Abstract
The retroviral oncoprotein Tax from Human T cell leukemia virus type 1 (HTLV-1), an etiological factor that causes adult T cell leukemia and lymphoma, plays a crucial role in initiating T lymphocyte transformation by inducing oncogenic signaling activation. We here report that Tax is a determining factor for dysregulation of autophagy in HTLV-1-transformed T cells and Tax-immortalized CD4 memory T cells. Tax facilitated autophagic process by activating IκB kinase complex, which subsequently recruited an autophagy molecular complex containing Beclin1 and Bif-1 to the lipid raft microdomains. Tax engaged a crosstalk between IκB kinase complex and autophagic molecule complex by directly interacting with both complexes, promoting assembly of LC3+ autophagosomes. Moreover, expression of lipid raft-targeted Bif-1 or Beclin1 was sufficient to induce formation of LC3+ autophagosomes, suggesting that Tax recruitment of autophagic molecules to lipid rafts is a dominant strategy to deregulate autophagy in the context of HTLV-1 transformation of T cells. Furthermore, depletion of autophagy molecules such as Beclin1 and PI3 kinase class III resulted in impaired growth of HTLV-1-transformed T cells, indicating a critical role of Tax-deregulated autophagy in promoting survival and transformation of virally infected T cells.
Keywords: HTLV-1 Tax, IKK, autophagy, lipid rafts, Beclin1, Bif-1
Introduction
Human T cell leukemia virus type 1 (HTLV-1) is the etiological factor that causes adult T cell leukemia and lymphoma (ATL). The HTLV-1 viral genome-encoded oncoprotein, Tax, plays a pivotal role for promoting viral replication and initiating malignant transformation of CD4+ T lymphocytes. Tax deregulates various oncogenic signaling including IκB kinase (IKK)/NF-κB, STAT3 and PI3KC1/Akt for aberrant proliferation of infected T cells 1-6. Notably, the constitutive activity of NF-κB is thought to be a prerequisite for induction of ATL. Tax activates NF-κB by stimulating the activity of the IKK complex, the key regulator of NF-κB signaling 1,7-9. The IKK complex is composed of two highly homologous catalytic subunits, IKKα and IKKβ, and a regulatory subunit, IKKγ 10-12. The IKK complex can be activated by divergent upstream kinases that connect it to signals from cell surface receptors such as T cell receptor (TCR) 13-19. Upon T cell activation, IκB kinases are recruited to the plasma membrane lipid raft microdomains where they become catalytically active. This detergent-resistant membrane structure is enriched with cholesterol and sphingolipids, which are generated in the Golgi and can be recycled from the Golgi to the plasma membrane 20. These unique membrane structures serve as crucial signal transduction platform. Distinct from TCR activation, Tax bypasses upstream kinases to target the IKK complex through direct interaction with IKKγ 1,21, recruiting this kinase complex to the lipid raft microdomains for activation 22.
The IKK complex has been implicated in playing an important role in starvation- or rapamycin-induced autophagy 23, though the underlying mechanism remains to be determined. Autophagy is a catabolic process and is evolutionarily conserved among living organisms 24,25. In response to metabolic stress, autophagosomes form and sequester aggregated cellular proteins and organelles, which are subsequently degraded through their fusion with lysosomes to generate autolysosomes. This process generates energy for the need of metabolically stressed cells and hence, the primary function of autophagy is pro-survival. Although the role of autophagy in oncogenesis remains controversial, it is known that autophagy contributes to chemotherapy resistance due to its cytoprotective function 26. Furthermore, autophagy is necessary for certain tumorigenic viruses for their productive replication and induction of oncogenesis 27-30. Hepatitis C virus (HCV)-induced autophagy promotes initiation of viral infection 31, and inhibition of autophagy represses HCV replication 32. Latent membrane protein 1 (LMP1), an oncogene product from Epstein-Barr virus (EBV), induces early or late stage autophagy depending on its expression levels 33. Inhibition of autophagy in EBV-infected cells suppresses transforming phenotypes resulting from accumulation of LMP1. Further, the oncogenic X protein from Hepatitis B Virus (HBV) sensitizes cells to starvation-induced autophagy by increasing Beclin1 (BECN1) expression 34.
The autophagic process is regulated by a variety of oncogenic signaling pathways 35-38. Given the evidence that HTLV-1 mediates oncogenic activation, it is conceivable that HTLV-1 may deregulate autophagy for its own benefits in viral oncogenesis. In our previous reports, we identified a novel feature of HTLV-1 Tax in dysregulation of autophagy 22, and examined the role of HTLV-2 Tax-deregulated autophagy in supporting survival of Tax2-immortalized, human memory CD4+ T cells 39. In the present study, we investigated the underlying mechanism of HTLV-1 Tax in deregulating autophagy in human T lymphocytes. Our data demonstrated that Tax deregulates autophagy by connecting the IKK complex to autophagy pathways in a unique mechanism that involves lipid raft recruitment of IκB kinases and autophagic molecular complexes. We further showed that Tax-deregulated autophagy is crucial for survival and proliferation of HTLV-1-transformed T cells.
Results
Tax deregulates autophagy in HTLV-1-transformed T cells
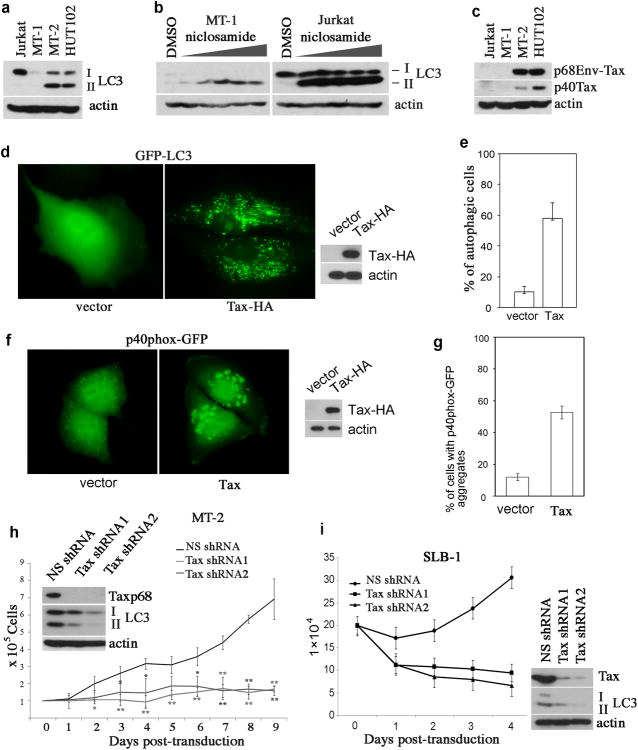
During the process of autophagy, LC3 is lipidated, resulting in a mobility shift from LC3-I to –II. The latter is associated with the autophagosome, which can be visualized by fluorescence imaging. Using these techniques, we evaluated the basal activity of autophagy in HTLV-1-transformed T cell lines including MT-1, MT-2 and HUT102. We observed high levels of LC3-II in MT-2 and HUT102, but not in non-HTLV-1-infected T cell line, Jurkat (Figure 1a). MT-1 cells expressed very low levels of both LC3-I and LC3-II (Figure 1a), indicating that these cells had a low basal activity of autophagy. Accumulation of LC3-II was observed in MT-1 cells and Jurkat T cells that were treated with niclosamide (Figure 1b), an inhibitor of mammalian target of rapamycin (mTOR) complex 40, which suggested that the autophagy pathway remained responsive to mTOR inhibition in these cells.
Figure 1.
