Abstract
According to Damasio’s somatic marker hypothesis, emotions are generated by conveying the current state of the body to the brain through interoceptive and proprioceptive afferent input. The resulting brain activation patterns represent unconscious emotions and correlate with subjective feelings. This proposition implies a corollary that the deliberate control of motor behavior could regulate feelings. We tested this possibility, hypothesizing that engaging in movements associated with a certain emotion would enhance that emotion and/or the corresponding valence. Furthermore, because motor imagery and observation are thought to activate the same mirror-neuron network engaged during motor execution, they might also activate the same emotional processing circuits, leading to similar emotional effects. Therefore, we measured the effects of motor execution, motor imagery and observation of whole-body dynamic expressions of emotions (happiness, sadness, fear) on affective state. All three tasks enhanced the corresponding affective state, indicating their potential to regulate emotions.
Keywords: Body expression of emotion, Nonverbal behavior, Emotion regulation, Embodiment, Simulation, Motor imagery
1. Introduction
Watching Charlie Chaplin acting in silent movies, we can easily comprehend his emotions based on his body language. Most people consider body language as the external manifestation of internal emotions through posture and movements. However, it has been suggested that the reversed process, i.e., that postures and movements can affect emotional state, is also true. This concept is based on Darwin’s ideas and the James-Lange theory, which at their extreme, propose that bodily responses to stimuli are necessary for emotional experience, and feelings are not the causes of autonomic system activation and emotional behavior, but rather are the consequence of these. Thus, we feel angry because we strike and afraid because we tremble, and not that we strike or tremble because we are angry or fearful (James, 1884).
In recent years, this theory has been re-formulated in neurophysiological terms by Antonio Damasio. According to Damasio, the current state of the body is conveyed to the brain through the processes of proprioception (afferent input representing muscle length and joint angle) and interoception (afferent input representing the physiological (e.g., thermal, metabolic) status of all body tissues), which create in the brain unique neural activation patterns. These neural activation patterns represent unconscious emotions that guide behavior and influence decisions, and they correlate with the conscious feelings of those emotions (Damasio, 1999; Damasio et al., 2000). The uncovering of neuronal underpinnings of interoception (Craig, 2002; Critchley, 2005) and the identification of anterior insular cortex as the brain region in which representation of internal bodily states becomes available to conscious awareness (Craig, 2009), provide plausible neurocircuits in support of this proposition. One important implication of Damasio’s proposition is the potential to regulate one’s feelings through deliberate control of motor behavior and its consequent proprioception and interoception (Riskind, 1984). Thus, by engaging in movements that are associated with a certain emotion, one should be able to generate or enhance that emotion and its corresponding feelings. Could we really get happier by skipping like a kid or sense fear when shrinking and retreating?
The effects of facial expression on corresponding affective state have been widely studied (for review see McIntosh, 1996) and smiling is now used in dialectical behavioral therapy as a behavioral intervention for mood regulation. Evidence suggesting that the effects of facial expressions on affective states are attained through proprioception come from studies suggesting that changes in proprioceptive feedback from facial expressions following botulinum toxin treatment may weaken emotional experience (Davis, Senghas, Brandt, & Ochsner, 2010) and attenuate neural activation in the amygdala (Hennenlotter et al., 2009). Surprisingly, although progressive muscle relaxation is widely used for tension reduction (and has scientific support: Vancampfort et al., 2011) and dance has been used for centuries to intensify joy in social settings, evidence for the impact of emotional bodily posture and movements on affective state is scarce. A handful of studies have shown that isometric arm flexion (associated with approach, e.g., bringing food towards one’s mouth) and arm extension (associated with rejection) affect evaluative cognitive processing, causing subjects, for example, to rate neutral novel stimuli more positively during arm flexion than during arm extension (Cacioppo, Priester, & Berntson, 1993). A few other studies have shown that assuming certain postures (e.g., upright, slumped, expansive) immediately induce corresponding feelings (pride, sadness, power, respectively) (Carney, Cuddy, & Yap, 2010; Duclos, Laird, Schneider, & Sexter, 1989; Riskind & Gotay, 1982; Stepper & Strack, 1993), and that inhibition of specific facial and motor behaviors reduce the corresponding feelings (Duclos & Laird, 2001). Moreover, holding for 2 min a posture that expresses power not only increased feelings of power, but also resulted in physiological responses: reduced cortisol and increased testosterone (Carney et al., 2010), and combining facial expressions with matching expressive bodily postures resulted in corresponding feelings which lasted several minutes after stopping these behaviors (Schnall & Laird, 2003), and were stronger than engaging in either the facial expressions or postures alone (Flack, Laird, & Cavallaro, 1999).
We live in a dynamic world, where people’s behavior is constantly modified to adjust to continuous changes in the environment. Thus, it is possible that engaging in whole-body dynamic movements which are associated with specific emotions (emotional movements) might have stronger effects on affective state than static postures, as it is more closely related to the ecological context in which emotion is experienced. Moreover, because brain response to unchanging stimuli diminishes over time due to neuronal adaptation, the consistently changing proprioceptive input from dynamic movements might create a stronger effect than the constant, unchanged proprioceptive input from a static posture. Indeed, perception of dynamic compared to static whole-body expressions of anger resulted in better recognition and stronger and more widespread emotion-specific brain activation (Pichon, de Gelder, & Grezes, 2008). Yet, to date, only one study has investigated the effects of whole-body emotional movements upon affective state (Duclos & Laird, 2001), and it was limited to movements designed to elicit only negative emotions. The first aim of our study was to examine the effects of engaging in emotional movements upon affective state, in particular, happy movements. We hypothesized that emotional movements would enhance corresponding affective state. We were especially interested to test whether happy movements could enhance positive feelings, because such novel finding might serve as a basis for developing a new, specific-movement based, emotion-regulation intervention for mood enhancement. Additional aims were to explore whether observation and imagery of emotional movements could also influence affective state.
