Abstract
Purpose
Test whether equivalent changes in moderate (walking) and vigorous exercise (running) produce equivalent weight loss under free-living, non-experimental conditions.
Methods
Regression analyses of changes (Δ) in BMI vs. exercise energy expenditure (ΔMETh/d, 1 metabolic equivalent or MET=3.5 ml O2•kg−1•min−1) from survey questionnaires completed at baseline and 6.2 years thereafter in 15,237 walkers and 32,216 runners.
Results
At baseline, walkers spent less energy walking than runners spent running (mean±SD males: 2.22±1.65 vs. 5.31±3.12, females: 2.15±1.63 vs. 4.76±3.03 METh/d) and walkers were significantly heavier than runners (males: 26.63±4.04 vs. 24.09±2.58, females: 25.44±5.14 vs. 21.61±2.49 kg/m2). During follow-up, energy expenditure declined less for walking in walkers than for running in runners (males: −0.19±1.92 vs. −1.27±2.87, females: −0.30±1.93 vs. −1.28±2.85 METh/d). ΔBMI was inversely related to both ΔMETh/d run and ΔMETh/d walked, but more strongly to ΔMETh/d run than walked in men, and in heavier women. Specifically, the regression coefficient for ΔBMI vs. ΔMETh/d was significantly more negative for running than walking in men in the 1st quartile (differences in slope±SE: −0.06±0.03, P=0.01), 2nd quartile (−0.10±0.03, P=0.001), 3rd quartile (−0.17±0.03, P<10−8) and 4th quartile of BMI (−0.14±0.03, P<10−4) and in the 4th BMI quartile of women (−0.32±0.04 kg/m2 per METh/d, P<10−17). This represented 90% greater weight loss per METh/d run than walked in the 4th BMI quartile for both sexes. Age-related weight gain was attenuated by running in both sexes (P<10−6), and by walking in women (P=0.005).
Conclusion
Although ΔBMI was significantly associated with both ΔMETh/d run and walked, the ΔBMI was significantly greater for Δrunning than Δwalking.
Keywords: Obesity, prevention, epidemiology, overweight
Introduction
Obesity in the US has risen to 35.5% among adult men and 35.8% among adult women, with some evidence suggesting that recent increases may be leveling off [9]. Randomized clinical trials show that exercise produces moderate weight loss, particularly as an adjunct to dieting [21]. Most weight loss studies are one year or less. This is significant because longer follow-up usually shows high rates of recidivism following weight loss [4]. Thus, whereas the short-term consequences of exercise-induced weight loss may be well understood, the long-term consequences, the ones that matter most, are not.
Prospective follow-up studies may identify behaviors that prevent long-term weight gain in the unregulated, free-living, non-experimental environments in which people reside [7,10,15,17,21,24,40]. Those investigating physical activity share some common challenges. First, there is the question of design. When baseline exercise levels are compared to weight change or incident obesity, it is assumed that baseline values reflect follow-up conditions [27], which may not be true [34]. Second, there is the question of exposure. Most epidemiological studies rely on energy expenditure from self-reported exercise duration [21], which overestimates expenditure relative to objectively measured values [22], particularly for cohorts selected to be representative of populations whose activity levels are characteristically low [18]. In addition, most combine different activities without regard to mode or intensity [21], because they have limited statistical power to subdivide their data. Third, is the question of quantile-specific effects. Cross-sectionally, we have shown that the potencies of obesity risk factors (e.g., deficient exercise levels, diet, family history, socio-economic status) are strongly dependent upon the percentile of the body weight distribution (i.e., more potent at the 90th than the 10th BMI percentile) [30,33,35,38,39]. Because body weight may affect the choice of activity (leaner runners vs. heavier walkers), quantile effects could distort the estimated efficacy of different physical activities in preventing weight gain.
The National Runners’ and Walkers’ Health Study cohorts are unique in their being created expressly for the purpose of comparing specific physical activities to health outcomes [39,38,30,33]. Walking and running provide an ideal test of the health benefit of moderate intensity exercise (i.e., exercise requiring between 3 and 6 times the energy expenditure of sitting at rest [19]) versus vigorous intensity exercise (i.e., requiring more than 6-fold resting energy expenditure [19]) because they involve the same muscle groups. In addition, energy expended by walking and running can be computed from distance rather than time, which has been demonstrated to be a superior metric, showing cross-sectional associations with body weight with twice the effect size as its time-based estimation [36,37]. The purpose of this paper is to test whether vigorous physical activity (running) has the same long-term effects on body weight and abdominal obesity as moderate intensity exercise (walking), while recognizing the issues of design, exposure, and quantile-specific effects. Although others have shown changes in exercise affect changes in weight prospectively [7,10,17,24,40], their power to compare moderate vs. vigorous exercise has been limited.
Methods and Procedures
National Runners’ Health Study II and the National Walkers’ Health Study were recruited primarily between 1998 and 2001. The 2006 partial re-survey of the National Runners’ Health Study II and the National Walkers’ Health Study [36,37] were obtained to identify and qualify approximately 50,000 runners and walkers for a proposed clinical trial, rather than a prospective follow-up study per se. These represented approximately a third of the original walkers (33.2%), and one-half of the original runners surveyed (51.7%). The difference in recruitment rates was due to the greater effort made to recruit runners (two mailings) than walkers (one mailing). Compared to non-responders, those that responded were slightly more likely to be female, younger, slightly less educated, weighed slightly more, were less likely to report taking medications for blood pressure, hypertension, or diabetes, but reported approximately the same number of km/day run if a runner or walked if a walker as reported on their original questionnaire [37].
