Abstract
Objective
While it is known that positive surgical margins increase the risk of cervical cancer recurrence, little is known about the effect of close surgical margins (CSM). Therefore, we set out to determine the impact of margin status on recurrence and survival in patients with early-stage cervical cancer.
Methods
A retrospective review was conducted of patients undergoing radical hysterectomy from 2000 to 2010 with Stage IA2-IIA cervical cancer. CSM were defined as ≤5 mm; association with other clinicopathologic factors as well as recurrence and survival was evaluated.
Results
Of the 119 patients, 75 (63%) with CSM had a recurrence rate of 24% compared to 9% without CSM. Though not independently associated with recurrence, CSM were significantly associated with positive lymph nodes (44% vs. 18%), positive parametria (33.3% vs. 2.3%), larger tumors (3.5 vs. 2.5 cm), greater depth of stromal invasion (DOI) (84% vs. 33%), and lymphovascular space invasion (LVSI) (61.3% vs. 34.1%). We failed to find an association between adjuvant therapy and recurrence in those with CSM. Exploratory analysis revealed that a surgical margin of ≤2 mm was significantly associated with an increased risk of overall recurrence (36% vs. 9%, p=0.009) as well as loco-regional recurrence (22% vs. 4%, p=0.0034).
Conclusions
Surgical margins of ≤5 mm on radical hysterectomy specimens are often associated with other high or intermediate risk factors for recurrence. While not a proven independent risk factor, the distance to surgical margin may warrant further investigation as an intermediate risk factor along with tumor size, DOI and LVSI.
Keywords: Cervical cancer, Surgical margins, Recurrence risk, Radical hysterectomy
Introduction
It is estimated that there will be over 12,000 cases of invasive cervical cancer in the United States and over 4000 deaths this year [1]. Based on the International Federation of Gynecology and Obstetrics (FIGO), 50–75% of cases in the United States are diagnosed as early-stage (Stage IA–IIA) disease. Treatment of early-stage disease can be with either radical hysterectomy or curative-intent radiation therapy (RT), but usually depends on age, medical comorbidities, and other clinicopathologic features [2-4]. Outcomes of radical hysterectomy and curative radiation therapy are comparable, with 85–90% 5-year survival in patients with stage IB and 65–75% for patients with IIA disease [5].
Certain clinicopathologic features have been identified as risk factors for recurrence following primary treatment with a radical hysterectomy and lymph node dissection. These factors include high risk factors such as lymph node metastasis, positive surgical resection margins, and parametrial extension [6]. When any one of these factors is present, the role of adjuvant therapy with concurrent chemoradiation has been conclusively demonstrated to provide a progression free (PFS) and overall survival (OS) advantage [7]. Intermediate risk factors are also predictive of recurrence and include deep stromal invasion, lymphovascular space invasion, and large tumor size [8]. When certain combinations of these intermediate risk factors are found, patients who receive adjuvant radiotherapy have a lower risk of recurrence and a trend toward lower mortality [8,9].
Unlike studies in vulvar cancer, in which surgical margins of 8 mm or more are consistently associated with decreased risk of local recurrence, the role of close surgical margins in cervical cancer are less clear [10,11]. To date, few studies have evaluated this finding as a prognostic factor for cervical cancer. In one series, it was noted that patients who had a close vaginal margin, defined as ≤5 mm from invasive tumor, had significantly lower recurrence rates (86% versus 13%, p<0.05) and improved 5-year survival (81% vs. 29%, p<0.01) when adjuvant radiation therapy was administered [12]. However, it should be noted that in total, only twenty-three patients (2%) had CSM and that six of the sixteen who were treated with adjuvant radiation also had other high-intermediate risk factors. In another study by Viswanathan et al., a close surgical margin, defined as <10 mm, was identified in 46 out of 284 (16%) women. Lymph node metastasis and LVSI were identified in 22% and 41%, respectively. While the authors didn’t find a difference in recurrence rate between patients with negative versus CSM, univariate analysis revealed that each mm increase in distance from the tumor was correlated with improved relapse-free survival (HR 0.002/increasing mm from cancer, p=0.03) [13].
