Abstract
Background
To test whether equivalent energy expenditure by moderate-intensity (e.g., walking) and vigorous-intensity exercise (e.g., running) provides equivalent health benefits.
Methods and Results
We used the National Runners’ (n=33,060) and Walkers’ (n=15,945) Health Study cohorts to examine the effect of differences in exercise mode and thereby exercise intensity on coronary heart disease (CHD) risk factors. Baseline expenditure (METhr/d) was compared to self-reported, physician-diagnosed incident hypertension, hypercholesterolemia, diabetes and CHD during 6.2 years follow-up. Running significantly decreased the risks for incident hypertension by 4.2% (P<10-7), hypercholesterolemia by 4.3% (P<10-14), diabetes by 12.1% (P<10-5), and CHD by 4.5% per METh/d run (P=0.05). The corresponding reductions for walking were 7.2% (P<10-6), 7.0% (P<10-8), 12.3% (P<10-4), and 9.3% (P=0.01). Relative to <1.8 METh/d, the risk reductions for 1.8 to 3.6, 3.6 to 5.4, 5.4 to 7.2, and ≥ 7.2 METh/d were: 1) 10.1%, 17.7%, 25.1% and 34.9% from running and 14.0%, 23.8%, 21.8% and 38.3% from walking for hypercholesterolemia; 2) 19.7%, 19.4%, 26.8% and 39.8% from running and 14.7%, 19.1%, 23.6% and 13.3% from walking for hypertension; 3) 43.5%, 44.1%, 47.7% and 68.2% from running and 34.1%, 44.2%, and 23.6% from walking for diabetes (too few cases for diabetes for walking >5.4 METh/d). The risk reductions were not significantly greater for running than walking for diabetes (P=0.94) or CHD (P=0.26), and only marginally greater for walking than running for hypertension (P=0.06) and hypercholesterolemia (P=0.04).
Conclusion
Equivalent energy expenditures by moderate (walking) and vigorous (running) exercise produced similar risk reductions for hypertension, hypercholesterolemia, diabetes, and CHD, but there is limited statistical power to evaluate CHD conclusively.
Adjustment for BMI attenuated more of the risk reduction for the runners’ than walkers’ hypertension, hypercholesterolemia, and diabetes,
Current physical activity guidelines postulate that different activities can be combined to achieve a minimum recommended dose, including activities of different intensities [1-7]. Activities that expend 3- to 6-fold the energy expenditure of sitting at rest (3 to 6 metabolic equivalents or METs, 1 MET=3.5 ml O2•kg-1•min-1) are defined as moderate, those that expend more as vigorous, and less as light intensity [1]. Walking is generally performed at moderate intensity [8] and is specifically recommended by the Centers for Disease Control [1], the American Heart Association [2], the American College of Sports Medicine [1], and others [6,7], but whether equivalent doses of moderate and vigorous physical activity yield the same long-term health benefits remains unresolved [9].
The current analyses examined whether equivalent energy expenditure by moderate and vigorous exercise produce similar reductions in coronary heart disease (CHD) risk factors. To this end, we examined the associations of incident hypertension, hypercholesterolemia (high cholesterol), and type 2 diabetes mellitis to reported exercise in two cohorts, the National Runners’ Health Study II and the National Walkers’ Health Study. Walking and running provide an ideal test of the health benefits of moderate-intensity versus vigorous-intensity exercise because they involve the same muscle groups. In addition, the National Runners’ and Walkers’ Health Studies assess running and walking energy expenditure from weekly distance run or walked, which appears to be a better metric than the traditional time-based measurements used by other studies [10-12].
Methods
The National Runners’ Health Study II and the National Walkers’ Health Study were started in 1998 and 1999, respectively, to examine the relationships between various amounts and intensity of physical activity in a large national cohort of 63,308 runners and 42,140 walkers. The original cohorts were partially resurveyed in 2006 to establish a population of approximately 50,000 runners and walkers for a proposed clinical trial, rather than a prospective follow-up study per se. These represented approximately a third of the original walker (33.2%), and one-half of the original runner surveyed (51.7%). The difference in recruitment rates was due to the greater effort made to recruit runners (two mailings) than walkers (one mailing). Compared to non-responders, those that responded were slightly more likely to be female, younger, slightly less educated, weighed slightly more, were less likely to report taking medications for blood pressure, hypertension, or diabetes at baseline, but reported approximately the same number of km/day run if a runner or walked if a walker as reported on their baseline questionnaire [35].
Participants completed baseline and follow-up questionnaires on height, current and past weight, diet, current and past cigarette use, and history of diseases. Intakes of meat and fruit were based on the questions “During an average week, how many servings of beef, lamb, or pork do you eat”, and “...pieces of fruit do you eat”. Alcohol intake was estimated from the corresponding questions for 4-oz (112 mL) glasses of wine, 12-oz (336 mL) bottles of beer, and mixed drinks and liqueurs. Alcohol was computed as 10.8 g/4-oz glass of wine, 13.2 g/12-oz bottle of beer, and 15.1 g/mixed drink. Education was solicited by requesting the participant provide “years of education (examples: HS=12; BS or BA = 16; MS or MA = 18; PhD or MD = 20).” Height and weight were determined by asking the participant, “What is your current height (in inches, without shoes)?” and, “What is your current weight (pre-pregnancy weight if pregnant)?” BMI was calculated as weight in kilograms divided by the square of height in meters. Elsewhere, we have reported the strong correlations between self-reported and clinically measured heights (r=0.96) and weights (r=0.96) [11]. The study protocol was reviewed by the University of California Berkeley committee for the protection of human subjects, and all subjects provided a signed statement of informed consent.