Constitutive autophagic activity in HTLV-1-transformed T cells. (a) LC3 immunoblot analysis of HTLV-1-transformed T cell lines including MT-1, MT-2 and HUT102. Non-HTLV-1-transformed T cell line, Jurkat, is used for control. (b) MT-1 and Jurkat T cells were treated with niclosamide at increasing doses (0.625, 1.25, 2.5, 5 and 10μM) for 6 hours, and total cell lysates were prepared for anti-LC3 immunoblot. (c) The expression status of the Tax protein in HTLV-1-transformed T cell lines, as examined by anti-Tax immunoblot. (d) GFP-LC3 was co-transfected with vector or with Tax in HeLa cells. 48 hours following transfection, the cells were analyzed with fluorescence microscopy (left panel) and the whole cell lysates were examined with immunoblot for detection of Tax (right panel). (e) Percentage of autophagic cells in transfected cells seen in (d). (f) p40phox-GFP was co-transfected with the control vector or with Tax in HeLa cells. The cytoplasmic aggregates of p40phox-GFP were detected by fluorescence imaging (left panel). The right panel showed Tax expression in transfected cells. (g) The percentage of transfected cells with p40phox-GFP aggregates was shown. (h) Tax was depleted in MT-2 cells by lentivirus transduction of Tax shRNAs, and the efficiency of Tax knockdown was determined by anti-Tax immunoblot (top panel), and the levels of LC3-I and LC3-II in Tax-depleted MT-2 cells were shown in the middle panel. β-actin was used for protein loading control. Trypan blue exclusion assay was performed to examine cell viability of MT-2 cells transduced with a non-specific shRNA (NS), and Tax-specific shRNA1 and shRNA2 via lentivirus transduction. * (p < 0.05) and ** (p < 0.01), as determined by Student's t-test, indicate cell viability differences in Tax shRNA-transfected cells as compared to NS shRNA-transfected control cells at the corresponding time point. (i) The same methods as (h) were used to analyze SLB-1 cells for Tax knockdown efficiency, LC3-II level and cell viability.
MT-2 and HUT102 cells are known to express Tax. Indeed, both MT-2 and HUT102 cells expressed two forms of Tax, a predominant form of the p68 Env-Tax chimeric protein and a minor form, the wild type p40Tax, whereas MT-1 cells did not express Tax (Figure 1c). Therefore, we reasoned that Tax might be a causative factor for induction of autophagy in HTLV-1-transformed T cells. To test this possibility, Tax was co-expressed with GFP-LC3 in an autophagy cell model, HeLa cells. The cytoplasmic GFP-LC3 punctate dots reminiscent of autophagosome foci were seen in Tax-expressing cells, whereas the GFP-LC3 fluorescence appeared to distribute evenly in vector-transfected cells (Figure 1d). At least a 5-fold increase of autophagic cells was detected in Tax-transfected cells as compared to the vector-transfected cells (Figure 1e). Tax also induced formation of p40phox-GFP aggregates with roughly 5-fold increase as compared to the vector-transfected cells (Figure 1f and 1g). This indicated that PI3 kinase class III (PI3KC3), a key autophagy mediator, was activated by Tax. Depletion of Tax via lentivirus transduction of Tax shRNAs impaired conversion of LC3-I to LC3-II (Figure 1g), and resulted in growth arrest of HTLV-1-transformed MT-2 T cells (Figure 1h). The p68Tax knockdown efficiency was shown in Figure 1h, and the wild type p40Tax in MT-2 cells was easily depleted by Tax shRNAs because it was expressed at a much lower level than p68Tax (Figure 1c). Similarly, in SLB-1 cells that only expressed the p40Tax protein, knockdown of Tax resulted in reduction of LC3-II and impaired growth (Figure 1i). Taken together, these results indicated that Tax is the determining factor for formation of increased LC3+ autophagosomes in HTLV-1-transformed T cells.
Tax promotes constitutive autophagy in human CD4 memory T cells
We next determined if Tax plays an essential role in deregulating autophagy in primary human T cells. Two Tax-expressing, human primary T cell lines, PTX4-1 and PL9-1, were established by stable expression of the Tax-GFP fusion protein via lentivirus transduction in human peripheral blood lymphocytes obtained from two healthy donors. These two cell lines exhibited a CD3+/TCRαβ+/CD4+/CD25+/CD45RO+/CD69+ immunophenotype, indicating that they were activated memory CD4+ T lymphocytes (Figure 2a). Surface expression of TCRαβ was slightly down-regulated in these cells (Figure 2a). Constitutive activation of various oncogenic signaling molecules including NF-κB, STAT3, AP-1 and NF-ATc were detected in Tax-GFP-established T cell lines (Figure 2b). Compared to primary human CD4+ T cells, significant accumulation of LC3-II was detected in PTX4-1 and PL9-1 cells, indicating that a constitutive, high level of autophagic activity occurred in Tax-established primary T cells (Figure 2c).
Figure 2.
Oncogenic activation and autophagy induction in Tax-GFP-established T cell lines. (a) Immunophenotype of Tax-GFP immortalized T cell lines, PTX4-1 and PL9-1, as determined by FACS analysis. (b) EMSA assay to detect the activities of NF-κB, Stat3, AP-1, NF-ATc and OCT-1 in Tax-established cells. Human primary CD4+ T cells, which were used as control, were isolated from healthy donor using anti-CD4 conjugated magnetic beads, and stimulated with PHA (1μg/ml) for 1 day, followed by adding IL-2 (100u/ml, every other day) for 2 weeks. (c) Anti-LC3 immunoblot to examine the conversion of LC3-I to LC3-II and Tax expression in Tax-GFP-established T cell lines, PTX4-1 and PL9-1.
Tax deregulates autophagy via the IKK complex
HTLV-1-transformed T cells express constitutively activated PI3KC1/Akt and IKK/NF-κB signaling molecules 7. Previous studies indicated that these two signaling pathways exhibit opposing activities in regulating autophagy 5. Although non-Tax-expressing MT-1 cells exhibited a slightly increased NF-κB activity, much higher levels of NF-κB activity were seen in MT-2, SLB-1 and HUT102 cells 41, which correlated with expression of Tax and high levels of autophagic activity in these cells. These results suggested that Tax could utilize an IκB kinase-dependent cellular mechanism that potentially overpowers Akt-mediated inhibition of autophagy, thereby facilitating autophagic process.
To investigate the notion that the activation of IKK is required for the Tax-deregulated autophagy, we generated Tax-GFP and its variant forms, M22-GFP and M47-GFP. M22 is defective in activating IKK while maintaining its ability to activate CREB 42. In contrast, M47 is unable to activate CREB but maintains the capacity to induce full-scale activation of IKK 42. We found that Tax and M47 induced NF-κB activity whereas M22 did not (Figure 3a). The subcellular distributions of Tax and its mutants also differed. Tax-GFP was distributed in both the nucleus and the cytoplasm with a perinuclear cluster pattern in transfected HT1080 autophagy model cells (Figure 3b), and this subcellular distribution pattern was similar to that seen in HTLV-1-transformed T cells 43. M22-GFP lost the perinuclear cluster pattern and was expressed in the nucleus and the cytoplasm, whereas M47-GFP was expressed predominantly in the perinuclear clusters (Figure 3b). When co-transfected with mKate2-LC3, a far-red, monomeric protein mKate2-tagged LC3, Tax-GFP or M47-GFP induced formation of the cytoplasmic mKate2-LC3 foci, while M22-GFP failed to do so (Figure 3c). Consistent with this finding, IKKβKA, a constitutively active form of IKKβ, induced formation of LC3+ foci (Figure 3c). Similar to the results from Tax mutants, IKKβKA, but not IKKβKM (a kinase mutant form of IKKβ), induced formation of LC3+ foci in HT1080 cells (Figure 3d). In addition, depletion of the catalytic subunits of the IKK complex, IKKα or IKKβ, led to reduced conversion of LC3-I to LC3-II in HTLV-1-transformed MT-2 cells (Figure 3e). Further, knockdown of IKKγ, the essential regulatory subunit of the IKK complex, caused reduction of the LC3-II level (Figure 3f). Together, these results strongly suggest a crucial role of the IKK complex in Tax-deregulated autophagy.
Figure 3.
Tax-induced autophagy is dependent on its ability to activate IκB kinase. (a) NF-κB luciferase reporter assay with transient co-transfection of pNF-κB luciferase plasmid with vector, Tax, M22 or M47 in 293 cells. The lower panel showed expression levels of Tax and its mutants as detected by anti-HA immunoblot. (b) Subcellular localization of Tax-GFP, M22-GFP and M47-GFP in transfected HT1080 cells. (c) HT1080 cells were co-transfected with mKate2-LC3, together with Tax-GFP, M22-GFP, M47-GFP, GFP alone (negative control) or GFP-IKKβKA. The transfected cells were analyzed by fluorescence imaging 48 hours following transfection. (d) Percentage of autophagic cells in GFP-LC3 co-transfected HT1080 cells with vector, IKKβKA or IKKβKM, a kinase mutant form of IKKβ. (e) Anti-LC3, IKKα and IKKβ immunoblots with cellular lysates from HTLV-1-transformed MT-2 T cells transduced with NS- (non-specific shRNA), IKKα- or IKKβ-specific shRNA. (f) Anti-LC3 and -IKKγ immunoblot with cellular lysates from MT-2 cells transduced with NS shRNA or with IKKγ-specific shRNA.
