Abstract
Purpose
To retrospectively assess the local control and intermediate- and long-term survival of patients with liver metastases from breast cancer who have undergone percutaneous ultrasonography (US)-guided radiofrequency (RF) ablation.
Materials and Methods
This study was approved by the hospital ethics committee, and all patients provided written informed consent. RF ablation was used to treat 87 breast cancer liver metastases (mean diameter, 2.5 cm) in 52 female patients (median age, 55 years). Inclusion criteria were as follows: fewer than five tumors, maximum tumor diameter of 5 cm or smaller, and disease either confined to the liver or stable with medical therapy. Forty-five (90%) of 50 patients had previously undergone chemotherapy, hormonal therapy, or both, and had no response or an incomplete response to the treatment. Contrast material–enhanced computed tomography and US were performed to evaluate complications and technical success and to assess for local tumor progression during follow-up. The Kaplan-Meier method was used to assess survival, and results were compared between groups with a log-rank test. Cox regression analysis was used to assess independent prognostic factors that affected survival.
Results
Complete tumor necrosis was achieved in 97% of tumors. Two (4%) minor complications occurred. Median time to follow-up from diagnosis of liver metastasis and from RF ablation was 37.2 and 19.1 months, respectively. Local tumor progression occurred in 25% of patients. New intrahepatic metastases developed in 53% of patients. From the time of first RF ablation, overall median survival time and 5-year survival rate were 29.9 months and 27%, respectively. From the time the first liver metastasis was diagnosed, overall median survival time was 42 months, and the 5-year survival rate was 32%. Patients with tumors 2.5 cm in diameter or larger had a worse prognosis (hazard ratio, 2.1) than did patients with tumors smaller than 2.5 cm in diameter.
Conclusion
Survival rates in selected patients with breast cancer liver metastases treated with RF ablation are comparable to those reported in the literature that were achieved with surgery or laser ablation.
© RSNA, 2009
Introduction
Breast cancer is the second leading cause of cancer death in women in the Western world; in fact, 40 480 deaths from breast cancer were anticipated in the United States in 2008, and 131 900 deaths from this disease were anticipated in Europe in 2006 (1,2). While breast cancer can spread to virtually any part of the body, the most common metastatic locations are the bones, lungs, and liver. Importantly, the liver is involved in the majority of patients who develop breast cancer metastases, but only 5%–18% of patients have disease confined to the liver at the time of presentation (3–5). Metastatic breast cancer to the liver is considered a systemic disease, and it is associated with a dismal prognosis. If left untreated, the median survival time of patients with liver metastases from breast cancer is only 4–8 months (3,6). Even with the standard treatment of systemic chemotherapy, hormonal therapy, or both, the median survival time reported in the literature is only 5–31 months (7–11).
The role of surgery in the care of patients with liver metastases from colon cancer is well established (12–15), but hepatic resection is more controversial in patients with metastatic breast cancer than in patients with metastatic colorectal cancer. Early research revealed only modest improvements in the survival time with use of resection instead of medical therapy (16). However, more recent studies have shown significantly increased survival time in selected patients who underwent hepatic resection for metastases that were confined to the liver or who had stable extrahepatic disease (17–19). Importantly, the results of surgical series show that despite metastatic breast cancer being a systemic disease, local therapies have the potential to improve survival time.
Percutaneous ablative therapies are effective treatments for certain primary and metastatic liver cancers (20–27) and are associated with significantly lower cost, morbidity, and mortality than is hepatic resection (28,29). Radiofrequency (RF) ablation is the most widely used ablative therapy, and it is an established treatment option for colorectal cancer liver metastases. Given the increasing rationale for surgical resection of hepatic metastases, there has been a concomitant increase in interest in the use of ablative techniques to treat liver metastases from breast cancer. To our knowledge, no data are available regarding the long-term survival of patients with liver metastases from breast cancer who have undergone RF ablation. Therefore, the purpose of our study was to retrospectively assess the local control and intermediate- and long-term survival of patients with liver metastases from breast cancer who have undergone percutaneous ultrasonography (US)-guided RF ablation.
Materials and Methods
Patients and Tumors
Approval for this study was obtained from our institutional ethics committee (Vimercate Hospital, Vimercate, Milan, Italy), and written informed consent was obtained from each patient prior to the procedure. Between January 1996 and January 2008, 57 consecutive female patients with liver metastases from breast cancer were treated with US-guided percutaneous RF ablation. Nine of these patients were also included in a previously published study (30). Five patients were excluded from our study: Three had more than five metastases, and two had a tumor that was larger than 5 cm in diameter. Therefore, the study population consisted of 52 female patients (median age, 55 years ± 14 [standard deviation]; interquartile range [IQR], 45.5–69.5 years) with 87 breast cancer liver metastases. Forty-four patients had metachronous liver metastases, and eight patients had synchronous liver metastases. Forty-five (90%) of 50 patients had previously undergone chemotherapy, hormonal therapy, or both, with either no response or an incomplete response, as determined with Response Evaluation Criteria in Solid Tumors and contrast material–enhanced computed tomography (CT). Systemic therapy was continued after RF ablation, as considered appropriate by the oncologist. The presence of extrahepatic metastases was considered a contraindication to RF ablation, unless the extrahepatic disease had been stable for a minimum of 6 months after chemotherapy. Inclusion criteria for the survival study were identical to those reported by Mack et al (31): The patients had fewer than five tumors and a maximum tumor diameter of 5 cm or less. The patient and tumor characteristics are listed in Table 1. The mean number of liver tumors per patient was 1.7 ± 1.0 (range, 1–5 liver tumors per patient), and the mean tumor diameter was 2.5 cm ± 1.3 (range, 0.7–5.0 cm). The total number of metastases in each liver segment was as follows: segment II, four tumors; segment III, nine tumors; segment IV, nine tumors; segment V, 24 tumors; segment VI, 17 tumors; segment VII, 13 tumors; and segment VIII, 11 tumors.
Table 1.
Patient and Disease Characteristics
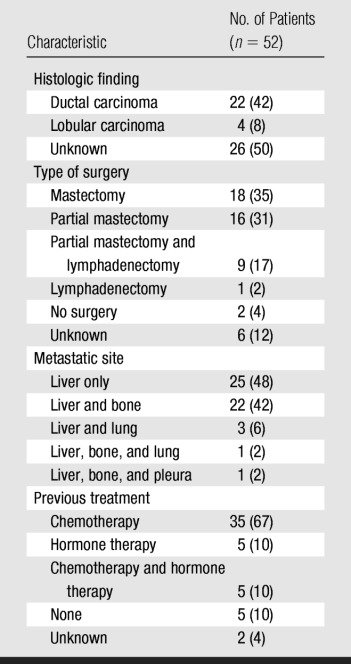
Note.—Data in parentheses are percentages.
Preablation Work-up
Preablation imaging work-up included an unenhanced and a biphasic contrast-enhanced CT scan of the abdomen (Somatom DRH, Siemens Medical Systems, Erlangen, Germany; PQ5000, Picker, Cleveland, Ohio; CT HiSpeed Advantage, GE Medical Systems, Milwaukee, Wis). CT scanning was performed during and immediately after injection of 2 mL per kilogram of body weight iopamidol (Iopamiro; Bracco, Milan, Italy) at a rate of 3 mL/sec. The liver was scanned 20 and 60 seconds (hepatic arterial and portal venous phases, respectively) after the initiation of contrast material injection. CT scanning was performed with 3-mm section thickness, 2.5-mm collimation, and 1:1 pitch. CT images were evaluated by two authors (M.F.M., T.L.) who had 2 and 14 years of experience reading abdominal CT images, respectively, at the beginning of the study and 15 and 27 years of experience, respectively, at the end.