It is now well established that observation of movements activates the same neural network that is active during execution of those same movements (i.e., the mirror neuron network). This mechanism of shared representations for perception and action of body and facial movements was proposed as the basis for action recognition (Rizzolatti, Fogassi, & Gallese, 2001), emotion recognition, and empathy (Carr, Iacoboni, Dubeau, Mazziotta, & Lenzi, 2003; Gallese, Keysers, & Rizzolatti, 2004). Both animal (Raos, Evangeliou, & Savaki, 2007) and human (Calvo-Merino, Grèzes, Glaser, Passingham, & Haggard, 2006) studies have suggested that movement observation simulates in the brain the neural motor commands used to initiate execution of the same movement. It has also been suggested that neural motor commands for a given movement generate in the brain an internal representation of the expected proprioceptive feedback from that movement (Christensen et al., 2010; Raos et al., 2007; Sommer &Wurtz, 2008), and that emotions such as disgust or pain can be induced not only by afferent input from the body, but also by brain simulation of that afferent input (Bastiaansen, Thioux, & Keysers, 2009). Thus, it is very likely that simulation of the expected proprioceptive input from an emotional movement can induce the corresponding emotion during observation of such movement, similar to its induction by real proprioceptive input during motor execution. Support for this idea comes from both monkey (Raos et al., 2007) and human (Gazzola & Keysers, 2009) studies that found regions in the somatosensory cortex which were activated during both the execution and observation of the same action. Other studies found that observing movements which express different emotions generated differential brain activation patterns in emotional processing regions (Peelen, Atkinson, Andersson, & Vuilleumier, 2007; Pichon, de Gelder, & Grèzes, 2009). In addition, observing emotional facial expressions caused subjects to experience the same emotions expressed by the stimuli (Lundqvist & Dimberg, 1995), with more intense expressions producing more intense feelings (Wild, Erb, & Bartels, 2001). Thus, we hypothesized that not only motor execution, but also observation of emotional movements would induce their corresponding feelings. Such emotion regulation effects of motor observation might be useful when actual motor execution is not feasible, and motor imagery could have a similar emotional response.
Several studies have shown that kinesthetic motor imagery (i.e., imagining oneself doing a movement) results in activation of motor circuits similar to their activation during motor execution (Decety & Grèzes, 2006), and that brain activation underlying motor execution and motor imagery differ primarily in inhibition processes that suppress motor output during imagery (Lotze et al., 1999). Motor imagery has been shown to elicit autonomic responses and sensory experience that are directly associated with the imagined movements (Decety, Jeannerod, Durozard, & Baverel, 1993; Naito et al., 2002), and Schwoebel et al. have suggested that motor imagery involves generation of the expected proprioceptive input from the imagined movement (Schwoebel, Boronat, & Branch Coslett, 2002). Moreover, Kim et al. found that imagery of emotional facial expressions elicited activation in the amygdala (Kim et al., 2007). We therefore hypothesized that similar to observation, imagery of emotional movements would also enhance the corresponding affective state.
In this study we measured the effects of motor execution, observation, and kinesthetic motor imagery of happy, sad, fearful, and emotionally neutral movements on affective state, in order to explore their potential for therapeutic application. We hypothesized that all three modalities of whole-body emotional movements will enhance corresponding affective state.
2. Materials and methods
2.1. Participants
Twenty-nine participants were recruited for the study. After giving informed consent, participants were screened using the Movement Imagery Questionnaire-Revised Scale (MIQ-RS) (Gregg, Hall, & Butler, 2007) and the Expression Manipulation Procedure (Duclos & Laird, 2001). The MIQ-RS assesses the ability and ease of motor imagery. Only subjects who scored >70, indicating adequate motor imagery ability, continued their participation in the study. The Expression Manipulation Procedure determines the extent to which people are focused on, and are emotionally affected by their own bodies and actions (personal cues), as opposed to social expectations (situational cues). Only participants who scored >10, indicating a personal trait of sufficient sensitivity to their bodily cues of emotion, continued the study. Five participants were found ineligible by the Expression Manipulation Procedure and one by the MIQ-RS. One additional subject withdrew due to difficulties learning the movements. Thus, 22 healthy adults (11 males, 11 females; mean age = 25.4 years, SD = 6.15) completed the study protocol. All had no history of neurological or psychiatric disease, and were not taking any medications. All procedures were approved by University of Michigan Institutional Review Board.
2.2. Stimuli
For motor execution, each participant was taught to perform four sequences of emotional movements: one happy, one sad, one fearful and one neutral. The movement sequences were modeled from short (3 s), grey scale movie clips of dynamic whole-body expressions of emotions and emotionally neutral actions such as bending forward to touch one’s toes. Movie clips were taken from a validated set (Atkinson, Dittrich, Gemmell, & Young, 2004) which included ten different motor sequences expressing each emotion (40 total). All movements in the clips were presented on a black background, and were performed by males and females actors who wore uniform dark grey, tight-fitting clothes and headwear, so that facial expressions were not visible (see Fig. 1). Because different people express emotions in slightly different ways, we decided to use several different movement sequences to express each emotion. Thus, out of ten different clips available for each emotion, the four best recognized were taught to the subjects so that for each emotion, two stimuli expressing that emotion were each taught to five subjects and another two stimuli expressing the same emotion were each taught to six subjects. Stimuli assignment was random, but all male participants learned movements acted in the clips by male actors, and all female participants learned movements acted by female actors. For motor imagery, subjects imagined themselves (kinesthetic imagery) moving the same motor sequences that they learned to execute. During the observation task, for each emotion subjects watched all available stimuli for that emotion, except for the stimulus that consisted of the motor sequence that they had learned to perform.
Fig. 1.
Snapshots from the emotional movements stimuli. Pictures created from the video clips that were used in this study, by freezing each short movie in the middle of the movement. The left picture was produced from a sad movement, the middle from a happy movement (a jump) and the right picture from a fearful movement.