As recurrent disease often results in death among women with cervical cancer, factors that improve outcomes after primary treatment are important to identify. Salvage options after definitive or adjuvant radiotherapy are limited to radical, usually exenterative, surgery and occasionally re-irradiation [5]. Given the limited options and poor salvage rate, it is important to identify characteristics of those patients at highest risk of recurrence and thus identify those who may benefit from adjuvant therapy after radical hysterectomy [14,15]. Therefore, the objective of this study was to evaluate close surgical margins as a risk factor for recurrent disease.
Methods
Approval to conduct this study was obtained from the Institutional Review Board at the Ohio State University Wexner Medical Center. All patients who underwent primary radical hysterectomy and pelvic lymph node dissection for FIGO clinical Stage IA2 to IIA cervical cancer at our institution from 1/2000 to 1/2010 were retrospectively identified from tumor registry databases. Only patients with squamous cell, adenocarcinoma, or adenosquamous carcinoma were included. Patients were required to have at least 6 months of follow-up data and were excluded if positive margins were noted or if surgical margin data was not included or not evaluable in the final pathology report.
Additional clinical information was obtained from patient medical records. This included patient demographics (age and race) and surgical data including stage, surgical procedures performed, tumor histology, lymph node counts and status, tumor size, margin status and distance from tumor, lymphovascular space invasion, parametrial involvement, and depth of stromal invasion. For the purposes of this study, we defined close surgical margins as invasive tumor ≤5 mm from the paracervical or paravaginal margin. Paravaginal margins were defined as the distance from the tumor to the edge of vaginal resection margins and were categorized as anterior and posterior. Paracervical margins included lateral margins as measured from the tumor to edge of resected parametria as well as anterior/posterior cervical soft tissue margins. Measurement of surgical margins is a routine portion of the histopathologic examination at our institution and a gynecologic pathologist reviews all specimens at initial evaluation and again at tumor board. Information regarding adjuvant treatment was also collected and, if applicable, details of recurrence including time to progression were recorded.
For statistical analysis, the Wilcoxon rank-sum test compared continuous variables and Fisher’s exact test compared categorical variables across margin status. Kaplan–Meier plots were used to display the progression free survival and log-rank tests were used to assess differences between the Kaplan–Meier curves. Cox proportional hazard model was used to determine the close surgical margin hazard ratio for time to recurrence. The model was adjusted for high and intermediate risk factors previously described. Surgical margin was analyzed as a dichotomous (≤2 mm, ≤5 mm, and ≤10 mm) and as a continuous variable. The method of fractional polynomials was used to assess the scale of the continuous variable to ensure that it was linear in the log-hazard and if a transformation is needed the results will be back transformed to the original units of mm. All confidence intervals (CI) are at the 95% level and p-values are two sided. All analyses were run using Stata 12.0, Stata Corporation, College Station, Texas.
Results
A total of 119 patients met study inclusion criteria. Clinicopathologic characteristics are listed in Table 1. The median age at diagnosis was 42 years. Eighty-seven patients (72%) had FIGO Stage IB1, 28 patients (24%) had Stage IB2, and the remaining 4 patients (4%) had either Stage IA2 or IIA cervical cancer. The majority of patients had squamous histology (68%), followed by adenocarcinoma (21%) and adenosquamous (11%). In addition to radical hysterectomy and pelvic lymph node dissection, other surgical procedures performed included bilateral salpingo-oophorectomy in 70 (59%) patients and para-aortic lymph node dissection in 56 (47%) patients. Additionally, 83% of surgical procedures were performed via laparotomy and the remaining 17% were performed robotically.
Table 1.
