-10-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cassidy PM, Hedberg K, Saulson A, McNelly E, Winthrop KL. Nontuberculous mycobacterial disease prevalence and risk factors: a changing epidemiology. Clin Infect Dis. 2009;49(12):e124–129. doi: 10.1086/648443. [DOI] [PubMed] [Google Scholar]
- 28.Thomson RM. Changing epidemiology of pulmonary nontuberculous mycobacteria infections. Emerg Infect Dis. 2010;16(10):1576–1583. doi: 10.3201/eid1610.091201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lai CC, Tan CK, Chou CH, Hsu HL, Liao CH, Huang YT, Yang PC, Luh KT, Hsueh PR. Increasing incidence of nontuberculous mycobacteria, Taiwan, 2000–2008. Emerging infectious diseases. 2010;16(2):294–296. doi: 10.3201/eid1602.090675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Houqani M, Jamieson F, Mehta M, Chedore P, May K, Marras TK. Aging, COPD, and other risk factors do not explain the increased prevalence of pulmonary Mycobacterium avium complex in Ontario. Chest. 2012;141(1):190–197. doi: 10.1378/chest.11-0089. [DOI] [PubMed] [Google Scholar]
- 31.Hoefsloot W, van Ingen J, Andrejak C, Angeby K, Bauriaud R, Bemer P, Beylis N, Boeree MJ, Cacho J, Chihota V, Chimara E, Churchyard G, Cias R, Dasa R, Daley CL, Dekhuijzen PN, Domingo D, Drobniewski F, Esteban J, Fauville-Dufaux M, Folkvardsen DB, Gibbons N, Gomez-Mampaso E, Gonzalez R, Hoffmann H, Hsueh PR, Indra A, Jagielski T, Jamieson F, Jankovic M, Jong E, Keane J, Koh WJ, Lange B, Leao S, Macedo R, Mannsaker T, Marras TK, Maugein J, Milburn HJ, Mlinko T, Morcillo N, Morimoto K, Papaventsis D, Palenque E, Paez-Pena M, Piersimoni C, Polanova M, Rastogi N, Richter E, Ruiz-Serrano MJ, Silva A, da Silva MP, Simsek H, van Soolingen D, Szabo N, Thomson R, Fernandez MT, Tortoli E, Totten SE, Tyrrell G, Vasankari T, Villar M, Walkiewicz R, Winthrop K, Wagner D for N-N. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: A NTM-NET collaborative study. Eur Respir J. 2013 doi: 10.1183/09031936.00149212. [DOI] [PubMed] [Google Scholar]
- 32.Inderlied CB, Kemper CA, Bermudez LE. The Mycobacterium avium complex. Clin Microbiol Rev. 1993;6(3):266–310. doi: 10.1128/cmr.6.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reich JM, Johnson RE. Mycobacterium avium complex pulmonary disease presenting as an isolated lingular or middle lobe pattern. The Lady Windermere syndrome. Chest. 1992;101(6):1605–1609. doi: 10.1378/chest.101.6.1605. [DOI] [PubMed] [Google Scholar]
- 34.O’Brien RJ, Geiter LJ, Snider DE., Jr The epidemiology of nontuberculous mycobacterial diseases in the United States. Results from a national survey. Am Rev Respir Dis. 1987;135(5):1007–1014. doi: 10.1164/arrd.1987.135.5.1007. [DOI] [PubMed] [Google Scholar]
- 35.Barber TW, Craven DE, Farber HW. Mycobacterium gordonae: a possible opportunistic respiratory tract pathogen in patients with advanced human immunodeficiency virus, type 1 infection. Chest. 1991;100(3):716–720. doi: 10.1378/chest.100.3.716. [DOI] [PubMed] [Google Scholar]
- 36.Jeong YJ, Lee KS, Koh WJ, Han J, Kim TS, Kwon OJ. Nontuberculous mycobacterial pulmonary infection in immunocompetent patients: comparison of thin-section CT and histopathologic findings. Radiology. 2004;231(3):880–886. doi: 10.1148/radiol.2313030833. [DOI] [PubMed] [Google Scholar]
- 37.Wallace RJ, Jr, Zhang Y, Brown BA, Dawson D, Murphy DT, Wilson R, Griffith DE. Polyclonal Mycobacterium avium complex infections in patients with nodular bronchiectasis. Am J Respir Crit Care Med. 1998;158(4):1235–1244. doi: 10.1164/ajrccm.158.4.9712098. [DOI] [PubMed] [Google Scholar]
