Abstract
Background
Based on review of patient data in case conferences over time, we hypothesized that clinically relevant data are omitted in routine soft tissue sarcoma staging.
Methods
We examined subsets of the Memorial Sloan-Kettering Cancer Center soft tissue sarcoma database with respect to criteria of the AJCC versions 6 (2002) and 7 (2010) staging systems and examined their clinical outcomes.
Results
Relapse-free survival decreases with increasing primary tumor size in four categories, versus two categories used in AJCC 6 and 7 staging. Disease-specific survival decreases over three categories. Conversely, omission of tumor depth as a prognostic factor in version 7 appears supported, since tumor depth is not an independent risk factor for disease-specific survival by multivariate analysis. Patients with nodal disease and no other metastases fare better than patients with other metastases, but have inferior outcomes compared to patients with large high-grade tumors without nodal metastasis. Multivariate analysis identified size, site, grade, age, nodal metastatic disease and other metastatic disease as independent risk factors for disease-specific survival. Version 6 and 7 criteria are tacit regarding anatomic site and histology for tumors with identical FNCLCC grade.
Conclusions
Improved patient risk assessment may be achieved by staging using a larger number of size categories. Staging system refinements come at the cost of a larger number of staging categories. Histology or site-specific staging systems, nomograms or Bayesian belief networks may provide more accurate means to assess clinical outcomes.
Introduction
Clinical staging of cancer is of fundamental importance in discussing patient outcomes. It is now possible to predict accurately the risk of cancer recurrence and mortality1, and thus to adapt therapy according to risk. Prognostic factors for outcome vary based on the endpoint, be it local recurrence, disease-specific survival, overall survival, or distant disease-free survival2. Tumor size, nodal status, and presence or absence of metastatic disease (TNM systems) have been employed most frequently to assess risk and stage patients. For soft tissue sarcomas, tumor grade is an important prognostic factor. Other factors, such as serum tumor markers, are part of staging systems for only a minority of cancers, e.g. testicular cancer. Tumor genetics are observed to impact clinical outcomes for patients with hematological malignancies3 and solid tumors, e.g. non-small cell lung adenocarcinoma4,5 and sarcomas such as gastrointestinal stromal tumors6,7 and rhabdomyosarcoma8, and debated in synovial sarcoma9,10, but are presently not routinely employed in soft tissue sarcoma staging.
Substantial efforts have been made to accurately grade and stage soft tissue sarcomas (STS)11–16. STS staging is hampered by its low incidence (<1% of all human cancers with more than 50 histological subtypes) and the use of varied systems for grade and stage. The Fédération Nationale des Centres de Lutte Contre le Cancer (FNCLCC) system of tumor grading better predicted outcome in comparison to a National Institutes of Health (NIH) staging system14. Two, three and four grade systems have been used to grade tumors, further complicating staging system comparisons.
The American Joint Committee on Cancer (AJCC) Cancer Staging Handbook, 7th edition17 was released in 2010 and updated the 6th edition18 adopted in 2002. In the newest staging system, the FNCLCC system was adopted for tumor grading13, eliminating the issue of 2 vs. 3 vs. 4 grade systems of STS grading of version 6. The depth of tumor is recorded in the 7th edition as in the 6th, but now does not impact tumor stage. Patients with draining node involvement are now included as stage III, a change from stage IV in version 6.
In reviewing the changes between AJCC versions 6 and 7, we could not identify supporting data to suggest the changes made, save for a publication demonstrating that N1M0 patients fare better than N1M1 patients, and concluded that such N1M0 patients had overall survival similar to AJCC version 6 G3T2N0 patients19. Conversely, data were presented between the publication of AJCC versions 6 and 7 that suggest that other changes in the staging system should be effected. In one study, the risk of tumor recurrence increased as primary size increased over 10 cm, which had been addressed neither in version 6 nor 715. STS are not presently staged based on anatomic primary site, even though primary site has an impact on outcome12. Finally, version 7 functionally eliminated the use of superficial vs. deep primary site, a known prognostic factor for recurrence of extremity sarcomas12,15. Histology is also an important prognostic factor, as highlighted by the new GIST staging system in version 7.
Based on the data above, as well as our own experience in weekly staging meetings, we hypothesized that outcomes from STS categorization by AJCC versions 6 and 7 did not accurately reflect patient outcomes. We investigated the impact of clinical variables on patient outcome using a prospectively collected single institution STS database.
Methods
Between July 1, 1982, and June 30, 2010, 8647 patients treated at Memorial Sloan-Kettering Cancer Center (MSKCC) were identified from an IRB-approved prospective STS database. Clinicopathologic data included age at diagnosis, sex, histologic type, tumor depth, grade, site, size, margin status, nodal status, presence of other metastatic disease, indication for RT, radiation dose, and use of concomitant chemotherapy. Tumor depth and grade were defined as previously reported20. Of note, the MSKCC database has not been coded by FNCLCC grade, and it is only possible in our analyses of grade to comment on low and high grade tumors (tumors classified as stage I and III in both versions 6 and 7), and not on intermediate grade tumors per se. Those tumors termed grade 2 (G2) on a three-grade scale are combined with G3 tumors, consistent with AJCC version 6. Sites of disease were defined as (1) extremity (upper and lower extremity), (2) abdomen or retroperitoneum (abdomen/RP), and (3) trunk (chest wall, proximal extremity/groin, thoracic, head and neck). Tumor size was recorded as the largest dimension and was also stratified as ≤5 cm, >5–10 cm, >10–15 cm and >15 cm. Margins of resection were defined as R0 (negative), R1 (microscopically positive), and R2 (grossly positive).