2.3. Design and procedures
The study had a within subject design and each subject performed all three tasks: motor execution, observation, and imagery of all four emotional movements. Testing took place in three sessions, separated by 1–7 (mean = 3) days from each other. During the first session, participants were taught by an experienced dance teacher the four emotional motor sequences in the order in which they were later tested. Subjects were told that the purpose of the study was to examine the effects of specific muscle activation patterns on brain function, and motor sequences were not identified as emotional, but were labeled with random letters, which were used during the second session as the cue prompting for motor execution and imagery of each sequence. Subjects’ training, using dance-teaching methodology, lasted 1–1.5 h, until both subject and teacher judged that the subject could perform the movements easily and accurately. Motor training of the sequences was followed by kinesthetic-imagery practice of the same movements. To ensure and enhance memory of the learned motor sequences, participants were asked to practice those sequences behaviorally and using imagery, for a few minutes each day in between testing sessions.
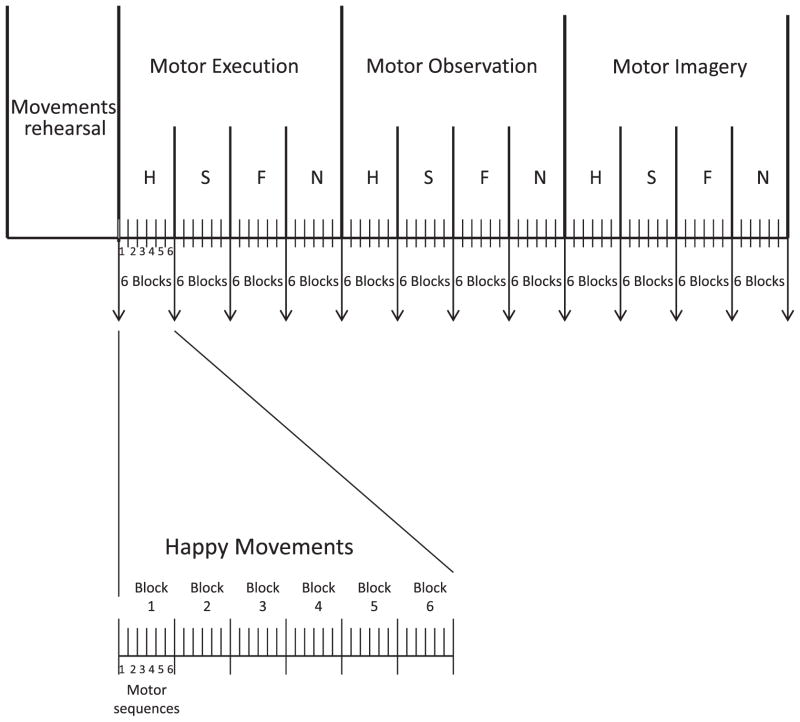
The second experimental session began with a short rehearsal of the learned movements, followed by testing motor execution, observation, and imagery effects. Before and after motor execution of each motor sequence, subjects performed a memory test of short lists of words (to disguise the purpose of the study), and they rated their physical tiredness/fatigue, muscle soreness, and affective state, using the PANAS (Positive and Negative Affect Schedule) (Crawford & Henry, 2004) and visual analog scales (16.5 cm line) for eight different emotions (to disguise which emotion we were interested in). Similar procedures were used to measure the effects on affective state of motor imagery and observation (see Fig. 2). During motor execution, each sequence was performed in six blocks of six consecutive repetitions, with a few seconds rest in between blocks. Since each movement lasted about 3 s plus 0.5 s to return to the starting position, block duration was approximately 21 s.
Fig. 2.
Experimental design. Example of experimental design of the second testing session for a representative subject. All subjects performed the motor execution task first. Half the subjects performed the motor observation task second and the motor imagery task third (as in this example), and half of the subjects performed the motor imagery second and the motor observation third. The order of the three emotional movements types (happy (H), sad (S), fearful (F)) within each task was randomized across subjects, but the neutral movements (N) were always the last. Each task was consisted of six blocks with a few seconds of rest in between blocks, and each block included six consecutive motor sequences. A memory test and physical fatigue, muscle soreness, and affective ratings were performed at the times represented by each of the downward pointing arrows.
The structure and duration of the observation and imagery tasks were based on the execution task, and they each included six blocks. In each observation block (21 s long), six different clips (3 s each) expressing the same emotion were shown, separated by 0.5 s of a cross sign. In some blocks, one or two of the clips repeated themselves. At the end of each block subjects were asked how many clips were repeated in that block. This design was employed to ensure that subjects paid attention to the movies. Motor imagery task consisted of six blocks of 21 s each, during which subjects were asked to imagine themselves performing repeatedly the sequence associated with the prompting letter. Because the experimenter could not know whether subjects indeed imagined themselves moving, to encourage subject to comply, after each block subjects were asked to rate the difficulty of motor imagery during that block.
All subjects performed the motor execution task first. Order of motor observation and motor imagery tasks was randomized and balanced across subjects. Order of emotional movements within each task was randomized and balanced across subjects, but the neutral movements were always the last, so that each new task would start from a neutral affective state. Order of emotions within each task stayed the same in all tasks for each subject. Subjects were videotaped during motor execution for later validation of the similarity of their movements to those in the modeled clip.
During the third testing session subjects were asked to identify the emotion expressed in each clip (forced choice) and to rate the intensity of that emotion on a scale of 1–7.