Tax recruits the autophagic molecular complex to lipid rafts through IKK
Our previous study showed that Tax associated with the lipid raft microdomains, hijacking the IKK complex to lipid rafts for activation 22. We reasoned that Tax might be able to direct lipid raft translocation of the autophagy molecules for their activation through the IKK complex. To test this possibility, we performed lipid raft fractionation analysis. BECN1 and Bif-1 are two key molecules that are involved in the initiation of vesicular nucleation during formation of autophagosomes 44. We found that Bif-1 and BECN1, together with IKK and Tax, were constitutively present in the lipid raft fraction in Tax-expressing T cell lines including MT-2, HUT102, SLB-1 and PTX4-1 (Figure 4a, 4b, 4c and 4d), while both autophagy molecules remained in the soluble fractions in non-Tax-expressing T cells including MT-1, Jurkat T cells and normal peripheral blood lymphocytes (PBLs) (Figure 4e, 4f and 4g). It was previously shown that the subunits of the IKK complex were in the soluble fractions in MT-1, Jurkat and PBLs. These results support the idea that Tax is capable of recruiting the autophagic molecules into lipid rafts.
Figure 4.
Autophagy molecules Bif-1 and Beclin1 are associated with the lipid raft microdomains in Tax-expressing T cells. Lipid raft fractionation assay was applied to examine cellular proteins associated with lipid rafts in HTLV-1-transformed T cell lines expressing Tax including HUT102 (a), MT-2 (b) and SLB-1 cells (c), Tax-GFP-established primary human T cell line (PTX4-) (d), HTLV-1-transformed T cell line with lack of Tax expression (MT-1)(e), non-HTLV-transformed T cell line (Jurkat)(f) and normal peripheral lymphocytes (PBLs (g) with various antibodies indicated in the figure. HRP-conjugated cholera toxin B that has specific affinity to lipid rafts to detect GM1 and anti-LAT immunoblot to detect LAT (a lipid raft marker protein) were used as indications of lipid raft fractions.
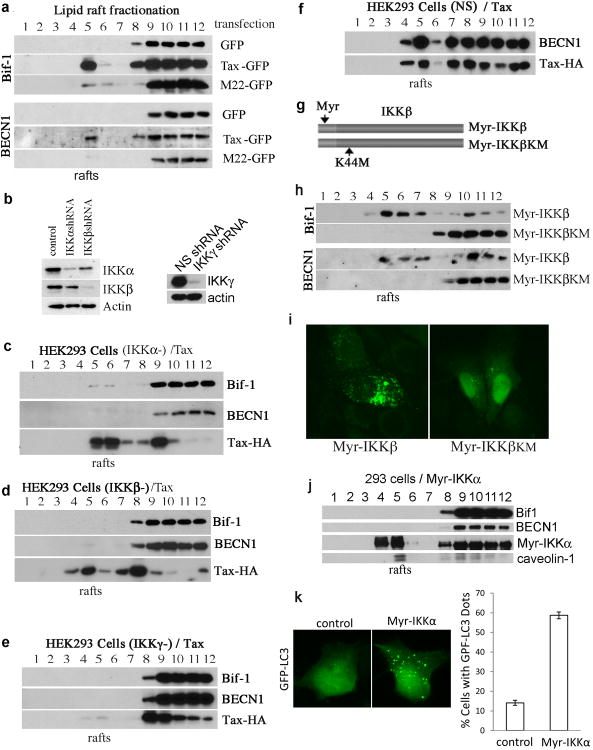
We next verified the role of Tax in directing the lipid raft translocation of BECN1 and Bif-1. In Tax-GFP-transfected cells, the autophagic molecules including BECN1 and Bif-1 were detected in the lipid raft fraction, whereas these molecules remained in the soluble fractions in GFP- or M22-GFP-transfected cells (Figure 5a). To validate the involvement of the IKK complex in Tax recruitment of BECN1 and Bif-1, we generated IKKα-, IKKβ- or IKKγ-depleted cells (Figure 5b), followed by transfection of Tax into these modified cells. We observed that depletion of IKKα, IKKβ or IKKγ impaired lipid raft translocation of BECN1 and Bif-1 by Tax (Figure 5c, 5d and 5e), whereas Tax was able to direct lipid raft translocation of BECN1 in the control cells transfected with Tax (Figure 5f). We also found that Tax failed to translocate into the lipid raft fractions in IKKγ-depleted HEK293 cells (Figure 5e), suggesting a crucial role of IKKγ in assisting lipid raft association of Tax.
Figure 5.
Tax recruits autophagy molecules into lipid rafts. (a) The presence of Bif-1 and BECN1 in lipid rafts in HEK293 cells-transfected with GFP, Tax-GFP or M22-GFP as examined by lipid raft fractionation analysis, followed by anti-Bif-1 and anti-BECN1 immunblots. (b) IKKα, IKKβ and IKKγ knockdown efficiency in HEK293 cells via lentivirus transduction as determined by anti-IKKα, anti-IKKβ and anti-IKKγ immunoblots. Lipid raft presence of autophagy molecules was shown in IKKα-depleted HEK293 cells transfected with Tax (c), in IKKβ-depleted HEK293 cells transfected with Tax (d), in IKKγ-depleted HEK293 cells transfected with Tax (e) and in non-specific (NS) shRNA-transduced HEK293 cells transfected with Tax (f). (g) Schematic structure of Myr-IKKβ and its kinase mutant form, Myr-IKKβKM. (h) Lipid raft presence of Bif-1 and BECN1 in HEK293 cells transfected with Myr-IKKβ or Myr-IKKβKM. (i) The presence of the cytoplasmic LC3+ foci in HeLa cells transfected with Myr-IKKβ or Myr-IKKβKM. (j) Lipid raft fractionation of Myr-IKKα-transfected HEK293 cells is analyzed with immunoblot using various antibodies as indicated in the figure. (k) Fluorescence imaging analysis of HT1080 cells co-transfected with GFP-LC3, together with mkate2 (control) or with Myr-IKKα (left panel), and statistic analysis of the percentage of autophagic cells (right panel).
To investigate further the role of IKK in autophagy induction, we generated lipid raft-targeted IKKβ (Myr-IKKβ) and its kinase mutant form, Myr-IKKβKM, as depicted in Figure 5g. Myr-IKKβ is catalytically active, whereas Myr-IKKβKM is not, as demonstrated by their abilities in activating NF-κB (data not shown). Myr-IKKβ, but not Myr-IKKβKM, induced lipid raft translocation of BECN1 and Bif-1 (Figure 5h). Expression of Myr-IKKβ, but not Myr-IKKβKM, induced formation of LC3+ autophagosomes in transfected HeLa cells (Figure 5i). In contrast, Myr-IKKα failed to recruit BECN1 and Bif-1 into lipid rafts (Figure 5j) but it was still able to induce formation of LC3+ cytoplasmic puncta (Figure 5k). These data, therefore, validated an important role of the IKK complex in Tax-mediated recruitment of the autophagy molecules to lipid rafts for induction of autophagy. In addition, our results showed that although both IKKα and IKKβ mediate autophagy, these kinases act in their distinct modes in regulating autophagic processes.