The primary end point of the analysis was disease-specific survival (DSS), defined as time from date of surgery to date of death as a result of disease or treatment complication. Recurrence free survival (RFS) was a secondary endpoint, defined as the date of surgery to the date of the event indicating disease recurrence. The influence of clinicopathologic features on DSS and RFS was analyzed using the Kaplan-Meier method and the log-rank test in the univariate setting and using a Cox proportional hazard regression analysis in the multivariate setting. A p-value <0.05 was considered significant.
Results
Patient outcome by tumor size
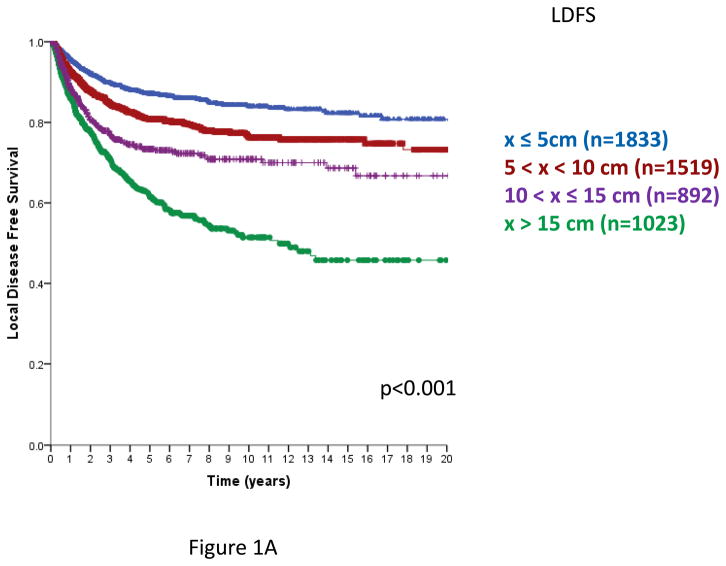
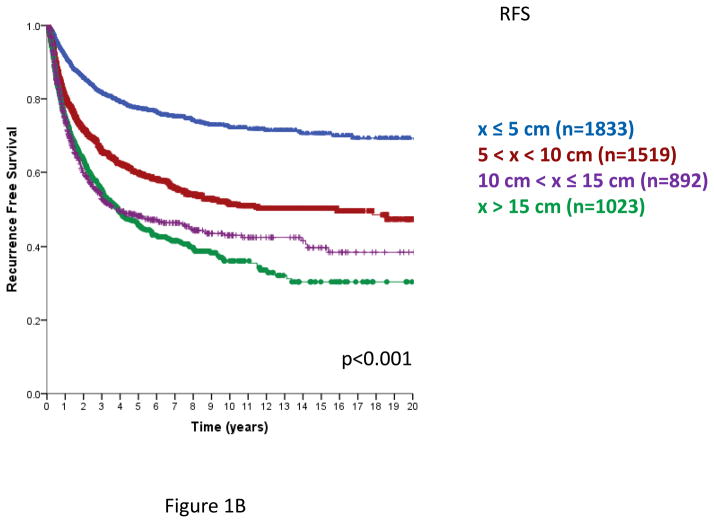
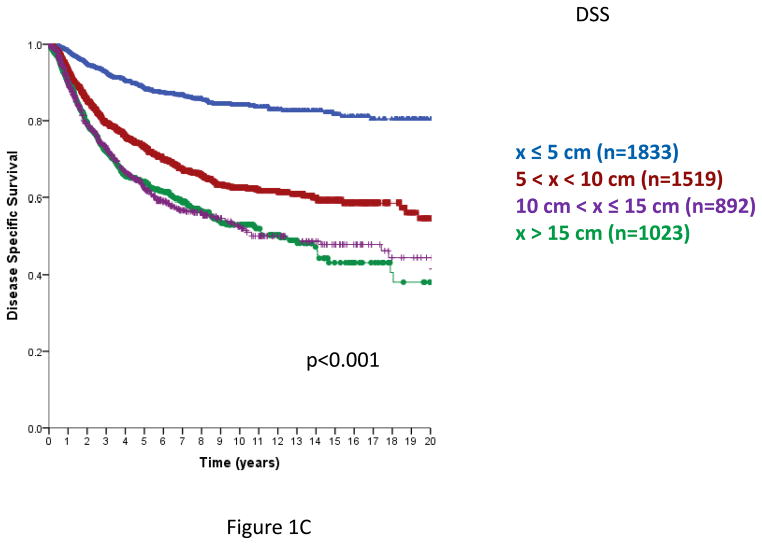
In AJCC versions 6 and 7, primary STS are segregated into sizes ≤5 cm or >5 cm. From this database, the risk of local recurrence increases with primary tumor size, and continues to increase from ≤ 5 cm to >5–10, >10–15 cm and >15 cm primary tumors. Employing four size classes, local recurrence-free survival (LRFS, Figure 1A) and overall recurrence free survival (RFS, Figure 1B) demonstrated a statistically significant decline with each size category. Disease-specific survival (DSS) also declined as primary tumor size increased, but was similar in the >10–15 cm and >15 cm categories (Figure 1C), p=0.91 for the comparison of >10–15 cm and >15 cm categories. Similar statistically significant differences in LRFS, RFS, and DSS are observed using three tumor size categories, ≤5 cm, >5–10 cm and >10 cm (data not shown).