2.4. Data analysis
To ensure that subjects executed the motor sequences correctly and that their performance was not compromised by physical fatigue, we compared their movements during representative sequences: the first motor sequence of the sixth (last) block of each type of emotional movements, with the movements in the corresponding original clips. To do that we extracted from the subject’s video short clips that included those representative motor sequences, and employed the EyesWeb software (Camurri, Lagerlöf, & Volpe, 2003) (http//www.infomus.dist.unige.it/), to obtain a ‘Quantity of Motion’ (QoM) value for each movie frame in the original stimulus clips and in the clips of the subjects performing those sequences. The EyesWeb software converts the body image into a ‘Silhouette Motion Image (SMI)’, which carries information about variation in the shape and position of the body silhouette across a moving window of a few frames (we used four) throughout the length of the movie. A QoM value, equivalent to the number of pixels of the SMI, was computed for each movie frame that this window moved across, and served as an estimate of the overall amount of detected motion. Since the duration of subjects’ sequences (3.095 ± 0.339 s) was not always exactly 3 s (the original clip duration), we either interpolated or deleted data points from each subject’s clip, in order to have the same number of data points (90) as in the original clip. We then correlated the QoM values from each subject clip to those from the corresponding original clip, as a measure of the similarity between the two motor sequences.
Emotion recognition (using arcsine-transformed proportion of correctly recognized clips of each emotion, to make the outcome normally distributed) and emotional intensity ratings of the happy, sad, fearful and neutral stimuli clips were compared using one-way repeated measure ANOVA, followed by pairwise comparisons corrected for multiple comparisons.
Even though we hypothesized that all three tasks: motor execution, motor observation, and kinesthetic imagery, would enhance the corresponding affect using a similar process involving internal representations of the expected afferent feedback, these three tasks are inherently different: observation in contrast to execution and imagery entails the additional differentiation between self and others, and imagery differs from execution and observation since under normal circumstances imagination and reality are not confused. In addition, several studies suggested that even within the mirror neurons network, brain activation during the three tasks is not completely identical. Differences have been found in activation magnitude (Raos et al., 2007), connectivity (Gao, Duan, & Chen, 2011), and some non-overlapping activation within the circuits that mediate the shared representations (Decety & Grèzes, 2006). Lastly, while during motor execution and imagery, subjects in our study continuously repeated one emotional motor sequence as a representative for each emotion, during the observation task they observed nine different motor sequences representing each emotion. Thus, one cannot compare the effects of one task to those of the other tasks, and separate analyses were performed for each task.
The effects of motor execution, observation and imagery of each type of emotional movements were therefore assessed with 1-tail paired t-tests, calculating separately for each emotional movement type within each task, the difference in affective score between post- and pre-task performance. For each emotional movement type, only the relevant affective ratings that were hypothesized to increase as a result of that movement were analyzed. These included the ratings of happiness and PANAS positive-summary-score (PANASp) to assess the effects of happy movements, the ratings of sadness and PANAS negative-summary-score (PANASn) to assess the effects of sad movements, the ratings of fear and PANASn to assess the effects of fearful movements, and the rating of neutral/not feeling any specific emotion, to assess the effects of neutral movements. Because the effects of each type of emotional movements (happy, sad, fearful) within each task were tested on two different emotional ratings, the results were corrected for these two multiple comparisons.
In addition to the paired t-tests described above, we also examined within each task for each affective rating measure, whether the effect of the relevant emotional movements on that affective rating was significantly different from the effect of the neutral movements on that affective rating. For example: whether during motor execution, the effects of happy movements on PANASp were significantly different from (greater than) the effects of neutral movements on PANASp. We examined these differences using post hoc comparisons from a repeated measures ANOVA analysis that we performed for each affective rating measure. The ANOVA included the factors: task, emotional movement type, and task by emotional movement interaction, and Bonferroni adjustment was used to correct for the multiple post hoc tests.
3. Results
Subjects executed the emotional motor sequences very similarly to the original validated ones (Atkinson et al., 2004). Fourteen out of 22 subjects (63%) achieved the strict criteria of significant correlations between frame-by-frame QoM during their movements and frame-by-frame QoM during the corresponding original clips, for 75% or more of their analyzed representative movements.
Ratings of each clip during the third session indicated that all emotions were recognized at above 89% correct (fear: 95%, happiness: 90%, neutral: 89%, sadness 91%). Repeated measures ANOVA showed no significant difference in recognition accuracy between emotions. Nonetheless, there was a significant difference between emotions in their rated intensity (Wilks’s Λ = 0.23, F(3,19) = 21.66, p < 0.01). The perceived emotional intensity of all emotional movements was significantly higher than that of the neutral movements. In addition, the perceived intensity of the happy movements (5.25) was significantly higher than that of the fear (4.85) and neutral (3.61) movements, but not different from that of the sad movements (5.12).
When rating the stimulus clips at the end of the study, four participants did not rate the supposedly happy motor sequence that they performed and imagined as happy, two participants did not rate the supposedly fearful sequence that they performed and imagined as fearful, and one participant did not rate the supposedly neutral sequence that he performed and imagined as neutral. We therefore performed the paired t-tests that examined the effects of motor execution and motor imagery for these emotions twice: once including all subjects in the analysis, and once excluding from each analysis those subjects who did not recognize correctly the emotional movement whose effects were tested. We found that both types of analyses generated very similar results and we therefore report only the results from the t-tests that included all subjects in the analysis. Because each block in the observation task included several different clips, we could not separate the response to correctly recognized clips from that to incorrectly recognized clips. Thus all subjects and all blocks were included in the analyses of the observation task.