It does not appear that the different recruitment rates between the runners and walkers affect the analyses. First, there was no difference between the first 33.2% of the runners recruited (corresponding to the recruitment rate in the walkers) and the second 18.5% (=51.7% - 33.2%) of the runners recruited for BMI (difference±SE, males: −0.02±0.03 kg/m2, P=0.50; females: −0.04±0.03 kg/m2, P=0.21), waist circumference (males: −0.09±0.10 cm, P=0.34; females: −0.11±0.13 cm, P=0.42), or running energy expenditure (males: 0.05±0.06 METh/d, P=0.36; females: 0.08±0.06 METh/d, P=0.18). Second, repeating the analyses using only the first 33.2% of the runners recruited (to match the 33.2% recruitment rate in the walkers) yielded results consistent with the entire sample (analyses not displayed).
The participants completed a four page survey on running and walking history (average weekly mileage over the preceding 5 years, minutes required to run or walk a mile, frequency of runs and walks per week >10 min, longest usual run or walk), height, current weight and body circumferences, diet (vegetarianism and the current weekly intakes of alcohol, red meat, fish, fruit), cigarette use, and history of diseases [30,33,38, 39]. Height and weight were determined by asking the participant, “What is your current height (in inches, without shoes)?” and, “What is your current weight (pre-pregnancy weight if pregnant)?” BMI was calculated as weight in kilograms divided by the square of height in meters. Self-reported waist circumference as elicited by the question, “Please provide, to the best of your ability, your body circumference in inches: waist___, hip___, and chest___,” without further instruction. Elsewhere, we have reported the strong correlations between self-reported and clinically measured heights (r=0.96) and weights (r=0.96) [33]. Self-reported waist circumferences were somewhat less precise, as indicated by their correlation with reported circumferences on a second questionnaire (r=0.84) and with their clinical measurements (r=0.68) [33]. The study protocol was approved by the University of California Berkeley committee for the protection of human subjects, and all subjects provided a signed statement of informed consent.
Walking (in walkers) and running (in runners) were reported in miles per week, while running (in walkers), walking (in runners), swimming, cycling, and other exercise were reported as hours per week. Usual pace (minutes per mile) was reported for walking in walkers, and for running in runners. Non-running energy expenditure in the runners, and non-walking energy expenditures in the walkers, was calculated using the metabolic equivalent (MET) tables published by Ainsworth et al. [1], where one MET is the energy expended sitting at rest (3.5 ml O2•kg−1•min−1). In walkers, METh/d walked was calculated by converting reported distance into duration (i.e., distance/mph) which was then multiplied by the MET value for the reported pace. The 8.7% of men and 8% of women who did not provided their usual walking pace had their values estimated by stochastic imputation using a sex-specific function of age. In runners, METh/d run was calculated as km run*1.02 METhr/km [37]. Strength exercise included lifting weights, circuit training, resistance or strength training, crunches, abdominal exercise, push-ups, pull-ups, sit-ups, leg lifts, upper body exercise, and unspecified calisthenics.
Statistical analyses
Statistical analyses were performed using the statistical software package JMP (SAS institute, Cary NC, version 5.1). Least-squares regression was used to estimate the relationships of ΔBMI and Δwaist circumferences to ΔMETh/d of walking, running, and other exercise. Covariates included adjustments for age (age and age2), education, follow-up duration, and intakes of meat, fruit, and alcohol, and changes in current smoking and menopausal status and parity. Linear contrasts were used to compare regression slopes for running, walking, and other exercise. Running in the walkers and walking in the runners, were included as other exercise because their values were time-based rather than distance-based. Quartiles for BMI and waist circumference were defined by the average of the baseline and follow-up values, because their average value is mathematically independent of the change data whereas the baseline value is not.
Results
Sample characteristics
Table 1 shows that at baseline the walkers were older than the runners, had slightly less education, and were more likely to smoke, drink less alcohol, eat more fruit, and be substantially heavier with substantially larger waistlines than the runners. Because baseline recruitment began later in the walkers than in the runners, their follow-up duration averaged 9.1 months less (all analyses were adjusted for follow-up duration). All groups increased waist circumference, and all but the male walkers gained weight, during follow-up. Changes in smoking status were very rare during the follow-up: only 26 started and 46 quit among male walkers, 93 started and 172 quit among female walkers, 91 started and 103 quit among male runners, 101 started and 133 quit among female runners. During follow-up period, there were 558 female walkers (4.6%) and 869 female runners (5.6%) who transitioned into menopause, and 426 female walkers (3.6%) and 2281 female runners (14.6%) who increased parity.
Table 1.