Figure 1. Local recurrence free survival (RFS, Figure 1A), Overall recurrence-free survival (Figure 1B) and disease specific survival (DSS, Figure 1C) by size category, ≤5, 5–10, 10–15, > 15 cm.
Figure 1A. Local recurrence-free survival (time from primary surgery to 1st local recurrence), n=5267 patients, excludes 75 patients with unknown size categories; log rank p<0.001.
Figure 1B. Recurrence-free survival (time from primary surgery to 1st local or distant recurrence), n=5267, excludes 75 patients with unknown size categories; log-rank p<0.001
Figure 1C. Disease-specific survival (time from primary surgery to death from disease), n=5267, excludes 75 patients with unknown size categories; log-rank p<0.001; log-rank p-value=0.91 comparing >10–15 and >15 cm groups.
Patient outcome by tumor depth
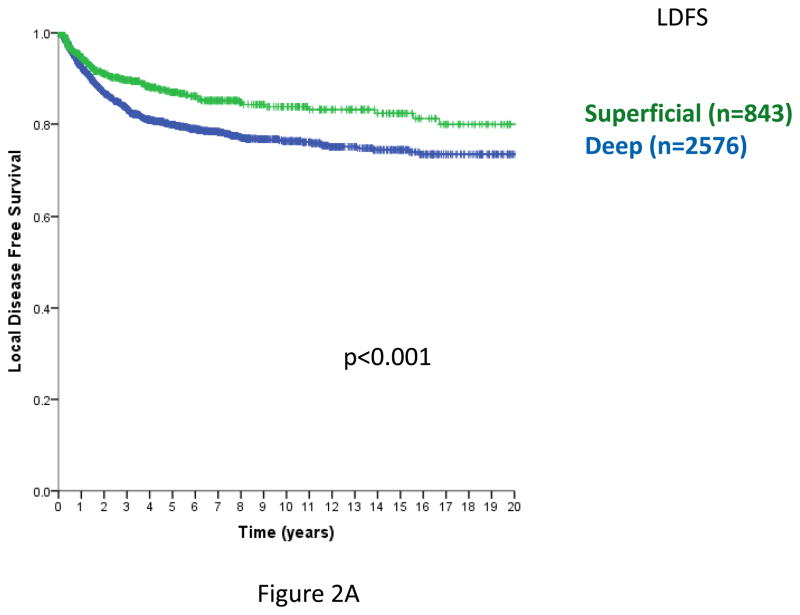
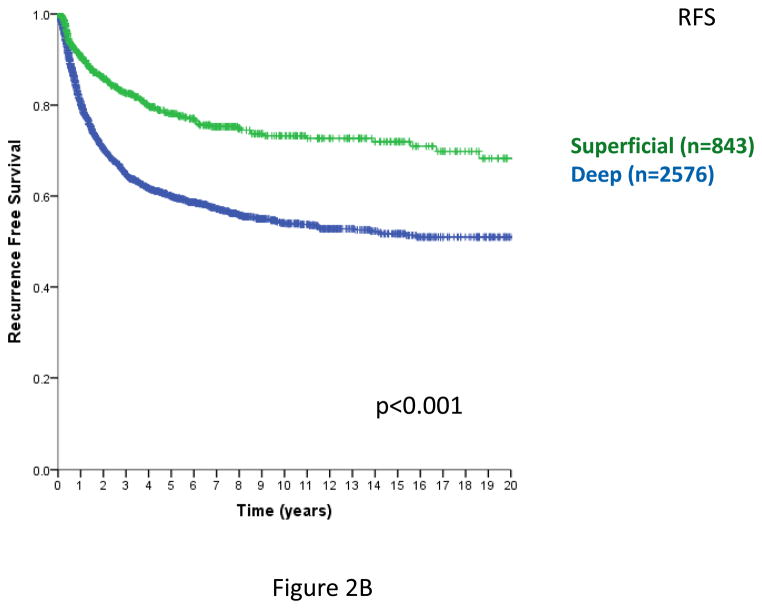
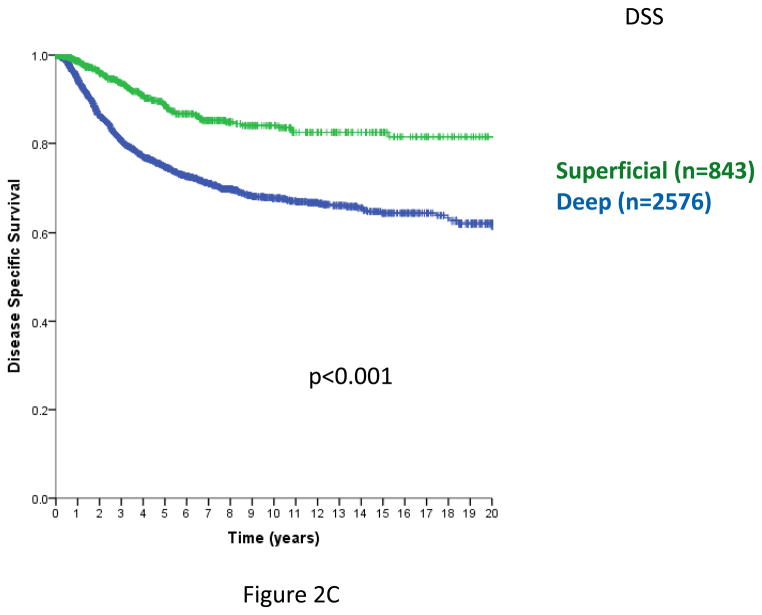
In prior analyses, tumor depth was shown to be a clinically important variable for patient outcomes11,12,15,21. Including STS with a primary site of extremity, trunk, and head/neck and specifically omitting intra-abdominal, retroperitoneal, and visceral primaries, where there are no superficial STS, depth remains a statistically significant predictor of local RFS (Figure 2A), overall RFS (Figure 2B), and DSS (Figure 2C) (log-rank p<0.001 in each case). Similar outcomes are observed examining only extremity and trunk primary sites (data not shown).
Figure 2. Figure 2A. Local relapse-free survival (time from primary surgery to 1st local relapse, trunk/extremity/H-N primary sites only). n=3419, excludes 6 patients with unknown size categories; log-rank p<0.001.
Figure 2B. Relapse-free survival (time from primary surgery to 1st local or distant relapse, trunk/extremity/H-N primary sites only). n=3419, excludes 6 patients with unknown size categories; log-rank p<0.001