- 38.Witty LA, Tapson VF, Piantadosi CA. Isolation of mycobacteria in patients with pulmonary alveolar proteinosis. Medicine (Baltimore) 1994;73(2):103–109. doi: 10.1097/00005792-199403000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Fowler SJ, French J, Screaton NJ, Foweraker J, Condliffe A, Haworth CS, Exley AR, Bilton D. Nontuberculous mycobacteria in bronchiectasis: Prevalence and patient characteristics. Eur Respir J. 2006;28(6):1204–1210. doi: 10.1183/09031936.06.00149805. [DOI] [PubMed] [Google Scholar]
- 40.Morita H, Usami I, Torii M, Nakamura A, Kato T, Kutsuna T, Niwa T, Katou K, Itoh M. Isolation of nontuberculous mycobacteria from patients with pneumoconiosis. J Infect Chemother. 2005;11(2):89–92. doi: 10.1007/s10156-004-0368-5. [DOI] [PubMed] [Google Scholar]
- 41.Fukuchi Y. The aging lung and chronic obstructive pulmonary disease: similarity and difference. Proceedings of the American Thoracic Society. 2009;6(7):570–572. doi: 10.1513/pats.200909-099RM. [DOI] [PubMed] [Google Scholar]
- 42.Sexton P, Harrison AC. Susceptibility to nontuberculous mycobacterial lung disease. Eur Respir J. 2008;31(6):1322–1333. doi: 10.1183/09031936.00140007. [DOI] [PubMed] [Google Scholar]
- 43.Dorman SE, Holland SM. Interferon-gamma and interleukin-12 pathway defects and human disease. Cytokine Growth Factor Rev. 2000;11(4):321–333. doi: 10.1016/s1359-6101(00)00010-1. [DOI] [PubMed] [Google Scholar]
- 44.Casanova JL, Abel L. Genetic dissection of immunity to mycobacteria: the human model. Annu Rev Immunol. 2002;20:581–620. doi: 10.1146/annurev.immunol.20.081501.125851. [DOI] [PubMed] [Google Scholar]
- 45.Mirsaeidi M. Personalized medicine approach in mycobacterial disease. International Journal of Mycobacteriology. 2012;1(2):59–64. doi: 10.1016/j.ijmyco.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Safdar A, Armstrong D, Murray HW. A novel defect in interferon-gamma secretion in patients with refractory nontuberculous pulmonary mycobacteriosis. Ann Intern Med. 2003;138(6):521. doi: 10.7326/0003-4819-138-6-200303180-00030. [DOI] [PubMed] [Google Scholar]
- 47.Greinert U, Schlaak M, Rusch-Gerdes S, Flad HD, Ernst M. Low in vitro production of interferon-gamma and tumor necrosis factor-alpha in HIV-seronegative patients with pulmonary disease caused by nontuberculous mycobacteria. J Clin Immunol. 2000;20(6):445–452. doi: 10.1023/a:1026407815946. [DOI] [PubMed] [Google Scholar]
- 48.Safdar A, White DA, Stover D, Armstrong D, Murray HW. Profound interferon gamma deficiency in patients with chronic pulmonary nontuberculous mycobacteriosis. Am J Med. 2002;113(9):756–759. doi: 10.1016/s0002-9343(02)01313-x. [DOI] [PubMed] [Google Scholar]
- 49.Horsburgh CR., Jr Epidemiology of disease caused by nontuberculous mycobacteria. Semin Respir Infect. 1996;11(4):244–251. [PubMed] [Google Scholar]
- 50.Mycobacterioses and the acquired immunodeficiency syndrome. Joint Position Paper of the American Thoracic Society and the Centers for Disease Control. Am Rev Respir Dis. 1987;136(2):492–496. doi: 10.1164/ajrccm/136.2.492. [DOI] [PubMed] [Google Scholar]
- 51.McCarthy KD, Cain KP, Winthrop KL, Udomsantisuk N, Lan NT, Sar B, Kimerling ME, Kanara N, Lynen L, Monkongdee P, Tasaneeyapan T, Varma JK. Nontuberculous mycobacterial disease in patients with HIV in Southeast Asia. Am J Respir Crit Care Med. 2012;185(9):981–988. doi: 10.1164/rccm.201107-1327OC. [DOI] [PubMed] [Google Scholar]
