Abstract
To study the complex cellular interactions involved in wound healing, it is essential to have an animal model that adequately mimics the human wound microenvironment. Currently available murine models are limited because wound contraction introduces bias into wound surface area measurements. The purpose of this study was to demonstrate utility of a human–mouse xenograft model for studying human wound healing. Normal human skin was harvested from elective abdominoplasty surgery, xenografted onto athymic nude (nu/nu) mice, and allowed to engraft for 3 months. The graft was then wounded using a 2‐mm punch biopsy. Wounds were harvested on sequential days to allow tissue‐based markers of wound healing to be followed sequentially. On the day of wound harvest, mice were injected with XenoLight RediJect cyclooxygenase‐2 (COX‐2) probe and imaged according to package instructions. Immunohistochemistry confirms that this human–mouse xenograft model is effective for studying human wound healing in vivo. Additionally, in vivo fluorescent imaging for inducible COX‐2 demonstrated upregulation from baseline to day 4 (P = 0·03) with return to baseline levels by day 10, paralleling the reepithelialisation of the wound. This human–mouse xenograft model, combined with in vivo fluorescent imaging provides a useful mechanism for studying molecular pathways of human wound healing.
Keywords: COX‐2, Mouse model, Wound healing, Xenograft
Introduction
Chronic wounds that have failed to heal after 3 months of appropriate wound care affect approximately 6·5 million people in the USA with a prevalence of 1% and cost an estimated $25 billion per year 1. In addition to the financial costs, these wounds significantly impact mortality 2 and cause considerable pain, affecting patient‐reported psychosocial well‐being and quality of life 3, 4.
There is an unmet need to identify new therapies to improve outcomes and quality of life for patients suffering from chronic wounds 5. Interactions between the many pathways contributing to the inflammatory state in chronic wounds are poorly understood and clinicians are constantly searching for new methods to transform wounds from the inflammatory to the proliferative phase 6, 7. Prolonged inflammation 8, fibroblast senescence 9, imbalance of regulatory growth factors and proinflammatory cytokines 10, 11, defective keratinocyte function 12, 13, 14, impaired angiogenesis and bacterial factors in the wound‐bed biofilm 15, 16 have all been postulated to contribute to delay in wound healing. To date, it has been difficult to study the interactions of these complex pathways methodically to identify key regulator points that might be amenable to interventions in order to improve healing.
One of the roadblocks to studying human wound healing is the lack of a good animal model that effectively mimics human wound healing. Numerous models involving in vitro cocultures, complex organotypic systems and animal models including those based on mice, pigs and non‐human primates have been used to study wound healing 17, 18. Porcine models have been extensively studied and are favoured as a model for human skin disease, as the epithelial architecture, matrix, vascularity and innervation are similar to those seen in human skin. However, swine are large, unwieldy and difficult to house, therefore swine models are not suitable for in vivo imaging experiments. Murine models have the advantage of using animals of a smaller size that are much easier to handle and house. However, there are well‐recognised limitations of using mouse models for studying wound healing because of the difference in dermal anatomy and physiology of mice compared with humans 17. Although mouse skin contains the same three layers (epidermis, dermis and hypodermis) seen in human skin, it also has a panniculosus carnosus, a layer of muscle that contracts in response to injury. This creates confounding variability when using pure murine models of wound healing, because there is an initial rapid decrease in wound surface area after wounding that is purely related to wound contraction rather than reepithelialisation. Thus, it is challenging to translate observations in murine models to the human wound environment. Another factor to be considered when using murine models is that mechanical transduction forces differ in human versus mouse skin (with mouse skin being more compliant), and this can lead to bias when using traditional mouse models to study wound repair 19. To overcome the challenges of wound contraction and differing mechanical forces, various wound splinting models have been used. While these models allow histological monitoring of wound‐bed granulation, one of the most important factors in healing of human chronic wounds 17, they too do not fully mimic human wound healing.