Figure 2C. Disease-specific survival (time from primary surgery to death from disease, trunk/extremity/H-N primary sites only). n=3419, excludes 6 patients with unknown size categories. log-rank p<0.005
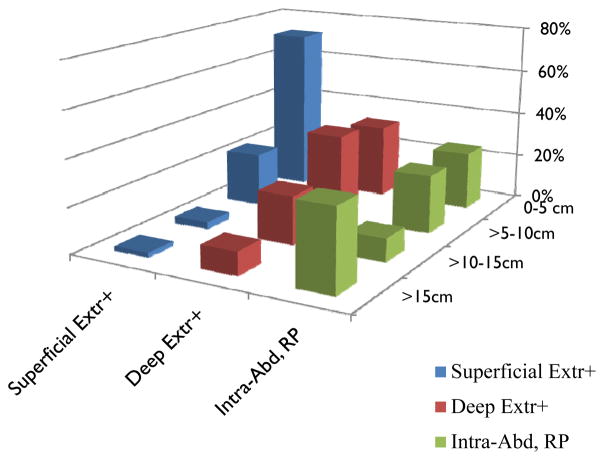
In a Cox proportional hazards multivariate analysis including anatomic site and four size categories as above (Table 1), statistically significant factors predicting disease specific survival include patient age above or below the median (54.4 years), primary tumor size, primary site, and grade, but not gender. Primary tumor depth is not an independent variable predicting overall survival. This finding is explained at least in part by the relative dearth of large superficial and of smaller deep sarcomas (Figure 3).
Table 1.
Cox proportional hazard regression analysis for disease-specific survival including all database patients. Tumors >10 cm are excluded if their exact sizes were not specified.
| Variable | Categories | p-value | HR | 95%CI for HR |
|---|---|---|---|---|
| Age | <54.4, ≥54.4 years (median) | <0.001 | 0.749 | (0.641, 0.874) |
| Gender | Male, female | 0.914 | - | - |
| Anatomic primary site | Other site, retroperitoneal and visceral, extremity | 0.005 | 1.221 | (1.061, 1.405) |
| Primary tumor Size (cm) | >15, >10–15, >5–10, <5 | <0.001 | 1.198 | (1.106, 1.299) |
| Depth | Superficial, deep | 0.166 | - | - |
| Grade | Low, high | 0.042 | 0.556 | (0.316, 0.978) |
| Metastatic disease | None, nodal metastases (N1M0), other metastases (N0M1), both nodal and other metastases (N1M1) | <0.001 | ||
| N0M0 vs N1M0 | 0.011 | 0.392 | (0.190, 0.807) | |
| N1M0 vs N0M1 | <0.001 | 0.197 | (0.109, 0.353) | |
| N1M0 vs N1M1 | 0.613 | - | - |
HR: Hazard ratio; 95%CI: 95% confidence interval; - : omitted since not statistically significant
Figure 3.