The cytoplasmic, lipid raft-associated Tax induces formation of autophagosome
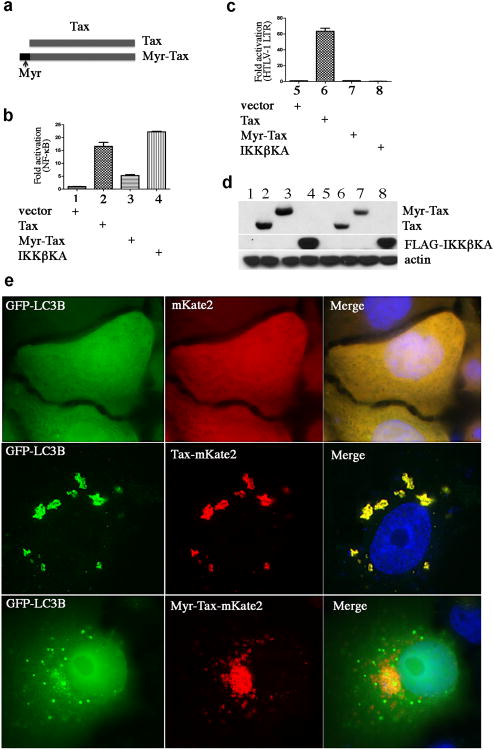
Tax is a biphasic protein, shuttling between the nucleus and the cytoplasm to mediate distinct cytoplasmic and nuclear functions. To exclude the involvement of the nuclear Tax protein, we constructed a lipid raft-targeted Tax, Myr-Tax, as depicted in Figure 6a. We found that this modified Tax protein was exclusively associated with lipid rafts (data not shown), and was able to increase NF-κB activity at least 5-fold over the vector control, though it was less potent than the wild type p40Tax (Figure 6b). The less potent activity of Myr-Tax in activating NF-κB might be caused by different protein processing from p40Tax. Unlike the wild type Tax, Myr-Tax failed to activate HTLV-1LTR (Figure 6c), though both p40Tax and Myr-Tax were expressed at comparable levels (Figure 6d). Tax-mKate2 co-localized with GFP-LC3 to form the cytoplasmic LC3+ foci, while Myr-Tax-mKate2 did not apparently co-localize with GFP-LC3 but was able to induce dramatic cytoplasmic LC3+ puncta (Figure 6e), thereby supporting the notion that the cytoplasmic Tax deregulates autophagy via activation of the IKK complex.
Figure 6.
Lipid raft-associated Tax is sufficient to induce LC3+ puncta. (a) Schematic structure of Myr-Tax and its lipid raft presence. HEK293 cells were transfected with NF-κB-luciferase reporter plasmid (b) or HTLV-1LTR-luciferase reporter plasmid (c), together with vector (control), Tax-HA, Myr-Tax-HA or FLAG-IKKβKA. 24 hours following transfection, luciferase activity was examined. (d) Immunoblot analysis of transfected cells seen in (b) and (c). (e) HeLa cells were co-tranfected with GFP-LC3, together with mKate2, Tax-mKate2 or Myr-Tax-mKate2. 48 hours following transfection, the transfected cells were analyzed with fluorescence imaging.
Lipid raft targeting of BECN1 or Bif-1 is sufficient to increase LC3+ autophagosomes
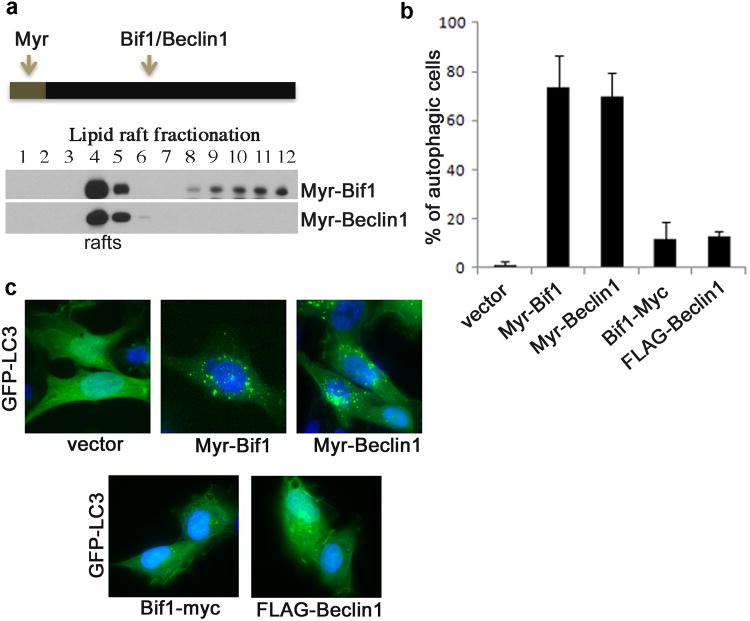
In Tax-expressing T cells, Bif-1 and BECN1 were constitutively present in lipid rafts (Figure 4). Similar phenotypes were observed in Tax-transiently transfected cells. To test the idea whether the lipid raft-associated Bif-1 or BECN1 is capable of promoting autophagy, we constructed Myr-Bif-1 and Myr-BECN1 as depicted in Figure 7a. Both Wild type Bif-1 and BECN1 were mainly localized in the soluble fractions (Figure 5a), and as expected, both Myr-Bif-1 and Myr-BECN1 accumulated in the lipid raft fractions (Figure 7a). Surprisingly, expression of Myr-Bif-1 or Myr-BECN1 alone significantly increased LC3+ autophagosomes (Figure 7b and 7c).
Figure 7.
Lipid raft-targeted Bif1 or Beclin1 mediates formation of the cytoplasmic LC3+ puncta. (a) Schematic structure of myristoylated Bif1 and Beclin1 (Myr-Bif1 and Myr-Beclin1) and lipid raft association of Myr-BECN1 and Myr-Bif-1 in transfected 293 cells using lipid raft fractionation assay. (b) Percentage of autophagic HT1080 cells co-transfected with GFP-LC3, together with vector, Myr-Bif1, Myr-Beclin1, Bif1-myc or FLAG-Beclin1. (c) LC3+ foci in the transfected cells from (b) with fluorescence imaging analysis.
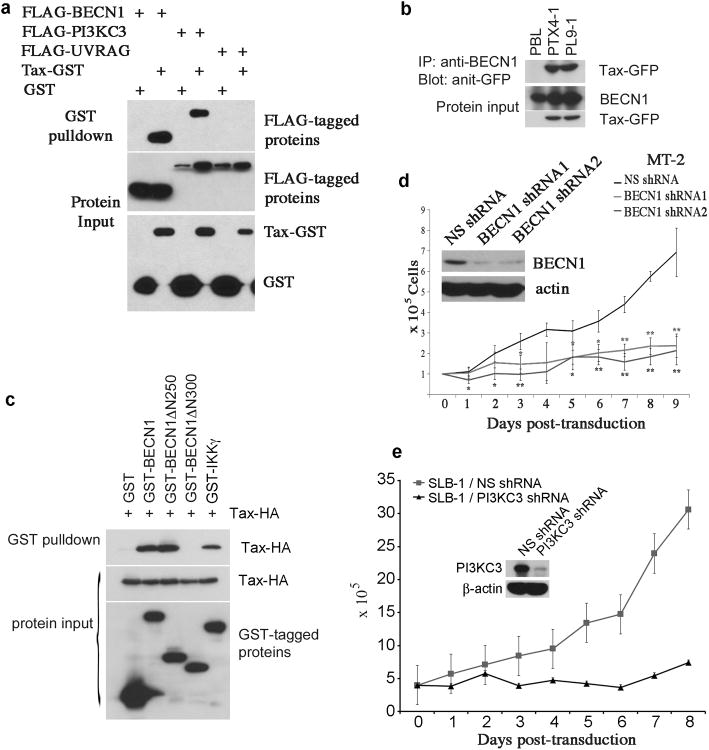
Tax interacts with the autophagy molecular complex containing BECN1 and PI3KC3
To understand the mechanistic nature of Tax-mediated autophagy, we examined a possible physical interaction between Tax and autophagic molecular complex. In co-transfected 293 cells, Tax was strongly co-precipitated with BECN1 and PI3KC3, but not UVRAG (Figure 8a). The Tax-BECN1 interaction was readily detected in Tax-immortalized T cells (Figure 8b). Tax apparently interacted with the domain situated at the amino acid sequence of BECN1 between aa250-aa300, as BECN1ΔN250 was still co-precipitated with Tax whereas BECN1ΔN300 was not (Figure 8c). The strength of physical interaction between Tax and BECN1 was comparable to that between Tax and IKKγ (Figure 8c). We further showed that depletion of Beclin1 with lentivirus transduction of specific shRNAs led to significant reduction of cell viability of MT-2 cells (Figure 8d). Similarly, depletion of PI3KC3 in SLB-1 cells resulted in growth retardation (Figure 8e). Together, these results validated a crucial role of Tax in dysregulation of autophagy. Tax-mediated autophagy functioned as pro-survival machinery in HTLV-1-infected T cells.