The use of human skin transplanted onto the back of immunocompromised mice has been described previously and shown to be an effective method for examining human skin healing in vivo 20, 21, 22. Immunohistochemical studies of these models have shown that these grafts maintain morphological, immunological and functional characteristics of human skin 20, 22. Excisional wound healing studies on human–mouse xenografts have shown that wounds generated on these grafts heal by secondary intention with reepithilalisation by typical human keratinocytes. Historically these models have been criticised, as there has been concern that the immunocompromised state of the mouse might impact the inflammatory and immune responses that are critical for human wound repair. However, to date this has not been studied methodically and in vivo imaging has not been used previously to monitor the influx of critical inflammatory molecules involved in wound repair in this human–mouse xenograft model.
Prostaglandins are critical proinflammatory mediators involved in the acute inflammatory phase of wound healing. Arachidonic acid is converted into prostaglandins by cyclooxygenase‐1 and ‐2 (COX‐1 and ‐2). COX‐1 is constitutively expressed under physiological conditions, whereas COX‐2 is induced in sites of inflammation 23. In wounds, prostaglandin activation causes increased microvascular permeability in the wound bed, allowing influx of inflammatory cells and other mediators that facilitate healing. Studies from COX‐2 deficient mice suggest that COX‐2 plays an important role in dermal wound healing 24. However, studies using COX‐2 inhibition in various animal models have shown mixed effects on healing, while some studies show transient retardation of early epithelialisation, there are no consistent differences in angiogenesis, collagen deposition or tensile strength 25, 26. In humans, COX‐2 expression has been used as a marker for wound age determination in forensic medicine with the highest ratio of COX‐2 positive neutrophils and macrophages are seen in wounds in the 8‐hour to 2‐day time frame 27. These findings suggest that differential COX‐2 expression in a wound may be an important biomarker of healing.
The purpose of this study was to refine established methodology for generating a reliable human–mouse xenograft model for investigating mechanisms of wound healing in normal human skin. To test our hypothesis, we performed wounding experiments on mice that had successful xenografts. We used a fluorescent probe to measure inducible COX2 in the wound bed and to demonstrate the utility of this system for studying human wound healing.
Methods
This study was approved by the Georgetown University Medical Center Animal Care and Use Committee (GUACUC# 2011–050). Unidentified human skin, that would otherwise be discarded, was harvested from elective abdominoplasty resections. The use of unidentified tissue is not considered under human subject research and therefore this research did not require prospective review or approval by the Georgetown University Medical Center Institutional Review Board (IRB).
Mouse acclimatisation
Athymic female ‘nude’ (nu/nu) mice were purchased from Harlan Laboratories (Indianapolis, IN) at 5 weeks of age. They were housed in the Georgetown University Division of Comparative Medicine (DCM) rodent barrier facility, which is maintained as a pathogen‐free environment under temperature and humidity control. Animals were housed in micro‐isolator cages under high‐efficiency particulate absorption (HEPA)‐filtered laminar flow racks using sterile bedding, water and food. Mice were allowed to acclimatise for 14 days prior to grafting and underwent ear tagging several days prior to the grafting surgery.
Human skin harvest
Unidentified normal human skin was harvested from elective abdominoplasty procedures and transported on ice to the laboratory. A 1·2‐cm punch biopsy (Acupunch, Acuderm Inc, Fort Lauderdale FL) was used to harvest uniform samples of skin for engraftment. The dermis was dissected from the underlying adipose tissue using sharp dissection with a scalpel; grafted tissue included the stratum germinativum. The punch biopsy specimens were dissected and xenografted within 1 hour of surgical harvest. This methodology was developed because specimens harvested using a dermatome set at 12/1000th inch had a much higher engraftment failure rate.