Gray-scale and color Doppler US of the liver were also performed (Technos and AU5, Esaote, Genoa, Italy; H21 and Logos, Esaote-Hitachi, Genoa, Italy). From 2002 onward, contrast-enhanced US was also performed. This technique, which was not available before 2002, yields complementary information for planning and monitoring RF ablation without exposing the patient to ionizing radiation. In particular, it enables one to target lesions that are difficult to depict with conventional US (32–35). Contrast-enhanced US was performed with an Acuson-Sequoia 512 unit (Siemens Medical Systems) equipped with a 4-MHz convex probe and contrast-specific imaging software. A 2.4-mL bolus of a sulfur hexafluoride–filled microbubble contrast agent (Sonovue; Bracco) was injected into an antecubital vein via a 20-gauge cannula and followed by a 10-mL normal saline flush. Contrast-enhanced US was performed with a low mechanical index for up to 5 minutes after the administration of contrast material.
Additional work-up for patients with metastatic disease included contrast-enhanced CT of the chest, whole-body bone scintigraphy, and CT of the brain if focal neurologic deficits were detected. The diagnosis of liver metastases was established on the basis of the presence of characteristic imaging patterns on US or CT images obtained in a patient with a known history of breast cancer. Needle biopsy was performed to confirm the diagnosis in one patient with a questionable lesion on the margin of a previous resection of a breast cancer liver metastasis.
RF Ablation
All patients were admitted to the hospital the day before RF ablation, and they underwent chest radiography, electrocardiography, routine blood work (complete blood cell count and measurement of prothrombin time and electrolyte, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, bilirubin, hemoglobin, and fibrinogen levels), US (with administration of contrast material from 2002 onward), and preoperative evaluation prior to anesthesia.
On the morning of the procedure, patients were given the intravenous antibiotic ceftriaxone (1 gram per day, Rocefin; Roche, Basil, Switzerland). Additional doses were administered 24 and 48 hours after ablation.
The majority of patients (n = 47) underwent general anesthesia and mechanical ventilation, in accordance with the usual practice at our institution. Five treatment sessions in five patients were performed with conscious sedation and analgesia because we anticipated only one or two electrode insertions would be required (n = 3) or because of a contraindication to general anesthesia (n = 2). After successful induction of anesthesia, patients were cleansed with iodized alcohol. All RF ablations were performed with US guidance and monitoring with a 3.5-MHz transducer (CA B411; Hitachi, Tokyo, Japan). In three patients, contrast-enhanced US was used during the procedure to target three tumors that were not visible with unenhanced US. After injection of the contrast material, the tumor was seen in the portal phase, and the electrode was rapidly inserted. All procedures were performed by two authors (M.F.M., T.L.) with 2 years of experience in ablation of focal liver lesions when the study began and 14 years of experience when it ended. RF ablations were performed with four systems that have been described elsewhere: (a) A CC-1 RF generator (Radionics, Burlington, Mass) was used in three patients. (b) A Cool-tip RF system (Valleylab, Boulder, Colo) with a single or cluster electrode was used in 44 and three patients, respectively. (c) An RF 3000 system (Boston Scientific, Natick, Mass) was used in one patient. (d) A bipolar Celon Power System (Olympus, Southend-on-sea, England) was used in one patient. In all patients except the woman in whom RF ablation was performed with the bipolar system, two return pads were placed on the thighs to complete the circuit. Track cauterization was performed with the CC-1 and Cool-tip RF systems before individual electrodes were removed by disabling electrode cooling, allowing the tip temperatures to reach 80°C, and retracting the electrode at a rate that maintained this target temperature.
Patients remained in the hospital for at least 2 days after the ablation procedure. A complete blood cell count was performed, and blood glucose, electrolyte, creatinine, bilirubin, and liver transaminase levels were checked 24 and 48 hours after ablation. All patients underwent contrast-enhanced CT of the abdomen and contrast-enhanced US (from 2002 onward) the day after the procedure to evaluate for complications and to assess completeness of the ablation. We considered ablations complete and technical success achieved if the ablation zone (nonenhancing region on CT images 24 hours after ablation) completely covered the tumor and there was no irregular enhancement at the treatment margin. The protocols for postablation scanning were the same as those used for preablation scanning. A second RF ablation was scheduled if residual tumor was detected at imaging (n = 1).
Follow-up
Follow-up consisted of contrast-enhanced CT scanning and contrast-enhanced US (from 2002 onward) beginning 1 month after ablation and continuing every 3–4 months thereafter. Lack of focal enhancement within or at the periphery of a tumor at follow-up contrast-enhanced imaging was considered radiographic evidence of complete necrosis. All follow-up CT images were interpreted prospectively by at least one of two investigators (M.F.M., T.L.) who had also performed the preablation evaluation. Contrast-enhanced US examinations were performed by an author (M.F.M.) who started performing this technique in 2002. The time from diagnosis of breast cancer to last follow-up ranged from 12.0 to 297.3 months (median, 73.0 months; IQR, 43.0–116.2 months). The time between RF ablation and last follow-up ranged from 2.9 to 110.3 months (median, 19.1 months; IQR, 8.8–45.8 months). The time between diagnosis of liver disease and last follow-up ranged from 5.0 to 138.2 months (median, 37.2 months; IQR, 17.9–55.6 months). One patient was lost to follow-up and excluded from survival analysis.
Statistical Analysis
The Shapiro-Wilk test was used to test whether the distribution of quantitative variables was normal. If they were distributed normally, the results were summarized by using the mean and standard deviation. Otherwise, the median and IQR (25th and 75th percentiles) were used. Follow-up started on the date of RF ablation or the date liver metastases or breast cancer was diagnosed and ended on the date of event occurrence or the date on which the last available examination results were obtained (censored observations). The Kaplan-Meier method was used to describe overall survival. The log-rank test was used for univariate comparisons. Cox regression analysis was used to identify independent predictors (age, number of tumors, diameter of the largest metastasis, time between first hepatic metastasis and RF ablation, time between diagnosis of breast cancer and development of hepatic metastasis, and presence of other metastases outside of the liver) of events. Hazard ratios and 95% confidence intervals (CIs) were calculated. Variables with a P value of less than .2 at univariate analysis were included in multivariate models. Proportional hazard assumption was verified graphically and with a test based on Schoenfeld residuals; calibration and discrimination variables were assessed for model validation. All tests were two sided. P < .05 was considered to indicate a significant difference. All computations were made with Stata software (version 9; Stata, College Station, Tex).
Results
Local Tumor Control and Ablation Zones
Parameters for the RF ablations are listed in Table 2. Complete coverage of the tumor by the tumor coagulation area (technical success) was achieved after one treatment session in 83 (95%) of 87 tumors (Figs 1, 2). One of the four incompletely treated tumors was re-treated 7 days after the first RF ablation. Therefore, primary technique effectiveness was 97% (84 of 87 tumors). Mean diameter of necrosis, which was based on CT findings obtained the day after RF ablation, was 3.9 cm ± 1.5 (range, 1.0–7.0 cm).
Table 2.