Sample characteristics (mean±SD or percent)
| Male | Female | |||
|---|---|---|---|---|
| Runners | Walkers | Runners | Walkers | |
| Sample (N) | 16,561 | 3,194 | 15,655 | 12,043 |
| Age (years) | 48.24±10.93 | 61.65±11.05 | 40.86±10.63 | 52.92±11.99 |
| Follow-up (years) | 6.31±0.91 | 5.59±1.16 | 6.55±0.94 | 5.69±1.27 |
| Education (years) | 16.81±2.45 | 16.36±2.70 | 16.36±2.31 | 15.29±2.54 |
| Current smokers (%) | 1.22 | 3.41 | 1.66 | 3.60 |
| Meat (servings/d) | 0.44±0.40 | 0.46±0.41 | 0.27±0.30 | 0.37±0.34 |
| Fish (servings/d) | 0.21±0.21 | 0.25±0.22 | 0.17±0.19 | 0.21±0.20 |
| Fruit (pieces/d) | 1.53±1.18 | 1.63±1.22 | 1.53±1.06 | 1.70±1.14 |
| Alcohol (g/d) | 9.88±13.45 | 9.27±13.40 | 5.89±8.22 | 4.98±9.14 |
| Running | ||||
| METh/d baseline | 5.31±3.12 | 4.76±3.03 | ||
| ΔMETh/d | −1.27±2.87 | −1.28±2.85 | ||
| Walking | ||||
| METh/d baseline | 2.22±1.65 | 2.15±1.63 | ||
| ΔMETh/d | −0.19±1.92 | -0.30±1.93 | ||
| Other exercise, vigorous | ||||
| METh/d baseline | 1.70±3.20 | 1.69±3.29 | 2.07±3.34 | 1.48±2.97 |
| ΔMETh/d | 0.36±3.52 | −0.26±3.57 | 0.15±3.77 | −0.12±3.34 |
| Other exercise, moderate | ||||
| METh/d baseline | 0.76±1.62 | 0.43±1.46 | 0.83±1.73 | 0.36±1.27 |
| ΔMETh/d | 0.63±2.43 | 0.05±2.04 | 0.91±2.67 | 0.06±1.72 |
| Other exercise, light | ||||
| METh/d baseline | 0.02±0.30 | 0.04±0.61 | 0.03±0.36 | 0.03±0.25 |
| ΔMETh/d | 0.00±0.40 | 0.01±0.56 | 0.04±0.47 | 0.03±0.42 |
| Other exercise, strength | ||||
| METh/d baseline | 0.53±1.26 | 0.21±0.86 | 0.54±1.25 | 0.20±0.76 |
| ΔMETh/d | −0.14±1.42 | 0.04±1.13 | −0.16±1.46 | 0.11±1.19 |
| BMI (kg/m2) | ||||
| Baseline | 24.09±2.58 | 26.63±4.04 | 21.61±2.49 | 25.44±5.14 |
| Change | 0.59±1.49 | 0.09±2.10 | 0.59±1.64 | 0.47±2.64 |
| Body weight (kg) | ||||
| Baseline | 24.09±2.58 | 26.63±4.04 | 21.61±2.49 | 25.44±5.13 |
| Change | 1.88±4.76 | 0.30±6.63 | 1.56±4.44 | 1.28±7.07 |
| Waist circumference (cm) | ||||
| Baseline | 84.88±6.23 | 93.47±9.82 | 70.02±6.70 | 78.61±12.07 |
| Change | 1.55±4.59 | 1.12±6.68 | 2.42±6.06 | 2.45±8.31 |
Table 1 shows that energy expended by walking in the walkers was less than half that reported for running in the runners. The proportions of total other exercise energy expenditure that was vigorous, moderate, and light exercises (i.e., exclusive of running in runners and walking in walkers) were 21.8%, 9.8%, and 0.3% in male runners, 38.6%, 9.8%, and 0.9% in male walkers, 26.9%, 10.8%, and 0.4% in female runners, 36.8%, 9.0%, and 0.7% in female walkers, respectively. This included strengthening exercise, which represented 6.8% and 7.1% of the energy expenditure in male and female runners, respectively, and 4.7% and 5.1% in male and female walkers, respectively.
Attenuating effects of exercise on weight gain
The traditional prospective study design compares baseline characteristics to changes in disease status during follow-up, e.g., baseline exercise vs. weight change or transition into obesity during follow-up. Such analyses are based on the assumption that during follow-up the independent variable (exercise) remains relatively constant. To adhere to this assumption, we restricted our analyses to men and women whose running or walking changed less than 0.73 METh/d between baseline and follow-up (±0.73 METh/d being the energy equivalent of ±5 km/wk run). The results, displayed in Table 2, show that: 1) greater running energy expenditure at baseline was associated with significantly less gains in BMI (males: P<10−6, females: P<10−7) and waist circumference during follow-up (males: P<10−4, females: P=0.0004); 2) greater walking energy expenditure at baseline also significantly attenuated increases in female BMI (P=0.005), but not increases in male BMI or male or female waist circumference during follow-up; and 3) other exercise at baseline showed no significant association with changes in BMI or waist circumference during follow-up. The attenuating effect per METh/d was greater for running than for other exercise (males: P=0.001 for BMI and P=0.02 for waist circumference; females: P<10−5 for BMI and P=0.02 for waist circumference).
Table 2.
Estimated effect per METh/d of running, walking, and other exercise at baseline on changes in BMI and waist circumferences in 5935 men and 9451 women whose running and walking remained relatively constant (±0.73 METh/d) during 6.2-years of follow-up.
| BMI (Δkg/m2 per METh/d) | Waist circumference (Δcm per METh/d) |
|||
|---|---|---|---|---|
| Males | Females | Males | Females | |
| Regression coefficients | ||||
| Running | −0.044±0.009§ | −0.073±0.013§ | −0.121±0.030§ | −0.174±0.049‡ |
| Walking | −0.006±0.029 | −0.071±0.025† | −0.122±0.107 | −0.096±0.096 |
| Other exercise | −0.011±0.007 | 0.000±0.009 | −0.040±0.023 | −0.039±0.033 |
| Differences between coefficients | ||||
| Run-Walk | −0.038±0.030 | −0.003±0.028 | 0.001±0.110 | −0.078±0.106 |
| Run-Other | −0.033±0.010‡ | −0.073±0.016§ | −0.081±0.036* | −0.135±0.058* |
| Walk-Other | 0.005±0.030 | −0.070±0.027† | −0.082±0.110 | −0.057±0.104 |
Adjusted for baseline age, education, follow-up duration, intakes of meat, fruit, and alcohol, smoking, and exercise group (Runners vs. Walkers). In addition, the women’s data were adjusted for baseline parity and menopause status. Significantly different from zero at:
P<0.05;
P<0.01;
P<0.001;
P<0.0001.