Mouse xenograft surgery
Mice were anaesthetised using inhalation of 1–3% isofluorane in oxygen generated by a non‐rebreathing nose‐cone system with an exhaust evacuator and F‐air canister. Adequate anaesthesia was determined by the absence of withdrawal reflex to toe‐pinch. Procedures were performed under sterile conditions. The skin on the dorsum of the mouse was sterilised using povidone iodine followed by isopropyl alcohol. Full thickness skin was removed from two graft beds, 2 cm in diameter, on each side of the mouse flank. Full thickness human skin xenografts, harvested using the methodology described in the previous section, were placed on each mouse wound bed (two per mouse). Xenografts were secured using three sterile Steri‐Strips™ (3M St Paul, Minnesota), with the mouse skin margin splinted 2–4 mm away from the border of the xenograft (Figure 1A). This method was developed owing to issues encountered in early grafting experiments, when contraction of the mouse wound resulted in acute failure of the human xenograft. The xenograft was dressed using a 1·5 cm strip of Telfa non‐adherent dressing (Kendall; Tyco Healthcare Covidien, Mansfield, MA) and held in place using 1 inch wide Coban (3M, Figure 1B). Postoperatively, mice were housed in individual cages to minimise disturbance to the dressings and possible loss of the xenograft.
Figure 1.
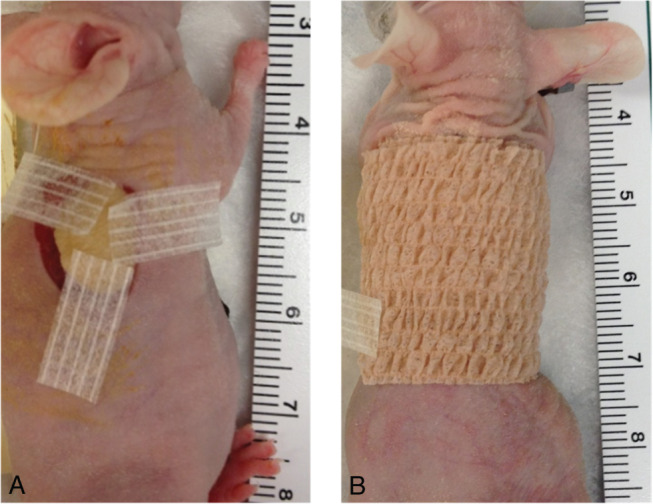
(A) Human xenograft was secured using three sterile Steri‐Strips™ (3M) with the mouse skin margin splinted 2–4 mm away from the border of the xenograft. (B) The xenograft was dressed using a 1·5 cm strip of Telfa non‐adherent dressing (Kendall, Tyco Healthcare, USA) and held in place using 1 inch wide Coban (3M, USA).
Dressings were changed on postoperative days 7 and 14, at which time the steri‐strips were removed. Xenografts remained dressed until day 25–30. Graft viability was assessed after 2 months.
Wounding surgery
Wounding experiments were performed using mice with xenografts older than 90 days. As human keratinocytes are known to change from stem cells to desquamation every 40–56 days 28, this 90‐day time period ensured that the xenograft was viable and that there was no ongoing rejection.
On the day of wounding, the mouse was anaesthetised using inhalation of 1–3% isoflurane in oxygen as described in the previous section. A 2‐mm punch biopsy was used to make a wound in the centre of each xenograft. Both xenografts on each mouse were wounded on the same day. Individual mice were wounded on different days to allow tissue‐based markers of wound healing to be followed sequentially. Three to six wounds were available for each data time point.
After wounding, the wound was dressed using a 1·5 cm strip of Telfa non‐adherent dressing held in place using Coban. The wound remained dressed until the day of harvest.
Quantitative in vivo imaging
The XenoLight RediJect COX‐2 probe (Perkin Elmer Waltham, MA) is a novel fluorescent imaging probe that specifically detects COX‐2 activity in vivo. This probe has been used in animal studies of malignancy and has been shown to have a high sensitivity and specificity; targeting only COX‐2 positive tumours, and not targeting COX‐2 negative tumours 29. Additional studies show that this probe has good signal‐to‐background ratios using a spectral imaging system.
Several weeks prior to the wounding experiments described in this study, control imaging was performed on a subset of mice in order to generate spectral libraries for the human–mouse xenografts. On the morning of imaging, 100 µl of the XenoLight RediJect COX‐2 probe (Perkin Elmer) was injected intra‐peritoneally into each mouse according to package instructions. Spectral fluorescence images were obtained using the Maestro™ In Vivo Imaging System (CRi, Inc, Woburn, MA) 8 hours after probe injection. A band‐pass filter appropriate for the probe of interest (wavelength 560–850) was used. The camera captured images at automatic exposure. Spectral libraries were generated by assigning peaks to the background fluorescence for unwounded human and mouse skin and fluorescence from the RediJect COX‐2 probe.