Walking and running were reported in miles per week. In addition, the questionnaires asked how many hours per week on average did respondents spend running, walking, swimming, cycling, and doing other exercises which they described in detail. They were also asked for their usual pace (minutes per mile) during walking and running. Time based calculations of METhr/d of vigorous, moderate, and light exercise were summed as the product of average daily hours spent on each activity and the activity's estimated energy expenditure [8]. The distance-based calculation of METhr/d walked converted distance into duration (i.e., distance/mph) and calculated the product of the average hours walked per day and the MET value for the reported pace. Running MET values were calculated as 1.02 MET•h per km [11]. Time-based calculation of METhr/d run was computed by converting the hours run into distance (i.e., hours*kmph).
New onset or ”incident” hypertension, hypercholesterolemia, diabetes, and CHD (myocardial infarction, coronary artery bypass graphs (CABG), percutaneous coronary intervention, and angina pectoris) were defined as physician diagnosis or starting medications for these conditions since the baseline questionnaire. Self-reported hypertension and hypercholesterolemia have been demonstrated as consistent by repeated surveys and reliable as confirmed by medical records [13] and have been used by the Nurses’ Health Study [14] and other major cohort studies [15].
Statistical analyses were performed using JMP (SAS institute, Cary NC, version 5.1) and Stata (StataCorp LP, College Station TX, version 11). Cox proportional hazard analyses were used to estimate the hazard rate per METhr/d of running, walking, and other vigorous, moderate, and light intensity exercise.
Results
There were 15,945 walkers (21.0% males), and 33,060 runners (51.4% males) eligible for analysis (Table 1). Baseline hypertension, hypercholesterolemia and diabetes excluded 3,271 walkers and 1,841 runners, 2,638 walkers and 2,148 runners, 716 walkers and 249 runners from the analyses of incident hypertension, hypercholesterolemia and diabetes, respectively. Energy expended by running in the runners was more than twice that reported for walking by walkers. The majority of the other exercise reported by runners and walkers was vigorous.
Table 1.
Sample characteristics
| Male | Female | |||
|---|---|---|---|---|
| Runners | Walkers | Runners | Walkers | |
| Sample (N) | 16,983 | 3,349 | 16,077 | 12,596 |
| Age (years) | 48.28±10.98 | 61.77±11.10 | 40.89±10.66 | 53.08±12.05 |
| Follow-up (years) | 6.30±0.91 | 5.60±1.17 | 6.55±0.94 | 5.69±1.26 |
| Education (years) | 16.79±2.46 | 16.31±2.72 | 16.35±2.31 | 15.27±2.54 |
| Current smokers (%) | 1.22 | 3.40 | 1.69 | 3.68 |
| Meat (servings/d) | 0.44±0.40 | 0.46±0.41 | 0.27±0.30 | 0.37±0.34 |
| Fruit (pieces/d) | 1.53±1.18 | 1.62±1.22 | 1.53±1.06 | 1.70±1.14 |
| Alcohol (g/d) | 9.85±13.47 | 9.16±13.40 | 5.88±8.21 | 4.93±9.09 |
| BMI (kg/m2) | 24.09±2.59 | 26.63±4.05 | 21.62±2.51 | 25.48±5.18 |
| Energy expenditure (METhr/d) | ||||
| Running | 5.29±3.12 | 4.74±3.03 | ||
| Walking | 2.20±1.66 | 2.14±1.63 | ||
| Other vigorous exercise | 1.70±3.21 | 1.69±3.34 | 2.06±3.34 | 1.46±2.95 |
| Other exercise, moderate | 0.76±1.63 | 0.43±1.49 | 0.83±1.73 | 0.36±1.26 |
| Other exercise, light | 0.02±0.30 | 0.04±0.59 | 0.03±0.36 | 0.03±0.25 |
| Other exercise, strength | 0.53±1.26 | 0.20±0.86 | 0.54±1.26 | 0.20±0.75 |
Runners vs. walkers
The runners had 38% lower risk for incident hypertension, 36% lower risk for hypercholesterolemia, and 71% lower risk for diabetes mellitis than walkers (Table 2). These differences were independent of the reported exercise energy expenditure, but were substantially reduced by adjustment for BMI, i.e., to 14%, 18%, and 41% lower risk for hypertension, hypercholesterolemia, and diabetes, respectively (Table 3).
Table 2.