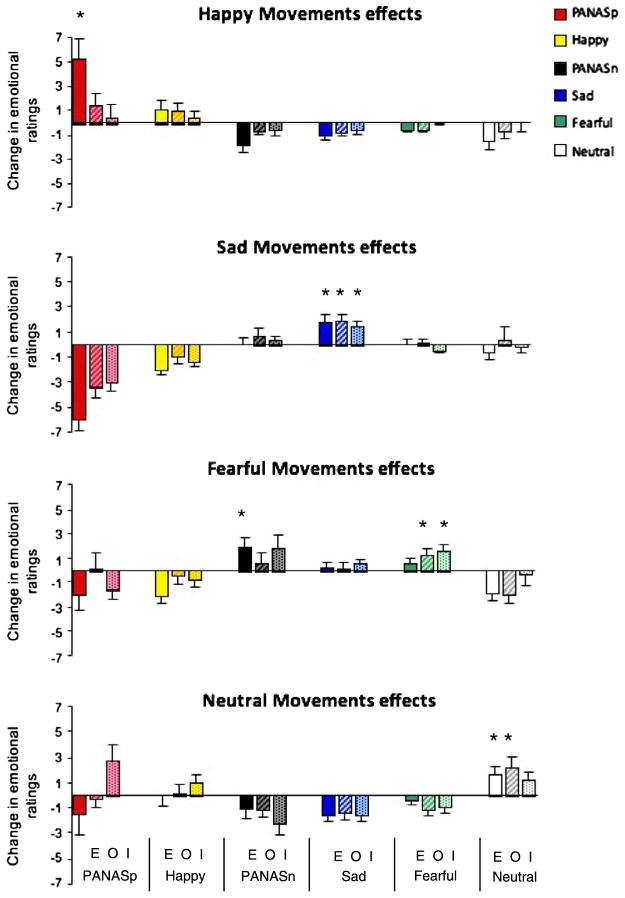
The paired t-tests revealed that motor execution of happy movements significantly increased positive affect, as measured by the PANAS positive affect ratings (PANASp) (t(21) = 3.06, p = 0.003). Execution of sad movements significantly increased the ratings of feeling sad (t(21) = 3.72, p < 0.001). Execution of fearful movements significantly increased negative affect, as measured by the PANAS negative affect (PANASn) (t(21) = 2.43, p = 0.012), and execution of neutral movements significantly increased neutral feelings, i.e., the ratings in the visual analog scale of feeling ‘neutral’/not feeling any other specific emotion (t(21) = 2.39, p = 0.026) (see Fig. 3).
Fig. 3.
Impact of emotional movements on affective state. Mean differences (changes) in affective ratings between post- and pre-task performance are presented to illustrate the effects of happy, sad, fearful and neutral movements on affective state. The effects of each type of emotional movements is presented in a separate graph. Within each graph, the effects of motor execution (E; solid bars), observation (O; striped bars) and kinesthetic imagery (I; dotted bars) of the emotional motor sequence on each affective rating measure is presented. For each emotional movement type, we present its effects on six different affective ratings: The positive summary score of the Positive and Negative Affect Schedule (PANASp), the level of happiness as measured by the visual analog scale, the negative summary score of the Positive and Negative Affect Schedule (PANASn), and the levels of sadness, fear, and no specific emotions/neutral feelings, as measured by the visual analog scale. Although all six affective ratings measures are presented, each emotional movement was hypothesized and tested only for its effects on the two affective measures which were relevant to that movement, and the neutral movement was tested and hypothesized to affect only the rating of feeling neutral/no specific emotion. Units are based on visual analog scores for the affective ratings, and error bars represent ±1SE.
Observation of happy movements had no significant effects on affective state, but observing sad movements significantly increased sadness (t(21) = 3.72, p < 0.001). Observing fearful movements significantly increased fear (t(21) = 2.29, p = 0.016) and observation of neutral movements significantly increased neutral feelings (t(20) = 2.66, p = 0.015). Motor imagery of happy movements had no significant effects. Imagery of sad movements significantly increased sadness (t(21) = 3.0, p = 0.0035), and imagery of fearful movements significantly increased fear (t(21) = 2.35, p = 0.0145).
Considering the significant effects of observation and imagery of sad and fearful movements on the corresponding feelings, and the significant effect of motor execution of happy movements on positive affect, the finding that observation and imagery of happy movements had no significant effects was unexpected. One possible explanation for the lack of observation and imagery effects on positive affect might be that after reporting their emotions several times during the motor execution condition, participants might have become tired of completing questionnaires, and therefore started reporting less positive affect. To test this hypothesis of an order effect of fatigue on ratings, we compared the mean difference in positive affect between pre- and post-observation and imagery of happy movements, in those subjects who performed the observation task before the imagery task with those who performed the observation task after the imagery task. We found that the difference in PANASp between pre- and post-observation of happy movements was significantly higher in those subjects who performed the observation task third compared to those who performed the observation task second (t(20) = −3.396), p = 0.003). A similar analysis for the difference in PANASp between before and after the imagery task revealed no significant difference between those subjects who performed the imagery task second and those who performed it third (t(14) = −0.388, p = 0.704). These results ruled out an order/fatigue effect.
The post hoc comparisons from the ANOVA analyses, which examined the effects of task by emotional-movement interaction on each affective rating measure, revealed that during motor execution the effect of happy movements on positive affect (measured by PANASp) was significantly greater than the effect of the neutral movements on positive affect (t(231) = 3.194, p = 0.010), and the effect of sad movements on sadness was significantly greater than the effect of neutral movements on sadness (t(231) = 4.735, p < 0.001). During motor observation, the effect of sad movements on sadness was significantly greater than the effect of neutral movements on sadness (t(231) = 4.610, p < 0.001), and the effect of fearful movements on fear was significantly greater than the effect of neutral movements on fear (t(231) = 5.009, p < 0.001). During motor imagery, the effect of sad movements on sad feelings was significantly greater than the effect of the neutral movements on sad feelings (t(231) = 4.103, p < 0.001). Likewise, the effect of fearful movements on fear was significantly greater than the effect of neutral movements on fear (t(231) = 3.909, p = 0.001).
4. Discussion
Our purpose in this study was to evaluate whether motor execution of emotional movements, in particular happy movements, could enhance corresponding affective state, and could therefore be used as a means for emotion regulation. We further explored whether similar effects could be obtained also by observation and imagery of the same movements. We found that all three tasks enhanced the corresponding affective state, indicating their potential to regulate emotions and raising the possibility that emotional movements may have therapeutic applications.