RF Ablation Parameters
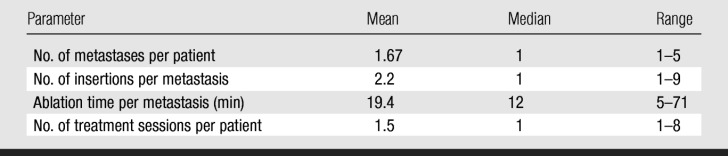
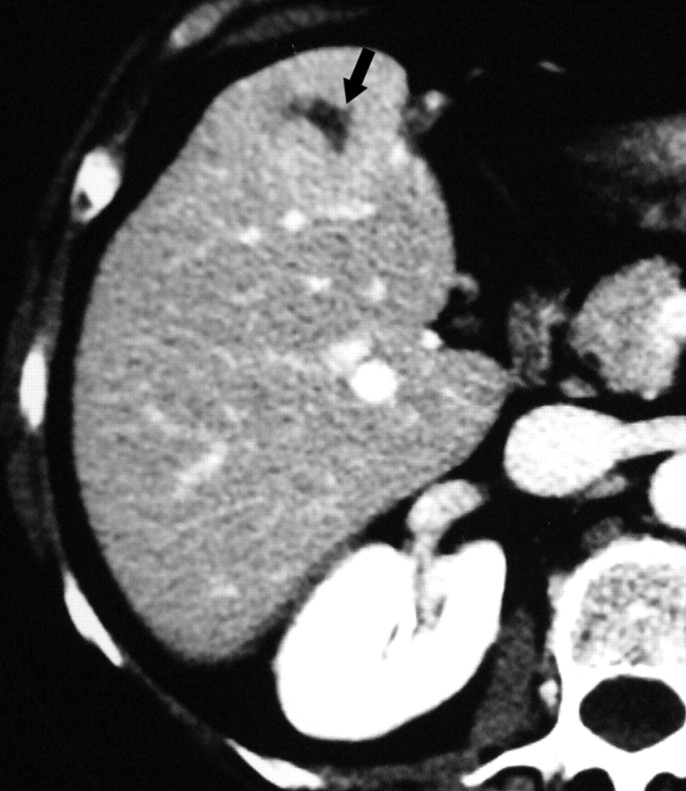
Figure 1a:
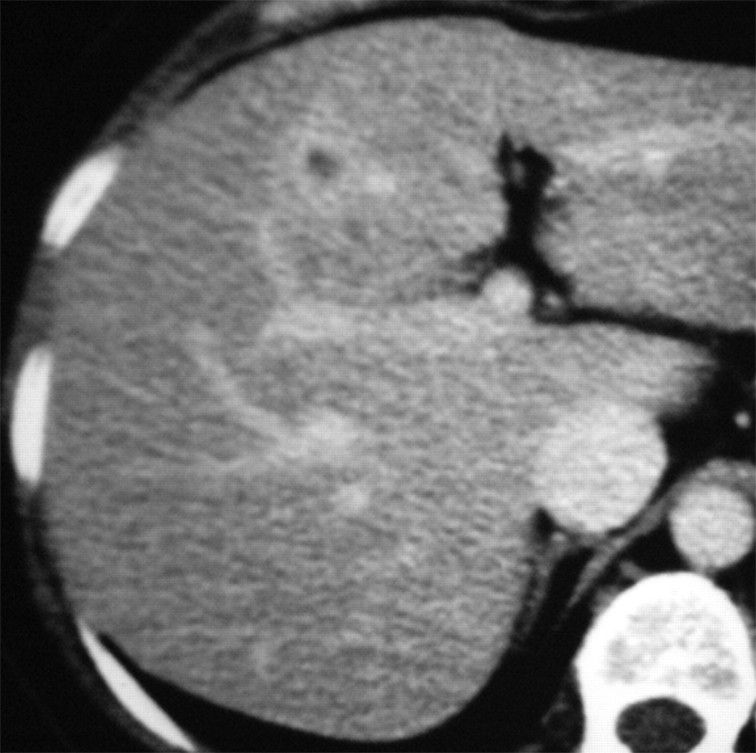
Images in a 57-year-old woman with a breast cancer liver metastasis located in segment V and treated with RF ablation with an internally cooled electrode needle. As of this writing, the patient has survived for 12 years. (a) Preablation portal venous phase CT image shows a 1.3-cm-diameter tumor (arrow) in the right anteroinferior segment. (b) Portal venous phase CT scan obtained the day after RF ablation shows a 2.9 × 3.7-cm nonperfused area (necrosis) at the tumor site. The ablation was considered complete. (c, d) CT images obtained at (c) 3- and (d) 6-year follow-up show progressive involution of coagulation.
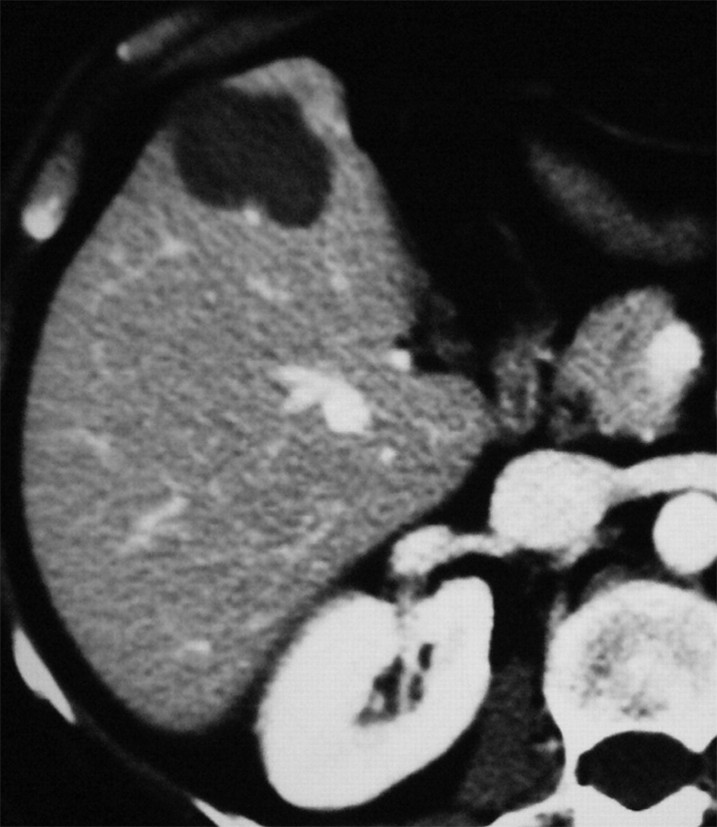
Figure 1b:
Images in a 57-year-old woman with a breast cancer liver metastasis located in segment V and treated with RF ablation with an internally cooled electrode needle. As of this writing, the patient has survived for 12 years. (a) Preablation portal venous phase CT image shows a 1.3-cm-diameter tumor (arrow) in the right anteroinferior segment. (b) Portal venous phase CT scan obtained the day after RF ablation shows a 2.9 × 3.7-cm nonperfused area (necrosis) at the tumor site. The ablation was considered complete. (c, d) CT images obtained at (c) 3- and (d) 6-year follow-up show progressive involution of coagulation.