Hazard ratios (95% confidence intervals) from Cox proportional hazard analyses of self-reported incident hypertension, hypercholesterolemia, diabetes and CHD.
| Hypertension | Hypercholesterolemia | Diabetes | CHD | |
|---|---|---|---|---|
| Sample size (N) | 43,341 | 44,216 | 48,116 | 47921 |
| Incident events | 3874 | 6637 | 647 | 530 |
| Runners (0,1) | 0.623§ (0.552, 0.704) | 0.640¶ (0.583, 0.702) | 0.294§ (0.214, 0.405) | 0.478§ (0.342, 0.666) |
| Energy expenditure at baseline (per METhr/d) | ||||
| Running | 0.958§ (0.944, 0.973) | 0.957¶ (0.946, 0.968) | 0.879§ (0.832, 0.929) | 0.955* (0.912, 1.000) |
| Walking | 0.928§ (0.899, 0.957) | 0.930§ (0.908, 0.953) | 0.877§ (0.824, 0.934) | 0.907† (0.839, 0.981) |
| Other vigorous | 0.983† (0.972, 0.994) | 0.986‡ (0.978, 0.994) | 0.980 (0.950, 1.007) | 0.994 (0.966, 1.024) |
| Other moderate | 0.997 (0.976, 1.018) | 0.998 (0.982, 1.014) | 0.969 (0.908, 1.024) | 0.984 (0.927, 1.044) |
| Other light | 0.886 (0.739, 1.006) | 1.011 (0.955, 1.061) | 0.992 (0.736, 1.121) | 0.983 (0.807, 1.197) |
Analyses of runners and walkers combined adjusted for baseline age (age, age2), sex, and race (self identified African-American, Hispanic, Asian, Native American), education, smoking, and intakes of red meat, fruit, and alcohol. Analyses of hypertension, hypercholesterolemia, and diabetes also included adjustment for preexisting CHD at baseline. Significance levels for individual coefficients are coded:
P<0.05
P<0.01
P<0.001
P<0.0001
P<10-14.
Table 3.
Survival analyses (Cox proportional hazard) of self-reported incident hypertension, hypercholesterolemia, diabetes, and CHD, adjusted for BMI.
| Hypertension | Hypercholesterolemia | Diabetes | CHD | |
|---|---|---|---|---|
| Sample size (N) | 42,853 | 43,683 | 47,584 | 47339 |
| Incident events | 3811 | 6520 | 629 | 509 |
| BMI (kg/m2) | 1.087¶ (1.079, 1.095) | 1.061¶ (1.055, 1.067) | 1.138¶ (1.125, 1.150) | 1.070§ (1.048, 1.093) |
| Runners (0,1) | 0.862* (0.759, 0.979) | 0.819§ (0.743, 0.903) | 0.587† (0.420, 0.821) | 0.569† (0.401, 0.808) |
| Energy expenditure at baseline (per METhr/d) | ||||
| Running | 0.977† (0.962, 0.992) | 0.968§ (0.957, 0.979) | 0.912‡ (0.861, 0.963) | 0.978 (0.934, 1.025) |
| Walking | 0.987 (0.957, 1.018) | 0.976* (0.952, 1.000) | 1.013 (0.950, 1.078) | 0.946 (0.873, 1.025) |
| Other vigorous | 0.988* (0.977, 0.999) | 0.990* (0.982, 0.998) | 0.995 (0.965, 1.022) | 0.997 (0.968, 1.027) |
| Other moderate | 0.995 (0.974, 1.016) | 0.996 (0.980, 1.013) | 0.965 (0.904, 1.020) | 0.983 (0.925, 1.044) |
| Other light | 0.920 (0.776, 1.034) | 1.026 (0.973, 1.075) | 1.040 (0.801, 1.158) | 0.998 (0.828, 1.204) |
Covariates for adjustment included baseline age (age, age2), sex, and race (self identified African-American, Hispanic, Asian, Native American), education, smoking, and intakes of red meat, fruit, and alcohol. Analyses of hypertension, hypercholesterolemia, and diabetes also included adjustment for preexisting CHD at baseline. Significance levels for individual coefficients are coded
P<0.05
P<0.01
P<0.001
P<0.0001
P<10-15.
Energy expended by running vs. walking
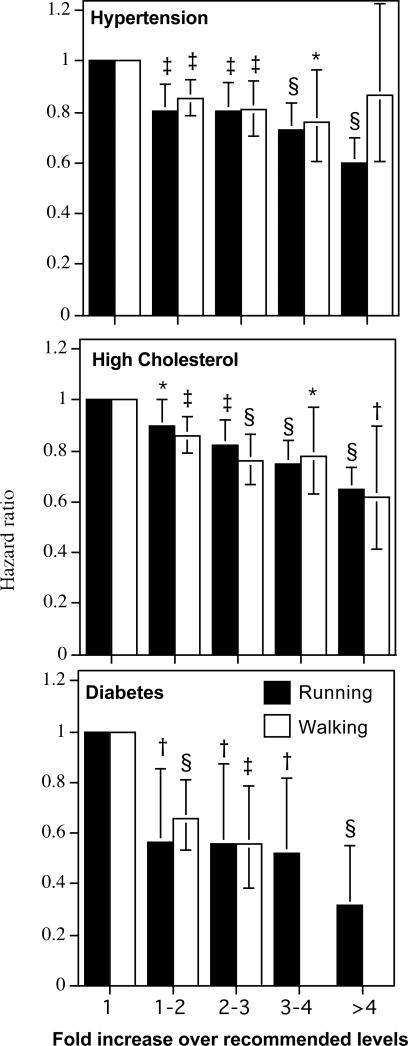
Equivalent energy spent running and walking was associated with comparable risk reductions for hypertension, hypercholesterolemia, and diabetes mellitis (Figure 1). Moreover, there were incremental reductions in risk at 2, 3, and 4-times the dose of exercise recommended by the American Heart Association and the American College of Sports Medicine [2]. Table 2 shows that greater METhr/d run or walked was associated with significantly lower risks, respectively, for incident hypertension (P<10-7 and P<10-6), hypercholesterolemia (P<10-14 and P<10-8), and diabetes (P<10-5 and P<10-4). The risk reductions per METhr/d were not significantly greater for running than walking for hypertension (running vs. walking, P=0.06), hypercholesterolemia (P=0.04, significantly greater for walking not running), or diabetes mellitis (P=0.94). The equivalent benefits per METhr/d run and METhr/d walked persisted even after adjustment for BMI for hypertension (running vs. walking: P=0.54) and hypercholesterolemia (P=0.56), but not for diabetes (running > walking, P=0.01, Table 3).