Effects of changing exercise levels
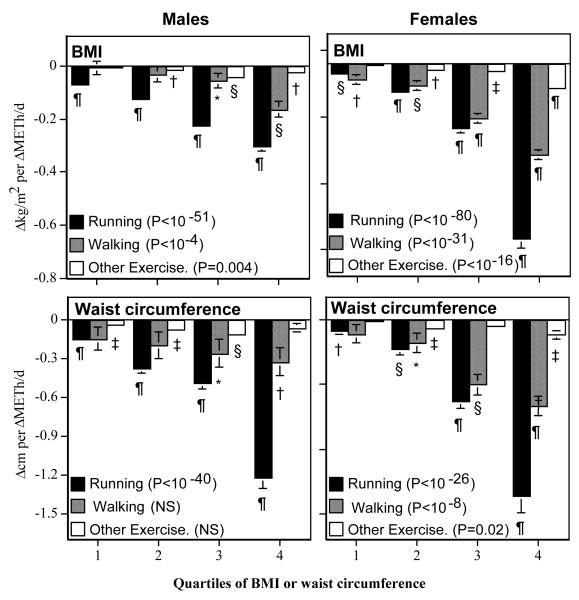
Figure 1 displays the regression slopes for ΔBMI and Δwaist circumference (dependent variables) vs. ΔMETh/d of reported recreational activity (independent variables) in all 19,755 men and 27,698 women, stratified by adiposity levels. Within each stratum, changes in running showed strongly significant inverse relationships with both ΔBMI and Δwaist circumference. For example, a 5 km (3.1 mi) increase in running distance was associated with a 0.34±0.03 kg/m2 BMI reduction in men who weighed ≤23.93 kg/m2 (P<10−33), 0.62±0.04 kg/m2 reduction in those weighing between 23.94 and 26.07 (P<10−58), 1.12±0.05 kg/m2 reduction in those weighed between 26.08 and 28.61 kg/m2 (P<10−97) and a 1.52±0.08 kg/m2 reduction in those ≥28.61 kg/m2 (P<10−97). When stratified by quartiles of waist circumference, the corresponding reductions in men’s waist circumference were 0.74±0.08 (P<10−17), 1.89±0.17 (P<10−28), 2.43±0.24 (P<10−53), and 6.13±0.39 cm (P<10−54). Similar reductions in weight and waist circumference per ΔMETh/d run were observed in women, except that the ΔBMI per ΔMETh/d run was substantially greater for females than males in the heaviest quartile (female vs. male slope±SE: −0.66±0.03 vs. −0.30±0.02 kg/m2 per METh/d run).
Figure 1.
Regression slopes for ΔBMI or ΔWaist circumference (dependent variables) vs. changes in exercise energy expenditure (ΔMETh/d, independent variable), stratified by quartiles of adiposity (Average of baseline and follow-up BMIs or waist circumferences). The significance levels associated with the key refer to the test for progressively increasing regression slopes with increasing adiposity. Significance of the individual regression slopes are coded: * P<0.05; † P<0.01; ‡ P<0.001; § P<0.0001; and ¶ P<10−15. The cut points for the 25th, 50th, and 75th quantiles were: 23.94, 26.08, and 28.62 kg/m2, respectively, for male BMI; 22.17, 26.08, and 27.90 kg/m2, respectively, for female BMI; 87.63, 92.71, and 99.06 cm, respectively, for male waist circumference; and 71.12, 77.47, and 85.72 cm, respectively, for female waist circumference.
Changes in walking were also inversely related to ΔBMI and Δwaist circumference, albeit not as much as running in men or in heavier women. In men, the weight loss (ΔBMI) was significantly greater per ΔMETh/d run than per ΔMETh/d walked in all quartiles: the 1st or leanest quantile (running-walking difference in slope ±SE: −0.06±0.03, P=0.01), 2nd (−0.10±0.03, P=0.001), 3rd (−0.17±0.03, P<10−8), and the 4th or heaviest quantile (−0.14±0.03, P<10−4). This was also true for the higher quartiles of men’s waist circumference, i.e., the 3rd quantile (slope differences±SE: −0.23±0.12, P=0.05) and the 4th or highest quantile (−0.90±0.13, P<10−11). In women, the significant differences between running and walking were restricted to the highest quartile of BMI (slope differences±SE: −0.32±0.04 kg/m2 per METh/d, P<10−17) and waist circumference (slope differences±SE: −0.70±0.15 cm per METh/d, P<10−5). Although ΔMETh/d for other exercise was also associated with ΔBMI and Δwaist circumference, its effect was generally much smaller than that of running or walking.
The preceding analyses were based on runners who averaged twelve to thirteen years younger than the walkers. Although the analyses of Figure 1 were adjusted for age, additional analyses were carried out to confirm that the older age of the walkers did not account the significantly greater effects of running than walking on ΔBMI and Δwaist circumference. Restricting the runners to ≥55 years old created a subset of male runners of approximately the same average age (61.19 years) as the male walkers. Compared to walking, the slope for ΔBMI per ΔMETh/d run was 4.1-fold greater for the 1st quartile (P=0.19), 9.2-fold greater for the 2nd quartile (P=0.002), 3.3-fold greater for the 3rd quartile (P<10−5), and 1.9-fold greater for the 4th quartile (P=0.002). A greater effect of walking than running was also true for the higher quartiles of men’s waist circumference, i.e., the 3rd quantile (2.8-fold greater effect per ΔMETh/d run than walked, P=0.04 for difference) and the 4th quantile (10.5-fold greater effect, P<10−7 for difference). Similarly, excluding female walkers ≥50 yielded a subset of female walkers of similar mean age (42.1 years) as the female runners. Compared to ΔMETh/d walked, the slopes for ΔBMI and Δwaist circumference per ΔMETh/d run were 38% larger (P<10−4) and 66% larger (P=0.002) in the highest BMI and waist circumference quintiles.