During the wounding experiments, on the day of wound harvest, mice were injected with the XenoLight RediJect COX‐2 probe and imaged 8 hours later using the same methodology as for the control images. Spectral fluorescent images of the wounds were captured and unmixed on the basis of their spectral patterns using commercially available software (Maestro; CRi). To evaluate signal intensities, regions of interest (ROI) were selected corresponding to the wound, and the total fluorescence signal from those areas was determined. Total signal in the ROI (in photons) measured at the surface of the wound was divided by the wound area (in pixels) as well as the exposure time. Statistical analysis of the data was conducted using Wilcoxon signed rank test, and data was expressed as mean and standard error of the mean (SEM). A two‐sided P value of less than 0·05 was considered to indicate statistical significance. Analysis was performed with SAS software, version 9.3.
Wound harvest
Following imaging, the mice were euthanised using 100% inhaled carbon dioxide at a rate of 1·5 l/minute until death. The entire xenograft including the region of the wound was harvested from each flank. Harvested wound tissue was fixed in formalin and then embedded in paraffin for histochemical analysis.
Immunohistochemistry
5 µm sections of formalin‐fixed paraffin‐embedded tissues were de‐paraffinised with xylenes and rehydrated through a graded alcohol series. Heat‐induced epitope retrieval (HIER) was performed by immersing the tissue sections at 98°C for 20 minutes in 10 mM citrate buffer (pH 6·0) with 0·05% Tween. Immunohistochemical staining was performed for the antibodies listed in Table 1 according to manufacturer's instructions. Serial sections stained with the secondary antibody only were used as negative controls. Images were captured using an Olympus DP25 camera on an Olympus BX41 microscope.
Table 1.
Source and dilution of primary antibodies used for immunohistochemistry
| Description | Cat. number | Company/Source | Dilution |
|---|---|---|---|
| Human major histocompatability complex‐1 | ab52922 | Abcam, Cambridge, MA | 1:250 |
| Human CD 31 (PECAM) | 2540‐1 | Epitomics, Burlingame, CA | 1:250 |
| Human involucrin | ab68 | Abcam | 1:4000 |
| Mouse PECAM | NBP2‐11848 | Novus Biological, Littleton, CO | 1:50 |
| Type I collagen | ab21286 | Abcam | 1:1600 |
| Cyclooxygenase‐2 | sc‐1747 | Santa Cruz Biotechnology, Dallas, TX | 1:800 |
Results
Demonstration of effective xenograft methods
Human keratinocytes are known to change from stem cells to desquamation every 40–56 days 28. Using the methods of xenografting described in the previous section, we were able to demonstrate viable human–mouse xenografts at 140 days after grafting (Figure 2A). Using haematoxylin and eosin (H + E) staining we were able to confirm clear pathological delineation of the human–mouse interface (Figure 2B). Based on histological examination of the punch biopsy specimen, the human xenografts exhibited normal dermal morphology and the presence of viable human tissue was further confirmed by positive staining for human major histocompatability complex‐1 (MHC‐1, Figure 2C). There were no signs of tissue rejection. This confirms that the xenograft methodology adapted in our laboratory successfully allows development of a viable and sustainable in vivo model for studying human skin diseases including human wound healing.
Figure 2.