Figure 1.
Reduction in the risks for hypertension, hypercholesterolemia, and diabetes vs. baseline METhr/d energy expended by walking or running. Energy expenditure (X-axis) is categorized in terms of the upper limit of the minimum recommended physical activity levels (750 METmin/wk=1.8 METhr/d [2]), e.g., 1 to 2-fold higher activity covers from 1.8 to 3.6 METhr/d, etc. The average energy expended by runners and walkers within each interval were 314 and 371 METmin/wk for <1-fold of the recommended levels (<1.8 METhr/d), respectively, 1208 and 1108 METmin/wk for 1 to 2-fold (1.8 to 3.6 METhr/d), respectively, 1927 and 1845 METmin/wk for 2- to 3-fold (3.6 to 5.4 METhr/d), respectively, 2684 and 2587 METmin/wk for 3- to 4-fold (5.4 to 7.2 METhr/d), respectively, and 4197 and 3436 METmin/wk for ≥4-fold (≥7.2 METhr/d). Analyses performed separately in runners and walkers, adjusted for age, sex, race, smoking, prior CHD, and intakes of red meat, fruit, and alcohol. Incident diabetes in walkers excluded for 3- to 4-fold and ≥4-fold due to the small number of cases. Error bars represent 95% confidence intervals. Significant levels relative to the least active runners and walkers coded: * P<0.05; † P<0.01, ‡ P<0.001, and § P<0.0001.
Other exercise
Higher levels of nonrunning vigorous exercises were also associated with lower risks of hypertension (P=0.003) and hypercholesterolemia (P=0.0008), but not diabetes (P=0.16). The METhr/d reductions in risk were significantly less for nonrunning vigorous exercise than for running for hypertension: (running - other vigorous exercise: P=0.006), hypercholesterolemia (P<10-4) and diabetes (P=0.0006). Other moderate exercise was not significantly related to hypertension (P=0.72), hypercholesterolemia (P=0.79), or diabetes mellitis (P=0.72), and its risk reduction was significantly less than that of walking (walking-other moderate exercise, hypertension: P=0.0002; hypercholesterolemia: P<10-5; and diabetes: P=0.03).
Whereas METhr/d for walking and running were calculated from distance and intensity, METhr/d for other exercises were calculated from time (duration) and intensity. In part, the weak effects of other exercise may be due to its method of estimation rather than the activities themselves. To show that time-based energy estimation underestimates the association of exercise with incident hypertension, hypercholesterolemia, and diabetes, the analyses of Table 2 were repeated for METhr/d run as calculated from reported time and intensity (not displayed), rather than distance (Table 2). This shows that the reductions in risk per METhr/d run were much less for the time-based than the distance-based calculations (52% less for hypertension, 29% less for hypercholesterolemia, and 63% less for diabetes mellitis, analyses not displayed). When the time-based METhr/d run and distance-based METhr/d run were included together in the same survival model so that their coefficient could be compared directly, the distance based estimates remained significant (hazard ratio, hypertension: HR=0.961, P=0.0001; hypercholesterolemia: HR=0.963, P=10-6; and diabetes: HR=0.876, P=0.0002), whereas the time-based estimates were not (hypertension: HR=0.997, P=0.68; hypercholesterolemia: HR=0.994, P=0.25; and diabetes: HR=1.003, P=0.88), and in every case the risk reduction for the distance-based estimate was significantly greater than that of the time-based estimate (hypertension: P=0.01; hypercholesterolemia: P=0.007; and diabetes: P=0.008). Thus, time-based estimates of exercise energy expenditure appear to substantially underestimate the reductions in hypertension, hypercholesterolemia, and diabetes risk.