Potency by BMI level
The progressively greater effect of Δexercise on ΔBMI and Δwaist circumference from the 1st to the 4th quartiles of adiposity (leanest to the heaviest subjects) was strongest for running. It was also significant for walking albeit less than running. Compared to the leanness quartile, the effects of running in the highest quartile of adiposity were 4.4-fold and 18.3-fold greater for male and female BMI, respectively, and 8.3-fold and 17.4-fold greater for male and female waist circumference. Similarly, the effects of walking in the highest quartile of adiposity were 28-fold and 6-fold greater for male and female BMI, respectively, and 2.2-fold and 6.2-fold greater for male and female waist circumference, compared to the leanness quartile.
Superiority of distance based vs. time based estimates of energy expenditure
Virtually all epidemiological studies calculate exercise energy expenditure from time and intensity. Running and walking are two well-defined activities whose energy expenditure can be calculated from distance rather than time [36,37]. Cross-sectional analyses show that the distance-based calculations provides a superior metric for epidemiological research over the time-based calculations [36,37], but whether this applies to longitudinal analyses of changing physical activity is not known. The runners’ data were therefore used to test whether ΔMETh/d was more strongly related to ΔBMI and Δwaist circumference when calculated from distance than from intensity and time. When the two estimates were used in separate regression models, the effect (Δkg/m2 or Δcm per ΔMETh/d adjusted for covariates) was greater when energy expenditure was calculated from distance than time for both ΔBMI (ΔMETh/ddistance vs. ΔMETh/dtime slope±SE males:-0.122±0.004, P<10−211 vs. −0.059±0.002, P<10−128; females:-0.086±0.005, P<10−77 vs. −0.044±0.003, P<10−55) and Δwaist circumference (males:−0.249±0.014, P<10−73 vs. −0.119±0.009, P<10−43; females:-0.212±0.019, P<10−26 vs. −0.111±0.012, P<10−19). When included simultaneously in the same regression models so that their effects can be compared directly, the effect was significantly greater when calculated from distance than time for both ΔBMI (slope±SE males: −0.099±0.004 vs. −0.028±0.003, P<10−28 for their difference; females:-0.066±0.005 vs. −0.024±0.003, difference P<10−7) and Δwaist circumference (males: −0.206±0.016 vs. −0.052±0.010, difference P<10−11; females:-0.161±0.023 vs. −0.061±0.014, difference P=0.002). Thus, consistent with previous cross-sectional analyses, the distance-based calculation of ΔMETh/d had at least a 2-fold larger effect on ΔBMI and Δwaist circumference than its time-based calculation.
Failure to stratify by adiposity
Table 3 presents the regression analyses of ΔBMI and Δwaist circumference vs. Δexercise, not stratified by adiposity. Although the unstratified analyses identified the inverse relationships correctly, i.e., that ΔBMI and Δwaist circumference were inversely related to Δrunning, Δwalking, and Δother exercise, it fails when comparing the runners’ and walkers’ regression slopes. This is because of the runners’ and walkers’ substantial difference in adiposity (Table 1), and the quantile effect of adiposity on the exercise-weight relationships (Figure 1). The 80th percentile of the runner’s BMI distribution corresponds to the 53rd and 40th percentiles of the male and female walker’s distribution, respectively, while the 80th percentile of the runners’ waist circumference corresponds to the walkers’ 38th and 46th percentiles, respectively. This greatly distorts (diminishes) the differences in the runners’ and walkers’ regression slopes by comparing runners, selected from the BMI range where exercise has a weaker effect, with walkers, selected from the range where exercise has a stronger effect. In women, the distortion is so great that an opposite conclusion is supported by the unstratified vis-á-vis the stratified analyses, i.e., making it appear that both ΔBMI and Δwaist circumference were more strongly affected by Δwalking than Δrunning (P<10−14 and P<10−21, respectively). The distortion of the unstratified analyses also obfuscates the significant difference between the men’s waist circumference slopes for walking and running (P=0.89) while retaining only some of the significance of their difference between their BMI slopes (P<10−4).
Table 3.
Estimated effect per ΔMETh/d in running, walking, and other exercise on changes in BMI and waist circumferences in 5935 men and 9451 women when analyzed without stratifying by body weight.
| BMI | Waist circumference | |||
|---|---|---|---|---|
| Males | Females | Males | Females | |
| Males | ||||
| Coefficients | ||||
| ΔRunning | −0.123±0.004¶ | −0.086±0.006¶ | −0.253±0.015¶ | −0.217±0.023¶ |
| ΔWalking | −0.062±0.014§ | −0.174±0.010¶ | −0.261±0.053§ | −0.363±0.040¶ |
| ΔOther exercise | −0.017±0.003§ | 0.021±0.003§ | −0.051±0.009§ | −0.043±0.011§ |
| Differences between coefficients | ||||
| ΔRun-ΔWalk | −0.062±0.015§ | 0.088±0.011§ | 0.008±0.055 | 0.146±0.045‡ |
| ΔRun-ΔOther | −0.106±0.005¶ | −0.065±0.006¶ | −0.202±0.016¶ | −0.174±0.025§ |
| ΔWalk-ΔOther | −0.044±0.014† | −0.153±0.010¶ | −0.209±0.053§ | −0.320±0.041§ |
Adjusted for baseline age, education, follow-up duration, exercise group (Runners vs. Walkers) and changes in the intakes of meat, fish, fruit, and alcohol, and smoking. In addition, the women’s data were adjusted for baseline parity and menopause status. Significantly different from zero at:
P≤0.05;
P≤0.01,
P≤0.001,
P≤0.0001, and
P<10-15.