Clinicopathologic characteristics by close surgical margin.
| Characteristic | Close surgical margin
|
Total | p-Value | |
|---|---|---|---|---|
| No (>5 min) | Yes (≤5 mm) | |||
| Count | 44 | 75 | 119 | |
| Median age in years (range) | 40.5 (33–48) | 42.0 (34–52) | 42 (33–52) | 0.48 |
| White race, (%) | 33 (75%) | 59 (79%) | 92 (77%) | 0.66 |
| Stage, (%) | ||||
| IA2 | 2 (5%) | 1 (1%) | 3 (3%) | 0.47 |
| IB1 | 34 (77%) | 53 (71%) | 87 (72%) | |
| IB2 | 8 (18%) | 20 (27%) | 28 (24%) | |
| IIA | 0 | 1 (1%) | 1 (1%) | |
| Histology, (%) | 0.18 | |||
| Squamous | 28 (63%) | 53 (71%) | 81 (68%) | |
| Adenocarcinoma | 13 (30%) | 12 (16%) | 25 (21%) | |
| Adenosquamous | 3 (7%) | 10 (13%) | 13 (11%) | |
| Surgery type, (%) | 0.005 | |||
| Laparotomy | 42 (95%) | 57 (76%) | 99 (83%) | |
| Robotic | 2 (5%) | 18 (24%) | 20(17%) | |
| Positive nodes, (%) | 8 (18%) | 33 (44%) | 41 (34%) | 0.005 |
| Positive parametria, (%) | 1 (2%) | 25 (33%) | 26 (22%) | <0.001 |
| Median tumor size (mm) | 25 (13–38) | 35 (30–50) | N/A | <0.001 |
| Median DOI (mm) | 6 (3–12) | 12 (11–16) | N/A | <0.001 |
| Median DOI (%) | 33 (20–50) | 84 (73–94) | N/A | <0.001 |
| LVSI, (%) | 15 (34%) | 46 (61%) | 61 (51%) | 0.005 |
| Adjuvant therapy, (%) | 12 (28%) | 48 (64%) | 60 (50%) | <0.001 |
LVSI – lymphovascular space invasion.
DOI – depth of cervical stromal invasion.
In regard to margin status, the median distance from tumor was 4 mm (range 0.5–21 mm). Seventy-five patients (63%) were found to have surgical margins within 5 mm of the tumor. Twelve (10%) patients had a vaginal margin≤5 mm and five (4%) had a parametrial margin≤5 mm. Anterior and posterior paracervical margins≤5 mm were found in 34 (29%) and 21 (18%) patients, respectively. The remainder of patients had close paracervical margins not otherwise specified. No statistical difference was found between patients with close margins compared to those without in regard to race, stage, grade, or histology. However, patients who underwent robotic surgery (18/20, 90%) were more likely to have close surgical margins compared to those undergoing laparotomy (57/99, 58%) (p=0.005). Patients with close margins were then examined for the frequency of high and intermediate risk factors (Table 1). Patients with close surgical margins were more likely to have positive lymph nodes and parametrial extension as well as larger tumors, greater depth of stromal invasion, and the presence of LVSI. In fact, only 5 patients (4.2%) who had close surgical margins were noted to have an absence of other high or intermediate risk factors for recurrence. Accordingly, 64% of patients with close surgical margins received adjuvant therapy as compared to 28% of those without CSM (p<0.001).
Recurrent cervical cancer was diagnosed in 18 (24%) of patients with close margins and only 4 (9%) of those without. Table 2 depicts the location of recurrences in each group. Despite the absolute difference of 15%, close surgical margins was not statistically significant in regard to rate or time to recurrence when compared to those without close surgical margins (Fig. 1). The unadjusted hazard ratio (HR) for recurrence was 2.75 (95% CI: 0.93–8.15; p=0.07). However, when adjusting for high and intermediate risk factors this trend was lost with HR of 1.61 (95% CI: 0.49–5.24; p=0.43). Univariate Cox proportional hazard regressions (Table 3) showed that significant predictors of recurrence included positive lymph nodes, parametrial extension, a 10 mm incremental increase in DOI, as well as a one percentage increase in DOI and LVSI. Close surgical margins approached significance with a HR of 2.83 (95% CI: 0.96–8.38, p=0.06); however the HR of 1.88 (95% CI: 0.82–4.30, p=0.13) for adjuvant therapy was not statistically associated with recurrence.
Table 2.