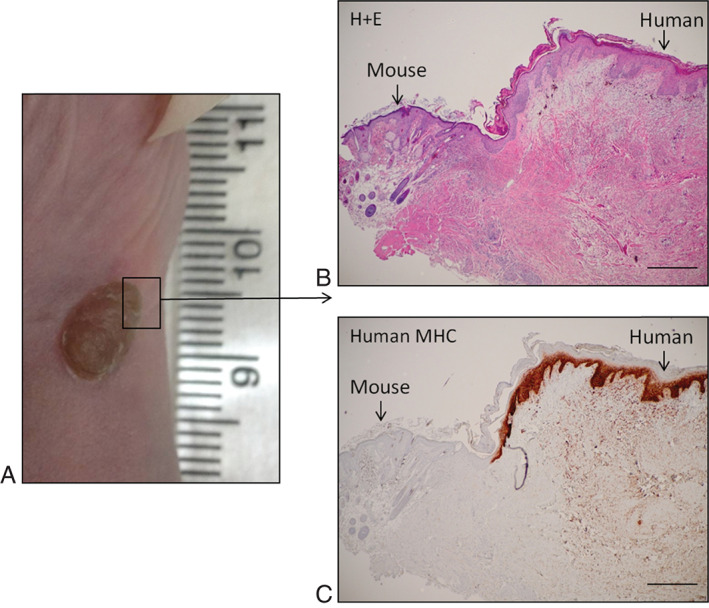
(A) Photograph demonstrating healthy human–mouse xenograft at 140 days after engraftment. (B) Haematoxylin and eosin staining demonstrating structural delineation of human and mouse tissue. (C) Confirmatory staining for human major histocompatability complex‐1 confirming the human origin of the keratinocytes with normal human skin structure (original magnification ×4, scale bars: 500 µm).
Reepithelialisation by human keratinocytes in response to wounding
Immunohistochemistry (IHC) of serial wound harvest specimens using H + E staining, as well as stains for human MHC‐1 and type I collagen were used to demonstrate that the xenografted tissue exhibited the four normal phases of human wound healing in response to a punch biopsy. As shown in Figure 3, the histopathology shows acute fibrin clot formation, followed by inflammatory infiltration, collagen deposition, granulation tissue formation and subsequent reepithelialisation. MHC‐1 staining clearly demonstrated wound reepithelialisation by regeneration of normal human keratinocytes resulting in wound healing by day 10.
Figure 3.
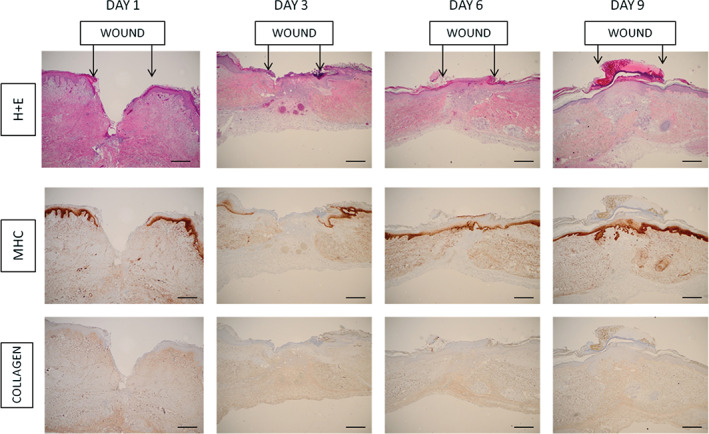
Representative immunohistochemistry of wound specimens harvested on serial days after wounding. Top panel demonstrates haematoxylin and eosin staining, middle panel demonstrates staining for human major histocompatability complex‐1 (MHC‐1) and bottom panel demonstrates staining for type I collagen. Arrows indicate the wound margins. By day 3, after wounding, the wound base is filled with collagen and granulation tissue. MHC staining clearly demonstrates that subsequent reepithelialisation (days 6–9) is with cells expressing human MHC‐1 signifying that they are of human origin. (Original magnification ×4, scale bars: 500 µm).
Quantitative in vivo fluorescent imaging
In vivo fluorescent imaging with a probe to detect COX‐2 activity was used to investigate whether this non‐invasive imaging method could be used to monitor wound healing in these human–mouse xenografts. We were able to demonstrate upregulation of inducible COX‐2 beginning on day 2 and maintained until day 4 after wounding. The mean (SEM) difference of COX‐2 signal between day 0 and day 4 was 8 725 045 ± 4 474 992 (P = 0·0313, Figure 4). By day 10 after wounding, the COX‐2 activity had returned close to baseline levels, with a mean (SEM) difference of 1 005 950 ± 766 414 (P = 0·3750) between day 0 and day 10 consistent with reepithelialisation of the wound was seen on IHC. This supports a role for in vivo fluorescent imaging using a probe for inducible COX‐2 for monitoring human wound healing in human–mouse xenograft models.