Discussion
The effects of increased physical activity on total and abdominal fat are well established [21]. Moderate intensity activity is recommended for weight loss and the prevention of weight regain [8], in part because it is preferred by the overweight and is therefore pragmatically useful [11]. Little attention has been given to the scientific question of how exercise intensity might affect weight loss for similar energy deficits [21], despite their being plausible reasons for greater weight loss from vigorous than moderate exercise. Specifically, increases in post-exercise metabolic rate and post-exercise appetite suppression are greater for vigorous exercise [6,13,14, 20], and the effects are nontrivial. For example, a reported 190 kcal mean extra increase in resting metabolic rate following a 519-kcal exercise session represents a 37% increase in their combined energy expenditure [13].
To date, clinical trials of exercise intensity seem to suggest that equivalent energy expenditure by moderate and vigorous exercise produce equivalent loss of total and abdominal fat [2,26,28,26]. In contrast, several cross-sectional and prospective studies suggest a greater effect of vigorous exercise [5,17,25,29]. Figure 1 of the current report shows highly significant differences between walking (a moderate intensity activity) and running (a vigorous intensity exercise). Specifically, the changes in BMI and waist circumference per ΔMETh/d were significantly greater for running than walking in men and in heavier women. In addition, among subjects who maintained a consistent level of physical activity during follow-up, Table 2 showed that running significantly attenuated age-related weight gain whereas walking did not. The large sample size of the current report versus the few hundred studied in the clinical trials may explain some of the differences.
These differences may also reflect a fundamental distinction in the effects being studied. As a tightly regulated experiment, controlled clinical trials most directly address the biological question of whether exercise intensity affects weight loss. This often requires substantial effort to control dietary intake and other extraneous variables that could potentially influence weight change. Observational studies, by contrast, examine the effects of exercise on body weight when other behaviors are allowed to change in free-living people in unregulated environments. In this regard, observational studies may be more á propos to the public health implications of changing physical activity.
This distinction is particularly relevant to weight loss. Even in clinical trials, long-term exercise regimens produce only about 30% of their predicted weight loss from their estimated calorie deficit [23]. Discrepancies between predicted and actual exercise-produced weight loss are reported to increase with the exercise dose [3]. King et al. reported that those that compensated for their exercise energy expenditure did so by increased calorie intake [12]. When asked to compensate exercise energy expenditure with food intake, even normal weight subjects will overcompensate by 2- to 3-fold [31].
In addition to the uniqueness of directly comparing running with walking, the current analyses are distinguished from other studies in their recognition of quantile effects, their use of distance-based energy estimation, and their exclusion of subjects who fail to maintain consistent exercise during follow-up in assessing the attenuating effects of exercise.
Quantile effects
Figure 1 shows that the effects of Δexercise on ΔBMI and Δwaist circumference became progressively greater with increasing body weight, and were in fact were over four-fold greater in the highest BMI tertile than its lowest tertile. These are consistent with earlier cross-sectional analyses showing that the inverse relationships between BMI with running and walking distances increased progressively with the percentile of the BMI distribution (i.e., from the leanness to the heaviest [30,33,38,39,38]). In this regard, they follow a pattern exhibited by other BMI risk factors (e.g., diet, family history, educational attainment) of acquiring progressively greater potency from the leanest to the heaviest subjects [35].
Distance- vs. time-based energy estimation
The analyses of Table 2 also benefited from the estimation of vigorous (running) and moderate intensity (walking) energy expenditures from distance and intensity rather than time and intensity. The estimates are entirely consistent with our previous cross-sectional analyses that showed that declines in BMI and waist circumference per METh/d run and walked were also over two-fold greater when calculated from reported distance than from time and intensity [36,37]. In this paper, we furthered these results by showing that ΔBMI and Δwaist circumferences per ΔMETh/d run were over two-fold greater than when calculated from distance than from time and intensity (the corresponding analyses in walkers were not possible because baseline walking duration was not collected).
Attenuating weight gain
Although exceptions exist, many cohort studies have had difficulty showing that baseline physical activity affects body weight prospectively [27]. Our findings suggest that this difficulty at least partly relates to the lack of consistency in physical activity over time, in violation of a principal premise of the analyses. Table 2 shows that when restricted to runners whose exercise changed within ±0.73 METh/d (corresponding to ±5 km/wk use in our earlier paper [32]), increases in BMI and waist circumference over time were attenuated in relation to the average running energy expenditure. These results provide independent confirmation of our initial report of the attenuating effect running on middle-aged weight gain, i.e., that men who ran≥ 48 km/wk had one half of the average annual weight gain as men who ran < 24 km/wk [32].
Caveats
The samples used in these analyses are likely to be healthier than the general population. The initial recruitment of participants was through footrace events and from exercise-oriented magazine subscribers. This strategy was pursued in order to include higher exercise doses than represented in other population studies. In addition, the current analyses use a sample of convenience that was obtained as part of an effort to recruit participants for a different study. However, we believe that the same bias would apply to both walkers and runners, and that the biological processes that relate running to body weight would not differ dramatically between the current sample and a less selected population.