- 52.Prince DS, Peterson DD, Steiner RM, Gottlieb JE, Scott R, Israel HL, Figueroa WG, Fish JE. Infection with Mycobacterium avium complex in patients without predisposing conditions. N Engl J Med. 1989;321(13):863–868. doi: 10.1056/NEJM198909283211304. [DOI] [PubMed] [Google Scholar]
- 53.Reich JM, Johnson RE. Mycobacterium avium complex pulmonary disease. Incidence, presentation, and response to therapy in a community setting. Am Rev Respir Dis. 1991;143(6):1381–1385. doi: 10.1164/ajrccm/143.6.1381. [DOI] [PubMed] [Google Scholar]
- 54.Huang JH, Kao PN, Adi V, Ruoss SJ. Mycobacterium avium-intracellulare pulmonary infection in HIV-negative patients without preexisting lung disease: diagnostic and management limitations. Chest. 1999;115(4):1033–1040. doi: 10.1378/chest.115.4.1033. [DOI] [PubMed] [Google Scholar]
- 55.Iseman MD, Buschman DL, Ackerson LM. Pectus excavatum and scoliosis. Thoracic anomalies associated with pulmonary disease caused by Mycobacterium avium complex. Am Rev Respir Dis. 1991;144(4):914–916. doi: 10.1164/ajrccm/144.4.914. [DOI] [PubMed] [Google Scholar]
- 56.Pomerantz M, Denton JR, Huitt GA, Brown JM, Powell LA, Iseman MD. Resection of the right middle lobe and lingula for mycobacterial infection. Ann Thorac Surg. 1996;62(4):990–993. doi: 10.1016/0003-4975(96)00534-6. [DOI] [PubMed] [Google Scholar]
- 57.Chan ED, Iseman MD. Slender, older women appear to be more susceptible to nontuberculous mycobacterial lung disease. Gender medicine. 2010;7(1):5–18. doi: 10.1016/j.genm.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 58.Guide SV, Holland SM. Host susceptibility factors in mycobacterial infection. Genetics and body morphotype. Infect Dis Clin North Am. 2002;16(1):163–186. doi: 10.1016/s0891-5520(03)00051-5. [DOI] [PubMed] [Google Scholar]
- 59.Iseman MD. That’s no lady. Chest. 1996;109(5):1411. doi: 10.1378/chest.109.5.1411. [DOI] [PubMed] [Google Scholar]
- 60.Kendall BA, Winthrop KL. Update on the epidemiology of pulmonary nontuberculous mycobacterial infections. Semin Respir Crit Care Med. 2013;34(1):87–94. doi: 10.1055/s-0033-1333567. [DOI] [PubMed] [Google Scholar]
- 61.Haverkamp MH, Arend SM, Lindeboom JA, Hartwig NG, van Dissel JT. Nontuberculous mycobacterial infection in children: a 2-year prospective surveillance study in the Netherlands. Clin Infect Dis. 2004;39(4):450–456. doi: 10.1086/422319. [DOI] [PubMed] [Google Scholar]
- 62.Baghaei P, Tabarsi P, Farnia P, Marjani M, Sheikholeslami FM, Chitsaz M, Gorji Bayani P, Shamaei M, Mansouri D, Masjedi MR, Velayati AA. Pulmonary disease caused by Mycobacterium simiae in Iran’s national referral center for tuberculosis. J Infect Dev Ctries. 2012;6(1):23–28. doi: 10.3855/jidc.1297. [DOI] [PubMed] [Google Scholar]
- 63.Mirsaeidi M, Hadid W, Ericsoussi B, Rodgers D, Sadikot RT. Non-tuberculous mycobacterial disease is common in patients with non-cystic fibrosis bronchiectasis. Int J Infect Dis. 2013;17(11):e1000–1004. doi: 10.1016/j.ijid.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Norman DC. Fever in the elderly. Clin Infect Dis. 2000;31(1):148–151. doi: 10.1086/313896. [DOI] [PubMed] [Google Scholar]
- 65.Waller EA, Roy A, Brumble L, Khoor A, Johnson MM, Garland JL. The expanding spectrum of Mycobacterium avium complex-associated pulmonary disease. Chest. 2006;130(4):1234–1241. doi: 10.1378/chest.130.4.1234. [DOI] [PubMed] [Google Scholar]
- 66.Field SK, Fisher D, Cowie RL. Mycobacterium avium complex pulmonary disease in patients without HIV infection. Chest. 2004;126(2):566–581. doi: 10.1378/chest.126.2.566. [DOI] [PubMed] [Google Scholar]