As hypothesized, motor execution of happy movements significantly increased positive affect, indicating the potential for using execution of these movements as a method for mood enhancement. In contrast to the effects of happy movements, execution of fearful movements significantly increased negative affect, indicating that the enhancement of positive affect following happy-movements execution was not due to the increased amount of physical activity per se, but due to the specific qualities of the happy movement, i.e., the specific muscle activation pattern and joints angle configuration which characterize those movements. Moreover, execution of sad movements enhanced sad feelings, suggesting that particular movements may enhance not only the same affective valence, but also a more specific corresponding emotion. The capability of execution of specific movements to enhance corresponding affect was further corroborated by the finding that execution of emotionally neutral movements increased neutral feelings, and the findings that compared to neutral movements, execution of happy movements significantly increased positive affect, while execution of sad movements significantly increased sadness.
Our results further suggest that feelings might be altered not only by active execution of certain movements, but also by stopping and/or avoiding other movements. Although we had no hypotheses regarding the effects of each type of emotional movement on non-corresponding emotions, we explored these effects by calculating the difference in those non-corresponding emotional ratings between post- and pre-task performance. Those differences are displayed in Fig. 3. Without specific hypothesis-testing we cannot draw any unequivocal conclusion. Nevertheless, the performance of sad movements was associated with a considerable reduction in positive affect and happy feelings, which suggest that sad movements might diminish happiness and therefore positive mood might be enhanced not only directly by executing happy movements, but also indirectly by consciously avoiding sad body expressions.
As hypothesized, observation and motor imagery of sad and fearful movements significantly enhanced corresponding feelings. These effects were further confirmed by comparing the affective changes following these respective emotional movements to those following the neutral movements. Observation and imagery of happy movements, however, did not produce such results. One possible explanation for the lack of observation and imagery effects on positive affect might be an order effect of fatigue on ratings, which might have caused the subjects to report less positive affect during motor observation and imagery, since they always followed motor execution. We tested this hypothesis and the results ruled out such an order/fatigue effect. Another possible explanation might be that the lack of observation and imagery effects of happy movements might have been caused by a ceiling effect in affective state. Our emotionally-healthy subjects had a positive baseline affective state, and the hypothesized neural signal of the simulated expected proprioceptive feedback during imagery and observation might have been too weak to increase positive affect even more. This was in contrast to motor execution, where the additional real proprioceptive feedback from the muscles and joints might have been strong enough to cause an effect. Support for this hypothesis comes from Raos et al. who found twice as much activation in the monkey’s forelimb somatosensory cortex during motor execution compared to motor observation. Raos et al. suggested that the higher level of activity observed in the sensory cortex during action execution might have reflected the anticipated sensory consequence of the movement (based on efference copy from the motor cortex) and the actual afferent feedback (signal from the muscles), whereas the 50% lower activity observed during action observation might have reflected the anticipated consequence of the movement only (Raos et al., 2007). If this explanation is true, then the neural signal from observation and imagery of happy movements might be strong enough to enhance affective state when the individual’s baseline mood is negative, such as during depression. This proposition will have to be tested in future studies. Observation of neutral movements significantly increased neutral feelings as hypothesized, but imagery of neutral movements seemed to increase positive feelings more than neutral feelings. Kinesthetic motor imagery is not a simple task and the more experience and motor memory one has of a certain movement, the easier it is to imagine oneself doing it. The neutral movements were the simplest and easiest to learn and perform accurately, and might have caused the subjects to feel more successful in accomplishing the imagery task, thus producing positive affect during imagery. In sum, our results suggest that apart from a couple of exceptions (which might have a sensible explanation), observation and imagery of emotional movements tend to induce the corresponding affective state, suggesting that our hypothesis regarding simulation of afferent input as the underlying mechanism might be correct.
In a recent paper, Gallese and Sinigaglia have proposed that embodied simulation using the mirror neuron system might be one of the mechanisms underlying social cognition. They suggested that during mirror mechanismdriven embodied simulation, people reuse their own mental states or processes involving representations that have bodily format, and functionally attribute them to others (Gallese & Sinigaglia, 2011). In line with this view, here we suggest (as explained in the introduction), that during motor observation and imagery, the simulation (representation) of the sensory input which is expected to be generated by the observed or imagined movement, produces the corresponding emotion and affective state. This emotion could then be “reused” to understand the other’s affective state and to emotionally empathize with her, by attributing that emotion to the observed other. To the best of our knowledge, this is the first time where observation and imagery of whole body movements have been shown to induce emotions and enhance corresponding affective state. This emotion induction by motor observation and motor imagery supports Gallese and Sinigaglia’s idea regarding the role of embodied simulation in social cognition.
One limitation of our study was that task order was not completely balanced and motor execution effects were always tested first. This design was chosen in order to give the subjects an additional opportunity to experience the movements before they had to imagine them, so that kinesthetic imagery would be easier. This strategy also mimics what would typically be used in training and practice. This strategy should be more, not less, likely to enhance the impact of imagery. Another limitation was that we could not control for subject compliance during imagery, and the evaluation of imagery ability was based on subjective report. Additional limitation was that given the repeated surveys of emotion, the subjects may have responded to demand characteristics of the study and judged their emotion based on what they anticipated (consciously or unconsciously) the experimenter expected. This is a common problem for studies involving subjective report of emotion and difficult to fully exclude. We believe this confound was relatively minimized by our use of a cover story and word-recognition task to mask the purpose of the study. In addition, in order to disguise which emotions we were interested in, we asked the subjects to rate a variety of emotions. Lastly, evaluation of the effects on physiological responses such as cortisol secretion or heart rate in addition to the subjective affective ratings would have strengthened our results. However, these physiological measures may be affected by engagement in the physical activity per se, and there are no existing tools that enable to distinguish the relative contribution of the quantity vs. quality of movements to physiological responses following the different emotional movements.