Figure 8.
Tax interacts with the autophagy molecular complex containing Beclin1 and PI3KC3. (a) Tax binds to BECN1 and PI3KC3. Tax-GST was co-transfected with the autophagy molecules including FLAG-Beclin1, FLAG-PI3KC3 or FLAG-UVRAG in HEK293 cells. 24 hours following transfection, GST pulldown assay was performed. (b) Co-immunoprecipitation of Tax-GFP and BECN1 in Tax-GFP-established, primary human CD4 T cell lines. (c) Tax binds to the domain situated at aa250-300 of BECN1. Tax-HA was co-transfected with GST-tagged BECN1, BECN1ΔN250, BECN1ΔN300 in HEK293 cells, followed with GST pulldown assay. (d) Depletion of BECN1 by lentivirus transduction of BECN1-specific shRNAs in MT-2 cells as examined by anti-BECN1 immunoblot (upper left panel), and Trypan blue exclusion assay to examine cell viability of MT-2 cells transduced with NS- or BECN1-specific shRNAs. * (p < 0.05) and ** (p < 0.01), as determined by Student's t-test, indicate cell viability differences in BECN1 shRNA-transfected cells as compared to NS shRNA-transfected control cells at corresponding time points. (e) PI3KC3 is depleted by PI3KC3-specific shRNA in SLB-1 cells (upper panel), and the cell viability of PI3KC3-depleted SLB-1 cells vs NS shRNA-transduced SLB-1 cells is shown in lower panel.
Discussion
In the present study, we demonstrated that increased autophagic activity occurs spontaneously in HTLV-1-transformed T cells. The viral oncoprotein Tax is the determining factor for dysregulation of autophagy in HTLV-1-transformed T cells and in Tax-immortalized CD4 memory T cells. Disruption of autophagic pathways results in growth retardation of HTLV-1-transformed T cells, thereby implicating a critical role of autophagy in promoting T cell survival and malignant transformation.
Both PI3KC1/Akt and IKK are activated in HTLV-1-transformed T cells, however, these two kinases have been reported to exhibit opposing activities in regulating autophagy 23,36. The overall outcome is in favor of autophagosome induction in HTLV-1-transformed T cells expressing the viral oncoprotein Tax. Similarly, TCR engagement results in activation of IKK and PI3KC1/Akt, and promotes autophagic process, causing T cell expansion 45,46. Our findings strongly suggest that autophagy is beneficial for the retrovirus-mediated oncogenesis by providing crucial survival machinery to HTLV-1-transformed T cells. Dysregulation of autophagy can occur during the process of tumorigenesis in a variety of human cancers in addition to virus-mediated oncogenesis 47-49. Tumor growth typically exceeds the rate of angiogenesis during the early stages of malignancy, causing the center of tumor to experience constant hypoxic and metabolic stresses. These stress signals trigger autophagy-mediated survival machinery in cancer cells 50. In addition, induction of autophagy by chemotherapeutic agent may contribute to the resistance of cancer cells to therapy 51,52. Indeed, cancer chemotherapy in conjunction with an inhibitor of autophagy results in an improved therapeutic efficacy 51, supporting a pro-survival role of autophagy for cancer cells. During the progression of cancer, activation of PI3KC1 and loss of autophagy mediators causes defective autophagy 53,54. Defects in both autophagy and apoptosis result in necrotic cell death in metabolically stressed tumor regions, leading to an inflammatory response, DNA damage and consequent tumor progression. In the context of HTLV-1-induced malignant transformation of T cells, Tax is required for initiating T cell transformation since HTLV-1 infectious clone with lack of the tax gene has no transforming activity on T cells 55,56. The finding that Tax expression is lost in roughly 50% of ATL cases suggests that Tax is no longer required at the late stage of leukemia. Accumulation of multiple oncogenic events is likely to replace Tax's functions in advanced disease. Intriguingly, loss of the beclin1 or bif1 gene showed hyper-proliferative and increased incidence of lymphoma and other malignancies in mice 44. Heterozygous loss of beclin1 is present in some types of human cancer 57. This may be attributable to the function of autophagy in limiting genome damage. Therefore, it is possible that a much lower autophagic activity in advanced ATL that lacks Tax expression could further enhance genome instability and accumulation of mutant cellular oncoproteins, thereby facilitating cancer progression.
Tax deregulates autophagy by increasing formation of autophagosomes, and some of them can reach the stage of autolysosome. This conclusion is supported by several experimental findings. When co-transfected with the acid-sensitive GFP-LC3, a majority of the Tax protein was found to co-localize with GFP-LC3 in the cytoplasmic puncta (Figure 6e), suggesting that Tax directly participated in the assembly of autophagosomes. However, Tax only partially co-localized with the cytoplasmic mKate2-LC3 red puncta (Figure 3c), which represents both autophagosomes and autolysosomes since mKate2 is acid-stable. Furthermore, Tax induced aggregation of p40phox-GFP, the substrate of PI3KC3, suggesting that this viral protein increases autophagic influx. A recent report showed that Tax increases autophagosomes by blocking fusion of autophagosomes with lysosomes. This process is IκB kinase-dependent 58. Although the underlying mechanism of this action is presently unclear, it was suggested that the increased autophagosomes are beneficial for supporting HTLV-1 replication by preventing the Tax protein from degradation in lysosome 58. Our study showed that Tax physically interacted with the autophagy molecular complex of Beclin1-PI3KC3 and participated in the assembly of the LC3+ autophagosomes. This process was dependent on the activity of the IKK complex. IκB kinases have been reported to play crucial roles in starvation and rapamycin-mediated autophagy, leading to the completion of the autophagic process. Recent reports further demonstrated a crosstalk between Beclin1 and the components of NF-κB signaling pathway, and autophagy induction may be necessary for activation of IκB kinases 59.
Our study demonstrated that Tax-deregulated autophagy was involved in the lipid raft recruitment of the autophagic molecular complex containing Beclin1 and Bif-1 and that this action was also dependent on the activity of IκB kinases. Similarly, the viral LMP1 protein from EBV associates with the lipid raft microdomains to activate NF-κB, and it also induces autophagy 33,60. However it is currently not clear if the lipid raft microdomain is involved in the LMP1-mediated autophagic process. The involvement of lipid raft association of the autophagy molecules in regulating autophagy has not been previously reported. Although the autophagy molecule LC3B is found to complex with Fas in lipid rafts to activate extrinsic apoptosis in cigarette smoke-induced emphysema 61, the role of lipid raft-associated autophagy mediators for induction of autophagy is presently not known. In the context of Tax-mediated oncogenesis, the following scenario may occur. Tax may utilize lipid rafts as a signaling platform to recruit both IκB kinases and autophagy molecules into this structure for activating both NF-κB and autophagy pathways. The lipid raft-associated autophagy molecules such as Beclin1 and Bif-1 gain their activity to facilitate the processes of autophagy and Tax-mediated oncogenesis. It is important to investigate further the role of lipid raft-associated Beclin1 and Bif-1 in HTLV-1-associated diseases.
Materials and Methods
Cell lines, antibodies and chemicals
MT-2 cell line was obtained from AIDS research and reference reagent program. HT1080 and Jurkat cell lines were from ATCC. HUT102 and MT-1 cells were described previously 62,63. Antibodies for IKKα, IKKβ and IKKγ were purchased from IMGENEX (San Diego CA). Antibodies for LAT, ERK1, BECN1, HA, GST and GFP were from Santa Cruz Biotechnology (Dallas, Texas), anti-LC3 from Cell Signaling (Danvers, MA), and anti-beta-actin and -FLAG from Sigma (St. Louis, MO). Monoclonal anti-Tax antibody was obtained from AIDS reagent program. Niclosamide was purchased from Sigma.