Figure 4.
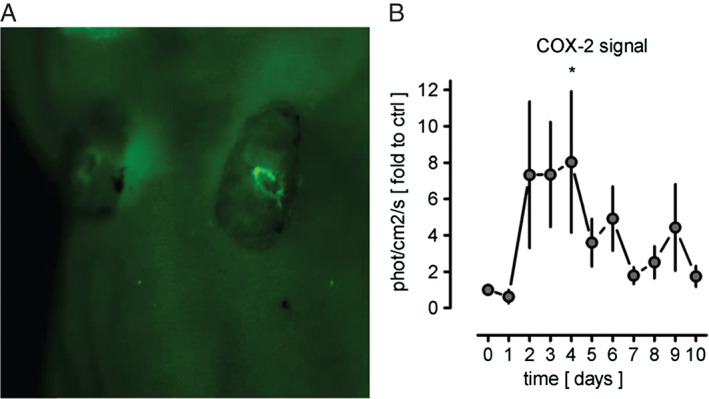
Quantification of cyclooxygenase‐2 activity in human xenografts before and after wounding. (A) Representative image using CRi Maestro™ in vivo fluorescent imaging. (B) Data is represented as mean ± standard error of the mean , expressed as a fold change compared with the baseline unwounded control skin (n = 3–12 animals/time point). Difference between day 0 and day 4, P = 0·0313; difference between day 0 and day 10, P = 0·3750.
Discussion
The present study confirms that the described human–mouse xenograft model is useful for studying the complex physiological interactions that contribute to normal human wound healing. We were able to demonstrate reepithelialisation of a 2‐mm punch biopsy wound by 9–10 days after wounding and confirm that the epithelial tissue is of human origin. IHC suggests that the findings seen in this wound model are very similar to those seen in an acute wound, and therefore this is a good model to study the complex interactions occurring during normal human wound healing.
Previous investigators who have used a similar model to investigate partial thickness wounds found that after wounding, human fibroblasts disappear from the wounded dermis. Invasion of mouse fibroblasts generates mouse granulation tissue, which provides a matrix for ingrowth of human keratinocytes 21. In the experiments described in this study, we used a full thickness biopsy to create the wound. No residual human tissue remained in the wound bed. Although we did not investigate fibroblast origin in this study, IHC suggests that the collagen and granulation tissue deposited in the wound bed is at least partially of murine origin and that this forms a matrix for human keratinocyte reepithelialisation. Taken together, the observations from the published studies and our own experience suggest that there must be important synergistic epithelial–mesenchymal interactions, which are not species‐specific, occurring in the wound bed and permitting reepithelialisation in this environment. This suggests that despite concerns regarding the murine origin of the granulation tissue and the reduced immune response in the mouse, there are very important interactions occurring that facilitate healing in this model which may provide interesting insights into possible therapeutic targets for the management of chronic wounds. Additionally, this human–mouse xenograft model may have potential for future study of mechanisms of other forms of dysfunctional wound healing including human keloid and hypertrophic scar.
A novel feature of the present study is that we harnessed near‐infrared imaging modalities that are currently used extensively in animal models of malignancy. We were able to demonstrate that in vivo imaging can be used in this human–mouse xenograft model to investigate inflammatory markers in the wound bed in real time. The near‐infrared imaging described has not previously been used in the study of human skin diseases. However, harnessing these imaging methods for the study of wound healing and other skin diseases in this human–mouse chimeric model greatly increases the experimental potential of this model, and adds a new angle for investigation which would not be possible in swine models. Although the near‐infrared imaging described is dependent on the half‐life of the probe selected, it opens the possibility of using other imaging probes to track inflammatory markers, critical cytokines and matrix metalloproteinases serially in wounds, rather than requiring wound harvest in order to track and interpret histological findings. This could potentially reduce the number of viable xenografts required for any individual experiment, thus greatly increasing the potential for this model in future.