Location of recurrence by surgical margin status.
| Surgical margin>5 mm N = 44 | Surgical margin<5 mm N = 75 | p-Value | |
|---|---|---|---|
| Recurrence | 4 (9%) | 18 (24%) | 0.05 |
| Vaginal recurrence | 1 (2%) | 5 (7%) | 0.48 |
| Pelvic recurrence | 3 (7%) | 7 (9%) | 0.79 |
| Distant recurrence | 2 (5%) | 11 (15%) | 0.18 |
Fig. 1.
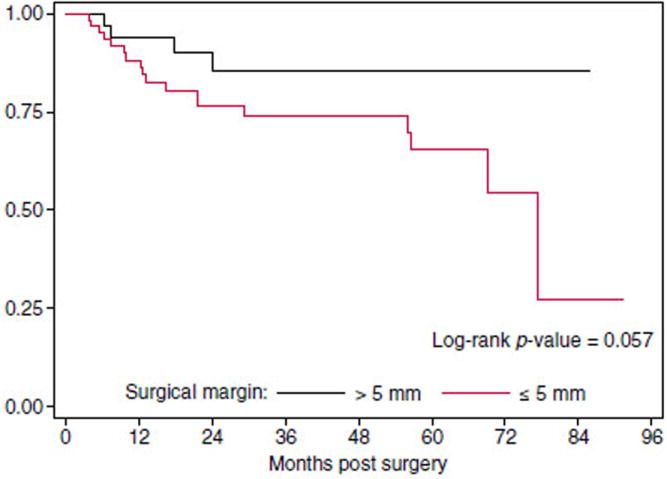
Progression-free survival for patients based on surgical margins.
Table 3.
Table based on univariate Cox proportional hazard regressions.
| Predictor | HR (95% CI) | p-Value |
|---|---|---|
| Close surgical margin, yes | 2.83 (0.96–8.38) | 0.06 |
| Positive lymph node, yes | 2.83 (1.25–6.43) | 0.01 |
| Positive parametria, % | 2.88 (1.24–6.67) | 0.01 |
| 10 mm increase in tumor size | 1.15 (0.90–1.48) | 0.26 |
| 10 mm increase in DOI | 1.86 (1.14–3.02) | 0.01 |
| 1% increase in SI | 1.03 (1.00–1.05) | 0.04 |
| 1% increase in LVSI | 5.81 (1.97–17.09) | 0.001 |
As a surgical margin of <5 mm was not predictive of recurrence, additional margin distance was evaluated to determine a correlation with recurrence. Univariate analysis of surgical margin status using ≤10 mm, ≤5 mm, and ≤2 mm is depicted in Table 4. Closer margins were associated with a higher hazard of recurrence with the hazard ratios of 1.51 (95% CI: 0.45–5.12, p=0.506), 2.83 (95% CI: 0.96–8.38, p=0.061), and 3.22 (95% CI: 1.37–7.55, p=0.007) for ≤10 mm, ≤5 mm, and ≤2 mm, respectively. Surgical margin was also analyzed as a continuous variable with a margin of 1 mm as the referent group. Hazards of recurrence were then generated for margins of 2 to 10 mm by increments of 1 mm. The scale of continuous margins was assessed using the methods of fractional polynomials to insure that margin was linear in the log-hazard. A reciprocal transformation was found to significantly reduce the model’s log-likelihood. When the model results were back transformed the hazard of recurrence decreased 66.2% for 2 mm margin relative to 1 mm (p<0.001). Similarly at 5 mm and 10 mm, the hazard of recurrence decreases 82.4% and 85.8% (both p<0.001), respectively.
Table 4.
Univariate analysis of surgical margin distance.
| Model | HR (95% CI) | p-Value |
|---|---|---|
| Margin≤10 mm | 1.51 (0.45–5.12) | 0.506 |
| Margin≤5 mm | 2.83 (0.96–8.38) | 0.061 |
| Margin≤2 mm | 3.22 (1.37–7.55) | 0.007 |
Patients that recurred had a median surgical margin of 2 mm as opposed to 5 mm in patients who did not recur (Fig. 2). When using 2 mm as a cut-off for surgical margins, patients were noted to have an increased risk of overall recurrence (36% vs. 9%, p=0.009) as well as loco-regional recurrence (22% vs. 4%, p=0.003). The HR for recurrence was significant at 3.22 (95% CI: 1.37–7.55, p=0.007) as depicted in Table 4. In patients with surgical margins≤2 mm, only the presence of LVSI was significantly associated with increased risk of recurrence (46% vs. 9%, p=0.03). Interestingly, adjuvant therapy did not decrease the risk of recurrence (38% vs. 35%, p=0.72).