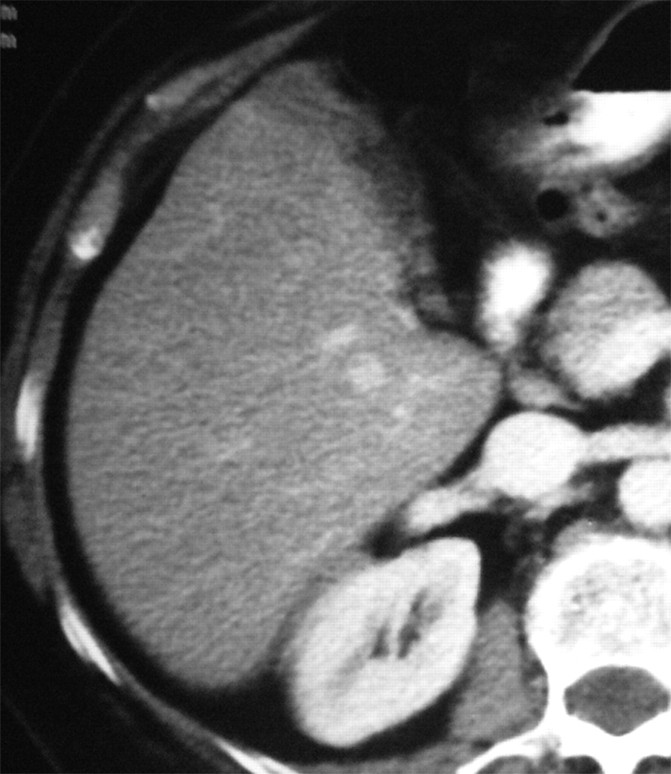
Figure 1c:
Images in a 57-year-old woman with a breast cancer liver metastasis located in segment V and treated with RF ablation with an internally cooled electrode needle. As of this writing, the patient has survived for 12 years. (a) Preablation portal venous phase CT image shows a 1.3-cm-diameter tumor (arrow) in the right anteroinferior segment. (b) Portal venous phase CT scan obtained the day after RF ablation shows a 2.9 × 3.7-cm nonperfused area (necrosis) at the tumor site. The ablation was considered complete. (c, d) CT images obtained at (c) 3- and (d) 6-year follow-up show progressive involution of coagulation.
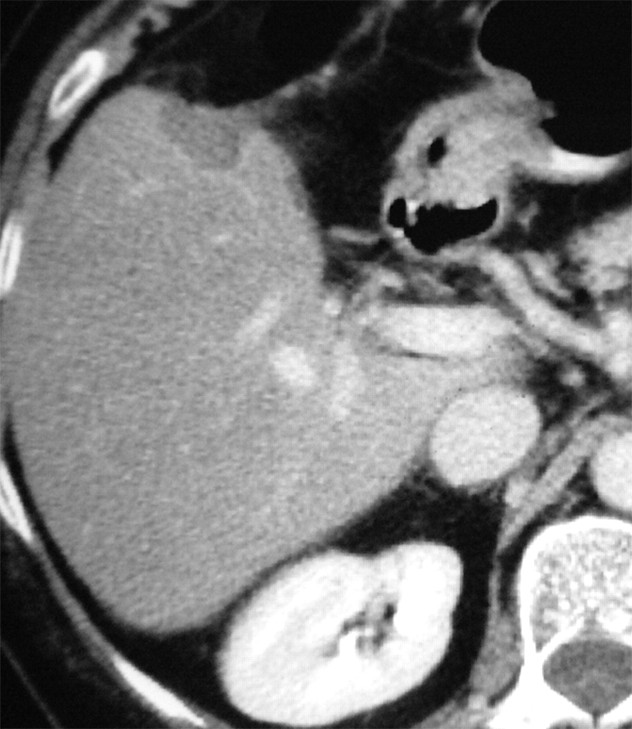
Figure 1d:
Images in a 57-year-old woman with a breast cancer liver metastasis located in segment V and treated with RF ablation with an internally cooled electrode needle. As of this writing, the patient has survived for 12 years. (a) Preablation portal venous phase CT image shows a 1.3-cm-diameter tumor (arrow) in the right anteroinferior segment. (b) Portal venous phase CT scan obtained the day after RF ablation shows a 2.9 × 3.7-cm nonperfused area (necrosis) at the tumor site. The ablation was considered complete. (c, d) CT images obtained at (c) 3- and (d) 6-year follow-up show progressive involution of coagulation.
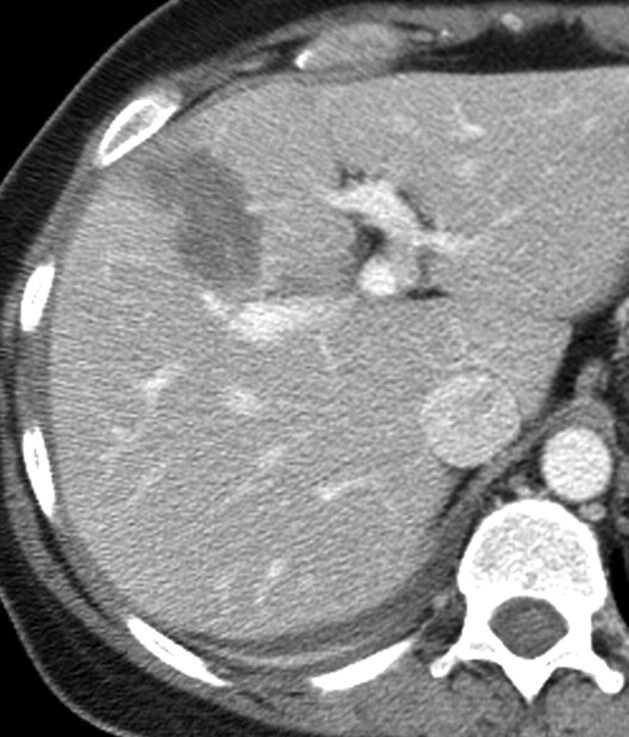
Figure 2a:
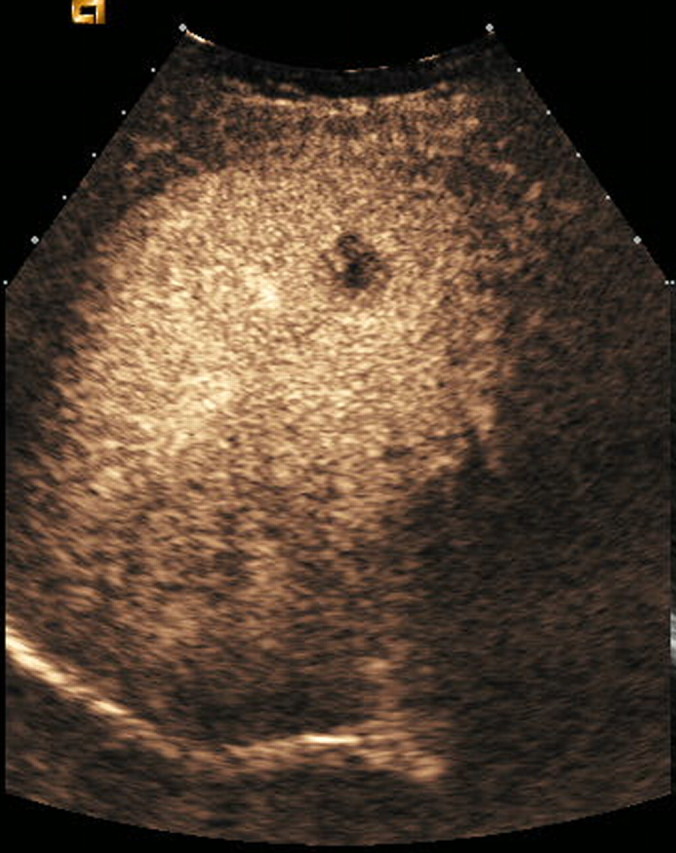
Contrast-enhanced US and CT images in a 45-year-old woman before and after RF ablation of a liver metastasis in segment IV. (a) Portal phase contrast-enhanced US image shows a 2-cm-diameter lesion (nonperfused black area). (b) Portal phase transverse CT image obtained before RF ablation enabled us to confirm contrast-enhanced US findings. This image shows a hypoattenuating metastasis with a hyperattenuating rim. (c, d) Portal phase contrast-enhanced (c) US and (d) CT images obtained the day after RF ablation show a large nonperfused area (4.0 × 2.6 cm) at the tumor site. Note the small right pleural effusion.