Strengthening exercise
When METhr/d of strengthening and non-strengthening exercises replaced other exercise in the analyses of Table 2, the effects of strengthening exercises and nonstrengthening exercise did not differ significantly from each other for incident hypertension (P=0.08), hypercholesterolemia (P=0.21), or diabetes (P=0.13). Specifically, the per METh/d effect of strengthening exercise was modestly significant for hypercholesterolemia (HR=0.973, 95%CI: 0.949 to 0.998, P=0.03) and diabetes (HR=0.902, 95%CI: 0.802 to 0.999, P=0.05), but not hypertension (HR=1.011, 95%CI: 0.982 to 1.040, P=0.49). Non-strengthening other exercise was significantly associated with hypertension (HR=0.983, 95%CI: 0.973 to 0.993, P=0.0007) and hypercholesterolemia risk (HR=0.990, 95%CI: 0.983 to 0.998, P=0.01), but not diabetes risk (HR=0.984, 95%CI: 0.957 to 1.009, P=0.21).
Running and walking intensity
Within both walkers and runners, faster pace (per m/s) was associated with lower risks of hypertension (runners: HR=0.609, 95%CI: 0.553 to 0.671, P<10-15; walkers: HR=0.758, 95%CI: 0.639 to 0.899, P=0.002), hypercholesterolemia (runners: HR= 0.667, 95%CI: 0.619 to 0.720, P<10-15; walkers: HR=0.823, 95%CI: 0.720 to 0.942, P=0.005), and diabetes (runners: HR= 0.433, 95%CI: 0.334 to 0.574, P<10-7; walkers: HR=0.427, 95%CI: 0.331 to 0.573, P<10-9), which were, for the most part, independent of exercise dose, but largely accounted for by BMI. There were no significant interactions between energy expended (METhr/d) and intensity (m/s) to suggest that the same energy expended at a faster pace produced a greater reduction in the risk of hypertension (significance of interaction, runners: P=0.13; walkers: P=0.33), hypercholesterolemia (runners: P=0.24; walkers: P=0.51) or diabetes (runners: P=0.98; walkers: P=0.71).
Coronary heart disease
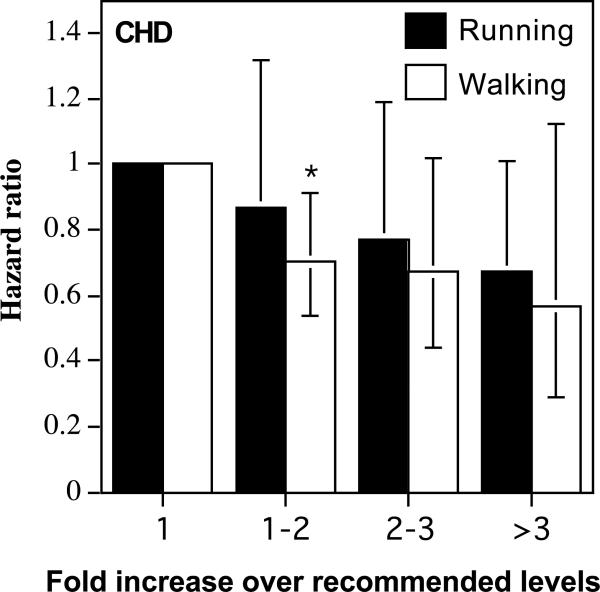
The limited number of incident cases (530) provides limited statistical power for testing whether running and walking were associated with equivalent reductions in CHD risk. Nevertheless, the results were at least consistent with their equivalent effects per METhr/d. There were 706 walkers (442 males, 264 females) and 370 runners (337 males, 33 females) excluded for pre-existing CHD, leaving 189 de novo myocardial infarctions (102 walkers, 87 runners), 122 CABGs (68 walkers, 54 runners), 185 angioplasties (93 walkers, 92 runners), and 34 angina cases (19 walkers, 15 runners). The runners, as a group, had 52% lower CHD risk than the walkers (P<10-5, Table 2), which was diminished somewhat by adjustment for BMI (P=0.002, Table 3). Table 2 shows that both METhr/d run and METhr/d walked were associated with significantly lower CHD risk (P=0.05 and P=0.01, respectively), which did not differ from each other (P=0.26). The hazard ratios of Figure 2 are consistent with equivalent CHD risk reductions for walking and running.
Figure 2.
Reduction in CHD risks per METhr/d energy expended by walking or running. Error bars represent 95% confidence intervals. Significant levels relative to the least active runners and walkers coded: * P<0.05; † P<0.01, ‡ P<0.001, and § P<0.0001.
Adjustment for recruitment
Different recruitment rates between the runners (51.7%) and walkers (33.2%) did not affect the analyses. Repeating the analyses using only the first 33.2% of the runners recruited (to match the 33.2% recruitment rate in the walkers) produced results entirely consistent with the complete sample, namely: 1) there were significant declines per METh/d run in risk for hypertension (4.2% lower per METh/d run, 95%CI: 2.4% to 6.0% lower, P<10-5), hypercholesterolemia (3.8% lower per METh/d run, 95%CI: 2.5% to 5.2% lower, P<10-7), and diabetes (11.4% lower per METh/d run, 95%CI: 4.4% to 16.1% lower, P=0.001) whose differences from those of the walkers differed little from the complete sample (P=0.08, P=0.02, and P=0.60, respectively), 2) adjustment for BMI did not eliminate the decline in risk for hypertension (2.4% lower per METh/d run, 95%CI: 0.6% to 4.3% lower, P=0.01), hypercholesterolemia (2.8% lower per METh/d run, 95%CI: 1.4% to 4.1% lower, P=0.0001), and diabetes (6.9% lower per METh/d run, 95%CI: 0.6% to 12.8% lower, P=0.03), and 3) declines in CHD risk that were consistent with the complete sample (HR: 0.057, 95% CI: 0.906 to 1.008 per METh/d run, P=0.10).