Clinical applications of our findings are potentially numerous. Happy movements could be used as activities in behavioral activation, and for positive mood induction in mood disorders and/or other circumstances or conditions in which mood might be affected (e.g., Parkinson’s disease). To induce the desired mood, patients could either execute those movements, or if their motor abilities are limited, they could watch and imagine themselves doing the movements. Emotional movements could also be used during dance-movement therapy sessions to either evoke specific emotions in patients through motor execution, or to help the therapist feel and understand the patient’s affective state by either mirroring the patient’s movements, or observing and imagining herself doing the same movements. The effects of emotional movements might be further enhanced by accompanying the movements with appropriate corresponding music (Murrock & Higgins, 2009), and this hypothesis will have to be investigated in future studies. Future studies should also discern which motor elements (e.g., the body part that moves, movement speed, movement direction, movement size, etc.) characterize and are common to all emotional movements which are associated with, and enhance a specific emotion. This knowledge will enable to personalize the emotional movements used by each patient to enhance a specific affective state, based on his/her movement style and ability, without lessening the effectiveness of the movements. Our results indicate that only about 2 min of moving happy movements significantly increase positive affect. Future studies will investigate the duration of the effects of emotional movements on affective state, and will generate the knowledge required to determine effective dosage and prescription guidelines.
5. Conclusions
In summary, our study demonstrates that motor execution, observation and imagery of whole body emotional movements can enhance the corresponding affective state, and could therefore be used to assist in regulation of one’s own emotions and recognition of others’ emotions. In addition, our finding that observation and imagery of whole body emotional expressions can enhance corresponding affective state supports the notion of embodied simulation as a plausible mechanism underlying emotion recognition and empathy.
Acknowledgments
Role of the funding source
This work was supported by the Phil F. Jenkins Foundation and Grant UL1RR024986 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
We thank John Thoresen and Lisong Ni for their help with the EyesWeb ‘QoM’ analysis, and Heng Wang for his excellent wide-ranging technical assistance. We are also grateful to the study participants and to Mark Everson, Dave Hsu, Kristine Konz, Janna Kryscynski, Gahl Liberzon, Brian Mickey, Steve Reily, Preeti Samudra and Sara Weisenbach for volunteering their time and feedback during the development of the experimental and motor-sequence teaching procedures.
References
- Atkinson AP, Dittrich WH, Gemmell AJ, Young AW. Emotion perception from dynamic and static body expressions in point-light and full-light displays. Perception. 2004;33(6):717–746. doi: 10.1068/p5096. [DOI] [PubMed] [Google Scholar]
- Bastiaansen JACJ, Thioux M, Keysers C. Evidence for mirror systems in emotions. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364(1528):2391–2404. doi: 10.1098/rstb.2009.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Priester JR, Berntson GG. Rudimentary determinants of attitudes: II. Arm flexion and extension have differential effects on attitudes. Journal of Personality and Social Psychology. 1993;65(1):5–17. doi: 10.1037//0022-3514.65.1.5. [DOI] [PubMed] [Google Scholar]
- Calvo-Merino B, Grèzes J, Glaser DE, Passingham RE, Haggard P. Seeing or doing? Influence of visual and motor familiarity in action observation. Current Biology. 2006;16(19):1905–1910. doi: 10.1016/j.cub.2006.07.065. [DOI] [PubMed] [Google Scholar]
- Camurri A, Lagerlöf I, Volpe G. Recognizing emotion from dance movement: Comparison of spectator recognition and automated techniques. International Journal of Human-Computer Studies. 2003;59(1–2):213–225. [Google Scholar]
- Carney DR, Cuddy AJC, Yap AJ. Power posing. Psychological Science. 2010;21(10):1363–1368. doi: 10.1177/0956797610383437. [DOI] [PubMed] [Google Scholar]
- Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: A relay from neural systems for imitation to limbic areas. PNAS. 2003;100(9):5497–5502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen MS, Lundbye-Jensen J, Grey MJ, Vejlby AD, Belhage B, Nielsen JB. Illusory sensation of movement induced by repetitive transcranial magnetic stimulation. PLoS ONE. 2010;5(10):e13301. doi: 10.1371/journal.pone.0013301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: The sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3(8):655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig ADB. How do you feel-now? The anterior insula and human awareness (Viewpoint essay) Nature Reviews Neuroscience. 2009;10(1) doi: 10.1038/nrn2555. [59 (12)] [DOI] [PubMed] [Google Scholar]
- Crawford JR, Henry JD. The Positive and Negative Affect Schedule (PANAS): Construct validity, measurement properties and normative data in a large non-clinical sample. British Journal of Clinical Psychology. 2004;43(3):245–265. doi: 10.1348/0144665031752934. [DOI] [PubMed] [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. The Journal of Comparative Neurology. 2005;493(1):154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The feeling of what happens: Body and emotion in the making of consciousness. New York: Harcourt Brace; 1999. [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LLB, Parvizi J, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nature Neuroscience. 2000;3(10):1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Davis JI, Senghas A, Brandt F, Ochsner KN. The effects of BOTOX injections on emotional experience. Emotion. 2010;10(3):433–440. doi: 10.1037/a0018690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Grèzes J. The power of simulation: Imagining one’s own and other’s behavior. Brain Research. 2006;1079(1):4–14. doi: 10.1016/j.brainres.2005.12.115. [DOI] [PubMed] [Google Scholar]