Frequency of different size categories by superficial or deep site. All intra-abdominal, retroperitoneal, and visceral tumors are deep, and are noted separately. Number of cases and percentages of each tumor class by size are indicated. Extr: extremity; H/N: head and neck; RP: retroperitoneal; visc: visceral
Patient outcome by nodal status and other metastatic disease
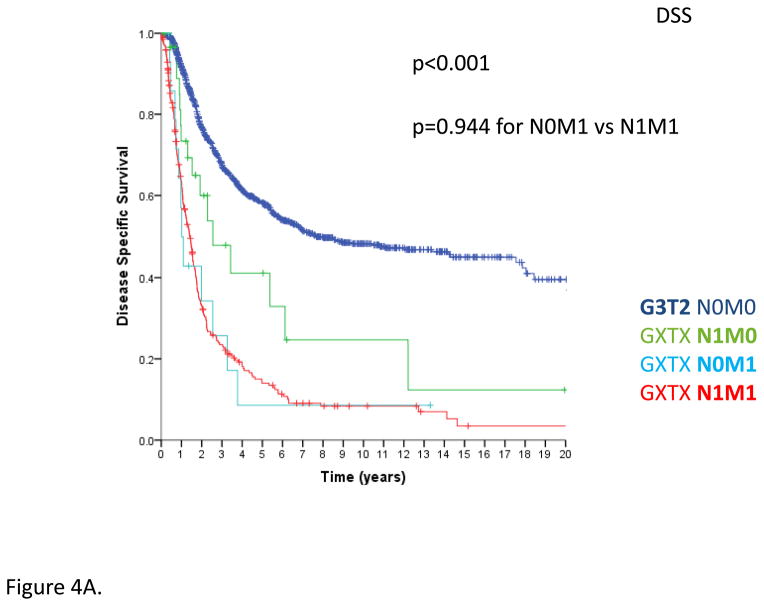
In AJCC version 6, node positive (N1) disease was combined with M1 disease as stage IV. Published series indicated that patients with node positive, metastasis negative (N1M0) disease may fare better than those with any node status, metastasis positive (NXM1) disease19,22, such that N1M0 STS were recoded as stage III in AJCC version 715,21. In examining this prospectively collected database, patients with any grade, any tumor size, node positive, metastasis negative (GXTXN1M0) disease demonstrated inferior DSS compared to people with other stage III tumors, i.e. grade 3, large primary, node negative, metastasis negative (G3T2N0M0) primary STS, but superior survival than those with N0M1 or N1M1 disease (p<0.001, Figure 4A).
Figure 4.
Figure 4A. Disease-specific survival comparing G3T2N0M0 primary STS to GXTXN1M0 and GXTXN1M1 STS, n=1440 total; G3T2N0M0 disease (n=1123), GXTXN1M0 (n=33), GXTXN1M1 (n=15), and GXTXN0M1 disease (n=269); log-rank p<0.001. Comparing GXTXN0M1 and GXTXN1M1 patients, log-rank p=0.944. 95% confidence intervals are noted at 5 years for the two largest groups; they are not meaningful for the smallest groups with so few events.
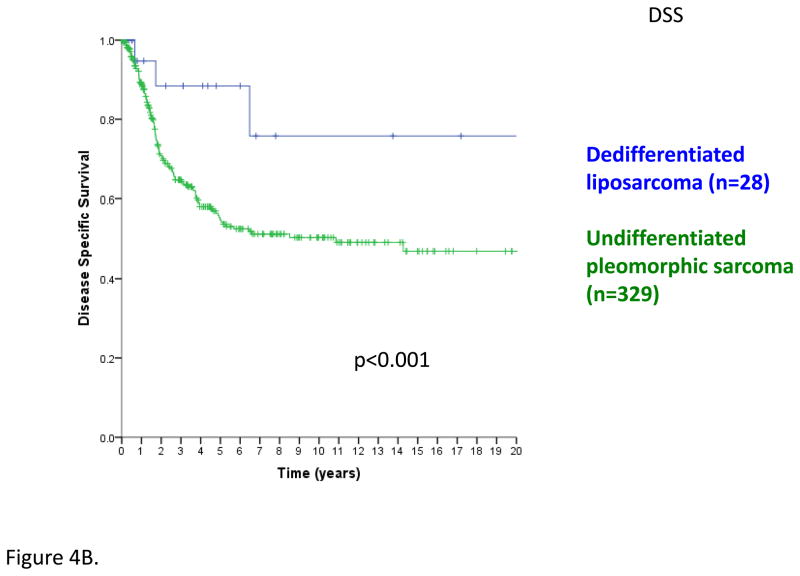
Figure 4B. Disease-specific survival comparing extremity dedifferentiated liposarcoma (n=28) and undifferentiated pleomorphic sarcoma (n=329); log rank p<0.001
Thus, the small proportion of patients with N1M0 disease have a DSS intermediate to those with localized disease and overt metastatic disease, with the implication that they represent a unique class of patient for staging purposes, inferior to G3T2 (N0M0) primaries but superior to patients with overt M1 disease. This is borne out in the multivariate analysis, in which nodal disease is significantly worse than no metastatic disease, and N0M1 metastatic disease is significantly worse than N1M0 disease (Table 1). In other words, N1M0 patients have outcomes intermediate to what are presently defined as AJCC version 7 stages III and IV disease.