Discussion
These results from these very large, prospective, cohorts suggest that equivalent doses of running (a vigorous exercise) and walking (a moderate exercise) are associated with equivalent reductions in the risks for new onset hypertension, hypercholesterolemia, and diabetes. These results also show continued reduction in risk for new onset hypertension, hypercholesterolemia and diabetes when the exercise-dose exceeds 450 to 750 MET minutes per week (1.1 to 1.8 METh/d), the amount of exertion currently recommended by the American Heart Association and the American College of Sports Medicine for health (Figure 1). Furthermore, it does not appear to matter whether these exercise doses are achieved by running or by walking. The equivalence of walking, the most commonly performed exercise, [16] and running has not to our knowledge been previously demonstrated prospectively in a large sample, nor has the dose-response relationship between walking and these endpoints been assessed prospectively over such a broad activity range. The additional health benefits of exceeding currently recommended exercise levels are consistent with cross-sectional data in runners and walkers [17,18]. The runners’ results showing increased benefit with increased running energy expenditure also provide confirmation in a new independent sample of a progressively beneficial dose-response relationship for this activity [19].
Activity in the present study was self-selected both with respect to the intensity, running vs walking, and the total exercise dose. The average exercise dose measured as estimated caloric expenditure was more than twice as great for those who chose running over those who chose walking. Specifically, there were substantially more walkers whose walking was at or below the guideline levels than runners whose running was at or below the guidelines (48.1% vs. 12.2%), and substantially fewer walkers than runners whose walking or running exceeded the guideline levels by 2-fold (15.4% vs. 61.1%), three fold (4.5% vs. 40.1%), and four fold (1.1% vs. 17.9%). This is likely due to the fact that runners can expend more calories in a given period of time. Our results suggest that this caloric expenditure is the key issue to reducing CHD risk factors and possibly CHD events.
Clinical trials are required to settle the role of exercise intensity on CHD risk, but clinical trials are necessarily restricted by sample size and duration. Available clinical trials on the influence of exercise intensity on new onset blood pressure, cholesterol, and blood glucose control or insulin sensitivity have yielded mixed results. Both moderate and vigorous-intensity training improve blood pressure with approximately equal effects [20], albeit greater benefits have been ascribed to both moderate [21] and vigorous intensity [9]. The ability of exercise to lower total and low-density lipoprotein cholesterol is not widely accepted [20,22,23] irrespective of intensity and some maintain that any reduction in LDL is due to plasma volume expansion [24]. Our results suggest that exercise does affect LDL levels and that this effect increases with increasing exercise doses, consistent with the suggestion that LDL concentrations are more responsive to the exercise quantity than intensity [25]. Both moderate and vigorous exercise have been associated with lower risk of type 2 diabetes [20]; however, studies of blood glucose control tend to achieve significant improvement for vigorous more than moderate physical activity [9]. The benefits of walking, in particular, in lowering type 2 diabetes risk are well-documented [26]. Prospective epidemiologic studies tend to show a greater CHD-risk reduction for vigorous than moderate intensity exercise [20]; however, vigorous physical activity is more accurately reported than moderate-intensity exercise [27], which could contribute to its stronger relationship to CHD, hypertension, hypercholesterolemia, and diabetes when studied prospectively in epidemiologic cohorts [20]. This may be less of an issue for the analyses presented here, which compares two specific activities, running and walking, quantified by distance rather than duration. The superiority of vigorous over moderate exercise in some studies may simply reflect the fact that more calories can be expended per minute of activity with vigorous exercise. Consequently, when exercise is compared by time spent in activity, vigorous exercise appears more beneficial.
This last point we believe to be of particular significance. In this paper we have shown that the effects of exercise on incident hypertension, hypercholesterolemia, and diabetes are at least two-fold greater when exercise energy expenditure calculated from distance than when exercise is measured by time. Similar results have been shown for using distance to assess energy expenditure cross-sectionally for body weight, hypertension, hypercholesterolemia, and diabetes [10-12] Presumably, deficiencies in time-based estimates of exercise energy expenditure apply to nonrunning and nonwalking activities as well, which may contribute to the significant differences between running and other vigorous exercise, and walking vs. other moderate exercise (Table 2).
The superiority of the distance-based vs. time-based estimation of exercise energy expenditure has other important implications. If runners and walkers substantially overestimate exercise duration for a sustained activity, it is reasonable to assume even greater bias for unsustained activities by more-sedentary populations. Most epidemiological studies estimate exercise dose by time and intensity [20], which our analyses would suggest substantially underestimates the true health benefits of physical activity. Moreover, all public health recommendations prescribe physical activity by duration [1-7,20], and if people overestimate exercise by time, then implementing time-based recommendations may be problematic.