An inherent limitation of comparing changes in exercise with changes in adiposity is the uncertainty of whether change in physical activity preceded change in body weight or whether the converse occurred. In addition, detailed dietary data, sleep, sedentary behaviors such as sitting, and other variables that could have affected body weight were not collected [27]. Finally, we note that the purported tendency for overweight individuals to overestimate their physical activity [16] would diminish their inverse relationship, and thus could not explain the associations of Table 2.
In conclusion, these data suggest that in free-living individuals whose running and walking change overtime, along with concomitant changes in other behaviors that naturally accompany such changes, both Δmoderate and Δvigorous exercise energy expenditure were inversely related to ΔBMI and Δabdominal obesity, but that the effects were greater for vigorous than moderate intensity exercise in men and in heavier women.
Acknowledgements
This research was supported by grant HL094717 from the National Heart, Lung, and Blood Institute and was conducted at the Ernest Orlando Lawrence Berkeley National Laboratory (Department of Energy DE-AC03-76SF00098 to the University of California). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Disclosure The author has no financial conflict of interest to disclose. The results of the present study do not constitute endorsement by ACSM.
The authors have declared that no competing interests exist.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498–516. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 2.Chambliss HO. Exercise duration and intensity in a weight-loss program. Clin J Sport Med. 2005;15:113–5. doi: 10.1097/01.jsm.0000151867.60437.5d. [DOI] [PubMed] [Google Scholar]
- 3.Church TS, Martin CK, Thompson AM, Earnest CP, Mikus CR, Blair SN. Changes in weight, waist circumference and compensatory responses with different doses of exercise among sedentary, overweight postmenopausal women. PLoS One. 2009;4(2):e4515. doi: 10.1371/journal.pone.0004515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report: National Institutes of Health. Obes Res. 1998;(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 5.Coakley EH, Rimm EB, Colditz G, Kawachi I, Willett W. Predictors of weight change in men: results from the Health Professionals Follow-up Study. Int J Obes Relat Metab Disord. 1998;22:89–96. doi: 10.1038/sj.ijo.0800549. [DOI] [PubMed] [Google Scholar]
- 6.Dionne I, Van Vugt S, Tremblay A. Postexercise macronutrient oxidation: a factor dependent on postexercise macronutrient intake. Am J Clin Nutr. 1999;69:927–30. doi: 10.1093/ajcn/69.5.927. [DOI] [PubMed] [Google Scholar]
- 7.Di Pietro L, Dziura J, Blair SN. Estimated change in physical activity level (PAL) and prediction of 5-year weight change in men: the Aerobics Center Longitudinal Study. Int J Obes Relat Metab Disord. 2004;28:1541–7. doi: 10.1038/sj.ijo.0802821. [DOI] [PubMed] [Google Scholar]
- 8.Donnelly JE, Blair SN, Jakicic JM, et al. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459–71. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 9.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307:491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 10.Haapanen N, Miilunpalo S, Pasanen M, Oja P, Vuori I. Association between leisure time physical activity and 10-yr body mass change among working-aged men and women. Int J Obes Relat Metab Disord. 1997;21:288–296. doi: 10.1038/sj.ijo.0800403. [DOI] [PubMed] [Google Scholar]
- 11.King AC, Haskell WL, Young DR, Oka RK, Stefanick ML. Long-term effects of varying intensities and formats of physical activity on participation rates, fitness, and lipoproteins in men and women aged 50 to 65 years. Circulation. 1991;91:2596–604. doi: 10.1161/01.cir.91.10.2596. [DOI] [PubMed] [Google Scholar]
- 12.King NA, Hopkins M, Caudwell P, Stubbs RJ, Blundell JE. Individual variability following 12 weeks of supervised exercise: identification and characterization of compensation for exercise-induced weight loss. Int J Obes (Lond) 2008;32:177–84. doi: 10.1038/sj.ijo.0803712. [DOI] [PubMed] [Google Scholar]
- 13.Knab AM, Shanely RA, Corbin KD, Jin F, Sha W, Nieman DC. A 45-minute vigorous exercise bout increases metabolic rate for 14 hours. Med Sci Sports Exerc. 2011;43:1643–8. doi: 10.1249/MSS.0b013e3182118891. [DOI] [PubMed] [Google Scholar]
- 14.LaForgia J, Withers RT, Gore CJ. Effects of exercise intensity and duration on the excess post-exercise oxygen consumption. J Sports Sci. 2006;24:1247–64. doi: 10.1080/02640410600552064. [DOI] [PubMed] [Google Scholar]
- 15.Lee IM, Djoussé L, Sesso HD, Wang L, Buring JE. Physical activity and weight gain prevention. JAMA. 2010;24:303–1173. doi: 10.1001/jama.2010.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lichtman SW, Pisarska K, Berman ER, et al. Discrepancy between self-reported and actual caloric intake and exercise in obese subjects. New Engl J Med. 1992;327:1893–8. doi: 10.1056/NEJM199212313272701. [DOI] [PubMed] [Google Scholar]
- 17.Littman AJ, Kristal AR, White E. Effects of physical activity intensity, frequency, and activity type on 10-y weight change in middle-aged men and women. Int J Obes (Lond) 2005;29:524–33. doi: 10.1038/sj.ijo.0802886. [DOI] [PubMed] [Google Scholar]