- Decety J, Jeannerod M, Durozard D, Baverel G. Central activation of autonomic effectors during mental simulation of motor actions in man. The Journal of Physisology. 1993;461:549–563. doi: 10.1113/jphysiol.1993.sp019528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclos SE, Laird JD. The deliberate control of emotional experience through control of expressions. Cognition and Emotion. 2001;15:27–56. [Google Scholar]
- Duclos SE, Laird JD, Schneider E, Sexter M. Emotion-specific effects of facial expressions and postures on emotional experience. Journal of Personality and Social Psychology. 1989;57(1):100–108. [Google Scholar]
- Flack WF, Laird JD, Cavallaro LA. Separate and combined effects of facial expressions and bodily postures on emotional feelings. European Journal of Social Psychology. 1999;29(2–3):203–217. [Google Scholar]
- Gallese V, Keysers C, Rizzolatti G. A unifying view of the basis of social cognition. Trends in Cognitive Sciences. 2004;8(9):396–403. doi: 10.1016/j.tics.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Gallese V, Sinigaglia C. What is so special about embodied simulation? Trends in Cognitive Sciences. 2011;15(11):512–519. doi: 10.1016/j.tics.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Gao Q, Duan X, Chen H. Evaluation of effective connectivity of motor areas during motor imagery and execution using conditional Granger causality. NeuroImage. 2011;54(2):1280–1288. doi: 10.1016/j.neuroimage.2010.08.071. [DOI] [PubMed] [Google Scholar]
- Gazzola V, Keysers C. The observation and execution of actions share motor and somatosensory voxels in all tested subjects: Single-subject analyses of unsmoothed fMRI data. Cerebral Cortex. 2009;19(6):1239–1255. doi: 10.1093/cercor/bhn181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg M, Hall C, Butler A. The MIQ-RS: A suitable option for examining movement imagery ability. eCAM. 2007:nem170. doi: 10.1093/ecam/nem170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennenlotter A, Dresel C, Castrop F, Ceballos-Baumann AO, Wohlschlager AM, Haslinger B. The link between facial feedback and neural activity within central circuitries of emotion – New insights from botulinum toxin-induced denervation of frown muscles. Cerebral Cortex. 2009;19(3):537–542. doi: 10.1093/cercor/bhn104. [DOI] [PubMed] [Google Scholar]
- James W. What is emotion. Mind. 1884;9:188–205. [Google Scholar]
- Kim SE, Kim JW, Kim JJ, Jeong BS, Choi EA, Jeong YG, et al. The neural mechanism of imagining facial affective expression. Brain Research. 2007;1145:128–137. doi: 10.1016/j.brainres.2006.12.048. [DOI] [PubMed] [Google Scholar]
- Lotze M, Montoya P, Erb M, Hulsmann E, Flor H, Klose UBN, et al. Activation of cortical and cerebellar motor areas during executed and imagined hand movements: An fMRI study. Journal of Cognitive Neuroscience. 1999;11(5):491. doi: 10.1162/089892999563553. [DOI] [PubMed] [Google Scholar]
- Lundqvist LO, Dimberg U. Facial expressions are contagious. Journal of Psychophysiology. 1995;9(3):203–211. [Google Scholar]
- McIntosh D. Facial feedback hypotheses: Evidence, implications, and directions. Motivation and Emotion. 1996;20(2):121–147. [Google Scholar]
- Murrock CJ, Higgins PA. The theory of music, mood and movement to improve health outcomes. Journal of Advanced Nursing. 2009;65(10):2249–2257. doi: 10.1111/j.1365-2648.2009.05108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito E, Kochiyama T, Kitada R, Nakamura S, Matsumura M, Yonekura Y, et al. Internally simulated movement sensations during motor imagery activate cortical motor areas and the cerebellum. Journal of Neuroscience. 2002;22(9):3683–3691. doi: 10.1523/JNEUROSCI.22-09-03683.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelen MV, Atkinson AP, Andersson F, Vuilleumier P. Emotional modulation of body-selective visual areas. Social cognitive and Affective Neuroscience. 2007;2(4):274–283. doi: 10.1093/scan/nsm023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichon S, de Gelder B, Grezes J. Emotional modulation of visual and motor areas by dynamic body expressions of anger. Social Neuroscience. 2008;3(3):199–212. doi: 10.1080/17470910701394368. [DOI] [PubMed] [Google Scholar]
- Pichon S, de Gelder B, Grèzes J. Two different faces of threat. Comparing the neural systems for recognizing fear and anger in dynamic body expressions. NeuroImage. 2009;47(4):1873–1883. doi: 10.1016/j.neuroimage.2009.03.084. [DOI] [PubMed] [Google Scholar]
- Raos V, Evangeliou MN, Savaki HE. Mental simulation of action in the service of action perception. Journal of Neuroscience. 2007;27(46):12675–12683. doi: 10.1523/JNEUROSCI.2988-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riskind JH. They stoop to conquer: Guiding and self-regulatory functions of physical posture after success and failure. Journal of Personality and Social Psychology. 1984;47(3):479–493. [Google Scholar]
- Riskind JH, Gotay CC. Physical posture: Could it have regulatory or feedback effects on motivation and emotion? Motivation and Emotion. 1982;6(3):273–298. [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nature Reviews Neuroscience. 2001;2(9):661–670. doi: 10.1038/35090060. [DOI] [PubMed] [Google Scholar]
- Schnall S, Laird JD. Keep smiling: Enduring effects of facial expressions and postures on emotional experience and memory. Cognition and Emotion. 2003;17(5):787–797. [Proceedings Paper] [Google Scholar]
- Schwoebel J, Boronat CB, Branch Coslett H. The man who executed “imagined” movements: Evidence for dissociable components of the body schema. Brain and Cognition. 2002;50(1):1–16. doi: 10.1016/s0278-2626(02)00005-2. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. Brain Circuits for the Internal Monitoring of Movements. Annual Review of Neuroscience. 2008;31(1):317–338. doi: 10.1146/annurev.neuro.31.060407.125627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepper S, Strack F. Proprioceptive determinants of emotional and nonemotional feelings. Journal of Personality and Social Psychology. 1993;64(2):211–220. [Google Scholar]
- Vancampfort D, De Hert M, Knapen J, Maurissen K, Raepsaet J, Deckx S, et al. Effects of progressive muscle relaxation on state anxiety and subjective well-being in people with schizophrenia: A randomized controlled trial. Clinical Rehabilitation. 2011;25(6):567–575. doi: 10.1177/0269215510395633. [DOI] [PubMed] [Google Scholar]
- Wild B, Erb M, Bartels M. Are emotions contagious? Evoked emotions while viewing emotionally expressive faces: Quality, quantity, time course and gender differences. Psychiatry Research. 2001;102(2):109–124. doi: 10.1016/s0165-1781(01)00225-6. [DOI] [PubMed] [Google Scholar]