Conclusions
In conclusion, our data demonstrates that this human–mouse xenograft model is useful for studying the dynamic process of human wound healing using in vivo imaging. Most importantly, this human–mouse xenograft model resolves the issue of wound contraction, which complicates other mouse wound healing models and can introduce bias into wounding experiments. Our study demonstrates that there is reepithelialisation of the wound with human keratinocytes. Unlike models in pigs and other larger mammals, this small animal model is suitable for in vivo fluorescent imaging to study the expression of inflammatory molecules including inducible COX‐2 and matrix metalloproteinases in the wound bed. Such imaging techniques may provide useful surrogate endpoints for intervention studies.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources, the National Cancer Institute or the National Institutes of Health.
Acknowledgements
This work was supported by award numbers KL2RR031974 and UL1TR000101 (previously UL1RR031975) from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through the Clinical and Translational Science Awards Program (CTSA). VKS, CEA, ET, SM and AW are supported by award R01NR013888 from the National Institute of Nursing Research. These studies were conducted in part at the Lombardi Comprehensive Cancer Center Histopathology and Tissue Shared Resource which is supported in part by the National Cancer Institute grant P30CA051008.
References
- 1. Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair and Regen 2009;17:763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Escandon J, Vivas AC, Tang J, Rowland KJ, Kirsner RS. High mortality in patients with chronic wounds. Wound Repair Regen 2011;19:526–8. [DOI] [PubMed] [Google Scholar]
- 3. Price P, Harding K. The impact of foot complications on health‐related quality of life in patients with diabetes. J Cutan Med Surg 2000;4:45–50. [DOI] [PubMed] [Google Scholar]
- 4. Price P, Harding K. Cardiff Wound Impact Schedule: the development of a condition‐specific questionnaire to assess health‐related quality of life in patients with chronic wounds of the lower limb. Int Wound J 2004;1:10–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Rijswijk L, Gray M. Evidence, research, and clinical practice: a patient‐centered framework for progress in wound care. J Wound Ostomy Continence Nurs 2012;39:35–44. [DOI] [PubMed] [Google Scholar]
- 6. Brem H, Tomic‐Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest 2007;117:1219–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schultz GS, Davidson JM, Kirsner RS, Bornstein P, Herman IM. Dynamic reciprocity in the wound microenvironment. Wound Repair Regen 2011;19:134–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol 2007;25:9–18. [DOI] [PubMed] [Google Scholar]
- 9. Harding KG, Moore K, Phillips TJ. Wound chronicity and fibroblast senescence – implications for treatment. Int Wound J 2005;2:364–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bader A, Lorenz K, Richter A, Scheffler K, Kern L, Ebert S, Giri S, Behrens M, Dornseifer U, Macchiarini P, Machens H-G. Interactive Role of Trauma Cytokines and Erythropoietin and Their Therapeutic Potential for Acute and Chronic Wounds. Rejuvenation Res 2011;14:57–66. [DOI] [PubMed] [Google Scholar]
- 11. Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic‐Canic M. Perspective article: growth factors and cytokines in wound healing. Wound Repair Regen 2008;16:585–601. [DOI] [PubMed] [Google Scholar]
- 12. Kirker KR, Secor PR, James GA, Fleckman P, Olerud JE, Stewart PS. Loss of viability and induction of apoptosis in human keratinocytes exposed to Staphylococcus aureus biofilms in vitro. Wound Repair Regen 2009;17:690–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pastar I, Stojadinovic O, Tomic‐Canic M. Role of keratinocytes in healing of chronic wounds. Surg Technol Int 2008;17:105–12. [PubMed] [Google Scholar]