Figure 2b:
Contrast-enhanced US and CT images in a 45-year-old woman before and after RF ablation of a liver metastasis in segment IV. (a) Portal phase contrast-enhanced US image shows a 2-cm-diameter lesion (nonperfused black area). (b) Portal phase transverse CT image obtained before RF ablation enabled us to confirm contrast-enhanced US findings. This image shows a hypoattenuating metastasis with a hyperattenuating rim. (c, d) Portal phase contrast-enhanced (c) US and (d) CT images obtained the day after RF ablation show a large nonperfused area (4.0 × 2.6 cm) at the tumor site. Note the small right pleural effusion.
Figure 2c:
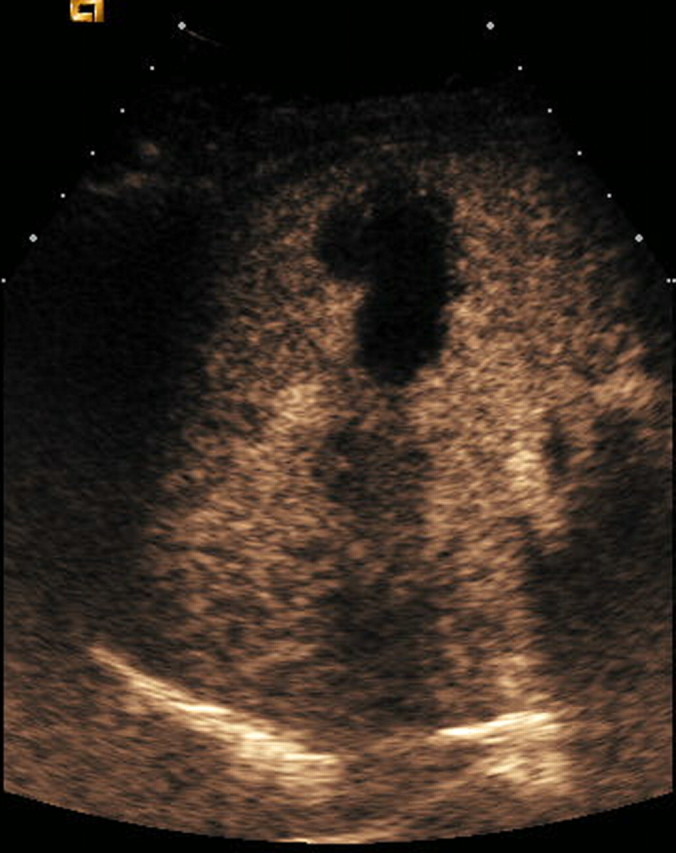
Contrast-enhanced US and CT images in a 45-year-old woman before and after RF ablation of a liver metastasis in segment IV. (a) Portal phase contrast-enhanced US image shows a 2-cm-diameter lesion (nonperfused black area). (b) Portal phase transverse CT image obtained before RF ablation enabled us to confirm contrast-enhanced US findings. This image shows a hypoattenuating metastasis with a hyperattenuating rim. (c, d) Portal phase contrast-enhanced (c) US and (d) CT images obtained the day after RF ablation show a large nonperfused area (4.0 × 2.6 cm) at the tumor site. Note the small right pleural effusion.
Figure 2d:
Contrast-enhanced US and CT images in a 45-year-old woman before and after RF ablation of a liver metastasis in segment IV. (a) Portal phase contrast-enhanced US image shows a 2-cm-diameter lesion (nonperfused black area). (b) Portal phase transverse CT image obtained before RF ablation enabled us to confirm contrast-enhanced US findings. This image shows a hypoattenuating metastasis with a hyperattenuating rim. (c, d) Portal phase contrast-enhanced (c) US and (d) CT images obtained the day after RF ablation show a large nonperfused area (4.0 × 2.6 cm) at the tumor site. Note the small right pleural effusion.
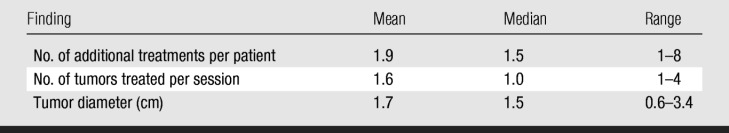
Local tumor progression of treated lesions was observed in 13 (25%) of the 51 patients (median time to detection, 4 months); therefore, the patient-based secondary technique effectiveness rate was 69% (35 of 51 patients). Twenty-seven (53%) patients developed new intrahepatic metastases during follow-up. Twenty-eight patients were not treated again, despite local tumor progression (n = 9) or new intrahepatic lesions (n = 19), as they developed extrahepatic metastases. Twelve patients underwent 25 subsequent RF treatment sessions for local tumor progression (n = 4) or new intrahepatic metastases (n = 8). Technique effectiveness was 75% (nine of 12 patients) for these subsequent ablations. Data about subsequent treatments are listed in Table 3.
Table 3.
Results of Subsequent Treatments
Follow-up and Survival
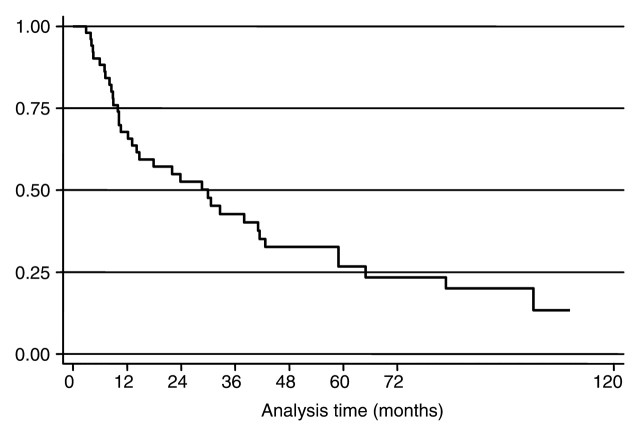
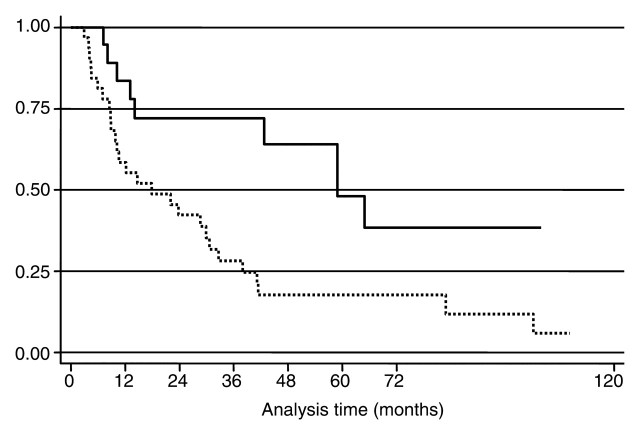
Overall median survival time from the time of the first RF ablation was 29.9 months (IQR, 9.9–64.9 months) (Fig 3). Median survival from the date of diagnosis of liver metastases was 42 months (IQR: 22.0–69.1 months). Overall median survival time from the date of diagnosis was calculated by using the date of breast surgery as a surrogate and was 84.5 months (IQR, 45.4–166.2 months). The overall 1-, 3-, and 5-year survival rates after the first RF ablation were 68% (95% CI: 53%, 79%), 43% (95% CI: 28%, 56%), and 27% (95% CI: 14%, 41%). The overall 1-, 3-, and 5-year survival rates after diagnosis of the first liver metastasis were 92% (95% CI: 80%, 97%), 60% (95% CI: 45%, 73%), and 32% (95% CI: 19%, 46%). As of this writing, 14 patients are alive and, of those, eight are disease free. The median follow-up time from diagnosis of breast cancer in the surviving patients was 94.5 months (IQR: 65.0–155.8 months). The only factor to demonstrate a prognostic value for survival from diagnosis of breast cancer, from diagnosis of first liver metastasis, and from the time of the first RF treatment was diameter of the metastasis, which was considered a continuous variable or used to divide patients into two groups, with a diameter of 2.5 cm serving as a cutoff (hazard ratio, 2.1; Fig 4). The period between breast surgery and detection of liver metastases, the presence of extrahepatic metastases, and the number of metastases did not significantly correlate with prognosis.