Lentivirus vector, viral production and transduction of primary CD4 T cells
The full-length tax cDNA from HTLV-1 was fused with enhanced green fluorescence protein (GFP), and the tax-gfp fusion fragment was cloned into the lentivirus vector pLCEF8 22, in which the human elongation factor 1 alpha promoter drives expression of Tax-GFP. The procedure for lentiviral production and concentration was described previously 64. Human peripheral blood lymphocytes were isolated from healthy blood donors, and stimulated with PHA (1μg/ml) for 24 hours, followed by adding recombinant IL-2 (100u/ml). The activated lymphocytes were cultured for 5-7 days, and the CD4+ cells were enriched by sorting with anti-CD4 magnetic beads (Invitrogen, Grand Island, NY). The purified CD4 T cells were then transduced with the lentivirus carrying the tax-gfp expression cassette. The transduced cells were cultured continuously in complete media containing 20% fetal bovine serum and 100u/ml of recombinant IL-2 (AIDS Reagent Program). Two Tax-established T cell lines, PTX4-1 and PL9-1, were developed.
Immunophenotype analysis, electrophoretic mobility gel shift assay (EMSA) and lipid raft fractionation assay
Tax-immortalized T cell lines were stained with allophycocyanin (APC) conjugated antibodies that included anti-CD3, -CD4, -CD25, -TCRαβ, -CD45RO and -CD69 (eBioscience, San Diego, CA) according to the manufacturer's instructions. The stained cells were subjected for FACS analysis.
Nuclear extracts were prepared from various T cell lines using NE-PER nuclear and cytoplasmic extraction reagents (Pierce, Rockford, IL). The oligonucleotide was 5′-end labeled with biotin (Integrated DNA Technologies, Coralville, Iowa) and annealed to its complementary strand. The probe sequences are reported previously 39, and the binding activities were examined by EMSA using Light Shift Chemiluminescent EMSA Kit (Pierce).
The lipid raft fractionation assay was performed with density gradient ultracentrifugation using a method described previously 22.
Plasmids, site-directed mutagenesis, immunoblot, co-immunoprecipitation and GST pulldown assay
The plasmids for FLAG-BECN1, FLAG-UVRAG, FLAG-PI3KC3, Bif-1-myc and GFP-LC3B were reported previously 44, and the BECN1ΔN250 and ΔN300 were generated using a PCR-based mutagenesis method. The myristoylation signal from human Lck was fused to the N-terminus of the full-length of IKKβ to generate a myristoylated IKKβ, Myr-IKKβ. The Myr-IKKβKM (K44M) kinase mutant was constructed using PCR-based site directed mutagenesis method. Myr-Tax, Myr-BECN1 and Myr-Bif1 were generated by adding the Lck myristoylation signal to the N-termini of their corresponding cDNAs. The Tax shRNAs were constructed in the lentivirus vector. Lentivirus vector shRNAs specific for IKKα, IKKβ and BECN1 were described previously 22,39. Lentivirus vectors expressing IKKγ-, BECN1- and PI3KC3-specific shRNAs were purchased from Open Biosystems (Pittsburgh, PA). The co-immunoprecipitation and GST pulldown assays were performed using the methods described previously 22.
Fluorescence imaging and autophagy assay
To construct fluorescence protein tagged proteins, mWasabi encoding a monomeric green fluorescent protein 65, or mKate2 encoding a monomeric far red fluorescent protein 66, was amplified from pTEC15 or pTEC20 (kindly provided by Lalita Ramakrishnan, Addgene plasmid 30174 or 30179 respectively) and was fused into the N-terminus of LC3 PCR fragment to generate mKate2-LC3, which was cloned in the mammalian expression vector pEF2. The mKate2 PCR fragment was fused to the C-terminus of Tax to generate Tax-mKate2. Transient co-transfection was performed in HT1080 and HeLa cells using FuGeneHD transfection reagent (Roche, Branford, CT). 48 hours post-transfection, the cells were fixed in 4% formaldehyde-PBS and mounted with DAPI. Fluorescent images were taken using an OLYMPUS IX81 deconvolution microscope and analyzed using SlideBook 5.0 software (Intelligent Imaging Innovations). For immunofluorescence staining, cells were fixed in 4% paraformaldehyde-PBS, blocked in 3% horse serum-PBS, stained with the indicated primary antibodies overnight at 4°C followed by incubation with fluorescent conjugated secondary antibodies and then mounted with DAPI (Invitrogen).
Acknowledgments
We thank Atsushi Koito and Takeo Ohsugi for MT-1 cell line, and Wen Dong, Li Chen, Dan Liu, Di Xiang and Huan Zhang for technical assistance. Research reported in this publication was supported by the National Institute of Allergy And Infectious Diseases of the National Institutes of Health under award number R01AI090113 to H. Cheng and was partly supported by the award CA129682 to H.G. Wang. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.Chu ZL, Shin YA, Yang JM, DiDonato JA, Ballard DW. IKKgamma mediates the interaction of cellular IkappaB kinases with the tax transforming protein of human T cell leukemia virus type 1. J Biol Chem. 1999;274:15297–15300. doi: 10.1074/jbc.274.22.15297. [DOI] [PubMed] [Google Scholar]
- 2.Carter RS, Geyer BC, Xie M, Acevedo-Suarez CA, Ballard DW. Persistent activation of NF-kappa B by the tax transforming protein involves chronic phosphorylation of IkappaB kinase subunits IKKbeta and IKKgamma. J Biol Chem. 2001;276:24445–24448. doi: 10.1074/jbc.C000777200. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura N, Fujii M, Tsukahara T, Arai M, Ohashi T, Wakao H, Kannagi M, Yamamoto N. Human T-cell leukemia virus type 1 Tax protein induces the expression of STAT1 and STAT5 genes in T-cells. Oncogene. 1999;18:2667–2675. doi: 10.1038/sj.onc.1202608. [DOI] [PubMed] [Google Scholar]
- 4.Horiuchi S, Yamamoto N, Dewan MZ, Takahashi Y, Yamashita A, Yoshida T, Nowell MA, Richards PJ, Jones SA, Yamamoto N. Human T-cell leukemia virus type-I Tax induces expression of interleukin-6 receptor (IL-6R): Shedding of soluble IL-6R and activation of STAT3 signaling. Int J Cancer. 2006;119:823–830. doi: 10.1002/ijc.21918. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y, Wang Y, Yamakuchi M, Masuda S, Tokioka T, Yamaoka S, Maruyama I, Kitajima I. Phosphoinositide-3 kinase-PKB/Akt pathway activation is involved in fibroblast Rat-1 transformation by human T-cell leukemia virus type I tax. Oncogene. 2001;20:2514–2526. doi: 10.1038/sj.onc.1204364. [DOI] [PubMed] [Google Scholar]
- 6.Tomita M, Semenza GL, Michiels C, Matsuda T, Uchihara JN, Okudaira T, Tanaka Y, Taira N, Ohshiro K, Mori N. Activation of hypoxia-inducible factor 1 in human T-cell leukaemia virus type 1-infected cell lines and primary adult T-cell leukaemia cells. Biochem J. 2007;406:317–323. doi: 10.1042/BJ20070286. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Chu ZL, DiDonato JA, Hawiger J, Ballard DW. The tax oncoprotein of human T-cell leukemia virus type 1 associates with and persistently activates IkappaB kinases containing IKKalpha and IKKbeta. J Biol Chem. 1998;273:15891–15894. doi: 10.1074/jbc.273.26.15891. [DOI] [PubMed] [Google Scholar]
- 8.Harhaj NS, Sun SC, Harhaj EW. Activation of NF-kappa B by the human T cell leukemia virus type I Tax oncoprotein is associated with ubiquitin-dependent relocalization of I kappa B kinase. J Biol Chem. 2007;282:4185–4192. doi: 10.1074/jbc.M611031200. [DOI] [PubMed] [Google Scholar]