Caveats
The subsample included in this report is a sample of convenience, since it was recruited to obtain approximately 50,000 subjects to determine their interest in a possible internet-based intervention, and therefore represent only a portion of the original National Runners’ Health Study II and the National Walkers’ Health Study participants. It is unlikely, however, that the biological interaction of between exercise and hypertension, hypercholesterolemia, and diabetes is different between the current and less selected populations. We cannot exclude the possibility that subjects who exercise have lower innate risks for hypertension, hypercholesterolemia, diabetes, or CHD. We have shown that men with higher high-density lipoprotein cholesterol at baseline (a CHD protective factor [22]) run longer distances when randomized to exercise training [28,29], and others have shown that selective breeding for fitness in rats produces substantial inherited differences in CHD risk factors even in the absence of training [30]. Diet and other variables that could have affected our results were not collected. We doubt the possibility that lower rates of new onset hypertension, hypercholesterolemia, and diabetes with greater exercise levels was due to less medical care contact in the more active men because more vigorously active participants in the Health Professional Study had more frequent medical check-ups than less active men [31] and there was no difference in the frequency of routine medical check-ups by activity level in the Nurses Health Study [32].
Our results probably provide among the best available answers to the important public health question as to what intensity of exercise is required to reduce CHD risk. Our results suggest similar benefit for similar energy expenditures. These results should be used to encourage physical activity in general regardless of its intensity. However, those who choose running achieved over twice the exercise doses as those that choose walking, and given the strong dose-response relationship higher exercise doses and lower risk factors, promoting more vigorous exercise is likely to produce greater health benefits.
Acknowledgements
Dr. Williams was responsible for the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript. Dr. Thompson was responsible for preparation, review, and approval of the manuscript. Dr. Williams had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
This research was supported by grant HL094717 from the National Heart, Lung, and Blood Institute and was conducted at the Ernest Orlando Lawrence Berkeley National Laboratory (Department of Energy DE-AC03-76SF00098 to the University of California).
Footnotes
Disclosures:
There are no financial conflicts of interest to report.
References
- 1.Pate RR, Pratt M, Blair SN, Haskell WL, Macera CA, Bouchard C, Buchner D, Ettinger W, Heath GW, King AC, Kriska A, Leon AS, Marcus BH, Morris J, Paffenbarger RS, Patrick K, Pollock ML, Rippe JM, Sallis J, Wilmore JH. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273:402–7. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 2.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A, American College of Sports Medicine. American Heart Association Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1423–34. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 3.Institute of Medicine . Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients) The National Academies Press; Washington DC.: 2005. pp. 880–935. (ISBN: 0309085373) [Google Scholar]
- 4.U.S. Department of Agriculture and U.S. Department of Health and Human Services . Dietary Guidelines for Americans, 2010. 7th Edition. U.S. Government Printing Office; Washington, DC: Dec, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Donovan G, Blazevich AJ, Boreham C, Cooper AR, Crank H, Ekelund U, Fox KR, Gately P, Giles-Corti B, Gill JM, Hamer M, McDermott I, Murphy M, Mutrie N, Reilly JJ, Saxton JM, Stamatakis E. The ABC of Physical Activity for Health: a consensus statement from the British Association of Sport and Exercise Sciences. J Sports Sci. 2010;28:573–91. doi: 10.1080/02640411003671212. [DOI] [PubMed] [Google Scholar]
- 6.US Department of Health and Human Services . 2008 Physical Activity Guidelines for Americans [Internet] Washington (DC): 2008. [2010 Oct 10]. p. 61. ODPHP Publication No. U0036 Available from: http://www.health.gov/paguidelines/pdf/paguide.pdf. [Google Scholar]
- 7.World Health Organization [Jan 19, 2012];Global Strategy on Diet, Physical Activity, and Health. 2006 http://www.who.int/dietphysicalactivity/en/index.html.