Figure 3:
Kaplan-Meier curve shows overall survival from the time of RF ablation. Censored data are shown. At 36 months, overall survival rate was 43%.
Figure 4:
Kaplan-Meier curves show survival from the time of RF ablation in patients with a maximum tumor diameter smaller than 2.5 cm (solid line) and those with a maximum tumor diameter of 2.5 cm or larger (dashed line). Survival was significantly longer in patients whose largest tumor diameter was smaller than 2.5 cm (log-rank test, P = .0077).
Complications
No deaths occurred as a result of the RF ablations. Two minor complications occurred in two (4%) patients: In one patient, an asymptomatic perirenal hematoma occurred. In the other, an asymptomatic infarct of the left lobe of the liver led to the formation of a biloma that was managed conservatively. Five (10%) patients developed a pleural effusion that was detected at imaging and did not require drainage or additional treatment. The following minor changes in blood test results were identified the day after the procedure: There was a transient increase in liver transaminase levels (in larger lesions, levels increased by a factor of up to four), a decrease in hemoglobin levels of approximately 1 g/L, and a decrease in the platelet count of about 10%. No tumor seeding occurred.
Discussion
Breast cancer is the most common cancer in women, and it is second to only lung cancer as the leading cause of cancer death in the United States. Unfortunately, breast cancer commonly metastasizes to the bones, lungs, and liver, at which point the cancer is considered a systemic disease and is associated with a poor prognosis. Importantly, liver metastases are present in the majority of patients; however, metastatic disease is confined to the liver in only 5%–18% of patients (3–5). Systemic chemotherapy is currently the standard treatment for liver metastases from breast cancer. Although a variety of chemotherapy regimens have been tried, median overall survival time for patients with metastatic breast cancer involving only the liver, regardless of the degree of hepatic involvement, is 22–27 months (5,7–10). Atalay et al (5) performed a retrospective analysis of two prospective randomized treatment trials in which researchers compared the survival rates of patients with breast cancer liver metastases that were treated with two different chemotherapy regimens. The median survival rates of the patients with only hepatic metastases were better than those of patients with multiple metastatic sites. Given the poor survival rates associated with the current treatments, there is increasing interest in the use of local therapies in conjunction with systemic therapy to improve the prognosis of patients with liver metastases from breast cancer.
The effectiveness of RF ablation in the treatment of primary liver tumors and colorectal liver metastases not amenable to surgery is well established (21,36–41). Several studies have shown that percutaneous RF ablation is safe and effective in the treatment of liver metastases from breast cancer (30,42,43). Livraghi et al (30) performed RF ablation in 24 patients with liver metastases from breast cancer and achieved complete necrosis in 59 of 64 lesions while experiencing only two minor complications. Despite the fact that 14 of the 24 patients developed new metastases (70% of which were hepatic), 23 patients were alive at the end of the study (median follow-up, 19 months). Furthermore, 10 of the 16 patients who had only liver metastases were disease-free at the end of the study. A recent study of 43 patients with characteristics similar to those of our study population and in whom RF ablation was performed with CT guidance revealed a median survival time of 58.6 months from the date of RF ablation (44).
The results of our study demonstrate that RF ablation can be used successfully in selected patients with liver metastases from breast cancer who have disease confined to the liver or who have stable extrahepatic disease to obtain local control of the disease. The local tumor progression rate (25%) was not negligible, and the number of patients who developed new intrahepatic tumors was high (53%); however, both findings were expected, given the natural course of the disease. Despite this fact, we achieved a median overall survival time of 42 months and a 5-year survival rate of 32% from the time of liver involvement in patients generally referred for RF ablation only after failure of multiple lines of chemotherapy. In addition, many of the patients seen early in this series received chemotherapy regimens now considered obsolete. The improved survival of patients in our study was likely due, in part, to the fact that RF ablation can be repeated if patients develop local tumor progression or new tumors. In addition, no treatment-related deaths or major complications were encountered, and the minor complication rate was 4%. As such, we believe that RF ablation should be considered a first-line therapy with which to achieve local control in patients with metastatic breast cancer confined to the liver and in patients whose extrahepatic disease is stable with systemic chemotherapy.
Other ablative therapies have been used to treat breast cancer liver metastases. To date, the largest study on the use of ablative therapies to treat breast cancer metastases was preformed by Mack et al (31), who treated 578 liver metastases from breast cancer in 232 female patients with magnetic resonance (MR)-guided laser thermotherapy. The exclusion criteria were the same as those adopted in this study. Mack et al achieved 3- and 5-year survival rates of 66% and 38%, respectively, from the time of treatment, with 1.5% of patients experiencing clinically relevant complications. In the subset of patients eligible for surgery, Mack et al reported a median survival time of 3.7 years from the diagnosis of the MR-guided laser thermotherapy–treated metastasis. When comparing the results of Mack et al with our results, it should be noted that Mack et al reported mean overall survival time whereas we report median overall survival time, which is more commonly used in survival studies. While their results are promising, MR-guided ablative therapy is more expensive than other techniques. RF ablation is generally performed with CT and/or US guidance, both of which are more readily accessible than open interventional MR imagers and do not require nonferromagnetic ablation devices.
Microwave ablation has also been used to treat breast cancer liver metastases. Abe et al (45) assessed the feasibility and efficacy of using MR-guided microwave thermocoagulation therapy in eight patients with liver metastasis from breast cancer, including patients with a maximum of five liver metastases smaller than 3 cm in diameter. Half of the patients had bone metastases, lung metastases, or both at enrollment. At the end of the study, after a mean observation period of 25.9 months, five (62%) of the eight patients were alive, and all of them had new metastases. No procedure-related deaths or major complications were encountered. While Abe et al found that MR-guided microwave ablation appeared to be a safe and feasible method for use in these patients, more experience is needed.
Interest in surgical resection of breast cancer liver metastases is also increasing. The results of several studies have shown prolonged survival in certain patients after curative resection (median survival time, 15–63 months) (17–19,46–52). Inclusion criteria are generally restricted to absence of extrahepatic disease or complete response of extrahepatic metastases (usually bone metastases) to systemic therapy, no brain metastases, normal performance status and liver function test results, and the possibility to perform curative resection. In two series, the authors attempted to identify prognostic factors for survival (17,18). The only significant factor identified was the time between primary breast surgery and the diagnosis of liver metastases, with a shorter interval being associated with a worse prognosis. However, the authors of a more recent series did not confirm this result (19). In the aforementioned surgical series (17,18), there was a high rate of new hepatic metastases during follow-up that ranged from 59% (50 of 85 patients) to 67% (12 of 18 patients) and was comparable with our results (53%) and expected, given the natural history of the disease. Adam et al (19) found that survival was worse when margins were macroscopically positive or small-volume disease remained in the remnant liver after resection. Adam et al concluded that surgery should be offered in combination with systemic therapy only to those patients with macroscopically resectable hepatic metastases. When compared with surgical resection, RF ablation is less invasive, less expensive, and has fewer contraindications. Moreover, since many patients will develop liver metastases after surgery, the test-of-time approach, which has already been proposed for colon cancer liver metastases (53,54), could be used in patients with breast cancer liver metastases. This approach would help avoid unnecessary surgery in patients who would develop new metastases.