- 67.Tillie-Leblond I, Grenouillet F, Reboux G, Roussel S, Chouraki B, Lorthois C, Dalphin JC, Wallaert B, Millon L. Hypersensitivity pneumonitis and metalworking fluids contaminated by mycobacteria. Eur Respir J. 2011;37(3):640–647. doi: 10.1183/09031936.00195009. [DOI] [PubMed] [Google Scholar]
- 68.Marras TK, Wallace RJ, Jr, Koth LL, Stulbarg MS, Cowl CT, Daley CL. Hypersensitivity pneumonitis reaction to Mycobacterium avium in household water. Chest. 2005;127(2):664–671. doi: 10.1378/chest.127.2.664. [DOI] [PubMed] [Google Scholar]
- 69.Schluger NW. Tuberculosis and nontuberculous mycobacterial infections in older adults. Clin Chest Med. 2007;28(4):773–781. vi. doi: 10.1016/j.ccm.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weiss CH, Glassroth J. Pulmonary disease caused by nontuberculous mycobacteria. Expert Rev Respir Med. 2012;6(6):597–612. doi: 10.1586/ers.12.58. quiz 613. [DOI] [PubMed] [Google Scholar]
- 71.Griffith DE, Girard WM, Wallace RJ., Jr Clinical features of pulmonary disease caused by rapidly growing mycobacteria. An analysis of 154 patients. Am Rev Respir Dis. 1993;147(5):1271–1278. doi: 10.1164/ajrccm/147.5.1271. [DOI] [PubMed] [Google Scholar]
- 72.Chick JF, Chauhan NR, Bair RJ, Chauhan VR. The Lady Windermere syndrome. Intern Emerg Med. 2013;8(1):83–85. doi: 10.1007/s11739-012-0870-1. [DOI] [PubMed] [Google Scholar]
- 73.Kim RD, Greenberg DE, Ehrmantraut ME, Guide SV, Ding L, Shea Y, Brown MR, Chernick M, Steagall WK, Glasgow CG, Lin J, Jolley C, Sorbara L, Raffeld M, Hill S, Avila N, Sachdev V, Barnhart LA, Anderson VL, Claypool R, Hilligoss DM, Garofalo M, Fitzgerald A, Anaya-O’Brien S, Darnell D, DeCastro R, Menning HM, Ricklefs SM, Porcella SF, Olivier KN, Moss J, Holland SM. Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med. 2008;178(10):1066–1074. doi: 10.1164/rccm.200805-686OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Falkinham JO. Impact of human activities on the ecology of nontuberculous mycobacteria. Future Microbiol. 2010;5(6):951–960. doi: 10.2217/fmb.10.53. [DOI] [PubMed] [Google Scholar]
- 75.Fujita J, Ohtsuki Y, Suemitsu I, Shigeto E, Yamadori I, Obayashi Y, Miyawaki H, Dobashi N, Matsushima T, Takahara J. Pathological and radiological changes in resected lung specimens in Mycobacterium avium intracellulare complex disease. Eur Respir J. 1999;13(3):535–540. doi: 10.1183/09031936.99.13353599. [DOI] [PubMed] [Google Scholar]
- 76.Kuznetcova TI, Sauty A, Herbort CP. Uveitis with occult choroiditis due to Mycobacterium kansasii: limitations of interferon-gamma release assay (IGRA) tests (case report and mini-review on ocular non-tuberculous mycobacteria and IGRA cross-reactivity) Int Ophthalmol. 2012;32(5):499–506. doi: 10.1007/s10792-012-9588-3. [DOI] [PubMed] [Google Scholar]
- 77.Kobashi Y, Obase Y, Fukuda M, Yoshida K, Miyashita N, Oka M. Clinical reevaluation of the QuantiFERON TB-2G test as a diagnostic method for differentiating active tuberculosis from nontuberculous mycobacteriosis. Clin Infect Dis. 2006;43(12):1540–1546. doi: 10.1086/509327. [DOI] [PubMed] [Google Scholar]
- 78.Salfinger M, Pfyffer GE. The new diagnostic mycobacteriology laboratory. Eur J Clin Microbiol Infect Dis. 1994;13(11):961–979. doi: 10.1007/BF02111498. [DOI] [PubMed] [Google Scholar]
- 79.Lipsky BA, Gates J, Tenover FC, Plorde JJ. Factors affecting the clinical value of microscopy for acid-fast bacilli. Rev Infect Dis. 1984;6(2):214–222. doi: 10.1093/clinids/6.2.214. [DOI] [PubMed] [Google Scholar]