- 9.Harhaj EW, Sun SC. IKKgamma serves as a docking subunit of the IkappaB kinase (IKK) and mediates interaction of IKK with the human T-cell leukemia virus Tax protein. J Biol Chem. 1999;274:22911–22914. doi: 10.1074/jbc.274.33.22911. [DOI] [PubMed] [Google Scholar]
- 10.Zandi E, Rothwarf DM, Delhase M, Hayakawa M, Karin M. The IkappaB kinase complex (IKK) contains two kinase subunits, IKKalpha and IKKbeta, necessary for IkappaB phosphorylation and NF-kappaB activation. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 11.Rothwarf DM, Zandi E, Natoli G, Karin M. IKK-gamma is an essential regulatory subunit of the IkappaB kinase complex. Nature. 1998;395:297–300. doi: 10.1038/26261. [DOI] [PubMed] [Google Scholar]
- 12.Mercurio F, Zhu H, Murray BW, Shevchenko A, Bennett BL, Li J, Young DB, Barbosa M, Mann M, Manning A, Rao A. IKK-1 and IKK-2: cytokine-activated IkappaB kinases essential for NF-kappaB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 13.Trushin SA, Pennington KN, Algeciras-Schimnich A, Paya CV. Protein kinase C and calcineurin synergize to activate IkappaB kinase and NF-kappaB in T lymphocytes. J Biol Chem. 1999;274:22923–22931. doi: 10.1074/jbc.274.33.22923. [DOI] [PubMed] [Google Scholar]
- 14.O'Neill LA, Greene C. Signal transduction pathways activated by the IL-1 receptor family: ancient signaling machinery in mammals, insects, and plants. J Leukoc Biol. 1998;63:650–657. [PubMed] [Google Scholar]
- 15.Chu W, Gong X, Li Z, Takabayashi K, Ouyang H, Chen Y, Lois A, Chen DJ, Li GC, Karin M, Raz E. DNA-PKcs is required for activation of innate immunity by immunostimulatory DNA. Cell. 2000;103:909–918. doi: 10.1016/s0092-8674(00)00194-x. [DOI] [PubMed] [Google Scholar]
- 16.Li Q, Van Antwerp D, Mercurio F, Lee KF, Verma IM. Severe liver degeneration in mice lacking the IkappaB kinase 2 gene. Science. 1999;284:321–325. doi: 10.1126/science.284.5412.321. [DOI] [PubMed] [Google Scholar]
- 17.Li ZW, Chu W, Hu Y, Delhase M, Deerinck T, Ellisman M, Johnson R, Karin M. The IKKbeta subunit of IkappaB kinase (IKK) is essential for nuclear factor kappaB activation and prevention of apoptosis. J Exp Med. 1999;189:1839–1845. doi: 10.1084/jem.189.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Makris C, Godfrey VL, Krähn-Senftleben G, Takahashi T, Roberts JL, Schwarz T, Feng L, Johnson RS, Karin M. Female mice heterozygous for IKK gamma/NEMO deficiencies develop a dermatopathy similar to the human X-linked disorder incontinentia pigmenti. Mol Cell. 2000;5:969–979. doi: 10.1016/s1097-2765(00)80262-2. [DOI] [PubMed] [Google Scholar]
- 19.Senftleben U, Li ZW, Baud V, Karin M. IKKbeta is essential for protecting T cells from TNFalpha-induced apoptosis. Immunity. 2001;14:217–230. doi: 10.1016/s1074-7613(01)00104-2. [DOI] [PubMed] [Google Scholar]
- 20.Verkade P, Simons K. Robert Feulgen Lecture 1997. Lipid microdomains and membrane trafficking in mammalian cells. Histochem Cell Biol. 1997;108:211–220. doi: 10.1007/s004180050161. [DOI] [PubMed] [Google Scholar]
- 21.Jin DY, Giordano V, Kibler KV, Nakano H, Jeang KT. Role of adapter function in oncoprotein-mediated activation of NF-kappaB. Human T-cell leukemia virus type I Tax interacts directly with IkappaB kinase gamma. J Biol Chem. 1999;274:17402–17405. doi: 10.1074/jbc.274.25.17402. [DOI] [PubMed] [Google Scholar]
- 22.Huang J, Ren T, Guan H, Jiang Y, Cheng H. HTLV-1 Tax is a critical lipid raft modulator that hijacks IkappaB kinases to the microdomains for persistent activation of NF-kappaB. J Biol Chem. 2009;284:6208–6217. doi: 10.1074/jbc.M806390200. [DOI] [PubMed] [Google Scholar]
- 23.Criollo A, Senovilla L, Authier H, Maiuri MC, Morselli E, Vitale I, Kepp O, Tasdemir E, Galluzzi L, Shen S, Tailler M, Delahaye N, Tesniere A, De Stefano D, Younes AB, Harper F, Pierron G, Lavandero S, Zitvogel L, Israel A, Baud V, Kroemer G. The IKK complex contributes to the induction of autophagy. EMBO J. 2010;29:619–631. doi: 10.1038/emboj.2009.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Omodei Zorini A. Considerations on primary carcinomatous caverns of the lung. Possibility of the intervention of a phenomenon of “autophagy of the neoplastic cells”. Lotta Tuberc. 1965;35:946–968. [PubMed] [Google Scholar]
- 25.Baudhuin P. Lysosomes and cellular autophagy. Brux Med. 1966;46:1059–1070. [PubMed] [Google Scholar]
- 26.Katayama M, Kawaguchi T, Berger MS, Pieper RO. DNA damaging agent-induced autophagy produces a cytoprotective adenosine triphosphate surge in malignant glioma cells. Cell Death Differ. 2007;14:548–558. doi: 10.1038/sj.cdd.4402030. [DOI] [PubMed] [Google Scholar]
- 27.Dreux M, Gastaminza P, Wieland SF, Chisari FV. The autophagy machinery is required to initiate hepatitis C virus replication. Proc Natl Acad Sci U S A. 2009;106:14046–14051. doi: 10.1073/pnas.0907344106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wen HJ, Yang Z, Zhou Y, Wood C. Enhancement of autophagy during lytic replication by the Kaposi's sarcoma-associated herpesvirus replication and transcription activator. J Virol. 2010;84:7448–7458. doi: 10.1128/JVI.00024-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan P, Qing G, Qu Z, Wu CC, Rabson A, Xiao G. Targeting autophagic regulation of NFkappaB in HTLV-I transformed cells by geldanamycin: implications for therapeutic interventions. Autophagy. 2007;3:600–603. doi: 10.4161/auto.4761. [DOI] [PubMed] [Google Scholar]
- 30.Sir D, Tian Y, Chen WL, Ann DK, Yen TS, Ou JH. The early autophagic pathway is activated by hepatitis B virus and required for viral DNA replication. Proc Natl Acad Sci U S A. 2010;107:4383–4388. doi: 10.1073/pnas.0911373107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dreux M, Chisari FV. Autophagy proteins promote hepatitis C virus replication. Autophagy. 2009;5:1224–1225. doi: 10.4161/auto.5.8.10219. [DOI] [PubMed] [Google Scholar]
- 32.Mizui T, Yamashina S, Tanida I, Takei Y, Ueno T, Sakamoto N, Ikejima K, Kitamura T, Enomoto N, Sakai T, Kominami E, Watanabe S. Inhibition of hepatitis C virus replication by chloroquine targeting virus-associated autophagy. J Gastroenterol. 2010;45:195–203. doi: 10.1007/s00535-009-0132-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee DY, Sugden B. The latent membrane protein 1 oncogene modifies B-cell physiology by regulating autophagy. Oncogene. 2008;27:2833–2842. doi: 10.1038/sj.onc.1210946. [DOI] [PubMed] [Google Scholar]
- 34.Tang H, Da L, Mao Y, Li Y, Li D, Xu Z, Li F, Wang Y, Tiollais P, Li T, Zhao M. Hepatitis B virus X protein sensitizes cells to starvation-induced autophagy via up-regulation of beclin 1 expression. Hepatology. 2009;49:60–71. doi: 10.1002/hep.22581. [DOI] [PubMed] [Google Scholar]
- 35.Furuta S, Hidaka E, Ogata A, Yokota S, Kamata T. Ras is involved in the negative control of autophagy through the class I PI3-kinase. Oncogene. 2004;23:3898–3904. doi: 10.1038/sj.onc.1207539. [DOI] [PubMed] [Google Scholar]
- 36.Petiot A, Ogier-Denis E, Blommaart EF, Meijer AJ, Codogno P. Distinct classes of phosphatidylinositol 3′-kinases are involved in signaling pathways that control macroautophagy in HT-29 cells. J Biol Chem. 2000;275:992–998. doi: 10.1074/jbc.275.2.992. [DOI] [PubMed] [Google Scholar]
- 37.Blommaart EF, Krause U, Schellens JP, Vreeling-Sindelarova H, Meijer AJ. The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. Eur J Biochem. 1997;243:240–246. doi: 10.1111/j.1432-1033.1997.0240a.x. [DOI] [PubMed] [Google Scholar]