Our study had several limitations. First, the long period of time over which the study was conducted can give rise to learning curve effects. Only patients examined after 2002 underwent contrast-enhanced US. This could have led to an improvement in the evaluation of technical effectiveness, local tumor progression, or both. However, we compared survival rates of patients treated before and after 2002 and found no significant difference. Another limitation was that no pathologic proof of liver metastases was obtained before treatment, except in one patient. Despite this limitation, the chance of having treated a benign lesion was low, as the radiologic appearance of metastases on CT and contrast-enhanced US images were typical. Moreover, the majority of the patients had tumors that were growing over time, despite medical treatment.
There were also some limitations related to chemotherapy. The study population was biased toward patients who had already received extensive chemotherapy, often up to third-line therapy. This leads to the subselection of patients with refractory disease. Another limitation related to chemotherapy was that early in our series, patients received drug regimens that were considered state-of-the-art at the time but would be considered outmoded by the standards of today. For example, early in our series, the hormone receptor status of many patients was not analyzed, and advanced histologic analysis was not performed. Therefore, there was not a consistent chemotherapy standard. In addition, several different RF ablation systems were used throughout the study; however, this did not appear to affect the technical success rate.
In conclusion, our results show that survival rates for percutaneous RF ablation in selected patients with breast cancer liver metastases, notably those with disease confined to the liver or with stable extrahepatic metastases, are comparable to those obtained with surgery or laser ablation and reported in the literature. Importantly, RF ablation is a safe technique that can be repeated if new hepatic metastases appear.
Advance in Knowledge.
-
•. Radiofrequency (RF) ablation of liver metastases from breast cancer in select patents results in intermediate- and long-term local control.
Implications for Patient Care.
-
•. RF ablation can be used successfully to obtain local control of the disease and improve survival in selected patients with liver metastases from breast cancer who have disease confined to the liver or who have stable extrahepatic disease.
-
•. RF ablation should be considered part of a multidisciplinary approach to the treatment of breast cancer liver metastases.
Acknowledgments
We thank Carlo Tinelli, MD, for his valuable contribution to the statistical analyses.
Received November 13, 2008; revision requested January 8, 2009; revision received April 5; accepted May 27; final version accepted June 15.
Authors stated no financial relationship to disclose.
Abbreviations:
- CI
- confidence interval
- IQR
- interquartile range
- RF
- radiofrequency
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin 2008;58:71–96. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol 2007;18:581–592. [DOI] [PubMed] [Google Scholar]
- 3.Hoe AL, Royle GT, Taylor I. Breast liver metastases: incidence, diagnosis and outcome. J R Soc Med 1991;84:714–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zinser JW, Hortobagyi GN, Buzdar AU, Smith TL, Fraschini G. Clinical course of breast cancer patients with liver metastases. J Clin Oncol 1987;5:773–782. [DOI] [PubMed] [Google Scholar]
- 5.Atalay G, Biganzoli L, Renard F, et al. Clinical outcome of breast cancer patients with liver metastases alone in the anthracycline-taxane era: a retrospective analysis of two prospective, randomised metastatic breast cancer trials. Eur J Cancer 2003;39:2439–2449. [DOI] [PubMed] [Google Scholar]
- 6.Elias D, Lasser P, Spielmann M, et al. Surgical and chemotherapeutic treatment of hepatic metastases from carcinoma of the breast. Surg Gynecol Obstet 1991;172:461–464. [PubMed] [Google Scholar]
- 7.Stockler M, Wilcken NR, Ghersi D, Simes RJ. Systematic reviews of chemotherapy and endocrine therapy in metastatic breast cancer. Cancer Treat Rev 2000;26:151–168. [DOI] [PubMed] [Google Scholar]
- 8.Miller KD, Sledge GW. The role of chemotherapy for metastatic breast cancer. Hematol Oncol Clin North Am 1999;13:415–434. [DOI] [PubMed] [Google Scholar]
- 9.Er O, Frye DK, Kau SC, et al. Clinical course of breast cancer patients with metastases limited to the liver treated with chemotherapy. Cancer J 2008;14:62–68. [DOI] [PubMed] [Google Scholar]
- 10.Pentheroudakis G, Fountzilas G, Bafaloukos D, et al. Metastatic breast cancer with liver metastases: a registry analysis of clinicopathologic, management and outcome characteristics of 500 women. Breast Cancer Res Treat 2006;97:237–244. [DOI] [PubMed] [Google Scholar]
- 11.Batist G, Harris L, Azarnia N, Lee LW, Daza-Ramirez P. Improved anti-tumor response rate with decreased cardiotoxicity of non-pegylated liposomal doxorubicin compared with conventional doxorubicin in first-line treatment of metastatic breast cancer in patients who had received prior adjuvant doxorubicin: results of a retrospective analysis. Anticancer Drugs 2006;17:587–595. [DOI] [PubMed] [Google Scholar]
- 12.Aloia TA, Vauthey J, Loyer EM, et al. Solitary colorectal liver metastasis: resection determines outcome. Arch Surg 2006;141:460–466. [DOI] [PubMed] [Google Scholar]
- 13.Curley SA.Outcomes after surgical treatment of colorectal cancer liver metastases. Semin Oncol 2005;32(6 suppl 9):S109–S111. [DOI] [PubMed] [Google Scholar]
- 14.Malafosse R, Penna C, Sa Cunha A, Nordlinger B. Surgical management of hepatic metastases from colorectal malignancies. Ann Oncol 2001;12:887–894. [DOI] [PubMed] [Google Scholar]
- 15.Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of indications for resection—registry of hepatic metastases. Surgery 1988;103:278–288. [PMC free article] [PubMed] [Google Scholar]
- 16.Schneebaum S, Walker MJ, Young D, Farrar WB, Minton JP. The regional treatment of liver metastases from breast cancer. J Surg Oncol 1994;55:26–31. [DOI] [PubMed] [Google Scholar]
- 17.Pocard M, Pouillart P, Asselain B, Falcou MC, Salmon RJ. Hepatic resection for breast cancer metastases: results and prognosis (65 cases). Ann Chir 2001;126:413–420. [DOI] [PubMed] [Google Scholar]
- 18.Selzner M, Morse MA, Vredenburgh JJ, Meyers WC, Clavien PA. Liver metastases from breast cancer: long-term survival after curative resection. Surgery 2000;127:383–389. [DOI] [PubMed] [Google Scholar]
- 19.Adam R, Aloia T, Krissat J, et al. Is liver resection justified for patients with hepatic metastases from breast cancer? Ann Surg 2006;244:897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gannon CJ, Curley SA. The role of focal liver ablation in the treatment of unresectable primary and secondary malignant liver tumors. Semin Radiat Oncol 2005;15:265–272. [DOI] [PubMed] [Google Scholar]