- 14. Stojadinovic O, Pastar I, Vukelic S, Mahoney MG, Brennan D, Krzyzanowska A, Golinko M, Brem H, Tomic-Canic M. Deregulation of keratinocyte differentiation and activation: a hallmark of venous ulcers. J Cell Mol Med 2008;12:2675–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. James GA, Swogger E, Wolcott R, Pulcini Ed, Secor P, Sestrich J, Costerton JW, Stewart PS. Biofilms in chronic wounds. Wound Repair Regen 2008;16:37–44. [DOI] [PubMed] [Google Scholar]
- 16. Secor P, James G, Fleckman P, Olerud J, McInnerney K, Stewart P. Staphylococcus aureus biofilm and planktonic cultures differentially impact gene expression, mapk phosphorylation, and cytokine production in human keratinocytes. BMC Microbiol 2011;11:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wong V, Sorkin M, Glotzbach J, Longaker M, Gurtner G. Surgical approaches to create murine models of human wound healing. J Biomed Biotechnol 2011;2011:969618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gottrup F, Ågren MS, Karlsmark T. Models for use in wound healing research: a survey focusing on in vitro and in vivo adult soft tissue. Wound Repair Regen 2000;8:83–96. [DOI] [PubMed] [Google Scholar]
- 19. Aarabi S, Bhatt KA, Shi Y, Paterno J, Chang EI, Loh SA, Holmes JW, Longaker MT, Yee H, Gurtner GC. Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB J 2007;21:3250–61. [DOI] [PubMed] [Google Scholar]
- 20. Démarchez M, Hartmann DJ, Herbage D, Ville G, Pruniéras M. Wound healing of human skin transplanted onto the nude mouse: II. An immunohistological and ultrastructural study of the epidermal basement membrane zone reconstruction and connective tissue reorganization. Dev Biol 1987;121:119–29. [DOI] [PubMed] [Google Scholar]
- 21. Rossio‐Pasquier P, Casanova D, Jomard A, Demarchez M. Wound healing of human skin transplanted onto the nude mouse after a superficial excisional injury: human dermal reconstruction is achieved in several steps by two different fibroblast subpopulations. Arch Dermatol Res 1999;291:591–9. [DOI] [PubMed] [Google Scholar]
- 22. Reed N, Manning D. Long‐term maintenance of normal human skin on congenitally athymic (nude) mice. Proc Soc Exp Biol Med 1973;143:350–3. [DOI] [PubMed] [Google Scholar]
- 23. Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, A. Van De Putte LB, Lipsky PE. Cyclooxygenase in biology and disease. FASEB J 1998;12:1063–73. [PubMed] [Google Scholar]
- 24. Laulederkind S, Thompson‐Jaeger S, Goorha S, Chen Q, Fu A, Rho J, Ballou L, Raghow R. Both constitutive and inducible prostaglandin H synthase affect dermal wound healing in mice. Lab Invest 2002;82:919–27. [DOI] [PubMed] [Google Scholar]
- 25. Muscará MN, McKnight W, Asfaha S, Wallace JL. Wound collagen deposition in rats: effects of an NO‐NSAID and a selective COX‐2 inhibitor. Br J Pharmacol 2000;129:681–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Muller‐Decker K, Hirschner W, Marks F, Furstenberger G. The effects of cyclooxygenase isozyme inhibition on incisional wound healing in mouse skin. J Invest Dermatol 2002;2002:1189–95. [DOI] [PubMed] [Google Scholar]
- 27. Ishida Y, Kimura A, Nosaka M, Kuninaka Y, Takayasu T, Eisenmenger W, Kondo T. Immunohistochemical analysis on cyclooxygenase-2 for wound age determination. Int J Legal Med 2012;126:435–40. [DOI] [PubMed] [Google Scholar]
- 28. Halprin K. Epidermal "turnover time" – a re‐examination. Br J Dermatol 1972;86:14–9. [DOI] [PubMed] [Google Scholar]
- 29. Uddin MJ, Crews BC, Blobaum AL, Kingsley PJ, Gorden DL, McIntyre JO, Matrisian LM, Subbaramaiah K, Dannenberg AJ, Piston DW, Marnett LJ, Selective Visualization of Cyclooxygenase-2 in Inflammation and Cancer by Targeted Fluorescent Imaging Agents. Cancer Res 2010;70:3618–27. [DOI] [PMC free article] [PubMed] [Google Scholar]