- 80.Tenover FC, Crawford JT, Huebner RE, Geiter LJ, Horsburgh CR, Jr, Good RC. The resurgence of tuberculosis: is your laboratory ready? J Clin Microbiol. 1993;31(4):767–770. doi: 10.1128/jcm.31.4.767-770.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stender H, Lund K, Petersen KH, Rasmussen OF, Hongmanee P, Miorner H, Godtfredsen SE. Fluorescence In situ hybridization assay using peptide nucleic acid probes for differentiation between tuberculous and nontuberculous mycobacterium species in smears of mycobacterium cultures. J Clin Microbiol. 1999;37(9):2760–2765. doi: 10.1128/jcm.37.9.2760-2765.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hongmanee P, Stender H, Rasmussen OF. Evaluation of a fluorescence in situ hybridization assay for differentiation between tuberculous and nontuberculous Mycobacterium species in smears of Lowenstein-Jensen and Mycobacteria Growth Indicator Tube cultures using peptide nucleic acid probes. J Clin Microbiol. 2001;39(3):1032–1035. doi: 10.1128/JCM.39.3.1032-1035.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mangione EJ, Huitt G, Lenaway D, Beebe J, Bailey A, Figoski M, Rau MP, Albrecht KD, Yakrus MA. Nontuberculous mycobacterial disease following hot tub exposure. Emerg Infect Dis. 2001;7(6):1039–1042. doi: 10.3201/eid0706.010623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim CJ, Kim NH, Song KH, Choe PG, Kim ES, Park SW, Kim HB, Kim NJ, Kim EC, Park WB, Oh MD. Differentiating rapid- and slow-growing mycobacteria by difference in time to growth detection in liquid media. Diagnostic microbiology and infectious disease. 2013;75(1):73–76. doi: 10.1016/j.diagmicrobio.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 85.van Ingen J. Diagnosis of nontuberculous mycobacterial infections. Semin Respir Crit Care Med. 2013;34(1):103–109. doi: 10.1055/s-0033-1333569. [DOI] [PubMed] [Google Scholar]
- 86.Yam WC, Yuen KY, Kam SY, Yiu LS, Chan KS, Leung CC, Tam CM, Ho PO, Yew WW, Seto WH, Ho PL. Diagnostic application of genotypic identification of mycobacteria. Journal of medical microbiology. 2006;55(Pt 5):529–536. doi: 10.1099/jmm.0.46298-0. [DOI] [PubMed] [Google Scholar]
- 87.Richardson ET, Samson D, Banaei N. Rapid Identification of Mycobacterium tuberculosis and nontuberculous mycobacteria by multiplex, real-time PCR. J Clin Microbiol. 2009;47(5):1497–1502. doi: 10.1128/JCM.01868-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jost KC, Jr, Dunbar DF, Barth SS, Headley VL, Elliott LB. Identification of Mycobacterium tuberculosis and M. avium complex directly from smear-positive sputum specimens and BACTEC 12B cultures by high-performance liquid chromatography with fluorescence detection and computer-driven pattern recognition models. J Clin Microbiol. 1995;33(5):1270–1277. doi: 10.1128/jcm.33.5.1270-1277.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Butler WR, Guthertz LS. Mycolic acid analysis by high-performance liquid chromatography for identification of Mycobacterium species. Clin Microbiol Rev. 2001;14(4):704–726. doi: 10.1128/CMR.14.4.704-726.2001. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Telenti A, Marchesi F, Balz M, Bally F, Bottger EC, Bodmer T. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J Clin Microbiol. 1993;31(2):175–178. doi: 10.1128/jcm.31.2.175-178.