Fig. 2.
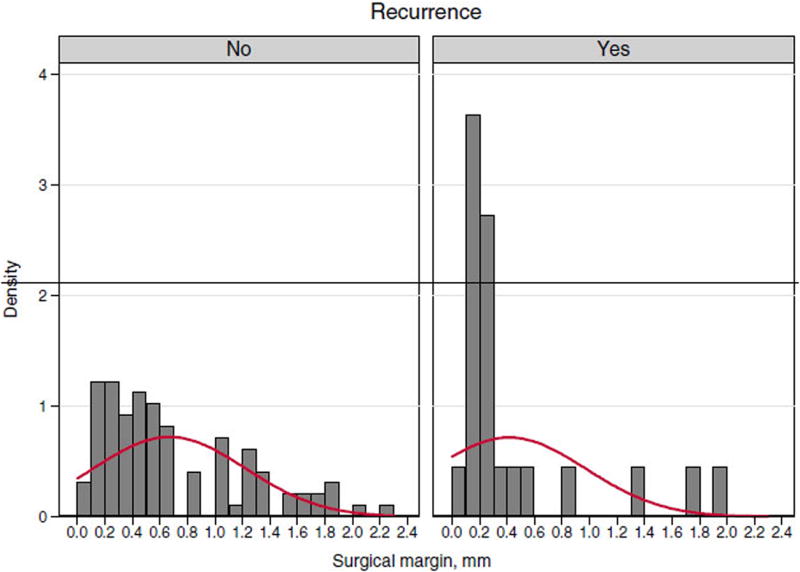
Descriptive summary of surgical margin and recurrence.
Discussion
Despite successful treatment of early-stage cervical cancer with either surgery, radiation or a combination of therapies, recurrence rates range from 10 to 20%. Along with parametrial, lymph node, and surgical margin involvement, factors including tumor size, lymphovascular space invasion and depth of stromal invasion increase the risk of recurrent disease. However, the impact of close surgical margins in cervical cancer has not been as extensively evaluated. In the present study, we analyzed the risk of recurrence in patients with early-stage cervical cancer after radical hysterectomy with close surgical margins defined as ≤5 mm. The data showed that patients with close margins were more likely to have larger tumor size, deep stromal invasion, as well as a higher rate of lymphovascular space invasion and positive nodal status. Though not significant, we noted an increased absolute recurrence rate in patients with close margins (24%) compared to those without close margins (9%). Interestingly, these rates are similar to those reported by Viswanathan and colleagues who also noted that close surgical margins were associated with a statistically significant higher rate of overall recurrence (20% vs. 11%) but not in the rate of local recurrence [13]. When analyses were adjusted for the presence of high and intermediate risk factors, the trend toward significance of close surgical margins was lost, most likely signifying the fact that close surgical margins are often associated with other known risk factors for recurrence.
The overall rate of close surgical margins in this study was higher than what is reported in the limited published literature on this topic. There are a few potential explanations for these findings. The papers by Estape et al. and Viswanathan et al. are the only other publications that address the question of CSM directly and both use different definitions. Specifically, Viswanathan et al. reported a 16% rate of CSM defined as <10 mm and didn’t include women with close vaginal margins alone [13]. On the other hand, Estape et al. focused their evaluation only on close vaginal margins and reported a 2% rate of CSM [12]. The comprehensive inclusion of all measurable surgical margins in our study is one possible explanation for the higher rate that we have observed. The advent of minimally invasive surgery, particularly robotic surgery, also has contributed to the higher rate of CSM in this study. We found that the majority of patients who were treated with robotic radical hysterectomy (18 out of 20) had CSM.