Tumor histology as a risk factor
AJCC version 7 STS staging deleted certain connective tissue malignancy subtypes (GIST, deep fibromatosis/desmoid tumor, and Kaposi sarcoma) and added others (angiosarcoma, extraskeletal Ewing sarcoma, and dermatofibrosarcoma protuberans) and encompasses all soft tissue tumors, even though some, such as extraskeletal Ewing sarcoma, receive chemotherapy while others often do not. As an example of the importance of histology, patients with dedifferentiated liposarcoma of the extremity have superior overall survival than those with pleomorphic sarcoma, even though both are G3 by FNCLCC (Figure 4B).
Discussion
The present data argue for greater use of histology- or site-specific staging systems, nomograms and Bayesian belief networks to more accurately assess patient risk than is currently feasible with AJCC staging. The development of cancer staging is an evolving process that attempts to account for both clinical and other variables in determining patient outcome, while at the same time retaining portability. The more accurate assessment of risk, as noted in this and other work since the publication of AJCC version 6, will hopefully allow for a clearer discussion with patients regarding treatment options. While there may not be as much impact in the choice of adjuvant radiation, in which the use of radiation for primaries over 5 cm is for most circumstances a standard of care, the choice to use adjuvant chemotherapy, which benefits patients modestly if at all, depending on the clinical situation, may be more greatly affected by the quantitation of increased metastatic risk inherent in larger sarcomas.
Refinements included in AJCC version 7 should help improve staging, in particular, the consistent use of FNCLCC grade and N1M0 disease as a separate tumor entity. Age, nodal status and M1 metastatic disease were each a statistically significant predictor for DSS, but patients with both nodal and other metastatic disease fared no worse than those with other metastases alone. However, with increasing categorization comes increasing complexity. Omitting histology, a staging system to incorporate all significant variables for DSS would involve four size categories, three grades, and a minimum of two categories each for site, age, nodal status and metastatic status, i.e. 192 categories, unwieldy by any measure.
Issues that arise from the updating of the AJCC criteria to include FNCLCC grading may merit more thorough study. For example, the terminology for sarcoma subtypes continues to evolve since the 1996 FNCLCC criteria were developed13, and newer subtypes have not been assigned differentiation scores. Malignant fibrous histiocytoma (MFH) is now termed high-grade undifferentiated pleomorphic sarcoma (UPS)23. New entities included in AJCC version 7 (“deep” angiosarcoma, dermatofibrosarcoma protuberans [DFSP]) are not included in the list of tumor differentiation score, but arguably should be 3 and 1, respectively. Low grade fibromyxoid sarcoma and sclerosing epithelioid fibrosarcoma are subtypes in AJCC version 7 that have no differentiation grade (grade 2 is suggested). The 2013 update of with the World Health Organisation (WHO) soft tissue sarcoma subtypes23 remains tacit on this the FNCLCC grade of these tumors. Another issue complicating staging is that the mitotic rate that defines FNCLCC high grade varies by histology. For example, myxoid-round cell liposarcoma is considered high grade if >5% of the tumor has round cell features24, which is not the case for other sarcomas.
AJCC version 7 eliminated depth as a variable for outcome and included only two size categories for the primary tumor, the latter of which is also the case for version 6. Interestingly, in a prior critique of AJCC 6, primary tumor size did not predict outcome when only retroperitoneal tumors were involved25. The lack of large superficial and small deep tumors11 also argues that retroperitoneal sarcomas should be staged separately26. Data provided in this study and other analyses27 imply the lack of independence of depth as a variable predicting clinical outcomes. This issue may best be examined in a dataset in which FNCLCC grade is homogeneously employed, a weakness of our analysis, which examined only high grade vs. low grade sarcomas. That being said, a number of low grade sarcoma would be reclassified in FNCLCC terms as intermediate grade, and the differences between low grade and high grade STS in that situation is likely to be more pronounced than the data presented here.
As a single institution study that confirms and extends other work, we also cannot claim that alternative means for staging sarcomas will be superior to AJCC version 6 or 7. However, even using the now decade-old nomogram for soft tissue sarcoma12 estimates risks for recurrence or death within ±8%, a claim that cannot be made with presently available AJCC staging. For example, the quoted AJCC 5-year overall survival data by stage17 do not include confidence intervals, and are based on data published with a previous AJCC staging system without re-characterization of tumors using new criteria.
We anticipate refinements to staging systems will allow for more accurate assessments of risk over time, as has now happened for rhabdomyosarcoma (an important sarcoma subtype as pertains to lymph node metastases28,29) and GIST8,30. While these issues are addressed, we suggest that the use of general or anatomic site specific nomograms for soft tissue sarcomas (e.g. www.mskcc.org/nomograms), or staging system for specific anatomic sites or subtypes12,26,31,32 when feasible. These newer methods allow integration of multiple variables into risk assessment with greatest facility, as may Bayesian strategies for risk classification in the future27. While efforts for accurate clinical staging may be swept aside someday through the greater use of the molecular classification of sarcomas33, for the time being it remains necessary to utilize clinical variables to inform treatment decisions.