- 8.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O'Brien WL, Bassett DR, Jr, Schmitz KH, Emplaincourt PO, Jacobs DR, Jr, Leon AS. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 9.Swain DP, Franklin BA. Comparison of cardioprotective benefits of vigorous versus moderate intensity aerobic exercise. Am J Cardiol. 2006;97:141–7. doi: 10.1016/j.amjcard.2005.07.130. [DOI] [PubMed] [Google Scholar]
- 10.Williams PT. Advantage of distance- versus time-based estimates of walking in predicting adiposity. Med Sci Sports Exerc. 2012 Apr 19; doi: 10.1249/MSS.0b013e318258af3f. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams PT. Non-exchangeability of running vs. other exercise in their association with adiposity, and its implications for public health recommendations. PLOSOne. 2012;7:e36360. doi: 10.1371/journal.pone.0036360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams PT. Effects of running and walking with osteoarthritis and hip replacement risk. Med Sci Sports Exerc. 2013;45:1292–7. doi: 10.1249/MSS.0b013e3182885f26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colditz G, Martin AP, Stampfer MJ, Willett WC, Sampson L, Rosner B, Hennekens CH, Speizer FE. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol. 1986;123:894–900. doi: 10.1093/oxfordjournals.aje.a114319. [DOI] [PubMed] [Google Scholar]
- 14.Huang Z, Willett WC, Manson JE, Rosner B, Stampfer MJ, Speizer FE, Colditz GA. Body weight, weight change, and risk for hypertension in women. Ann Intern Med. 1998;128:81–8. doi: 10.7326/0003-4819-128-2-199801150-00001. 15. [DOI] [PubMed] [Google Scholar]
- 15.Paffenbarger RS, Jr, Wing AL, Hyde RT, Jung DL. Physical activity and incidence of hypertension in college alumni. Am J Epidemiol. 1983;117:245–57. doi: 10.1093/oxfordjournals.aje.a113537. [DOI] [PubMed] [Google Scholar]
- 16.Tudor-Locke C, Johnson WD, Katzmarzyk PT. Frequently reported activities by intensity for U.S. adults: the American Time Use Survey. Am J Prev Med. 2010;39:e13–20. doi: 10.1016/j.amepre.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 17.Williams PT. Reduced diabetic, hypertensive, and cholesterol medication use with walking. Med Sci Sports Exerc. 2008;40:433–43. doi: 10.1249/MSS.0b013e31815f38f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams PT, Franklin B. Vigorous exercise and diabetic, hypertensive, and hypercholesterolemia medication use. Med Sci Sports Exerc. 2007;39:1933–41. doi: 10.1249/mss.0b013e318145b337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams PT. Vigorous exercise, fitness and incident hypertension, high cholesterol, and diabetes. Med Sci Sports Exerc. 2008;40:998–1006. doi: 10.1249/MSS.0b013e31816722a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Physical Activity Guidelines Advisory Committee . Physical Activity Guidelines Advisory Committee Report, 2008. U.S. Department of Health and Human Services; Washington, DC: 2008. [DOI] [PubMed] [Google Scholar]
- 21.Kelley GA, Sharpe KK. Aerobic exercise and resting blood pressure in older adults: a meta-analytic review of randomized controlled trials. J.Gerontol.A Biol.Sci.Med.Sci. 2001;56:M298–M303. doi: 10.1093/gerona/56.5.m298. [DOI] [PubMed] [Google Scholar]
- 22.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 23.Durstine JL, Grandjean PW, Davis PG, Ferguson MA, Alderson NL, DuBose KD. Blood lipid and lipoprotein adaptations to exercise: A quantitative analysis. Sports Medicine. 2001;31:1033–62. doi: 10.2165/00007256-200131150-00002. [DOI] [PubMed] [Google Scholar]
- 24.Cullinane EM, Sady SP, Vadeboncoeur L, Burke M, Thompson PD. Cardiac size and VO2max do not decrease after short-term exercise cessation. Med Sci Sports Exerc. 1986;18:420–4. [PubMed] [Google Scholar]
- 25.Kraus WE, Houmard JA, Duscha BD, Knetzger KJ, Wharton MB, McCartney JS, Bales CW, Henes S, Samsa GP, Otvos JD, Kulkarni KR, Slentz CA. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002;347:1483–92. doi: 10.1056/NEJMoa020194. [DOI] [PubMed] [Google Scholar]
- 26.Jeon CY, Lokken RP, Hu FB, van Dam RM. Physical activity of moderate intensity and risk of type 2 diabetes: a systematic review. Diabetes Care. 2007;30:744–52. doi: 10.2337/dc06-1842. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs DR, Jr, Ainsworth BE, Hartman TJ, Leon AS. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Med Sci Sports Exerc. 1993;25:81–91. doi: 10.1249/00005768-199301000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Williams PT, Wood PD, Haskell WL, Vranizan K. The effects of running mileage and duration on plasma lipoprotein levels. JAMA. 1982;24:2674–9. [PubMed] [Google Scholar]
- 29.Williams PT, Stefanick ML, Vranizan KM, Wood PD. The effects of weight loss by exercise or by dieting on high-density lipoprotein (HDL) levels in men with low, intermediate, and normal-to-high HDL at baseline. Metabolism. 1994;43:917–24. doi: 10.1016/0026-0495(94)90277-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wisløff U, Najjar SM, Ellingsen O, Haram PM, Swoap S, Al-Share Q, Fernström M, Rezaei K, Lee SJ, Koch LG, Britton SL. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science. 2005;307:418–20. doi: 10.1126/science.1108177. [DOI] [PubMed] [Google Scholar]
- 31.Leitzmann MF, Giovannucci EL, Rimm EB, Stampfer MJ, Spiegelman D, Wing AL, Willett WC. The relation of physical activity to risk for symptomatic gallstone disease in men. Ann Intern Med. 1998;128:417–25. doi: 10.7326/0003-4819-128-6-199803150-00001. [DOI] [PubMed] [Google Scholar]
- 32.Leitzmann MF, Rimm EB, Willett WC, Spiegelman D, Grodstein F, Stampfer MJ, Colditz GA, Giovannucci E. Recreational physical activity and the risk of cholecystectomy in women. N Engl J Med. 1999;341:777–84. doi: 10.1056/NEJM199909093411101. [DOI] [PubMed] [Google Scholar]