- 21.Livraghi T, Meloni F, Di Stasi M, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology 2008;47:82–89. [DOI] [PubMed] [Google Scholar]
- 22.Gillams AR.Image guided tumour ablation. Cancer Imaging 2005;5:103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lencioni R, Crocetti L, Cioni D, Della Pina C, Bartolozzi C. Percutaneous radiofrequency ablation of hepatic colorectal metastases: technique, indications, results, and new promises. Invest Radiol 2004;39:689–697. [DOI] [PubMed] [Google Scholar]
- 24.Winter TC, Laeseke PF, Lee FT. Focal tumor ablation: a new era in cancer therapy. Ultrasound Q 2006;22:195–217. [DOI] [PubMed] [Google Scholar]
- 25.Clark HP, Carson WF, Kavanagh PV, Ho CP, Shen P, Zagoria RJ. Staging and current treatment of hepatocellular carcinoma. RadioGraphics 2005;25(suppl 1):S3–S23. [DOI] [PubMed] [Google Scholar]
- 26.Al-Asfoor A, Fedorowicz Z, Lodge M. Resection versus no intervention or other surgical interventions for colorectal cancer liver metastases. Cochrane Database Syst Rev 2008;April 16;(2):CD006039. [DOI] [PubMed] [Google Scholar]
- 27.Garrean S, Hering J, Saied A, Helton WS, Espat NJ. Radiofrequency ablation of primary and metastatic liver tumors: a critical review of the literature. Am J Surg 2008;195:508–520. [DOI] [PubMed] [Google Scholar]
- 28.Gazelle GS, Hunink MGM, Kuntz KM, et al. Cost-effectiveness of hepatic metastasectomy in patients with metastatic colorectal carcinoma: a state-transition Monte Carlo decision analysis. Ann Surg 2003;237:544–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gazelle GS, McMahon PM, Beinfeld MT, Halpern EF, Weinstein MC. Metastatic colorectal carcinoma: cost-effectiveness of percutaneous radiofrequency ablation versus that of hepatic resection. Radiology 2004;233:729–739. [DOI] [PubMed] [Google Scholar]
- 30.Livraghi T, Goldberg SN, Solbiati L, Meloni F, Ierace T, Gazelle GS. Percutaneous radio-frequency ablation of liver metastases from breast cancer: initial experience in 24 patients. Radiology 2001;220:145–149. [DOI] [PubMed] [Google Scholar]
- 31.Mack MG, Straub R, Eichler K, Söllner O, Lehnert T, Vogl TJ. Breast cancer metastases in liver: laser-induced interstitial thermotherapy—local tumor control rate and survival data. Radiology 2004;233:400–409. [DOI] [PubMed] [Google Scholar]
- 32.Lu MD, Yu XL, Li AH, et al. Comparison of contrast enhanced ultrasound and contrast enhanced CT or MRI in monitoring percutaneous thermal ablation procedure in patients with hepatocellular carcinoma: a multi-center study in China. Ultrasound Med Biol 2007;33:1736–1749. [DOI] [PubMed] [Google Scholar]
- 33.Solbiati L, Ierace T, Tonolini M, Cova L. Guidance and monitoring of radiofrequency liver tumor ablation with contrast-enhanced ultrasound. Eur J Radiol 2004;51(suppl):S19–S23. [DOI] [PubMed] [Google Scholar]
- 34.Kim CK, Choi D, Lim HK, et al. Therapeutic response assessment of percutaneous radiofrequency ablation for hepatocellular carcinoma: utility of contrast-enhanced agent detection imaging. Eur J Radiol 2005;56:66–73. [DOI] [PubMed] [Google Scholar]
- 35.Meloni MF, Livraghi T, Filice C, Lazzaroni S, Calliada F, Perretti L. Radiofrequency ablation of liver tumors: the role of microbubble ultrasound contrast agents. Ultrasound Q 2006;22:41–47. [PubMed] [Google Scholar]
- 36.Poon RT.Is radiofrequency ablation the treatment of choice for patients with small hepatocellular carcinoma? Nat Clin Pract Gastroenterol Hepatol 2008;5:492–493. [DOI] [PubMed] [Google Scholar]
- 37.Gillams AR, Lees WR. Radiofrequency ablation of colorectal liver metastases. Abdom Imaging 2005;30:419–426. [DOI] [PubMed] [Google Scholar]
- 38.de Meijer VE, Ijzermans JN, Verhoef C. A place for radiofrequency ablation in the treatment of resectable colorectal liver metastases? Ann Surg Oncol 2008;15:2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pereira PL.Actual role of radiofrequency ablation of liver metastases. Eur Radiol 2007;17:2062–2070. [DOI] [PubMed] [Google Scholar]
- 40.Rossi S, Di Stasi M, Buscarini E, et al. Percutaneous RF interstitial thermal ablation in the treatment of hepatic cancer. AJR Am J Roentgenol 1996;167:759–768. [DOI] [PubMed] [Google Scholar]
- 41.Solbiati L, Livraghi T, Goldberg SN, et al. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology 2001;221:159–166. [DOI] [PubMed] [Google Scholar]
- 42.Gunabushanam G, Sharma S, Thulkar S, et al. Radiofrequency ablation of liver metastases from breast cancer: results in 14 patients. J Vasc Interv Radiol 2007;18:67–72. [DOI] [PubMed] [Google Scholar]
- 43.Sofocleous CT, Nascimento RG, Gonen M, et al. Radiofrequency ablation in the management of liver metastases from breast cancer. AJR Am J Roentgenol 2007;189:883–889. [DOI] [PubMed] [Google Scholar]
- 44.Jakobs TF, Hoffmann RT, Schrader A, et al. CT-guided radiofrequency ablation in patients with hepatic metastases from breast cancer. Cardiovasc Intervent Radiol 2009;32:38–46. [DOI] [PubMed] [Google Scholar]
- 45.Abe H, Kurumi Y, Naka S, et al. Open-configuration MR-guided microwave thermocoagulation therapy for metastatic liver tumors from breast cancer. Breast Cancer 2005;12:26–31. [DOI] [PubMed] [Google Scholar]
- 46.Vlastos G, Smith DL, Singletary SE, et al. Long-term survival after an aggressive surgical approach in patients with breast cancer hepatic metastases. Ann Surg Oncol 2004;11:869–874. [DOI] [PubMed] [Google Scholar]
- 47.Raab R, Nussbaum KT, Behrend M, Weimann A. Liver metastases of breast cancer: results of liver resection. Anticancer Res 1998;18:2231–2233. [PubMed] [Google Scholar]
- 48.Yoshimoto M, Tada T, Saito M, et al. Surgical treatment of hepatic metastases from breast cancer. Breast Cancer Res Treat 2000;59:177–184. [DOI] [PubMed] [Google Scholar]
- 49.Kondo S, Katoh H, Omi M, et al. Hepatectomy for metastases from breast cancer offers the survival benefit similar to that in hepatic metastases from colorectal cancer. Hepatogastroenterology 2000;47:1501–1503. [PubMed] [Google Scholar]
- 50.Caralt M, Bilbao I, Cortés J, et al. Hepatic resection for liver metastases as part of the “oncosurgical” treatment of metastatic breast cancer. Ann Surg Oncol 2008;15:2804–2810. [DOI] [PubMed] [Google Scholar]
- 51.d'Annibale M, Piovanello P, Cerasoli V, Campioni N. Liver metastases from breast cancer: the role of surgical treatment. Hepatogastroenterology 2005;52:1858–1862. [PubMed] [Google Scholar]
- 52.Lubrano J, Roman H, Tarrab S, Resch B, Marpeau L, Scotté M. Liver resection for breast cancer metastasis: does it improve survival? Surg Today 2008;38:293–299. [DOI] [PubMed] [Google Scholar]
- 53.Lambert LA, Colacchio TA, Barth RJ. Interval hepatic resection of colorectal metastases improves patient selection. Arch Surg 2000;135:473–479. [DOI] [PubMed] [Google Scholar]
- 54.Livraghi T, Solbiati L, Meloni F, Ierace T, Goldberg SN, Gazelle GS. Percutaneous radiofrequency ablation of liver metastases in potential candidates for resection: the “test-of-time approach”. Cancer 2003;97:3027–3035. [DOI] [PubMed] [Google Scholar]