Synopsis.
Current staging systems omit potentially useful information assessing soft tissue sarcoma outcomes. Inclusion of more detailed data regarding primary tumor size, site, histology, and reassessing the role of nodal positivity may yield more accurate risk assessment.
Acknowledgments
Research support: NCI grants CA47179, CA140146, CA142860
Footnotes
Presented in part at an oral session at the ASCO 2012 Annual Meeting, Chicago, IL
Conflicts of Interest: None
References
- 1.Ravdin PM, Siminoff LA, Davis GJ, et al. Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol. 2001 Feb 15;19(4):980–991. doi: 10.1200/JCO.2001.19.4.980. [DOI] [PubMed] [Google Scholar]
- 2.Pisters PW, Leung DH, Woodruff J, Shi W, Brennan MF. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol. 1996 May;14(5):1679–1689. doi: 10.1200/JCO.1996.14.5.1679. [DOI] [PubMed] [Google Scholar]
- 3.Byrd JC, Mrozek K, Dodge RK, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002 Dec 15;100(13):4325–4336. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 4.Bell DW, Lynch TJ, Haserlat SM, et al. Epidermal growth factor receptor mutations and gene amplification in non-small-cell lung cancer: molecular analysis of the IDEAL/INTACT gefitinib trials. J Clin Oncol. 2005 Nov 1;23(31):8081–8092. doi: 10.1200/JCO.2005.02.7078. [DOI] [PubMed] [Google Scholar]
- 5.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007 Aug 2;448(7153):561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 6.Desai J, Shankar S, Heinrich MC, et al. Clonal evolution of resistance to imatinib in patients with metastatic gastrointestinal stromal tumors. Clin Cancer Res. 2007 Sep 15;13(18 Pt 1):5398–5405. doi: 10.1158/1078-0432.CCR-06-0858. [DOI] [PubMed] [Google Scholar]
- 7.Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003 Dec 1;21(23):4342–4349. doi: 10.1200/JCO.2003.04.190. [DOI] [PubMed] [Google Scholar]
- 8.Missiaglia E, Williamson D, Chisholm J, et al. PAX3/FOXO1 fusion gene status is the key prognostic molecular marker in rhabdomyosarcoma and significantly improves current risk stratification. J Clin Oncol. 2012 May 10;30(14):1670–1677. doi: 10.1200/JCO.2011.38.5591. [DOI] [PubMed] [Google Scholar]
- 9.Ladanyi M, Antonescu CR, Leung DH, et al. Impact of SYT-SSX fusion type on the clinical behavior of synovial sarcoma: a multi-institutional retrospective study of 243 patients. Cancer Res. 2002 Jan 1;62(1):135–140. [PubMed] [Google Scholar]
- 10.Kawai A, Woodruff J, Healey JH, Brennan MF, Antonescu CR, Ladanyi M. SYT-SSX gene fusion as a determinant of morphology and prognosis in synovial sarcoma. N Engl J Med. 1998 Jan 15;338(3):153–160. doi: 10.1056/NEJM199801153380303. [DOI] [PubMed] [Google Scholar]
- 11.Brennan MF. Staging of soft tissue sarcomas. Ann Surg Oncol. 1999 Jan-Feb;6(1):8–9. doi: 10.1007/s10434-999-0008-5. [DOI] [PubMed] [Google Scholar]
- 12.Kattan MW, Leung DH, Brennan MF. Postoperative nomogram for 12-year sarcoma-specific death. J Clin Oncol. 2002 Feb 1;20(3):791–796. doi: 10.1200/JCO.2002.20.3.791. [DOI] [PubMed] [Google Scholar]
- 13.Coindre JM, Terrier P, Bui NB, et al. Prognostic factors in adult patients with locally controlled soft tissue sarcoma. A study of 546 patients from the French Federation of Cancer Centers Sarcoma Group. J Clin Oncol. 1996 Mar;14(3):869–877. doi: 10.1200/JCO.1996.14.3.869. [DOI] [PubMed] [Google Scholar]
- 14.Guillou L, Coindre JM, Bonichon F, et al. Comparative study of the National Cancer Institute and French Federation of Cancer Centers Sarcoma Group grading systems in a population of 410 adult patients with soft tissue sarcoma. J Clin Oncol. 1997 Jan;15(1):350–362. doi: 10.1200/JCO.1997.15.1.350. [DOI] [PubMed] [Google Scholar]
- 15.Lahat G, Tuvin D, Wei C, et al. New perspectives for staging and prognosis in soft tissue sarcoma. Ann Surg Oncol. 2008 Oct;15(10):2739–2748. doi: 10.1245/s10434-008-9970-6. [DOI] [PubMed] [Google Scholar]
- 16.Lahat G, Tuvin D, Wei C, et al. Molecular prognosticators of complex karyotype soft tissue sarcoma outcome: a tissue microarray-based study. Ann Oncol. 2010 May;21(5):1112–1120. doi: 10.1093/annonc/mdp459. [DOI] [PubMed] [Google Scholar]