Given the increased risk of complications with radiation therapy following radical hysterectomy and consequences of recurrence, it is critical that patients who are at increased risk of recurrence are identified and managed appropriately [9,13,15]. Though our study does not demonstrate that CSM are an independent predictor of recurrence, this finding is consistent with the current literature [12,13]. This is likely due to the fact that CSM are often associated with other risk factors for recurrence. In fact, only 5 (4.2%) patients in this study had close surgical margins in the absence of other intermediate or high-risk factors for recurrence, which is similar to the 2% in the study by Estape et al. [12]. Furthermore, Viswanathan et al. established that among patients with close margins, each millimeter increase in the distance from the tumor to the margin significantly increased relapse-free survival [13]. This is consistent with the results of the exploratory analysis of this study where a margin of ≤2 mm was found to be associated with a statistically significant increase in recurrence. However, there was no difference in the rate of recurrence among patients that received adjuvant therapy and those that did not. It is possible that the study was not powered to detect such a difference, but does raise suspicion that perhaps there are other factors such as tumor biology that negates the effect of adjuvant therapy. Since the cut-off margin of ≤2 mm was not pre-defined, it is difficult to draw conclusions from this analysis.
Interestingly, we found that patients who were treated with robotic radical hysterectomy and pelvic lymph node dissection had higher rates of CSM (90%) than patients treated with laparotomy (58%). One possible explanation is that the magnification provided by robotic surgery gives an overestimation of margin status. Thus, it is imperative that the surgeon examines the surgical specimen before sending to the pathology lab and obtains additional margins as necessary. One may hypothesize that given the ingenuity of robotic surgery that less challenging cases would initially be selected. However, this did not appear to be the case as the median tumor size of patients treated with robotic surgery was 34 mm. Similar to the general population of patients with close surgical margins, patients treated with robotic radical hysterectomy and pelvic lymph node dissection had a recurrence rate of 25%.
As a retrospective study, limitations of data analyses including selection bias and lack of pathology re-review should be acknowledged. Secondly, as a single-institution review, a limited sample size, and the exclusion of patients with missing data present difficulties in making definitive conclusions. Furthermore, excluding patients with risk factors that result in the recommendation for adjuvant therapy would have resulted in a significantly smaller sample size. However, we made efforts to include all patients who met study inclusion criteria and our study results parallel the current literature [12,13]. Though a larger sample size from multiple institutions may have allowed for some of the observed trends to be statistically significant, our study demonstrates that close surgical margins warrant further attention. Despite the above weaknesses, this is the only study to include a comprehensive evaluation of surgical margins after radical hysterectomy and the first to examine patients treated with robotic radical hysterectomy.
In summary, CSM after radical hysterectomy are often observed with other high and intermediate-risk factors for recurrence. As an isolated entity, it may be associated with an increased risk of recurrence, but understanding the true role of CSM as a prognostic factor remains elusive. Given the high association with other risk factors for recurrence, it is unlikely that the exact risk of an isolated close surgical margin can be studied in a prospective setting. However, consideration of close surgical margins as an intermediate risk factor, along with tumor size, depth of invasion, and lymphovascular space invasion, may be warranted.
HIGHLIGHTS.
-
►
Close surgical margins (≤5 mm) after surgery for cervical cancer are associated with other known risk factors for recurrence.
-
►
Surgical margins (≤2 mm) were associated with an increase risk of recurrence.