- 18.Luke A, Dugas LR, Durazo-Arvizu RA, Cao G, Cooper RS. Assessing Physical Activity and its Relationship to Cardiovascular Risk Factors: NHANES 2003-2006. BMC Public Health. 2011;11:387. doi: 10.1186/1471-2458-11-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pate RR, Pratt M, Blair SN, et al. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273:402–7. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 20.Phelain JF, Reinke E, Harris MA, Melby CL. Postexercise energy expenditure and substrate oxidation in young women resulting from exercise bouts of different intensity. J Am Coll Nutr. 1997;16:140–6. doi: 10.1080/07315724.1997.10718664. [DOI] [PubMed] [Google Scholar]
- 21.Physical Activity Guidelines Advisory Committee . Physical Activity Guidelines Advisory Committee Report, 2008. U.S. Department of Health and Human Services; Washington, DC: 2008. pp. 1–683. [Google Scholar]
- 22.Prince SA, Adamo KB, Hamel ME, et al. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56. doi: 10.1186/1479-5868-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ross R, Janssen I. Physical activity, total and regional obesity: dose-response considerations. Med Sci Sports Exerc. 2001;33:S521–7. doi: 10.1097/00005768-200106001-00023. [DOI] [PubMed] [Google Scholar]
- 24.Schmitz KH, Jacobs DRJ, Leon AS, Schreiner PJ, Sternfeld B. Physical activity and body weight: associations over ten years in the CARDIA Study. Coronary Artery Risk Development In Young Adults. Int J Obes Relat Metab Disord. 2000;24:1475–87. doi: 10.1038/sj.ijo.0801415. [DOI] [PubMed] [Google Scholar]
- 25.Sherwood NE, Jeffery RW, French SA, Hannan PJ, Murray DM. Predictors of weight gain in the Pound of Prevention study. Int. J. Obes. 2000;24:395–403. doi: 10.1038/sj.ijo.0801169. [DOI] [PubMed] [Google Scholar]
- 26.Slentz CA, Aiken LB, Houmard JA, et al. Inactivity, exercise, and visceral fat. STRRIDE: a randomized,controlled study of exercise intensity and amount. J Appl Physiol. 2005;99:1613–8. doi: 10.1152/japplphysiol.00124.2005. [DOI] [PubMed] [Google Scholar]
- 27.Summerbell CD, Douthwaite W, Whittaker V, et al. The association between diet and physical activity and subsequent excess weight gain and obesity assessed at 5 years of age or older: a systematic review of the epidemiological evidence. Int J Obes (Lond) 2009;33(Suppl 3):S1–92. doi: 10.1038/ijo.2009.80. [DOI] [PubMed] [Google Scholar]
- 28.Swain DP, Franklin BA. Comparison of cardioprotective benefits of vigorous versus moderate intensity aerobic exercise. Am J Cardiol. 2006;1:97–141. doi: 10.1016/j.amjcard.2005.07.130. [DOI] [PubMed] [Google Scholar]
- 29.Tremblay A, Després JP, Leblanc C, et al. Effect of intensity of physical activity on body fatness and fat distribution. Am J Clin Nutr. 1990;51:153–7. doi: 10.1093/ajcn/51.2.153. [DOI] [PubMed] [Google Scholar]
- 30.Williams PT. Nonlinear relationships between weekly walking distance and adiposity in 27,596 women. Med Sci Sports Exerc. 2005;37:1893–901. doi: 10.1249/01.mss.0000175860.51204.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willbond SM, Laviolette MA, Duval K, Doucet E. Normal weight men and women overestimate exercise energy expenditure. J Sports Med Phys Fitness. 2010;50:377–84. [PubMed] [Google Scholar]
- 32.Williams PT. Maintaining vigorous activity attenuates 7-yr weight gain in 8340 runners. Med Sci Sports Exerc. 2007;39:801–809. doi: 10.1249/mss.0b013e31803349b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams PT. Association between walking distance and percentiles of body mass index in older and younger men. Br J Sports Med. 2008;42:352–6. doi: 10.1136/bjsm.2007.041822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams PT. Asymmetric weight gain and loss from increasing and decreasing exercise. Med Sci Sports Exerc. 2008;40:296–302. doi: 10.1249/mss.0b013e31815b6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams PT. Evidence that obesity risk factor potencies are weight dependent, a phenomenon that may explain accelerated weight gain in western societies. PLoS One. 2011;6:e27657. doi: 10.1371/journal.pone.0027657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams PT. Advantage of distance-versus time-based estimates of walking in predicting adiposity. Med Sci Sports Exer. 2012;44:1728–37. doi: 10.1249/MSS.0b013e318258af3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams PT. Non-exchangeability of running vs. other exercise in their association with adiposity, and its implications for public health recommendations. PloS One. 2012;7:e36360. doi: 10.1371/journal.pone.0036360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams PT, Pate RR. Cross-sectional relationships of exercise and age to adiposity in 60,617 male runners. Med Sci Sports Exerc. 2005;37:1329–37. doi: 10.1249/01.mss.0000174894.05236.45. [DOI] [PubMed] [Google Scholar]
- 39.Williams PT, Satariano WA. Relationships of age and weekly running distance to BMI and circumferences in 41,582 physically active women. Obes Res. 2005;13:1370–80. doi: 10.1038/oby.2005.166. [DOI] [PubMed] [Google Scholar]
- 40.Williamson DF, Madans J, Anda RF, Kleinman JC, Kahn HS, Byers T. Recreational physical activity and ten-year weight change in a US national cohort. Int J Obes Relat Metab Disord. 1993;17:279–86. [PubMed] [Google Scholar]