- 38.Maddodi N, Huang W, Havighurst T, Kim K, Longley BJ, Setaluri V. Induction of autophagy and inhibition of melanoma growth in vitro and in vivo by hyperactivation of oncogenic BRAF. J Invest Dermatol. 2010;130:1657–1667. doi: 10.1038/jid.2010.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ren T, Dong W, Takahashi Y, Xiang D, Yuan Y, Liu X, Loughran TP, Jr, Sun SC, Wang HG, Cheng H. HTLV-2 Tax immortalizes human CD4+ memory T lymphocytes by oncogenic activation and dysregulation of autophagy. J Biol Chem. 2012;287:34683–34693. doi: 10.1074/jbc.M112.377143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balgi AD, Fonseca BD, Donohue E, Tsang TC, Lajoie P, Proud CG, Nabi IR, Roberge M. Screen for chemical modulators of autophagy reveals novel therapeutic inhibitors of mTORC1 signaling. PLoS One. 2009;4:e7124. doi: 10.1371/journal.pone.0007124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mori N, Murakami S, Oda S, Prager D, Eto S. Production of interleukin 8 in adult T-cell leukemia cells: possible transactivation of the interleukin 8 gene by human T-cell leukemia virus type I tax. Cancer Res. 1995;55:3592–3597. [PubMed] [Google Scholar]
- 42.Adya N, Giam CZ. Distinct regions in human T-cell lymphotropic virus type I tax mediate interactions with activator protein CREB and basal transcription factors. J Virol. 1995;69:1834–1841. doi: 10.1128/jvi.69.3.1834-1841.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith MR, Greene WC. Identification of HTLV-I tax trans-activator mutants exhibiting novel transcriptional phenotypes. Genes Dev. 1990;4:1875–1885. doi: 10.1101/gad.4.11.1875. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi Y, Coppola D, Matsushita N, Cualing HD, Sun M, Sato Y, Liang C, Jung JU, Cheng JQ, Mulé JJ, Pledger WJ, Wang HG. Bif-1 interacts with Beclin 1 through UVRAG and regulates autophagy and tumorigenesis. Nat Cell Biol. 2007;9:1142–1151. doi: 10.1038/ncb1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paul S, Kashyap AK, Jia W, He YW, Schaefer BC. Selective autophagy of the adaptor protein Bcl10 modulates T cell receptor activation of NF-kappaB. Immunity. 2012;36:947–958. doi: 10.1016/j.immuni.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.von Willebrand M, Baier G, Couture C, Burn P, Mustelin T. Activation of phosphatidylinositol-3-kinase in Jurkat T cells depends on the presence of the p56lck tyrosine kinase. Eur J Immunol. 1994;24:234–238. doi: 10.1002/eji.1830240137. [DOI] [PubMed] [Google Scholar]
- 47.Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 48.Mathew R, Karantza-Wadsworth V, White E. Assessing metabolic stress and autophagy status in epithelial tumors. Methods Enzymol. 2009;453:53–81. doi: 10.1016/S0076-6879(08)04004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karantza-Wadsworth V, Patel S, Kravchuk O, Chen G, Mathew R, Jin S, White E. Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis. Genes Dev. 2007;21:1621–1635. doi: 10.1101/gad.1565707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, Chen G, Mukherjee C, Shi Y, Gélinas C, Fan Y, Nelson DA, Jin S, White E. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10:51–64. doi: 10.1016/j.ccr.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carew JS, Nawrocki ST, Cleveland JL. Modulating autophagy for therapeutic benefit. Autophagy. 2007;3:464–467. doi: 10.4161/auto.4311. [DOI] [PubMed] [Google Scholar]
- 52.Song J, Qu Z, Guo X, Zhao Q, Zhao X, Gao L, Sun K, Shen F, Wu M, Wei L. Hypoxia-induced autophagy contributes to the chemoresistance of hepatocellular carcinoma cells. Autophagy. 2009;5:1131–1144. doi: 10.4161/auto.5.8.9996. [DOI] [PubMed] [Google Scholar]
- 53.Scott RC, Schuldiner O, Neufeld TP. Role and regulation of starvation-induced autophagy in the Drosophila fat body. Dev Cell. 2004;7:167–178. doi: 10.1016/j.devcel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 54.Wei H, Wei S, Gan B, Peng X, Zou W, Guan JL. Suppression of autophagy by FIP200 deletion inhibits mammary tumorigenesis. Genes Dev. 2011;25:1510–1527. doi: 10.1101/gad.2051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weiss RA, Schulz TF. Transforming properties of the HTLV-I tax gene. Cancer Cells. 1990;2:281–283. [PubMed] [Google Scholar]
- 56.Hatanaka M. Transformation by the tax gene of HTLV-1. Uirusu. 1992;42:13–20. doi: 10.2222/jsv.42.13. [DOI] [PubMed] [Google Scholar]
- 57.Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H, Troxel A, Rosen J, Eskelinen EL, Mizushima N, Ohsumi Y, Cattoretti G, Levine B. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest. 2003;112:1809–1820. doi: 10.1172/JCI20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang SW, Chen CY, Klase Z, Zane L, Jeang KT. The cellular autophagy pathway modulates human T-cell leukemia virus type 1 replication. J Virol. 2012;87:1699–707. doi: 10.1128/JVI.02147-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Niso-Santano M, Criollo A, Malik SA, Michaud M, Morselli E, Mariño G, Lachkar S, Galluzzi L, Maiuri MC, Kroemer G. Direct molecular interactions between Beclin 1 and the canonical NFkappaB activation pathway. Autophagy. 2012;8:268–270. doi: 10.4161/auto.8.2.18845. [DOI] [PubMed] [Google Scholar]
- 60.Valentin-Acevedo A, Sinquett FL, Covey LR. c-Rel deficiency increases caspase-4 expression and leads to ER stress and necrosis in EBV-transformed cells. PLoS One. 2011;6:e25467. doi: 10.1371/journal.pone.0025467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen ZH, Lam HC, Jin Y, Kim HP, Cao J, Lee SJ, Ifedigbo E, Parameswaran H, Ryter SW, Choi AM. Autophagy protein microtubule-associated protein 1 light chain-3B (LC3B) activates extrinsic apoptosis during cigarette smoke-induced emphysema. Proc Natl Acad Sci U S A. 2010;107:18880–18885. doi: 10.1073/pnas.1005574107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Robb RJ, Greene WC, Rusk CM. Low and high affinity cellular receptors for interleukin 2. Implications for the level of Tac antigen. J Exp Med. 1984;160:1126–1146. doi: 10.1084/jem.160.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsudo M, Uchiyama T, Uchino H, Yodoi J. Failure of regulation of Tac antigen/TCGF receptor on adult T-cell leukemia cells by anti-Tac monoclonal antibody. Blood. 1983;61:1014–1016. [PubMed] [Google Scholar]
- 64.Cheng H, Cenciarelli C, Nelkin G, Tsan R, Fan D, Cheng-Mayer C, Fidler IJ. Molecular mechanism of hTid-1, the human homolog of Drosophila tumor suppressor l(2)Tid, in the regulation of NF-kappaB activity and suppression of tumor growth. Mol Cell Biol. 2005;25:44–59. doi: 10.1128/MCB.25.1.44-59.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ai HW, Olenych SG, Wong P, Davidson MW, Campbell RE. Hue-shifted monomeric variants of Clavularia cyan fluorescent protein: identification of the molecular determinants of color and applications in fluorescence imaging. BMC Biol. 2008;6:13. doi: 10.1186/1741-7007-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shcherbo D, Murphy CS, Ermakova GV, Solovieva EA, Chepurnykh TV, Shcheglov AS, Verkhusha VV, Pletnev VZ, Hazelwood KL, Roche PM, Lukyanov S, Zaraisky AG, Davidson MW, Chudakov DM. Far-red fluorescent tags for protein imaging in living tissues. Biochem J. 2009;418:567–574. doi: 10.1042/BJ20081949. [DOI] [PMC free article] [PubMed] [Google Scholar]