- 17.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A III, editors. AJCC Cancer Staging Manual. 7. New York: Springer; 2010. Soft Tissue Sarcoma; pp. 291–298. [Google Scholar]
- 18.Greene FL, Page DL, Fleming ID, et al., editors. AJCC Cancer Staging Manual. 6. New York: Springer; 2002. Soft Tissue Sarcoma; pp. 193–200. [Google Scholar]
- 19.Riad S, Griffin AM, Liberman B, et al. Lymph node metastasis in soft tissue sarcoma in an extremity. Clin Orthop Relat Res. 2004 Sep;(426):129–134. doi: 10.1097/01.blo.0000141660.05125.46. [DOI] [PubMed] [Google Scholar]
- 20.Hajdu SI, Shiu MH, Brennan MF. The role of the pathologist in the management of soft tissue sarcomas. World J Surg. 1988 Jun;12(3):326–331. doi: 10.1007/BF01655665. [DOI] [PubMed] [Google Scholar]
- 21.Brennan MF. Staging of soft tissue sarcoma: what is new? Ann Surg Oncol. 2008 Oct;15(10):2643. doi: 10.1245/s10434-008-0093-x. [DOI] [PubMed] [Google Scholar]
- 22.Behranwala KA, A’Hern R, Omar AM, Thomas JM. Prognosis of lymph node metastasis in soft tissue sarcoma. Ann Surg Oncol. 2004 Jul;11(7):714–719. doi: 10.1245/ASO.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 23.Fletcher CDM, Bridge JA, Hogendoorn P, Mertens F. WHO Classification of Tumours of Soft Tissue and Bone. 4. Vol. 5. Geneva: World Health Organisation; 2013. [Google Scholar]
- 24.Antonescu CR, Tschernyavsky SJ, Decuseara R, et al. Prognostic impact of P53 status, TLS-CHOP fusion transcript structure, and histological grade in myxoid liposarcoma: a molecular and clinicopathologic study of 82 cases. Clin Cancer Res. 2001 Dec;7(12):3977–3987. [PubMed] [Google Scholar]
- 25.Nathan H, Raut CP, Thornton K, et al. Predictors of survival after resection of retroperitoneal sarcoma: a population-based analysis and critical appraisal of the AJCC staging system. Ann Surg. 2009 Dec;250(6):970–976. doi: 10.1097/SLA.0b013e3181b25183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ardoino I, Miceli R, Berselli M, et al. Histology-specific nomogram for primary retroperitoneal soft tissue sarcoma. Cancer. 2010 May 15;116(10):2429–2436. doi: 10.1002/cncr.25057. [DOI] [PubMed] [Google Scholar]
- 27.Forsberg JA, Healey JH, Brennan MF. A Probabilistic Analysis of Completely Excised High-grade Soft Tissue Sarcomas of the Extremity: An Application of a Bayesian Belief Network. Ann Surg Oncol. 2012 Apr 20; doi: 10.1245/s10434-012-2345-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fong Y, Coit DG, Woodruff JM, Brennan MF. Lymph node metastasis from soft tissue sarcoma in adults. Analysis of data from a prospective database of 1772 sarcoma patients. Ann Surg. 1993 Jan;217(1):72–77. doi: 10.1097/00000658-199301000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neville HL, Andrassy RJ, Lobe TE, et al. Preoperative staging, prognostic factors, and outcome for extremity rhabdomyosarcoma: a preliminary report from the Intergroup Rhabdomyosarcoma Study IV (1991–1997) J Pediatr Surg. 2000 Feb;35(2):317–321. doi: 10.1016/s0022-3468(00)90031-9. [DOI] [PubMed] [Google Scholar]
- 30.Crist WM, Anderson JR, Meza JL, et al. Intergroup rhabdomyosarcoma study-IV: results for patients with nonmetastatic disease. J Clin Oncol. 2001 Jun 15;19(12):3091–3102. doi: 10.1200/JCO.2001.19.12.3091. [DOI] [PubMed] [Google Scholar]
- 31.Canter RJ, Qin LX, Maki RG, Brennan MF, Ladanyi M, Singer S. A synovial sarcoma-specific preoperative nomogram supports a survival benefit to ifosfamide-based chemotherapy and improves risk stratification for patients. Clin Cancer Res. 2008 Dec 15;14(24):8191–8197. doi: 10.1158/1078-0432.CCR-08-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gold JS, Gonen M, Gutierrez A, et al. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: a retrospective analysis. Lancet Oncol. 2009 Nov;10(11):1045–1052. doi: 10.1016/S1470-2045(09)70242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lagarde P, Perot G, Kauffmann A, et al. Mitotic checkpoints and chromosome instability are strong predictors of clinical outcome in gastrointestinal stromal tumors. Clin Cancer Res. 2012 Feb 1;18(3):826–838. doi: 10.1158/1078-0432.CCR-11-1610. [DOI] [PubMed] [Google Scholar]