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Roth A, Reischl U, Streubel A, Naumann L, Kroppenstedt RM, Habicht M, Fischer M, Mauch H. Novel diagnostic algorithm for identification of mycobacteria using genus-specific amplification of the 16S–23S rRNA gene spacer and restriction endonucleases. J Clin Microbiol. 2000;38(3):1094–1104. doi: 10.1128/jcm.38.3.1094-1104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim BJ, Lee KH, Park BN, Kim SJ, Bai GH, Kook YH. Differentiation of mycobacterial species by PCR-restriction analysis of DNA (342 base pairs) of the RNA polymerase gene (rpoB) J Clin Microbiol. 2001;39(6):2102–2109. doi: 10.1128/JCM.39.6.2102-2109.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Diagnosis and treatment of disease caused by nontuberculous mycobacteria. This official statement of the American Thoracic Society was approved by the Board of Directors, March 1997. Medical Section of the American Lung Association. Am J Respir Crit Care Med. 1997;156(2 Pt 2):S1–25. doi: 10.1164/ajrccm.156.2.atsstatement. [DOI] [PubMed] [Google Scholar]
- 94.Wallace RJ, Jr, Brown BA, Griffith DE. Drug intolerance to high-dose clarithromycin among elderly patients. Diagn Microbiol Infect Dis. 1993;16(3):215–221. doi: 10.1016/0732-8893(93)90112-k. [DOI] [PubMed] [Google Scholar]
- 95.Kuroishi S, Nakamura Y, Hayakawa H, Shirai M, Nakano Y, Yasuda K, Suda T, Nakamura H, Chida K. Mycobacterium avium complex disease: prognostic implication of high-resolution computed tomography findings. Eur Respir J. 2008;32(1):147–152. doi: 10.1183/09031936.00074207. [DOI] [PubMed] [Google Scholar]
- 96.Lam PK, Griffith DE, Aksamit TR, Ruoss SJ, Garay SM, Daley CL, Catanzaro A. Factors related to response to intermittent treatment of Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2006;173(11):1283–1289. doi: 10.1164/rccm.200509-1531OC. [DOI] [PubMed] [Google Scholar]
- 97.Kitada S, Uenami T, Yoshimura K, Tateishi Y, Miki K, Miki M, Hashimoto H, Fujikawa T, Mori M, Matsuura K, Kuroyama M, Maekura R. Long-term radiographic outcome of nodular bronchiectatic Mycobacterium avium complex pulmonary disease. Int J Tuberc Lung Dis. 2012;16(5):660–664. doi: 10.5588/ijtld.11.0534. [DOI] [PubMed] [Google Scholar]
- 98.Horsburgh CR, Jr, Mason UG, 3rd, Heifets LB, Southwick K, Labrecque J, Iseman MD. Response to therapy of pulmonary Mycobacterium avium-intracellulare infection correlates with results of in vitro susceptibility testing. Am Rev Respir Dis. 1987;135(2):418–421. doi: 10.1164/arrd.1987.135.2.418. [DOI] [PubMed] [Google Scholar]
- 99.Huang CW, Chen JH, Hu ST, Huang WC, Lee YC, Huang CC, Shen GH. Synergistic activities of tigecycline with clarithromycin or amikacin against rapidly growing mycobacteria in Taiwan. International journal of antimicrobial agents. 2013;41(3):218–223. doi: 10.1016/j.ijantimicag.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 100.Huang YC, Liu MF, Shen GH, Lin CF, Kao CC, Liu PY, Shi ZY. Clinical outcome of Mycobacterium abscessus infection and antimicrobial susceptibility testing. Journal of microbiology, immunology, and infection = Wei mian yu gan ran za zhi. 2010;43(5):401–406. doi: 10.1016/S1684-1182(10)60063-1. [DOI] [PubMed] [Google Scholar]
- 101.van Ingen J, Boeree MJ, van Soolingen D, Mouton JW. Resistance mechanisms and drug susceptibility testing of nontuberculous mycobacteria. Drug resistance updates: reviews and commentaries in antimicrobial and anticancer chemotherapy. 2012;15(3):149–161. doi: 10.1016/j.drup.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 102.Woods, et al. CLSI document M24-A2. Clinical and Laboratory Standards Institute; Wayne, PA: 2011. Susceptibility testing of mycobacteria, nocardia, and other aerobic actinomycetes; approved standard. avaiable at: Highted version of Review NTM Elderly R1.docx. [PubMed] [Google Scholar]