Footnotes
Conflict of interest statement
All of the authors have completed the disclosure form and no author has a conflict of interest with the content of the manuscript.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Newton M. Radical hysterectomy or radiotherapy for stage I cervical cancer. A prospective comparison with 5 and 10 years follow-up. Am J Obstet Gynecol. 1975;123(5):535–42. doi: 10.1016/0002-9378(75)90041-1. [DOI] [PubMed] [Google Scholar]
- 3.Morley GW, Seski JC. Radical pelvic surgery versus radiation therapy for stage I carcinoma of the cervix (exclusive of microinvasion) Am J Obstet Gynecol. 1976;126(7):785–98. doi: 10.1016/0002-9378(76)90668-2. [DOI] [PubMed] [Google Scholar]
- 4.Hoskins WJ, Ford JH, Jr, Lutz MH, Averette HE. Radical hysterectomy and pelvic lymphadenectomy for the management of early invasive cancer of the cervix. Gynecol Oncol. 1976;4(3):278–90. doi: 10.1016/0090-8258(76)90033-0. [DOI] [PubMed] [Google Scholar]
- 5.Barakat RR, Markman M, Randall M. Principles and practice of gynecologic oncology. 5. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2009. [Google Scholar]
- 6.Samlal RA, van der Velden J, Schilthuis MS, Gonzalez Gonzalez D, Ten Kate FJ, Hart AA, et al. Identification of high-risk groups among node-positive patients with stage IB and IIA cervical carcinoma. Gynecol Oncol. 1997;64(3):463–7. doi: 10.1006/gyno.1996.4576. [DOI] [PubMed] [Google Scholar]
- 7.Fuller AF, Jr, Elliott N, Kosloff C, Hoskins WJ, Lewis JL., Jr Determinants of increased risk for recurrence in patients undergoing radical hysterectomy for stage IB and IIA carcinoma of the cervix. Gynecol Oncol. 1989;33(1):34–9. doi: 10.1016/0090-8258(89)90598-2. [DOI] [PubMed] [Google Scholar]
- 8.Delgado G, Bundy B, Zaino R, Sevin BU, Creasman WT, Major F. Prospective surgical-pathological study of disease-free interval in patients with stage IB squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol Oncol. 1990;38(3):352–7. doi: 10.1016/0090-8258(90)90072-s. [DOI] [PubMed] [Google Scholar]
- 9.Rotman M, Sedlis A, Piedmonte MR, Bundy B, Lentz SS, Muderspach LI, et al. A phase III randomized trial of postoperative pelvic irradiation in Stage IB cervical carcinoma with poor prognostic features: follow-up of a gynecologic oncology group study. Int J Radiat Oncol Biol Phys. 2006;65(1):169–76. doi: 10.1016/j.ijrobp.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 10.Heaps JM, Fu YS, Montz FJ, Hacker NF, Berek JS. Surgical-pathologic variables predictive of local recurrence in squamous cell carcinoma of the vulva. Gynecol Oncol. 1990;38(3):309–14. doi: 10.1016/0090-8258(90)90064-r. [DOI] [PubMed] [Google Scholar]
- 11.Chan JK, Sugiyama V, Pham H, Gu M, Rutgers J, Osann K, et al. Margin distance and other clinico-pathologic prognostic factors in vulvar carcinoma: a multivariate analysis. Gynecol Oncol. 2007;104(3):636–41. doi: 10.1016/j.ygyno.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Estape RE, Angioli R, Madrigal M, Janicek M, Gomez C, Penalver M, et al. Close vaginal margins as a prognostic factor after radical hysterectomy. Gynecol Oncol. 1998;68(3):229–32. doi: 10.1006/gyno.1998.4960. [DOI] [PubMed] [Google Scholar]
- 13.Viswanathan A, Lee H, Hanson E, Berkowitz R, Crum C. Influence of margin status and radiation on recurrence after radical hysterectomy in Stage IB cervical cancer. Int J Radiat Oncol Biol Phys. 2006;65(5):1501–7. doi: 10.1016/j.ijrobp.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 14.Hong JH, Tsai CH, Chang TC, Wang CC, Chou HH, Lee SP, et al. Recurrent squamous cell carcinoma of cervix after definitive radiotherapy. Int J Radiat Oncol Biol Phys. 2004;60(1):249–57. doi: 10.1016/j.ijrobp.2004.02.044. [DOI] [PubMed] [Google Scholar]
- 15.Bandy LC, Clarke-Pearson DL, Soper JT, Mutch DG, MacMillan J, Creasman WT. Long-term effects on bladder function following radical hysterectomy with and without postoperative radiation. Gynecol Oncol. 1987;26(2):160–8. doi: 10.1016/0090-8258(87)90269-1. [DOI] [PubMed] [Google Scholar]