- 103.Westphal JF. Macrolide - induced clinically relevant drug interactions with cytochrome P-450A (CYP) 3A4: an update focused on clarithromycin, azithromycin and dirithromycin. British journal of clinical pharmacology. 2000;50(4):285–295. doi: 10.1046/j.1365-2125.2000.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kharasch ED, Thummel KE. Identification of cytochrome P450 2E1 as the predominant enzyme catalyzing human liver microsomal defluorination of sevoflurane, isoflurane, and methoxyflurane. Anesthesiology. 1993;79(4):795–807. doi: 10.1097/00000542-199310000-00023. [DOI] [PubMed] [Google Scholar]
- 105.Gooderham MJ, Bolli P, Fernandez PG. Concomitant digoxin toxicity and warfarin interaction in a patient receiving clarithromycin. The Annals of pharmacotherapy. 1999;33(7–8):796–799. doi: 10.1345/aph.18330. [DOI] [PubMed] [Google Scholar]
- 106.Mergenhagen KA, Olbrych PM, Mattappallil A, Krajewski MP, Ott MC. Effect of azithromycin on anticoagulation-related outcomes in geriatric patients receiving warfarin. Clinical therapeutics. 2013;35(4):425–430. doi: 10.1016/j.clinthera.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 107.Delavenne X, Ollier E, Basset T, Bertoletti L, Accassat S, Garcin A, Laporte S, Zufferey P, Mismetti P. A semi-mechanistic absorption model to evaluate drug-drug interaction with dabigatran: application with clarithromycin. British journal of clinical pharmacology. 2013;76(1):107–113. doi: 10.1111/bcp.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Baciewicz AM, Chrisman CR, Finch CK, Self TH. Update on rifampin, rifabutin, and rifapentine drug interactions. Current medical research and opinion. 2013;29(1):1–12. doi: 10.1185/03007995.2012.747952. [DOI] [PubMed] [Google Scholar]
- 109.Davey PG. Overview of drug interactions with the quinolones. The Journal of antimicrobial chemotherapy. 1988;22 (Suppl C):97–107. doi: 10.1093/jac/22.supplement_c.97. [DOI] [PubMed] [Google Scholar]
- 110.Hayashi M, Takayanagi N, Kanauchi T, Miyahara Y, Yanagisawa T, Sugita Y. Prognostic factors of 634 HIV-negative patients with Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2012;185(5):575–583. doi: 10.1164/rccm.201107-1203OC. [DOI] [PubMed] [Google Scholar]
- 111.Mirsaeidi M, Machado RF, Garcia JGN, Schraufnagel DE. Nontuberculous Mycobacterial Disease Mortality in the United States, 1999–2010; A Population-Based Comparative Study. PloS one. 2014 doi: 10.1371/journal.pone.0091879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chou CH, Chen HY, Chen CY, Huang CT, Lai CC, Hsueh PR. Clinical features and outcomes of disseminated infections caused by non-tuberculous mycobacteria in a university hospital in Taiwan, 2004–2008. Scand J Infect Dis. 2011;43(1):8–14. doi: 10.3109/00365548.2010.519345. [DOI] [PubMed] [Google Scholar]
- 113.Rybicki BA, Iannuzzi MC, Frederick MM, Thompson BW, Rossman MD, Bresnitz EA, Terrin ML, Moller DR, Barnard J, Baughman RP, DePalo L, Hunninghake G, Johns C, Judson MA, Knatterud GL, McLennan G, Newman LS, Rabin DL, Rose C, Teirstein AS, Weinberger SE, Yeager H, Cherniack R. Familial aggregation of sarcoidosis. A case-control etiologic study of sarcoidosis (ACCESS) Am J Respir Crit Care Med. 2001;164(11):2085–2091. doi: 10.1164/ajrccm.164.11.2106001. [DOI] [PubMed] [Google Scholar]


